b3312fc91c0f5670ea830c8837223e6e.ppt
- Количество слайдов: 92
 Envr 725 l l Tues. and Thurs- 3 credit hours 11 am to 12: 15 pm snow days call me at 942 4880 or cell 919 614 4730 l room 0015 MHRC l http: //www. unc. edu/courses/2007 spring/envr/725/ 001/Envr 725. html l Rich Kamens; 966 5452 kamens@unc. edu http: //airsite. unc. edu/~kamens/ 1
Envr 725 l l Tues. and Thurs- 3 credit hours 11 am to 12: 15 pm snow days call me at 942 4880 or cell 919 614 4730 l room 0015 MHRC l http: //www. unc. edu/courses/2007 spring/envr/725/ 001/Envr 725. html l Rich Kamens; 966 5452 kamens@unc. edu http: //airsite. unc. edu/~kamens/ 1
 Introduction to Environmental Physical Organic Chemistry l Environmental chemistry may be defined as "the study of sources, reactions, transport, effects, and fates of chemical species in water, soil, and air environments, and the effects of technology thereon. ” Manahan, 1994 2
Introduction to Environmental Physical Organic Chemistry l Environmental chemistry may be defined as "the study of sources, reactions, transport, effects, and fates of chemical species in water, soil, and air environments, and the effects of technology thereon. ” Manahan, 1994 2
 Class objectives: l Highlight some important areas in environmental chemistry l present some of the common techniques that environmental chemists use to quantify process that occur in the environment u It is assumed that everyone has courses in organic and physical chemistry. 3
Class objectives: l Highlight some important areas in environmental chemistry l present some of the common techniques that environmental chemists use to quantify process that occur in the environment u It is assumed that everyone has courses in organic and physical chemistry. 3
 Class objectives: u Partitioning is a thread that runs through the course u Linear free energy relationships will be used to help quantify equilibrium and kinetic processes 4
Class objectives: u Partitioning is a thread that runs through the course u Linear free energy relationships will be used to help quantify equilibrium and kinetic processes 4
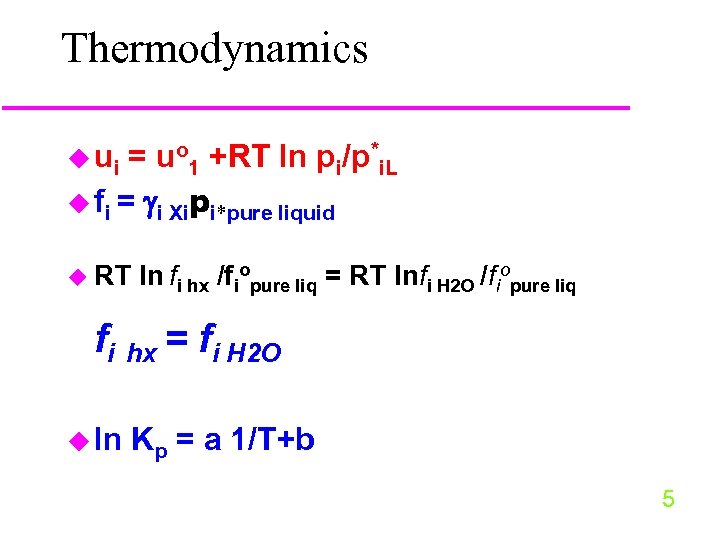 Thermodynamics u ui = uo 1 +RT ln pi/p*i. L u fi = i Xipi*pure liquid u RT ln fi hx /fiopure liq = RT lnfi H 2 O /fiopure liq fi hx = fi H 2 O u ln Kp = a 1/T+b 5
Thermodynamics u ui = uo 1 +RT ln pi/p*i. L u fi = i Xipi*pure liquid u RT ln fi hx /fiopure liq = RT lnfi H 2 O /fiopure liq fi hx = fi H 2 O u ln Kp = a 1/T+b 5
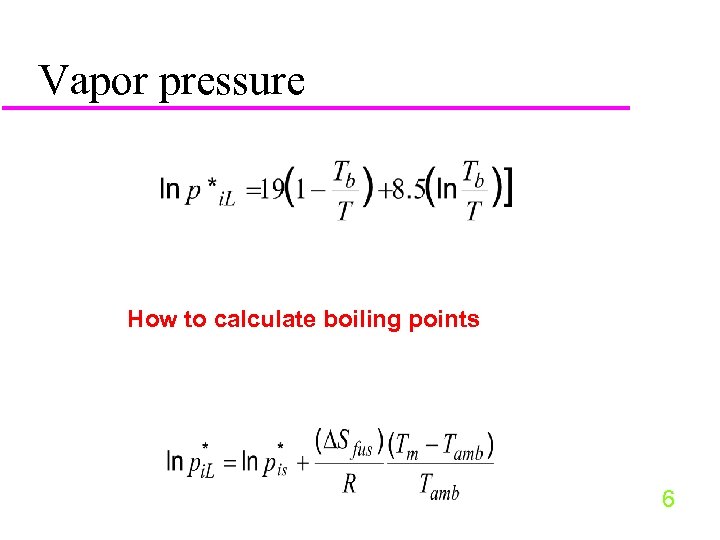 Vapor pressure How to calculate boiling points 6
Vapor pressure How to calculate boiling points 6
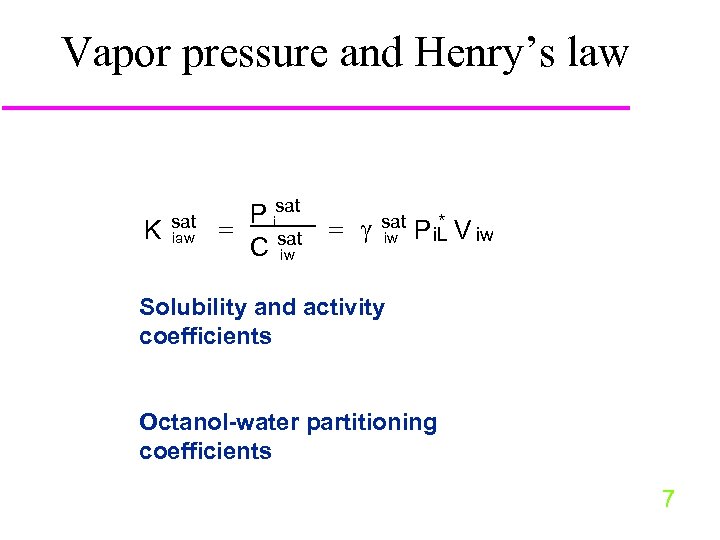 Vapor pressure and Henry’s law K sat iaw P isat C sat iw * P i. L V iw Solubility and activity coefficients Octanol-water partitioning coefficients 7
Vapor pressure and Henry’s law K sat iaw P isat C sat iw * P i. L V iw Solubility and activity coefficients Octanol-water partitioning coefficients 7
 Additional Principles u Organic Acid-bases and LFERs u diffusion u chemical u Organic u Solidu spills and mass transfer reactions in the environment liquid interactions photochemistry 8
Additional Principles u Organic Acid-bases and LFERs u diffusion u chemical u Organic u Solidu spills and mass transfer reactions in the environment liquid interactions photochemistry 8
 Homework, quizzes, exams u To insure that most of us stay reasonably current with the lectures and readings, an option is to have 6 -8 unannounced quizzes throughout the semester. u They will take ~10 minutes. The first quiz will be on Chapter 2 since we will not cover Chapter 2. Quizzes will count 10% of your grade. 9
Homework, quizzes, exams u To insure that most of us stay reasonably current with the lectures and readings, an option is to have 6 -8 unannounced quizzes throughout the semester. u They will take ~10 minutes. The first quiz will be on Chapter 2 since we will not cover Chapter 2. Quizzes will count 10% of your grade. 9
 u Another option is a set of short questions to be answered and handed in before most lectures (5% of grade) —your choice! 10
u Another option is a set of short questions to be answered and handed in before most lectures (5% of grade) —your choice! 10
 u There will be a homework problem set associated with each assigned chapter of the book. It is due a week after the completion of the book chapter. u These problem sets should take between 3 and 10 hrs. Answers will graded and returned to you as soon as possible. These will count for 25% of your grade. 11
u There will be a homework problem set associated with each assigned chapter of the book. It is due a week after the completion of the book chapter. u These problem sets should take between 3 and 10 hrs. Answers will graded and returned to you as soon as possible. These will count for 25% of your grade. 11
 u In addition, you are expected to work through the illustrative examples and problems which have answers in the test on your own. u Some of these could appear on exams u There will be three exams (70% of your grade ), 25% homeworks, 5%? ? ? 12
u In addition, you are expected to work through the illustrative examples and problems which have answers in the test on your own. u Some of these could appear on exams u There will be three exams (70% of your grade ), 25% homeworks, 5%? ? ? 12
 Important Environmental Issues l Global warming and stratospheric ozone depletion l Concentration of environmental pollutants at the poles; pesticides in foods, etc. l Buildup of environmental chemicals in the oceans; contamination of soil and ground water l Particle exposure, photochemical oxidant exposure, acid deposition l Energy shortages 13
Important Environmental Issues l Global warming and stratospheric ozone depletion l Concentration of environmental pollutants at the poles; pesticides in foods, etc. l Buildup of environmental chemicals in the oceans; contamination of soil and ground water l Particle exposure, photochemical oxidant exposure, acid deposition l Energy shortages 13
 Why the interest? u There are more than 70, 000 synthetic chemicals that are in daily use: – solvents – components of detergents – dyes and varnishes – additives in plastics and textiles – chemicals used for construction – antifouling agents – herbicides, insecticides, fungicides
Why the interest? u There are more than 70, 000 synthetic chemicals that are in daily use: – solvents – components of detergents – dyes and varnishes – additives in plastics and textiles – chemicals used for construction – antifouling agents – herbicides, insecticides, fungicides
 Some examples of environmental chemicals Polynuclear Aromatic HC (PAHs) u Dioxins u Ketones u PCBs u CFCs u DDT u O 3, NO 2, aerosols, SO 2 u
Some examples of environmental chemicals Polynuclear Aromatic HC (PAHs) u Dioxins u Ketones u PCBs u CFCs u DDT u O 3, NO 2, aerosols, SO 2 u
 PAHs u Formed from small ethylene radicals “building blocks” produced when carbon based fuels are burned u Sources u in are all types of burning Chiang. Mai, Thailand: a) 2 -stroke motorcycle engines b) cars- light diesels c) open burning d) barbecued meat? ?
