989ced87916bd0bb2bb8ba8eeaecfc2e.ppt
- Количество слайдов: 42

Ethical aspects of biobanks and progress status of the Genome Project in Latvia Valdis Pīrāgs Seminar for the Research Ethics Committees of the Baltic countries Skolas iela 3, Riga May 9 -10, 2008

Background • Biomedical research utilising stored human biological materials is a powerful tool to improve human health and healthcare systems • The primary intention of research undertaken on human biological materials is to advance knowledge so that patients in general may benefit Recommendation on research on human biological materials, Council of Europe, 2005
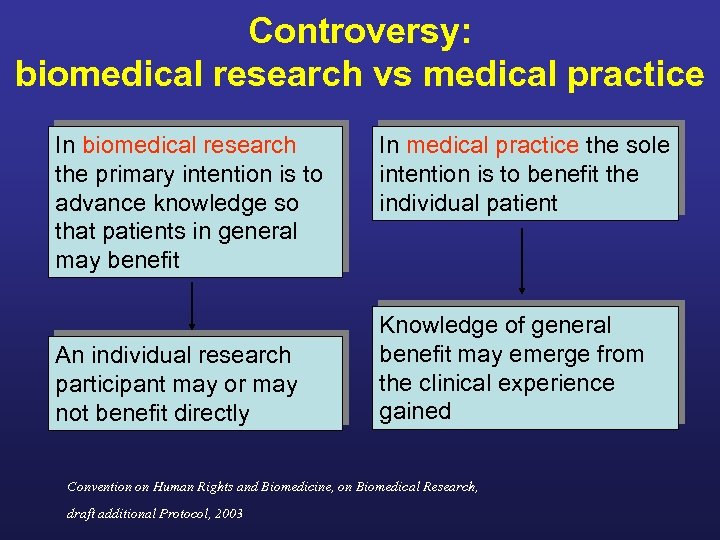
Controversy: biomedical research vs medical practice In biomedical research the primary intention is to advance knowledge so that patients in general may benefit An individual research participant may or may not benefit directly In medical practice the sole intention is to benefit the individual patient Knowledge of general benefit may emerge from the clinical experience gained Convention on Human Rights and Biomedicine, on Biomedical Research, draft additional Protocol, 2003

Ethical problem: How to make knowledge of general benefit gained from the biomedical research useful for every individual patient? Solution: Population based health and genome databases Risks: Social or psychological risks to the gene donors

1. Introduction to the population based Genome Projects

Development and transformation of the Latvian Genome Project 1. Initiative group for the human genome research in Latvia created (1999). First proposal of the Latvian Genome Project submitted to the government 2. Collaboration programme “Genetic Studies of Latvian Population, Application for Diagnosis and Prevention of Human Pathology” funded by the Latvian Council of Science (since 2001) 3. Pilot project “Genome Database of the Latvian Population” funded directly by the government (2003) 4. National Research Programme “Study of the main pathologies threatening the life expectancy and quality of the Latvian population” supervised by the Ministry of Education and Science (2006 -2009)
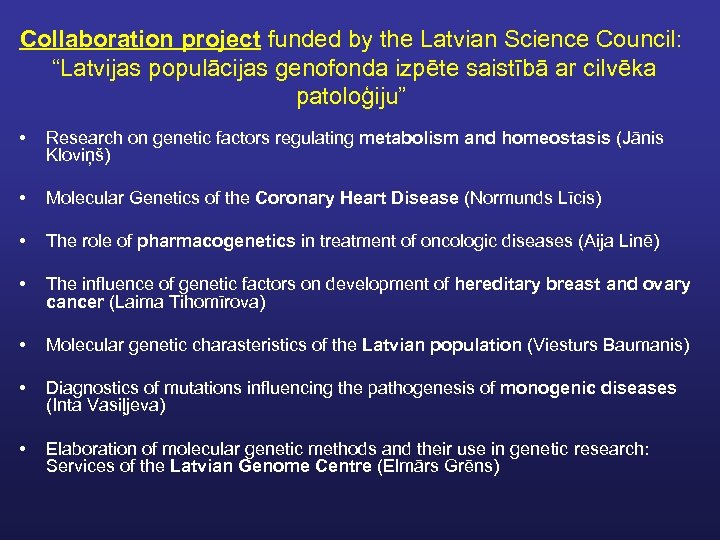
Collaboration project funded by the Latvian Science Council: “Latvijas populācijas genofonda izpēte saistībā ar cilvēka patoloģiju” • Research on genetic factors regulating metabolism and homeostasis (Jānis Kloviņš) • Molecular Genetics of the Coronary Heart Disease (Normunds Līcis) • The role of pharmacogenetics in treatment of oncologic diseases (Aija Linē) • The influence of genetic factors on development of hereditary breast and ovary cancer (Laima Tihomīrova) • Molecular genetic charasteristics of the Latvian population (Viesturs Baumanis) • Diagnostics of mutations influencing the pathogenesis of monogenic diseases (Inta Vasiļjeva) • Elaboration of molecular genetic methods and their use in genetic research: Services of the Latvian Genome Centre (Elmārs Grēns)

