b0cfd50ee098f29ead5fdfc04d5a21d3.ppt
- Количество слайдов: 27
 ession 2: Flow Chemistry Chris Rayner
ession 2: Flow Chemistry Chris Rayner
 Flow Chemistry within i. PRD Presentation focuses on: § Track record/in house expertise (highlights) § Current projects § Future perspectives/targets Discussion welcomed on: § Comments/suggestions on project portfolio § Identification of interested partners/ consortia for collaboration, application or information exchange
Flow Chemistry within i. PRD Presentation focuses on: § Track record/in house expertise (highlights) § Current projects § Future perspectives/targets Discussion welcomed on: § Comments/suggestions on project portfolio § Identification of interested partners/ consortia for collaboration, application or information exchange
 Flow Chemistry within i. PRD § Continuous Synthetic Chemistry § Thermolytic reactions § Hazardous reagents § Photochemistry § Equipment and modelling § Rotating tube and spinning disc reactors § Reactor and Reaction Modelling § Microchannel reactors § In-process analytics § Supercritical fluids § Continuous reactions and product isolation
Flow Chemistry within i. PRD § Continuous Synthetic Chemistry § Thermolytic reactions § Hazardous reagents § Photochemistry § Equipment and modelling § Rotating tube and spinning disc reactors § Reactor and Reaction Modelling § Microchannel reactors § In-process analytics § Supercritical fluids § Continuous reactions and product isolation
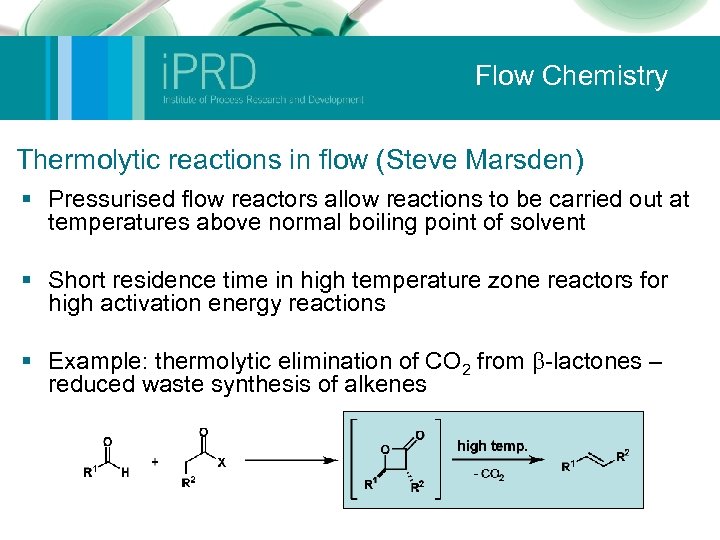 Flow Chemistry Thermolytic reactions in flow (Steve Marsden) § Pressurised flow reactors allow reactions to be carried out at temperatures above normal boiling point of solvent § Short residence time in high temperature zone reactors for high activation energy reactions § Example: thermolytic elimination of CO 2 from b-lactones – reduced waste synthesis of alkenes
Flow Chemistry Thermolytic reactions in flow (Steve Marsden) § Pressurised flow reactors allow reactions to be carried out at temperatures above normal boiling point of solvent § Short residence time in high temperature zone reactors for high activation energy reactions § Example: thermolytic elimination of CO 2 from b-lactones – reduced waste synthesis of alkenes
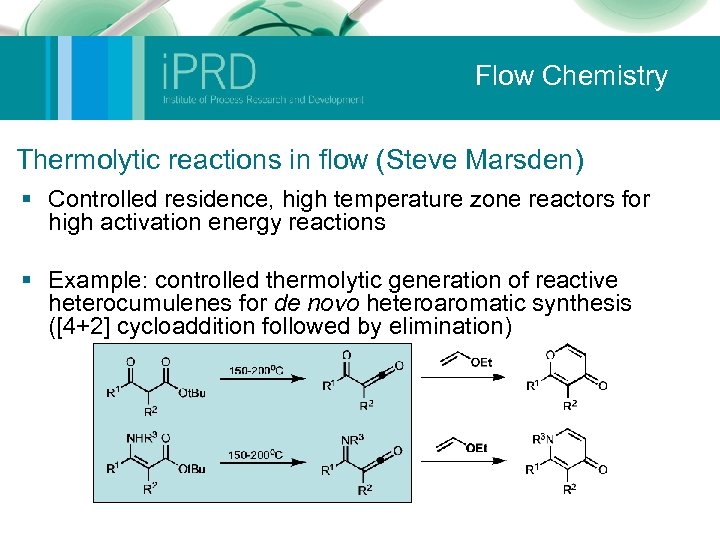 Flow Chemistry Thermolytic reactions in flow (Steve Marsden) § Controlled residence, high temperature zone reactors for high activation energy reactions § Example: controlled thermolytic generation of reactive heterocumulenes for de novo heteroaromatic synthesis ([4+2] cycloaddition followed by elimination)
Flow Chemistry Thermolytic reactions in flow (Steve Marsden) § Controlled residence, high temperature zone reactors for high activation energy reactions § Example: controlled thermolytic generation of reactive heterocumulenes for de novo heteroaromatic synthesis ([4+2] cycloaddition followed by elimination)
