da10742aac1ee809472e96f31b1c28e3.ppt
- Количество слайдов: 67
 ELISA AND RELATED TECHNOLOGIES CORE MODULE 1 : Laboratory Methods and Instrumentation Dr Brian Jones, Clinical Immunology Division bmjones@ha. org. hk
ELISA AND RELATED TECHNOLOGIES CORE MODULE 1 : Laboratory Methods and Instrumentation Dr Brian Jones, Clinical Immunology Division bmjones@ha. org. hk
 ENZYME-LINKED IMMUNOSORBENT ASSAY • • • valuable tools for use in clinical labs can measure antibodies or antigens inexpensive, rapid, quantitative, specific sensitive (pg/ml) expensive equipment not required (but helps!) can be automated
ENZYME-LINKED IMMUNOSORBENT ASSAY • • • valuable tools for use in clinical labs can measure antibodies or antigens inexpensive, rapid, quantitative, specific sensitive (pg/ml) expensive equipment not required (but helps!) can be automated
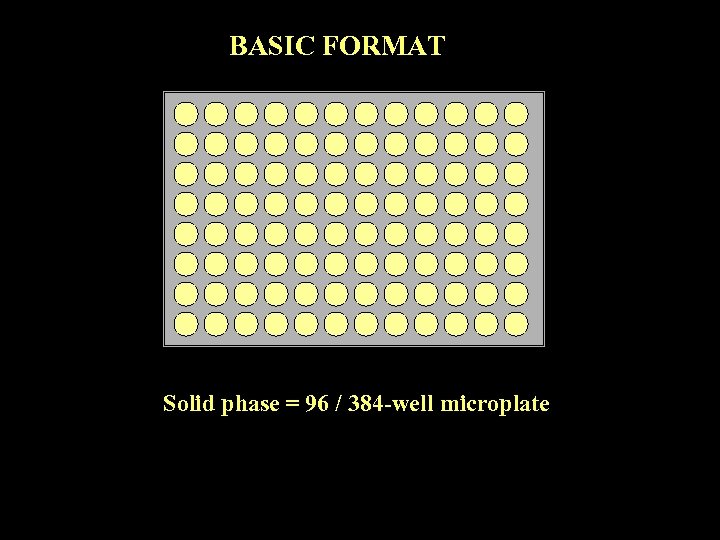 BASIC FORMAT Solid phase = 96 / 384 -well microplate
BASIC FORMAT Solid phase = 96 / 384 -well microplate
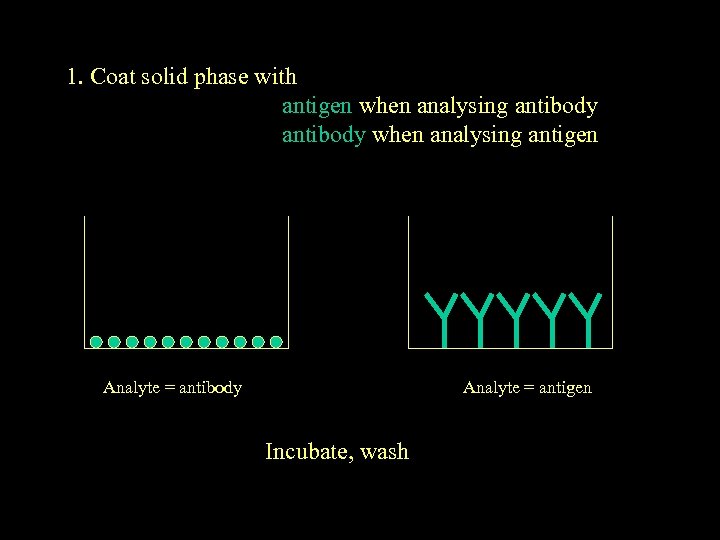 1. Coat solid phase with antigen when analysing antibody when analysing antigen Analyte = antibody Analyte = antigen Incubate, wash
1. Coat solid phase with antigen when analysing antibody when analysing antigen Analyte = antibody Analyte = antigen Incubate, wash
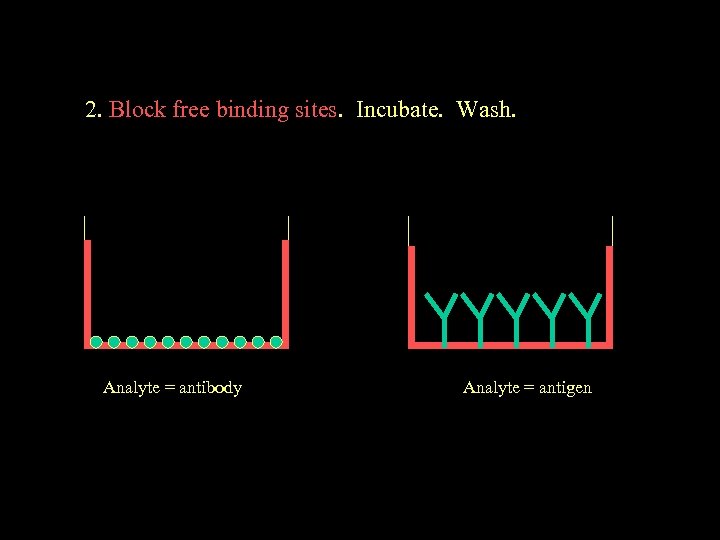 2. Block free binding sites. Incubate. Wash. Analyte = antibody Analyte = antigen
2. Block free binding sites. Incubate. Wash. Analyte = antibody Analyte = antigen
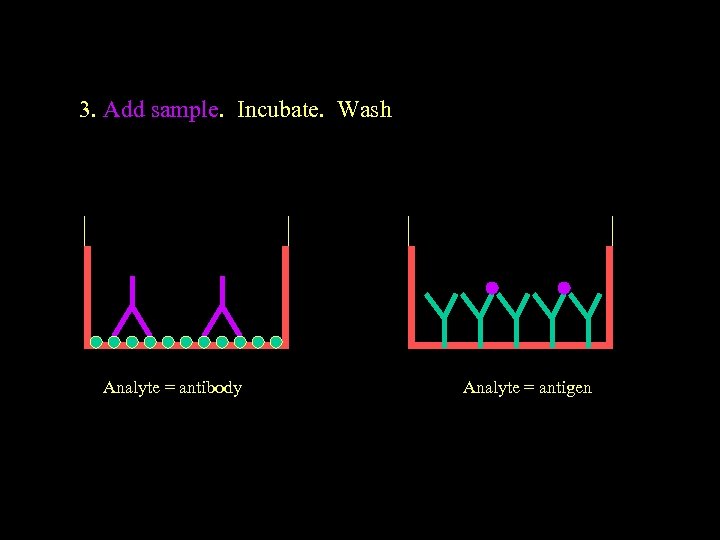 3. Add sample. Incubate. Wash Analyte = antibody Analyte = antigen
3. Add sample. Incubate. Wash Analyte = antibody Analyte = antigen
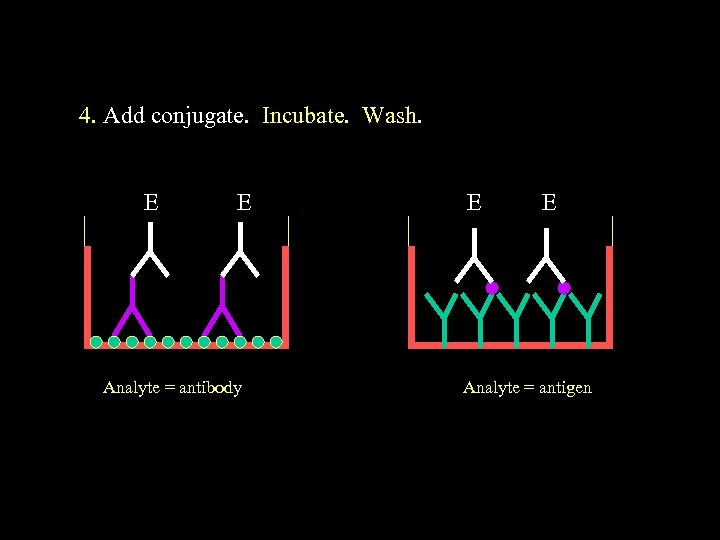 4. Add conjugate. Incubate. Wash. E E Analyte = antibody E E Analyte = antigen
4. Add conjugate. Incubate. Wash. E E Analyte = antibody E E Analyte = antigen
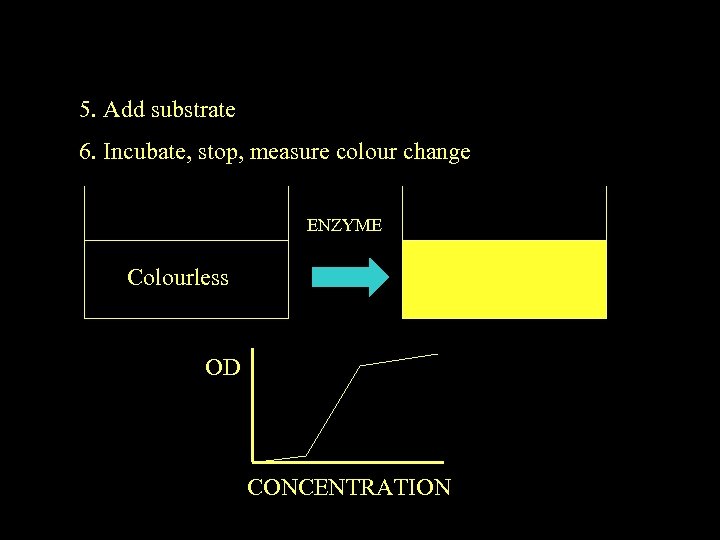 5. Add substrate 6. Incubate, stop, measure colour change ENZYME Colourless OD CONCENTRATION
5. Add substrate 6. Incubate, stop, measure colour change ENZYME Colourless OD CONCENTRATION
 COATING THE PLATE • protein-binding 96 (384)-well polystyrene plate eg Immulon-2 (Dynatech) • buffer = 0. 1 M Na 2 CO 3/Na. HCO 3 p. H 9. 6 0. 1 M tris-HCl p. H 7. 6 0. 01 M PBS p. H 7. 3 etc. • antigen or antibody at 0. 5 - 20 g/ml • 100 l/well, 4 o. C overnight
