Enzymes Professor of Novosibirsk State Agrarian University Korotkevich


Enzymes Professor of Novosibirsk State Agrarian University Korotkevich O.S.
Contents: The structure of enzymes Factors affecting enzymatic reactions Mechanism of enzymes
Enzyme structure Enzymes are proteins, which themselves are polymers of amino acids. Some enzymes have extra molecules associated with them, besides amino acids, that assist in the reaction they carry out. The protein portion of an enzyme is called the apoenzyme. A cofactor is the non-protein part of an enzyme. Cofacors can be loosely bound, coenzymes or tightly bound, prosthetic groups. The complete enzyme (apoprotein + cofactor) is termed the holoenzyme.
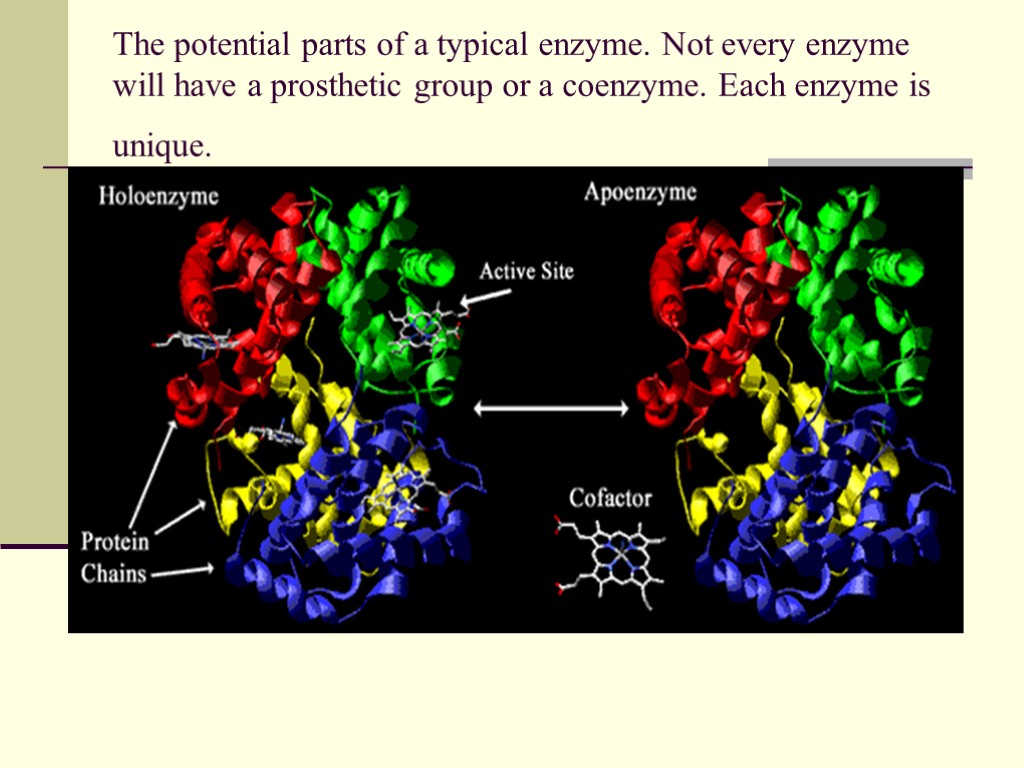
The potential parts of a typical enzyme. Not every enzyme will have a prosthetic group or a coenzyme. Each enzyme is unique.
Red blood cells contain the protein hemoglobin which has heme (the oxygen carrier in our blood) as a prosthetic group. Many of the vitamins are coenzymes. For example, niacin is a precursor used in the synthesis of NAD+ and pantothenic acid is a part of coenzyme A. Vitamins are crucial to catabolism, but they are only needed in trace amounts. Taking them in excess as proposed by some "health experts" usually has no benefit and in some cases can even be harmful.
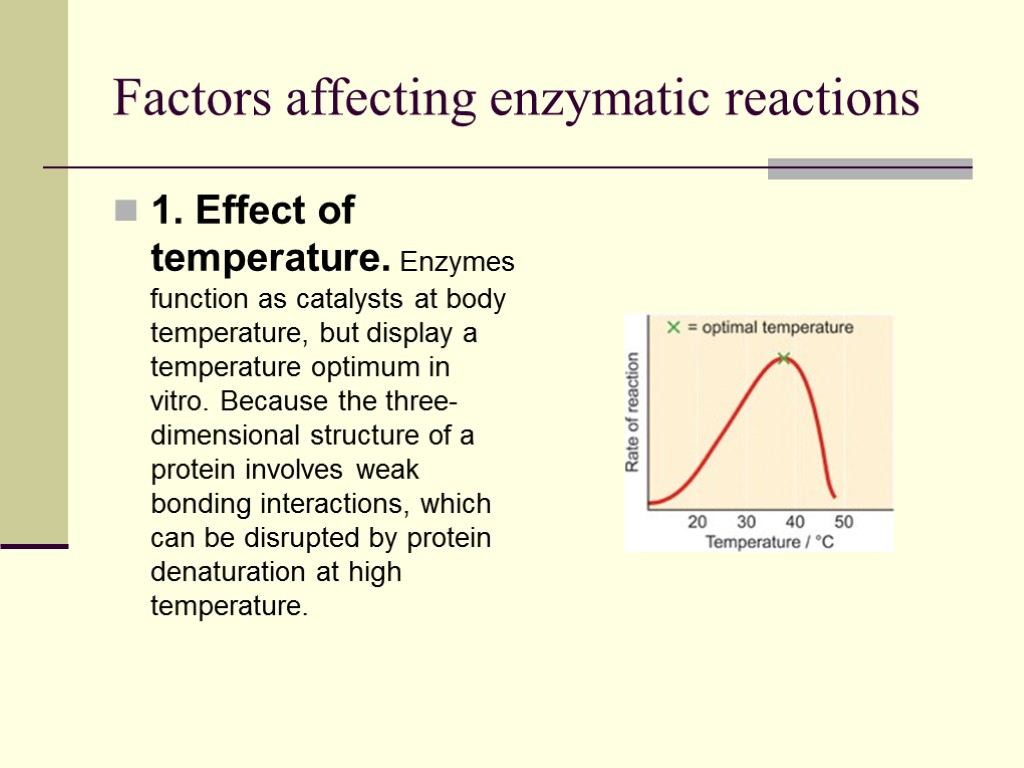
Factors affecting enzymatic reactions 1. Effect of temperature. Enzymes function as catalysts at body temperature, but display a temperature optimum in vitro. Because the three-dimensional structure of a protein involves weak bonding interactions, which can be disrupted by protein denaturation at high temperature.