Genome Database of the Latvian Population – the main project • November 1, 2006. The Cabinet of Ministers authorized the Latvian Biomedical Research and Study Centre to create the Genome Database of the Latvian Population. • March 22, 2007. The Central Medical Ethics Committee approved the protocols and documents used by the Genome Database of the Latvian Population • September 20, 2007. The Latvian Genome Centre started to collect the samples and data in collaboration with the general practitioners and largest hospitals

Latvian Genome Database Recruitment Update: 5, 732 (May 2008)

National Research Programme in Medicine (2006 -2009) • Multidisciplinary research consortium on the main pathologies threatening the life expectancy and the quality of life of the Latvian Population • The recruitment target for the years 20082009 is 4, 000 randomly selected and carefully phenotyped individuals from all parts of Latvia
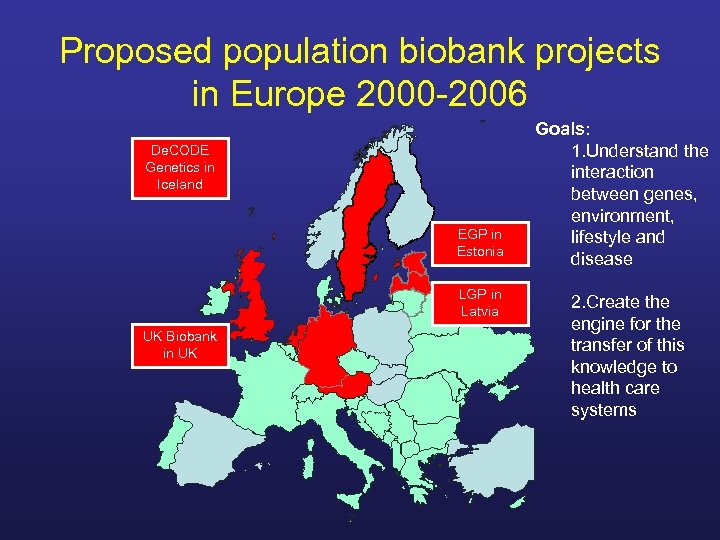
Proposed population biobank projects in Europe 2000 -2006 De. CODE Genetics in Iceland EGP in Estonia LGP in Latvia UK Biobank in UK Goals: 1. Understand the interaction between genes, environment, lifestyle and disease 2. Create the engine for the transfer of this knowledge to health care systems
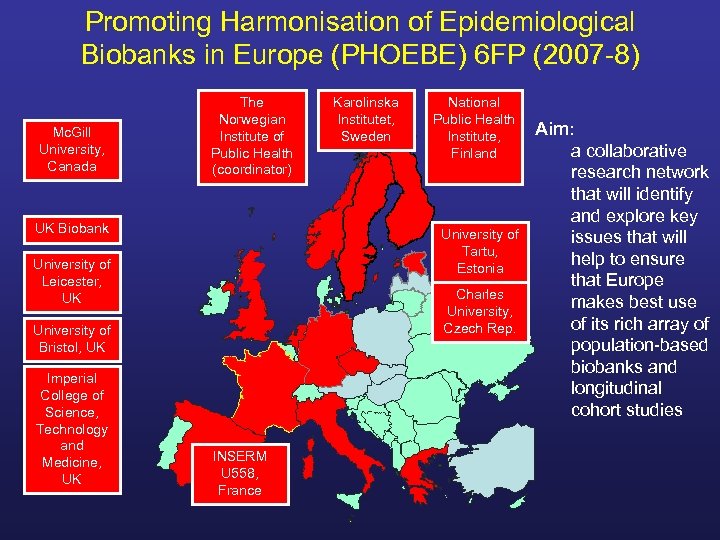
Promoting Harmonisation of Epidemiological Biobanks in Europe (PHOEBE) 6 FP (2007 -8) Mc. Gill University, Canada The Norwegian Institute of Public Health (coordinator) UK Biobank National Public Health Institute, Finland University of Tartu, Estonia University of Leicester, UK Charles University, Czech Rep. University of Bristol, UK Imperial College of Science, Technology and Medicine, UK Karolinska Institutet, Sweden INSERM U 558, France Aim: a collaborative research network that will identify and explore key issues that will help to ensure that Europe makes best use of its rich array of population-based biobanks and longitudinal cohort studies

