dd40d55e4385b8370edec6eddf5609e6.ppt
- Количество слайдов: 36
Epitope prediction algorithms Urmila Kulkarni-Kale Bioinformatics Centre University of Pune October 2 K 5 © Bioinformatics Centre, Uo. P
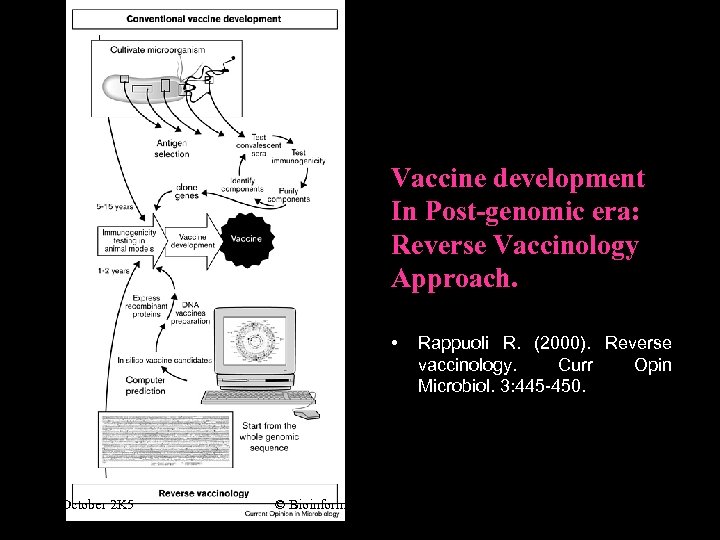
Vaccine development In Post-genomic era: Reverse Vaccinology Approach. • October 2 K 5 Rappuoli R. (2000). Reverse vaccinology. Curr Opin Microbiol. 3: 445 -450. © Bioinformatics Centre, Uo. P 2
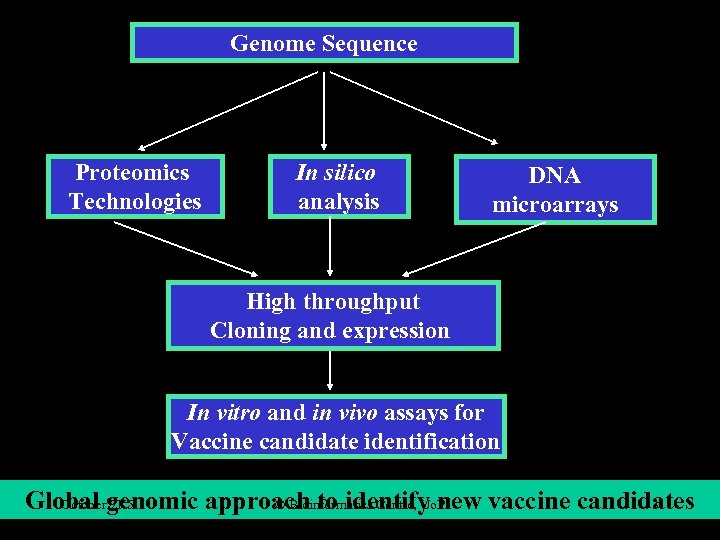
Genome Sequence Proteomics Technologies In silico analysis DNA microarrays High throughput Cloning and expression In vitro and in vivo assays for Vaccine candidate identification Global genomic approach to identify new vaccine candidates October 2 K 5 © Bioinformatics Centre, Uo. P 3
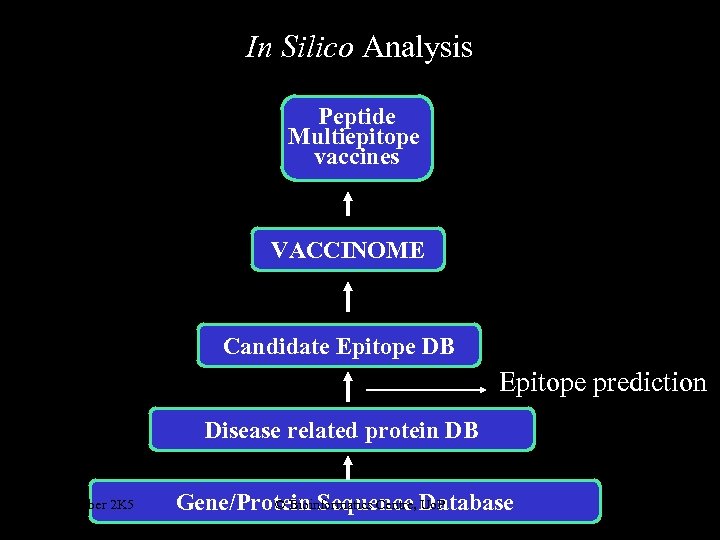
In Silico Analysis Peptide Multiepitope vaccines VACCINOME Candidate Epitope DB Epitope prediction Disease related protein DB October 2 K 5 © Bioinformatics Centre, Uo. P Gene/Protein Sequence Database 4

October 2 K 5 © Bioinformatics Centre, Uo. P 5
Types of Epitopes • Sequential / Continuous epitopes: • recognized by Th cells • linear peptide fragments • amphipathic helical 9 -12 mer • Conformational / Discontinuous epitopes: • recognized by both Th & B cells • non-linear discrete amino acid sequences, come together due to folding • exposed 15 -22 mer October 2 K 5 © Bioinformatics Centre, Uo. P 6

Properties of Epitopes • They occur on the surface of the protein and are more flexible than the rest of the protein. • They have high degree of exposure to the solvent. • The amino acids making the epitope are usually charged and hydrophilic. October 2 K 5 © Bioinformatics Centre, Uo. P 7