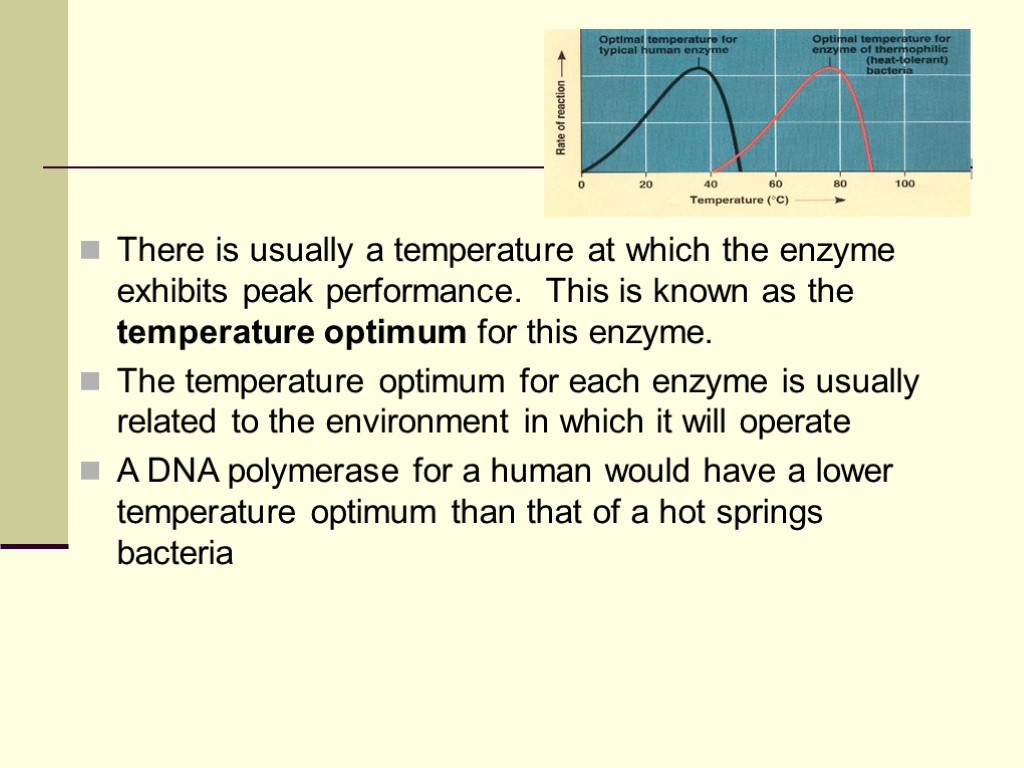
There is usually a temperature at which the enzyme exhibits peak performance. This is known as the temperature optimum for this enzyme. The temperature optimum for each enzyme is usually related to the environment in which it will operate A DNA polymerase for a human would have a lower temperature optimum than that of a hot springs bacteria
![2. pH - a measure of [H+] - acidic and basic conditions Like temperature, 2. pH - a measure of [H+] - acidic and basic conditions Like