Genome banks and networks in Europe (2008) • • • Biobanks for health in Norway http: //www. fhi. no/eway/default 0. asp? e=0&pi d=225 • Public Health Genomics (PHGEN) http: //www. phgen. nrw. de/typo 3/index. php • The German National Genome Research Network http: //www. ngfn. de/englisch/index. htm • UK Biobank http: //www. ukbiobank. ac. uk/ • Molecular Phenotyping to Accelerate Genomic Epidemiology Mol. PAGE http: //molpage. org/pubs. asp • European Network of Genomic and Genetic Epidemiology (ENGAGE) http: //www. euengage. org/science. html Genome Database of the Latvian Population http: //biomed. lu. lv/gene/ Estonian Genome Project Foundation http: //www. geenivaramu. ee/index. php? lang =eng&show=main • Genome. EUtwin http: //www. genomeutwin. org/ • Public Population Projects in Genomics (P 3 G) http: //www. p 3 gconsortium. org/ • P 3 G Observatory http: //www. p 3 gobservatory. org
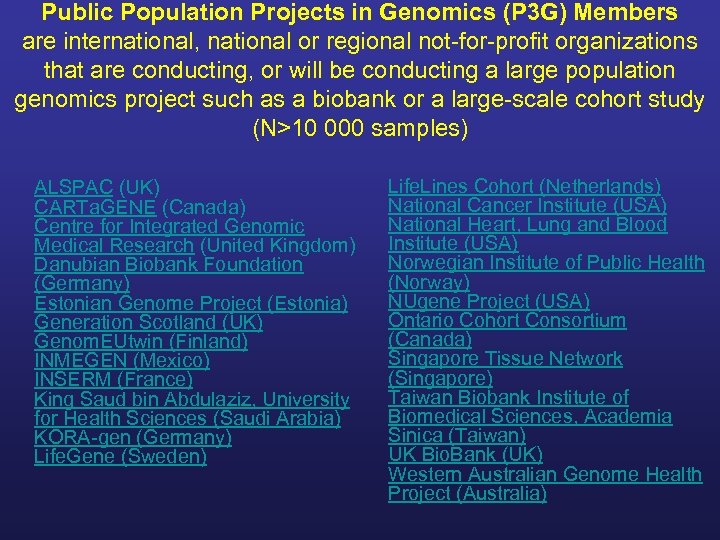
Public Population Projects in Genomics (P 3 G) Members are international, national or regional not-for-profit organizations that are conducting, or will be conducting a large population genomics project such as a biobank or a large-scale cohort study (N>10 000 samples) ALSPAC (UK) CARTa. GENE (Canada) Centre for Integrated Genomic Medical Research (United Kingdom) Danubian Biobank Foundation (Germany) Estonian Genome Project (Estonia) Generation Scotland (UK) Genom. EUtwin (Finland) INMEGEN (Mexico) INSERM (France) King Saud bin Abdulaziz, University for Health Sciences (Saudi Arabia) KORA-gen (Germany) Life. Gene (Sweden) Life. Lines Cohort (Netherlands) National Cancer Institute (USA) National Heart, Lung and Blood Institute (USA) Norwegian Institute of Public Health (Norway) NUgene Project (USA) Ontario Cohort Consortium (Canada) Singapore Tissue Network (Singapore) Taiwan Biobank Institute of Biomedical Sciences, Academia Sinica (Taiwan) UK Bio. Bank (UK) Western Australian Genome Health Project (Australia)

