48da118b7626bdec7a7457076fe226ae.ppt
- Количество слайдов: 21
 Enhanced Strategies to Improve the Quality of HIV Testing ART in Pregnancy, Breastfeeding and Beyond June 18 -20, 2012 Mireille Kalou, CGH/ILB Keisha Jackson, CGH/ILB Omotayo Bolu, CDC, Cameroon
Enhanced Strategies to Improve the Quality of HIV Testing ART in Pregnancy, Breastfeeding and Beyond June 18 -20, 2012 Mireille Kalou, CGH/ILB Keisha Jackson, CGH/ILB Omotayo Bolu, CDC, Cameroon
 Why Enhanced Focus on the Quality of HIV Rapid Testing? q First step to HIV prevention, care and treatment, and surveillance (ALL PROGRAMS) q Several reports of testing errors q >40 million people tested by RT in 2011 § 1% error = 400, 000 wrong diagnosis § 5% error = 2 million wrong diagnosis § 10% error = 4 million wrong diagnosis q Impact of false HIV diagnosis at the individual and program level
Why Enhanced Focus on the Quality of HIV Rapid Testing? q First step to HIV prevention, care and treatment, and surveillance (ALL PROGRAMS) q Several reports of testing errors q >40 million people tested by RT in 2011 § 1% error = 400, 000 wrong diagnosis § 5% error = 2 million wrong diagnosis § 10% error = 4 million wrong diagnosis q Impact of false HIV diagnosis at the individual and program level
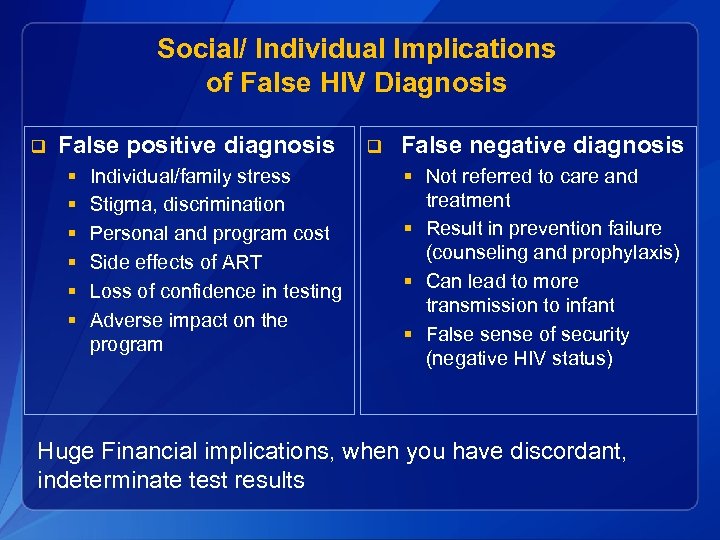 Social/ Individual Implications of False HIV Diagnosis q False positive diagnosis § § § Individual/family stress Stigma, discrimination Personal and program cost Side effects of ART Loss of confidence in testing Adverse impact on the program q False negative diagnosis § Not referred to care and treatment § Result in prevention failure (counseling and prophylaxis) § Can lead to more transmission to infant § False sense of security (negative HIV status) Huge Financial implications, when you have discordant, indeterminate test results
Social/ Individual Implications of False HIV Diagnosis q False positive diagnosis § § § Individual/family stress Stigma, discrimination Personal and program cost Side effects of ART Loss of confidence in testing Adverse impact on the program q False negative diagnosis § Not referred to care and treatment § Result in prevention failure (counseling and prophylaxis) § Can lead to more transmission to infant § False sense of security (negative HIV status) Huge Financial implications, when you have discordant, indeterminate test results
