a8d0e983d918ed990aae17d4e26b15ba.ppt
- Количество слайдов: 45
 Endoscope Reprocessing: Current Status of Disinfection Recommendations William A. Rutala, Ph. D. , M. P. H. University of North Carolina (UNC) Health Care System and UNC at Chapel Hill Copyright © 2004 WA Rutala
Endoscope Reprocessing: Current Status of Disinfection Recommendations William A. Rutala, Ph. D. , M. P. H. University of North Carolina (UNC) Health Care System and UNC at Chapel Hill Copyright © 2004 WA Rutala
 Endoscope Reprocessing Lecture Goals Background l Infections related to endoscopy l Reprocessing of endoscopes and accessories l Cleaning n High-level disinfection/sterilization n Automated endoscope reprocessing n l Quality control Copyright © 2004 WA Rutala
Endoscope Reprocessing Lecture Goals Background l Infections related to endoscopy l Reprocessing of endoscopes and accessories l Cleaning n High-level disinfection/sterilization n Automated endoscope reprocessing n l Quality control Copyright © 2004 WA Rutala
 GI ENDOSCOPES l l l Widely used diagnostic and therapeutic procedure Endoscope contamination during use (109 in/105 out) Semicritical items require high-level disinfection minimally Inappropriate cleaning and disinfection has lead to cross-transmission In the inanimate environment, although the incidence remains very low, endoscopes represent a risk of disease transmission Copyright © 2004 WA Rutala
GI ENDOSCOPES l l l Widely used diagnostic and therapeutic procedure Endoscope contamination during use (109 in/105 out) Semicritical items require high-level disinfection minimally Inappropriate cleaning and disinfection has lead to cross-transmission In the inanimate environment, although the incidence remains very low, endoscopes represent a risk of disease transmission Copyright © 2004 WA Rutala
 TRANSMISSION OF INFECTION l Gastrointestinal endoscopy n n n l >300 infections transmitted 70% agents Salmonella sp. and P. aeruginosa Clinical spectrum ranged from colonization to death (~4%) Bronchoscopy n n 90 infections transmitted M. tuberculosis, atypical Mycobacteria, P. aeruginosa Spach DH et al Ann Intern Med 1993: 118: 117 -128 and Weber DJ, Rutala WA Gastroint Dis 2002 Copyright © 2004 WA Rutala
TRANSMISSION OF INFECTION l Gastrointestinal endoscopy n n n l >300 infections transmitted 70% agents Salmonella sp. and P. aeruginosa Clinical spectrum ranged from colonization to death (~4%) Bronchoscopy n n 90 infections transmitted M. tuberculosis, atypical Mycobacteria, P. aeruginosa Spach DH et al Ann Intern Med 1993: 118: 117 -128 and Weber DJ, Rutala WA Gastroint Dis 2002 Copyright © 2004 WA Rutala
 Nosocomial Infections via GI Endoscopes l Observations Number of reported infections is small, suggesting a very low incidence n Endemic transmission may go unrecognized (e. g. inadequate surveillance, low frequency, asymptomatic infections) n Spach DH. Ann Int Med 1993; 118: 117 and Weber DJ, Rutala, WA. Gastroint Dis 2002 Copyright © 2004 WA Rutala
Nosocomial Infections via GI Endoscopes l Observations Number of reported infections is small, suggesting a very low incidence n Endemic transmission may go unrecognized (e. g. inadequate surveillance, low frequency, asymptomatic infections) n Spach DH. Ann Int Med 1993; 118: 117 and Weber DJ, Rutala, WA. Gastroint Dis 2002 Copyright © 2004 WA Rutala
 Nosocomial Infections via GI Endoscopes l Infections traced to deficient practices n Inadequate cleaning (clean all channels) n Inappropriate/ineffective disinfection (time exposure, perfuse channels, test concentration, ineffective disinfectant, inappropriate disinfectant) n Failure to follow recommended disinfection practices (tapwater rinse) n Flaws is design of endoscopes or AERs Copyright © 2004 WA Rutala
Nosocomial Infections via GI Endoscopes l Infections traced to deficient practices n Inadequate cleaning (clean all channels) n Inappropriate/ineffective disinfection (time exposure, perfuse channels, test concentration, ineffective disinfectant, inappropriate disinfectant) n Failure to follow recommended disinfection practices (tapwater rinse) n Flaws is design of endoscopes or AERs Copyright © 2004 WA Rutala