UK Biobank Recruitment Update: 114, 217 (9 pm Thursday 8 May 2008) • UK Biobank is a major UK medical research initiative, and a registered charity in its own right, with the aim of improving the prevention, diagnosis and treatment of a wide range of serious and life-threatening illnesses – including – – – cancer, heart diseases, diabetes, arthritis forms of dementia • UK Biobank is now recruiting 500, 000 people aged 40 -69 from across the country to take part in this project • UK Biobank Ethics and Governance Council

Estonian Genome Project Recruitment Update: 18, 141 (May 2008)
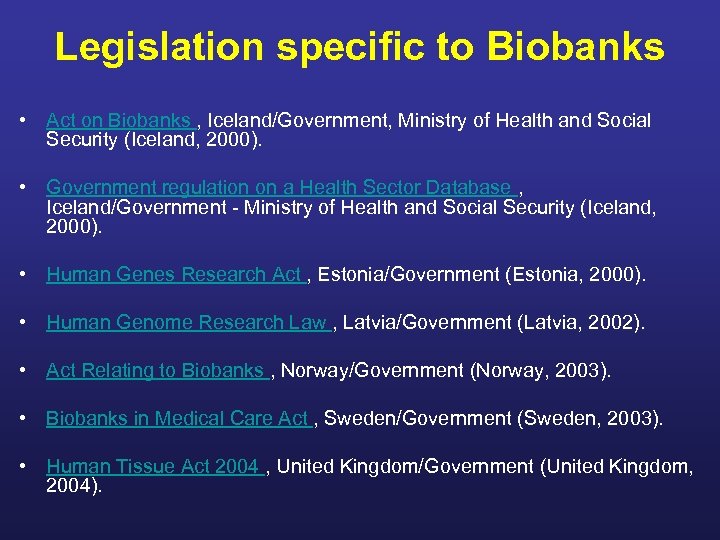
Legislation specific to Biobanks • Act on Biobanks , Iceland/Government, Ministry of Health and Social Security (Iceland, 2000). • Government regulation on a Health Sector Database , Iceland/Government - Ministry of Health and Social Security (Iceland, 2000). • Human Genes Research Act , Estonia/Government (Estonia, 2000). • Human Genome Research Law , Latvia/Government (Latvia, 2002). • Act Relating to Biobanks , Norway/Government (Norway, 2003). • Biobanks in Medical Care Act , Sweden/Government (Sweden, 2003). • Human Tissue Act 2004 , United Kingdom/Government (United Kingdom, 2004).

Selected guidelines and reports • Australian Law Reform Commission (2003): “Essentially Yours: The Protection of Human Genetic Information in Australia” - contains 144 recommandations of the commission • WHO's European Partnership on Patients' Rights and Citizens' Empowerment (2004): “Genetic databases: assessing the benefits and the impact on human and patient rights” - the ethical, social and legal issues that surround the creation and operation of databases containing human genetic material • Fonds de la recherche en santé du Québec (2006): “Governance Framework for Data Banks and Biobanks Used for Health Research” – the final report by the Advisory Group on the collection, processing, utilization, storage and management of data banks and biobanks for health research purposes • the Bioethics Advisory Committee, Singapore (2007): “Personal Information in Biomedical Research” - a report discussing the need to use personal information in biomedical research and makes recommendations aimed at establishing principles for data protection and confidentiality