PAHs u Formed from small ethylene radicals “building blocks” produced when carbon based fuels are burned u Sources u in are all types of burning Chiang. Mai, Thailand: a) 2 -stroke motorcycle engines b) cars- light diesels c) open burning d) barbecued meat? ?
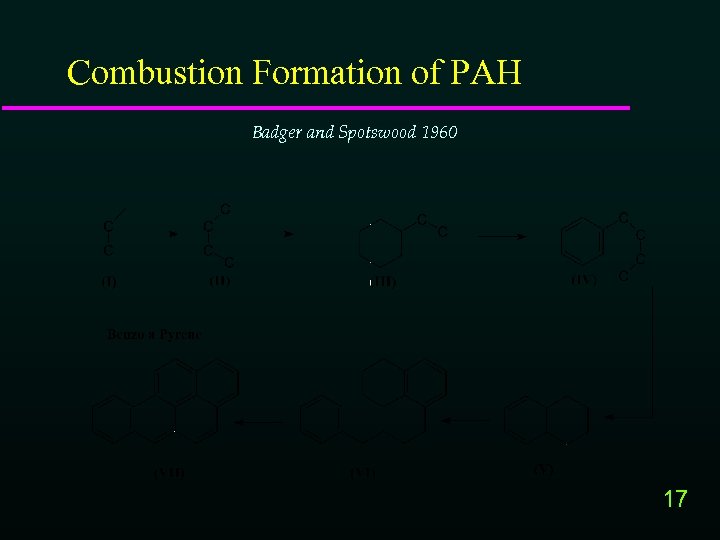 Combustion Formation of PAH Badger and Spotswood 1960 17
Combustion Formation of PAH Badger and Spotswood 1960 17
![Some PAH structures naphthalene anthracene fluoranthene benz(a)anthracene phenanthrene benzo(a)pyrene [Ba. P] 18 Some PAH structures naphthalene anthracene fluoranthene benz(a)anthracene phenanthrene benzo(a)pyrene [Ba. P] 18](https://present5.com/presentation/b3312fc91c0f5670ea830c8837223e6e/image-18.jpg) Some PAH structures naphthalene anthracene fluoranthene benz(a)anthracene phenanthrene benzo(a)pyrene [Ba. P] 18
Some PAH structures naphthalene anthracene fluoranthene benz(a)anthracene phenanthrene benzo(a)pyrene [Ba. P] 18
 PAHs u. Naphthalene, phenanthrene and anthracene are found in the gas phase pyrene and fluoranthene are in both the gas and particle phase u u. Ba. A and Ba. P are mostly on the particles, Why? ? ?
PAHs u. Naphthalene, phenanthrene and anthracene are found in the gas phase pyrene and fluoranthene are in both the gas and particle phase u u. Ba. A and Ba. P are mostly on the particles, Why? ? ?
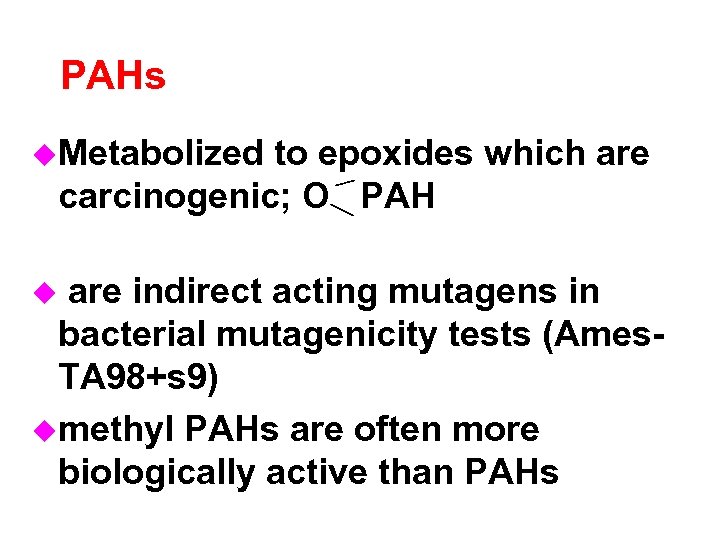 PAHs u. Metabolized to epoxides which are carcinogenic; O PAH are indirect acting mutagens in bacterial mutagenicity tests (Ames. TA 98+s 9) umethyl PAHs are often more biologically active than PAHs u
PAHs u. Metabolized to epoxides which are carcinogenic; O PAH are indirect acting mutagens in bacterial mutagenicity tests (Ames. TA 98+s 9) umethyl PAHs are often more biologically active than PAHs u
 Carcinogenic tests with PAHs u. Professor Gernot Grimmer extracted different types of smoke particles u He then took the extract and applied it to mouse skin u and implanted it into rat lungs u How did he obtain extracts? u How did he fractionate his extracts? ?
Carcinogenic tests with PAHs u. Professor Gernot Grimmer extracted different types of smoke particles u He then took the extract and applied it to mouse skin u and implanted it into rat lungs u How did he obtain extracts? u How did he fractionate his extracts? ?