COATING THE PLATE • protein-binding 96 (384)-well polystyrene plate eg Immulon-2 (Dynatech) • buffer = 0. 1 M Na 2 CO 3/Na. HCO 3 p. H 9. 6 0. 1 M tris-HCl p. H 7. 6 0. 01 M PBS p. H 7. 3 etc. • antigen or antibody at 0. 5 - 20 g/ml • 100 l/well, 4 o. C overnight
 WASHING THE PLATE • buffer + 0. 05% Tween 20 • 200 l/well • 3 - 6 washes with 1 minute soak • automated washer or “flood and flick” (biohazard) or multichannel pipette for dispensing, manifold connected to vacuum pump (for safe disposal of wash fluid) • •
WASHING THE PLATE • buffer + 0. 05% Tween 20 • 200 l/well • 3 - 6 washes with 1 minute soak • automated washer or “flood and flick” (biohazard) or multichannel pipette for dispensing, manifold connected to vacuum pump (for safe disposal of wash fluid) • •
 BLOCKING THE PLATE • 0. 25% - 2% bovine serum albumin 2% non-fat dried milk 5 - 10% foetal calf serum in buffer + 0. 05% Tween 20 • 100 l/well, 37 o. C, > 60 min • Wash x 3 with buffer-Tween 20
BLOCKING THE PLATE • 0. 25% - 2% bovine serum albumin 2% non-fat dried milk 5 - 10% foetal calf serum in buffer + 0. 05% Tween 20 • 100 l/well, 37 o. C, > 60 min • Wash x 3 with buffer-Tween 20
 SAMPLE • Dilute in buffer-Tween 20 • include known positive and negative samples • standards……. recombinant protein international standard antibody double-dilute from 10 pg/ml - 10 ng/ml • 100 l/well, duplicates • 2 - 4 hours 20/37 o. C or overnight 4 o. C • 3 - 6 washes with buffer-Tween 20
SAMPLE • Dilute in buffer-Tween 20 • include known positive and negative samples • standards……. recombinant protein international standard antibody double-dilute from 10 pg/ml - 10 ng/ml • 100 l/well, duplicates • 2 - 4 hours 20/37 o. C or overnight 4 o. C • 3 - 6 washes with buffer-Tween 20
 CONJUGATE • For assays of (human) antibodies use : anti-(human) Ig-enzyme Ig. G / A / M / E / subclass-specific • For assays of antigens use enzyme-conjugated antibody: against a different epitope to the one recognized by capture antibody often monoclonal capture antibody polyclonal detection antibody
CONJUGATE • For assays of (human) antibodies use : anti-(human) Ig-enzyme Ig. G / A / M / E / subclass-specific • For assays of antigens use enzyme-conjugated antibody: against a different epitope to the one recognized by capture antibody often monoclonal capture antibody polyclonal detection antibody