Comparison of Latvian and Estonian Genome projects Latvian Genome Project Estonian Genome Project Main aim To create a national system of genomic and health information storage and data processing To establish a Gene Bank, that contains health and genetic data on the people of Estonia Legal basis The Human Genes Research Act passed by parliament in June 2002, amended in June 2003 The Human Genes Research Act passed by parliament in December 2000 Ownership The genome database is operated The gene bank belongs to the by the state scientific institution Estonian Genome Project authorized by the government Foundation and controlled by the Council of Genome Database
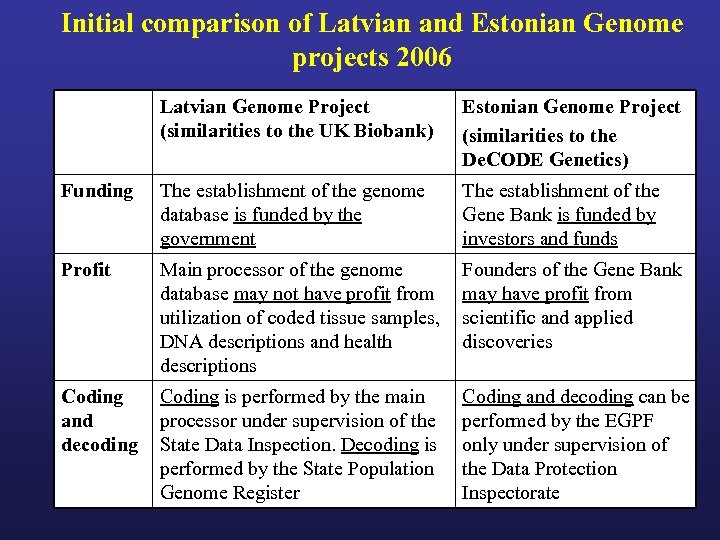
Initial comparison of Latvian and Estonian Genome projects 2006 Latvian Genome Project (similarities to the UK Biobank) Estonian Genome Project (similarities to the De. CODE Genetics) Funding The establishment of the genome database is funded by the government The establishment of the Gene Bank is funded by investors and funds Profit Main processor of the genome database may not have profit from utilization of coded tissue samples, DNA descriptions and health descriptions Founders of the Gene Bank may have profit from scientific and applied discoveries Coding and decoding Coding is performed by the main processor under supervision of the State Data Inspection. Decoding is performed by the State Population Genome Register Coding and decoding can be performed by the EGPF only under supervision of the Data Protection Inspectorate

2. Ethical aspects of population biobanks: - what is the best way of data protection? - ownership of biological materials and data

Long tradition of data collection: Latvian Folklore Repository has more than 1 500 000 entries, circa 400 000 folk songs, including 4 000 “naughty” dainas

More than 5 000 DNA samples and associated data (including potentially sensitive data) are filed in the Latvian Genome Data Base in 2008
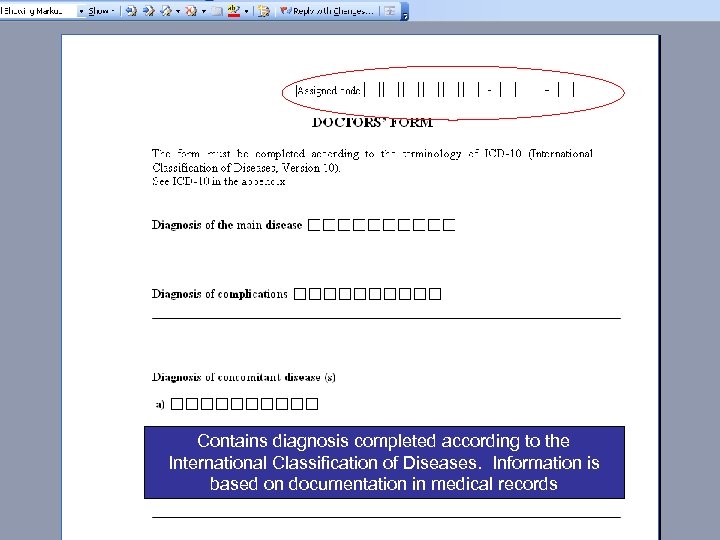
Contains diagnosis completed according to the International Classification of Diseases. Information is based on documentation in medical records

Contains 45 questions including information on ethnicity, life stile, family, anthropometry, disease status (more detailed on cancer, cardiovascular and endocrine diseases)

Identified data in the Latvian Folklore Repository No. 34, 343 received from R. Bērziņš living in Džūkste

