5f2705a7c6bb904ad00363bc991944d7.ppt
- Количество слайдов: 38
 Enantiomeric Excess of POPs in the Environment Hiramoto S 1, Tsurukawa M 2, Matsumura C 2, Nakano T 2, Kunugi M 3 1 H yogo Environmental Advancement Association 2 H y o g o Prefectural Institute of Public Health Association 3 N a t i o n a l I n s t i t u t e f o r E n v i r o n m e n t a l S t u d i e s
Enantiomeric Excess of POPs in the Environment Hiramoto S 1, Tsurukawa M 2, Matsumura C 2, Nakano T 2, Kunugi M 3 1 H yogo Environmental Advancement Association 2 H y o g o Prefectural Institute of Public Health Association 3 N a t i o n a l I n s t i t u t e f o r E n v i r o n m e n t a l S t u d i e s
 Introduction
Introduction
 Purpose To identify the behavior of POPs Enantiomeric compositions of chiral POPs in seawater and air were investigated. The results of chiral analysis were shown using Enantiomeric Excess (EE). * In this study, (+) or (-) enantiomer does not identified.
Purpose To identify the behavior of POPs Enantiomeric compositions of chiral POPs in seawater and air were investigated. The results of chiral analysis were shown using Enantiomeric Excess (EE). * In this study, (+) or (-) enantiomer does not identified.
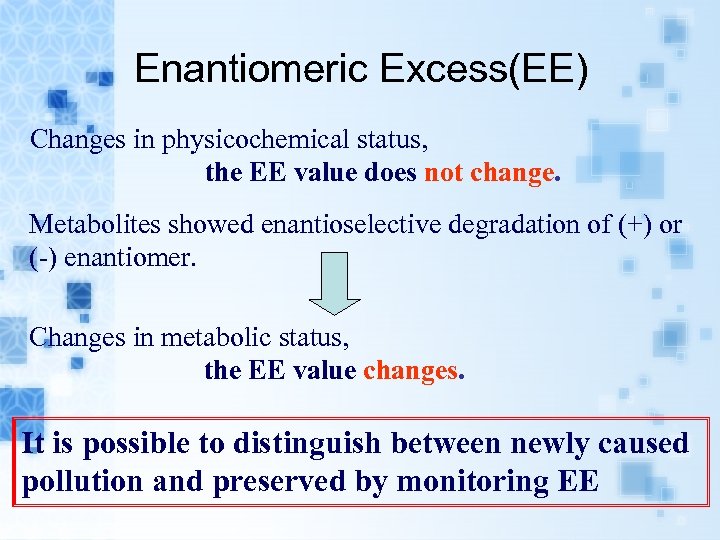 Enantiomeric Excess(EE) Changes in physicochemical status, the EE value does not change. Metabolites showed enantioselective degradation of (+) or (-) enantiomer. Changes in metabolic status, the EE value changes. It is possible to distinguish between newly caused pollution and preserved by monitoring EE