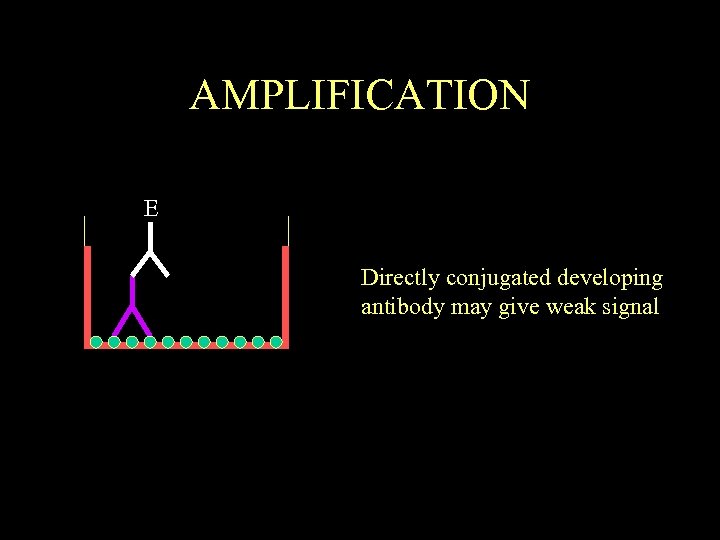 AMPLIFICATION E Directly conjugated developing antibody may give weak signal
AMPLIFICATION E Directly conjugated developing antibody may give weak signal
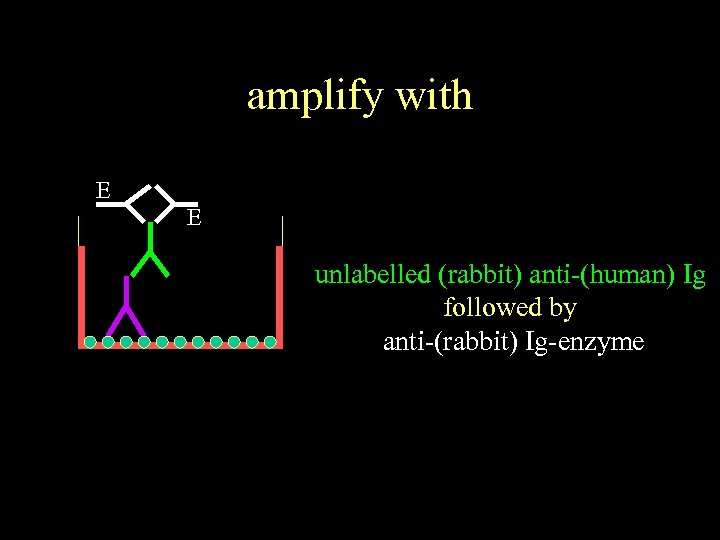 amplify with E E unlabelled (rabbit) anti-(human) Ig followed by anti-(rabbit) Ig-enzyme
amplify with E E unlabelled (rabbit) anti-(human) Ig followed by anti-(rabbit) Ig-enzyme
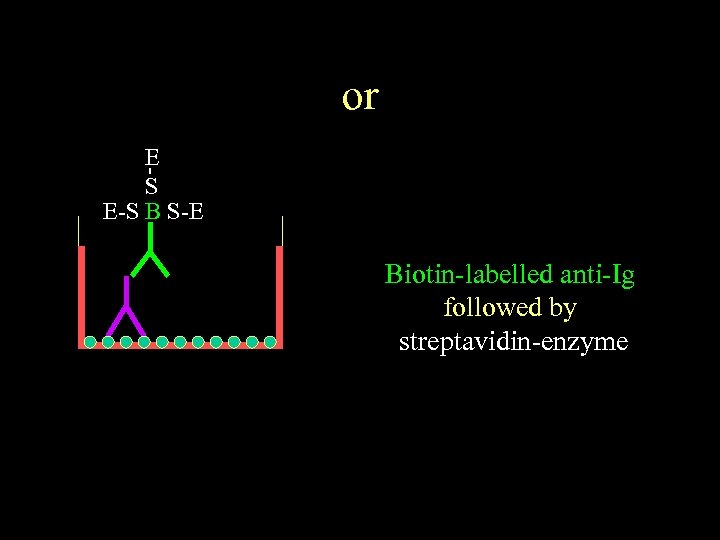 or E S E-S B S-E Biotin-labelled anti-Ig followed by streptavidin-enzyme
or E S E-S B S-E Biotin-labelled anti-Ig followed by streptavidin-enzyme
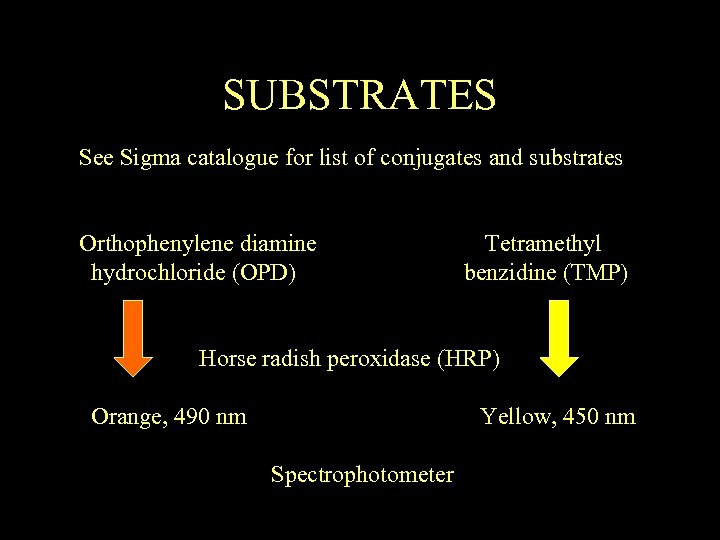 SUBSTRATES See Sigma catalogue for list of conjugates and substrates Orthophenylene diamine hydrochloride (OPD) Tetramethyl benzidine (TMP) Horse radish peroxidase (HRP) Orange, 490 nm Yellow, 450 nm Spectrophotometer
SUBSTRATES See Sigma catalogue for list of conjugates and substrates Orthophenylene diamine hydrochloride (OPD) Tetramethyl benzidine (TMP) Horse radish peroxidase (HRP) Orange, 490 nm Yellow, 450 nm Spectrophotometer
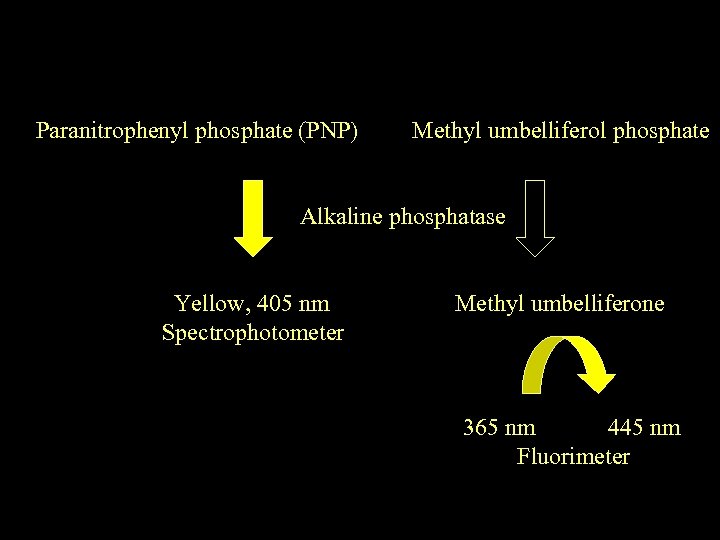 Paranitrophenyl phosphate (PNP) Methyl umbelliferol phosphate Alkaline phosphatase Yellow, 405 nm Spectrophotometer Methyl umbelliferone 365 nm 445 nm Fluorimeter
Paranitrophenyl phosphate (PNP) Methyl umbelliferol phosphate Alkaline phosphatase Yellow, 405 nm Spectrophotometer Methyl umbelliferone 365 nm 445 nm Fluorimeter
 INDIRECT ELISA TO DETECT SPECIFIC ANTIBODIES • • screening hybridoma supernatants detecting clinically important antibodies - autoantibodies - anti-pathogens - anti-allergens 1. Antigen
INDIRECT ELISA TO DETECT SPECIFIC ANTIBODIES • • screening hybridoma supernatants detecting clinically important antibodies - autoantibodies - anti-pathogens - anti-allergens 1. Antigen
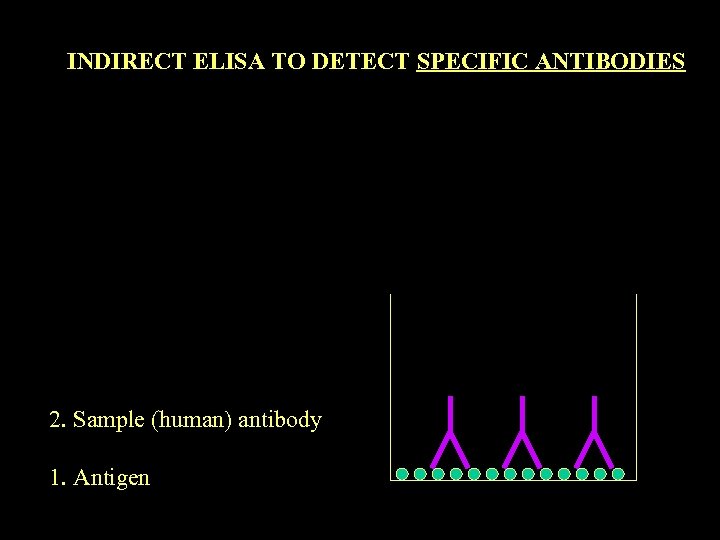 INDIRECT ELISA TO DETECT SPECIFIC ANTIBODIES 2. Sample (human) antibody 1. Antigen
INDIRECT ELISA TO DETECT SPECIFIC ANTIBODIES 2. Sample (human) antibody 1. Antigen
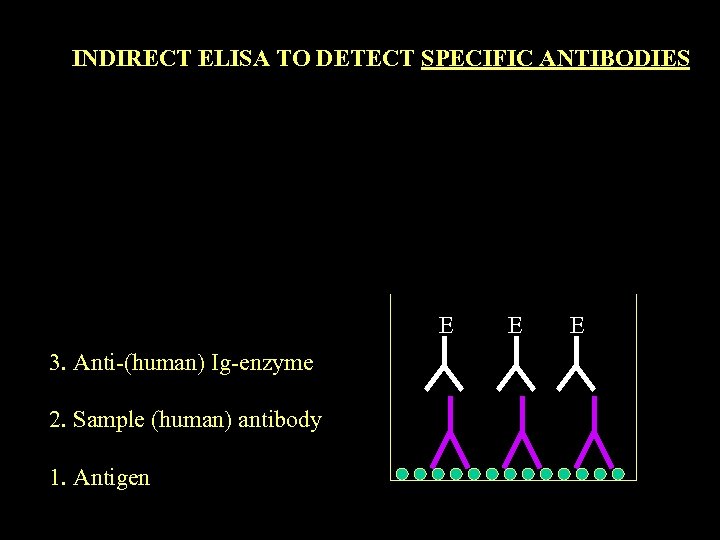 INDIRECT ELISA TO DETECT SPECIFIC ANTIBODIES E 3. Anti-(human) Ig-enzyme 2. Sample (human) antibody 1. Antigen E E
INDIRECT ELISA TO DETECT SPECIFIC ANTIBODIES E 3. Anti-(human) Ig-enzyme 2. Sample (human) antibody 1. Antigen E E
