w1L1Chap1 Course overview_Itskos_Fall_15.pptx
- Количество слайдов: 27
 Engineering Materials Lecture 1: Course Overview & Introduction 1
Engineering Materials Lecture 1: Course Overview & Introduction 1
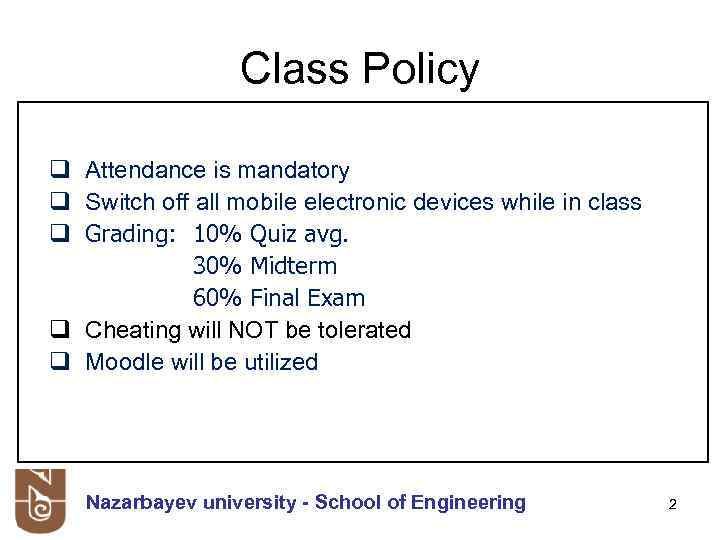 Class Policy q Attendance is mandatory q Switch off all mobile electronic devices while in class q Grading: 10% Quiz avg. 30% Midterm 60% Final Exam q Cheating will NOT be tolerated q Moodle will be utilized Nazarbayev university - School of Engineering 2
Class Policy q Attendance is mandatory q Switch off all mobile electronic devices while in class q Grading: 10% Quiz avg. 30% Midterm 60% Final Exam q Cheating will NOT be tolerated q Moodle will be utilized Nazarbayev university - School of Engineering 2
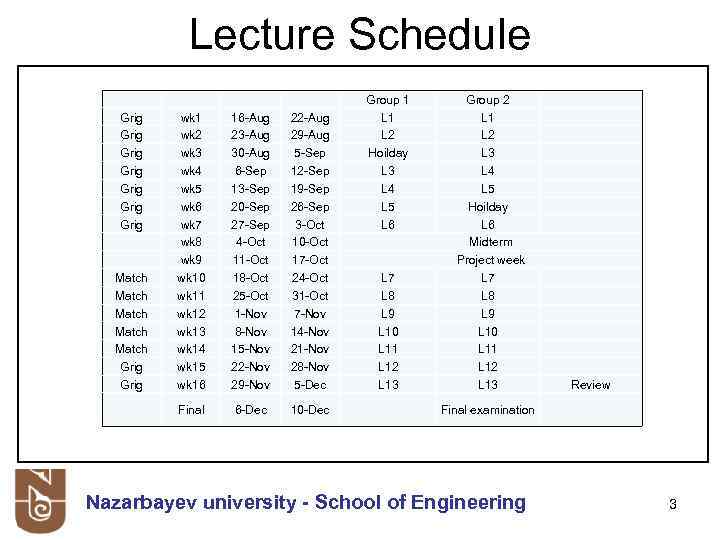 Lecture Schedule Match Match Grig 16 -Aug 23 -Aug 30 -Aug 6 -Sep 13 -Sep 20 -Sep 27 -Sep 4 -Oct 11 -Oct 18 -Oct 25 -Oct 1 -Nov 8 -Nov 15 -Nov 22 -Nov 29 -Nov 22 -Aug 29 -Aug 5 -Sep 12 -Sep 19 -Sep 26 -Sep 3 -Oct 10 -Oct 17 -Oct 24 -Oct 31 -Oct 7 -Nov 14 -Nov 21 -Nov 28 -Nov 5 -Dec Group 1 L 2 Hoilday L 3 L 4 L 5 L 6 Final Grig Grig wk 1 wk 2 wk 3 wk 4 wk 5 wk 6 wk 7 wk 8 wk 9 wk 10 wk 11 wk 12 wk 13 wk 14 wk 15 wk 16 L 7 L 8 L 9 L 10 L 11 L 12 L 13 Group 2 L 1 L 2 L 3 L 4 L 5 Hoilday L 6 Midterm Project week L 7 L 8 L 9 L 10 L 11 L 12 L 13 6 -Dec 10 -Dec Review Final examination Nazarbayev university - School of Engineering 3
Lecture Schedule Match Match Grig 16 -Aug 23 -Aug 30 -Aug 6 -Sep 13 -Sep 20 -Sep 27 -Sep 4 -Oct 11 -Oct 18 -Oct 25 -Oct 1 -Nov 8 -Nov 15 -Nov 22 -Nov 29 -Nov 22 -Aug 29 -Aug 5 -Sep 12 -Sep 19 -Sep 26 -Sep 3 -Oct 10 -Oct 17 -Oct 24 -Oct 31 -Oct 7 -Nov 14 -Nov 21 -Nov 28 -Nov 5 -Dec Group 1 L 2 Hoilday L 3 L 4 L 5 L 6 Final Grig Grig wk 1 wk 2 wk 3 wk 4 wk 5 wk 6 wk 7 wk 8 wk 9 wk 10 wk 11 wk 12 wk 13 wk 14 wk 15 wk 16 L 7 L 8 L 9 L 10 L 11 L 12 L 13 Group 2 L 1 L 2 L 3 L 4 L 5 Hoilday L 6 Midterm Project week L 7 L 8 L 9 L 10 L 11 L 12 L 13 6 -Dec 10 -Dec Review Final examination Nazarbayev university - School of Engineering 3
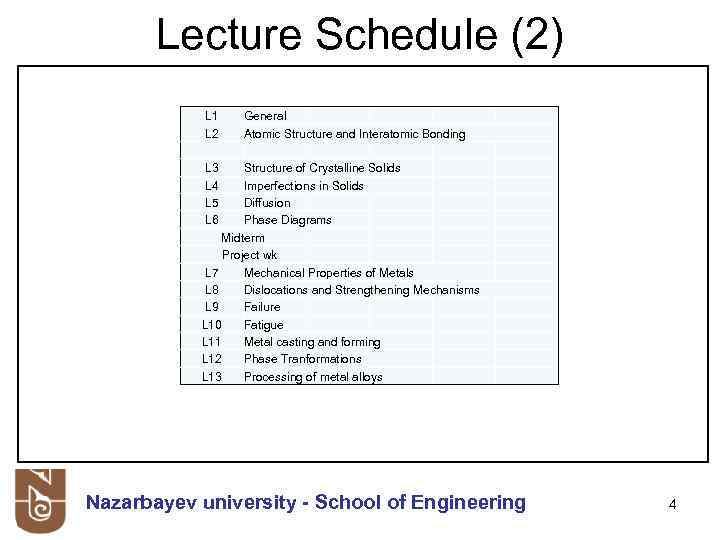 Lecture Schedule (2) L 1 L 2 General Atomic Structure and Interatomic Bonding L 3 L 4 L 5 L 6 Structure of Crystalline Solids Imperfections in Solids Diffusion Phase Diagrams Midterm Project wk L 7 Mechanical Properties of Metals L 8 Dislocations and Strengthening Mechanisms L 9 Failure L 10 Fatigue L 11 Metal casting and forming L 12 Phase Tranformations L 13 Processing of metal alloys Nazarbayev university - School of Engineering 4