Enantiomeric Excess(EE) Changes in physicochemical status, the EE value does not change. Metabolites showed enantioselective degradation of (+) or (-) enantiomer. Changes in metabolic status, the EE value changes. It is possible to distinguish between newly caused pollution and preserved by monitoring EE
 Materials and Methods
Materials and Methods
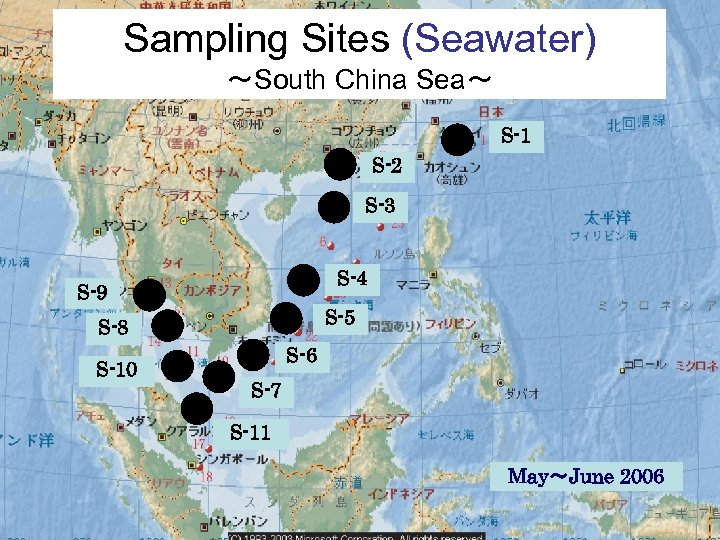 Sampling Sites (Seawater) ~South China Sea~ S-1 S-2 S-3 S-4 S-9 S-5 S-8 S-10 S-6 S-7 S-11 May~June 2006
Sampling Sites (Seawater) ~South China Sea~ S-1 S-2 S-3 S-4 S-9 S-5 S-8 S-10 S-6 S-7 S-11 May~June 2006
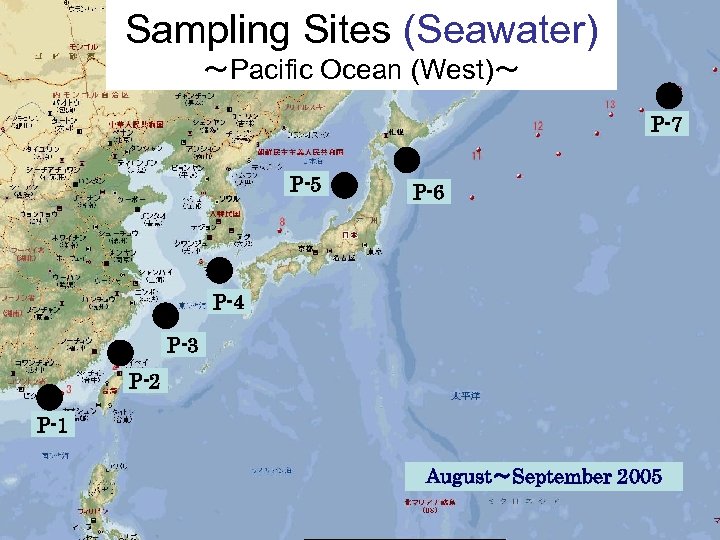 Sampling Sites (Seawater) ~Pacific Ocean (West)~ P-7 P-5 P-6 P-4 P-3 P-2 P-1 August~September 2005
Sampling Sites (Seawater) ~Pacific Ocean (West)~ P-7 P-5 P-6 P-4 P-3 P-2 P-1 August~September 2005
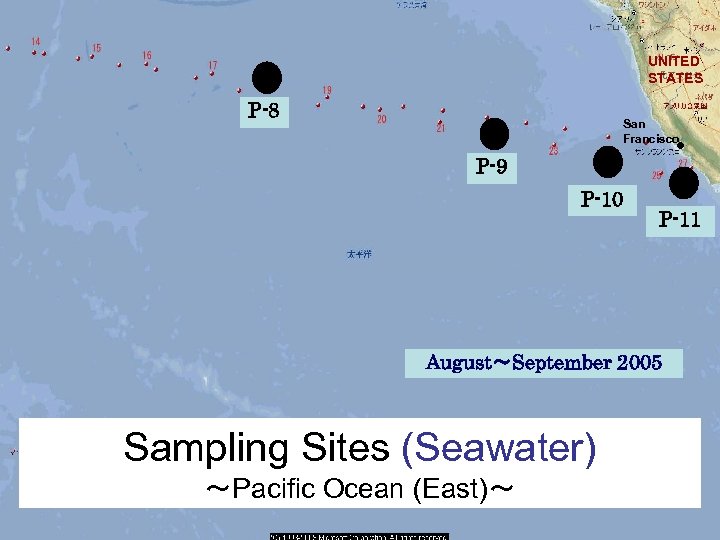 UNITED STATES P-8 San Francisco P-9 P-10 P-11 August~September 2005 Sampling Sites (Seawater) ~Pacific Ocean (East)~
UNITED STATES P-8 San Francisco P-9 P-10 P-11 August~September 2005 Sampling Sites (Seawater) ~Pacific Ocean (East)~
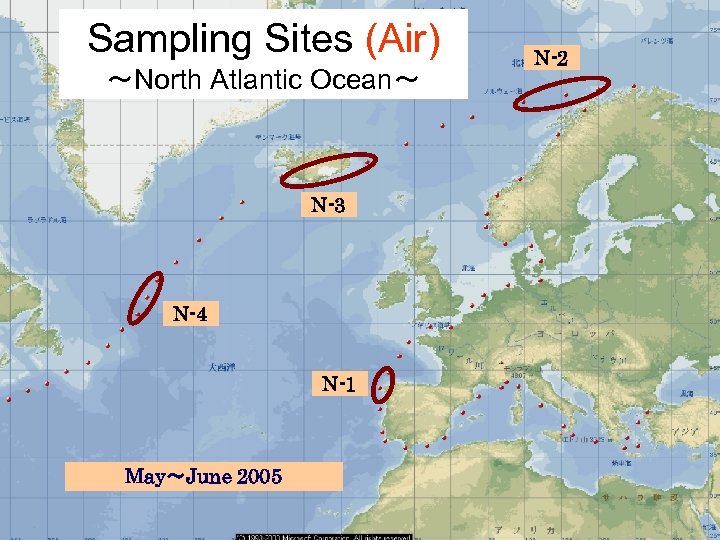 Sampling Sites (Air) ~North Atlantic Ocean~ N-3 N-4 N-1 May~June 2005 N-2
Sampling Sites (Air) ~North Atlantic Ocean~ N-3 N-4 N-1 May~June 2005 N-2
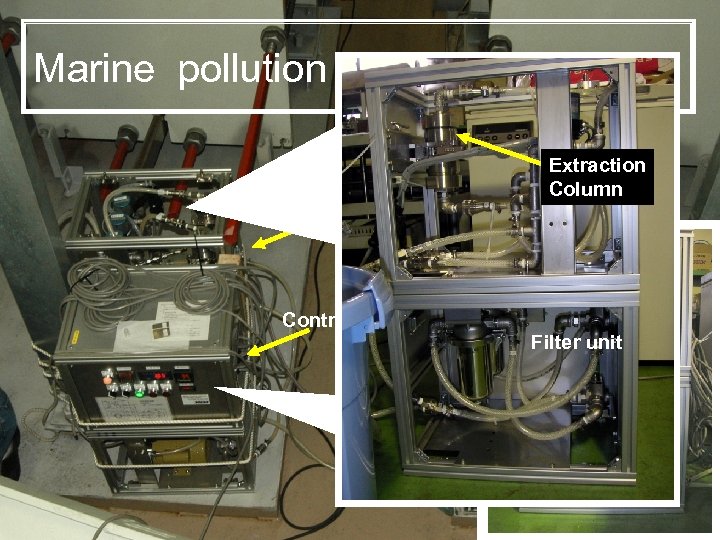 Marine pollution observation system Solid Phase Extraction unit Extraction Column Control Unit Filter unit
Marine pollution observation system Solid Phase Extraction unit Extraction Column Control Unit Filter unit