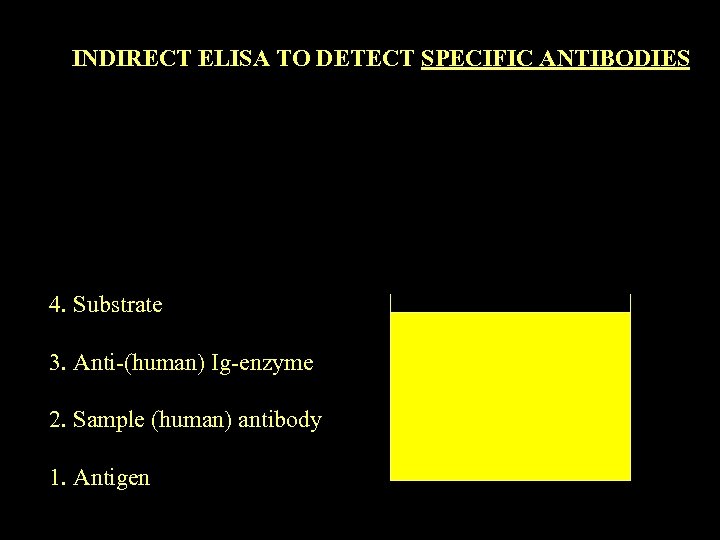 INDIRECT ELISA TO DETECT SPECIFIC ANTIBODIES 4. Substrate 3. Anti-(human) Ig-enzyme 2. Sample (human) antibody 1. Antigen E E E
INDIRECT ELISA TO DETECT SPECIFIC ANTIBODIES 4. Substrate 3. Anti-(human) Ig-enzyme 2. Sample (human) antibody 1. Antigen E E E
 ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES Useful when pure antigen not available or antigen coats poorly 1. Specific antibody
ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES Useful when pure antigen not available or antigen coats poorly 1. Specific antibody
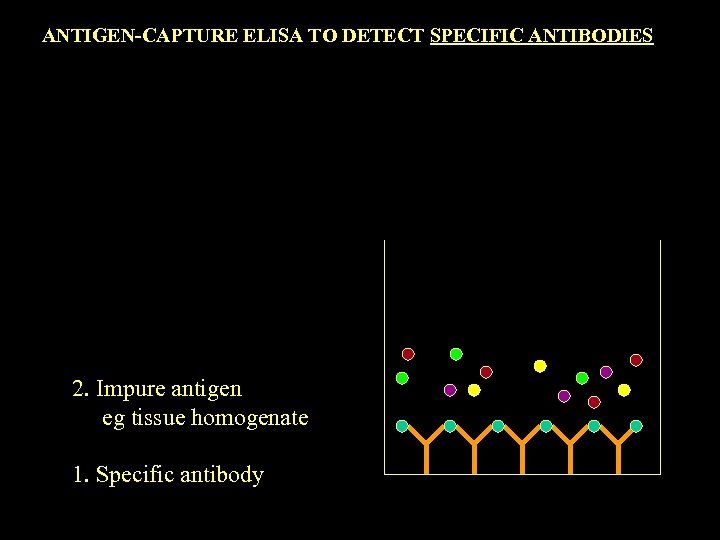 ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 2. Impure antigen eg tissue homogenate 1. Specific antibody
ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 2. Impure antigen eg tissue homogenate 1. Specific antibody
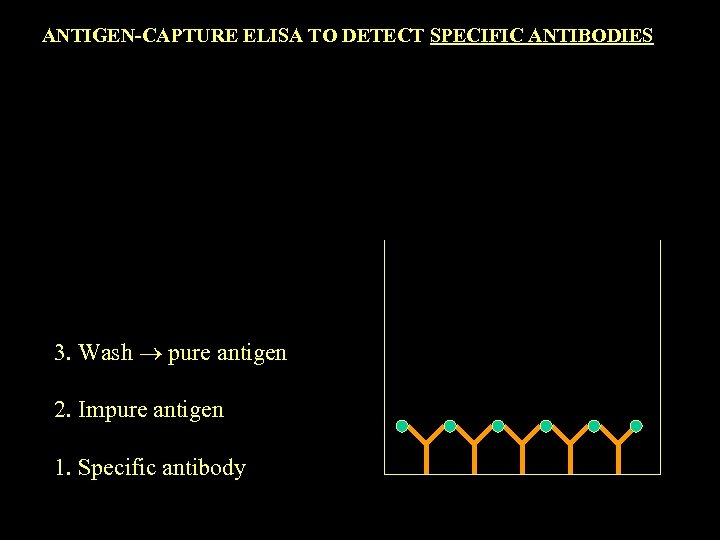 ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 3. Wash pure antigen 2. Impure antigen 1. Specific antibody
ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 3. Wash pure antigen 2. Impure antigen 1. Specific antibody
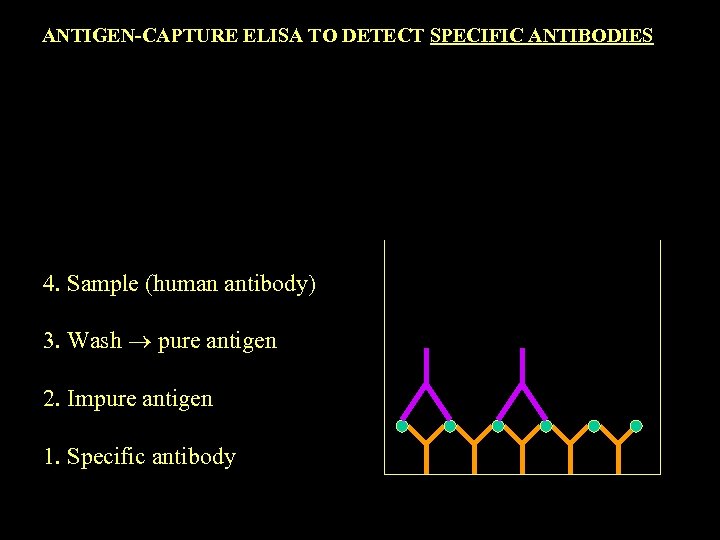 ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 4. Sample (human antibody) 3. Wash pure antigen 2. Impure antigen 1. Specific antibody
ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 4. Sample (human antibody) 3. Wash pure antigen 2. Impure antigen 1. Specific antibody
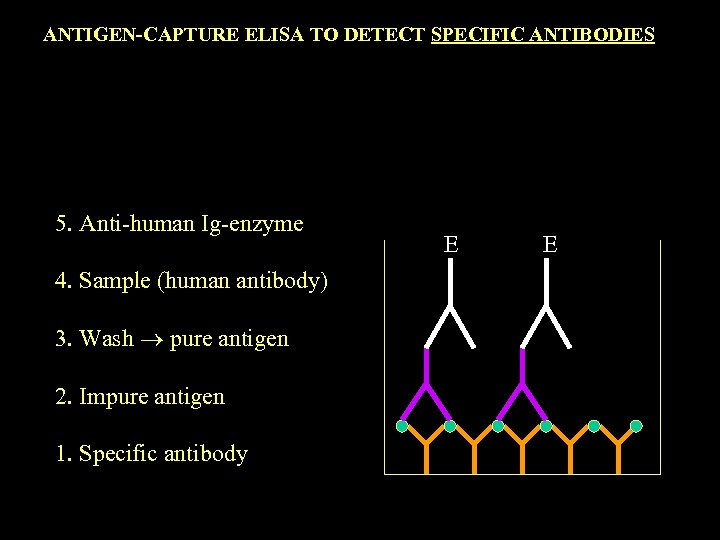 ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 5. Anti-human Ig-enzyme 4. Sample (human antibody) 3. Wash pure antigen 2. Impure antigen 1. Specific antibody E E
ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 5. Anti-human Ig-enzyme 4. Sample (human antibody) 3. Wash pure antigen 2. Impure antigen 1. Specific antibody E E
 ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 6. Substrate 5. Anti-human Ig-enzyme 4. Sample (human antibody) 3. Wash pure antigen 2. Impure antigen 1. Specific antibody
ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 6. Substrate 5. Anti-human Ig-enzyme 4. Sample (human antibody) 3. Wash pure antigen 2. Impure antigen 1. Specific antibody
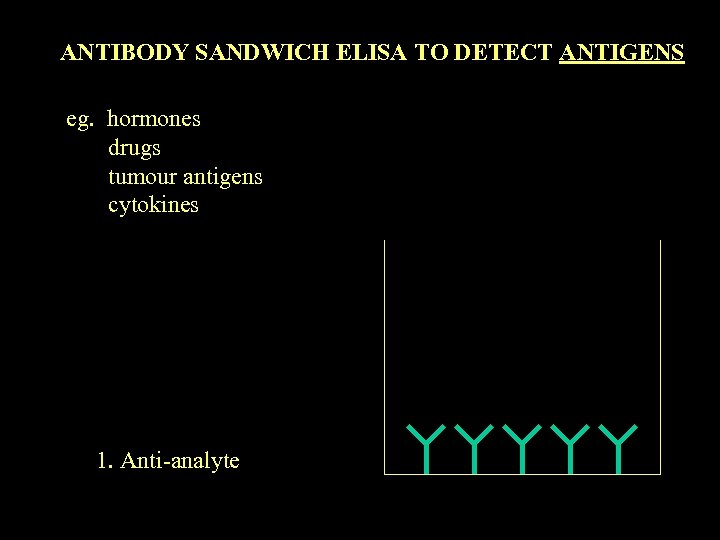 ANTIBODY SANDWICH ELISA TO DETECT ANTIGENS eg. hormones drugs tumour antigens cytokines 1. Anti-analyte
ANTIBODY SANDWICH ELISA TO DETECT ANTIGENS eg. hormones drugs tumour antigens cytokines 1. Anti-analyte
