b241007668da1cd8b08c938ec8971e24.ppt
- Количество слайдов: 20
 EQASs for TTI Diagnostics as Tool for Elucidating and Solving Problems of Commercial and In-house Tests H. Zeichhardt 1, 3, V. Lindig 1, 2 and H. -P. Grunert 1, 2, 3 1 CharitéCentrum für diagnostische und präventive Labormedizin, Institut für Virologie, Campus Benjamin Franklin, Berlin 2 Institut für Biotechnologische Diagnostik (GBD), Berlin 3 INSTAND e. V. , Düsseldorf Gesellschaft zur Förderung der Qualitätssicherung in medizinischen Laboratorien Collaborating Centers of International Consortium for Blood Safety (ICBS), New York Koch-Metchnikov-Forum of the Petersburg Dialogue INSTAND GBD ICBS
EQASs for TTI Diagnostics as Tool for Elucidating and Solving Problems of Commercial and In-house Tests H. Zeichhardt 1, 3, V. Lindig 1, 2 and H. -P. Grunert 1, 2, 3 1 CharitéCentrum für diagnostische und präventive Labormedizin, Institut für Virologie, Campus Benjamin Franklin, Berlin 2 Institut für Biotechnologische Diagnostik (GBD), Berlin 3 INSTAND e. V. , Düsseldorf Gesellschaft zur Förderung der Qualitätssicherung in medizinischen Laboratorien Collaborating Centers of International Consortium for Blood Safety (ICBS), New York Koch-Metchnikov-Forum of the Petersburg Dialogue INSTAND GBD ICBS
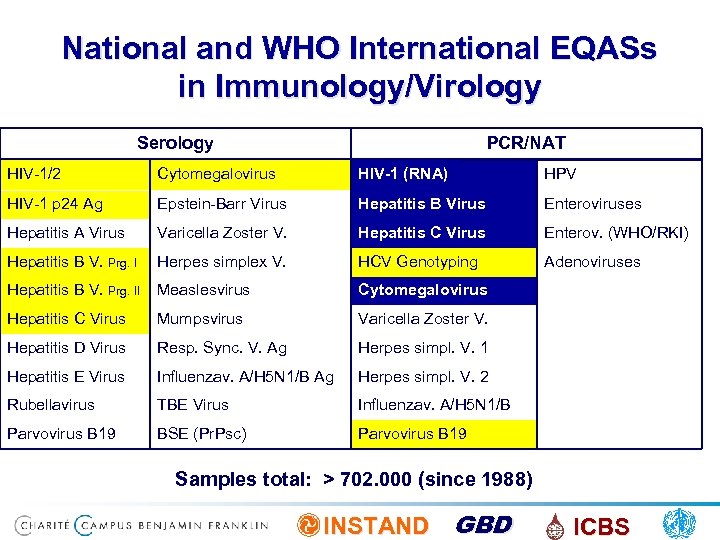 National and WHO International EQASs in Immunology/Virology Serology PCR/NAT HIV-1/2 Cytomegalovirus HIV-1 (RNA) HPV HIV-1 p 24 Ag Epstein-Barr Virus Hepatitis B Virus Enteroviruses Hepatitis A Virus Varicella Zoster V. Hepatitis C Virus Enterov. (WHO/RKI) Hepatitis B V. Prg. I Herpes simplex V. HCV Genotyping Adenoviruses Hepatitis B V. Prg. II Measlesvirus Cytomegalovirus Hepatitis C Virus Mumpsvirus Varicella Zoster V. Hepatitis D Virus Resp. Sync. V. Ag Herpes simpl. V. 1 Hepatitis E Virus Influenzav. A/H 5 N 1/B Ag Herpes simpl. V. 2 Rubellavirus TBE Virus Influenzav. A/H 5 N 1/B Parvovirus B 19 BSE (Pr. Psc) Parvovirus B 19 Samples total: > 702. 000 (since 1988) INSTAND GBD ICBS