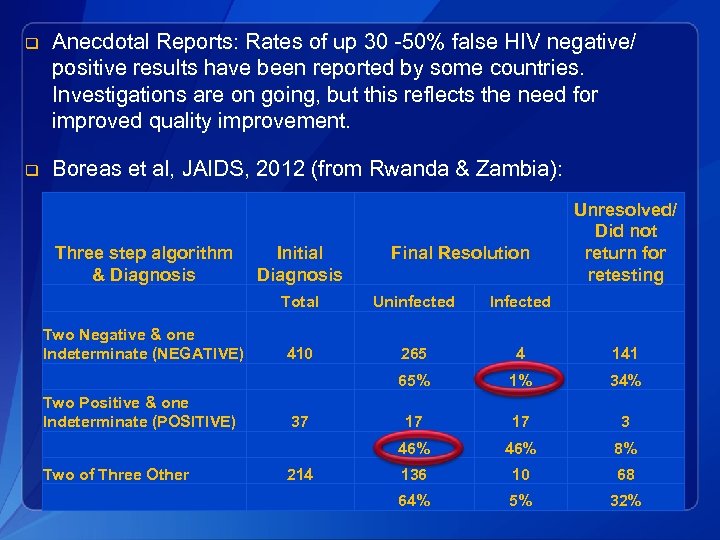 q Anecdotal Reports: Rates of up 30 -50% false HIV negative/ positive results have been reported by some countries. Investigations are on going, but this reflects the need for improved quality improvement. q Boreas et al, JAIDS, 2012 (from Rwanda & Zambia): Three step algorithm Initial & Diagnosis Final Resolution Unresolved/ Did not return for retesting Total Uninfected Infected Two Negative & one Indeterminate (NEGATIVE) 410 265 4 141 65% 1% 34% 37 17 17 3 46% 8% 214 136 10 68 64% 5% 32% Two Positive & one Indeterminate (POSITIVE) Two of Three Other
q Anecdotal Reports: Rates of up 30 -50% false HIV negative/ positive results have been reported by some countries. Investigations are on going, but this reflects the need for improved quality improvement. q Boreas et al, JAIDS, 2012 (from Rwanda & Zambia): Three step algorithm Initial & Diagnosis Final Resolution Unresolved/ Did not return for retesting Total Uninfected Infected Two Negative & one Indeterminate (NEGATIVE) 410 265 4 141 65% 1% 34% 37 17 17 3 46% 8% 214 136 10 68 64% 5% 32% Two Positive & one Indeterminate (POSITIVE) Two of Three Other
 Factors Impacting the Quality of HIV Testing q q q q Use of sub-optimal test kits Control specimens not used Procedures not followed Use of expired test kits Deviation from country’s testing algorithm Testing personnel not trained or under trained Test results improperly recorded
Factors Impacting the Quality of HIV Testing q q q q Use of sub-optimal test kits Control specimens not used Procedures not followed Use of expired test kits Deviation from country’s testing algorithm Testing personnel not trained or under trained Test results improperly recorded