 Endoscope Reprocessing: Current Status of Cleaning and Disinfection l Guidelines n n n n n Multi-Society Guideline, 11 professional organizations, 2003 Society of Gastroenterology Nurses and Associates, 2000 European Society of Gastrointestinal Endoscopy, 2000 British Society of Gastroenterology Endoscopy, 1998 Gastroenterological Society of Australia, 1999 Gastroenterological Nurses Society of Australia, 1999 American Society for Gastrointestinal Endoscopy, 1996 Association for Professional in Infection Control and Epidemiology, 2000 Centers for Disease Control and Prevention, 2004 (in press) Copyright © 2004 WA Rutala
Endoscope Reprocessing: Current Status of Cleaning and Disinfection l Guidelines n n n n n Multi-Society Guideline, 11 professional organizations, 2003 Society of Gastroenterology Nurses and Associates, 2000 European Society of Gastrointestinal Endoscopy, 2000 British Society of Gastroenterology Endoscopy, 1998 Gastroenterological Society of Australia, 1999 Gastroenterological Nurses Society of Australia, 1999 American Society for Gastrointestinal Endoscopy, 1996 Association for Professional in Infection Control and Epidemiology, 2000 Centers for Disease Control and Prevention, 2004 (in press) Copyright © 2004 WA Rutala
 Endoscope Reprocessing, Worldwide l Worldwide, endoscopy reprocessing varies greatly India, of 133 endoscopy centers, only 1/3 performed even a minimum disinfection (1% glut for 2 min) n Brazil, “a high standard …occur only exceptionally” n Western Europe, >30% did not adequately disinfect n Japan, found “exceedingly poor” disinfection protocols Copyright © 2004 WA Rutala n US, 25% of endoscopes revealed >100, 000 bacteria n
Endoscope Reprocessing, Worldwide l Worldwide, endoscopy reprocessing varies greatly India, of 133 endoscopy centers, only 1/3 performed even a minimum disinfection (1% glut for 2 min) n Brazil, “a high standard …occur only exceptionally” n Western Europe, >30% did not adequately disinfect n Japan, found “exceedingly poor” disinfection protocols Copyright © 2004 WA Rutala n US, 25% of endoscopes revealed >100, 000 bacteria n
 Endoscopes Copyright © 2004 WA Rutala
Endoscopes Copyright © 2004 WA Rutala
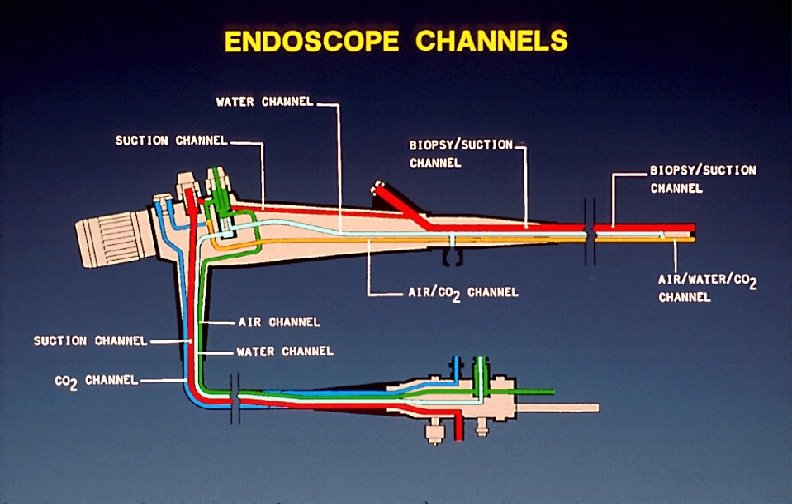 Copyright © 2004 WA Rutala
Copyright © 2004 WA Rutala
 ENDOSCOPE DISINFECTION l l CLEAN-mechanically cleaned with water and enzymatic cleaner HLD/STERILIZE-immerse scope and perfuse HLD/sterilant through all channels for exposure time RINSE-scope and channels rinsed with sterile water, filtered water, or tap water followed by alcohol DRY-use forced air to dry insertion tube and Copyright © 2004 WA Rutala channels
ENDOSCOPE DISINFECTION l l CLEAN-mechanically cleaned with water and enzymatic cleaner HLD/STERILIZE-immerse scope and perfuse HLD/sterilant through all channels for exposure time RINSE-scope and channels rinsed with sterile water, filtered water, or tap water followed by alcohol DRY-use forced air to dry insertion tube and Copyright © 2004 WA Rutala channels
 ENDOSCOPE REPROCESSING l Source of contamination for infections (36 outbreaks) transmitted by GI endoscopes from 1974 -2001: n Cleaning-3 (12%) n Disinfection-19 (73%) n Rinse, Dry, Store-3 (12%) n Etiology unknown-11 Copyright © 2004 WA Rutala
ENDOSCOPE REPROCESSING l Source of contamination for infections (36 outbreaks) transmitted by GI endoscopes from 1974 -2001: n Cleaning-3 (12%) n Disinfection-19 (73%) n Rinse, Dry, Store-3 (12%) n Etiology unknown-11 Copyright © 2004 WA Rutala
 ENDOSCOPE DISINFECTION l Cleaning (results in dramatic decrease in bioburden, 4 -5 log 10 reduction) n No brushing biopsy channel. (Schousboe M. NZ Med J 1980; 92: 275) n No precleaning before AER. (Hawkey PM. J Hosp Inf 1981; 2: 373) n Biopsy-suction channel not cleaned with a brush. (Bronowicki JP. NEJM 1997; 337: 237) Copyright © 2004 WA Rutala