 Flow Chemistry Hazardous reactions (Rob Hammond) § Use of hazardous reagents particularly problematic in large scale batch processes § Azide and cyanide widely used in synthetic chemistry for heterocycle synthesis, introduction of N-functionality, C-1 synthon etc. § Flow techniques required § Hazardous scale-up, particularly if require parent acids which are highly toxic, volatile and potentially explosive § Use of flow reactor to generate HCN or HN 3 in small quantities under highly controlled conditions § Objective is to maximise safety and efficiency aspects in flow reactor § Long term goal is to develop a reaction system that can allow large scale synthesis of intermediates using hazardous reagents
Flow Chemistry Hazardous reactions (Rob Hammond) § Use of hazardous reagents particularly problematic in large scale batch processes § Azide and cyanide widely used in synthetic chemistry for heterocycle synthesis, introduction of N-functionality, C-1 synthon etc. § Flow techniques required § Hazardous scale-up, particularly if require parent acids which are highly toxic, volatile and potentially explosive § Use of flow reactor to generate HCN or HN 3 in small quantities under highly controlled conditions § Objective is to maximise safety and efficiency aspects in flow reactor § Long term goal is to develop a reaction system that can allow large scale synthesis of intermediates using hazardous reagents
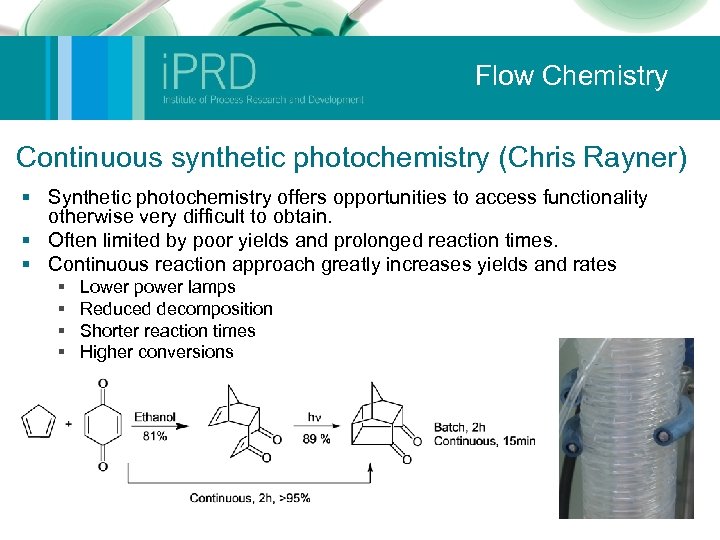 Flow Chemistry Continuous synthetic photochemistry (Chris Rayner) § Synthetic photochemistry offers opportunities to access functionality otherwise very difficult to obtain. § Often limited by poor yields and prolonged reaction times. § Continuous reaction approach greatly increases yields and rates § § Lower power lamps Reduced decomposition Shorter reaction times Higher conversions
Flow Chemistry Continuous synthetic photochemistry (Chris Rayner) § Synthetic photochemistry offers opportunities to access functionality otherwise very difficult to obtain. § Often limited by poor yields and prolonged reaction times. § Continuous reaction approach greatly increases yields and rates § § Lower power lamps Reduced decomposition Shorter reaction times Higher conversions
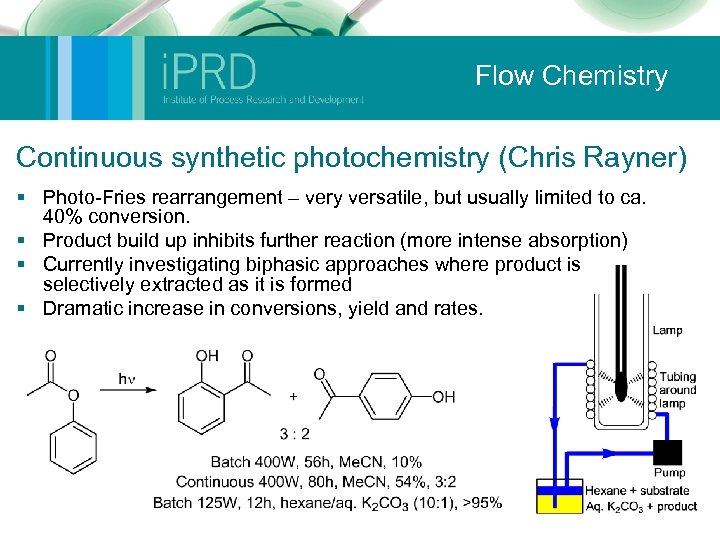 Flow Chemistry Continuous synthetic photochemistry (Chris Rayner) § Photo-Fries rearrangement – very versatile, but usually limited to ca. 40% conversion. § Product build up inhibits further reaction (more intense absorption) § Currently investigating biphasic approaches where product is selectively extracted as it is formed § Dramatic increase in conversions, yield and rates.