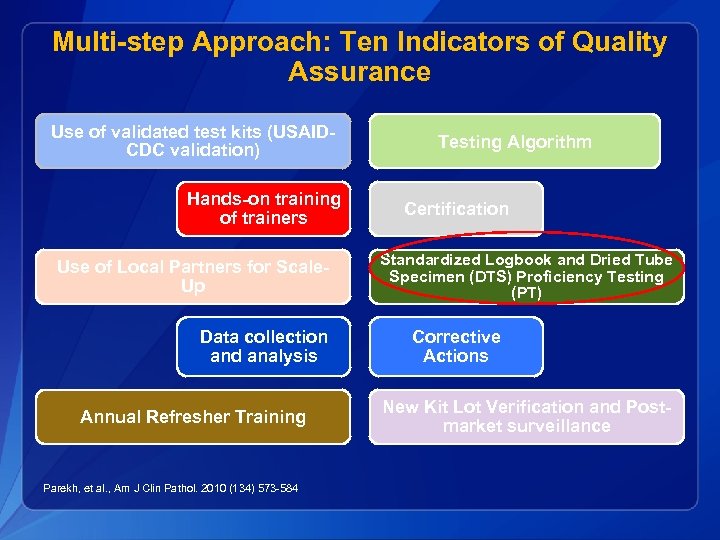 Multi-step Approach: Ten Indicators of Quality Assurance Use of validated test kits (USAIDCDC validation) Hands-on training of trainers Use of Local Partners for Scale. Up Data collection and analysis Annual Refresher Training Parekh, et al. , Am J Clin Pathol. 2010 (134) 573 -584 Testing Algorithm Certification Standardized Logbook and Dried Tube Specimen (DTS) Proficiency Testing (PT) Corrective Actions New Kit Lot Verification and Postmarket surveillance
Multi-step Approach: Ten Indicators of Quality Assurance Use of validated test kits (USAIDCDC validation) Hands-on training of trainers Use of Local Partners for Scale. Up Data collection and analysis Annual Refresher Training Parekh, et al. , Am J Clin Pathol. 2010 (134) 573 -584 Testing Algorithm Certification Standardized Logbook and Dried Tube Specimen (DTS) Proficiency Testing (PT) Corrective Actions New Kit Lot Verification and Postmarket surveillance
 Approaches used for Improving Quality of RT ü Proficiency testing using Dried Tube Specimen (DTS) ü Standardized Log Book ü Training Curriculum on Improving the Quality of RT
Approaches used for Improving Quality of RT ü Proficiency testing using Dried Tube Specimen (DTS) ü Standardized Log Book ü Training Curriculum on Improving the Quality of RT
 I. Proficiency Testing Program Using Dried Tube Specimens q Dried tube specimens (DTS) concept § § § q Developed in the Serology laboratory, CDC/DGHA/ILB Cost effective and practical alternative for proficiency testing programs Easy to prepare and stable at room temperature for at least 4 weeks compared with traditional approach of shipping PT panels that require a cold chain system Objectives § § § Panels of coded specimens sent to multiple test sites by reference laboratory Test sites perform tests and report results Results indicate quality of personnel performance and test site operations
I. Proficiency Testing Program Using Dried Tube Specimens q Dried tube specimens (DTS) concept § § § q Developed in the Serology laboratory, CDC/DGHA/ILB Cost effective and practical alternative for proficiency testing programs Easy to prepare and stable at room temperature for at least 4 weeks compared with traditional approach of shipping PT panels that require a cold chain system Objectives § § § Panels of coded specimens sent to multiple test sites by reference laboratory Test sites perform tests and report results Results indicate quality of personnel performance and test site operations