National and WHO International EQASs in Immunology/Virology Serology PCR/NAT HIV-1/2 Cytomegalovirus HIV-1 (RNA) HPV HIV-1 p 24 Ag Epstein-Barr Virus Hepatitis B Virus Enteroviruses Hepatitis A Virus Varicella Zoster V. Hepatitis C Virus Enterov. (WHO/RKI) Hepatitis B V. Prg. I Herpes simplex V. HCV Genotyping Adenoviruses Hepatitis B V. Prg. II Measlesvirus Cytomegalovirus Hepatitis C Virus Mumpsvirus Varicella Zoster V. Hepatitis D Virus Resp. Sync. V. Ag Herpes simpl. V. 1 Hepatitis E Virus Influenzav. A/H 5 N 1/B Ag Herpes simpl. V. 2 Rubellavirus TBE Virus Influenzav. A/H 5 N 1/B Parvovirus B 19 BSE (Pr. Psc) Parvovirus B 19 Samples total: > 702. 000 (since 1988) INSTAND GBD ICBS
 Partnership for National and International Quality Control Systems in Virus Diagnostics and Safety Testing of Blood • • CharitéCentrum für diagnostische und präventive Labormedizin Campus Benjamin Franklin, Institut für Virologie, Berlin Institute for Standardization and Documentation in Medical Laboratories (INSTAND), Düsseldorf WHO Collaborating Center for Quality Assurance and Standardization in Laboratory Medicine • • • Institut für Biotechnologische Diagnostik der GBD, Berlin German Medical Association (Bundesärztekammer) German Association against Virus Diseases (DVV) Society of Virology (Gf. V) Paul Ehrlich Institute Diagnostic Council of DVV and Gf. V Robert Koch Institute National and International Reference Institutes Friedrich-Loeffler-Institute World Health Organization, Geneva and Lyon International Consortium for Blood Safety (ICBS), New York INSTAND GBD ICBS
Partnership for National and International Quality Control Systems in Virus Diagnostics and Safety Testing of Blood • • CharitéCentrum für diagnostische und präventive Labormedizin Campus Benjamin Franklin, Institut für Virologie, Berlin Institute for Standardization and Documentation in Medical Laboratories (INSTAND), Düsseldorf WHO Collaborating Center for Quality Assurance and Standardization in Laboratory Medicine • • • Institut