 u Extraction by soxhlet extraction starts with solvent (Me. Cl 2) in a flask 22
u Extraction by soxhlet extraction starts with solvent (Me. Cl 2) in a flask 22
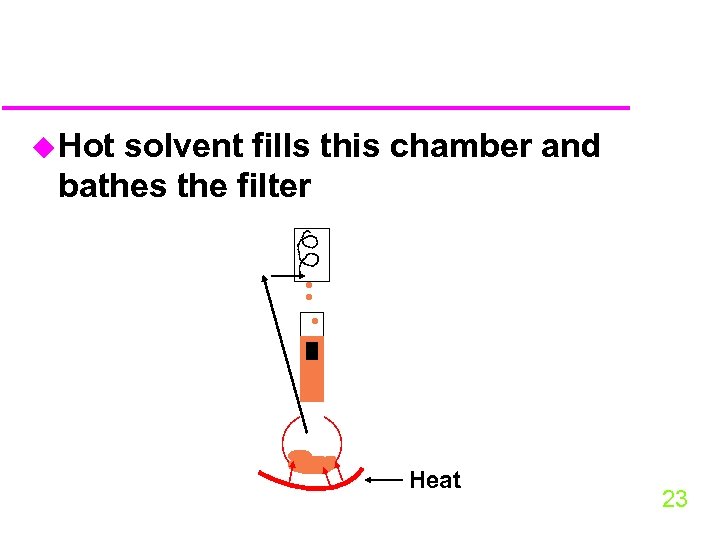 u Hot solvent fills this chamber and bathes the filter Heat 23
u Hot solvent fills this chamber and bathes the filter Heat 23
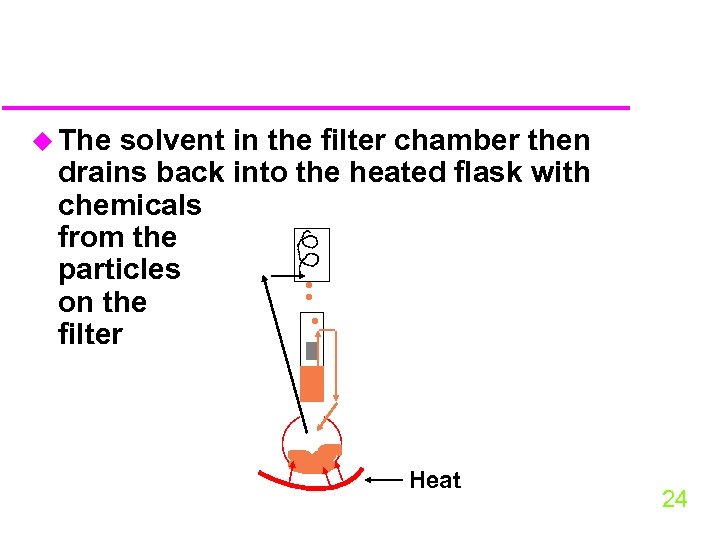 u The solvent in the filter chamber then drains back into the heated flask with chemicals from the particles on the filter Heat 24
u The solvent in the filter chamber then drains back into the heated flask with chemicals from the particles on the filter Heat 24
 u The organic liquid in the soxhlet flask can be concentrated by evaporation by a dry nitrogen stream or rotary evaporation u the extract can then be fractionated into different polarity compound groups 25
u The organic liquid in the soxhlet flask can be concentrated by evaporation by a dry nitrogen stream or rotary evaporation u the extract can then be fractionated into different polarity compound groups 25
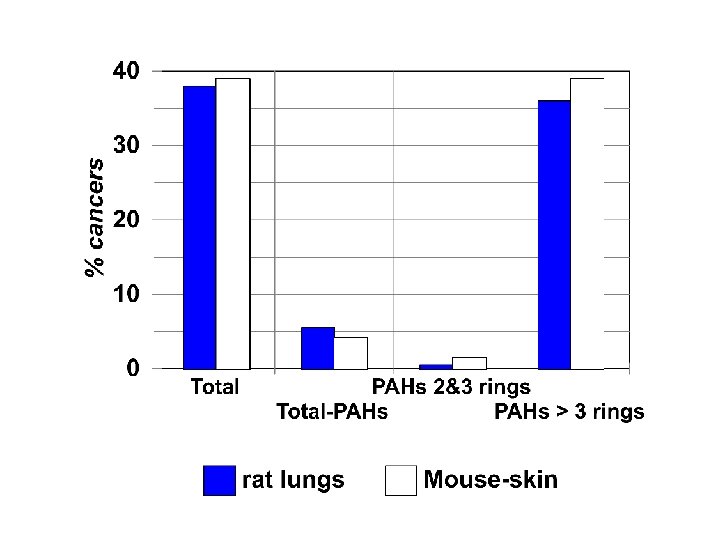
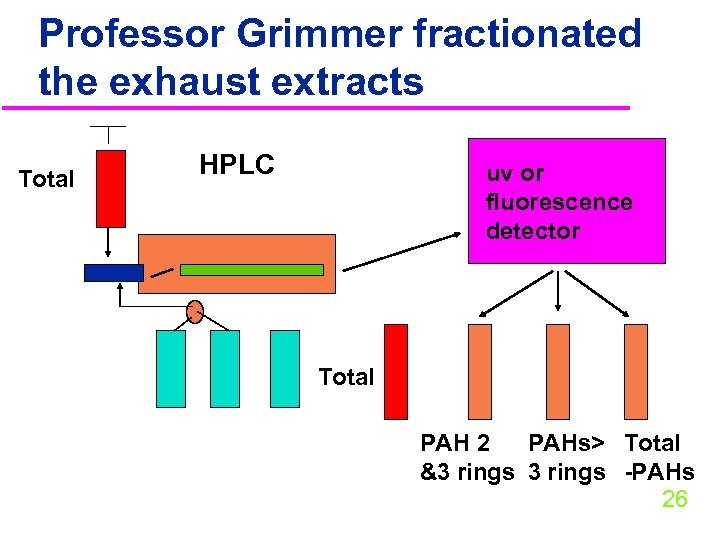 Professor Grimmer fractionated the exhaust extracts Total HPLC uv or fluorescence detector Total PAH 2 PAHs> Total &3 rings -PAHs 26
Professor Grimmer fractionated the exhaust extracts Total HPLC uv or fluorescence detector Total PAH 2 PAHs> Total &3 rings -PAHs 26
 What did Grimmer see when exposed rats and mice to the different fractions? u skin painted mice u implanted rat lungs 27
What did Grimmer see when exposed rats and mice to the different fractions? u skin painted mice u implanted rat lungs 27
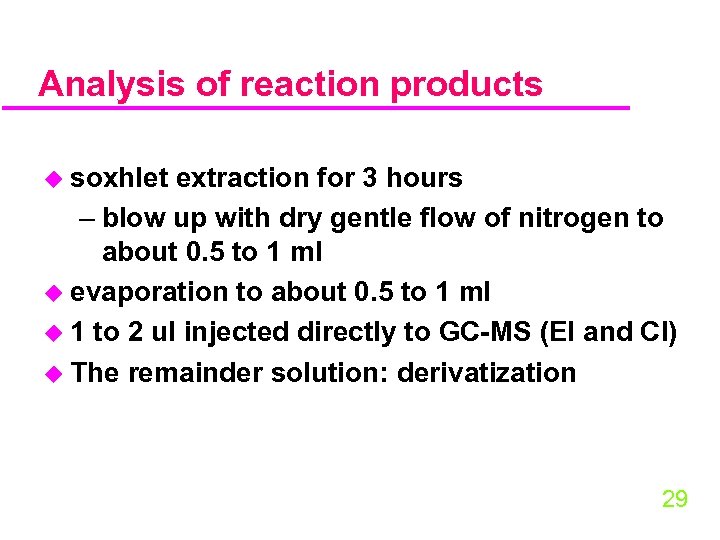 Analysis of reaction products u soxhlet extraction for 3 hours – blow up with dry gentle flow of nitrogen to about 0. 5 to 1 ml u evaporation to about 0. 5 to 1 ml u 1 to 2 ul injected directly to GC-MS (EI and CI) u The remainder solution: derivatization 29
Analysis of reaction products u soxhlet extraction for 3 hours – blow up with dry gentle flow of nitrogen to about 0. 5 to 1 ml u evaporation to about 0. 5 to 1 ml u 1 to 2 ul injected directly to GC-MS (EI and CI) u The remainder solution: derivatization 29
 In environmental samples why don’t we see some large highly oxygenated compounds that form in the atmophere? ? Reverse reactions to the original aldehyde parent structures can occur during sample work up/solvent extraction procedures; 30
