Enzymes (continuation).ppt
- Количество слайдов: 30

Enzymes (continuation) Professor of Novosibirsk State Agrarian University Korotkevich O. S.

Contents: n Enzyme inhibition n Enzyme in clinical diagnosis and isoenzymes n Classification of enzymes
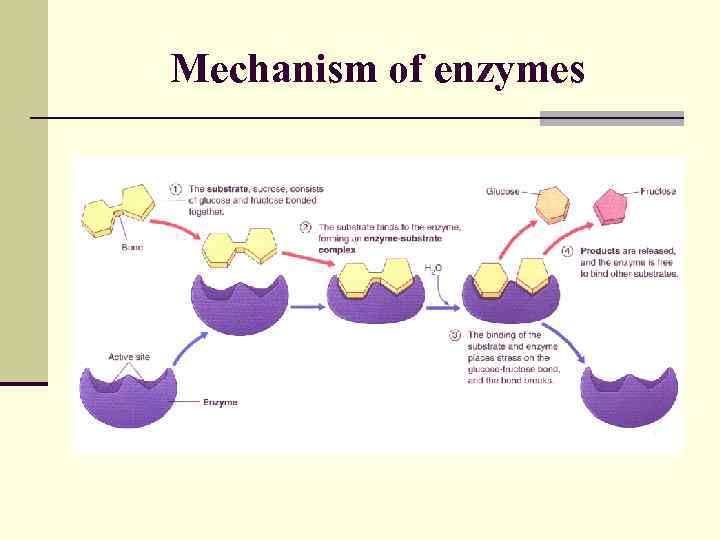
Mechanism of enzymes
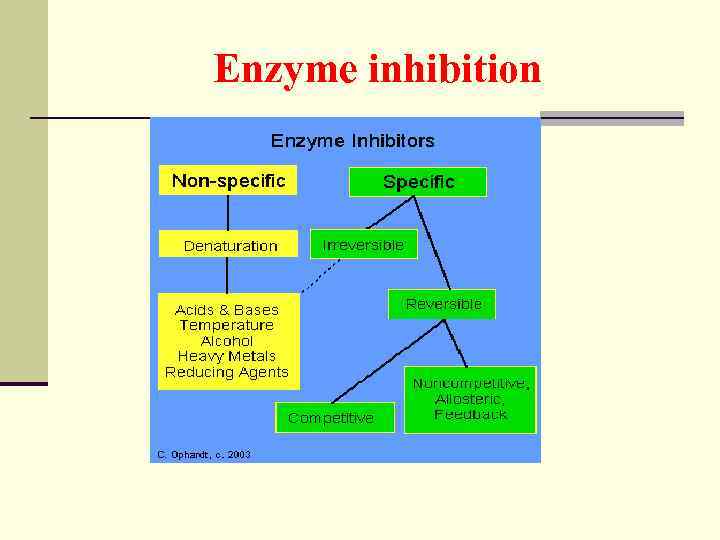
Enzyme inhibition

n An inhibitor, as the name implies, is a substance that interferes with the action of an enzyme and slows the rate of a reaction. n A good deal of information about enzymatic reactions can be obtained by observing the changes in the reaction caused by the presence of inhibitors.