für Biotechnologische Diagnostik der GBD, Berlin German Medical Association (Bundesärztekammer) German Association against Virus Diseases (DVV) Society of Virology (Gf. V) Paul Ehrlich Institute Diagnostic Council of DVV and Gf. V Robert Koch Institute National and International Reference Institutes Friedrich-Loeffler-Institute World Health Organization, Geneva and Lyon International Consortium for Blood Safety (ICBS), New York INSTAND GBD ICBS
 INSTAND EQAS - Reference Laboratories • • • • • Prof. Braun / Prof. Enders, Stuttgart Prof. Doerr / Prof. Rabenau / Dr. Berger, Frankfurt Prof. Fleckenstein / Dr. Huber / Dr. Korn, Erlangen Prof. Gerlich / Dr. Willems, Gießen Prof. Gürtler, Greifswald Dr. Heckler, Hannover Dr. Heim, Hannover Prof. Klenk / Prof. Radsak, Marburg Prof. Krüger / Dr. Meisel / Dr. Hofmann, Berlin Prof. Kurth / Prof. Pauli / Prof. Schreier / Dr. Mankertz / Dr. Schweiger / Dr. Tischer, RKI, Berlin Prof. Liebert / Prof. Pustowoit, Leipzig Prof. Löwer / Dr. Chudy / Dr. Nick / Dr. Nübling / Dr. Scheiblauer / Dr. Unger, PEI, Langen Prof. Mettenleiter / Prof. Groschup / Dr. Buschmann / PD Dr. Harder / Dr. Ziegler, FLI, Greifswald Insel Riems Prof. Müller-Lantzsch / Dr. Gärtner, Homburg/Saar Prof. Neumann-Haefelin / Dr. Huzly, Freiburg Prof. Pfister / Dr. Wieland, Köln Prof. Roggendorf / PD Dr. Ross / Dr. Fiedler, Essen Dr. Vornwald, Berlin Prof. Wutzler / Prof. Faber / Dr. Sauerbrei, Jena INSTAND GBD ICBS
INSTAND EQAS - Reference Laboratories • • • • • Prof. Braun / Prof. Enders, Stuttgart Prof. Doerr / Prof. Rabenau / Dr. Berger, Frankfurt Prof. Fleckenstein / Dr. Huber / Dr. Korn, Erlangen Prof. Gerlich / Dr. Willems, Gießen Prof. Gürtler, Greifswald Dr. Heckler, Hannover Dr. Heim, Hannover Prof. Klenk / Prof. Radsak, Marburg Prof. Krüger / Dr. Meisel / Dr. Hofmann, Berlin Prof. Kurth / Prof. Pauli / Prof. Schreier / Dr. Mankertz / Dr. Schweiger / Dr. Tischer, RKI, Berlin Prof. Liebert / Prof. Pustowoit, Leipzig Prof. Löwer / Dr. Chudy / Dr. Nick / Dr. Nübling / Dr. Scheiblauer / Dr. Unger, PEI, Langen Prof. Mettenleiter / Prof. Groschup / Dr. Buschmann / PD Dr. Harder / Dr. Ziegler, FLI, Greifswald Insel Riems Prof. Müller-Lantzsch / Dr. Gärtner, Homburg/Saar Prof. Neumann-Haefelin / Dr. Huzly, Freiburg Prof. Pfister / Dr. Wieland, Köln Prof. Roggendorf / PD Dr. Ross / Dr. Fiedler, Essen Dr. Vornwald, Berlin Prof. Wutzler / Prof. Faber / Dr. Sauerbrei, Jena INSTAND GBD ICBS
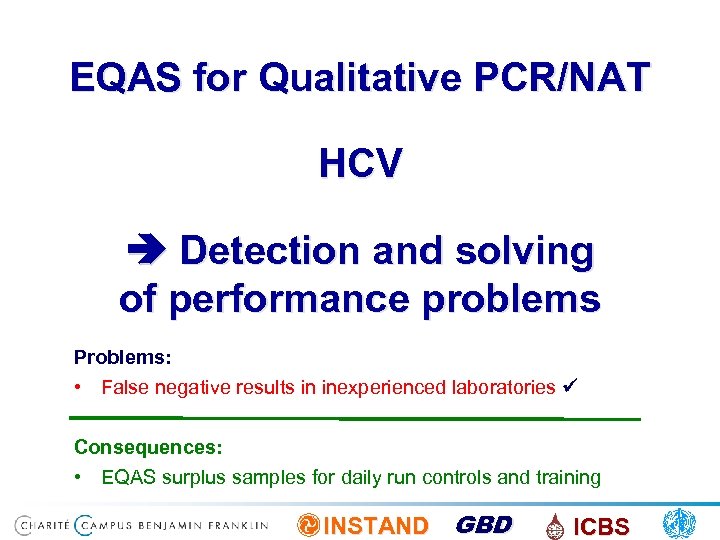 EQAS for Qualitative PCR/NAT HCV Detection and solving of performance problems Problems: • False negative results in inexperienced laboratories Consequences: • EQAS surplus samples for daily run controls and training INSTAND GBD ICBS
EQAS for Qualitative PCR/NAT HCV Detection and solving of performance problems Problems: • False negative results in inexperienced laboratories Consequences: • EQAS surplus samples for daily run controls and training INSTAND GBD ICBS
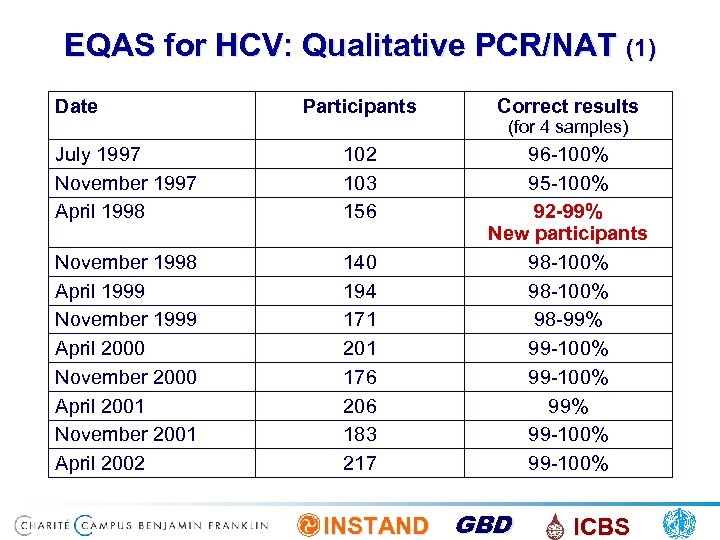 EQAS for HCV: Qualitative PCR/NAT (1) Date Participants Correct results July 1997 November 1997 April 1998 102 103 156 November 1998 April 1999 November 1999 April 2000 November 2000 April 2001 November 2001 April 2002 140 194 171 201 176 206 183 217 96 -100% 95 -100% 92 -99% New participants 98 -100% 98 -99% 99 -100% (for 4 samples) INSTAND GBD ICBS
EQAS for HCV: Qualitative PCR/NAT (1) Date Participants Correct results July 1997 November 1997 April 1998 102 103 156 November 1998 April 1999 November 1999 April 2000 November 2000 April 2001 November 2001 April 2002 140 194 171 201 176 206 183 217 96 -100% 95 -100% 92 -99% New participants 98 -100% 98 -99% 99 -100% (for 4 samples) INSTAND GBD ICBS
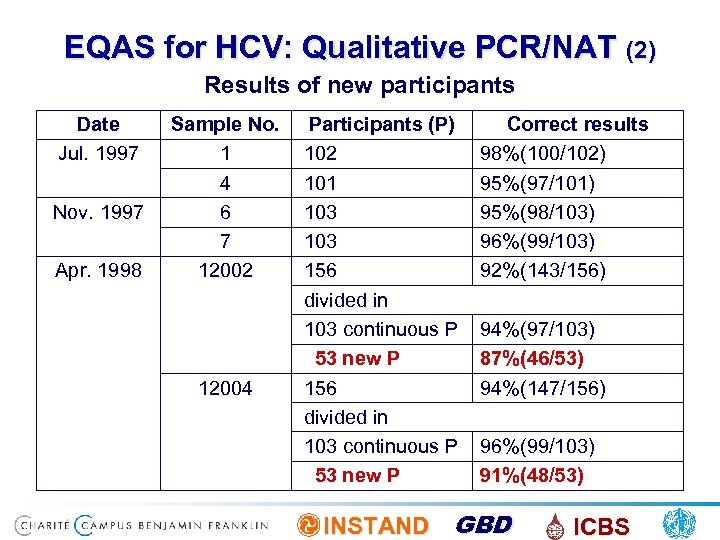 EQAS for HCV: Qualitative PCR/NAT (2) Results of new participants Date Jul. 1997 Nov. 1997 Apr. 1998 Sample No. 1 4 6 7 12002 12004 Participants (P) 102 101 103 156 divided in 103 continuous P 53 new P Correct results 98%(100/102) 95%(97/101) 95%(98/103) 96%(99/103) 92%(143/156) 94%(97/103) 87%(46/53) 94%(147/156) 96%(99/103) 91%(48/53) INSTAND GBD ICBS