Flow Chemistry Continuous synthetic photochemistry (Chris Rayner) § Photo-Fries rearrangement – very versatile, but usually limited to ca. 40% conversion. § Product build up inhibits further reaction (more intense absorption) § Currently investigating biphasic approaches where product is selectively extracted as it is formed § Dramatic increase in conversions, yield and rates.
 Flow Chemistry New continuous reactors (Chris Rayner/Roshan Jachuck) § § § Opportunity for new reactor designs Rotating tube reactors (Roshan Jachuck, Clarkson, NY) Residence time of seconds to several minutes (or batch) Highly sheered films (100 -200 microns) Immiscible liquids of different densities (e. g. water/organic) form 2 independent micron scale layers § Ideal for biphasic photochemistry and other two phase reactions § Not prone to blocking; also good for reactions involving gases
Flow Chemistry New continuous reactors (Chris Rayner/Roshan Jachuck) § § § Opportunity for new reactor designs Rotating tube reactors (Roshan Jachuck, Clarkson, NY) Residence time of seconds to several minutes (or batch) Highly sheered films (100 -200 microns) Immiscible liquids of different densities (e. g. water/organic) form 2 independent micron scale layers § Ideal for biphasic photochemistry and other two phase reactions § Not prone to blocking; also good for reactions involving gases
 Flow Chemistry Reaction modelling (Annette Taylor) § Fundamental kinetic and thermodynamic analysis of reaction processes in complex systems (practical and theoretical) § Modelling of features generic to any reaction containing feedback through autocatalysis or heat (thermal runaway) § Examples of feedback in organic chemistry include: § Addition of dialkylzinc reagents to pyrimidinecarbaldehyde (the Soai reaction) § Formaldehyde-sulfite addition § Polymerisations (e. g. vinyl acetate) § Continuous photochemistry (e. g. [2+2] cycloadditions) § Mechanistic studies on CO 2 capture and release (CCS)
Flow Chemistry Reaction modelling (Annette Taylor) § Fundamental kinetic and thermodynamic analysis of reaction processes in complex systems (practical and theoretical) § Modelling of features generic to any reaction containing feedback through autocatalysis or heat (thermal runaway) § Examples of feedback in organic chemistry include: § Addition of dialkylzinc reagents to pyrimidinecarbaldehyde (the Soai reaction) § Formaldehyde-sulfite addition § Polymerisations (e. g. vinyl acetate) § Continuous photochemistry (e. g. [2+2] cycloadditions) § Mechanistic studies on CO 2 capture and release (CCS)
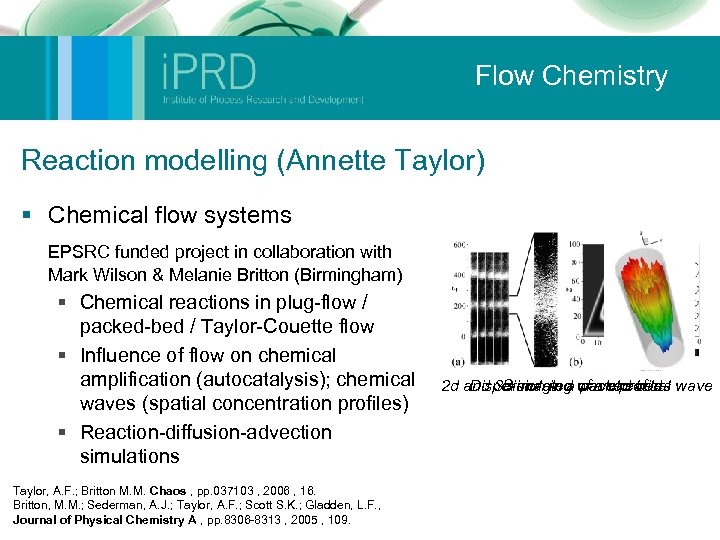 Flow Chemistry Reaction modelling (Annette Taylor) § Chemical flow systems EPSRC funded project in collaboration with Mark Wilson & Melanie Britton (Birmingham) § Chemical reactions in plug-flow / packed-bed / Taylor-Couette flow § Influence of flow on chemical amplification (autocatalysis); chemical waves (spatial concentration profiles) § Reaction-diffusion-advection simulations Taylor, A. F. ; Britton M. M. Chaos , pp. 037103 , 2006 , 16. Britton, M. M. ; Sederman, A. J. ; Taylor, A. F. ; Scott S. K. ; Gladden, L. F. , Journal of Physical Chemistry A , pp. 8306 -8313 , 2005 , 109. 2 d and 3 d imaging of a chemical wave Simulated packed bed Dispersion in a wave profiles
Flow Chemistry Reaction modelling (Annette Taylor) § Chemical flow systems EPSRC funded project in collaboration with Mark Wilson & Melanie Britton (Birmingham) § Chemical reactions in plug-flow / packed-bed / Taylor-Couette flow § Influence of flow on chemical amplification (autocatalysis); chemical waves (spatial concentration profiles) § Reaction-diffusion-advection simulations Taylor, A. F. ; Britton M. M. Chaos , pp. 037103 , 2006 , 16. Britton, M. M. ; Sederman, A. J. ; Taylor, A. F. ; Scott S. K. ; Gladden, L. F. , Journal of Physical Chemistry A , pp. 8306 -8313 , 2005 , 109. 2 d and 3 d imaging of a chemical wave Simulated packed bed Dispersion in a wave profiles