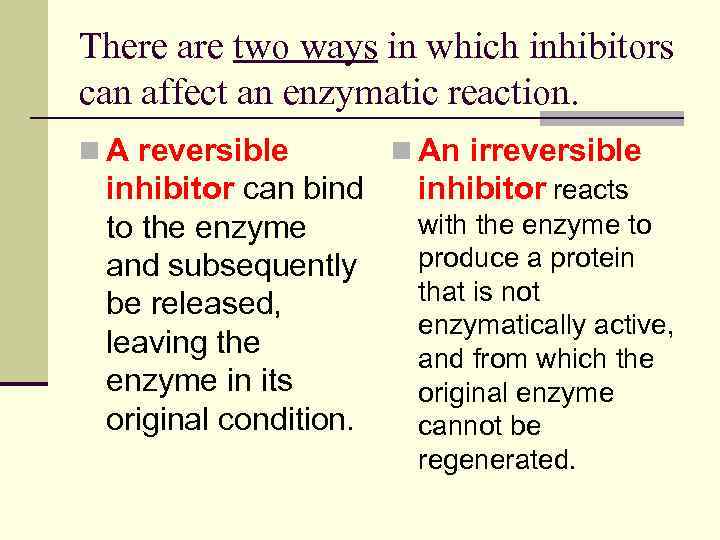
There are two ways in which inhibitors can affect an enzymatic reaction. n A reversible n An irreversible inhibitor can bind inhibitor reacts with the enzyme to to the enzyme produce a protein and subsequently that is not be released, enzymatically active, leaving the and from which the enzyme in its original enzyme original condition. cannot be regenerated.
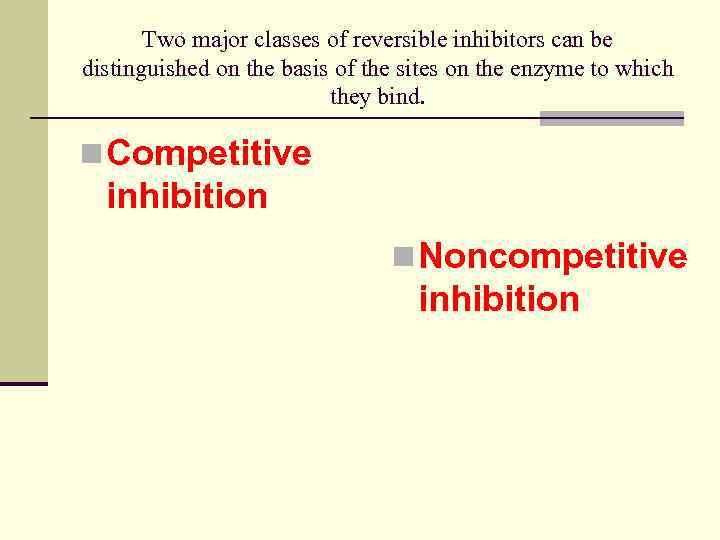
Two major classes of reversible inhibitors can be distinguished on the basis of the sites on the enzyme to which they bind. n Competitive inhibition n Noncompetitive inhibition

Competitive inhibition n One class consists of compounds very similar in structure to the substrate. In this case the inhibitor can bind to the active site and block the substrate’s access to it. This mode of action is called competitive inhibition because the inhibitor competes with the substrate for the active site on the enzyme.