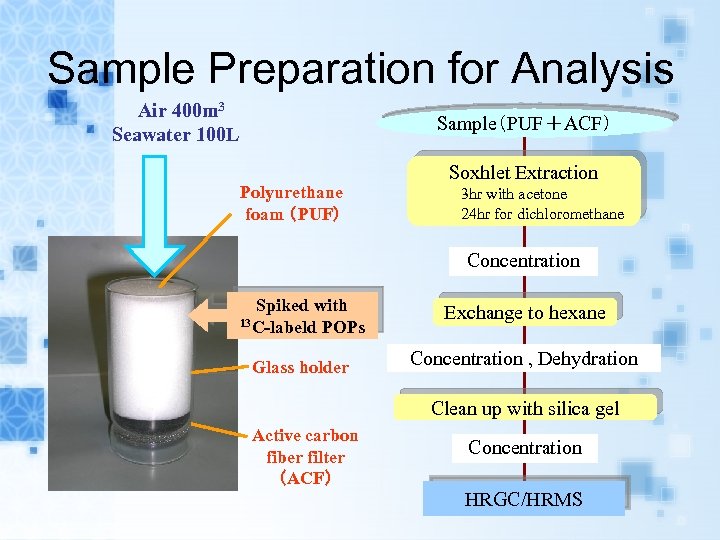 Sample Preparation for Analysis Air 400 m 3 Seawater 100 L Sample(PUF+ACF) Polyurethane foam (PUF) Soxhlet Extraction 3 hr with acetone 24 hr for dichloromethane Concentration Spiked with 13 C-labeld POPs Glass holder Exchange to hexane Concentration , Dehydration Clean up with silica gel Active carbon fiber filter (ACF) Concentration HRGC/HRMS
Sample Preparation for Analysis Air 400 m 3 Seawater 100 L Sample(PUF+ACF) Polyurethane foam (PUF) Soxhlet Extraction 3 hr with acetone 24 hr for dichloromethane Concentration Spiked with 13 C-labeld POPs Glass holder Exchange to hexane Concentration , Dehydration Clean up with silica gel Active carbon fiber filter (ACF) Concentration HRGC/HRMS
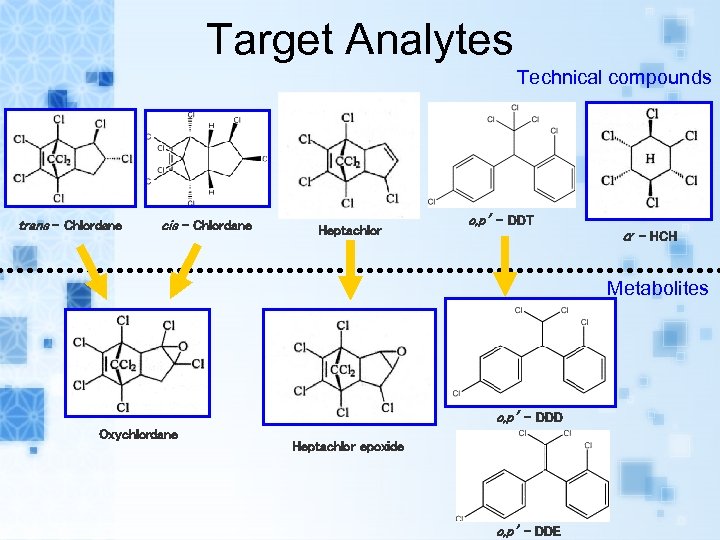 Target Analytes Technical compounds trans - Chlordane cis - Chlordane Heptachlor o, p’ - DDT α - HCH Metabolites o, p’ - DDD Oxychlordane Heptachlor epoxide o, p’ - DDE
Target Analytes Technical compounds trans - Chlordane cis - Chlordane Heptachlor o, p’ - DDT α - HCH Metabolites o, p’ - DDD Oxychlordane Heptachlor epoxide o, p’ - DDE
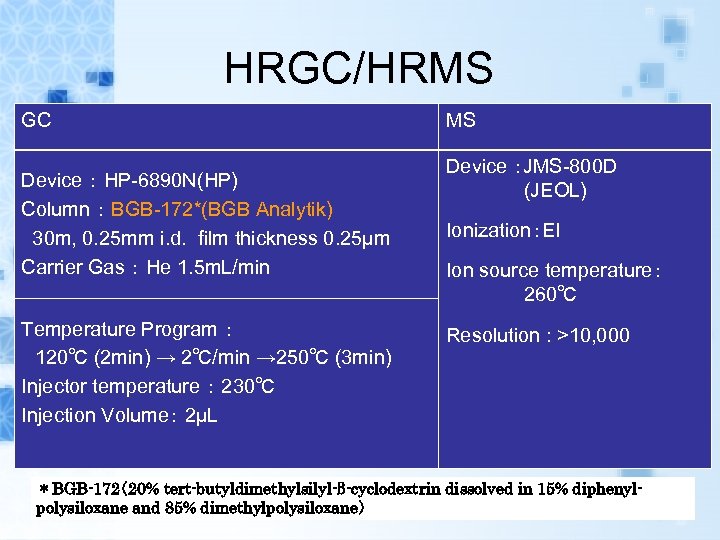 HRGC/HRMS GC Device : HP-6890 N(HP) Column : BGB-172*(BGB Analytik) 30 m, 0. 25 mm i. d. film thickness 0. 25μm Carrier Gas : He 1. 5 m. L/min Temperature Program : 120℃ (2 min) → 2℃/min → 250℃ (3 min) Injector temperature : 230℃ Injection Volume: 2μL MS Device :JMS-800 D (JEOL) Ionization:EI Ion source temperature: 260℃ Resolution : >10, 000 *BGB-172(20% tert-butyldimethylsilyl-β-cyclodextrin dissolved in 15% diphenylpolysiloxane and 85% dimethylpolysiloxane)