 Flow Chemistry Microfluidics (Nik Kapur and Mark Wilson) Micromixing for reaction: The aim here was to use computational methods to develop a mixing device for a specific set of flow conditions. The device is currently being manufactured. Mixing by chaotic advection: This was a study of a small geometry where oscillation of the free surface (one roll speed is perturbed) causes mixing of the fluid. Shown on the movie are small tracer particles within the flow illustrating the mixing. The experiments on the left provide validation. Ambient fluid capture: The following slide shows how, using the control of eddies within flow, particles or reagents can be trapped. Autocatalytic reaction: This slide illustrates a simple autocatalytic scheme within the same geometry. Its possible to build a series of reactions into these models to couple flow and kinetics. 2 Phase droplet: An illustration of a 2 -phase simulation similar to that found within a microemulsion device. This sort of method can be used to optimise geometric and flow conditions for robust droplet formation. The group also has considerable experience in experiments in this area (2 -phase flow).
Flow Chemistry Microfluidics (Nik Kapur and Mark Wilson) Micromixing for reaction: The aim here was to use computational methods to develop a mixing device for a specific set of flow conditions. The device is currently being manufactured. Mixing by chaotic advection: This was a study of a small geometry where oscillation of the free surface (one roll speed is perturbed) causes mixing of the fluid. Shown on the movie are small tracer particles within the flow illustrating the mixing. The experiments on the left provide validation. Ambient fluid capture: The following slide shows how, using the control of eddies within flow, particles or reagents can be trapped. Autocatalytic reaction: This slide illustrates a simple autocatalytic scheme within the same geometry. Its possible to build a series of reactions into these models to couple flow and kinetics. 2 Phase droplet: An illustration of a 2 -phase simulation similar to that found within a microemulsion device. This sort of method can be used to optimise geometric and flow conditions for robust droplet formation. The group also has considerable experience in experiments in this area (2 -phase flow).
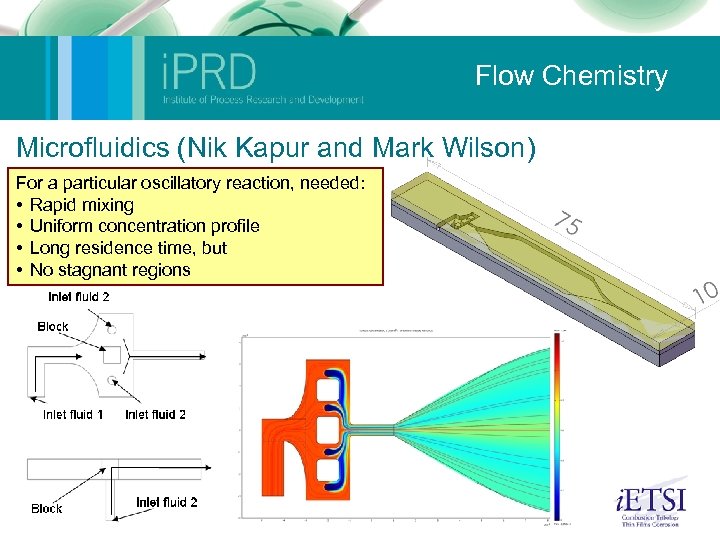 Flow Chemistry Microfluidics (Nik Kapur and Mark Wilson) For a particular oscillatory reaction, needed: • Rapid mixing • Uniform concentration profile • Long residence time, but • No stagnant regions
Flow Chemistry Microfluidics (Nik Kapur and Mark Wilson) For a particular oscillatory reaction, needed: • Rapid mixing • Uniform concentration profile • Long residence time, but • No stagnant regions
 Flow Chemistry Micromixing for reaction: The aim here was to use computational methods to develop a mixing device for a specific set of flow conditions. The device is currently being manufactured. Mixing by chaotic advection: This was a study of a small geometry where oscillation of the free surface (one roll speed is perturbed) causes mixing of the fluid. Shown on the movie are small tracer particles within the flow illustrating the mixing. The experiments on the left provide validation. Ambient fluid capture: The following slide shows how, using the control of eddies within flow, particles or reagents can be trapped. Autocatalytic reaction: This slide illustrates a simple autocatalytic scheme within the same geometry. Its possible to build a series of reactions into these models to couple flow and kinetics. 2 Phase droplet: An illustration of a 2 -phase simulation similar to that found within a microemulsion device. This sort of method can be used to optimise geometric and flow conditions for robust droplet formation. The group also has considerable experience in experiments in this area (2 -phase flow).