Identifiability of biological materials and associated data • Biological materials and associated data should be anonymised as far as appropriate to the research activities concerned. • Identifiable materials and data should not be utilised if there are less intrusive means to reach similar results. • Where there are identifiers, it is necessary to have a well -developed framework of protections to ensure that the risks to sources of human biological materials and data are minimised. • However, it must be kept in mind that in some cases, unlinked anonymisation might not be appropriate in the interests of the persons concerned or for scientific reasons. Recommendation on research on human biological materials, Council of Europe, 2005

Identifiability of biological materials and associated data • Any use of biological materials and associated data in an identified, coded, or linked anonymised form should be justified by the researcher • Ethics committees should ask researchers proposing to use unlinked anonymised biological materials to justify this choice in terms of the inevitable consequence that it means that there will be no possibility of giving health-related feedback resulting from the research to persons concerned Recommendation on research on human biological materials, Council of Europe, 2005

Organisation for Economic Cooperation and Development DRAFT GUIDELINES FOR HUMAN BIOBANKS AND GENETIC RESEARCH DATABASES 2008

The aim of the Guidelines • to provide guidance on the establishment, governance, management and use of Human Biobanks and Genetic Research Databases (HBGRDs) used for purposes of genetic research.

1. HBGRDs Generally • The objective of a HBGRD should be to foster research within legal norms and ethical principles. . • The HBGRD should consider and minimise risks to individuals, their families and potentially identifiable populations or groups whose specimens and data are included in the HBGRD and used for research.

2. Establishment of HBGRDs • The purpose(s), both current and future, of the HBGRD should be clearly formulated, and communicated as early and as widely as possible, especially to potential participants and potential users. • In the establishment of the HBGRD, the initiators should carry out consultations with stakeholders and the general public. • The HBGRD should develop a business plan, including a financial model, that it intends to adopt over its lifespan in order to ensure its sustainability. • The HBGRD should be explicit and transparent about the nature and source of its financing/funding.

3. Governance, Management, and Oversight • The HBGRD should be governed by the principles of transparency and accountability. • The initiators of the HBGRD should clearly formulate the governance structure and management responsibilities applicable to the HBGRD and should make available information to participants, stakeholders and the general public. • The governance structure should ensure that the rights and well-being of the participant prevail over the research interests of the initiators and users of the HBGRD. • Within its governance structure, the HBGRD should have a mechanism to review applications for access to the human biological materials and/or data

4. Terms of Participation • HBGRDs should obtain prior, free and informed consent from each participant. Where applicable, HBGRDs should provide for obtaining consent or authorisation from the appropriate substitute decision-maker. • HBGRDs should give careful consideration to any special issues related to the participation of vulnerable populations or groups, including children, individuals with impaired decisionmaking capacity, and prisoners. • During the informed consent process, HBGRDs should provide potential participants with sufficient information on the nature, implications, foreseeable risks and benefits of their participation, so that they can realistically assess the implications of their participation and can make an informed decision on whether to participate. This information should be presented so as to not constitute an improper inducement to participate in the research.

4. Terms of Participation • HBGRDs should have a clearly articulated policy on the nature of the feedback that will be provided to participants, taking into account any domestic legal requirements. This policy should cover the feedback of individual-level results, if any, as well as aggregate results arising from research carried out using human biological materials and/or data from the HBGRD. • Participants should be provided with the opportunity to decide on whether or not to receive feedback of individual-level results arising from research. • As a general rule, non-validated results from scientific research using a HBGRDs’ human biological materials and data should not be reported back to the participants and this should be explained to participants during the consent process. • Where a HBGRD has offered and the participant has elected to receive feedback of individual-level results, and depending on the nature of the feedback, it may be appropriate for a trained professional to provide this feedback to participants or for counselling to be available to participants. • HBGRDs should have a clearly articulated policy on whether participants will be re-contacted during the course of the HBGRD’s existence, the situations for which re-contact will be permitted, and the conditions that will govern recontact.