HRGC/HRMS GC Device : HP-6890 N(HP) Column : BGB-172*(BGB Analytik) 30 m, 0. 25 mm i. d. film thickness 0. 25μm Carrier Gas : He 1. 5 m. L/min Temperature Program : 120℃ (2 min) → 2℃/min → 250℃ (3 min) Injector temperature : 230℃ Injection Volume: 2μL MS Device :JMS-800 D (JEOL) Ionization:EI Ion source temperature: 260℃ Resolution : >10, 000 *BGB-172(20% tert-butyldimethylsilyl-β-cyclodextrin dissolved in 15% diphenylpolysiloxane and 85% dimethylpolysiloxane)
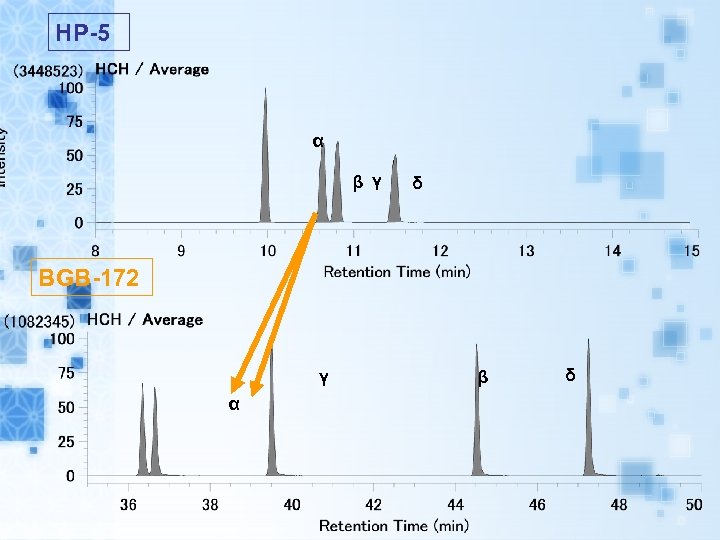 HP-5 α β γ δ BGB-172 γ α β δ
HP-5 α β γ δ BGB-172 γ α β δ
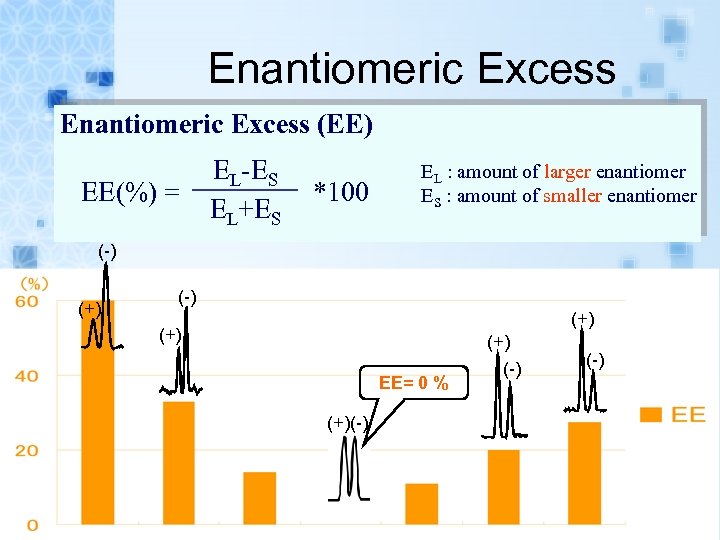 Enantiomeric Excess (EE) EE(%) = EL-ES EL+ES *100 EL : amount of larger enantiomer ES : amount of smaller enantiomer (-) (+) (+) EE= 0 % (+)(-) (-)
Enantiomeric Excess (EE) EE(%) = EL-ES EL+ES *100 EL : amount of larger enantiomer ES : amount of smaller enantiomer (-) (+) (+) EE= 0 % (+)(-) (-)
 Results
Results
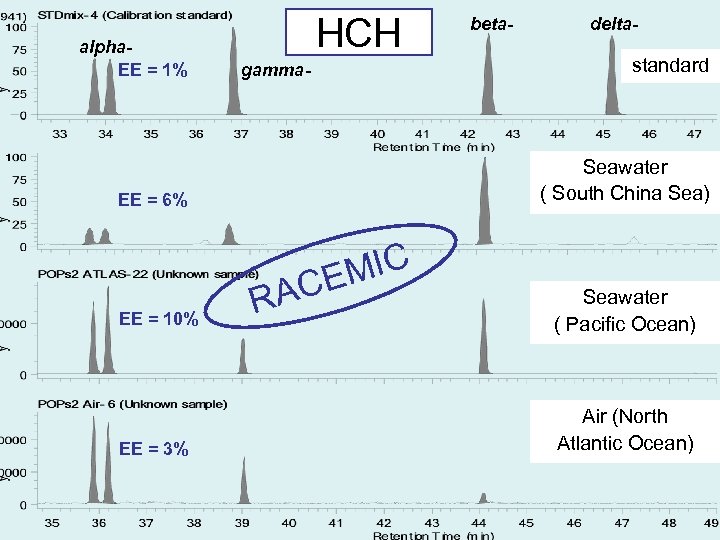 alpha. EE = 1% HCH gamma- MIC CE EE = 3% delta- standard Seawater ( South China Sea) EE = 6% EE = 10% beta- RA Seawater ( Pacific Ocean) Air (North Atlantic Ocean)
alpha. EE = 1% HCH gamma- MIC CE EE = 3% delta- standard Seawater ( South China Sea) EE = 6% EE = 10% beta- RA Seawater ( Pacific Ocean) Air (North Atlantic Ocean)
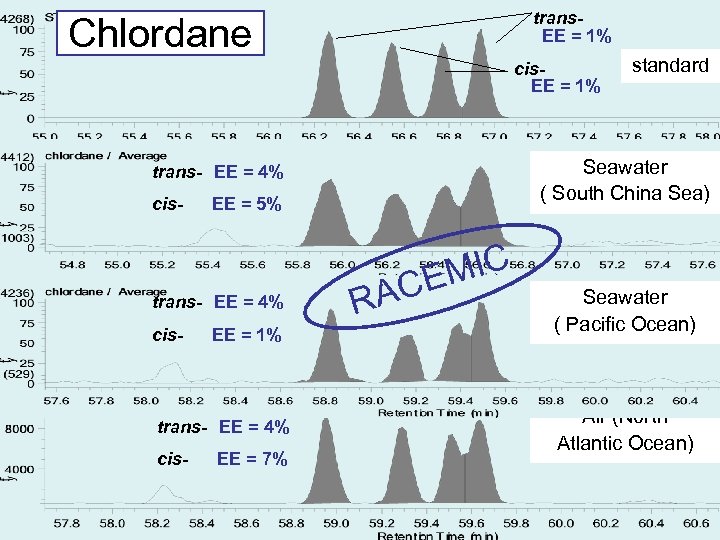 trans. EE = 1% Chlordane cis. EE = 1% Seawater ( South China Sea) trans- EE = 4% cis- EE = 5% trans- EE = 4% cis- EE = 1% trans- EE = 4% cis- EE = 7% standard RA MIC CE Seawater ( Pacific Ocean) Air (North Atlantic Ocean)
trans. EE = 1% Chlordane cis. EE = 1% Seawater ( South China Sea) trans- EE = 4% cis- EE = 5% trans- EE = 4% cis- EE = 1% trans- EE = 4% cis- EE = 7% standard RA MIC CE Seawater ( Pacific Ocean) Air (North Atlantic Ocean)