Lecture Schedule (2) L 1 L 2 General Atomic Structure and Interatomic Bonding L 3 L 4 L 5 L 6 Structure of Crystalline Solids Imperfections in Solids Diffusion Phase Diagrams Midterm Project wk L 7 Mechanical Properties of Metals L 8 Dislocations and Strengthening Mechanisms L 9 Failure L 10 Fatigue L 11 Metal casting and forming L 12 Phase Tranformations L 13 Processing of metal alloys Nazarbayev university - School of Engineering 4
 Major Topics Ø Effects of structure on material properties; Ø Mechanical properties, failure and strengthening mechanisms; Ø Applications and processing of common engineering materials such as metals & nonferrous alloys, ceramics, polymers, and composites; Ø Economic, environmental and social issues of material usage and considerations for materials selection in designs. Nazarbayev university - School of Engineering 5
Major Topics Ø Effects of structure on material properties; Ø Mechanical properties, failure and strengthening mechanisms; Ø Applications and processing of common engineering materials such as metals & nonferrous alloys, ceramics, polymers, and composites; Ø Economic, environmental and social issues of material usage and considerations for materials selection in designs. Nazarbayev university - School of Engineering 5
 Learning Outcomes On completion of the course the students will be able to: Ø Explain the influences of microscopic structure and defects on material properties, including dislocation and strengthening mechanisms Ø Design and control heat treatment procedures to achieve a set of desirable mechanical characteristics Ø Understand the applications and processing of common engineering materials including metals & nonferrous alloys, ceramics, polymers, and composites Ø Utilize the knowledge in materials selection processes taking further considerations of the economic, environmental and social issues Nazarbayev university - School of Engineering 6
Learning Outcomes On completion of the course the students will be able to: Ø Explain the influences of microscopic structure and defects on material properties, including dislocation and strengthening mechanisms Ø Design and control heat treatment procedures to achieve a set of desirable mechanical characteristics Ø Understand the applications and processing of common engineering materials including metals & nonferrous alloys, ceramics, polymers, and composites Ø Utilize the knowledge in materials selection processes taking further considerations of the economic, environmental and social issues Nazarbayev university - School of Engineering 6