ENDOSCOPE DISINFECTION l Cleaning (results in dramatic decrease in bioburden, 4 -5 log 10 reduction) n No brushing biopsy channel. (Schousboe M. NZ Med J 1980; 92: 275) n No precleaning before AER. (Hawkey PM. J Hosp Inf 1981; 2: 373) n Biopsy-suction channel not cleaned with a brush. (Bronowicki JP. NEJM 1997; 337: 237) Copyright © 2004 WA Rutala
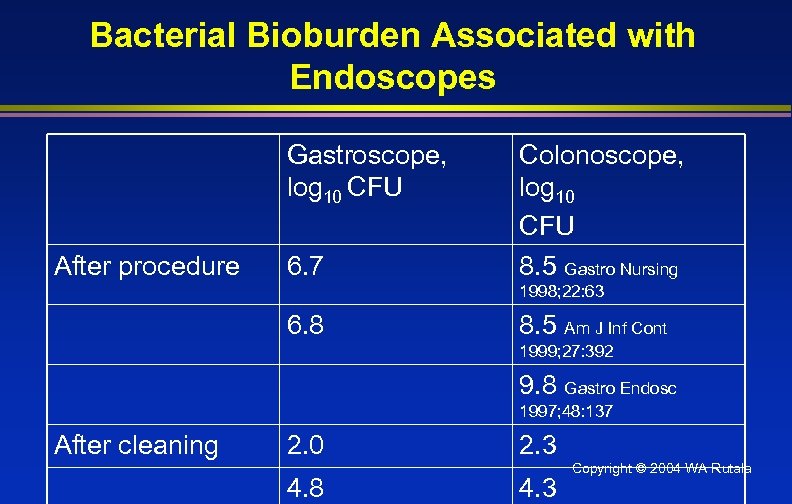 Bacterial Bioburden Associated with Endoscopes Gastroscope, log 10 CFU After procedure 6. 7 Colonoscope, log 10 CFU 8. 5 Gastro Nursing 1998; 22: 63 6. 8 8. 5 Am J Inf Cont 1999; 27: 392 9. 8 Gastro Endosc 1997; 48: 137 After cleaning 2. 0 4. 8 2. 3 4. 3 Copyright © 2004 WA Rutala
Bacterial Bioburden Associated with Endoscopes Gastroscope, log 10 CFU After procedure 6. 7 Colonoscope, log 10 CFU 8. 5 Gastro Nursing 1998; 22: 63 6. 8 8. 5 Am J Inf Cont 1999; 27: 392 9. 8 Gastro Endosc 1997; 48: 137 After cleaning 2. 0 4. 8 2. 3 4. 3 Copyright © 2004 WA Rutala
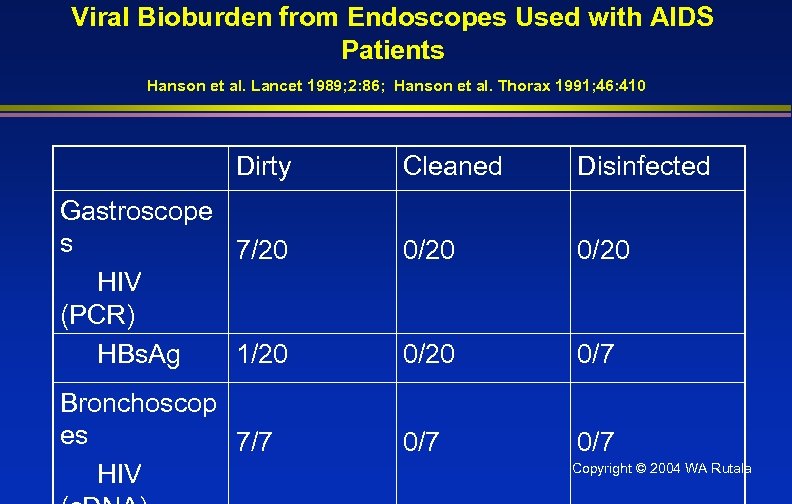 Viral Bioburden from Endoscopes Used with AIDS Patients Hanson et al. Lancet 1989; 2: 86; Hanson et al. Thorax 1991; 46: 410 Dirty Gastroscope s 7/20 HIV (PCR) HBs. Ag 1/20 Bronchoscop es 7/7 HIV Cleaned Disinfected 0/20 0/7 0/7 Copyright © 2004 WA Rutala
Viral Bioburden from Endoscopes Used with AIDS Patients Hanson et al. Lancet 1989; 2: 86; Hanson et al. Thorax 1991; 46: 410 Dirty Gastroscope s 7/20 HIV (PCR) HBs. Ag 1/20 Bronchoscop es 7/7 HIV Cleaned Disinfected 0/20 0/7 0/7 Copyright © 2004 WA Rutala
 ENDOSCOPE REPROCESSING l Precleaning After removal from patient, wipe the insertion tube with a wet cloth and alternate suctioning the enzymatic cleaner and air through the biopsy/suction channel until solution clean. The air-water channel is flushed or blown out per instructions. n Transport the endoscope to the reprocessing area. n Enyzmatic cleaner should be prepared per instructions. Some data suggest enzymes are more effective cleaners than detergents. Enyzmatic WA Rutala Copyright © 2004 cleaners must be changed after use. n
ENDOSCOPE REPROCESSING l Precleaning After removal from patient, wipe the insertion tube with a wet cloth and alternate suctioning the enzymatic cleaner and air through the biopsy/suction channel until solution clean. The air-water channel is flushed or blown out per instructions. n Transport the endoscope to the reprocessing area. n Enyzmatic cleaner should be prepared per instructions. Some data suggest enzymes are more effective cleaners than detergents. Enyzmatic WA Rutala Copyright © 2004 cleaners must be changed after use. n
 ENDOSCOPE REPROCESSING l Cleaning Immerse in a compatible low-sudsing, enzymatic cleaner n Wash all debris from exterior by brushing and wiping n Remove all removal parts of the endoscope and clean each reusable part separately n After exterior cleaning, brush accessible channels with appropriate-sized cleaning brush (bristles Copyright © 2004 WA Rutala contact all surfaces) n
ENDOSCOPE REPROCESSING l Cleaning Immerse in a compatible low-sudsing, enzymatic cleaner n Wash all debris from exterior by brushing and wiping n Remove all removal parts of the endoscope and clean each reusable part separately n After exterior cleaning, brush accessible channels with appropriate-sized cleaning brush (bristles Copyright © 2004 WA Rutala contact all surfaces) n
 ENDOSCOPE REPROCESSING l Cleaning (continued) n n After each passage, rinse the brush, remove debris before reinserting. Continue until no visible debris on brush. Attach cleaning adapters for each channel per manufacturer’s instructions and flush with enzymatic cleaner to remove debris. After cleaning is complete, rinse the endoscope with clean water. Purge water from channels using forced air. Dry exterior of the endoscope with a soft, lint-free cloth. Copyright © 2004 WA Rutala