5. Contents of HBGRDs • HBGRDs should have a clearly articulated policy and should communicate to potential participants which human biological materials and data will be collected from them or from other sources, stored and used for research purposes. • HBGRDs should have a clearly articulated policy on whether data will be accessed from health or other records, independently assembled, and whether or not these data will be linked with or stored in the HBGRD. Such a policy should also address the issue of secondary use of health and other records, especially when combined with other data. Where HBGRDs intend to access data from health or other records, it should ensure that participants are duly informed and that their informed consent for accessing such records is obtained.

6. Protection of Human Biological Materials and Data • HBGRDs should be established, managed and governed in such a way as to prevent any inappropriate or unauthorised uses of participants’ human biological materials and data. • HBGRDs should establish policies and procedures for the protection of the human biological materials and data, especially those potentially permitting, whether directly or indirectly, the identification of the participant. • The HBGRD should ensure that the data and information contained within its databases should be protected in accordance with applicable domestic law, especially in regard to the protection of privacy.

7. Access • • • HBGRDs should develop clear policies and procedures for accessing human biological materials and data in their databases, which should be based on objective and clearly articulated criteria. Access and use of human biological material and data should be consistent with the terms of participation and should respect the privacy of the participant and confidentiality of the human biological materials and data. HBGRDs should provide participants with explicit information on whether or not their human biological materials and data, in whole or in part, will be made accessible to third parties for non-research purposes. The HBGRD should not make accessible or disclose participants’ human biological materials or data obtained for health research purposes to third parties for non-research purposes, including to insurance providers, employers, or to law enforcement agencies, except where required by law. The informed consent document should be transparent and explicit about any legal requirements to share human biological materials or data with third parties. Unless strictly necessary, researchers should be provided access only to human biological materials, data or information that is coded such that the participant cannot be identified and researchers should be required to not attempt to reidentify participants. Such exceptional conditions should be clearly disclosed to research participants during the informed consent process.

7. Access • 7. F HBGRDs should have a clearly articulated policy on whether researchers using its database(s) will be allowed to contact participants directly. • 7. G Given the potentially finite nature of human biological materials, HBGRDs should formulate criteria for prioritising applications for access to the human biological materials. • 7. H The terms of access for researchers to the whole or a part of the database(s) of an HBGRD should be set out in an access agreement. • 7. I Where a HBGRD intends to provide access to the specimens and samples collected from participants, they should develop a material transfer agreement or other agreement appropriate for that purpose. • 7. J HBGRDs should only transfer specimens and data when there adequate standards in place regarding privacy of the participant and confidentiality of the data, safety and good laboratory methods and in accordance with applicable law and regulations.

8. Qualification, Education and Training • The HBGRD should ensure that all of its personnel are knowledgeable about its goals and mission. • The managing person(s) of the HBGRD should be qualified by training and experience to carry out its mandate. • The managing person of the HBGRD should ensure that personnel have the appropriate professional qualifications that meet recognised standards, underpinned by experience, education and training and are assigned responsibilities commensurate with their capabilities. • The managing person(s) of the HBGRD should develop and implement employee training programmes.

9. Custodianship, Benefitsharing and Intellectual Property • HBGRDs should have a clearly articulated policy on whether participants retain any rights in the human biological materials and data, and the nature of these rights. • Where the HBGRD intends to retain rights to the participant’s human biological materials and/or data, it should have a clearly articulated policy, which should be explicitly indicated to the participant and should be included in the consent document(s). Such policy should be consistent with applicable law, and regulatory and ethical best practices. • Benefits arising from research using the HBGRDs resources should be shared as broadly as possible. Benefits may be shared in different ways including the sharing of information, licensing, or transferring of technology or materials.

10. Demise of the HBGRD and Disposal of Materials and Data • The HBGRD should plan for an unforeseen demise, such as the end of its funding. • The initiators of a HBGRD should consider a possible end date for this endeavour. • In accordance with applicable law, a HBGRD should have a detailed policy setting out the manner in which the human biological materials and data that it holds will be dealt with in the event of its demise. • Once a HBGRD is no longer required or is no longer of scientific value, the human biological materials and data should be disposed of in an appropriate way, consistent with the principles of consent and privacy.
989ced87916bd0bb2bb8ba8eeaecfc2e.ppt