Methods to identify epitopes 1. Immunochemical methods • • • ELISA : Enzyme linked immunosorbent assay Immunoflurorescence Radioimmunoassay 2. X-ray crystallography: Ag-Ab complex is crystallized and the structure is scanned for contact residues between Ag and Ab. The contact residues on the Ag are considered as the epitope. 3. Prediction methods: Based on the X-ray crystal data available for Ag-Ab complexes, the propensity of an amino acid to lie in an epitope is calculated. October 2 K 5 © Bioinformatics Centre, Uo. P 8
Antigen-Antibody (Ag-Ab) complexes • Non-obligatory heterocomplexes that are made and broken according to the environment • Involve proteins (Ag & Ab) that must also exist independently • Remarkable feature: – high affinity and strict specificity of antibodies for their antigens. • Ab recognize the unique conformations and spatial locations on the surface of Ag • Epitopes & paratopes are relational entities October 2 K 5 © Bioinformatics Centre, Uo. P 9
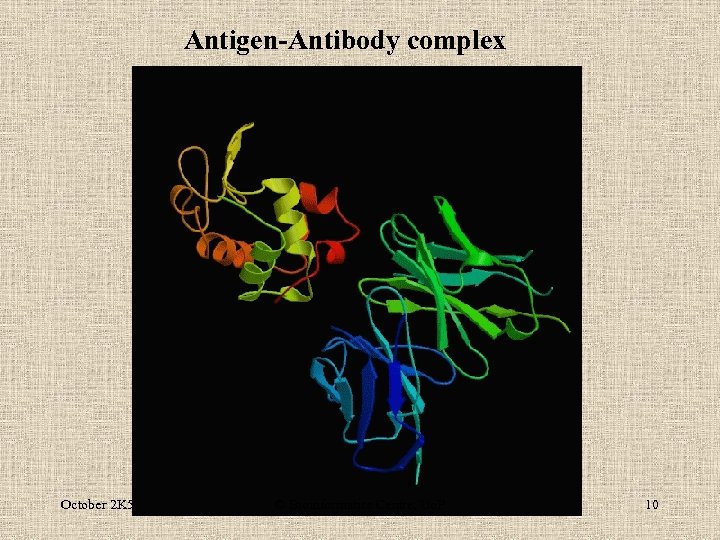
Antigen-Antibody complex October 2 K 5 © Bioinformatics Centre, Uo. P 10
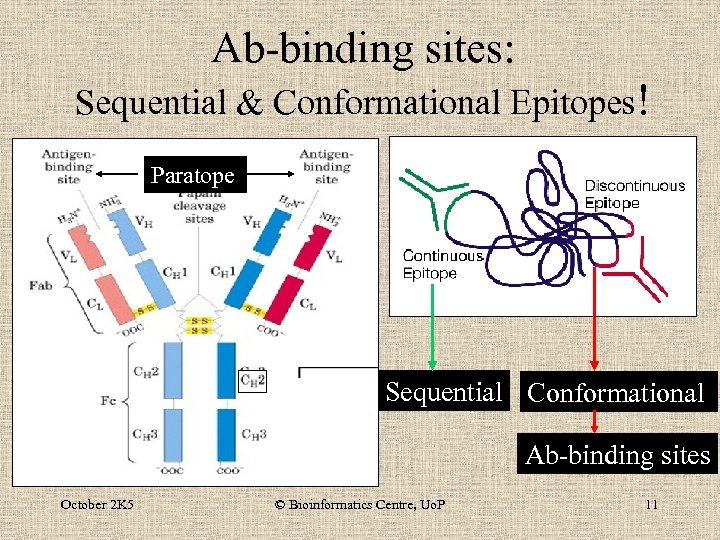
Ab-binding sites: Sequential & Conformational Epitopes! Paratope Sequential Conformational Ab-binding sites October 2 K 5 © Bioinformatics Centre, Uo. P 11
B cell epitope prediction algorithms : • • • Hopp and Woods – 1981 Welling et al – 1985 Parker & Hodges - 1986 Kolaskar & Tongaonkar – 1990 Kolaskar & Urmila Kulkarni - 1999 T cell epitope prediction algorithms : • • Margalit, Spouge et al - 1987 Rothbard & Taylor – 1988 Stille et al – 1987 Tepitope -1999 October 2 K 5 © Bioinformatics Centre, Uo. P 12