EQAS for HCV: Qualitative PCR/NAT (2) Results of new participants Date Jul. 1997 Nov. 1997 Apr. 1998 Sample No. 1 4 6 7 12002 12004 Participants (P) 102 101 103 156 divided in 103 continuous P 53 new P Correct results 98%(100/102) 95%(97/101) 95%(98/103) 96%(99/103) 92%(143/156) 94%(97/103) 87%(46/53) 94%(147/156) 96%(99/103) 91%(48/53) INSTAND GBD ICBS
 EQAS for Quantitative PCR/NAT HIV and HCV Good lab performance with robust test: Benefit of EQAS samples run in dublicate in one scheme INSTAND GBD ICBS
EQAS for Quantitative PCR/NAT HIV and HCV Good lab performance with robust test: Benefit of EQAS samples run in dublicate in one scheme INSTAND GBD ICBS
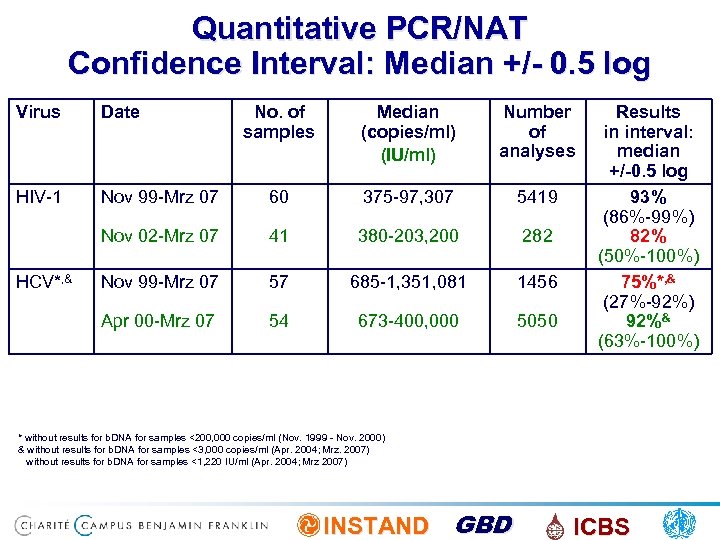
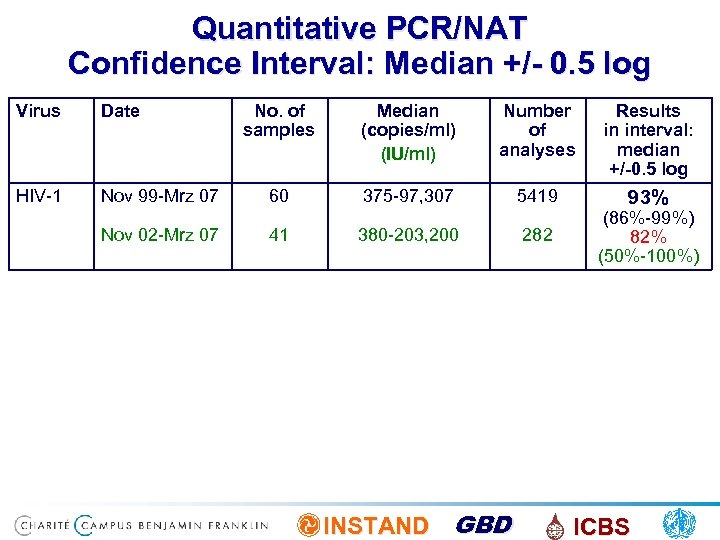 Quantitative PCR/NAT Confidence Interval: Median +/- 0. 5 log Virus Date HIV-1 Nov 99 -Mrz 07 Nov 02 -Mrz 07 No. of samples Median (copies/ml) (IU/ml) Number of analyses Results in interval: median +/-0. 5 log 60 375 -97, 307 5419 93% 41 380 -203, 200 INSTAND GBD 282 (86%-99%) 82% (50%-100%) ICBS
Quantitative PCR/NAT Confidence Interval: Median +/- 0. 5 log Virus Date HIV-1 Nov 99 -Mrz 07 Nov 02 -Mrz 07 No. of samples Median (copies/ml) (IU/ml) Number of analyses Results in interval: median +/-0. 5 log 60 375 -97, 307 5419 93% 41 380 -203, 200 INSTAND GBD 282 (86%-99%) 82% (50%-100%) ICBS
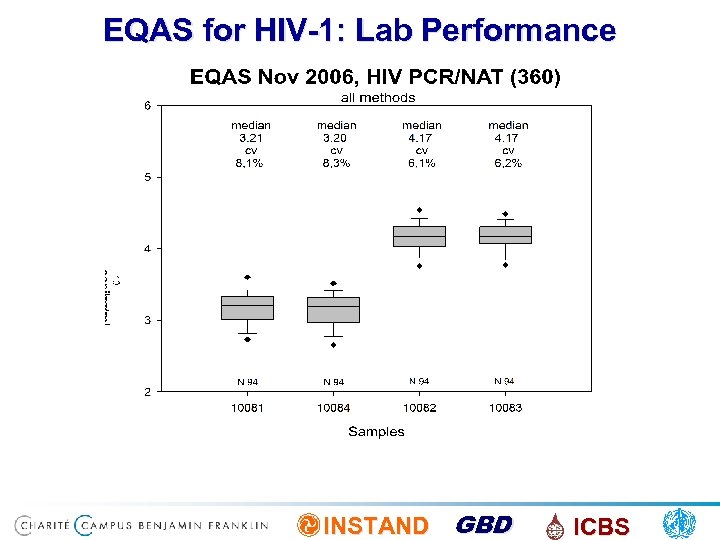 EQAS for HIV-1: Lab Performance INSTAND GBD ICBS
EQAS for HIV-1: Lab Performance INSTAND GBD ICBS
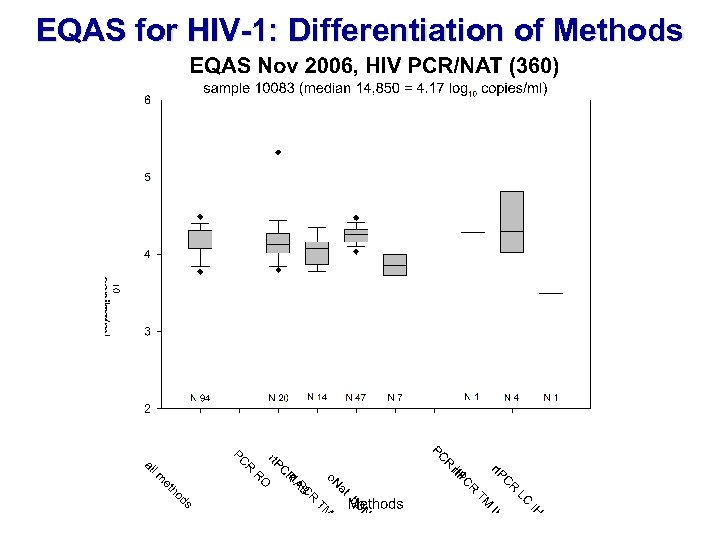 EQAS for HIV-1: Differentiation of Methods INSTAND GBD ICBS
EQAS for HIV-1: Differentiation of Methods INSTAND GBD ICBS
 Quantitative PCR/NAT Confidence Interval: Median +/- 0. 5 log Virus Date HIV-1 Median (copies/ml) (IU/ml) Number of analyses Nov 99 -Mrz 07 60 375 -97, 307 5419 Nov 02 -Mrz 07 41 380 -203, 200 282 Nov 99 -Mrz 07 57 685 -1, 351, 081 1456 Apr 00 -Mrz 07 HCV*, & No. of samples 54 673 -400, 000 5050 Results in interval: median +/-0. 