ENDOSCOPE REPROCESSING l Cleaning (continued) n n After each passage, rinse the brush, remove debris before reinserting. Continue until no visible debris on brush. Attach cleaning adapters for each channel per manufacturer’s instructions and flush with enzymatic cleaner to remove debris. After cleaning is complete, rinse the endoscope with clean water. Purge water from channels using forced air. Dry exterior of the endoscope with a soft, lint-free cloth. Copyright © 2004 WA Rutala
 ENDOSCOPE DISINFECTION l l CLEAN-mechanically cleaned with water and enzymatic detergent HLD/STERILIZE-immerse scope and perfuse HLD/sterilant through all channels for exposure time RINSE-scope and channels rinsed with sterile water, filtered water, or tap water followed by alcohol DRY-use forced air to dry insertion tube and Copyright © 2004 WA Rutala channels
ENDOSCOPE DISINFECTION l l CLEAN-mechanically cleaned with water and enzymatic detergent HLD/STERILIZE-immerse scope and perfuse HLD/sterilant through all channels for exposure time RINSE-scope and channels rinsed with sterile water, filtered water, or tap water followed by alcohol DRY-use forced air to dry insertion tube and Copyright © 2004 WA Rutala channels
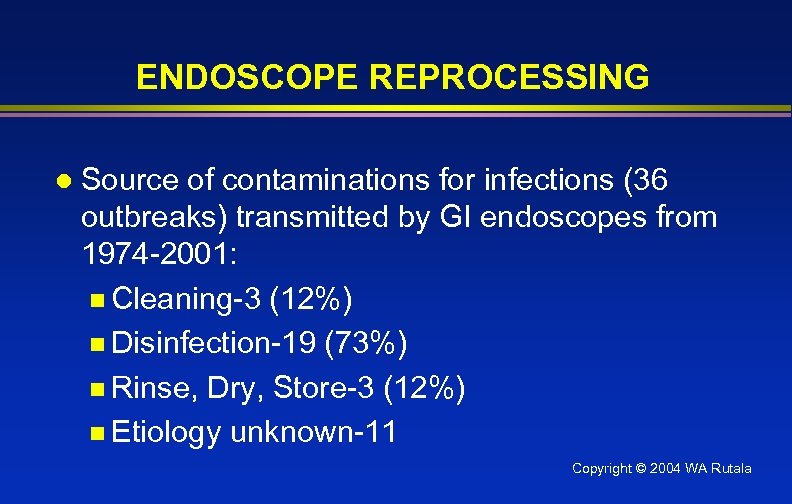 ENDOSCOPE REPROCESSING l Source of contaminations for infections (36 outbreaks) transmitted by GI endoscopes from 1974 -2001: n Cleaning-3 (12%) n Disinfection-19 (73%) n Rinse, Dry, Store-3 (12%) n Etiology unknown-11 Copyright © 2004 WA Rutala
ENDOSCOPE REPROCESSING l Source of contaminations for infections (36 outbreaks) transmitted by GI endoscopes from 1974 -2001: n Cleaning-3 (12%) n Disinfection-19 (73%) n Rinse, Dry, Store-3 (12%) n Etiology unknown-11 Copyright © 2004 WA Rutala
 ENDOSCOPE REPROCESSING Unacceptable Disinfectants for HLD l l l l Benzalkonium chloride Iodophor Hexachlorophene Alcohol Chlorhexidine gluconate Cetrimide Quaternary ammonium compounds Glutaraldehyde (0. 13%) with phenol Copyright © 2004 WA Rutala
ENDOSCOPE REPROCESSING Unacceptable Disinfectants for HLD l l l l Benzalkonium chloride Iodophor Hexachlorophene Alcohol Chlorhexidine gluconate Cetrimide Quaternary ammonium compounds Glutaraldehyde (0. 13%) with phenol Copyright © 2004 WA Rutala
 ENDOSCOPE REPROCESSING l Inappropriate disinfectants n n n n Benzalkonium chloride (Greene WH. Gastroenterol 1974; 67: 912) 70% alcohol (Elson CO. Gastroenterol 1975; 69: 507) QUAT (Tuffnell PG. Canad J Publ Health 1976; 67: 141) Hexachlorophene (Dean AG. Lancet 1977; 2: 134) Hexachlorophene (Beecham HJ. JAMA 1979; 1013) 70% alcohol (Parker HW. Gastro Endos 1979; 25; 102) Povidone-iodine (Low DE. Arch Intern Med 1980; 1076) Cetrimonium bromide. (Schliessler KH. Lancet 1980; 2: 1246) Copyright © 2004 WA Rutala
ENDOSCOPE REPROCESSING l Inappropriate disinfectants n n n n Benzalkonium chloride (Greene WH. Gastroenterol 1974; 67: 912) 70% alcohol (Elson CO. Gastroenterol 1975; 69: 507) QUAT (Tuffnell PG. Canad J Publ Health 1976; 67: 141) Hexachlorophene (Dean AG. Lancet 1977; 2: 134) Hexachlorophene (Beecham HJ. JAMA 1979; 1013) 70% alcohol (Parker HW. Gastro Endos 1979; 25; 102) Povidone-iodine (Low DE. Arch Intern Med 1980; 1076) Cetrimonium bromide. (Schliessler KH. Lancet 1980; 2: 1246) Copyright © 2004 WA Rutala
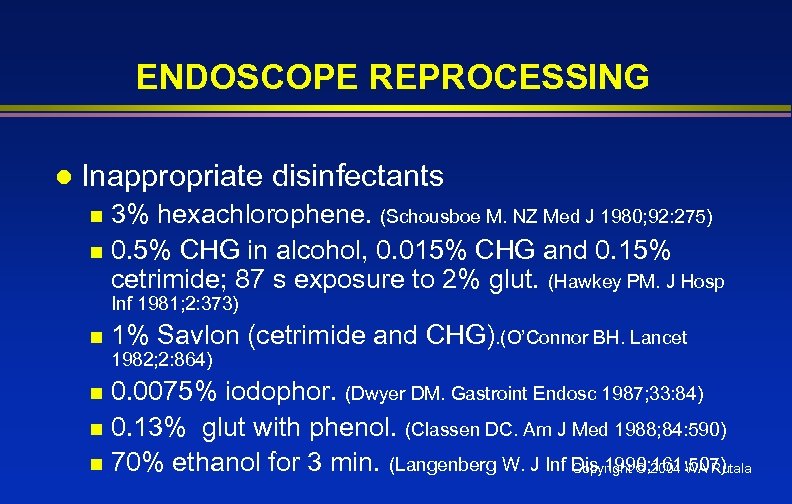 ENDOSCOPE REPROCESSING l Inappropriate disinfectants 3% hexachlorophene. (Schousboe M. NZ Med J 1980; 92: 275) n 0. 5% CHG in alcohol, 0. 015% CHG and 0. 15% cetrimide; 87 s exposure to 2% glut. (Hawkey PM. J Hosp n Inf 1981; 2: 373) n 1% Savlon (cetrimide and CHG). (O’Connor BH. Lancet 1982; 2: 864) 0. 0075% iodophor. (Dwyer DM. Gastroint Endosc 1987; 33: 84) n 0. 13% glut with phenol. (Classen DC. Am J Med 1988; 84: 590) n 70% ethanol for 3 min. (Langenberg W. J Inf Dis 1990; 161: 507) Copyright © 2004 WA Rutala n
ENDOSCOPE REPROCESSING l Inappropriate disinfectants 3% hexachlorophene. (Schousboe M. NZ Med J 1980; 92: 275) n 0. 5% CHG in alcohol, 0. 015% CHG and 0. 15% cetrimide; 87 s exposure to 2% glut. (Hawkey PM. J Hosp n Inf 1981; 2: 373) n 1% Savlon (cetrimide and CHG). (O’Connor BH. Lancet 1982; 2: 864) 0. 0075% iodophor. (Dwyer DM. Gastroint Endosc 1987; 33: 84) n 0. 13% glut with phenol. (Classen DC. Am J Med 1988; 84: 590) n 70% ethanol for 3 min. (Langenberg W. J Inf Dis 1990; 161: 507) Copyright © 2004 WA Rutala n