 Textbooks Materials Science and Engineering: An Introduction, 8 th Edition William D. Callister, David G. Rethwisch Engineering Materials 1: An Introduction to Properties, Applications and Design, 4 th Edition Michael F. Ashby, D R H Jones Nazarbayev university - School of Engineering 7
Textbooks Materials Science and Engineering: An Introduction, 8 th Edition William D. Callister, David G. Rethwisch Engineering Materials 1: An Introduction to Properties, Applications and Design, 4 th Edition Michael F. Ashby, D R H Jones Nazarbayev university - School of Engineering 7
 L 1. Course Overview & Introduction Classification of Materials Based on functions and applications: Ø Aerospace Ø Biomedical Ø Electronic Materials Ø Energy Technology and Environment Ø Magnetic Materials Ø Optical and Photonic Materials Ø “Smart” Materials Ø Structural Materials Nazarbayev university - School of Engineering 8
L 1. Course Overview & Introduction Classification of Materials Based on functions and applications: Ø Aerospace Ø Biomedical Ø Electronic Materials Ø Energy Technology and Environment Ø Magnetic Materials Ø Optical and Photonic Materials Ø “Smart” Materials Ø Structural Materials Nazarbayev university - School of Engineering 8
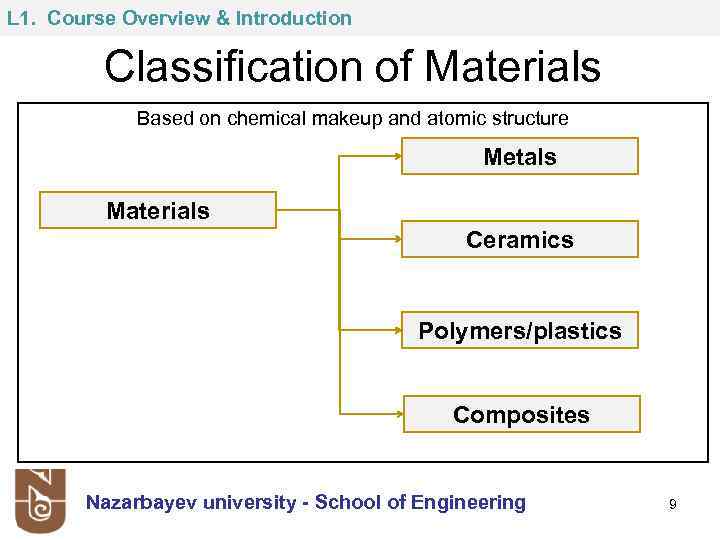 L 1. Course Overview & Introduction Classification of Materials Based on chemical makeup and atomic structure Metals Materials Ceramics Polymers/plastics Composites Nazarbayev university - School of Engineering 9
L 1. Course Overview & Introduction Classification of Materials Based on chemical makeup and atomic structure Metals Materials Ceramics Polymers/plastics Composites Nazarbayev university - School of Engineering 9
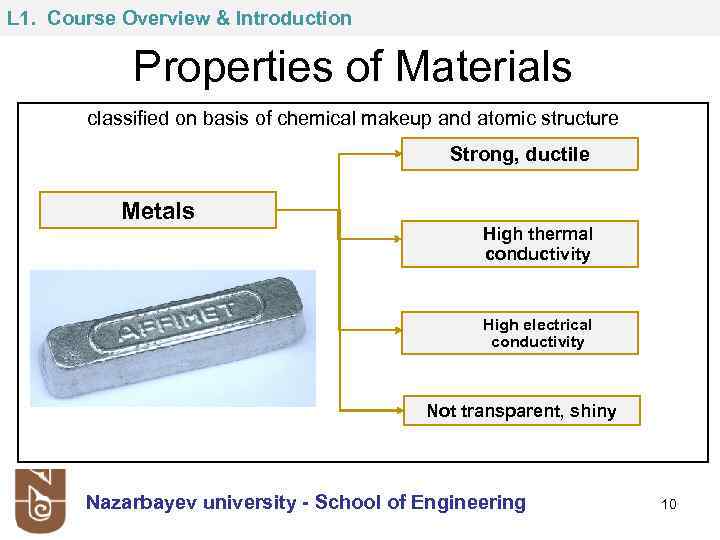 L 1. Course Overview & Introduction Properties of Materials classified on basis of chemical makeup and atomic structure Strong, ductile Metals High thermal conductivity High electrical conductivity Not transparent, shiny Nazarbayev university - School of Engineering 10
L 1. Course Overview & Introduction Properties of Materials classified on basis of chemical makeup and atomic structure Strong, ductile Metals High thermal conductivity High electrical conductivity Not transparent, shiny Nazarbayev university - School of Engineering 10
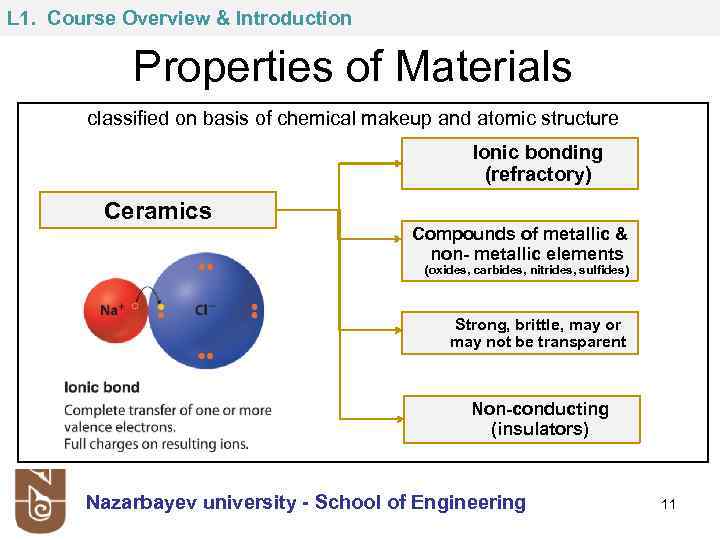 L 1. Course Overview & Introduction Properties of Materials classified on basis of chemical makeup and atomic structure Ionic bonding (refractory) Ceramics Compounds of metallic & non- metallic elements (oxides, carbides, nitrides, sulfides) Strong, brittle, may or may not be transparent Non-conducting (insulators) Nazarbayev university - School of Engineering 11