5 log 93% (86%-99%) 82% (50%-100%) 75%*, & (27%-92%) 92%& (63%-100%) * without results for b. DNA for samples <200, 000 copies/ml (Nov. 1999 - Nov. 2000) & without results for b. DNA for samples <3, 000 copies/ml (Apr. 2004; Mrz. 2007) without results for b. DNA for samples <1, 220 IU/ml (Apr. 2004; Mrz 2007) INSTAND GBD ICBS
Quantitative PCR/NAT Confidence Interval: Median +/- 0. 5 log Virus Date HIV-1 Median (copies/ml) (IU/ml) Number of analyses Nov 99 -Mrz 07 60 375 -97, 307 5419 Nov 02 -Mrz 07 41 380 -203, 200 282 Nov 99 -Mrz 07 57 685 -1, 351, 081 1456 Apr 00 -Mrz 07 HCV*, & No. of samples 54 673 -400, 000 5050 Results in interval: median +/-0. 5 log 93% (86%-99%) 82% (50%-100%) 75%*, & (27%-92%) 92%& (63%-100%) * without results for b. DNA for samples <200, 000 copies/ml (Nov. 1999 - Nov. 2000) & without results for b. DNA for samples <3, 000 copies/ml (Apr. 2004; Mrz. 2007) without results for b. DNA for samples <1, 220 IU/ml (Apr. 2004; Mrz 2007) INSTAND GBD ICBS
 EQAS for Quantitative PCR/NAT HBV Training with reliable tests: Benefit of identical EQAS samples for multiple testing INSTAND GBD ICBS
EQAS for Quantitative PCR/NAT HBV Training with reliable tests: Benefit of identical EQAS samples for multiple testing INSTAND GBD ICBS
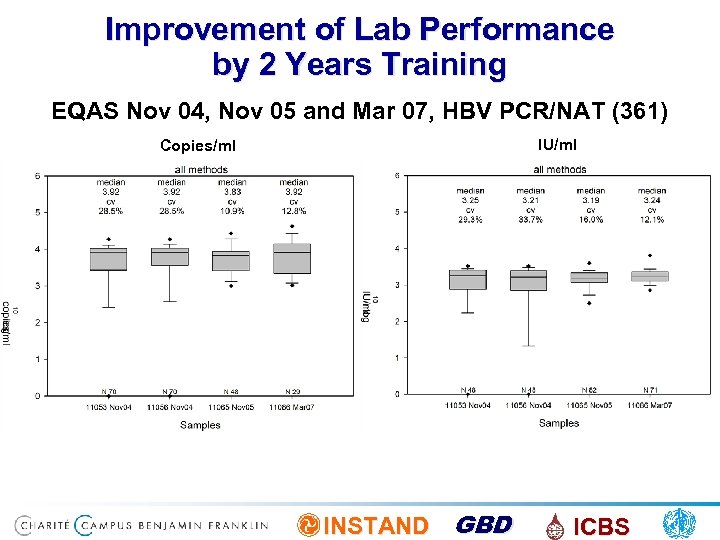 Improvement of Lab Performance by 2 Years Training EQAS Nov 04, Nov 05 and Mar 07, HBV PCR/NAT (361) IU/ml Copies/ml INSTAND GBD ICBS
Improvement of Lab Performance by 2 Years Training EQAS Nov 04, Nov 05 and Mar 07, HBV PCR/NAT (361) IU/ml Copies/ml INSTAND GBD ICBS
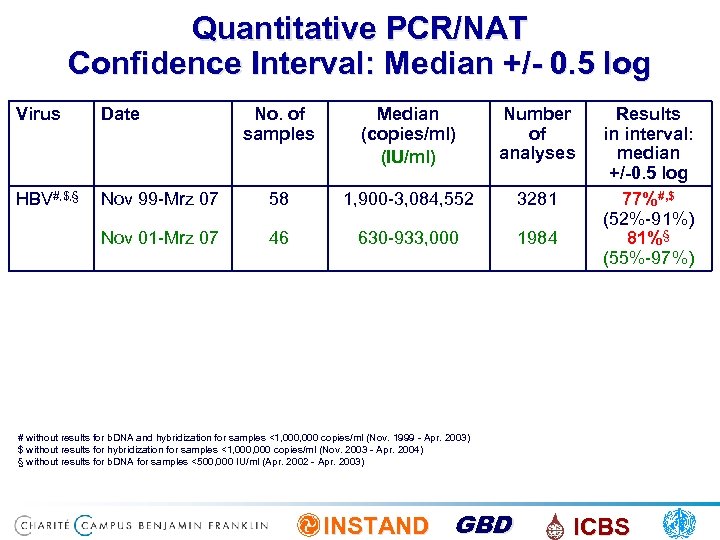 Quantitative PCR/NAT Confidence Interval: Median +/- 0. 5 log Virus Date No. of samples Median (copies/ml) (IU/ml) Number of analyses HBV#, $, § Nov 99 -Mrz 07 58 1, 900 -3, 084, 552 3281 Nov 01 -Mrz 07 46 630 -933, 000 1984 Results in interval: median +/-0. 5 log 77%#, $ (52%-91%) 81%§ (55%-97%) # without results for b. DNA and hybridization for samples <1, 000 copies/ml (Nov. 1999 - Apr. 2003) $ without results for hybridization for samples <1, 000 copies/ml (Nov. 2003 - Apr. 2004) § without results for b. DNA for samples <500, 000 IU/ml (Apr. 2002 - Apr. 2003) INSTAND GBD ICBS