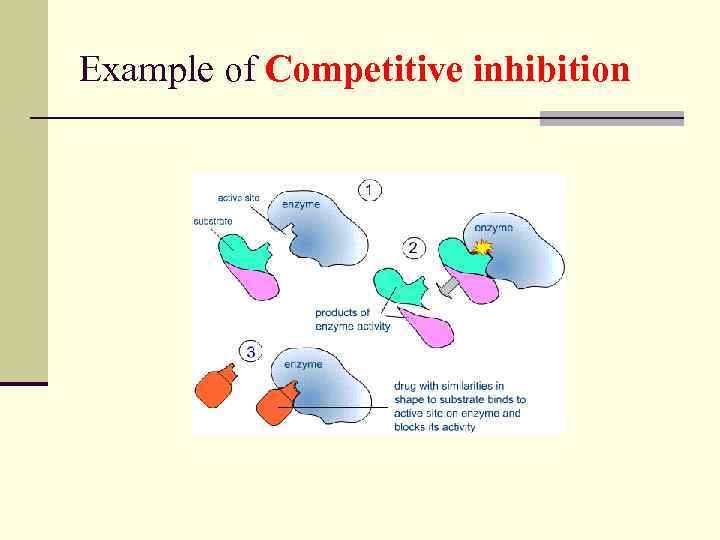
Example of Competitive inhibition
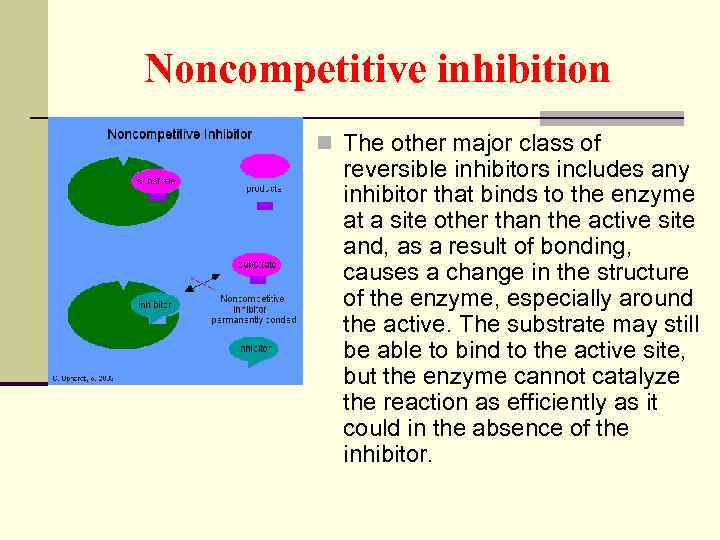
Noncompetitive inhibition n The other major class of reversible inhibitors includes any inhibitor that binds to the enzyme at a site other than the active site and, as a result of bonding, causes a change in the structure of the enzyme, especially around the active. The substrate may still be able to bind to the active site, but the enzyme cannot catalyze the reaction as efficiently as it could in the absence of the inhibitor.
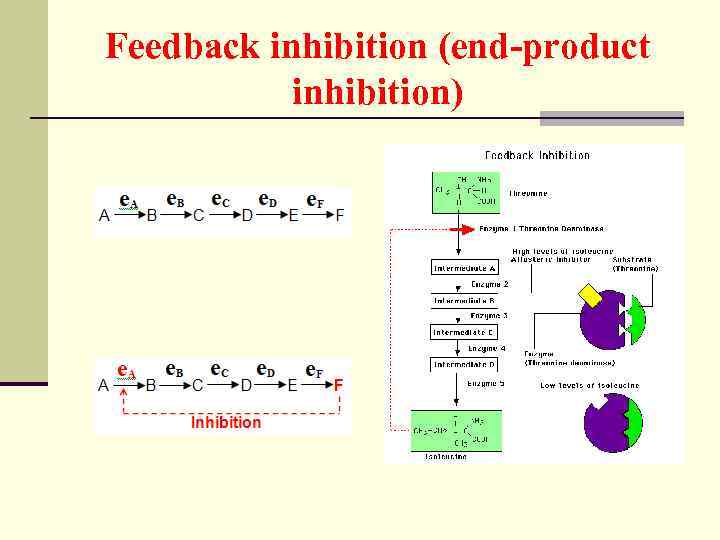
Feedback inhibition (end-product inhibition)
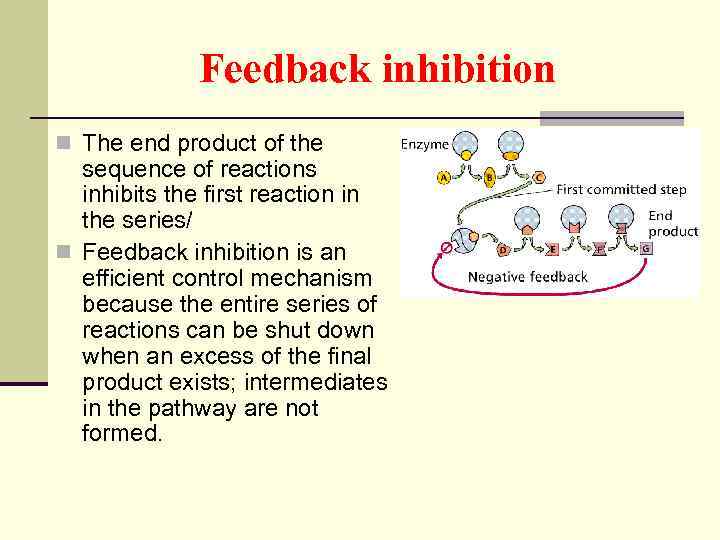
Feedback inhibition n The end product of the sequence of reactions inhibits the first reaction in the series/ n Feedback inhibition is an efficient control mechanism because the entire series of reactions can be shut down when an excess of the final product exists; intermediates in the pathway are not formed.