Hopp & Woods method • Pioneering work • Based on the fact that only the hydrophilic nature of amino acids is essential for an sequence to be an antigenic determinant • Local hydrophilicity values are assigned to each amino acid by the method of repetitive averaging using a window of six • Not very accurate October 2 K 5 © Bioinformatics Centre, Uo. P 13
Welling’s method • Based on the % of each aa present in known epitopes compared with the % of aa in the avg. composition of a protein. • assigns an antigenicity value for each amino acid from the relative occurrence of the amino acid in an antigenic determinant site. • regions of 7 aa with relatively high antigenicity are extended to 11 -13 aa depending on the antigenicity values of neighboring residues. October 2 K 5 © Bioinformatics Centre, Uo. P 14

Parker & Hodges method • Utilizes 3 parameters : – Hydrophilicity : HPLC – Accessibility : Janin’s scale – Flexibility : Karplus & Schultz • Hydrophilicity parameter was calculated using HPLC from retention co-efficients of model synthetic peptides. • Surface profile was determined by summing the parameters for each residue of a seven-residue segment and assigning the sum to the fourth residue. • One of the most useful prediction algorithms October 2 K 5 © Bioinformatics Centre, Uo. P 15
Kolaskar & Tongaonkar’s method • Semi-empirical method which uses physiological properties of amino acid residues • frequencies of occurrence of amino acids in experimentally known epitopes. • Data of 169 epitopes from 34 different proteins was collected of which 156 which have less than 20 aa per determinant were used. • Antigen: EMBOSS October 2 K 5 © Bioinformatics Centre, Uo. P 16