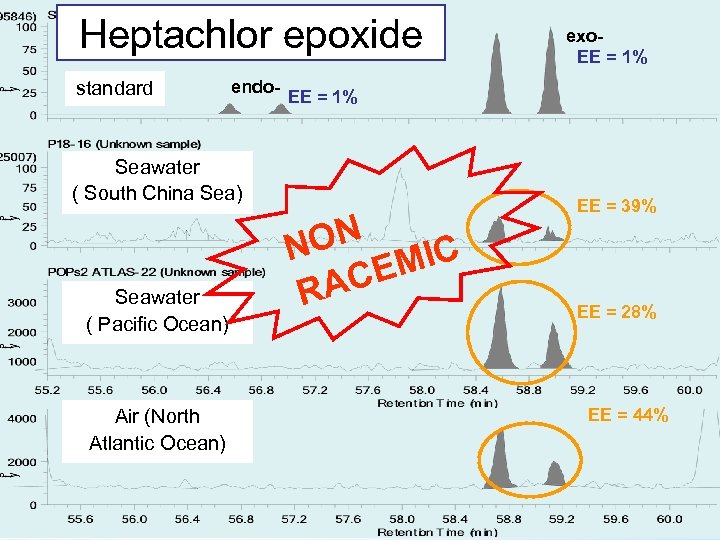 Heptachlor epoxide standard endo- EE = 1% Seawater ( South China Sea) Seawater ( Pacific Ocean) Air (North Atlantic Ocean) exo. EE = 1% ON N MIC CE RA EE = 39% EE = 28% EE = 44%
Heptachlor epoxide standard endo- EE = 1% Seawater ( South China Sea) Seawater ( Pacific Ocean) Air (North Atlantic Ocean) exo. EE = 1% ON N MIC CE RA EE = 39% EE = 28% EE = 44%
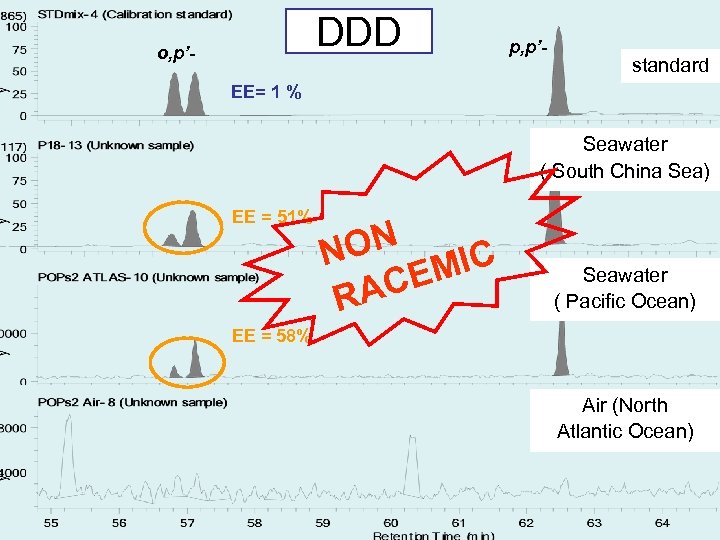 DDD o, p’- p, p’- standard EE= 1 % Seawater ( South China Sea) EE = 51% ON N MIC CE RA Seawater ( Pacific Ocean) EE = 58% Air (North Atlantic Ocean)
DDD o, p’- p, p’- standard EE= 1 % Seawater ( South China Sea) EE = 51% ON N MIC CE RA Seawater ( Pacific Ocean) EE = 58% Air (North Atlantic Ocean)
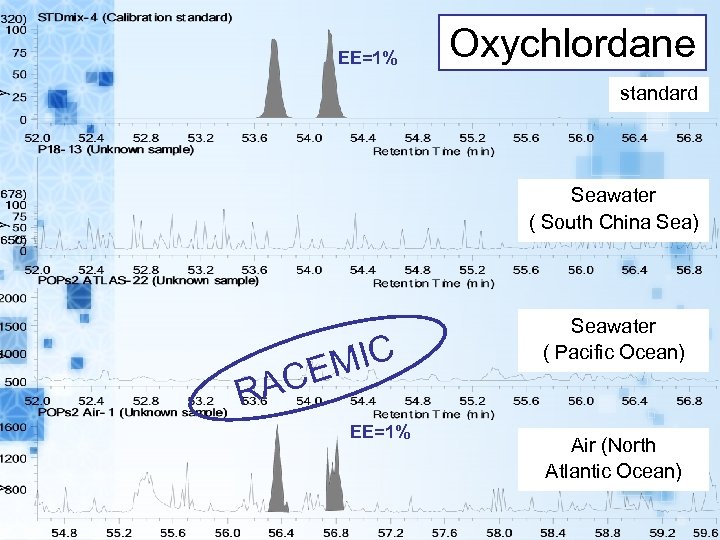 EE=1% Oxychlordane standard Seawater ( South China Sea) MIC CE Seawater ( Pacific Ocean) RA EE=1% Air (North Atlantic Ocean)
EE=1% Oxychlordane standard Seawater ( South China Sea) MIC CE Seawater ( Pacific Ocean) RA EE=1% Air (North Atlantic Ocean)
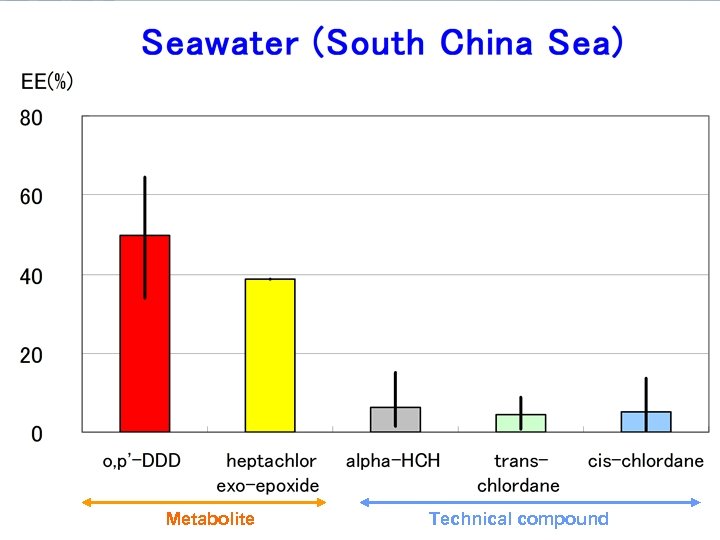 Metabolite Technical compound
Metabolite Technical compound
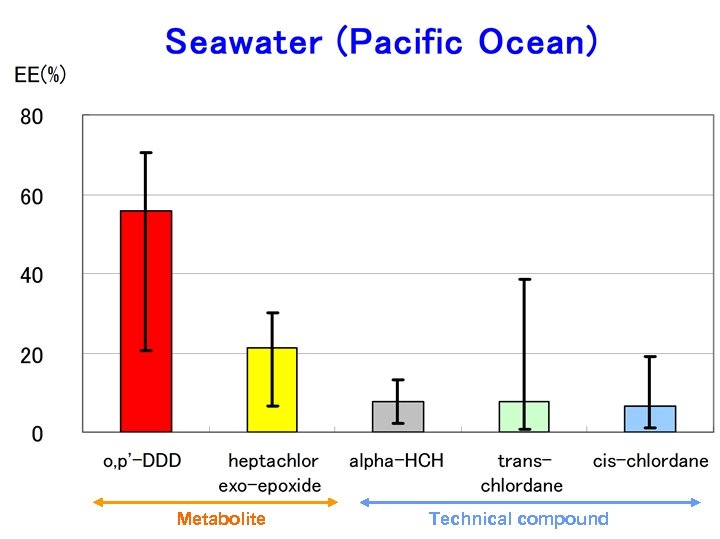 Metabolite Technical compound
Metabolite Technical compound
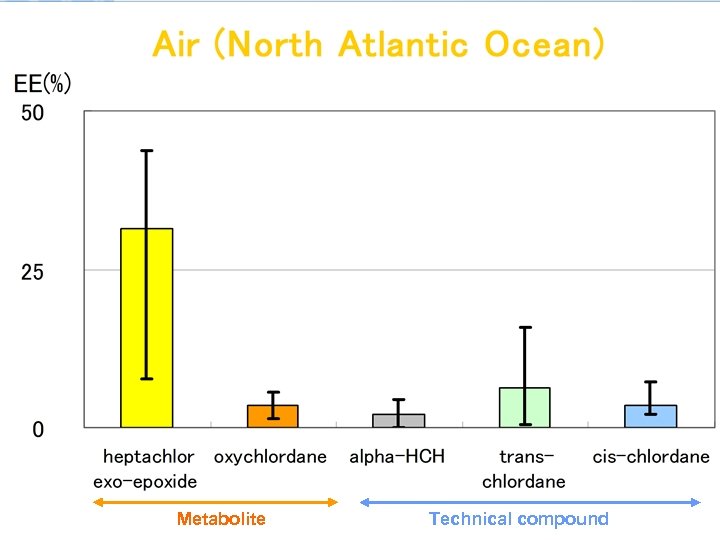 Metabolite Technical compound
Metabolite Technical compound
 Conclusion(1) EE of chiral POPs in seawater and air samples have been investigated. Enatiomeric composition of alpha-HCH, trans-, cis-chlordane were near racemic. The metabolites; heptachlor exoepoxide and o, p’-DDD exist non racemic. However, oxychlordane is the metabolite, it exists as racemic.