In environmental samples why don’t we see some large highly oxygenated compounds that form in the atmophere? ? Reverse reactions to the original aldehyde parent structures can occur during sample work up/solvent extraction procedures; 30
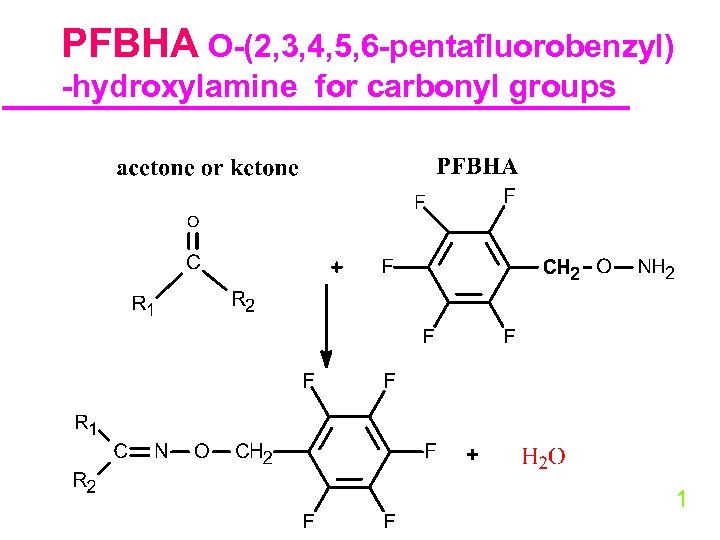 PFBHA O-(2, 3, 4, 5, 6 -pentafluorobenzyl) -hydroxylamine for carbonyl groups 31
PFBHA O-(2, 3, 4, 5, 6 -pentafluorobenzyl) -hydroxylamine for carbonyl groups 31
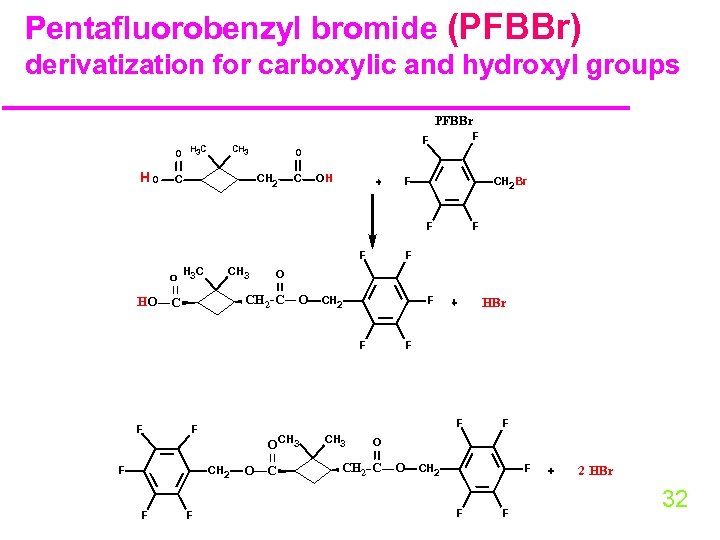 Pentafluorobenzyl bromide (PFBBr) derivatization for carboxylic and hydroxyl groups PFBBr H 3 C O HO O CH 2 C F F CH 3 C OH F CH 2 Br F F O HO H 3 C CH 3 F O CH 2 C O C CH 2 F F F O CH 3 CH 2 F F HBr F F F O C CH 3 F O CH 2 C O CH 2 F F F 2 HBr 32
Pentafluorobenzyl bromide (PFBBr) derivatization for carboxylic and hydroxyl groups PFBBr H 3 C O HO O CH 2 C F F CH 3 C OH F CH 2 Br F F O HO H 3 C CH 3 F O CH 2 C O C CH 2 F F F O CH 3 CH 2 F F HBr F F F O C CH 3 F O CH 2 C O CH 2 F F F 2 HBr 32
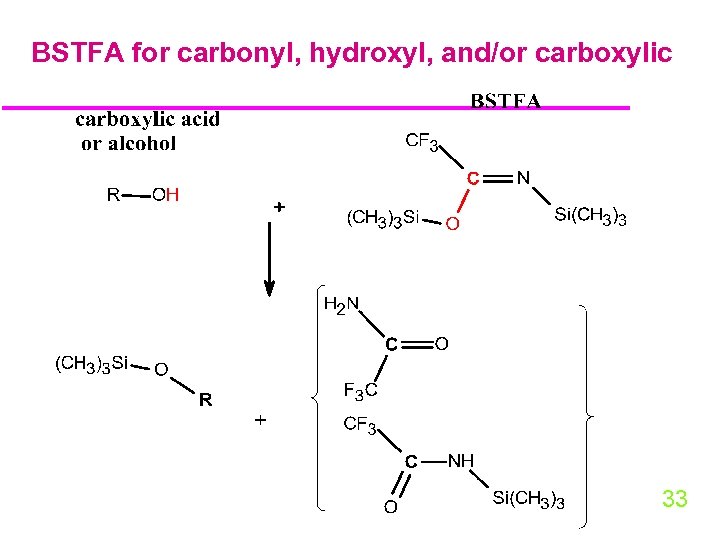 BSTFA for carbonyl, hydroxyl, and/or carboxylic 33
BSTFA for carbonyl, hydroxyl, and/or carboxylic 33
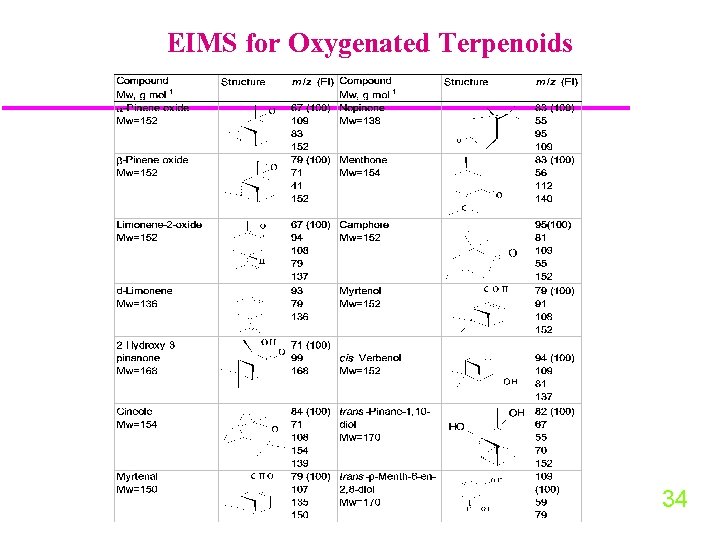 GC-EIMS for Oxygenated Terpenoids 34
GC-EIMS for Oxygenated Terpenoids 34
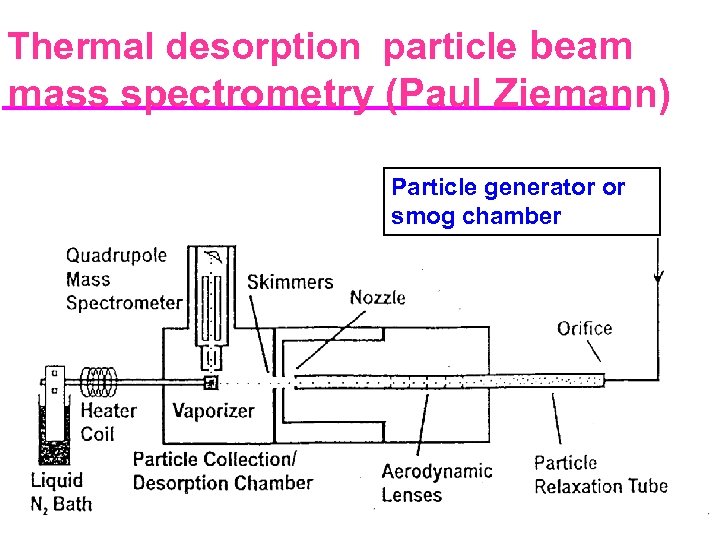 Thermal desorption particle beam mass spectrometry (Paul Ziemann) Particle generator or smog chamber 35
Thermal desorption particle beam mass spectrometry (Paul Ziemann) Particle generator or smog chamber 35
 Chlorinated dibenzo dioxins and Furans u These are some of the most toxic organics in the environment - LD 50 u Created by burning organics which have chlorine; incineration is a big source of atmospheric dioxins and furans u bleaching in making paper is another source
Chlorinated dibenzo dioxins and Furans u These are some of the most toxic organics in the environment - LD 50 u Created by burning organics which have chlorine; incineration is a big source of atmospheric dioxins and furans u bleaching in making paper is another source
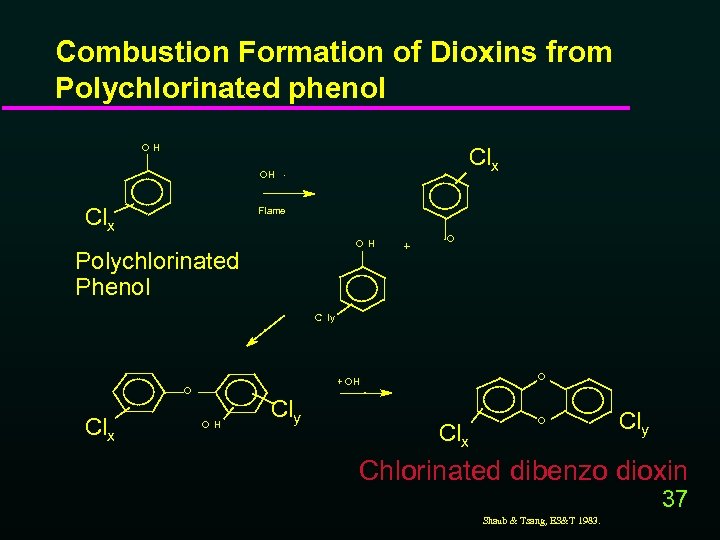 Combustion Formation of Dioxins from Polychlorinated phenol OH Clx OH. Clx Flame OH Polychlorinated Phenol + . O C ly Clx O + OH O OH Cly Clx O Cly Chlorinated dibenzo dioxin 37 Shaub & Tsang, ES&T 1983.
Combustion Formation of Dioxins from Polychlorinated phenol OH Clx OH. Clx Flame OH Polychlorinated Phenol + . O C ly Clx O + OH O OH Cly Clx O Cly Chlorinated dibenzo dioxin 37 Shaub & Tsang, ES&T 1983.