Flow Chemistry Micromixing for reaction: The aim here was to use computational methods to develop a mixing device for a specific set of flow conditions. The device is currently being manufactured. Mixing by chaotic advection: This was a study of a small geometry where oscillation of the free surface (one roll speed is perturbed) causes mixing of the fluid. Shown on the movie are small tracer particles within the flow illustrating the mixing. The experiments on the left provide validation. Ambient fluid capture: The following slide shows how, using the control of eddies within flow, particles or reagents can be trapped. Autocatalytic reaction: This slide illustrates a simple autocatalytic scheme within the same geometry. Its possible to build a series of reactions into these models to couple flow and kinetics. 2 Phase droplet: An illustration of a 2 -phase simulation similar to that found within a microemulsion device. This sort of method can be used to optimise geometric and flow conditions for robust droplet formation. The group also has considerable experience in experiments in this area (2 -phase flow).
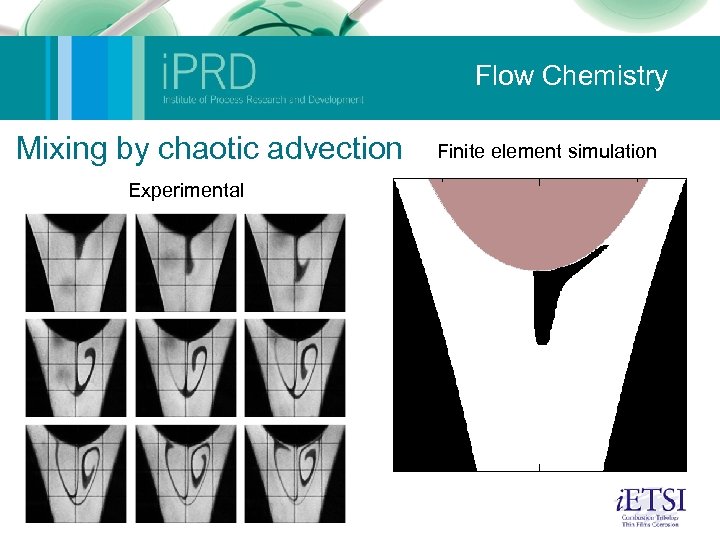 Flow Chemistry Mixing by chaotic advection Experimental Finite element simulation
Flow Chemistry Mixing by chaotic advection Experimental Finite element simulation
 Flow Chemistry Micromixing for reaction: The aim here was to use computational methods to develop a mixing device for a specific set of flow conditions. The device is currently being manufactured. Mixing by chaotic advection: This was a study of a small geometry where oscillation of the free surface (one roll speed is perturbed) causes mixing of the fluid. Shown on the movie are small tracer particles within the flow illustrating the mixing. The experiments on the left provide validation. Ambient fluid capture: The following slide shows how, using the control of eddies within flow, particles or reagents can be trapped. Autocatalytic reaction: This slide illustrates a simple autocatalytic scheme within the same geometry. Its possible to build a series of reactions into these models to couple flow and kinetics. 2 Phase droplet: An illustration of a 2 -phase simulation similar to that found within a microemulsion device. This sort of method can be used to optimise geometric and flow conditions for robust droplet formation. The group also has considerable experience in experiments in this area (2 -phase flow).
Flow Chemistry Micromixing for reaction: The aim here was to use computational methods to develop a mixing device for a specific set of flow conditions. The device is currently being manufactured. Mixing by chaotic advection: This was a study of a small geometry where oscillation of the free surface (one roll speed is perturbed) causes mixing of the fluid. Shown on the movie are small tracer particles within the flow illustrating the mixing. The experiments on the left provide validation. Ambient fluid capture: The following slide shows how, using the control of eddies within flow, particles or reagents can be trapped. Autocatalytic reaction: This slide illustrates a simple autocatalytic scheme within the same geometry. Its possible to build a series of reactions into these models to couple flow and kinetics. 2 Phase droplet: An illustration of a 2 -phase simulation similar to that found within a microemulsion device. This sort of method can be used to optimise geometric and flow conditions for robust droplet formation. The group also has considerable experience in experiments in this area (2 -phase flow).