temperature,](https://present5.com/customparser/140611395_128688320 --- enzymes.ppt/slide_8.jpg)
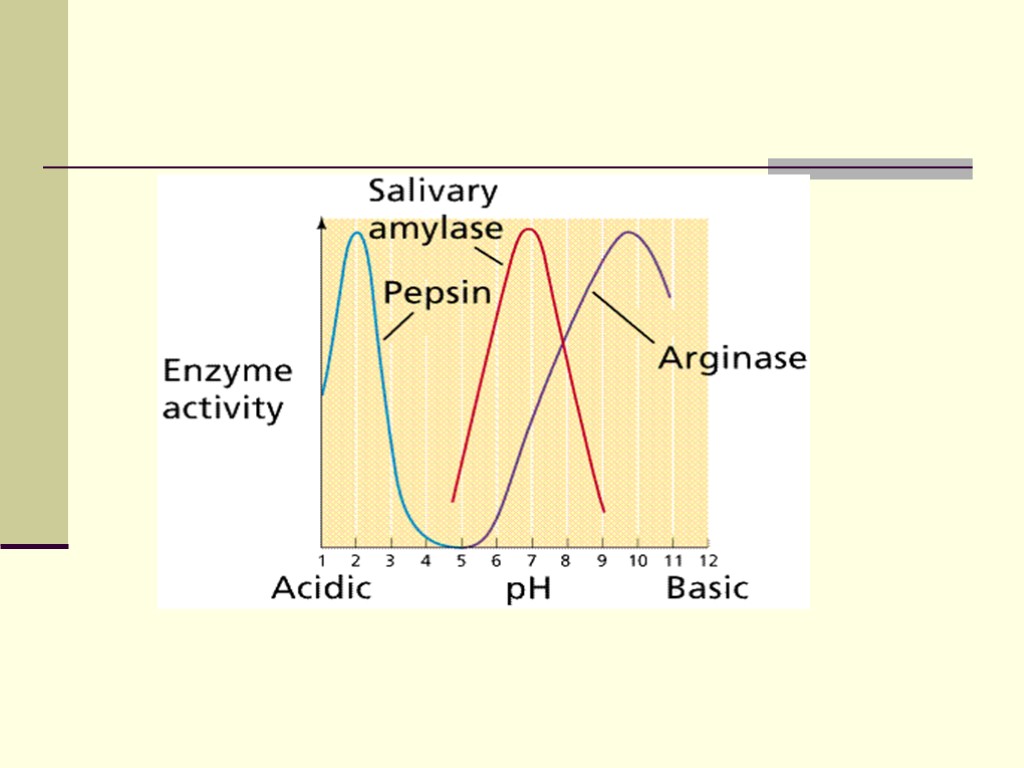
2. pH - a measure of [H+] - acidic and basic conditions Like temperature, most enzymes have a pH at which they perform at peak efficiency - the pH optimum Also like temperature, the pH optimum is related to the conditions in which it will be found At extreme pH's, the enzyme may denature
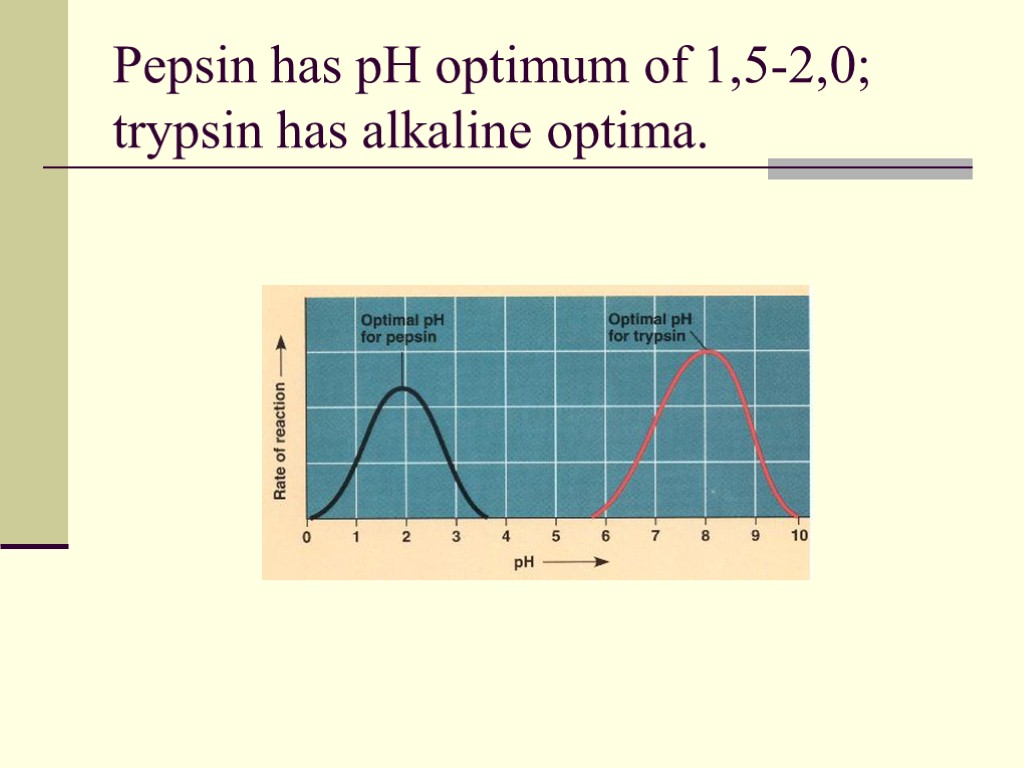
Pepsin has pH optimum of 1,5-2,0; trypsin has alkaline optima.