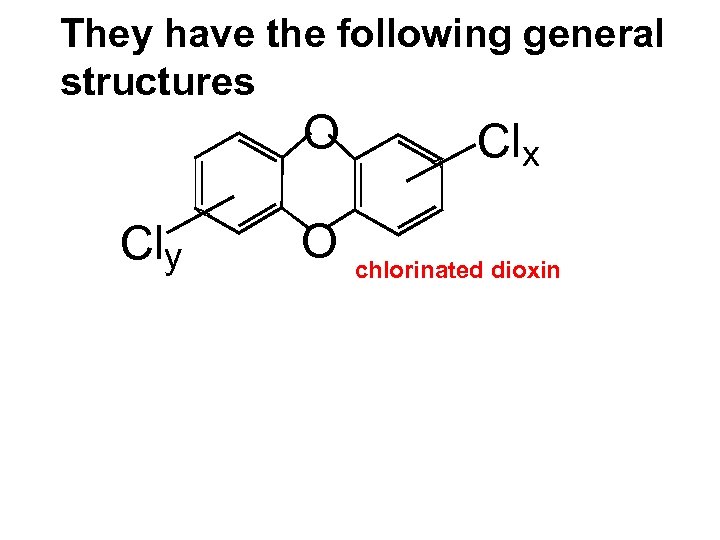 They have the following general structures O Cly O Clx chlorinated dioxin
They have the following general structures O Cly O Clx chlorinated dioxin
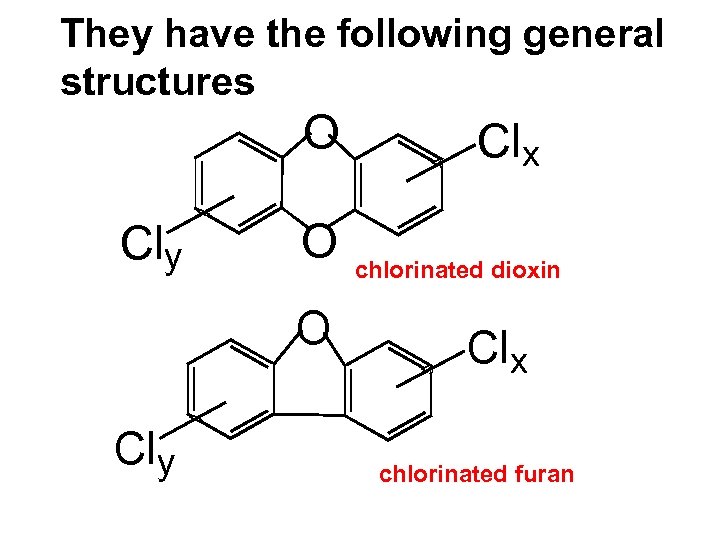 They have the following general structures O Cly O O Cly Clx chlorinated dioxin Clx chlorinated furan
They have the following general structures O Cly O O Cly Clx chlorinated dioxin Clx chlorinated furan
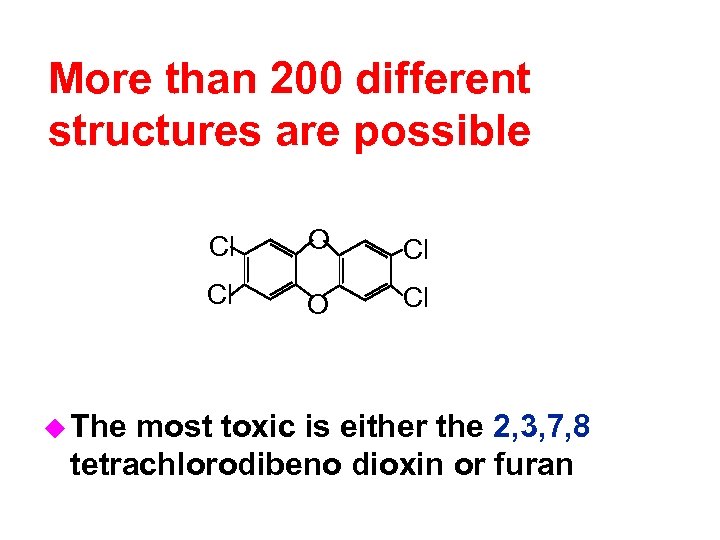 More than 200 different structures are possible Cl Cl Cl u The O O Cl most toxic is either the 2, 3, 7, 8 tetrachlorodibeno dioxin or furan
More than 200 different structures are possible Cl Cl Cl u The O O Cl most toxic is either the 2, 3, 7, 8 tetrachlorodibeno dioxin or furan
 u These types of compounds produce toxic enzymes: arylhydrocarbon hydroxylase and 7 -ethoxyresorufin deethylase u. At low concentrations they may behave as environmental estrogens
u These types of compounds produce toxic enzymes: arylhydrocarbon hydroxylase and 7 -ethoxyresorufin deethylase u. At low concentrations they may behave as environmental estrogens
 u Environmentally, they are unreactive and can be transported long distances u They did not start to show up in the environment until the 1920 s when there was a big increase in the production of chloro-organics (Professor Ron Hites, and students)
u Environmentally, they are unreactive and can be transported long distances u They did not start to show up in the environment until the 1920 s when there was a big increase in the production of chloro-organics (Professor Ron Hites, and students)
 Environmental Fate of Chlorinated Dioxins and Furans (Czuczwa and Hites, 1984) u Collected core sediment samples from southern Lake Huron in the USA u Based on sedimentation rates they established age vs. concentration profiles for chlorinated dioxins and furans 43
Environmental Fate of Chlorinated Dioxins and Furans (Czuczwa and Hites, 1984) u Collected core sediment samples from southern Lake Huron in the USA u Based on sedimentation rates they established age vs. concentration profiles for chlorinated dioxins and furans 43
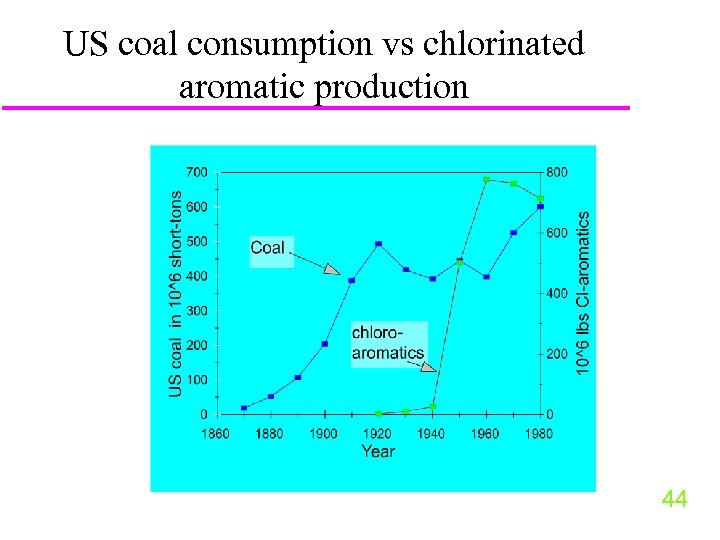 US coal consumption vs chlorinated aromatic production 44
US coal consumption vs chlorinated aromatic production 44
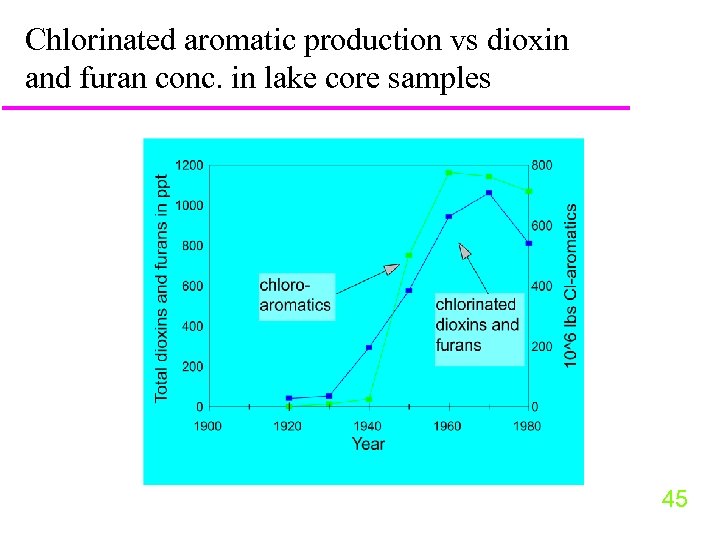 Chlorinated aromatic production vs dioxin and furan conc. in lake core samples 45
Chlorinated aromatic production vs dioxin and furan conc. in lake core samples 45
 PCBs in the U. S. Great Lakes u PCBs were banned in the early 1970 s u In 1980 Eisenreich and co-workers estimated that still 85% of the PCBs in the US great lakes came from atmospheric sources. 46
PCBs in the U. S. Great Lakes u PCBs were banned in the early 1970 s u In 1980 Eisenreich and co-workers estimated that still 85% of the PCBs in the US great lakes came from atmospheric sources. 46
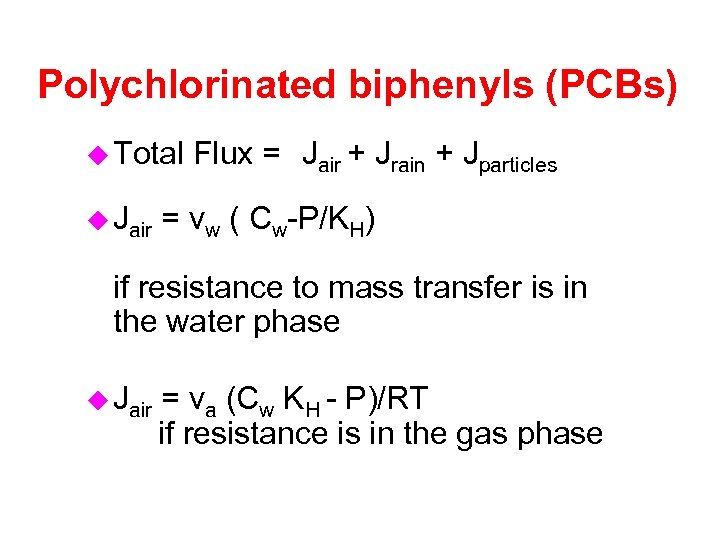 Polychlorinated biphenyls (PCBs) u Total u Jair Flux = Jair + Jrain + Jparticles = vw ( Cw-P/KH) if resistance to mass transfer is in the water phase u Jair = va (Cw KH - P)/RT if resistance is in the gas phase
Polychlorinated biphenyls (PCBs) u Total u Jair Flux = Jair + Jrain + Jparticles = vw ( Cw-P/KH) if resistance to mass transfer is in the water phase u Jair = va (Cw KH - P)/RT if resistance is in the gas phase
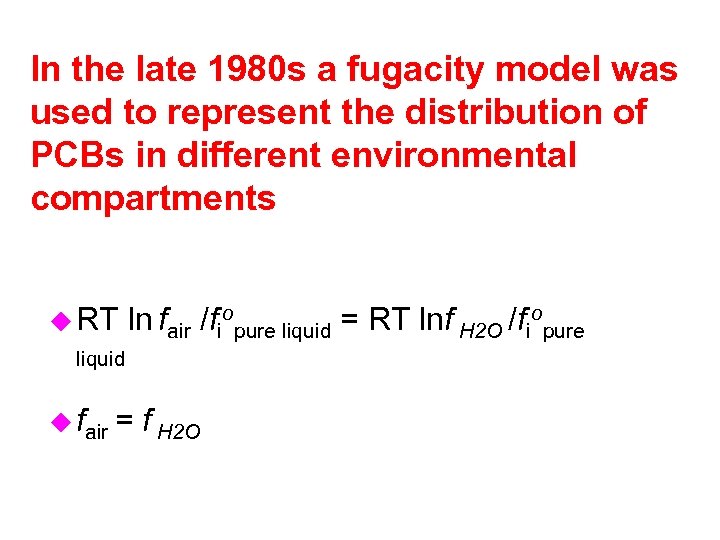 In the late 1980 s a fugacity model was used to represent the distribution of PCBs in different environmental compartments u RT ln fair /fiopure liquid = RT lnf H 2 O /fiopure liquid u fair = f H 2 O
In the late 1980 s a fugacity model was used to represent the distribution of PCBs in different environmental compartments u RT ln fair /fiopure liquid = RT lnf H 2 O /fiopure liquid u fair = f H 2 O
 u In 1990 Eisenreich and co-workers reported that ambient measurements over the great lakes were generally constant for the past 10 years. u For the past 15 years sources to the lakes had declined because of the PCB ban. u Based on mass transfer calculations it was proposed that during the summer months the lakes were actually a source of atmospheric PCBs. 49