Quantitative PCR/NAT Confidence Interval: Median +/- 0. 5 log Virus Date No. of samples Median (copies/ml) (IU/ml) Number of analyses HBV#, $, § Nov 99 -Mrz 07 58 1, 900 -3, 084, 552 3281 Nov 01 -Mrz 07 46 630 -933, 000 1984 Results in interval: median +/-0. 5 log 77%#, $ (52%-91%) 81%§ (55%-97%) # without results for b. DNA and hybridization for samples <1, 000 copies/ml (Nov. 1999 - Apr. 2003) $ without results for hybridization for samples <1, 000 copies/ml (Nov. 2003 - Apr. 2004) § without results for b. DNA for samples <500, 000 IU/ml (Apr. 2002 - Apr. 2003) INSTAND GBD ICBS
 EQAS for Quantitative PCR/NAT Cytomegalovirus Performance of commercial and in-house tests INSTAND GBD ICBS
EQAS for Quantitative PCR/NAT Cytomegalovirus Performance of commercial and in-house tests INSTAND GBD ICBS
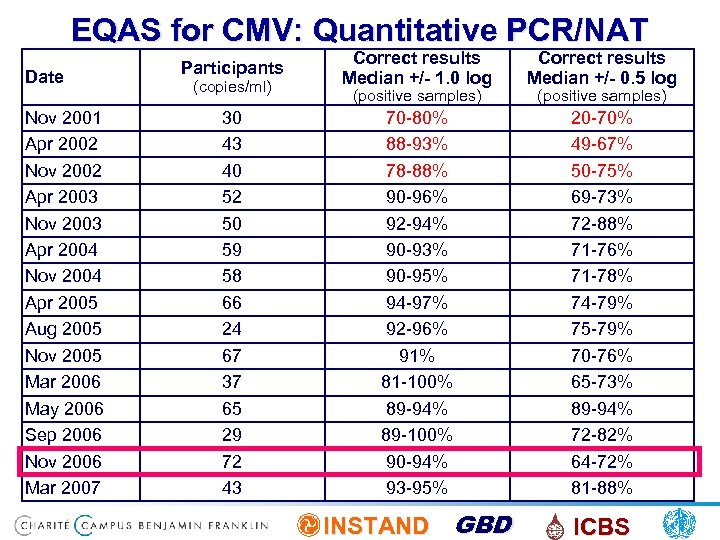 EQAS for CMV: Quantitative PCR/NAT Date Nov 2001 Apr 2002 Nov 2002 Apr 2003 Nov 2003 Apr 2004 Nov 2004 Apr 2005 Aug 2005 Nov 2005 Mar 2006 May 2006 Sep 2006 Nov 2006 Mar 2007 (copies/ml) Correct results Median +/- 1. 0 log Correct results Median +/- 0. 5 log 30 43 40 52 50 59 58 66 24 67 37 65 29 72 43 70 -80% 88 -93% 78 -88% 90 -96% 92 -94% 90 -93% 90 -95% 94 -97% 92 -96% 91% 81 -100% 89 -94% 89 -100% 90 -94% 93 -95% 20 -70% 49 -67% 50 -75% 69 -73% 72 -88% 71 -76% 71 -78% 74 -79% 75 -79% 70 -76% 65 -73% 89 -94% 72 -82% 64 -72% 81 -88% INSTAND GBD ICBS Participants (positive samples)
EQAS for CMV: Quantitative PCR/NAT Date Nov 2001 Apr 2002 Nov 2002 Apr 2003 Nov 2003 Apr 2004 Nov 2004 Apr 2005 Aug 2005 Nov 2005 Mar 2006 May 2006 Sep 2006 Nov 2006 Mar 2007 (copies/ml) Correct results Median +/- 1. 0 log Correct results Median +/- 0. 5 log 30 43 40 52 50 59 58 66 24 67 37 65 29 72 43 70 -80% 88 -93% 78 -88% 90 -96% 92 -94% 90 -93% 90 -95% 94 -97% 92 -96% 91% 81 -100% 89 -94% 89 -100% 90 -94% 93 -95% 20 -70% 49 -67% 50 -75% 69 -73% 72 -88% 71 -76% 71 -78% 74 -79% 75 -79% 70 -76% 65 -73% 89 -94% 72 -82% 64 -72% 81 -88% INSTAND GBD ICBS Participants (positive samples)