CEP Server • Predicts the conformational epitopes from X -ray crystals of Ag-Ab complexes. • uses percent accessible surface area and distance as criteria October 2 K 5 © Bioinformatics Centre, Uo. P 17
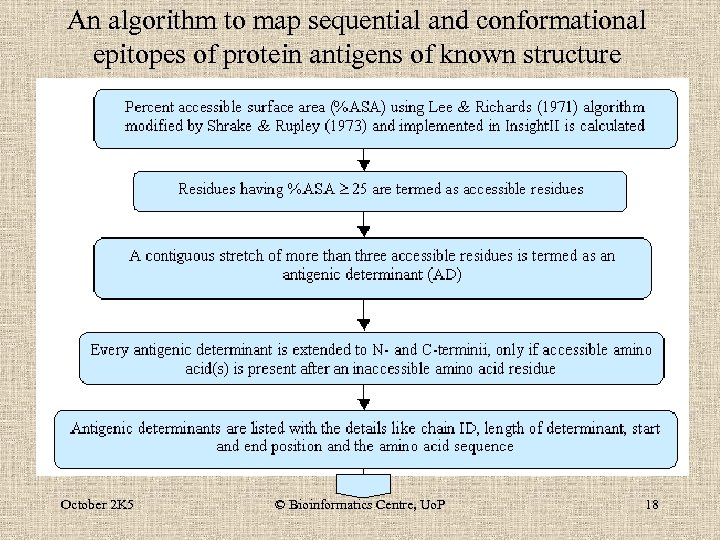
An algorithm to map sequential and conformational epitopes of protein antigens of known structure October 2 K 5 © Bioinformatics Centre, Uo. P 18
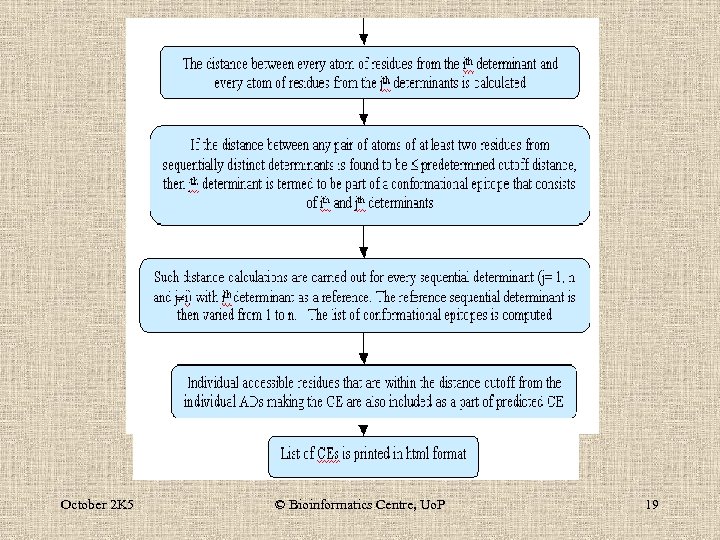
October 2 K 5 © Bioinformatics Centre, Uo. P 19
CE: Beyond validation • High accuracy: – Limited data set to evaluate the algorithm – Non-availability of true negative data sets • Prediction of false positives? – Are they really false positives? • Limitation: Different Abs (Hy. HEL 10 & D 1. 3) have over-lapping binding sites – Limited by the availability of 3 D structure data of antigens October 2 K 5 © Bioinformatics Centre, Uo. P 20
CE: Features • The first algorithm for the prediction of conformational epitopes or antibody binding sites of protein antigens • Maps both: sequential & conformational epitopes • Prerequisite: 3 D structure of an antigen October 2 K 5 © Bioinformatics Centre, Uo. P 21
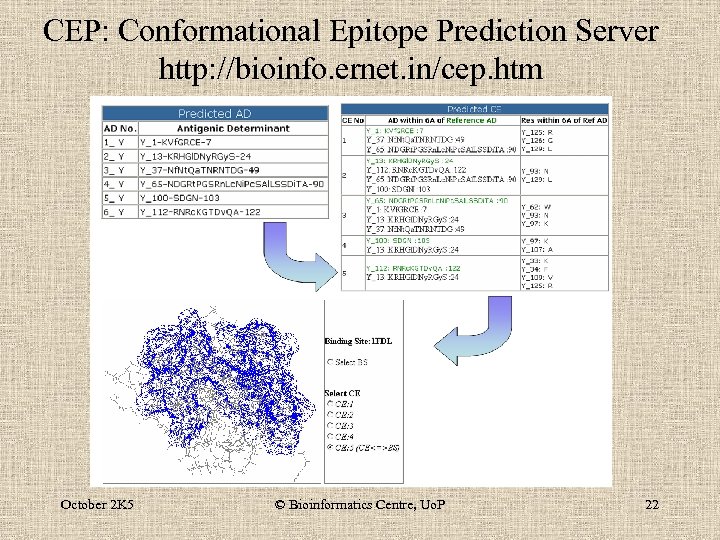
CEP: Conformational Epitope Prediction Server http: //bioinfo. ernet. in/cep. htm October 2 K 5 © Bioinformatics Centre, Uo. P 22
T-cell epitope prediction algorithms • Considers amphipathic helix segments, tetramer and pentamer motifs (charged residues or glycine) followed by 2 -3 hydrophobic residues and then a polar residue. • Sequence motifs of immunodominant secondary structure capable of binding to MHC with high affinity. • Virtual matrices which are used for predicting MHC polymorphism and anchor residues. October 2 K 5 © Bioinformatics Centre, Uo. P 23