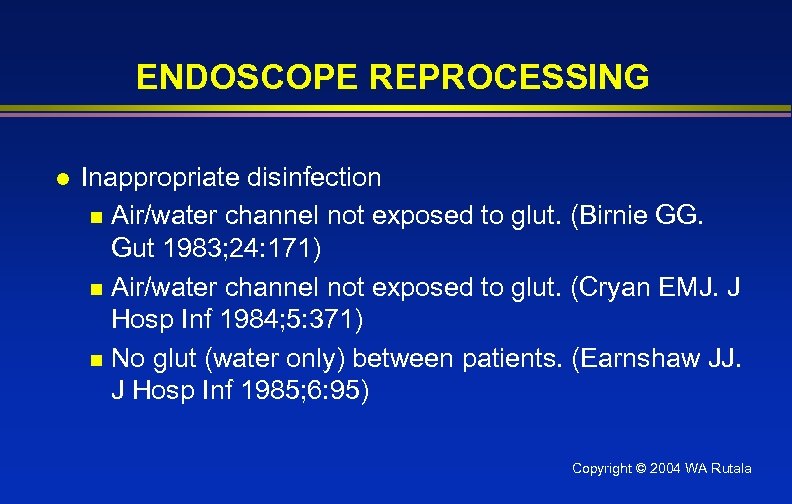 ENDOSCOPE REPROCESSING l Inappropriate disinfection n Air/water channel not exposed to glut. (Birnie GG. Gut 1983; 24: 171) n Air/water channel not exposed to glut. (Cryan EMJ. J Hosp Inf 1984; 5: 371) n No glut (water only) between patients. (Earnshaw JJ. J Hosp Inf 1985; 6: 95) Copyright © 2004 WA Rutala
ENDOSCOPE REPROCESSING l Inappropriate disinfection n Air/water channel not exposed to glut. (Birnie GG. Gut 1983; 24: 171) n Air/water channel not exposed to glut. (Cryan EMJ. J Hosp Inf 1984; 5: 371) n No glut (water only) between patients. (Earnshaw JJ. J Hosp Inf 1985; 6: 95) Copyright © 2004 WA Rutala
 High Level Disinfection of “Semicritical Objects” Exposure Time > 12 m-30 m, 20 o. C Germicide Concentration_____ Glutaraldehyde Ortho-phthalaldehyde (12 m) Hydrogen peroxide* Hydrogen peroxide and peracetic acid* 1. 0%/0. 08% Hydrogen peroxide and peracetic acid* 7. 5%/0. 23% Hypochlorite (free chlorine)* > 2. 0% 0. 55% 7. 5% Copyright © 2004 WA Rutala 650 -675
High Level Disinfection of “Semicritical Objects” Exposure Time > 12 m-30 m, 20 o. C Germicide Concentration_____ Glutaraldehyde Ortho-phthalaldehyde (12 m) Hydrogen peroxide* Hydrogen peroxide and peracetic acid* 1. 0%/0. 08% Hydrogen peroxide and peracetic acid* 7. 5%/0. 23% Hypochlorite (free chlorine)* > 2. 0% 0. 55% 7. 5% Copyright © 2004 WA Rutala 650 -675
 New FDA-Cleared Sterilants l “Old” n l New n n n l > 2% Glut, 7. 5% HP, 1. 0% HP and 0. 08% PA 1. 21% glut and 1. 93% phenol/phenate (HLD-20 m at 25 o. C) 0. 55% ortho-phthalaldehyde (HLD-12 m) 7. 35% HP and 0. 23% PA (HLD-15 m) 2. 5% Glut (HLD-5 m at 35 o. C) Hypochlorite (650 -675 ppm free chlorine) Ensure antimicrobial activity and material. Copyright © 2004 WA Rutala compatibility
New FDA-Cleared Sterilants l “Old” n l New n n n l > 2% Glut, 7. 5% HP, 1. 0% HP and 0. 08% PA 1. 21% glut and 1. 93% phenol/phenate (HLD-20 m at 25 o. C) 0. 55% ortho-phthalaldehyde (HLD-12 m) 7. 35% HP and 0. 23% PA (HLD-15 m) 2. 5% Glut (HLD-5 m at 35 o. C) Hypochlorite (650 -675 ppm free chlorine) Ensure antimicrobial activity and material. Copyright © 2004 WA Rutala compatibility
 Glutaraldehyde l l Advantages n Numerous use studies published n Relatively inexpensive n Excellent materials compatibility Disadvantages n Respiratory irritation from vapor n Pungent and irritating odor n Relatively slow mycobactericidal activity n Coagulate blood and fix tissues to surfaces n Allergic contact dermatitis Copyright © 2004 WA Rutala
Glutaraldehyde l l Advantages n Numerous use studies published n Relatively inexpensive n Excellent materials compatibility Disadvantages n Respiratory irritation from vapor n Pungent and irritating odor n Relatively slow mycobactericidal activity n Coagulate blood and fix tissues to surfaces n Allergic contact dermatitis Copyright © 2004 WA Rutala
 Ortho-phthalaldehyde Advantages l Fast acting HLD l No activation l Excellent materials compatibility l Not a known irritant to eyes and nasal passages l Weak odor l No ACGIH or OSHA limit Disadvantages l Stains protein gray l Cost ($30/gal); but lower reprocessing costs-soak time, devices per gal l Slow sporicidal activity l Hypersensitivity in some patients with a history of bladder cancer Copyright © 2004 WA Rutala
Ortho-phthalaldehyde Advantages l Fast acting HLD l No activation l Excellent materials compatibility l Not a known irritant to eyes and nasal passages l Weak odor l No ACGIH or OSHA limit Disadvantages l Stains protein gray l Cost ($30/gal); but lower reprocessing costs-soak time, devices per gal l Slow sporicidal activity l Hypersensitivity in some patients with a history of bladder cancer Copyright © 2004 WA Rutala