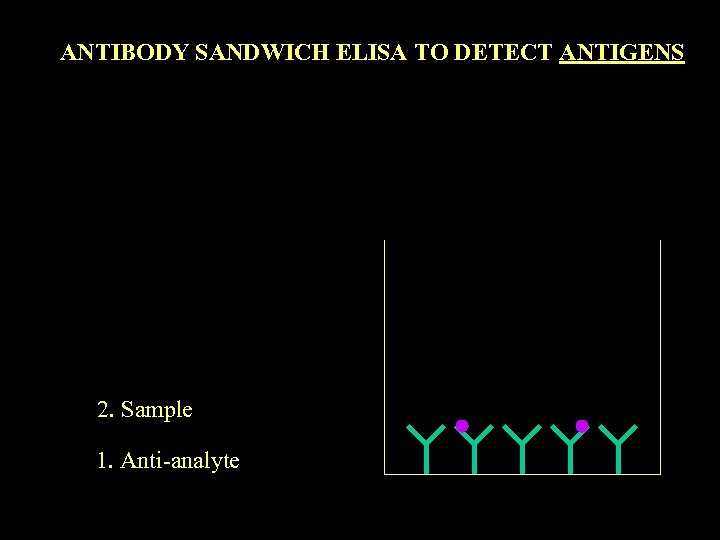 ANTIBODY SANDWICH ELISA TO DETECT ANTIGENS 2. Sample 1. Anti-analyte
ANTIBODY SANDWICH ELISA TO DETECT ANTIGENS 2. Sample 1. Anti-analyte
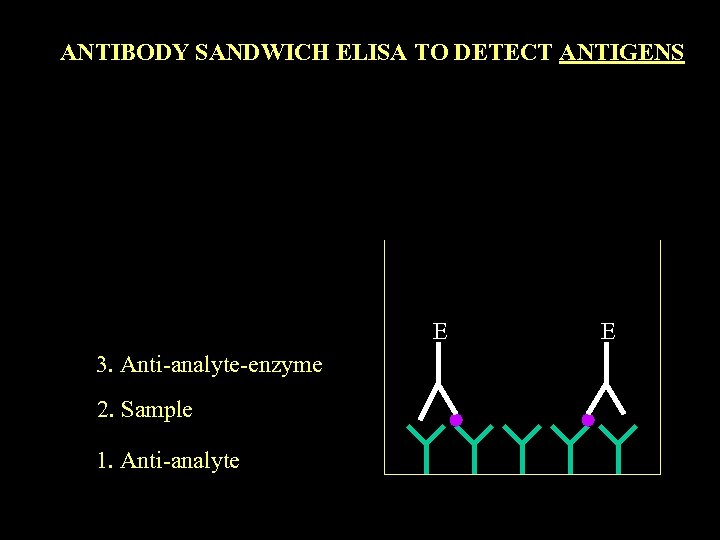 ANTIBODY SANDWICH ELISA TO DETECT ANTIGENS E 3. Anti-analyte-enzyme 2. Sample 1. Anti-analyte E
ANTIBODY SANDWICH ELISA TO DETECT ANTIGENS E 3. Anti-analyte-enzyme 2. Sample 1. Anti-analyte E
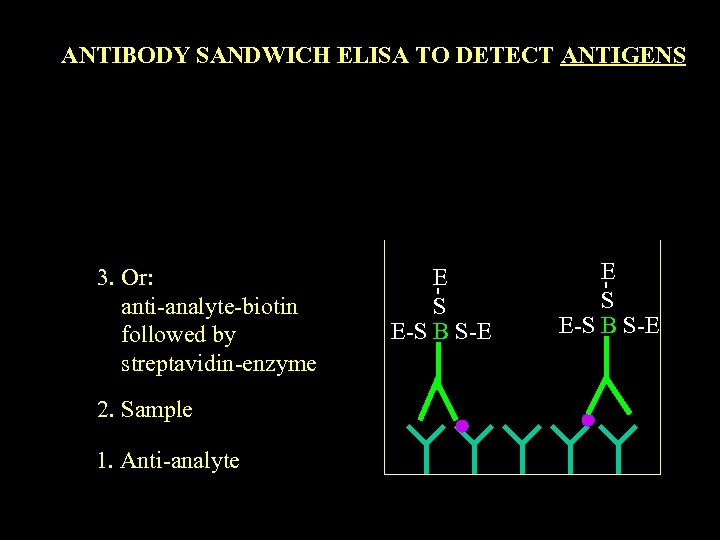 ANTIBODY SANDWICH ELISA TO DETECT ANTIGENS 3. Or: anti-analyte-biotin followed by streptavidin-enzyme 2. Sample 1. Anti-analyte E S E-S B S-E
ANTIBODY SANDWICH ELISA TO DETECT ANTIGENS 3. Or: anti-analyte-biotin followed by streptavidin-enzyme 2. Sample 1. Anti-analyte E S E-S B S-E
 ANTIBODY SANDWICH ELISA TO DETECT ANTIGENS 4. Substrate 3. Or: anti-analyte-biotin followed by streptavidin-enzyme 2. Sample 1. Anti-analyte
ANTIBODY SANDWICH ELISA TO DETECT ANTIGENS 4. Substrate 3. Or: anti-analyte-biotin followed by streptavidin-enzyme 2. Sample 1. Anti-analyte
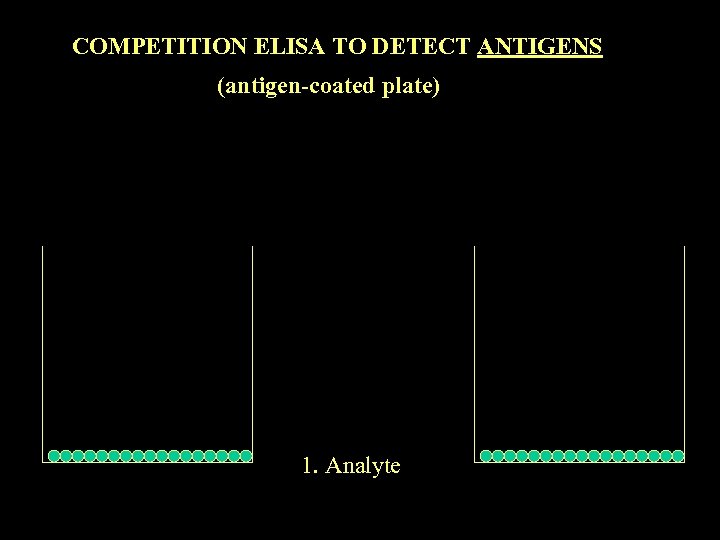 COMPETITION ELISA TO DETECT ANTIGENS (antigen-coated plate) 1. Analyte
COMPETITION ELISA TO DETECT ANTIGENS (antigen-coated plate) 1. Analyte
![COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] E High [analyte] E E E E COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] E High [analyte] E E E E](https://present5.com/presentation/da10742aac1ee809472e96f31b1c28e3/image-35.jpg) COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] E High [analyte] E E E E 2. Anti-analyte-E + sample 1. Analyte E E
COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] E High [analyte] E E E E 2. Anti-analyte-E + sample 1. Analyte E E
![COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 3. Wash E E E COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 3. Wash E E E](https://present5.com/presentation/da10742aac1ee809472e96f31b1c28e3/image-36.jpg) COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 3. Wash E E E 2. Anti-analyte-E + sample 1. Analyte E
COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 3. Wash E E E 2. Anti-analyte-E + sample 1. Analyte E
![COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 4. Substrate 3. Wash 2. COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 4. Substrate 3. Wash 2.](https://present5.com/presentation/da10742aac1ee809472e96f31b1c28e3/image-37.jpg) COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 4. Substrate 3. Wash 2. Anti-analyte-E + sample 1. Analyte
COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 4. Substrate 3. Wash 2. Anti-analyte-E + sample 1. Analyte
 COMPETITION ELISA TO DETECT ANTIGENS (antibody-coated plate) 1. Anti-analyte
COMPETITION ELISA TO DETECT ANTIGENS (antibody-coated plate) 1. Anti-analyte
![COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 2. Analyte-E + sample 1. COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 2. Analyte-E + sample 1.](https://present5.com/presentation/da10742aac1ee809472e96f31b1c28e3/image-39.jpg) COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 2. Analyte-E + sample 1. Anti-analyte
COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 2. Analyte-E + sample 1. Anti-analyte
![COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 3. Wash E E 2. COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 3. Wash E E 2.](https://present5.com/presentation/da10742aac1ee809472e96f31b1c28e3/image-40.jpg) COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 3. Wash E E 2. Analyte-E E + sample 1. Anti-analyte E E
COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 3. Wash E E 2. Analyte-E E + sample 1. Anti-analyte E E
![COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 4. Substrate 3. Wash 2. COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 4. Substrate 3. Wash 2.](https://present5.com/presentation/da10742aac1ee809472e96f31b1c28e3/image-41.jpg) COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 4. Substrate 3. Wash 2. Analyte-E + sample 1. Analyte
COMPETITION ELISA TO DETECT ANTIGENS Low [analyte] High [analyte] 4. Substrate 3. Wash 2. Analyte-E + sample 1. Analyte
 MICROPARTICLE ENZYME IMMUNOASSAY (ABBOTT Ax. SYM) Automated measurement down to 1 ng/ml Eg. tumour markers (AFP, CEA, PSA, CA 15. 3) immune activation marker 2 M Ig. E
MICROPARTICLE ENZYME IMMUNOASSAY (ABBOTT Ax. SYM) Automated measurement down to 1 ng/ml Eg. tumour markers (AFP, CEA, PSA, CA 15. 3) immune activation marker 2 M Ig. E
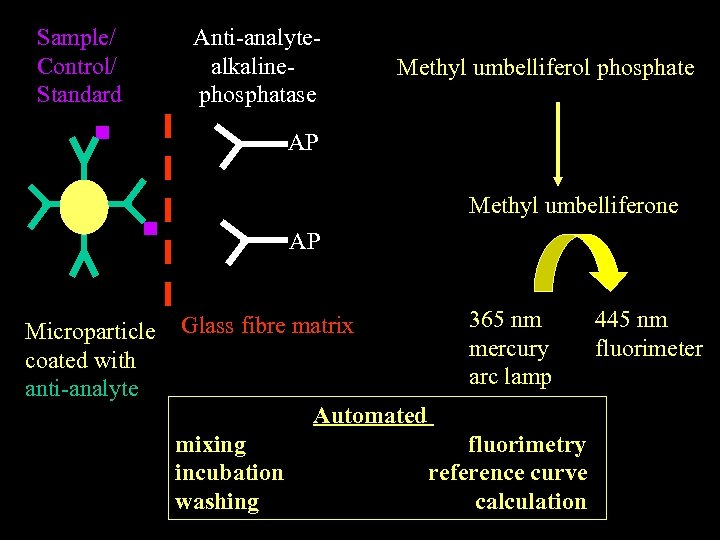 Sample/ Control/ Standard Anti-analytealkalinephosphatase Methyl umbelliferol phosphate AP Methyl umbelliferone AP Microparticle coated with anti-analyte Glass fibre matrix 365 nm mercury arc lamp Automated mixing incubation washing fluorimetry reference curve calculation 445 nm fluorimeter