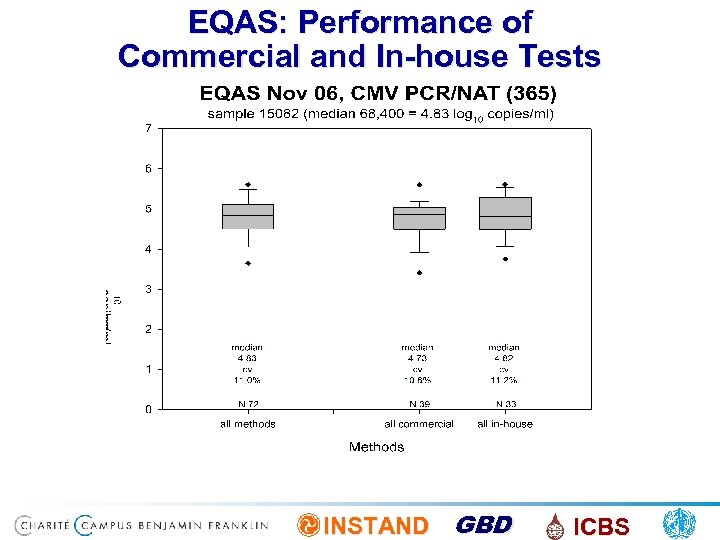 EQAS: Performance of Commercial and In-house Tests INSTAND GBD ICBS
EQAS: Performance of Commercial and In-house Tests INSTAND GBD ICBS
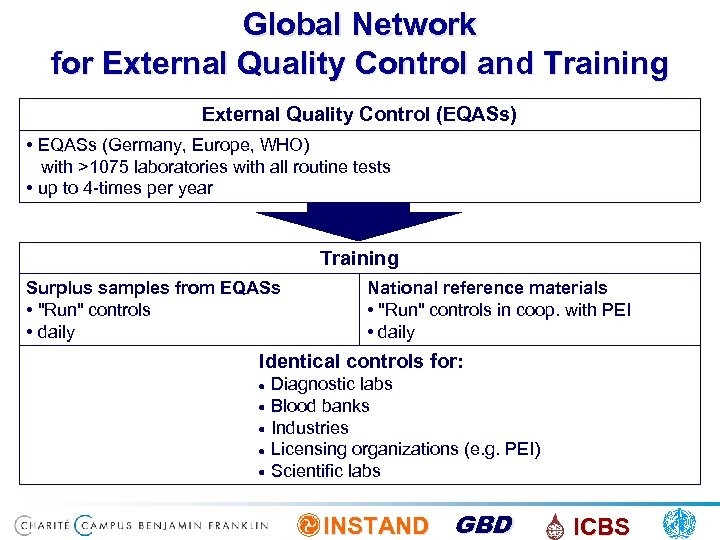 Global Network for External Quality Control and Training External Quality Control (EQASs) • EQASs (Germany, Europe, WHO) with >1075 laboratories with all routine tests • up to 4 -times per year Training Surplus samples from EQASs • "Run" controls • daily National reference materials • "Run" controls in coop. with PEI • daily Identical controls for: Diagnostic labs Blood banks Industries Licensing organizations (e. g. PEI) Scientific labs INSTAND GBD ICBS
Global Network for External Quality Control and Training External Quality Control (EQASs) • EQASs (Germany, Europe, WHO) with >1075 laboratories with all routine tests • up to 4 -times per year Training Surplus samples from EQASs • "Run" controls • daily National reference materials • "Run" controls in coop. with PEI • daily Identical controls for: Diagnostic labs Blood banks Industries Licensing organizations (e. g. PEI) Scientific labs INSTAND GBD ICBS
 Contact Hans-Peter Grunert Heinz Zeichhardt CharitéCentrum für diagnostische und präventive Labormedizin Institut für Virologie Campus Benjamin Franklin Hindenburgdamm 27 12203 Berlin Tel. : +49 -30 -8445 3624 or 3625 Fax: +49 -30 -8445 3626 Email: Hans-Peter. Grunert@charite. de Heinz. Zeichhardt@charite. de INSTAND GBD ICBS
Contact Hans-Peter Grunert Heinz Zeichhardt CharitéCentrum für diagnostische und präventive Labormedizin Institut für Virologie Campus Benjamin Franklin Hindenburgdamm 27 12203 Berlin Tel. : +49 -30 -8445 3624 or 3625 Fax: +49 -30 -8445 3626 Email: Hans-Peter. Grunert@charite. de Heinz. Zeichhardt@charite. de INSTAND GBD ICBS