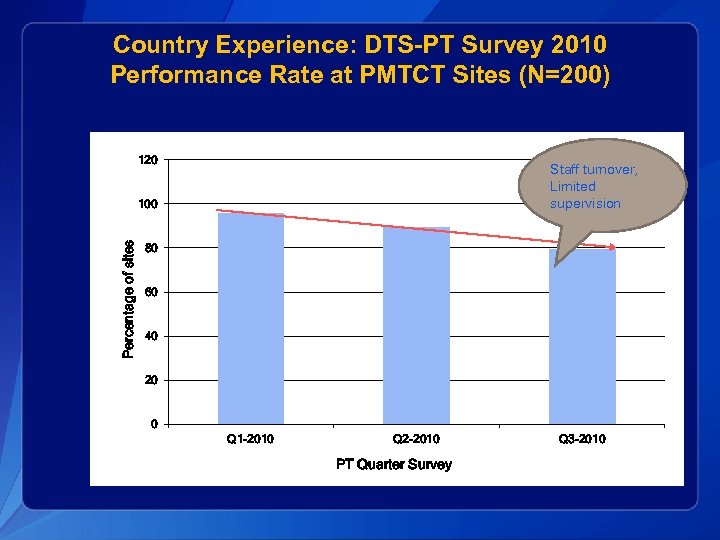 Country Experience: DTS-PT Survey 2010 Performance Rate at PMTCT Sites (N=200) 120 Staff turnover, Limited supervision Percentage of sites 100 80 60 40 20 0 Q 1 -2010 Q 2 -2010 PT Quarter Survey Q 3 -2010
Country Experience: DTS-PT Survey 2010 Performance Rate at PMTCT Sites (N=200) 120 Staff turnover, Limited supervision Percentage of sites 100 80 60 40 20 0 Q 1 -2010 Q 2 -2010 PT Quarter Survey Q 3 -2010
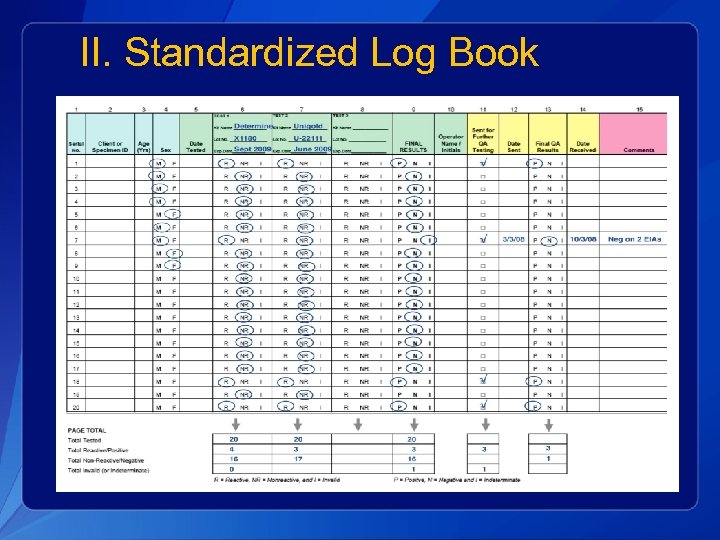 II. Standardized Log Book
II. Standardized Log Book
 Critical Variables to Add q HIV Test 1, 2, 3: § kit name, lot number, expiration date q q Operator doing the test Final QA Results
Critical Variables to Add q HIV Test 1, 2, 3: § kit name, lot number, expiration date q q Operator doing the test Final QA Results
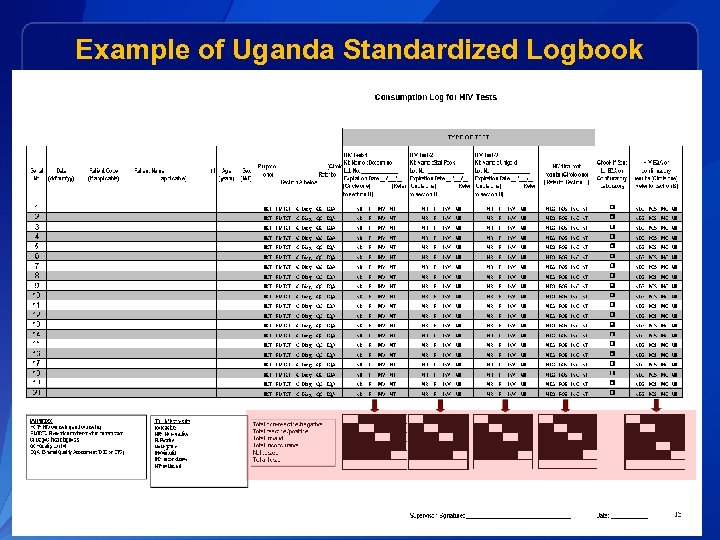 Example of Uganda Standardized Logbook
Example of Uganda Standardized Logbook
 Implementation of Standardized logbook at Testing Sites q q q Revision of existing logbooks to include key QA elements Training of supervisors and end-users Monthly review of logbook data Monthly supervision and corrective actions, if any Feedback to MOH, CDC HQ and in Country programs, key stakeholder
Implementation of Standardized logbook at Testing Sites q q q Revision of existing logbooks to include key QA elements Training of supervisors and end-users Monthly review of logbook data Monthly supervision and corrective actions, if any Feedback to MOH, CDC HQ and in Country programs, key stakeholder
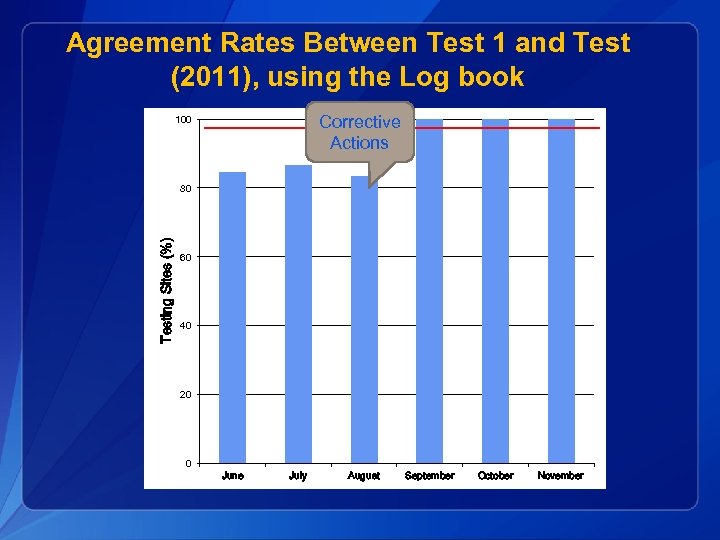 Agreement Rates Between Test 1 and Test (2011), using the Log book Corrective Actions 100 Testing Sites (%) 80 60 40 20 0 June July August September October November
Agreement Rates Between Test 1 and Test (2011), using the Log book Corrective Actions 100 Testing Sites (%) 80 60 40 20 0 June July August September October November
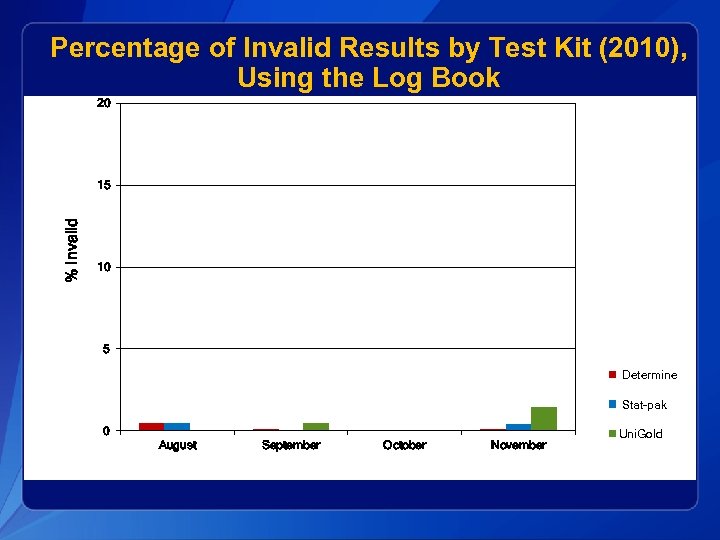 Percentage of Invalid Results by Test Kit (2010), Using the Log Book 20 % Invalid 15 10 5 Determine Stat-pak 0 August September October November Uni. Gold