 Ortho-phthalaldehyde (OPA) New Contraindications for OPA l l l Repeated exposure to OPA, following manual reprocessing of urological instruments, may have resulted in hypersensitivity in some patients with a history of bladder cancer undergoing repeated cystoscopy. Out of approximately 1 million urological procedures, there have been reports of 24 patients who have experience ‘anaphylaxis-like’ reactions after repeated cystoscopy (typically after 4 -9 treatments). Risk control measures: residues of OPA minimized; and contraindicated for reprocessing of urological instruments used on patients with history of bladder cancer. Copyright © 2004 WA Rutala
Ortho-phthalaldehyde (OPA) New Contraindications for OPA l l l Repeated exposure to OPA, following manual reprocessing of urological instruments, may have resulted in hypersensitivity in some patients with a history of bladder cancer undergoing repeated cystoscopy. Out of approximately 1 million urological procedures, there have been reports of 24 patients who have experience ‘anaphylaxis-like’ reactions after repeated cystoscopy (typically after 4 -9 treatments). Risk control measures: residues of OPA minimized; and contraindicated for reprocessing of urological instruments used on patients with history of bladder cancer. Copyright © 2004 WA Rutala
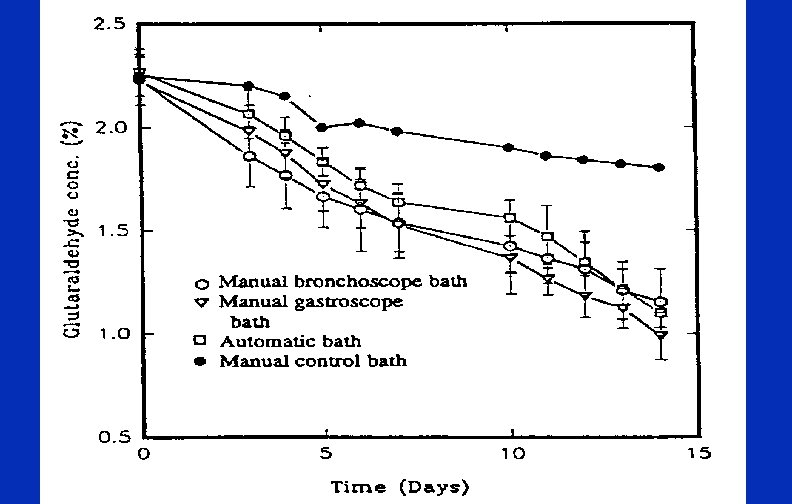
 Minimum Effective Concentration (MEC) High Level Disinfectant (HLD) Dilution of HLD occurs during use Test strips are available for monitoring MEC For example, test strips for glutaraldehyde monitor 1. 5% l Test strip not used to extend the use-life beyond the expiration date (date test strips when opened) l Testing frequency based on how frequently the solutions are used (used daily, test at least Copyright © 2004 WA Rutala daily) l l l
Minimum Effective Concentration (MEC) High Level Disinfectant (HLD) Dilution of HLD occurs during use Test strips are available for monitoring MEC For example, test strips for glutaraldehyde monitor 1. 5% l Test strip not used to extend the use-life beyond the expiration date (date test strips when opened) l Testing frequency based on how frequently the solutions are used (used daily, test at least Copyright © 2004 WA Rutala daily) l l l
 ENDOSCOPE DISINFECTION l l l CLEAN-mechanically cleaned with water and enzymatic detergent HLD/STERILIZE-immerse scope and perfuse HLD/sterilant through all channels for exposure time RINSE-scope and channels rinsed with sterile water, filtered water, or tap water followed by alcohol. Inadequate rinsing of HLD has caused colitis. DRY-use forced air to dry insertion tube and channels Copyright © 2004 WA Rutala STORE-prevent recontamination
ENDOSCOPE DISINFECTION l l l CLEAN-mechanically cleaned with water and enzymatic detergent HLD/STERILIZE-immerse scope and perfuse HLD/sterilant through all channels for exposure time RINSE-scope and channels rinsed with sterile water, filtered water, or tap water followed by alcohol. Inadequate rinsing of HLD has caused colitis. DRY-use forced air to dry insertion tube and channels Copyright © 2004 WA Rutala STORE-prevent recontamination
 ENDOSCOPE REPROCESSING l Rinse, Dry, Store n Irrigating water bottle. (Doherty DE. Dig Dis Sci 1982; 27: 169) n Inadequate drying (no alcohol). (Allen JI. Gastroenterol 1987; 92: 759) n Inadequate drying (no alcohol). (Classen DC. Am J Med 1988; 84: 590) Copyright © 2004 WA Rutala
ENDOSCOPE REPROCESSING l Rinse, Dry, Store n Irrigating water bottle. (Doherty DE. Dig Dis Sci 1982; 27: 169) n Inadequate drying (no alcohol). (Allen JI. Gastroenterol 1987; 92: 759) n Inadequate drying (no alcohol). (Classen DC. Am J Med 1988; 84: 590) Copyright © 2004 WA Rutala
 ENDOSCOPE DISINFECTION l l l CLEAN-mechanically cleaned with water and enzymatic detergent HLD/STERILIZE-immerse scope and perfuse HLD/sterilant through all channels for exposure time RINSE-scope and channels rinsed with sterile water, filtered water, or tap water followed by alcohol DRY-purge channels with air, flush with alcohol (assists drying), purge channels with air, dry the exterior Copyright © 2004 WA Rutala STORE-prevent recontamination