• Case study: Design & development of peptide vaccine against Japanese encephalitis virus October 2 K 5 © Bioinformatics Centre, Uo. P 24
We Have Chosen JE Virus, Because · JE virus is endemic in South-east Asia including India. · JE virus causes encephalitis in children between 5 -15 years of age with fatality rates between 21 -44%. · Man is a "DEAD END" host. October 2 K 5 © Bioinformatics Centre, Uo. P 25

We Have Chosen JE Virus, Because • Killed virus vaccine purified from mouse brain is used presently which requires storage at specific temperatures and hence not cost effective in tropical countries. • Protective prophylactic immunity is induced only after administration of 2 -3 doses. • Cost of vaccination, transportation is high. October 2 K 5 © Bioinformatics Centre, Uo. P storage and 26
Predicted structure of JEVS Mutations: JEVN/JEVS October 2 K 5 © Bioinformatics Centre, Uo. P 27
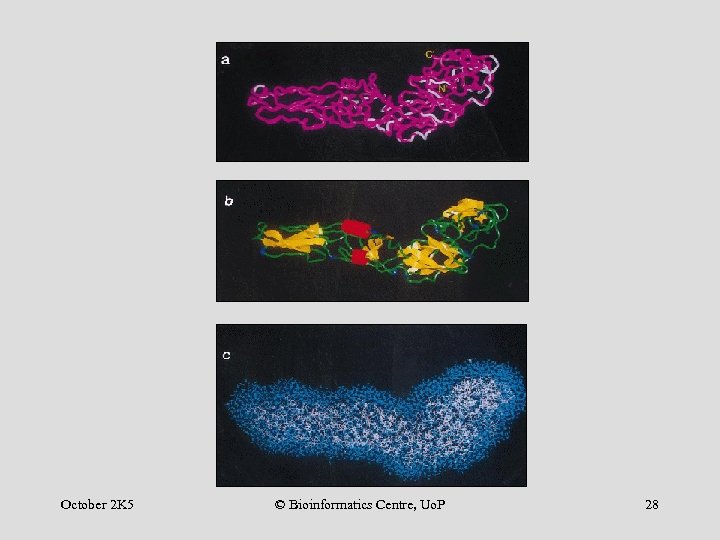
October 2 K 5 © Bioinformatics Centre, Uo. P 28
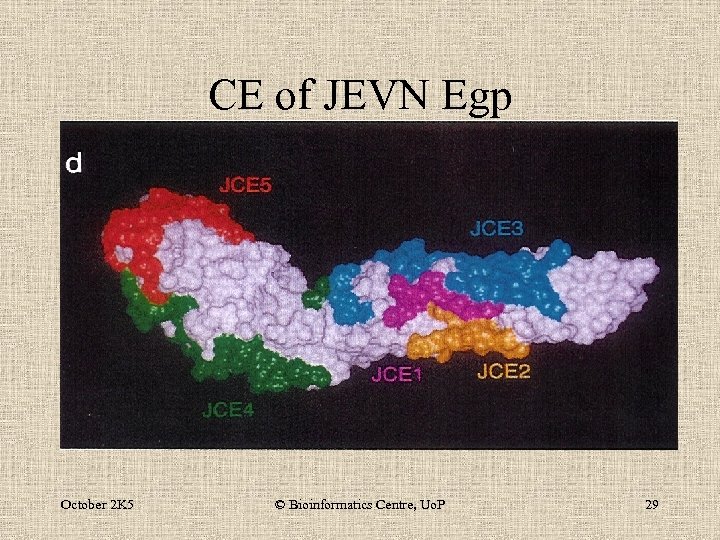
CE of JEVN Egp October 2 K 5 © Bioinformatics Centre, Uo. P 29
Species and Strain specific properties: TBEV/ JEVN/JEVS • Loop 1 in TBEV: LA EEH QGGT • Loop 1 in JEVN: HN EKR ADSS • Loop 1 in JEVS: HN KKR ADSS Antibodies recognising TBEV and JEVN would require exactly opposite pattern of charges in their CDR regions. Further, modification in CDR is required to recognise strain-specific region of JEVS. October 2 K 5 © Bioinformatics Centre, Uo. P 30

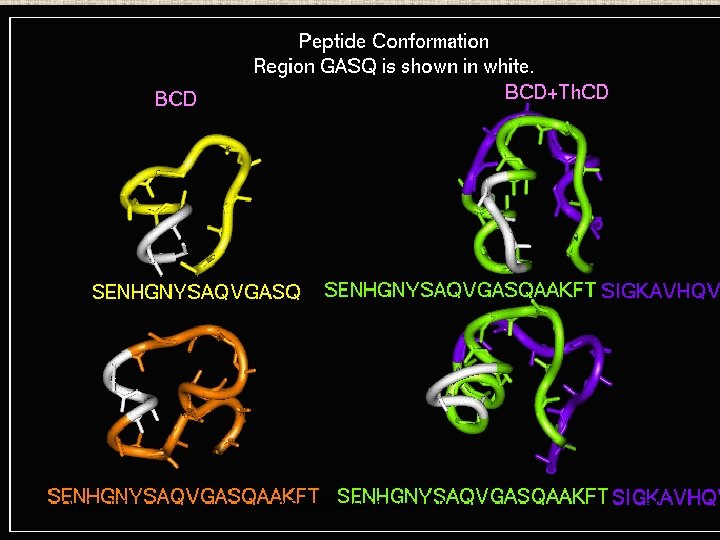