u In 1990 Eisenreich and co-workers reported that ambient measurements over the great lakes were generally constant for the past 10 years. u For the past 15 years sources to the lakes had declined because of the PCB ban. u Based on mass transfer calculations it was proposed that during the summer months the lakes were actually a source of atmospheric PCBs. 49
 Polychlorinated biphenyls (PCBs) u used as coolants - insulation fluids in transformers, capacitors , plastercisers, additives to epoxy paints u are thermally stable and biologically stable u can exist in the gas and particle phases
Polychlorinated biphenyls (PCBs) u used as coolants - insulation fluids in transformers, capacitors , plastercisers, additives to epoxy paints u are thermally stable and biologically stable u can exist in the gas and particle phases
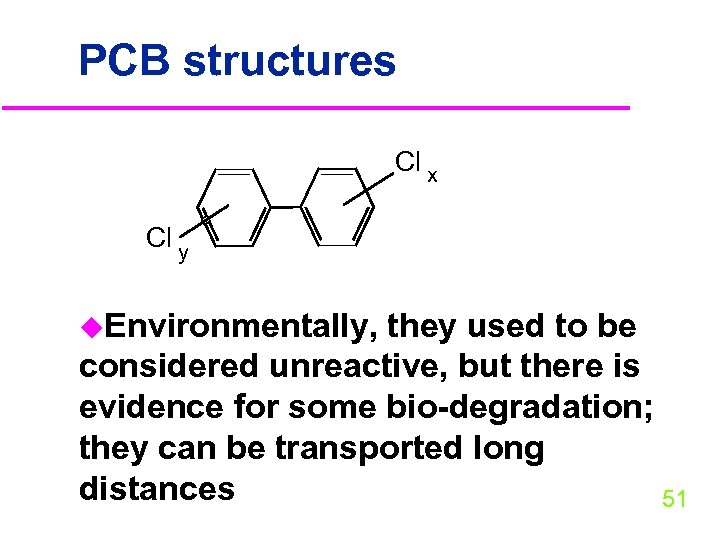 PCB structures Cl x Cl y u. Environmentally, they used to be considered unreactive, but there is evidence for some bio-degradation; they can be transported long distances 51
PCB structures Cl x Cl y u. Environmentally, they used to be considered unreactive, but there is evidence for some bio-degradation; they can be transported long distances 51
 u Up until the 1970 s there was a lot of dumping of industrial wastes in the USA u In one example, from 1950 to 1975 there were two capacitor manufacturing plants on the Hudson river in New York State, which discharged into the river.
u Up until the 1970 s there was a lot of dumping of industrial wastes in the USA u In one example, from 1950 to 1975 there were two capacitor manufacturing plants on the Hudson river in New York State, which discharged into the river.
 u Levels in the river sediments downstream from the plants exhibited concentrations of 10 ppm which was a factor of two higher than commonly found. u Dredging was considered financially impossible u it was also believed that is very difficult to bio-degrade PCBs with multiple chlorine atoms
u Levels in the river sediments downstream from the plants exhibited concentrations of 10 ppm which was a factor of two higher than commonly found. u Dredging was considered financially impossible u it was also believed that is very difficult to bio-degrade PCBs with multiple chlorine atoms
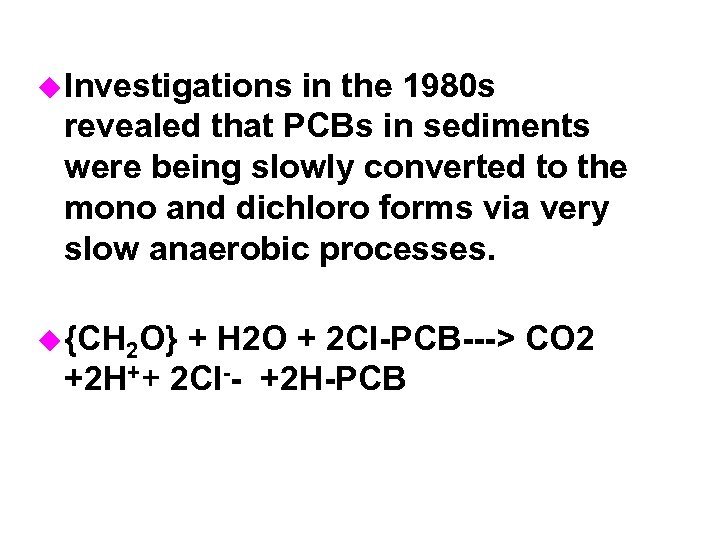 u Investigations in the 1980 s revealed that PCBs in sediments were being slowly converted to the mono and dichloro forms via very slow anaerobic processes. u {CH 2 O} + H 2 O + 2 Cl-PCB---> CO 2 +2 H++ 2 Cl-- +2 H-PCB
u Investigations in the 1980 s revealed that PCBs in sediments were being slowly converted to the mono and dichloro forms via very slow anaerobic processes. u {CH 2 O} + H 2 O + 2 Cl-PCB---> CO 2 +2 H++ 2 Cl-- +2 H-PCB
 What do we do now, when new compounds are introduced into the environment. . . ? ? u toxicity? ? u low concentration health effects? u damage to the ecosystem ? u where will it show up in the environment? u how is it transported in the environment and what is its lifetime? 55
What do we do now, when new compounds are introduced into the environment. . . ? ? u toxicity? ? u low concentration health effects? u damage to the ecosystem ? u where will it show up in the environment? u how is it transported in the environment and what is its lifetime? 55
 An example is a new compound called D 5. It is a silicon-oxygen compound u It is used to make silicone plastics. u It is possible that it could be used to replace toxic solvents like toluene and dichloro-methane. u Before it can be put into use in the US, we need to know its toxicity, chemical reactivity , environmental half-life, etc. 56
An example is a new compound called D 5. It is a silicon-oxygen compound u It is used to make silicone plastics. u It is possible that it could be used to replace toxic solvents like toluene and dichloro-methane. u Before it can be put into use in the US, we need to know its toxicity, chemical reactivity , environmental half-life, etc. 56
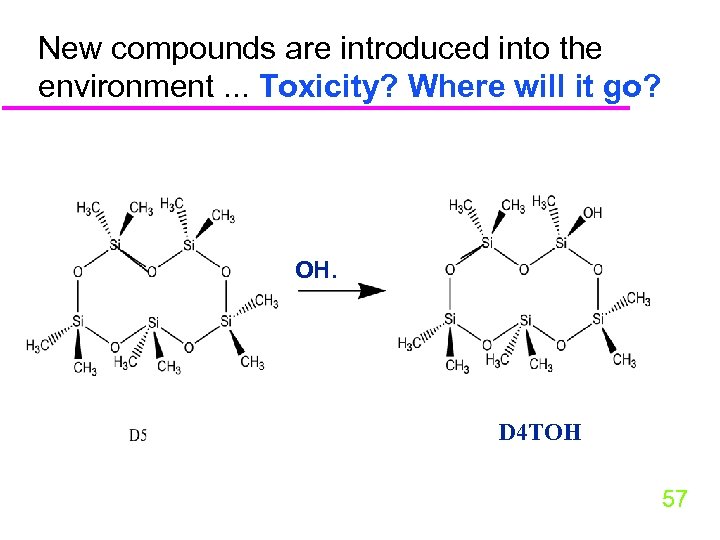 New compounds are introduced into the environment. . . Toxicity? Where will it go? OH. D 4 TOH 57
New compounds are introduced into the environment. . . Toxicity? Where will it go? OH. D 4 TOH 57
 Some examples of environmental exposures u In 1976 there was a significant industrial explosion in the town of Seveso, Italy that spewed out chlorinated dioxins. u 735 people were evacuated from the immediate vicinity. u Now excess cancers are showing up.