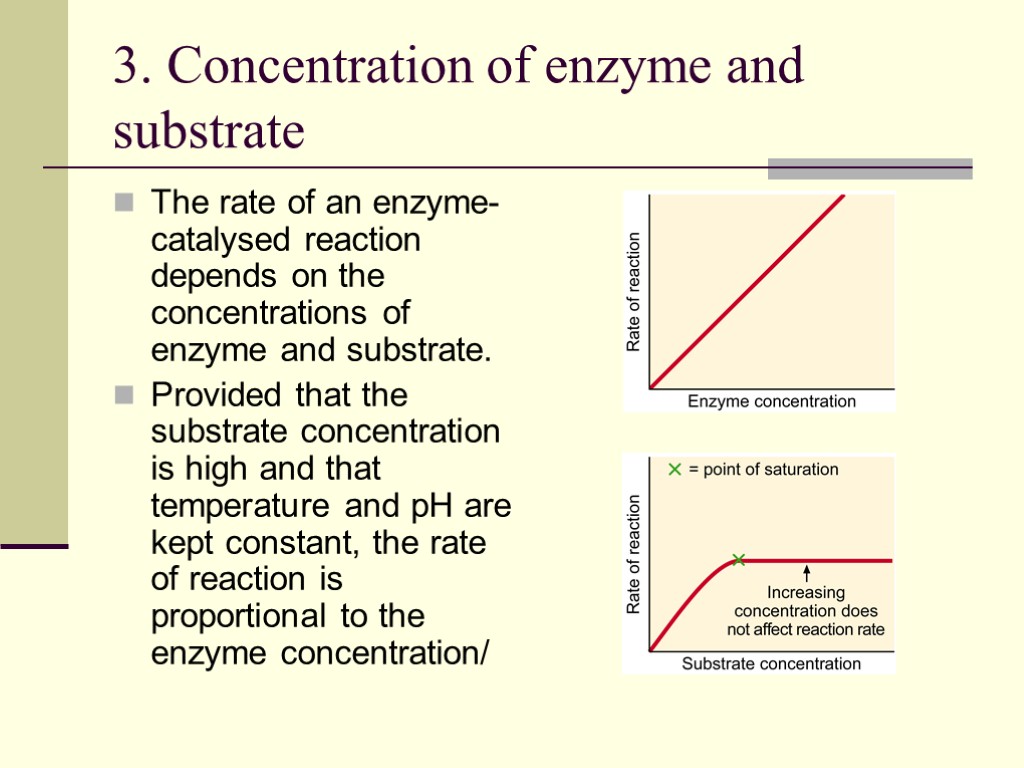
3. Concentration of enzyme and substrate The rate of an enzyme-catalysed reaction depends on the concentrations of enzyme and substrate. Provided that the substrate concentration is high and that temperature and pH are kept constant, the rate of reaction is proportional to the enzyme concentration/
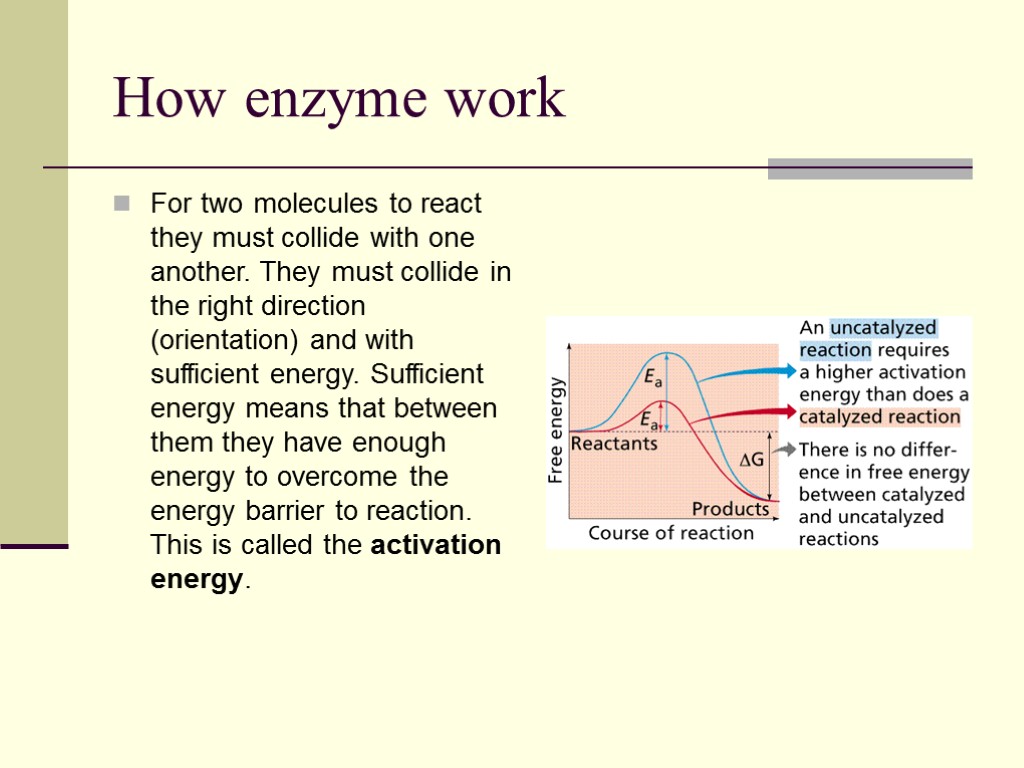
How enzyme work For two molecules to react they must collide with one another. They must collide in the right direction (orientation) and with sufficient energy. Sufficient energy means that between them they have enough energy to overcome the energy barrier to reaction. This is called the activation energy.