L 1. Course Overview & Introduction Properties of Materials classified on basis of chemical makeup and atomic structure Ionic bonding (refractory) Ceramics Compounds of metallic & non- metallic elements (oxides, carbides, nitrides, sulfides) Strong, brittle, may or may not be transparent Non-conducting (insulators) Nazarbayev university - School of Engineering 11
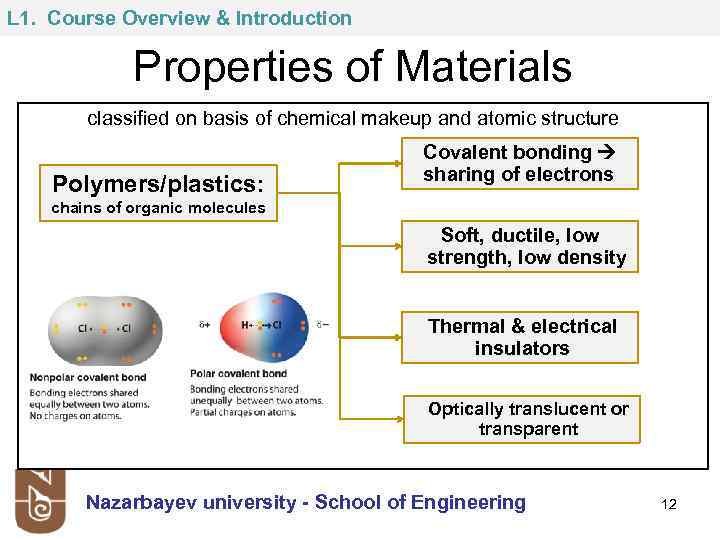 L 1. Course Overview & Introduction Properties of Materials classified on basis of chemical makeup and atomic structure Polymers/plastics: Covalent bonding sharing of electrons chains of organic molecules Soft, ductile, low strength, low density Thermal & electrical insulators Optically translucent or transparent Nazarbayev university - School of Engineering 12
L 1. Course Overview & Introduction Properties of Materials classified on basis of chemical makeup and atomic structure Polymers/plastics: Covalent bonding sharing of electrons chains of organic molecules Soft, ductile, low strength, low density Thermal & electrical insulators Optically translucent or transparent Nazarbayev university - School of Engineering 12
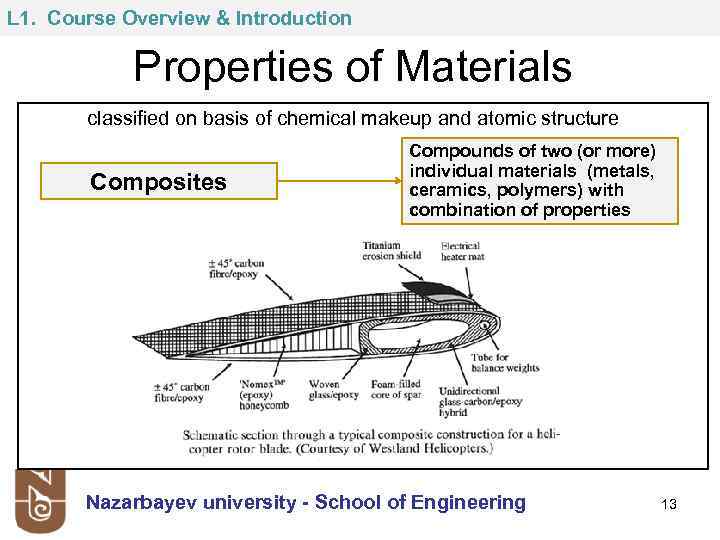 L 1. Course Overview & Introduction Properties of Materials classified on basis of chemical makeup and atomic structure Composites Compounds of two (or more) individual materials (metals, ceramics, polymers) with combination of properties Nazarbayev university - School of Engineering 13
L 1. Course Overview & Introduction Properties of Materials classified on basis of chemical makeup and atomic structure Composites Compounds of two (or more) individual materials (metals, ceramics, polymers) with combination of properties Nazarbayev university - School of Engineering 13
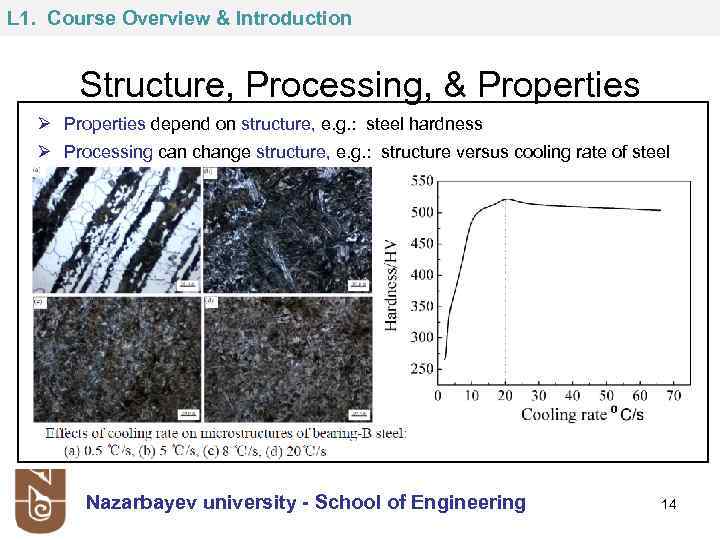 L 1. Course Overview & Introduction Structure, Processing, & Properties Ø Properties depend on structure, e. g. : steel hardness Ø Processing can change structure, e. g. : structure versus cooling rate of steel Nazarbayev university - School of Engineering 14
L 1. Course Overview & Introduction Structure, Processing, & Properties Ø Properties depend on structure, e. g. : steel hardness Ø Processing can change structure, e. g. : structure versus cooling rate of steel Nazarbayev university - School of Engineering 14
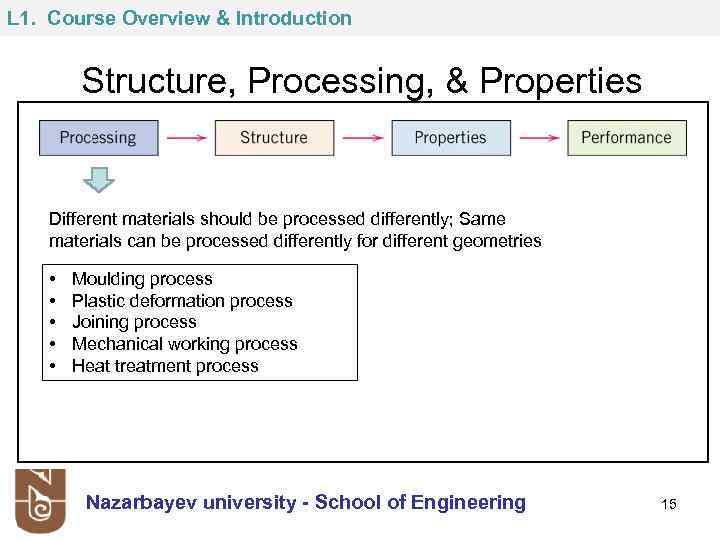 L 1. Course Overview & Introduction Structure, Processing, & Properties Different materials should be processed differently; Same materials can be processed differently for different geometries • • • Moulding process Plastic deformation process Joining process Mechanical working process Heat treatment process Nazarbayev university - School of Engineering 15