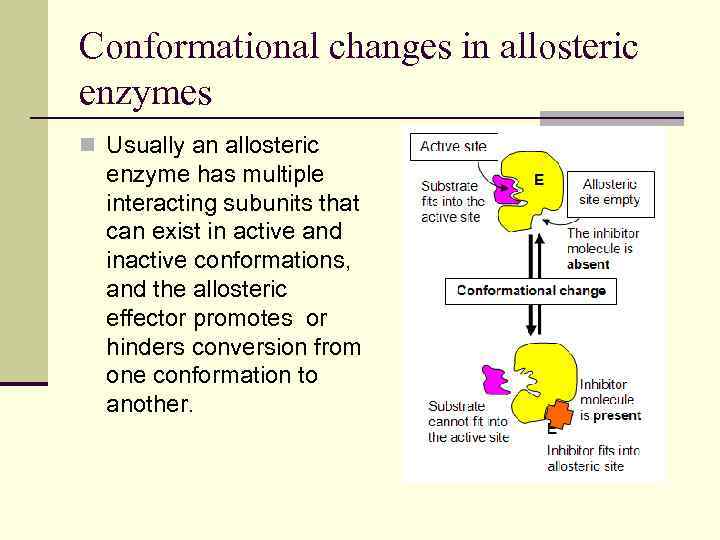
Conformational changes in allosteric enzymes n Usually an allosteric enzyme has multiple interacting subunits that can exist in active and inactive conformations, and the allosteric effector promotes or hinders conversion from one conformation to another.
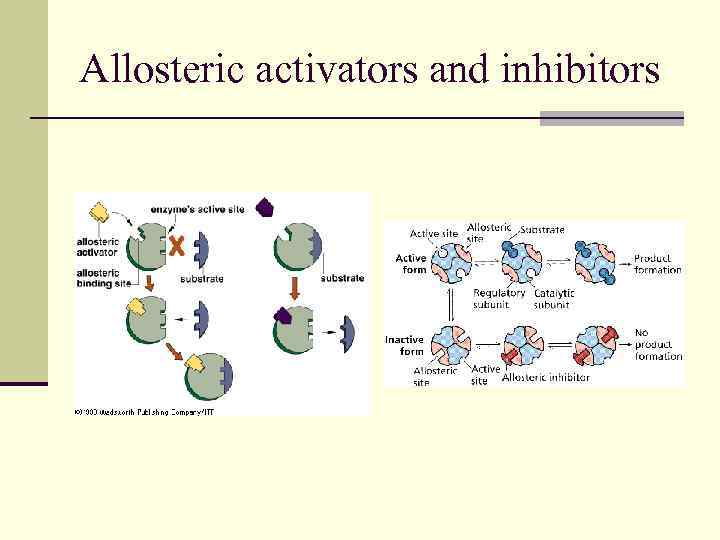
Allosteric activators and inhibitors
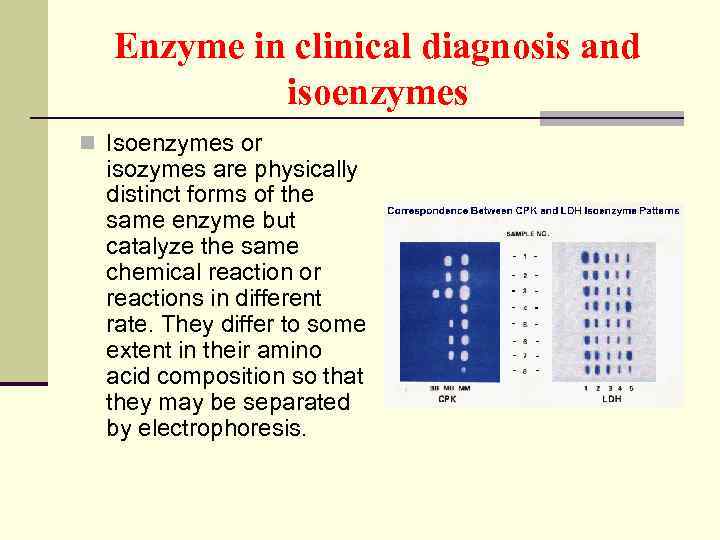
Enzyme in clinical diagnosis and isoenzymes n Isoenzymes or isozymes are physically distinct forms of the same enzyme but catalyze the same chemical reaction or reactions in different rate. They differ to some extent in their amino acid composition so that they may be separated by electrophoresis.

LDH Isoenzymes n LDH is a tetramer composed of two different subunits, M and H. Five different isoenzymes are possible: M 4, M 3 H, M 2 H 2, MH 3, and H 4, and all five are present in different tissue in different relative amounts. The five different isoenzymes are resolved by electrophoresis and determination of the LDH isoenzyme distribution by electrophoresis is a commonly performed clinical laboratory test.
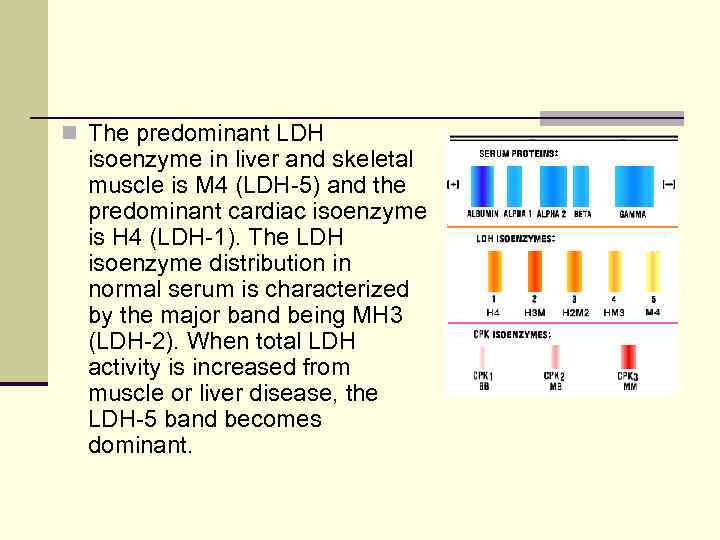
n The predominant LDH isoenzyme in liver and skeletal muscle is M 4 (LDH-5) and the predominant cardiac isoenzyme is H 4 (LDH-1). The LDH isoenzyme distribution in normal serum is characterized by the major band being MH 3 (LDH-2). When total LDH activity is increased from muscle or liver disease, the LDH-5 band becomes dominant.
n Increased LDH activity from MI is characterized by a predominance of the LDH 1 band. The change from the dominance of the LDH-2 band in normal serum to the dominance of the LDH-1 band is called the "LDH flip" and its occurrence is considered diagnostic for MI. However, the major LDH isoenzyme in erythrocytes is LDH-1 so that hemolysis, either intravascular or in the collected blood specimen, may also cause the LDH flip

Electrophoresis
Classification of enzymes n Enzymes are commonly named by adding a suffix "-ase" to the root name of the substrate molecule it is acting upon. For example, Lipase catalyzes the hydrolysis of a lipid triglyceride. Sucrase catalyzes the hydrolysis of sucrose into glucose and fructose. n A few enzymes discovered before this naming system was devised are known by common names. Examples are pepsin, trypsin, and chymotrypsin which catalyzes the hydrolysis of proteins.