Percentage of Invalid Results by Test Kit (2010), Using the Log Book 20 % Invalid 15 10 5 Determine Stat-pak 0 August September October November Uni. Gold
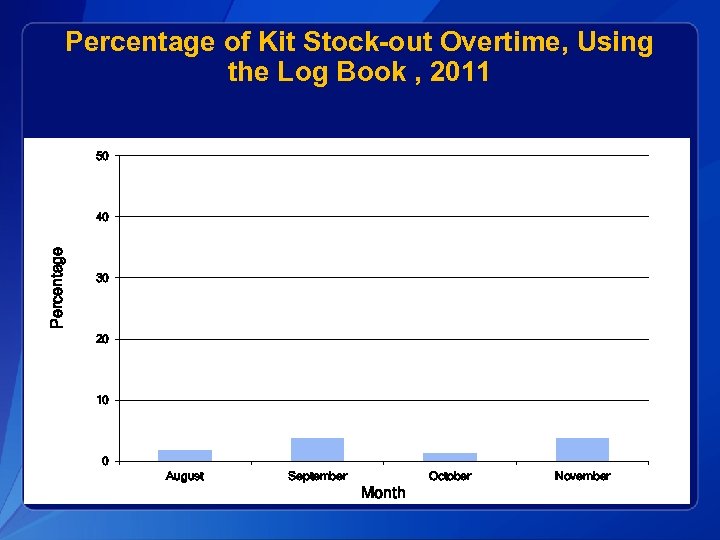 Percentage of Kit Stock-out Overtime, Using the Log Book , 2011 50 Percentage 40 30 20 10 0 August September October Month November
Percentage of Kit Stock-out Overtime, Using the Log Book , 2011 50 Percentage 40 30 20 10 0 August September October Month November
 III. RT Quality Improvement Training Curriculum q q q Requires some adaptation Country Experiences (Katy Yao et al, AJCP, 2010): Uganda, Botswana: § All lab and non lab staff performed RT well § For non-lab staff regular supervision was critical § Staff not conducting test regularly did not do so well on proficiency test
III. RT Quality Improvement Training Curriculum q q q Requires some adaptation Country Experiences (Katy Yao et al, AJCP, 2010): Uganda, Botswana: § All lab and non lab staff performed RT well § For non-lab staff regular supervision was critical § Staff not conducting test regularly did not do so well on proficiency test
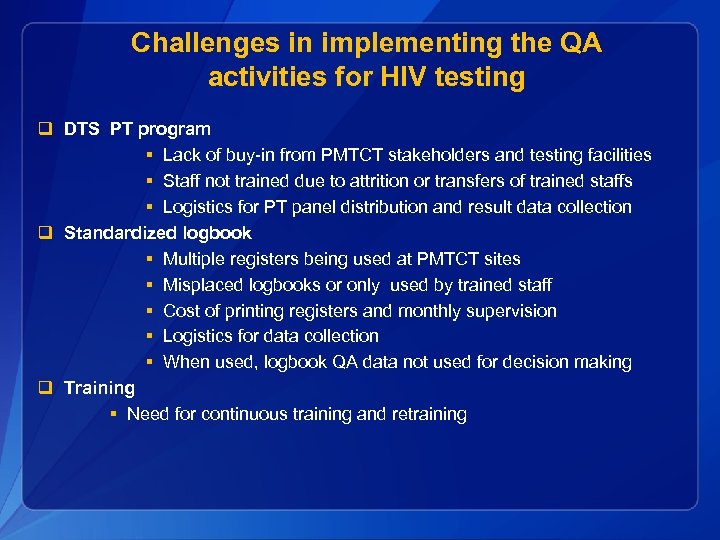 Challenges in implementing the QA activities for HIV testing q DTS PT program § Lack of buy-in from PMTCT stakeholders and testing facilities § Staff not trained due to attrition or transfers of trained staffs § Logistics for PT panel distribution and result data collection q Standardized logbook § Multiple registers being used at PMTCT sites § Misplaced logbooks or only used by trained staff § Cost of printing registers and monthly supervision § Logistics for data collection § When used, logbook QA data not used for decision making q Training § Need for continuous training and retraining
Challenges in implementing the QA activities for HIV testing q DTS PT program § Lack of buy-in from PMTCT stakeholders and testing facilities § Staff not trained due to attrition or transfers of trained staffs § Logistics for PT panel distribution and result data collection q Standardized logbook § Multiple registers being used at PMTCT sites § Misplaced logbooks or only used by trained staff § Cost of printing registers and monthly supervision § Logistics for data collection § When used, logbook QA data not used for decision making q Training § Need for continuous training and retraining