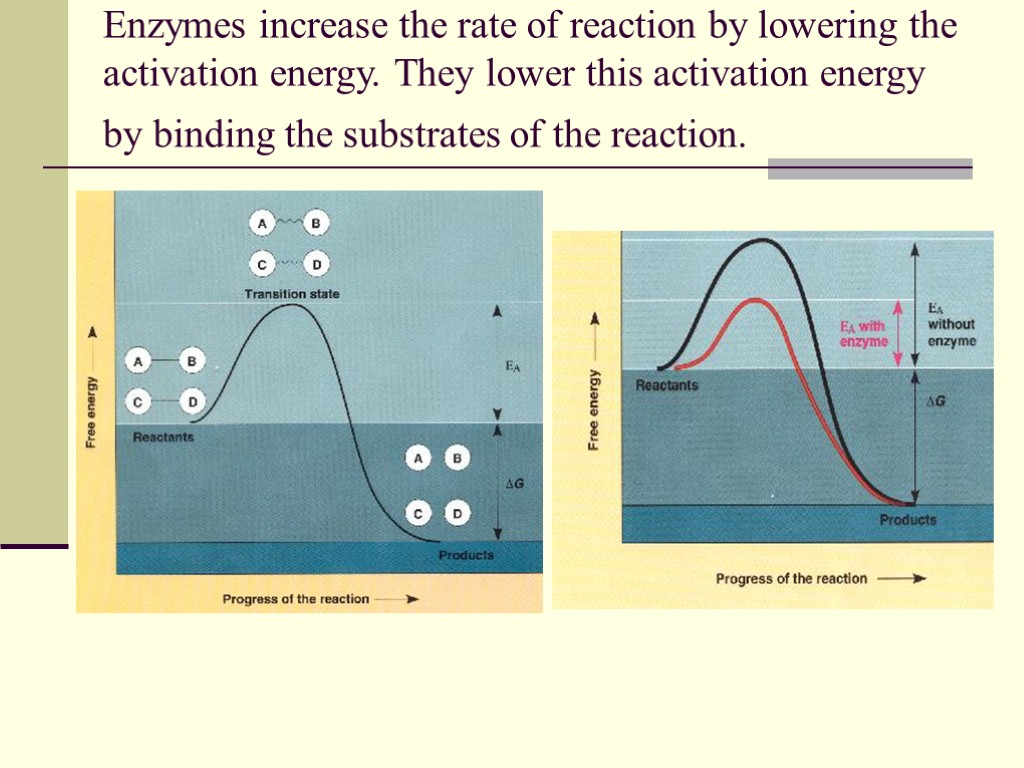
Enzymes increase the rate of reaction by lowering the activation energy. They lower this activation energy by binding the substrates of the reaction.

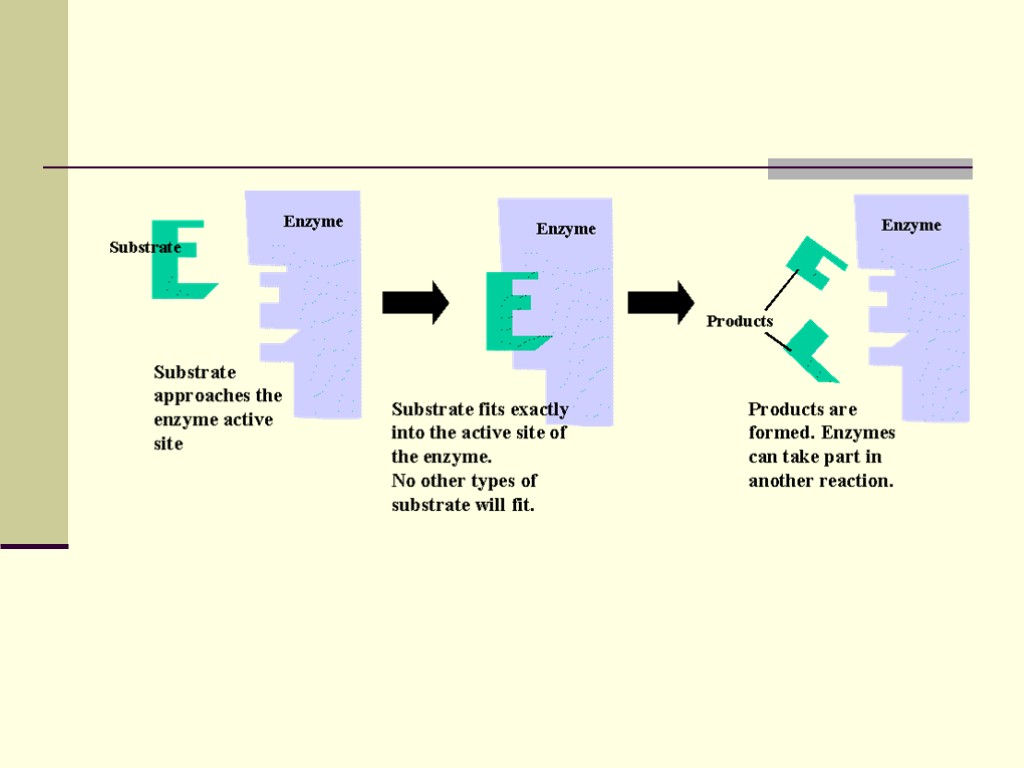
This causes a reaction to occur for two reasons. Enzymes bind their substrates at what is called the active site. When bound at the active site the substrates are brought into close proximity to one another and will react. The enzyme will bend the substrates in such a manner to make them more reactive. In biochemistry speak, the enzyme binds the substrates in a transition state complex. (Hey, a big word that you can use to make friends and influence people.) This makes it possible for substrates to react and form products.