Some examples of environmental exposures u In 1976 there was a significant industrial explosion in the town of Seveso, Italy that spewed out chlorinated dioxins. u 735 people were evacuated from the immediate vicinity. u Now excess cancers are showing up.
 Seveso, Italy Dioxin release u Over the past eight years the birth ratio has changed from 106 males: 100 females to 26: 48 u observed u decline increases in cancers in number of males born 59
Seveso, Italy Dioxin release u Over the past eight years the birth ratio has changed from 106 males: 100 females to 26: 48 u observed u decline increases in cancers in number of males born 59
 A similar observation has been made in the bird population u In the Great Lake region of the USA during the 1980’s, hatchlings of crested cormorants with a crossed bill deformity were almost always female u Male u birds did not show the deformity Scientist speculate that the chemicals causing the deformity were also killing the males before they hatched. 60
A similar observation has been made in the bird population u In the Great Lake region of the USA during the 1980’s, hatchlings of crested cormorants with a crossed bill deformity were almost always female u Male u birds did not show the deformity Scientist speculate that the chemicals causing the deformity were also killing the males before they hatched. 60
 1. There is a general concern that if we observe abnormalities in wildlife, similar kinds of mechanisms may exist in humans. 61
1. There is a general concern that if we observe abnormalities in wildlife, similar kinds of mechanisms may exist in humans. 61
 Mercury poisoning off the coast of Minamata, Japan is an example u Fishermen in the 1950 s noticed sea birds were dying and feral cats that scavenged fish from the docks were “stiff legged” u Cerebral palsy and mental retardation started showing up in children. 62
Mercury poisoning off the coast of Minamata, Japan is an example u Fishermen in the 1950 s noticed sea birds were dying and feral cats that scavenged fish from the docks were “stiff legged” u Cerebral palsy and mental retardation started showing up in children. 62
 2. Toxic loads u Scientists have hypothesized that the fetus is sharing the mother’s toxic load, and may actually provide some protection to the mother by reducing her internal exposure. 63
2. Toxic loads u Scientists have hypothesized that the fetus is sharing the mother’s toxic load, and may actually provide some protection to the mother by reducing her internal exposure. 63
 2. Toxic loads u Children get 12% of their lifetime exposure to dioxins during the 1 st year. u Their exposure is 50 times greater than an adult during a very critical developmental period. 64
2. Toxic loads u Children get 12% of their lifetime exposure to dioxins during the 1 st year. u Their exposure is 50 times greater than an adult during a very critical developmental period. 64
 2. Toxic loads u. Firstborns from dolphins off the coast of Florida usually die before they separate from their mothers 65
2. Toxic loads u. Firstborns from dolphins off the coast of Florida usually die before they separate from their mothers 65
 2. Toxic loads u It is speculated that mother dolphins unload 80% of their accumulated pollutants into their calves, probably during nursing. u The greatest exposures occurs with the 1 st born u Does this have any implications for humans? 66
2. Toxic loads u It is speculated that mother dolphins unload 80% of their accumulated pollutants into their calves, probably during nursing. u The greatest exposures occurs with the 1 st born u Does this have any implications for humans? 66
 3. Pesticide exposures u Children of farm families in the western Minnesota area of the US have significantly higher rates of birth defects than the general population. u The highest rates are among children conceived in the spring when spraying of pesticides is most intense; male babies had far more birth defects than females 67
3. Pesticide exposures u Children of farm families in the western Minnesota area of the US have significantly higher rates of birth defects than the general population. u The highest rates are among children conceived in the spring when spraying of pesticides is most intense; male babies had far more birth defects than females 67
 4. The end points may not only be cancer, but compromised immune systems and generally poorer health. 68
4. The end points may not only be cancer, but compromised immune systems and generally poorer health. 68
 4. Immune systems & Mother’s milk u In the Netherlands researchers have found that children with higher levels of dioxins and PCBs in their bodies have more health problems (immune system and hormonal changes) than children with lower levels. u This was linked to levels of PCBs in Mother’s milk. 69
4. Immune systems & Mother’s milk u In the Netherlands researchers have found that children with higher levels of dioxins and PCBs in their bodies have more health problems (immune system and hormonal changes) than children with lower levels. u This was linked to levels of PCBs in Mother’s milk. 69
 4. Mother’s milk u Overall, however, it was concluded that nursing was still of greater benefit than bottle feeding babies, but that even mild exposures may weaken immunity 70
4. Mother’s milk u Overall, however, it was concluded that nursing was still of greater benefit than bottle feeding babies, but that even mild exposures may weaken immunity 70
 4. Mother’s milk u Mother’s milk from Inuit Indians in the Canadian Arctic has 7 times the PCBs as mother’s milk from women in the urban industrialized areas of southern Quebec. 71
4. Mother’s milk u Mother’s milk from Inuit Indians in the Canadian Arctic has 7 times the PCBs as mother’s milk from women in the urban industrialized areas of southern Quebec. 71
 4. Mother’s milk u During the first year, Inuit babies suffer through 20 times more colds than babies in southern Quebec. u Acute ear infections are rampant. 72
4. Mother’s milk u During the first year, Inuit babies suffer through 20 times more colds than babies in southern Quebec. u Acute ear infections are rampant. 72
 4. Mother’s milk u Babies nursed by mothers with the highest contamination levels in their milk are afflicted with more acute ear infections than bottle fed Inuit babies. u Many of these children don’t seem to produce enough antibodies for childhood vaccinations to take. 73
4. Mother’s milk u Babies nursed by mothers with the highest contamination levels in their milk are afflicted with more acute ear infections than bottle fed Inuit babies. u Many of these children don’t seem to produce enough antibodies for childhood vaccinations to take. 73
 5. PCBs and lower intelligence u There is evidence of lower intelligence in babies exposed to PCBs. u In adults, a blood-brain barrier insulates the brain from many potentially harmful chemicals circulating through the body u In a human child this barrier is not fully developed until 6 months after birth. 74
5. PCBs and lower intelligence u There is evidence of lower intelligence in babies exposed to PCBs. u In adults, a blood-brain barrier insulates the brain from many potentially harmful chemicals circulating through the body u In a human child this barrier is not fully developed until 6 months after birth. 74
 5. PCBs and lower intelligence u In 1979 in Taiwan, more than 2000 people were exposed to PCBcontaminated cooking oil. u In the 1 st 3 months many babies died outright. As the surviving children grew up, many were slower intellectually than other kids their age, were hyperactive and had behavioral problems. 75
5. PCBs and lower intelligence u In 1979 in Taiwan, more than 2000 people were exposed to PCBcontaminated cooking oil. u In the 1 st 3 months many babies died outright. As the surviving children grew up, many were slower intellectually than other kids their age, were hyperactive and had behavioral problems. 75
 5. PCBs and lower intelligence u Similar observations were made in "high-PCB kids" in the Lake Michigan area. u This was associated with mothers eating salmon and trout from the Lake during the years before their children were born. 76
5. PCBs and lower intelligence u Similar observations were made in "high-PCB kids" in the Lake Michigan area. u This was associated with mothers eating salmon and trout from the Lake during the years before their children were born. 76
 5. PCBs and lower intelligence u At age 4 the high exposure group had poor short term memories. At age 11 the 30 most highly exposed kids had average IQ scores that were 6 points lower than the lowest-exposed group. u biomarker-metabolites? ? ? 77
5. PCBs and lower intelligence u At age 4 the high exposure group had poor short term memories. At age 11 the 30 most highly exposed kids had average IQ scores that were 6 points lower than the lowest-exposed group. u biomarker-metabolites? ? ? 77
 6. DDT and immune system damage u In a recent study (1998), residents whose homes are within a mile of Aberdeen, Texas pesticide sites show elevated DDE levels in their blood. 78
6. DDT and immune system damage u In a recent study (1998), residents whose homes are within a mile of Aberdeen, Texas pesticide sites show elevated DDE levels in their blood. 78
 6. DDT and immune system damage u DDE is a byproduct of the body’s attempt to break down the pesticide DDT, which has been banned in the USA since 1972. u “Levels of plasma DDE in the study population overall were low (6 ppb) compared to nationwide levels between 1976 and 1980, just after the DDT ban, ” (UNC, Prof. Vine) 79
6. DDT and immune system damage u DDE is a byproduct of the body’s attempt to break down the pesticide DDT, which has been banned in the USA since 1972. u “Levels of plasma DDE in the study population overall were low (6 ppb) compared to nationwide levels between 1976 and 1980, just after the DDT ban, ” (UNC, Prof. Vine) 79
 6. DDT and immune system damage u Younger Aberdeen residents – those between ages 18 and 40 – and people who lived there before 1985 when the plants were operating did show a two- to three-fold increased risk of herpes zoster, u or shingles, which indicates modest suppression of the body’s immune system 80
6. DDT and immune system damage u Younger Aberdeen residents – those between ages 18 and 40 – and people who lived there before 1985 when the plants were operating did show a two- to three-fold increased risk of herpes zoster, u or shingles, which indicates modest suppression of the body’s immune system 80
 7. Sexual impairment u There is evidence for sexual impairment in both animals and humans from high PCB exposures and other environmental chemicals. u Male beluga whales in the very polluted St. Lawrence River have exhibited female organs. 81
7. Sexual impairment u There is evidence for sexual impairment in both animals and humans from high PCB exposures and other environmental chemicals. u Male beluga whales in the very polluted St. Lawrence River have exhibited female organs. 81
 7. Sexual impairment u Highly exposed humans, alligators and panthers exhibit smaller male sex organs and low sperm counts. u Testicular cancers have nearly doubled among older teenagers in the US between 1973 and 1992. u In previous lectures I have said these have been linked to toxic exposures. . long way 82 from finding proof.