Conclusion(1) EE of chiral POPs in seawater and air samples have been investigated. Enatiomeric composition of alpha-HCH, trans-, cis-chlordane were near racemic. The metabolites; heptachlor exoepoxide and o, p’-DDD exist non racemic. However, oxychlordane is the metabolite, it exists as racemic.
 Conclusion(2) By monitoring enantiomeric composition, better understanding for the mechanism of environmental pollution will be provided. Further studies about global scale observations of chiral signatures are necessary.
Conclusion(2) By monitoring enantiomeric composition, better understanding for the mechanism of environmental pollution will be provided. Further studies about global scale observations of chiral signatures are necessary.
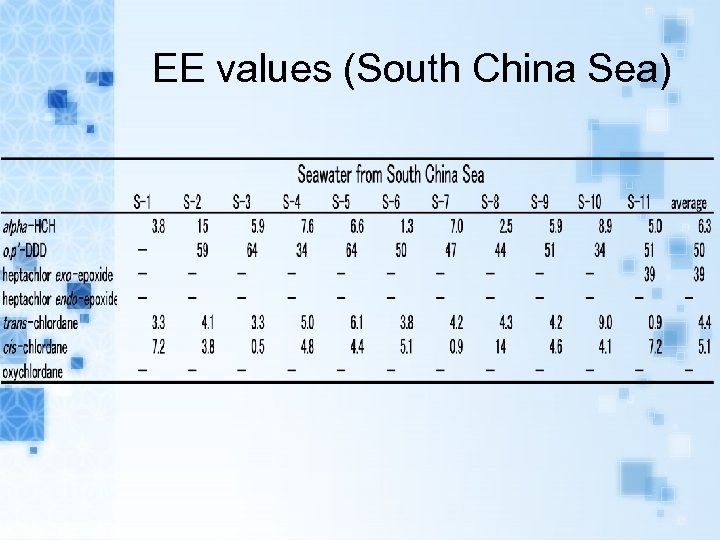 EE values (South China Sea)
EE values (South China Sea)
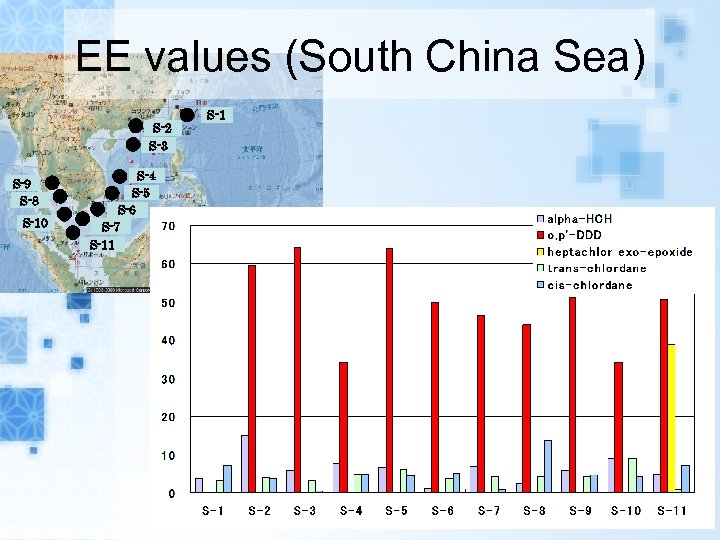 EE values (South China Sea) S-2 S-3 S-9 S-8 S-10 S-4 S-5 S-6 S-7 S-11 S-1
EE values (South China Sea) S-2 S-3 S-9 S-8 S-10 S-4 S-5 S-6 S-7 S-11 S-1
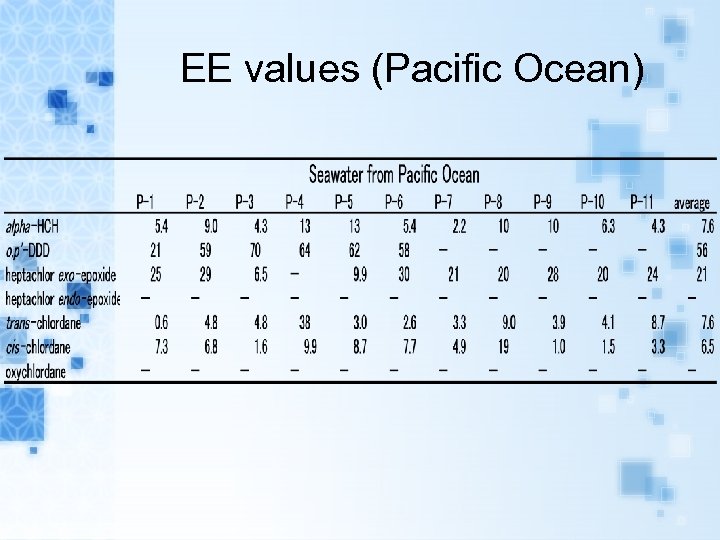 EE values (Pacific Ocean)
EE values (Pacific Ocean)
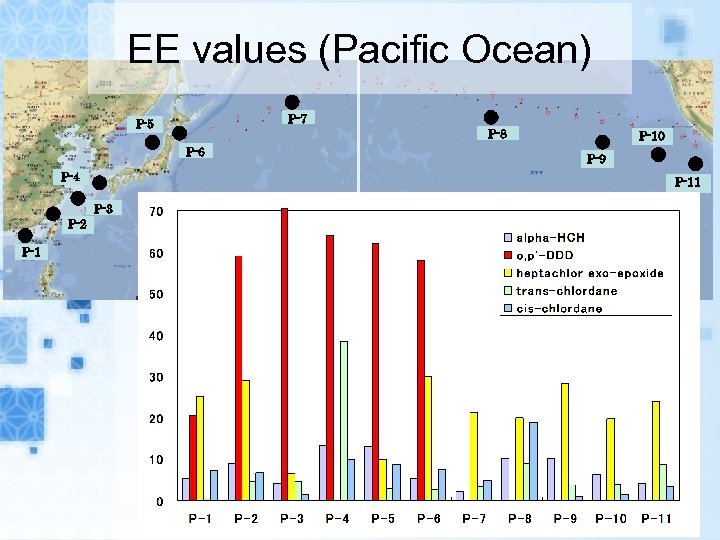 EE values (Pacific Ocean) P-7 P-5 P-8 P-6 P-4 P-1 P-9 P-11 P-3 P-2 P-10