Sample/ Control/ Standard Anti-analytealkalinephosphatase Methyl umbelliferol phosphate AP Methyl umbelliferone AP Microparticle coated with anti-analyte Glass fibre matrix 365 nm mercury arc lamp Automated mixing incubation washing fluorimetry reference curve calculation 445 nm fluorimeter
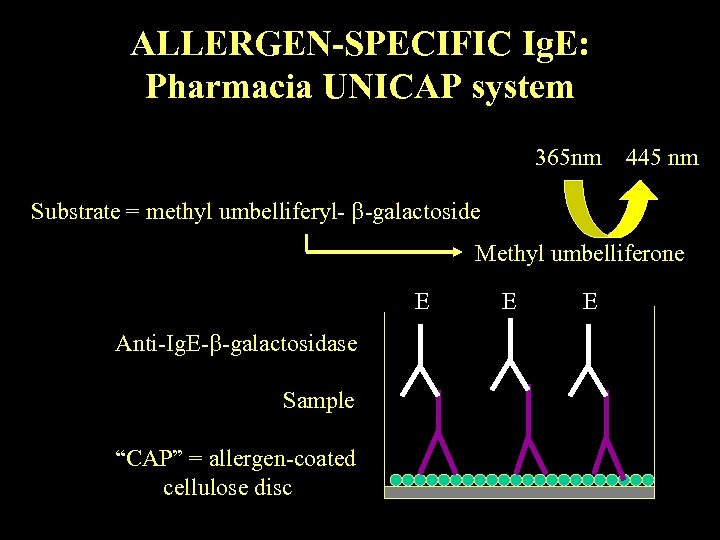 ALLERGEN-SPECIFIC Ig. E: Pharmacia UNICAP system 365 nm 445 nm Substrate = methyl umbelliferyl- -galactoside Methyl umbelliferone E Anti-Ig. E- -galactosidase Sample “CAP” = allergen-coated cellulose disc E E
ALLERGEN-SPECIFIC Ig. E: Pharmacia UNICAP system 365 nm 445 nm Substrate = methyl umbelliferyl- -galactoside Methyl umbelliferone E Anti-Ig. E- -galactosidase Sample “CAP” = allergen-coated cellulose disc E E
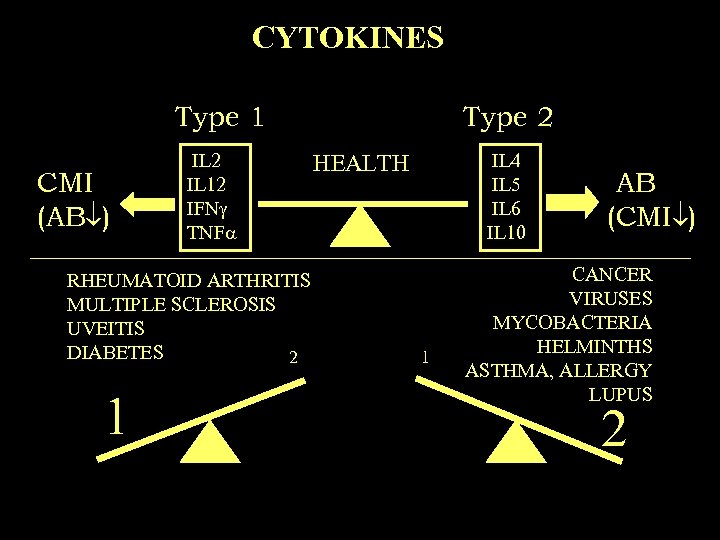 CYTOKINES Type 1 CMI (AB ) IL 2 IL 12 IFN TNF RHEUMATOID ARTHRITIS MULTIPLE SCLEROSIS UVEITIS DIABETES 2 1 Type 2 IL 4 IL 5 IL 6 IL 10 HEALTH 1 AB (CMI ) CANCER VIRUSES MYCOBACTERIA HELMINTHS ASTHMA, ALLERGY LUPUS 2
CYTOKINES Type 1 CMI (AB ) IL 2 IL 12 IFN TNF RHEUMATOID ARTHRITIS MULTIPLE SCLEROSIS UVEITIS DIABETES 2 1 Type 2 IL 4 IL 5 IL 6 IL 10 HEALTH 1 AB (CMI ) CANCER VIRUSES MYCOBACTERIA HELMINTHS ASTHMA, ALLERGY LUPUS 2
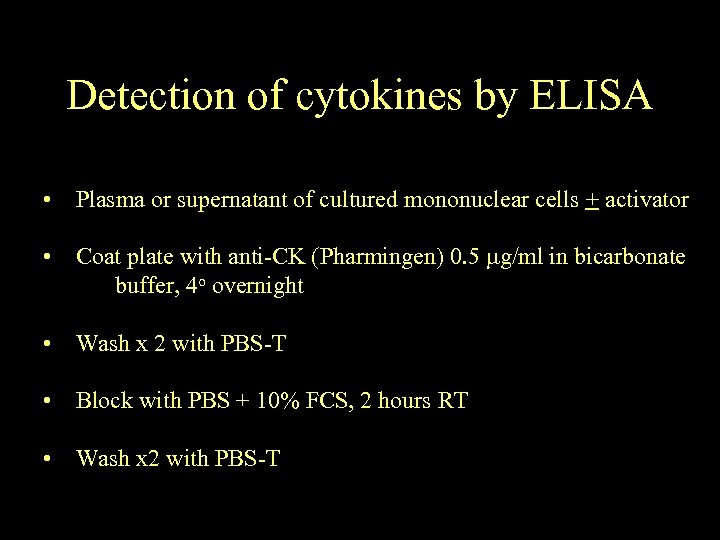 Detection of cytokines by ELISA • Plasma or supernatant of cultured mononuclear cells + activator • Coat plate with anti-CK (Pharmingen) 0. 5 g/ml in bicarbonate buffer, 4 o overnight • Wash x 2 with PBS-T • Block with PBS + 10% FCS, 2 hours RT • Wash x 2 with PBS-T
Detection of cytokines by ELISA • Plasma or supernatant of cultured mononuclear cells + activator • Coat plate with anti-CK (Pharmingen) 0. 5 g/ml in bicarbonate buffer, 4 o overnight • Wash x 2 with PBS-T • Block with PBS + 10% FCS, 2 hours RT • Wash x 2 with PBS-T
 • Add standards (recombinant CK 10 pg-10 ng /ml), controls and samples • 4 o overnight • Wash x 3 with PBS-T • Add biotinylated anti-CK (Pharmingen) 0. 5 g/ml • RT 60 minutes • Wash x 6 with PBS-T • Add streptavidin-peroxidase • RT 30 min
• Add standards (recombinant CK 10 pg-10 ng /ml), controls and samples • 4 o overnight • Wash x 3 with PBS-T • Add biotinylated anti-CK (Pharmingen) 0. 5 g/ml • RT 60 minutes • Wash x 6 with PBS-T • Add streptavidin-peroxidase • RT 30 min