L 1. Course Overview & Introduction Structure, Processing, & Properties Different materials should be processed differently; Same materials can be processed differently for different geometries • • • Moulding process Plastic deformation process Joining process Mechanical working process Heat treatment process Nazarbayev university - School of Engineering 15
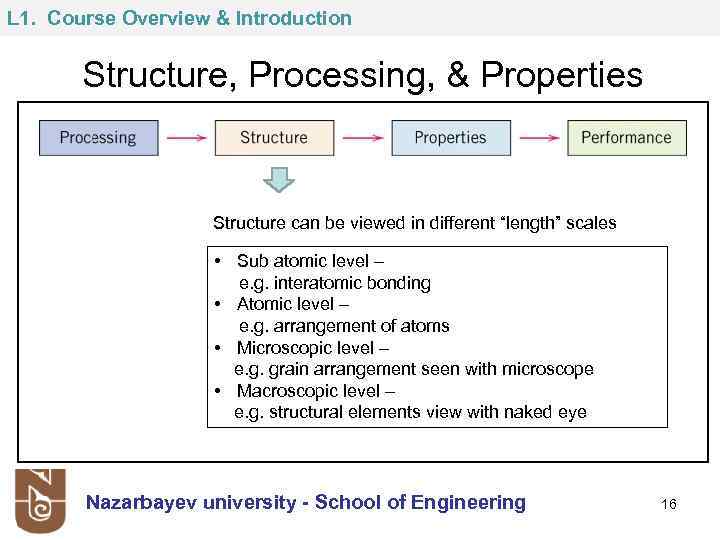 L 1. Course Overview & Introduction Structure, Processing, & Properties Structure can be viewed in different “length” scales • Sub atomic level – e. g. interatomic bonding • Atomic level – e. g. arrangement of atoms • Microscopic level – e. g. grain arrangement seen with microscope • Macroscopic level – e. g. structural elements view with naked eye Nazarbayev university - School of Engineering 16
L 1. Course Overview & Introduction Structure, Processing, & Properties Structure can be viewed in different “length” scales • Sub atomic level – e. g. interatomic bonding • Atomic level – e. g. arrangement of atoms • Microscopic level – e. g. grain arrangement seen with microscope • Macroscopic level – e. g. structural elements view with naked eye Nazarbayev university - School of Engineering 16
 L 1. Course Overview & Introduction Structure, Processing, & Properties Property is a material trait in terms of the type and magnitude of response to a specific imposed stimulus. • • • Mechanical – response to applied load, e. g. ultimate strength Electrical – response to electric field, e. g. resistivity Thermal – response to heat, e. g. thermal conductivity Magnetic – response to magnetic field, e. g. magnetic permeability Optical – response to light, e. g. transparent, translucent, opaque Deteriorative – relates to chemical reactivity, e. g. heat treatment can slow crack speed of some alloy in salt water Nazarbayev university - School of Engineering 17
L 1. Course Overview & Introduction Structure, Processing, & Properties Property is a material trait in terms of the type and magnitude of response to a specific imposed stimulus. • • • Mechanical – response to applied load, e. g. ultimate strength Electrical – response to electric field, e. g. resistivity Thermal – response to heat, e. g. thermal conductivity Magnetic – response to magnetic field, e. g. magnetic permeability Optical – response to light, e. g. transparent, translucent, opaque Deteriorative – relates to chemical reactivity, e. g. heat treatment can slow crack speed of some alloy in salt water Nazarbayev university - School of Engineering 17