EE values (Pacific Ocean) P-7 P-5 P-8 P-6 P-4 P-1 P-9 P-11 P-3 P-2 P-10
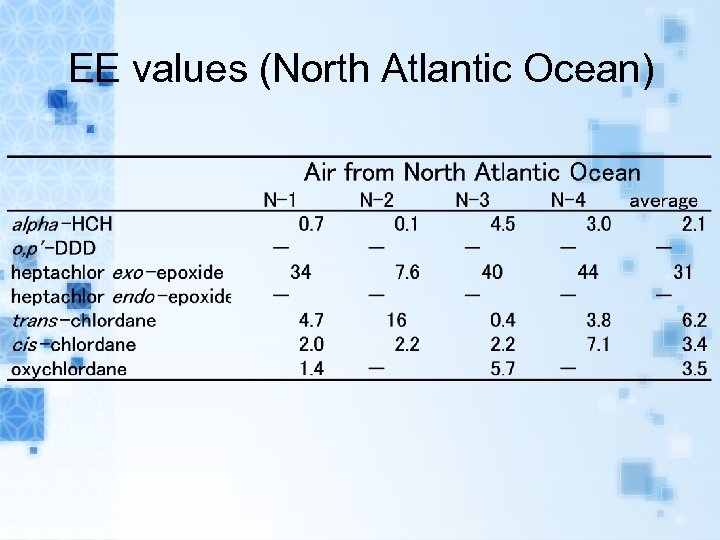 EE values (North Atlantic Ocean)
EE values (North Atlantic Ocean)
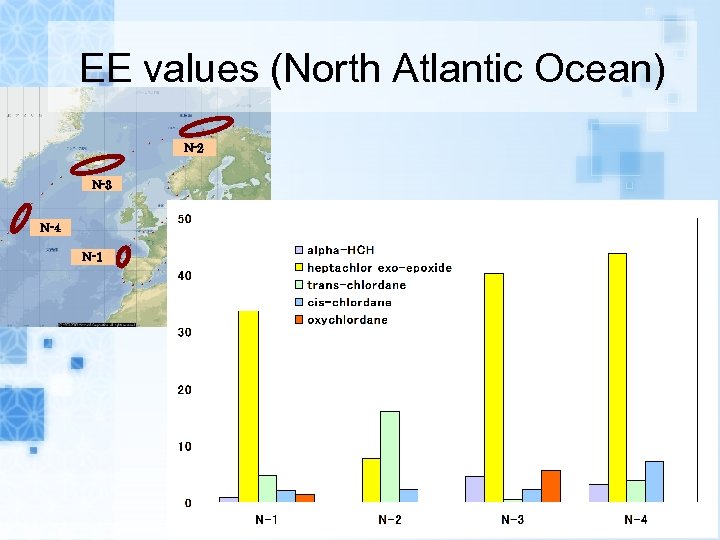 EE values (North Atlantic Ocean) N-2 N-3 N-4 N-1
EE values (North Atlantic Ocean) N-2 N-3 N-4 N-1
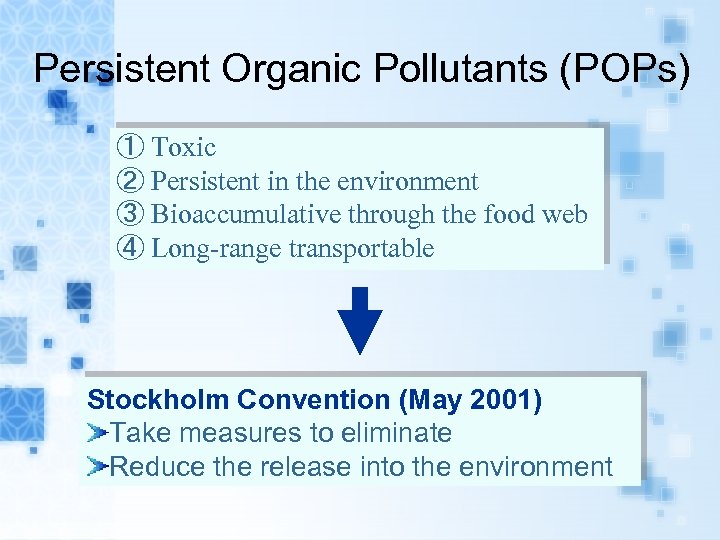 Persistent Organic Pollutants (POPs) ① Toxic ② Persistent in the environment ③ Bioaccumulative through the food web ④ Long-range transportable Stockholm Convention (May 2001) Take measures to eliminate Reduce the release into the environment
Persistent Organic Pollutants (POPs) ① Toxic ② Persistent in the environment ③ Bioaccumulative through the food web ④ Long-range transportable Stockholm Convention (May 2001) Take measures to eliminate Reduce the release into the environment
 Results EEs of alpha-HCH, trans-, cis-chlordane in air samples and seawater samples range from 0. 1 to 15. 9% and 0. 5 to 38. 4%, respectively. EEs of metabolites heptachlor exoepoxide in air samples and seawater samples range from 7. 6 to 43. 8% and 6. 5 to 38. 8%, respectively. The range of EEs of metabolites o, p’DDD in seawater samples were from 20. 7 to 64. 4%.