 Considerations for National Scale up q Advocacy to allocate resources to national and regional level to implement EQA approaches q Use of a combination of indigenous NGOs and a decentralized approach for the EQA program q National Reference Laboratory to provide oversight , coordinate supervisory visits and corrective actions q Involvement of in-country USG PMTCT team and national key stakeholders Development of strategies for corrective actions q § § § i. e. Standardized site visit tools which include testing QA elements Decision tree for correctives Include as part of other supportive supervisory visit (clinical/ lab related visits)
Considerations for National Scale up q Advocacy to allocate resources to national and regional level to implement EQA approaches q Use of a combination of indigenous NGOs and a decentralized approach for the EQA program q National Reference Laboratory to provide oversight , coordinate supervisory visits and corrective actions q Involvement of in-country USG PMTCT team and national key stakeholders Development of strategies for corrective actions q § § § i. e. Standardized site visit tools which include testing QA elements Decision tree for correctives Include as part of other supportive supervisory visit (clinical/ lab related visits)
 We need to have both lab and program teams working together to achieve better results PMTCT Cascade is achieved, when we work together as a team PMTCT Cascade is NOT achieved
We need to have both lab and program teams working together to achieve better results PMTCT Cascade is achieved, when we work together as a team PMTCT Cascade is NOT achieved
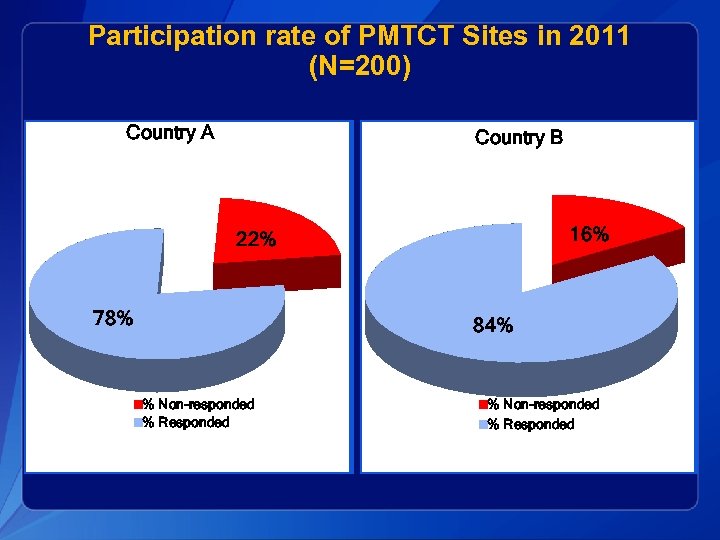 Participation rate of PMTCT Sites in 2011 (N=200) Country A Country B 16% 22% 78% 84% % Non-responded % Responded
Participation rate of PMTCT Sites in 2011 (N=200) Country A Country B 16% 22% 78% 84% % Non-responded % Responded