Multiple alignment of Predicted TH-cell epitope in the JE_Egp with corresponding epitopes in Egps of other Flaviviruses 426 457 JE DFGSIGGVFNSIGKAVHQVFGGAFRTLFGGMS MVE DFGSVGGVFNSIGKAVHQVFGGAFRTLFGGMS WNE DFGSVGGVFTSVGKAIHQVFGGAFRSLFGGMS KUN DFGSVGGVFTSVGKAVHQVFGGAFRSLFGGMS SLE DFGSIGGVFNSIGKAVHQVFGGAFRTLFGGMS DEN 2 DFGSLGGVFTSIGKALHQVFGAIYGAAFSGVS YF DFSSAGGFFTSVGKGIHTVFGSAFQGLFGGLN TBE DFGSAGGFLSSIGKAVHTVLGGAFNSIFGGVG COMM DF S GG S GK H V G F G Multiple alignment of JE_Egp with Egps of other Flaviviruses in the YSAQVGASQ region. 151 183 JE SENHGNYSAQVGASQAAKFTITPNAPSITLKLG MVE STSHGNYSTQIGANQAVRFTISPNAPAITAKMG WNE VESHG‑‑‑‑KIGATQAGRFSITPSAPSYTLKLG KUN VESHGNYFTQTGAAQAGRFSITPAAPSYTLKLG SLE STSHGNYSEQIGKNQAARFTISPQAPSFTANMG DEN 2 HAVGNDTG‑‑‑‑‑KHGKEIKITPQSSTTEAELT YF QENWN‑‑‑‑TDIKTLKFDALSGSQEVEFI October 2 K 5 © Bioinformatics Centre, Uo. P 31 TBE VAANETHS‑‑‑‑GRKTASFTIS‑‑SEKTILTMG
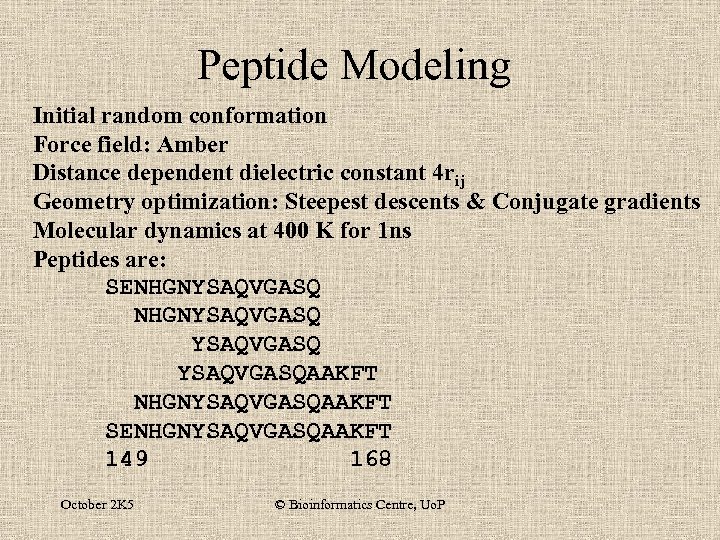
Peptide Modeling Initial random conformation Force field: Amber Distance dependent dielectric constant 4 rij Geometry optimization: Steepest descents & Conjugate gradients Molecular dynamics at 400 K for 1 ns Peptides are: SENHGNYSAQVGASQ YSAQVGASQAAKFT NHGNYSAQVGASQAAKFT SENHGNYSAQVGASQAAKFT 149 168 October 2 K 5 © Bioinformatics Centre, Uo. P
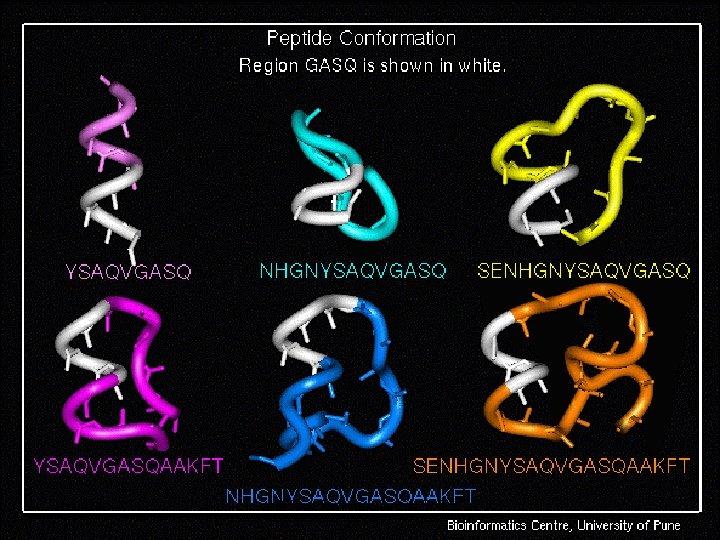
October 2 K 5 © Bioinformatics Centre, Uo. P 33
October 2 K 5 © Bioinformatics Centre, Uo. P 34
Relevant Publications & Patent • Urmila Kulkarni-Kale, Shriram Bhosale, G. Sunitha Manjari, Ashok Kolaskar, (2004). Vir. Gen: A comprehensive viral genome resource. Nucleic Acids Research 32: 289 -292. • Urmila Kulkarni-Kale & A. S. Kolaskar (2003). Prediction of 3 D structure of envelope glycoprotein of Sri Lanka strain of Japanese encephalitis virus. In Yi-Ping Phoebe Chen (ed. ), Conferences in research and practice in information technology. 