ENDOSCOPE DISINFECTION l l l CLEAN-mechanically cleaned with water and enzymatic detergent HLD/STERILIZE-immerse scope and perfuse HLD/sterilant through all channels for exposure time RINSE-scope and channels rinsed with sterile water, filtered water, or tap water followed by alcohol DRY-purge channels with air, flush with alcohol (assists drying), purge channels with air, dry the exterior Copyright © 2004 WA Rutala STORE-prevent recontamination
 ENDOSCOPE DISINFECTION l l l CLEAN-mechanically cleaned with water and enzymatic detergent HLD/STERILIZE-immerse scope and perfuse HLD/sterilant through all channels for exposure time RINSE-scope and channels rinsed with sterile water, filtered water, or tap water followed by alcohol DRY-use forced air to dry insertion tube and channels STORE-prevent recontamination (e. g. , hang © 2004 WA Rutala Copyright the endoscope vertically in a cabinet or clean
ENDOSCOPE DISINFECTION l l l CLEAN-mechanically cleaned with water and enzymatic detergent HLD/STERILIZE-immerse scope and perfuse HLD/sterilant through all channels for exposure time RINSE-scope and channels rinsed with sterile water, filtered water, or tap water followed by alcohol DRY-use forced air to dry insertion tube and channels STORE-prevent recontamination (e. g. , hang © 2004 WA Rutala Copyright the endoscope vertically in a cabinet or clean
 Nosocomial Outbreaks via GI Endoscopes Infections Associated with Accessories l Infections associated with biopsy forceps n n n l Contaminated biopsy forceps. (Dwyer DM. Gastroint Endosc 1987; 33: 84) Contaminated biopsy forceps (no cleaning between cases). Graham DY. Am J Gastroenterol 1988; 83: 974) Biopsy forceps not sterilized (glut exposed, ? time) Bronowicki JP. NEJM 1997; 334: 237) Reusable endoscopic accessories that break the mucosal barrier should be mechanically cleaned and Copyright © 2004 WA Rutala sterilized between patients
Nosocomial Outbreaks via GI Endoscopes Infections Associated with Accessories l Infections associated with biopsy forceps n n n l Contaminated biopsy forceps. (Dwyer DM. Gastroint Endosc 1987; 33: 84) Contaminated biopsy forceps (no cleaning between cases). Graham DY. Am J Gastroenterol 1988; 83: 974) Biopsy forceps not sterilized (glut exposed, ? time) Bronowicki JP. NEJM 1997; 334: 237) Reusable endoscopic accessories that break the mucosal barrier should be mechanically cleaned and Copyright © 2004 WA Rutala sterilized between patients
 Automated Endoscope Reprocessors (AERs) l l Advantages: automate and standardize reprocessing steps, reduce personnel exposure to chemicals, filtered tap water Disadvantages: failure of AERs linked to outbreaks, does not eliminate precleaning, does not monitor HLD concentration Problems: incompatible AER (side-viewing duodenoscope); biofilm buildup; contaminated AER; inadequate channel connectors Copyright © 2004 WA Rutala MMWR 1999; 48: 557. Used wrong set-up or connector
Automated Endoscope Reprocessors (AERs) l l Advantages: automate and standardize reprocessing steps, reduce personnel exposure to chemicals, filtered tap water Disadvantages: failure of AERs linked to outbreaks, does not eliminate precleaning, does not monitor HLD concentration Problems: incompatible AER (side-viewing duodenoscope); biofilm buildup; contaminated AER; inadequate channel connectors Copyright © 2004 WA Rutala MMWR 1999; 48: 557. Used wrong set-up or connector
 Disinfection of Emerging Pathogens Copyright © 2004 WA Rutala
Disinfection of Emerging Pathogens Copyright © 2004 WA Rutala
 Disinfection and Sterilization of Emerging Pathogens l l l l l Hepatitis C virus Clostridium difficile Cryptosporidium Helicobacter pylori E. coli 0157: H 7 SARS coronavirus Noroviruses Antibiotic-resistant microbes (MDR-TB, VRE, MRSA) Creutzfeldt-Jakob disease (no brain, eye, spinal cord contact) Copyright © 2004 WA Rutala