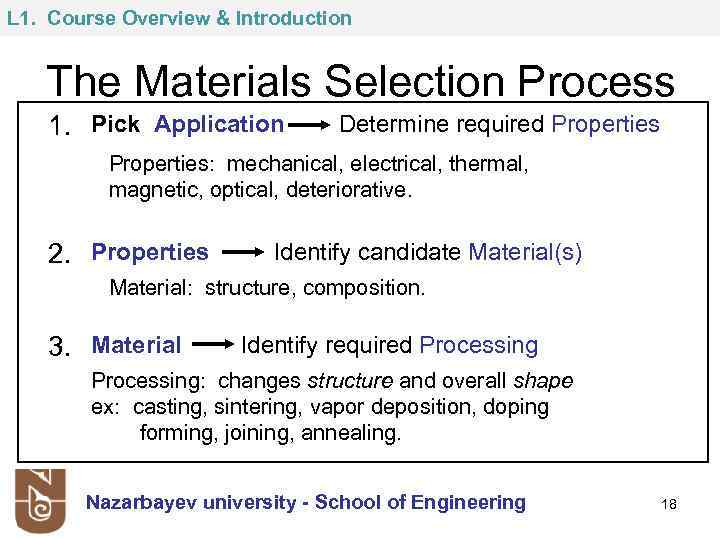 L 1. Course Overview & Introduction The Materials Selection Process 1. Pick Application Determine required Properties: mechanical, electrical, thermal, magnetic, optical, deteriorative. 2. Properties Identify candidate Material(s) Material: structure, composition. 3. Material Identify required Processing: changes structure and overall shape ex: casting, sintering, vapor deposition, doping forming, joining, annealing. Nazarbayev university - School of Engineering 18
L 1. Course Overview & Introduction The Materials Selection Process 1. Pick Application Determine required Properties: mechanical, electrical, thermal, magnetic, optical, deteriorative. 2. Properties Identify candidate Material(s) Material: structure, composition. 3. Material Identify required Processing: changes structure and overall shape ex: casting, sintering, vapor deposition, doping forming, joining, annealing. Nazarbayev university - School of Engineering 18
 L 1. Course Overview & Introduction Materials Selection Considerations Ø Cost Ø “Green” – environmentally friendly Ø Sustainability: e. g. bamboo bicycle frame Ø Easily available commercially in large quantities Ø Political: e. g. sanctions on nuclear materials Ø Technology: e. g. heat shield for space shuttle Ø Degradation during service: e. g. corrosion Nazarbayev university - School of Engineering 19
L 1. Course Overview & Introduction Materials Selection Considerations Ø Cost Ø “Green” – environmentally friendly Ø Sustainability: e. g. bamboo bicycle frame Ø Easily available commercially in large quantities Ø Political: e. g. sanctions on nuclear materials Ø Technology: e. g. heat shield for space shuttle Ø Degradation during service: e. g. corrosion Nazarbayev university - School of Engineering 19
 L 1. Course Overview & Introduction Module Summary You will learn in this module about: Ø material structure Ø how structure dictates properties Ø how processing can change structure Ø commonly used engineering materials This module will help you to: ü Use the right material for the job. ü Understand the relation between properties, structure, and processing. ü Recognize new design opportunities offered by materials selection. Nazarbayev university - School of Engineering 20
L 1. Course Overview & Introduction Module Summary You will learn in this module about: Ø material structure Ø how structure dictates properties Ø how processing can change structure Ø commonly used engineering materials This module will help you to: ü Use the right material for the job. ü Understand the relation between properties, structure, and processing. ü Recognize new design opportunities offered by materials selection. Nazarbayev university - School of Engineering 20