19: 87 -96. • A. S. Kolaskar & Urmila Kulkarni-Kale (1999) Prediction of threedimensional structure and mapping of conformational antigenic determinants of envelope glycoprotein of Japanese encephalitis virus. Virology. 261: 31 -42. Patent: Chimeric T helper-B cell peptide as a vaccine for Flaviviruses. Dr. M. M. Gore, Dr. S. S. Dewasthaly, Prof. A. S. Kolaskar, Urmila Kulkarni-Kale Sangeeta Sawant WO 02/053182 A 1 October 2 K 5 © Bioinformatics Centre, Uo. P 35
Important references • • • Hopp, Woods, 1981, Prediction of protein antigenic determinants from amino acid sequences, PNAS U. S. A 78, 3824 -3828 Parker, Hodges et al, 1986, New hydrophilicity scale derived from high performance liquid chromatography peptide retention data: Correlation of predicted surface residues with antigenicity and X-ray derived accessible sites, Biochemistry: 25, 5425 -32 Kolaskar, Tongaonkar, 1990, A semi empirical method for prediction of antigenic determinants on protein antigens, FEBS 276, 172 -174 Men‚ndez-Arias, L. & Rodriguez, R. (1990), A BASIC microcomputer program forprediction of B and T cell epitopes in proteins, CABIOS, 6, 101 -105 Peter S. Stern (1991), Predicting antigenic sites on proteins, TIBTECH, 9, 163 -169 A. S. Kolaskar and Urmila Kulkarni-Kale, 1999 - Prediction of threedimensional structure and mapping of conformational epitopes of envelope glycoprotein of Japanese encephalitis virus, Virology, 261, 31 -42 October 2 K 5 © Bioinformatics Centre, Uo. P 36
dd40d55e4385b8370edec6eddf5609e6.ppt