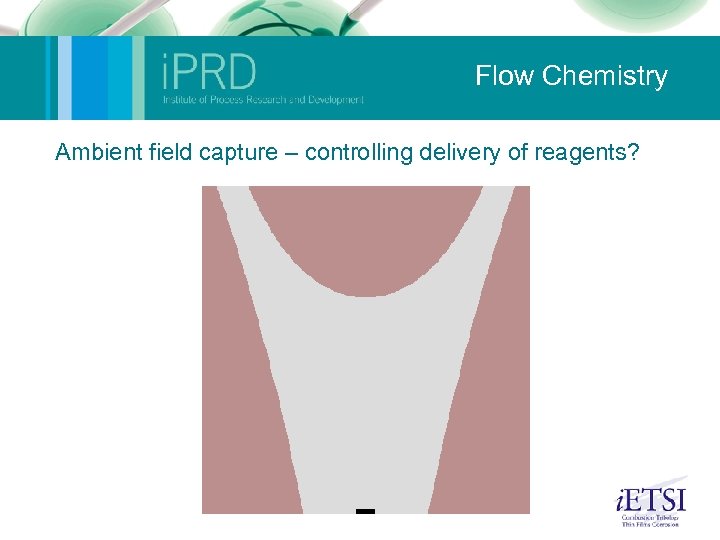 Flow Chemistry Ambient field capture – controlling delivery of reagents?
Flow Chemistry Ambient field capture – controlling delivery of reagents?
 Flow Chemistry Micromixing for reaction: The aim here was to use computational methods to develop a mixing device for a specific set of flow conditions. The device is currently being manufactured. Mixing by chaotic advection: This was a study of a small geometry where oscillation of the free surface (one roll speed is perturbed) causes mixing of the fluid. Shown on the movie are small tracer particles within the flow illustrating the mixing. The experiments on the left provide validation. Ambient fluid capture: The following slide shows how, using the control of eddies within flow, particles or reagents can be trapped. Autocatalytic reaction: This slide illustrates a simple autocatalytic scheme within the same geometry. Its possible to build a series of reactions into these models to couple flow and kinetics. 2 Phase droplet: An illustration of a 2 -phase simulation similar to that found within a microemulsion device. This sort of method can be used to optimise geometric and flow conditions for robust droplet formation. The group also has considerable experience in experiments in this area (2 -phase flow).
Flow Chemistry Micromixing for reaction: The aim here was to use computational methods to develop a mixing device for a specific set of flow conditions. The device is currently being manufactured. Mixing by chaotic advection: This was a study of a small geometry where oscillation of the free surface (one roll speed is perturbed) causes mixing of the fluid. Shown on the movie are small tracer particles within the flow illustrating the mixing. The experiments on the left provide validation. Ambient fluid capture: The following slide shows how, using the control of eddies within flow, particles or reagents can be trapped. Autocatalytic reaction: This slide illustrates a simple autocatalytic scheme within the same geometry. Its possible to build a series of reactions into these models to couple flow and kinetics. 2 Phase droplet: An illustration of a 2 -phase simulation similar to that found within a microemulsion device. This sort of method can be used to optimise geometric and flow conditions for robust droplet formation. The group also has considerable experience in experiments in this area (2 -phase flow).
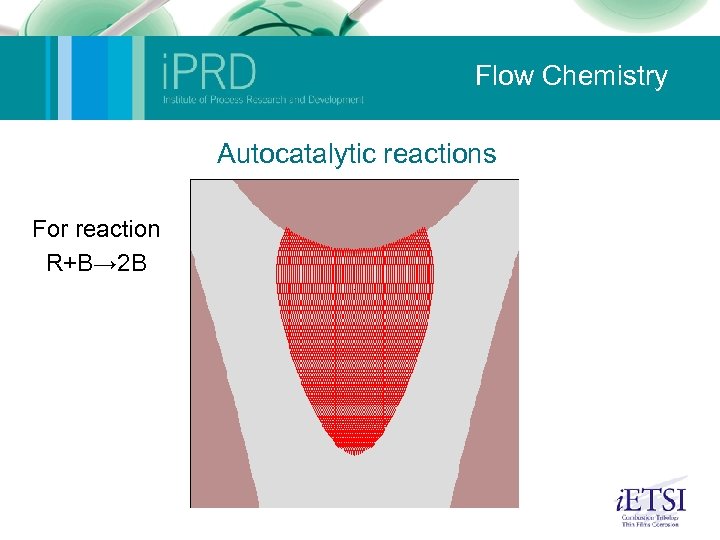 Flow Chemistry Autocatalytic reactions For reaction R+B→ 2 B
Flow Chemistry Autocatalytic reactions For reaction R+B→ 2 B
 Flow Chemistry Micromixing for reaction: The aim here was to use computational methods to develop a mixing device for a specific set of flow conditions. The device is currently being manufactured. Mixing by chaotic advection: This was a study of a small geometry where oscillation of the free surface (one roll speed is perturbed) causes mixing of the fluid. Shown on the movie are small tracer particles within the flow illustrating the mixing. The experiments on the left provide validation. Ambient fluid capture: The following slide shows how, using the control of eddies within flow, particles or reagents can be trapped. Autocatalytic reaction: This slide illustrates a simple autocatalytic scheme within the same geometry. Its possible to build a series of reactions into these models to couple flow and kinetics. 2 Phase droplet: An illustration of a 2 -phase simulation similar to that found within a microemulsion device. This sort of method can be used to optimise geometric and flow conditions for robust droplet formation. The group also has considerable experience in experiments in this area (2 -phase flow).