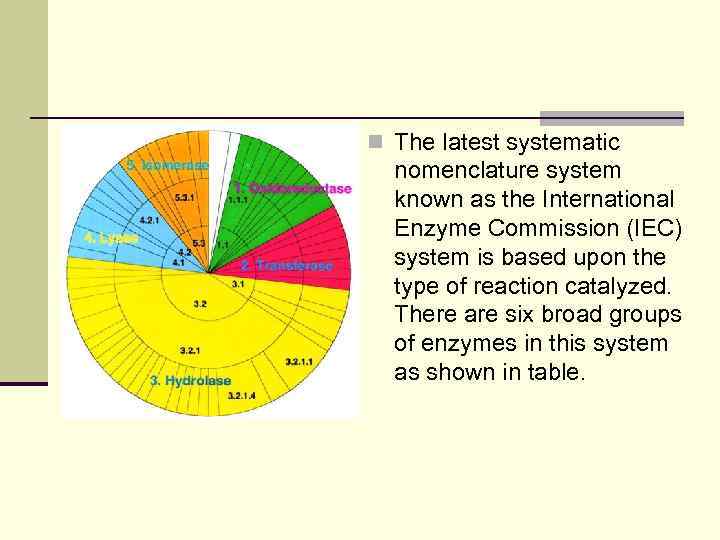
n The latest systematic nomenclature system known as the International Enzyme Commission (IEC) system is based upon the type of reaction catalyzed. There are six broad groups of enzymes in this system as shown in table.
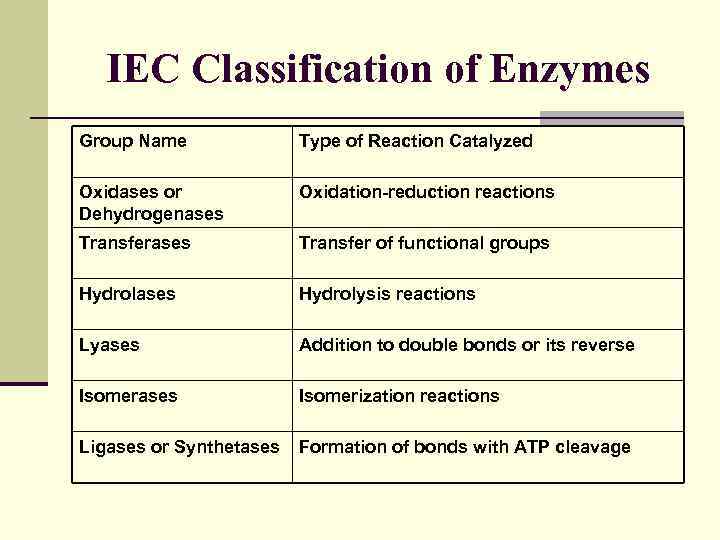
IEC Classification of Enzymes Group Name Type of Reaction Catalyzed Oxidases or Dehydrogenases Oxidation-reduction reactions Transferases Transfer of functional groups Hydrolases Hydrolysis reactions Lyases Addition to double bonds or its reverse Isomerases Isomerization reactions Ligases or Synthetases Formation of bonds with ATP cleavage
1. Oxidoreductases n catalyze a variety of oxidation-reduction reactions. Common names include dehydrogenase, oxidase, reductase and catalase.
2. Transferases n catalyze transfers of groups (acetyl, methyl, phosphate, etc. ). Common names include acetyltransferase, methylase, protein kinase and polymerase. The first three subclasses play major roles in the regulation of cellular processes. The polymerase is essential for the synthesis of DNA and RNA.