 L 1. Course Overview & Introduction Approach Ø Study microstructures, starting with the atom, atomic bonding, and how different classes of materials are bonded together Ø Look at the effect of composition on microstructure Ø Look at the effect of processing on microstructure Ø Connect how microstructure relates to properties We shall first do some revision Nazarbayev university - School of Engineering 21
L 1. Course Overview & Introduction Approach Ø Study microstructures, starting with the atom, atomic bonding, and how different classes of materials are bonded together Ø Look at the effect of composition on microstructure Ø Look at the effect of processing on microstructure Ø Connect how microstructure relates to properties We shall first do some revision Nazarbayev university - School of Engineering 21
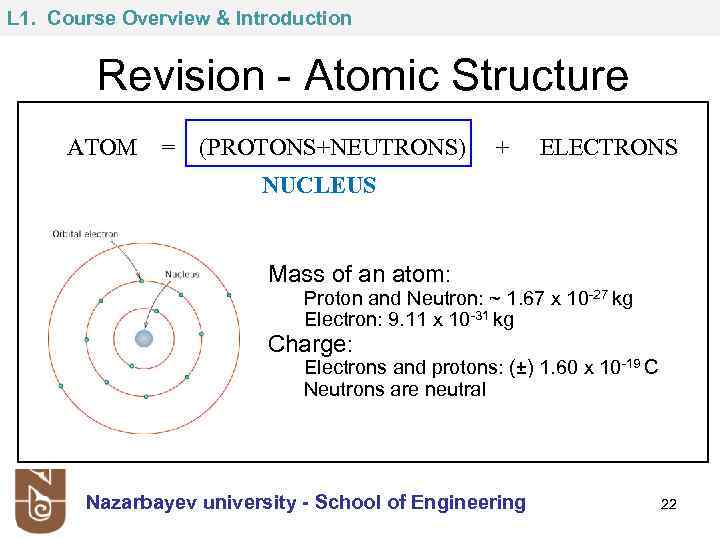 L 1. Course Overview & Introduction Revision - Atomic Structure ATOM = (PROTONS+NEUTRONS) + ELECTRONS NUCLEUS Mass of an atom: Proton and Neutron: ~ 1. 67 x 10 -27 kg Electron: 9. 11 x 10 -31 kg Charge: Electrons and protons: (±) 1. 60 x 10 -19 C Neutrons are neutral Nazarbayev university - School of Engineering 22
L 1. Course Overview & Introduction Revision - Atomic Structure ATOM = (PROTONS+NEUTRONS) + ELECTRONS NUCLEUS Mass of an atom: Proton and Neutron: ~ 1. 67 x 10 -27 kg Electron: 9. 11 x 10 -31 kg Charge: Electrons and protons: (±) 1. 60 x 10 -19 C Neutrons are neutral Nazarbayev university - School of Engineering 22
 L 1. Course Overview & Introduction Revision - Atomic Structure Z = Atomic number = number of protons in nucleus This is used to identify element N = number of neutrons in nucleus This is used to identify isotopes, written as (Z+N)XZ : ( e. g. 14 C 6 and 12 C 6 ) where: A = Atomic mass unit (amu) 1 amu is defined as the 1/12 of the atomic mass 12 C 6 Atomic mass of 12 C 6 is 12 amu: 6 protons (Z=6) + 6 neutrons (N=6) This is approximately the total mass of protons + total mass of neutrons Therefore 1 amu = Massproton ~ Massneutron = 1. 67 x 10 -27 kg and A = Atomic Mass = Z + N NAV = 1 mole = 6. 023 x 1023 molecules or atoms (Avogadro’s number) Atomic weight is expressed in amu/atom, i. e. 1 amu/atom = 1 g/mol Nazarbayev university - School of Engineering 23