Flow Chemistry Micromixing for reaction: The aim here was to use computational methods to develop a mixing device for a specific set of flow conditions. The device is currently being manufactured. Mixing by chaotic advection: This was a study of a small geometry where oscillation of the free surface (one roll speed is perturbed) causes mixing of the fluid. Shown on the movie are small tracer particles within the flow illustrating the mixing. The experiments on the left provide validation. Ambient fluid capture: The following slide shows how, using the control of eddies within flow, particles or reagents can be trapped. Autocatalytic reaction: This slide illustrates a simple autocatalytic scheme within the same geometry. Its possible to build a series of reactions into these models to couple flow and kinetics. 2 Phase droplet: An illustration of a 2 -phase simulation similar to that found within a microemulsion device. This sort of method can be used to optimise geometric and flow conditions for robust droplet formation. The group also has considerable experience in experiments in this area (2 -phase flow).
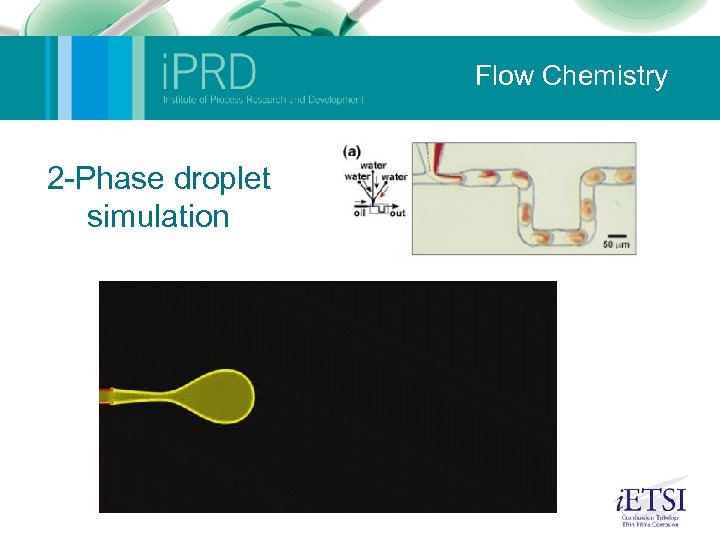 Flow Chemistry 2 -Phase droplet simulation
Flow Chemistry 2 -Phase droplet simulation
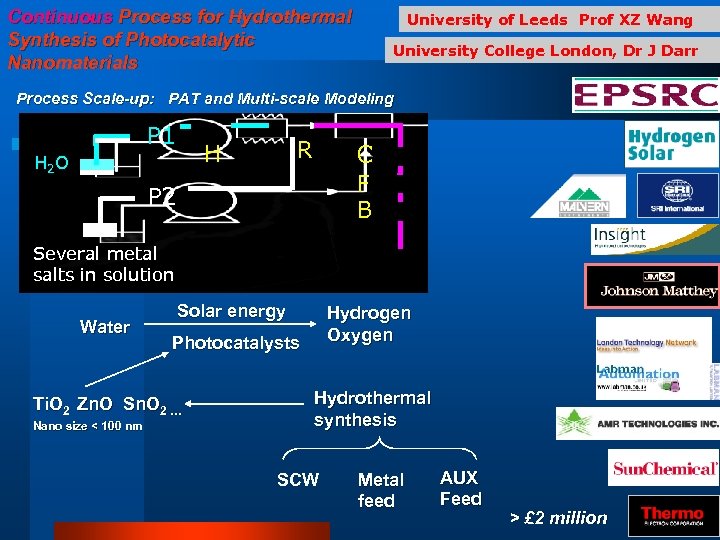 Continuous Process for Hydrothermal Synthesis of Photocatalytic Nanomaterials University of Leeds Prof XZ Wang University College London, Dr J Darr Process Scale-up: PAT and Multi-scale Modeling P 1 R H H 2 O C F B P 2 PID Several metal salts in solution Water Solar energy Photocatalysts Ti. O 2 Zn. O Sn. O 2 … Nano size < 100 nm Hydrogen Oxygen Hydrothermal synthesis SCW Metal feed AUX Feed > £ 2 million
Continuous Process for Hydrothermal Synthesis of Photocatalytic Nanomaterials University of Leeds Prof XZ Wang University College London, Dr J Darr Process Scale-up: PAT and Multi-scale Modeling P 1 R H H 2 O C F B P 2 PID Several metal salts in solution Water Solar energy Photocatalysts Ti. O 2 Zn. O Sn. O 2 … Nano size < 100 nm Hydrogen Oxygen Hydrothermal synthesis SCW Metal feed AUX Feed > £ 2 million
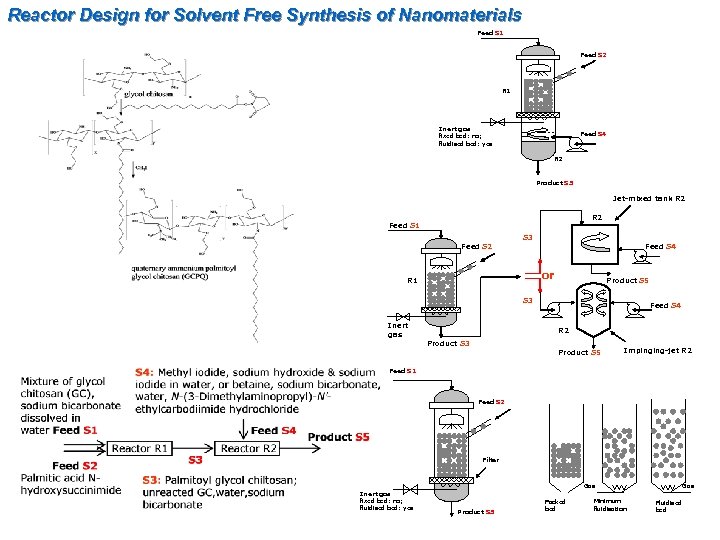 Reactor Design for Solvent Free Synthesis of Nanomaterials Feed S 1 Feed S 2 R 1 Inert gas fixed bed: no; fluidised bed: yes Feed S 4 R 2 Product S 5 Jet-mixed tank R 2 Feed S 1 S 3 Feed S 2 Feed S 4 or R 1 Product S 5 S 3 Inert gas Feed S 4 R 2 Product S 3 Product S 5 Impinging-jet R 2 Feed S 1 Feed S 2 Filter Gas Inert gas fixed bed: no; fluidised bed: yes Product S 3 Packed bed Minimum fluidisation Gas Fluidised bed
Reactor Design for Solvent Free Synthesis of Nanomaterials Feed S 1 Feed S 2 R 1 Inert gas fixed bed: no; fluidised bed: yes Feed S 4 R 2 Product S 5 Jet-mixed tank R 2 Feed S 1 S 3 Feed S 2 Feed S 4 or R 1 Product S 5 S 3 Inert gas Feed S 4 R 2 Product S 3 Product S 5 Impinging-jet R 2 Feed S 1 Feed S 2 Filter Gas Inert gas fixed bed: no; fluidised bed: yes Product S 3 Packed bed Minimum fluidisation Gas Fluidised bed