Disinfection and Sterilization of Emerging Pathogens l l l l l Hepatitis C virus Clostridium difficile Cryptosporidium Helicobacter pylori E. coli 0157: H 7 SARS coronavirus Noroviruses Antibiotic-resistant microbes (MDR-TB, VRE, MRSA) Creutzfeldt-Jakob disease (no brain, eye, spinal cord contact) Copyright © 2004 WA Rutala
 Disinfection and Sterilization of Emerging Pathogens Standard disinfection and sterilization procedures for patient care equipment are adequate to sterilize or disinfect instruments or devices contaminated with blood and other body fluids from persons infected with emerging pathogens Copyright © 2004 WA Rutala
Disinfection and Sterilization of Emerging Pathogens Standard disinfection and sterilization procedures for patient care equipment are adequate to sterilize or disinfect instruments or devices contaminated with blood and other body fluids from persons infected with emerging pathogens Copyright © 2004 WA Rutala
 ENDOSCOPE SAFETY Quality Control Ensure protocols equivalent to guidelines from professional organizations (APIC, SGNA, ASGE) l Are the staff who reprocess the endoscope specifically trained in that job? l Are the staff competency tested at least annually? l Conduct IC rounds to ensure compliance 2004 WA Rutala Copyright © with l
ENDOSCOPE SAFETY Quality Control Ensure protocols equivalent to guidelines from professional organizations (APIC, SGNA, ASGE) l Are the staff who reprocess the endoscope specifically trained in that job? l Are the staff competency tested at least annually? l Conduct IC rounds to ensure compliance 2004 WA Rutala Copyright © with l
 Conclusions Endoscopes represent a nosocomial hazard l Proper cleaning and disinfection will prevent nosocomial transmission l Current guidelines should be strictly followed l Compliance must be monitored l Safety and efficacy of new technologies must be validated l Copyright © 2004 WA Rutala
Conclusions Endoscopes represent a nosocomial hazard l Proper cleaning and disinfection will prevent nosocomial transmission l Current guidelines should be strictly followed l Compliance must be monitored l Safety and efficacy of new technologies must be validated l Copyright © 2004 WA Rutala
 Endoscope Reprocessing Lecture Goals Background l Infections related to endoscopy l Reprocessing of endoscopes and accessories l Cleaning n High-level disinfection/sterilization n Automated endoscope reprocessing n l Quality control Copyright © 2004 WA Rutala
Endoscope Reprocessing Lecture Goals Background l Infections related to endoscopy l Reprocessing of endoscopes and accessories l Cleaning n High-level disinfection/sterilization n Automated endoscope reprocessing n l Quality control Copyright © 2004 WA Rutala
 Thank you Copyright © 2004 WA Rutala
Thank you Copyright © 2004 WA Rutala
 References l l l Rutala WA, Weber DJ. Disinfection of endoscopes: Review of new chemical sterilants for high-level disinfection. Infect Control Hosp Epidemiol 1999; 20: 69 -76. Nelson DB, Jarvis WR, Rutala WA, et al. Multi-society guideline for reprocessing flexible gastrointestinal endoscopes. AJIC 2003; 31: 309 -315. Posters: www. olympusamerica. com/msg_section/msg_Reprocessing. as p Questions/Slides: www. disinfectionandsterilization. org (WA Rutala) Weber DJ, Rutala WA, Di. Marino AJ. Prevention of infection following gastrointestinal endoscopy. In Di. Marino AJ. Gastro Copyright © 2004 WA Rutala Dis. 2002; 87 -107 Rutala WA, Weber DJ. Reprocessing endoscopes: United
References l l l Rutala WA, Weber DJ. Disinfection of endoscopes: Review of new chemical sterilants for high-level disinfection. Infect Control Hosp Epidemiol 1999; 20: 69 -76. Nelson DB, Jarvis WR, Rutala WA, et al. Multi-society guideline for reprocessing flexible gastrointestinal endoscopes. AJIC 2003; 31: 309 -315. Posters: www. olympusamerica. com/msg_section/msg_Reprocessing. as p Questions/Slides: www. disinfectionandsterilization. org (WA Rutala) Weber DJ, Rutala WA, Di. Marino AJ. Prevention of infection following gastrointestinal endoscopy. In Di. Marino AJ. Gastro Copyright © 2004 WA Rutala Dis. 2002; 87 -107 Rutala WA, Weber DJ. Reprocessing endoscopes: United