L 1. Course Overview & Introduction Revision - Atomic Structure Z = Atomic number = number of protons in nucleus This is used to identify element N = number of neutrons in nucleus This is used to identify isotopes, written as (Z+N)XZ : ( e. g. 14 C 6 and 12 C 6 ) where: A = Atomic mass unit (amu) 1 amu is defined as the 1/12 of the atomic mass 12 C 6 Atomic mass of 12 C 6 is 12 amu: 6 protons (Z=6) + 6 neutrons (N=6) This is approximately the total mass of protons + total mass of neutrons Therefore 1 amu = Massproton ~ Massneutron = 1. 67 x 10 -27 kg and A = Atomic Mass = Z + N NAV = 1 mole = 6. 023 x 1023 molecules or atoms (Avogadro’s number) Atomic weight is expressed in amu/atom, i. e. 1 amu/atom = 1 g/mol Nazarbayev university - School of Engineering 23
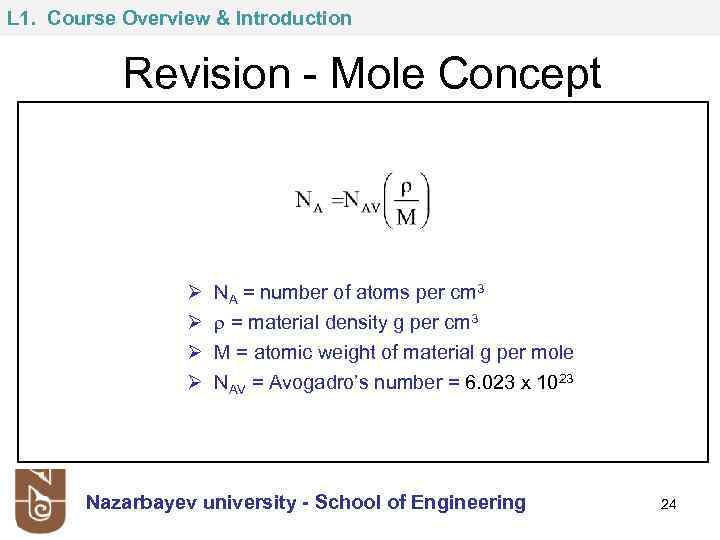 L 1. Course Overview & Introduction Revision - Mole Concept Ø Ø NA = number of atoms per cm 3 = material density g per cm 3 M = atomic weight of material g per mole NAV = Avogadro’s number = 6. 023 x 1023 Nazarbayev university - School of Engineering 24
L 1. Course Overview & Introduction Revision - Mole Concept Ø Ø NA = number of atoms per cm 3 = material density g per cm 3 M = atomic weight of material g per mole NAV = Avogadro’s number = 6. 023 x 1023 Nazarbayev university - School of Engineering 24
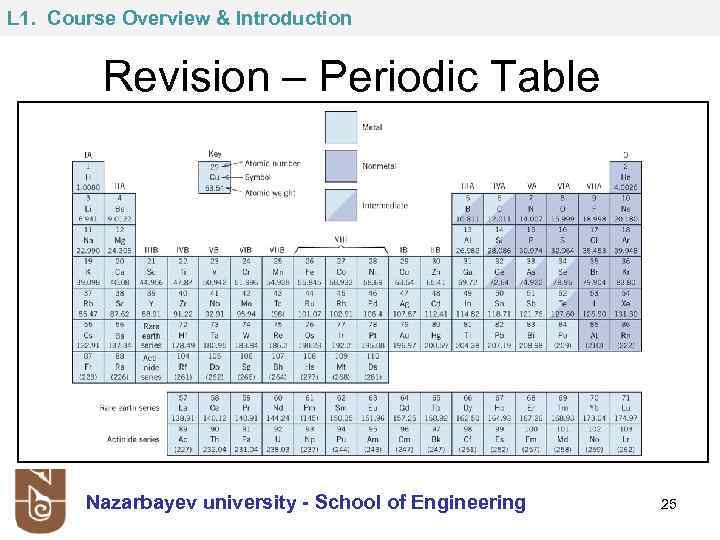 L 1. Course Overview & Introduction Revision – Periodic Table Nazarbayev university - School of Engineering 25
L 1. Course Overview & Introduction Revision – Periodic Table Nazarbayev university - School of Engineering 25
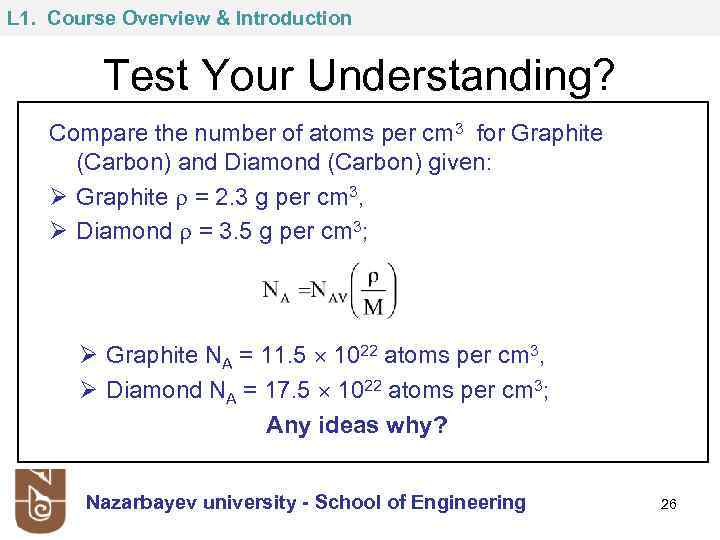 L 1. Course Overview & Introduction Test Your Understanding? Compare the number of atoms per cm 3 for Graphite (Carbon) and Diamond (Carbon) given: Ø Graphite = 2. 3 g per cm 3, Ø Diamond = 3. 5 g per cm 3; Ø Graphite NA = 11. 5 1022 atoms per cm 3, Ø Diamond NA = 17. 5 1022 atoms per cm 3; Any ideas why? Nazarbayev university - School of Engineering 26
L 1. Course Overview & Introduction Test Your Understanding? Compare the number of atoms per cm 3 for Graphite (Carbon) and Diamond (Carbon) given: Ø Graphite = 2. 3 g per cm 3, Ø Diamond = 3. 5 g per cm 3; Ø Graphite NA = 11. 5 1022 atoms per cm 3, Ø Diamond NA = 17. 5 1022 atoms per cm 3; Any ideas why? Nazarbayev university - School of Engineering 26
 L 1. Course Overview & Introduction Announcements Reading: • Chapter 1 in Materials Science & Engineering for this lecture • Chapter 2 in Materials Science & Engineering for next lecture Self-help problems: • None Nazarbayev university - School of Engineering 27
L 1. Course Overview & Introduction Announcements Reading: • Chapter 1 in Materials Science & Engineering for this lecture • Chapter 2 in Materials Science & Engineering for next lecture Self-help problems: • None Nazarbayev university - School of Engineering 27