7. Sexual impairment u Highly exposed humans, alligators and panthers exhibit smaller male sex organs and low sperm counts. u Testicular cancers have nearly doubled among older teenagers in the US between 1973 and 1992. u In previous lectures I have said these have been linked to toxic exposures. . long way 82 from finding proof.
 7 a. Sexual impairment u In a new study (Hardwell et al, Environ Presp, 2003) woman who’ve had substantial exposure to certain environmental pollutants are more likely to bear sons who develop testicular cancers (men ~ 30 years of age) u From 1973 -1999 testicular cancers up 67% u Men with test-cancers had high cis nona chloridane, not PCBs, etc u Mothers, however, had high PCBs, HCB (hexachlorobenzenes) and cis nona chloridane 83
7 a. Sexual impairment u In a new study (Hardwell et al, Environ Presp, 2003) woman who’ve had substantial exposure to certain environmental pollutants are more likely to bear sons who develop testicular cancers (men ~ 30 years of age) u From 1973 -1999 testicular cancers up 67% u Men with test-cancers had high cis nona chloridane, not PCBs, etc u Mothers, however, had high PCBs, HCB (hexachlorobenzenes) and cis nona chloridane 83
 7 b. Sexual impairment u These same mothers probably had high exposures when environmental contaminets peaked in Scandinavia in the 1970 s u Richard Sharpe of Edinburogh and Niels Skakkebek (Denmark) propose that exposure to endocrine disruptors before birth can alter testicular-cell development and some of these cells may be cancerous after puberty. u This may also may explain rising rates of male infertility, and other sexual deformities 84
7 b. Sexual impairment u These same mothers probably had high exposures when environmental contaminets peaked in Scandinavia in the 1970 s u Richard Sharpe of Edinburogh and Niels Skakkebek (Denmark) propose that exposure to endocrine disruptors before birth can alter testicular-cell development and some of these cells may be cancerous after puberty. u This may also may explain rising rates of male infertility, and other sexual deformities 84
 8. Endocrine disrupters u These studies have led to the notion of environmental "endocrine disrupters". u In the lock and key relationship between hormone and receptor molecules, these "hormone impostors" can: 85
8. Endocrine disrupters u These studies have led to the notion of environmental "endocrine disrupters". u In the lock and key relationship between hormone and receptor molecules, these "hormone impostors" can: 85
 8. Endocrine disrupters u bind with receptors and trigger biological processes u or bind with receptors and tie up an active hormone site u Some of these have been called environmental estrogens 86
8. Endocrine disrupters u bind with receptors and trigger biological processes u or bind with receptors and tie up an active hormone site u Some of these have been called environmental estrogens 86
 9. Other chemicals u From a historical perspective, everyone is now carrying at last 250 measurable chemicals that were not part of human chemistry before the 1920 s (Peter Myers, 1996) u The most basic toxicity testing results cannot be found in the public record for nearly 75% of the top volume chemicals in commercial use in the USA 87
9. Other chemicals u From a historical perspective, everyone is now carrying at last 250 measurable chemicals that were not part of human chemistry before the 1920 s (Peter Myers, 1996) u The most basic toxicity testing results cannot be found in the public record for nearly 75% of the top volume chemicals in commercial use in the USA 87
 9. Other chemicals u In other words, the public cannot tell whether a large majority of the highest-use chemicals in the United States pose health hazards or not (Amicus Journal, p 23, Spring 1998). u An example are phthalates that go into many types of plastics which have been shown to reduce the sperm counts in mice. 88
9. Other chemicals u In other words, the public cannot tell whether a large majority of the highest-use chemicals in the United States pose health hazards or not (Amicus Journal, p 23, Spring 1998). u An example are phthalates that go into many types of plastics which have been shown to reduce the sperm counts in mice. 88
 9. Other chemicals u Bisphenol-A (BPA) is an additive in polycarbonate plastics used in food liners, dental sealants, and dental fillings. u BPA causes increased prostate size in mice exposed to tiny doses while in the womb. These doses were 25, 000 times smaller than the EPA threshold. 89
9. Other chemicals u Bisphenol-A (BPA) is an additive in polycarbonate plastics used in food liners, dental sealants, and dental fillings. u BPA causes increased prostate size in mice exposed to tiny doses while in the womb. These doses were 25, 000 times smaller than the EPA threshold. 89
 9. Phthalates u Exposure of female rates to 200 to 1000 mg/kg body weight results in much lower testosterone in male offspring ( L. Earl Gray. Jr. EPA, RTP, J. Tox and Ind. Health, Mar, 1999). u Exposures to the herbicide linuron made the epididymis (sperm-storing organ in rats) much smaller in male rats. 90
9. Phthalates u Exposure of female rates to 200 to 1000 mg/kg body weight results in much lower testosterone in male offspring ( L. Earl Gray. Jr. EPA, RTP, J. Tox and Ind. Health, Mar, 1999). u Exposures to the herbicide linuron made the epididymis (sperm-storing organ in rats) much smaller in male rats. 90
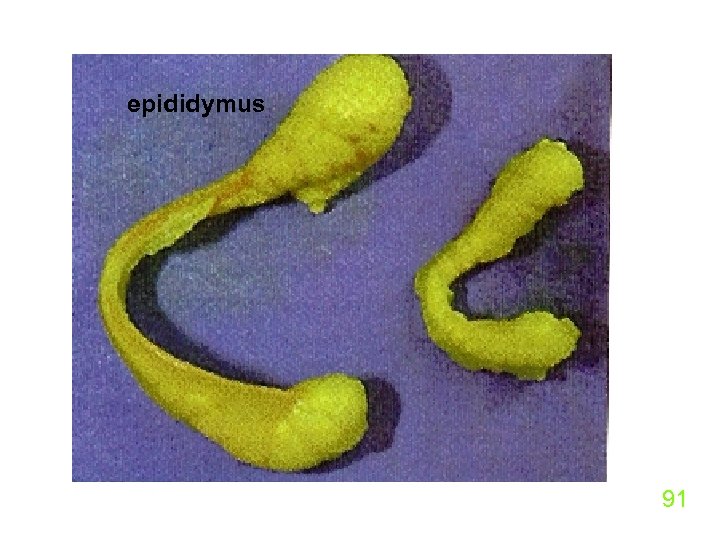 epididymus 91
epididymus 91
 Recommendations u During the insecticide spraying season, farmers should not try to have children. u Limit exposures to pesticides around the home. u When possible, buy foods that were grown without pesticides. u Governments must try to limit PCB introduction into the environment. u If incineration is used, chlorinated plastics should be removed, along with modern technology. 92
Recommendations u During the insecticide spraying season, farmers should not try to have children. u Limit exposures to pesticides around the home. u When possible, buy foods that were grown without pesticides. u Governments must try to limit PCB introduction into the environment. u If incineration is used, chlorinated plastics should be removed, along with modern technology. 92