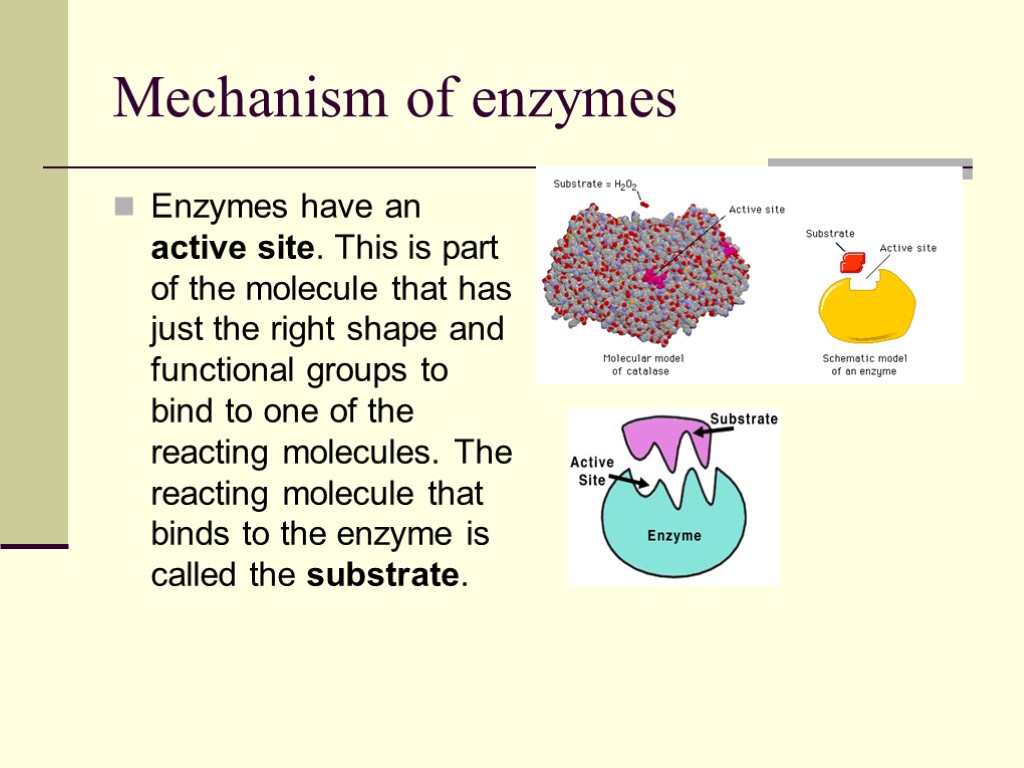
Mechanism of enzymes Enzymes have an active site. This is part of the molecule that has just the right shape and functional groups to bind to one of the reacting molecules. The reacting molecule that binds to the enzyme is called the substrate.
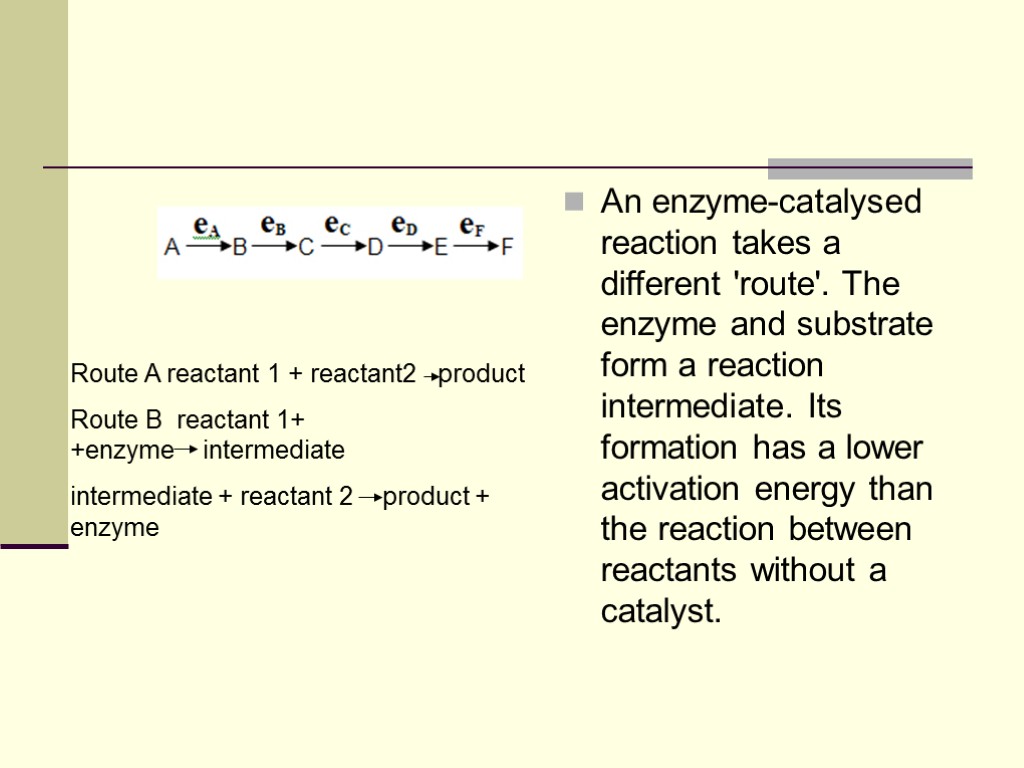
An enzyme-catalysed reaction takes a different 'route'. The enzyme and substrate form a reaction intermediate. Its formation has a lower activation energy than the reaction between reactants without a catalyst. Route A reactant 1 + reactant2 product Route B reactant 1+ +enzyme intermediate intermediate + reactant 2 product + enzyme
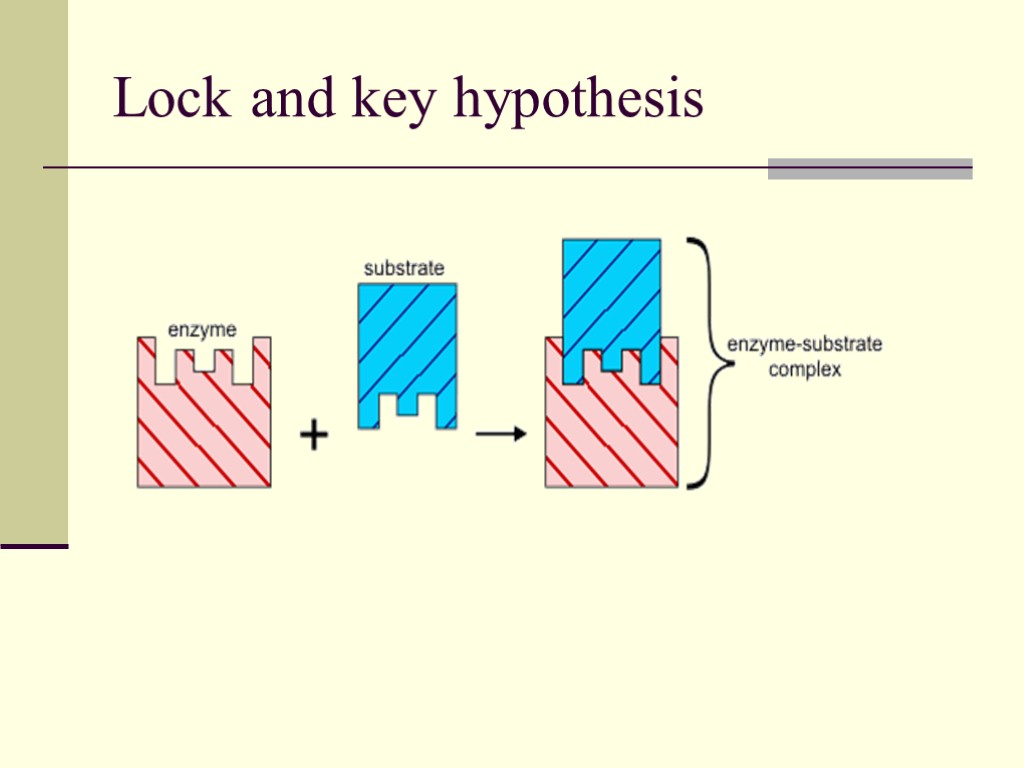