Results EEs of alpha-HCH, trans-, cis-chlordane in air samples and seawater samples range from 0. 1 to 15. 9% and 0. 5 to 38. 4%, respectively. EEs of metabolites heptachlor exoepoxide in air samples and seawater samples range from 7. 6 to 43. 8% and 6. 5 to 38. 8%, respectively. The range of EEs of metabolites o, p’DDD in seawater samples were from 20. 7 to 64. 4%.
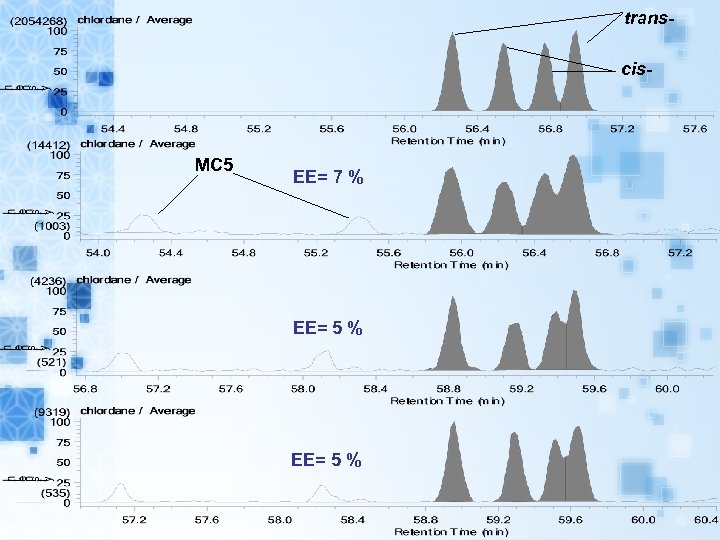 transcis- MC 5 EE= 7 % EE= 5 %
transcis- MC 5 EE= 7 % EE= 5 %
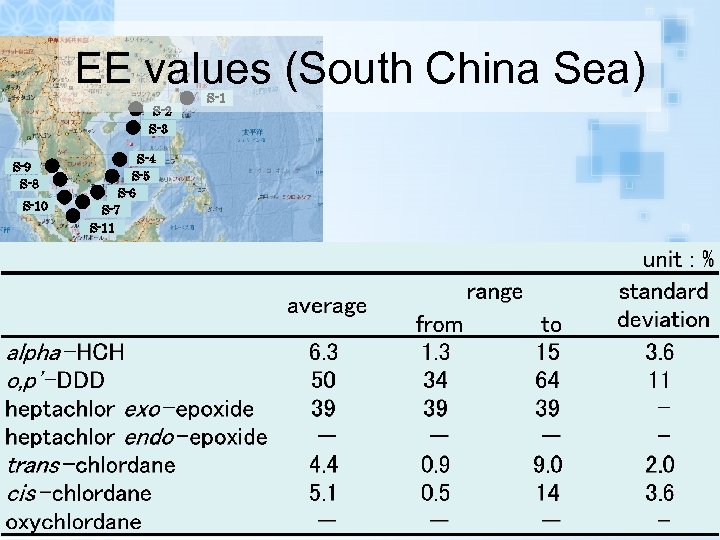 EE values (South China Sea) S-2 S-3 S-9 S-8 S-10 S-4 S-5 S-6 S-7 S-11 S-1
EE values (South China Sea) S-2 S-3 S-9 S-8 S-10 S-4 S-5 S-6 S-7 S-11 S-1
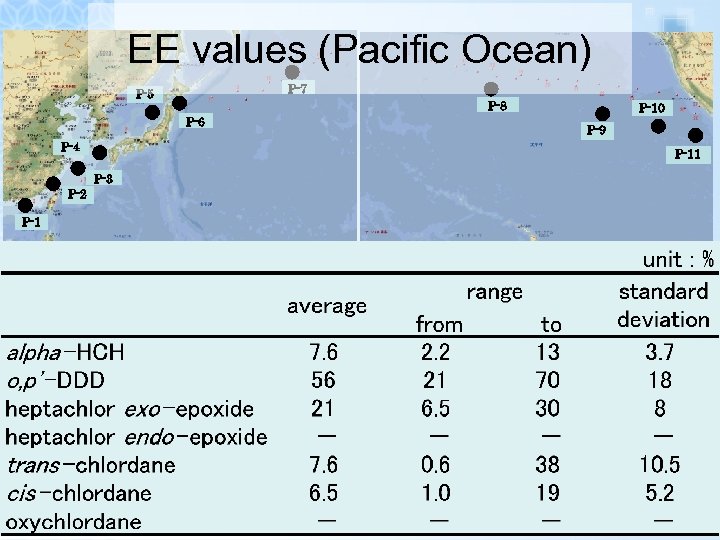 EE values (Pacific Ocean) P-7 P-5 P-8 P-6 P-4 P-1 P-9 P-11 P-3 P-2 P-10
EE values (Pacific Ocean) P-7 P-5 P-8 P-6 P-4 P-1 P-9 P-11 P-3 P-2 P-10
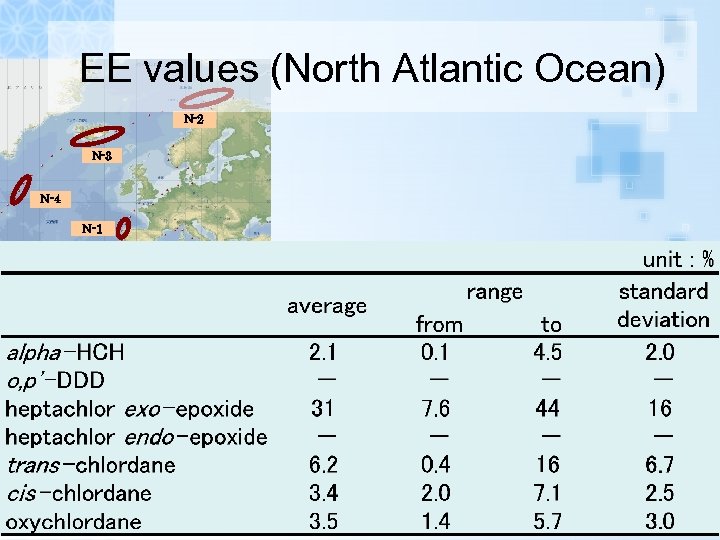 EE values (North Atlantic Ocean) N-2 N-3 N-4 N-1
EE values (North Atlantic Ocean) N-2 N-3 N-4 N-1