3. Hydrolases n catalyze hydrolysis reactions where a molecule is split into two or more smaller molecules by the addition of water. Common examples are given below. n Proteases splits protein molecules. Examples: HIV protease and caspase. HIV protease is essential for HIV replication. Caspase plays a major role in apoptosis.
n Nucleases splits nucleic acids (DNA and RNA). Based on the substrate type, they are divided into RNase and DNase. RNase catalyzes the hydrolysis of RNA and DNase acts on DNA. They may also be divided into exonuclease and endonuclease. The exonuclease progressively splits off single nucleotides from one end of DNA or RNA. The endonuclease splits DNA or RNA at internal sites. n Phosphatase catalyzes dephosphorylation (removal of phosphate groups). Example: calcineurin. The immunosuppressive drugs FK 506 and Cyclosporin A are the inhibitors of calcineurin.
4. Lyases n catalyze the cleavage of C-C, C-O, C-S and C-N bonds by means other than hydrolysis or oxidation. Common names include decarboxylase and aldolase.
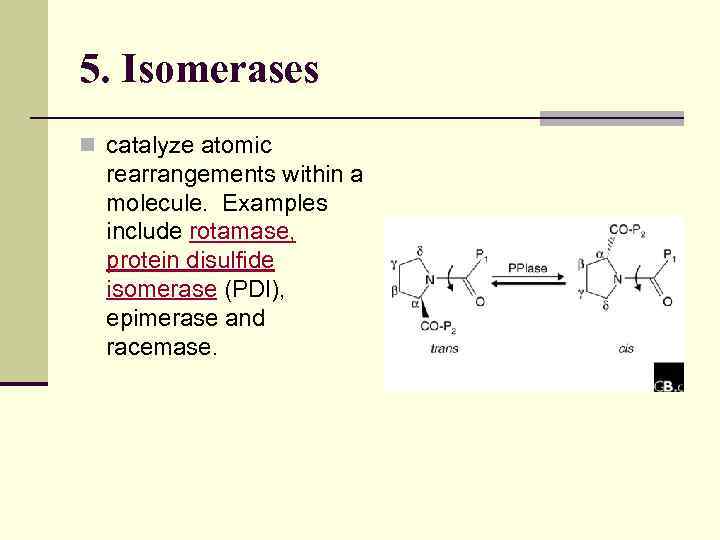
5. Isomerases n catalyze atomic rearrangements within a molecule. Examples include rotamase, protein disulfide isomerase (PDI), epimerase and racemase.
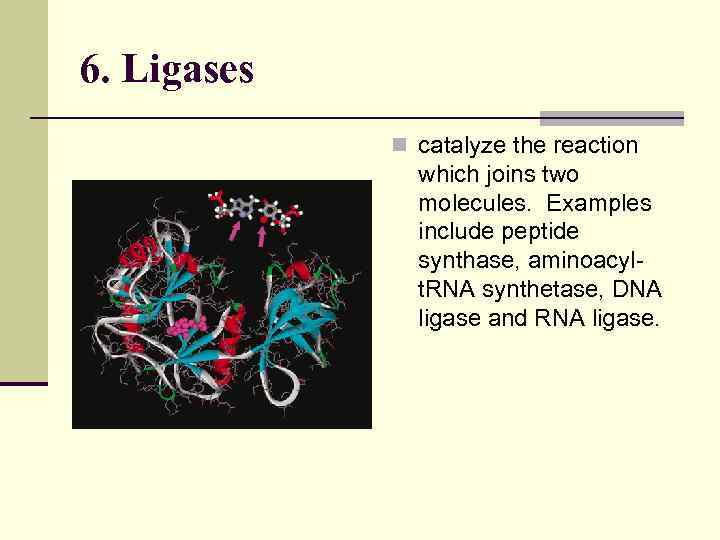
6. Ligases n catalyze the reaction which joins two molecules. Examples include peptide synthase, aminoacylt. RNA synthetase, DNA ligase and RNA ligase.

Enzymes (continuation).ppt