Lock and key hypothesis
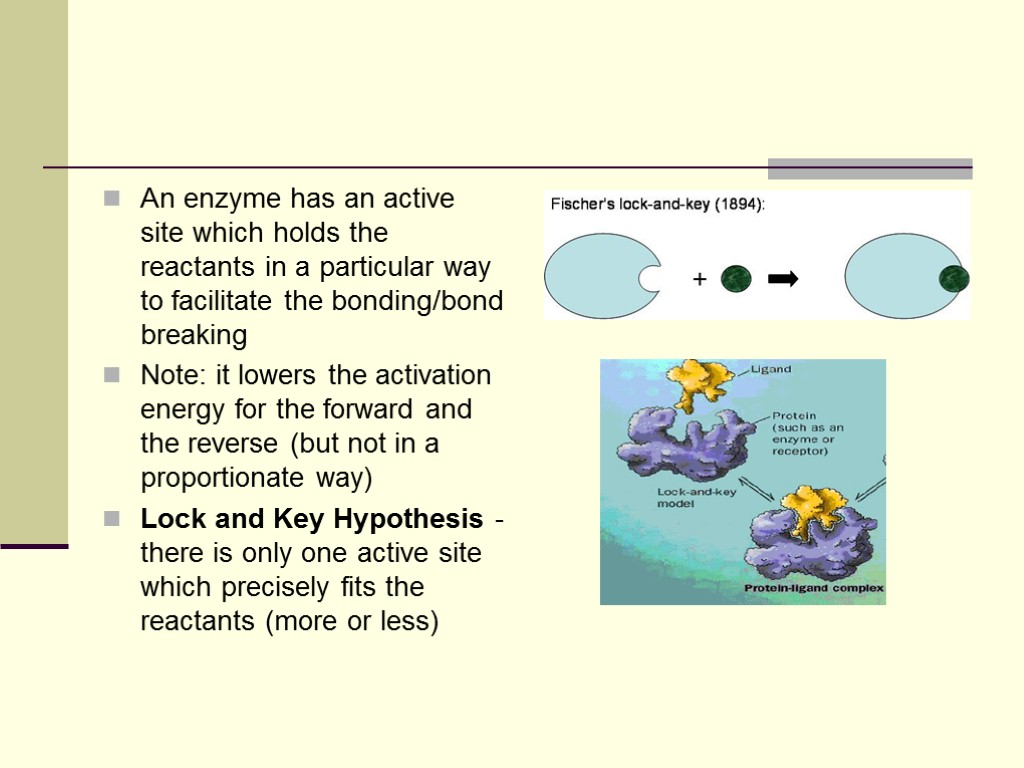
An enzyme has an active site which holds the reactants in a particular way to facilitate the bonding/bond breaking Note: it lowers the activation energy for the forward and the reverse (but not in a proportionate way) Lock and Key Hypothesis - there is only one active site which precisely fits the reactants (more or less)

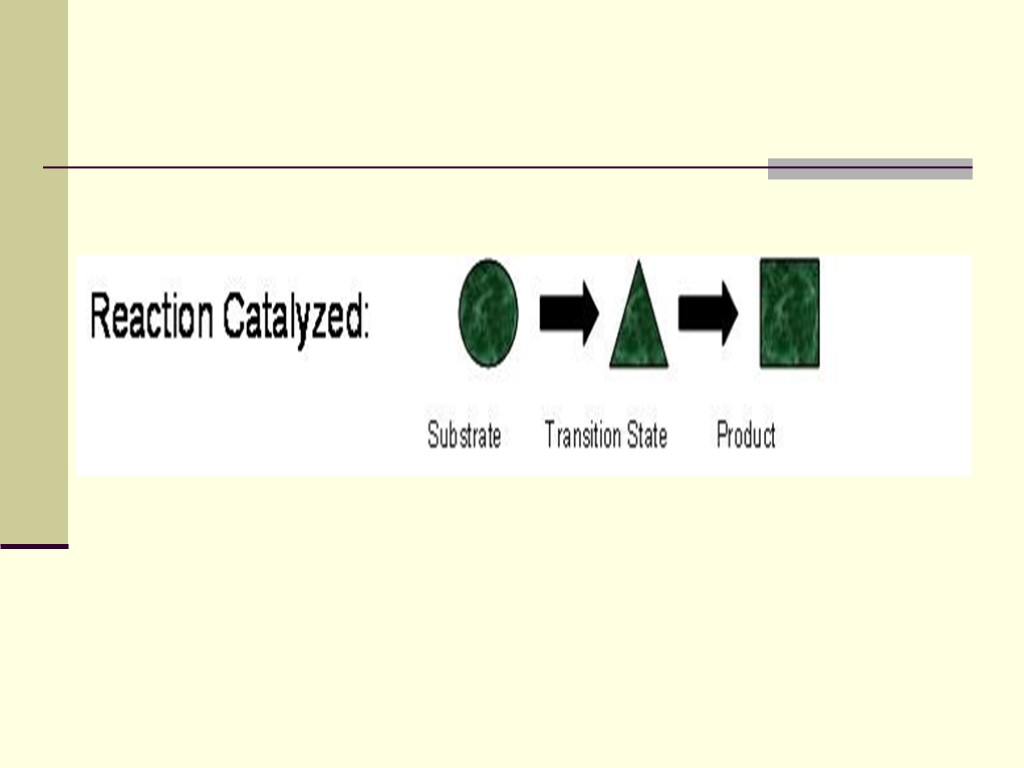
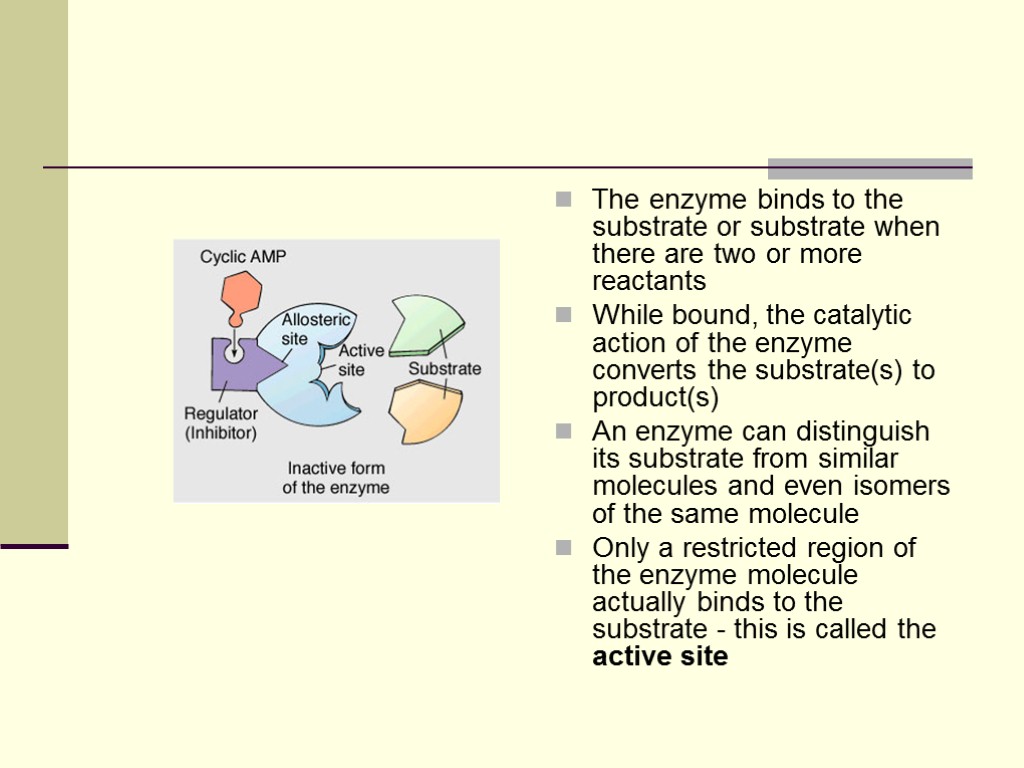
The enzyme binds to the substrate or substrate when there are two or more reactants While bound, the catalytic action of the enzyme converts the substrate(s) to product(s) An enzyme can distinguish its substrate from similar molecules and even isomers of the same molecule Only a restricted region of the enzyme molecule actually binds to the substrate - this is called the active site

Allosteric site - receptor site on some part of the enzyme remote from the active site can speed up or slow down enzyme function (enhancers and noncompetitive inhibitors) Example - enzymes of catabolic pathways have allosteric sites which can bind ATP and AMP ATP is an inhibitor, AMP is an enhancer When ATP prodction is greater than use, ATP will accumulate and then slow down or shut off the pathway When ATP production lags behind use, AMP will accumulate and enhance the pathway, creating more ATP

Thank you for your attention
enzymes.ppt
- Количество слайдов: 24