 Flow Chemistry sc. CO 2 Flow Chemistry (Chris Rayner) § Extensive expertise in reactions in s. CO 2 § Unique solvent, inexpensive, easy disposal and no solvent residues § Mostly done in batch in Leeds § Excellent understanding of likely problems (solubility, reactivity, high pressure) § sc. CO 2 (or liquid CO 2) flow methods in reactions, product isolation and purification. § E. g. stripping and/or recycling of dipolar aprotic solvents (DMF) § Crystallisation and drying
Flow Chemistry sc. CO 2 Flow Chemistry (Chris Rayner) § Extensive expertise in reactions in s. CO 2 § Unique solvent, inexpensive, easy disposal and no solvent residues § Mostly done in batch in Leeds § Excellent understanding of likely problems (solubility, reactivity, high pressure) § sc. CO 2 (or liquid CO 2) flow methods in reactions, product isolation and purification. § E. g. stripping and/or recycling of dipolar aprotic solvents (DMF) § Crystallisation and drying
 Flow Chemistry SCW Oxidation of Organics (Paul Williams) § Supercritical water oxidation very efficient for destruction of organics (Tc 374 ºC, Pc 221 bar) § Extensive experience in supercritical water technology (mainly batch) § Continuous SCW system to be commissioned shortly (Mojtaba Ghadiri/Yulong Ding) § Current projects include § SCW oxidation of organic wastes § SCW gasification of food wastes
Flow Chemistry SCW Oxidation of Organics (Paul Williams) § Supercritical water oxidation very efficient for destruction of organics (Tc 374 ºC, Pc 221 bar) § Extensive experience in supercritical water technology (mainly batch) § Continuous SCW system to be commissioned shortly (Mojtaba Ghadiri/Yulong Ding) § Current projects include § SCW oxidation of organic wastes § SCW gasification of food wastes
 Flow Chemistry Continuous reactions and purification (i. PRD) § Coupling reaction chemistry to product purification § Batch or continuous reactions § Batch or continuous purification methods (e. g. crystallisation) § Interdependence of purification and reaction chemistry § Improve reproducibility and quality of process and product § Extensive experience is all relevant areas
Flow Chemistry Continuous reactions and purification (i. PRD) § Coupling reaction chemistry to product purification § Batch or continuous reactions § Batch or continuous purification methods (e. g. crystallisation) § Interdependence of purification and reaction chemistry § Improve reproducibility and quality of process and product § Extensive experience is all relevant areas
 Flow Chemistry Points for discussion § Comments and suggestions on project portfolio § Identification of interested partners to form consortia for collaboration, application or information exchange
Flow Chemistry Points for discussion § Comments and suggestions on project portfolio § Identification of interested partners to form consortia for collaboration, application or information exchange