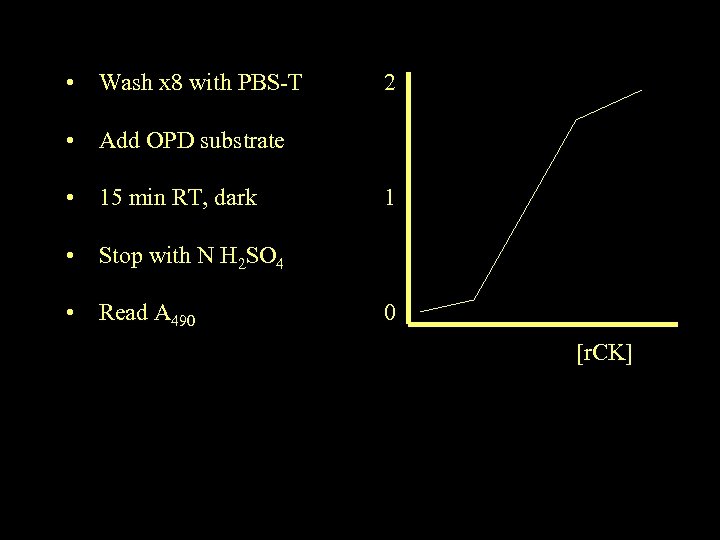 • Wash x 8 with PBS-T 2 • Add OPD substrate • 15 min RT, dark 1 • Stop with N H 2 SO 4 • Read A 490 0 [r. CK]
• Wash x 8 with PBS-T 2 • Add OPD substrate • 15 min RT, dark 1 • Stop with N H 2 SO 4 • Read A 490 0 [r. CK]
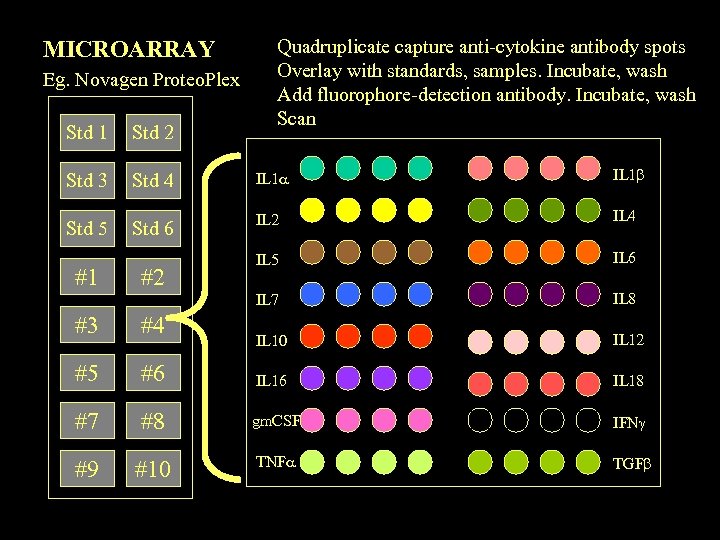 MICROARRAY Eg. Novagen Proteo. Plex Quadruplicate capture anti-cytokine antibody spots Overlay with standards, samples. Incubate, wash Add fluorophore-detection antibody. Incubate, wash Scan Std 1 Std 2 Std 3 Std 4 IL 1 Std 5 Std 6 IL 2 IL 4 IL 5 IL 6 IL 7 IL 8 IL 10 IL 12 #1 #2 #3 #4 #5 #6 IL 18 #7 #8 gm. CSF IFN #9 #10 TNF TGF
MICROARRAY Eg. Novagen Proteo. Plex Quadruplicate capture anti-cytokine antibody spots Overlay with standards, samples. Incubate, wash Add fluorophore-detection antibody. Incubate, wash Scan Std 1 Std 2 Std 3 Std 4 IL 1 Std 5 Std 6 IL 2 IL 4 IL 5 IL 6 IL 7 IL 8 IL 10 IL 12 #1 #2 #3 #4 #5 #6 IL 18 #7 #8 gm. CSF IFN #9 #10 TNF TGF
![S I G N A L [CK] S I G N A L [CK]](https://present5.com/presentation/da10742aac1ee809472e96f31b1c28e3/image-50.jpg) S I G N A L [CK]
S I G N A L [CK]
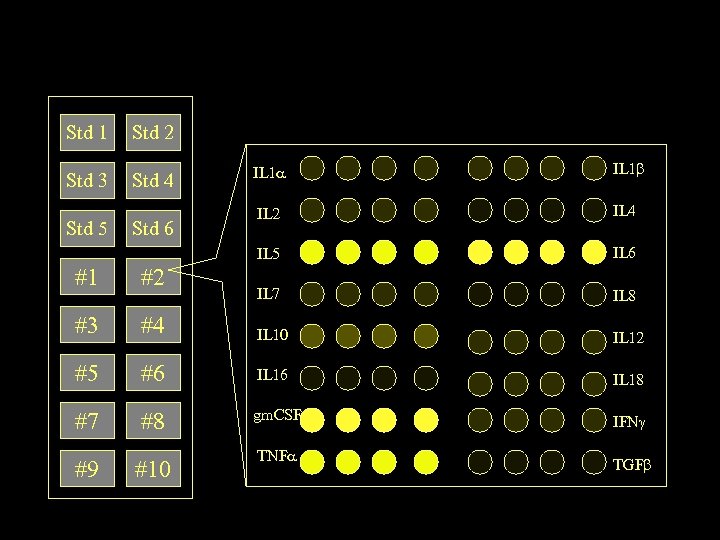 Std 1 Std 2 Std 3 Std 4 Std 6 IL 1 IL 2 IL 4 IL 5 Std 5 IL 1 IL 6 IL 7 IL 8 #1 #2 #3 #4 IL 10 IL 12 #5 #6 IL 18 #7 #8 gm. CSF IFN #9 #10 TNF TGF
Std 1 Std 2 Std 3 Std 4 Std 6 IL 1 IL 2 IL 4 IL 5 Std 5 IL 1 IL 6 IL 7 IL 8 #1 #2 #3 #4 IL 10 IL 12 #5 #6 IL 18 #7 #8 gm. CSF IFN #9 #10 TNF TGF
 CYTOMETRIC BEAD ASSAYS IL 1 IL 2 IL 4 IL 5 IL 6 IL 7 IL 8 IL 10 IL 12 IL 16 IL 18 gm. CSF IFN TNF TGF Mixture of beads with (a) Anti-cytokine capture antibody (b) Individual fluorescence properties
CYTOMETRIC BEAD ASSAYS IL 1 IL 2 IL 4 IL 5 IL 6 IL 7 IL 8 IL 10 IL 12 IL 16 IL 18 gm. CSF IFN TNF TGF Mixture of beads with (a) Anti-cytokine capture antibody (b) Individual fluorescence properties
 IL 1 IL 2 IL 4 IL 5 IL 6 IL 7 IL 8 IL 10 IL 12 IL 16 IL 18 gm. CSF IFN TNF TGF Add sample
IL 1 IL 2 IL 4 IL 5 IL 6 IL 7 IL 8 IL 10 IL 12 IL 16 IL 18 gm. CSF IFN TNF TGF Add sample
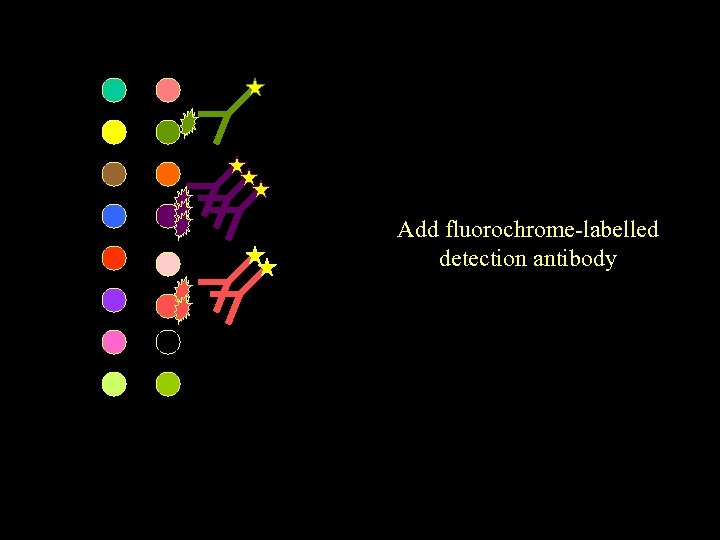 Add fluorochrome-labelled detection antibody
Add fluorochrome-labelled detection antibody
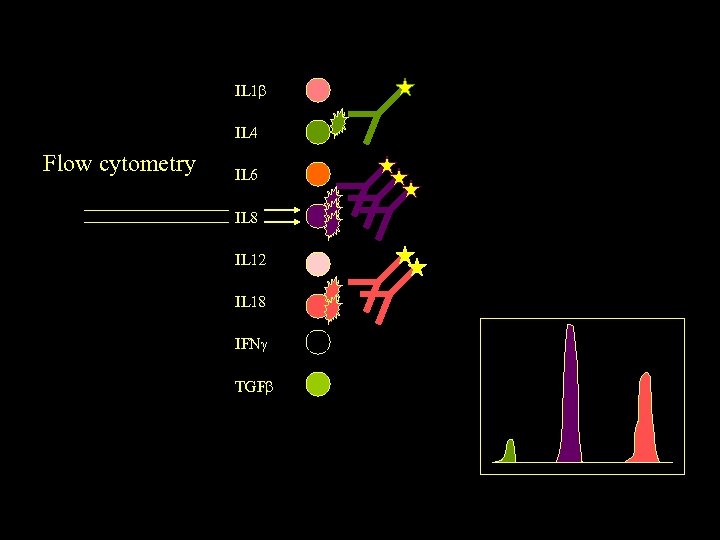 IL 1 IL 4 Flow cytometry IL 6 IL 8 IL 12 IL 18 IFN TGF
IL 1 IL 4 Flow cytometry IL 6 IL 8 IL 12 IL 18 IFN TGF
 DETECTION OF CYTOKINESECRETING CELLS BY ELISPOT ASSAY Czerkinsky et al, 1983; Sedgwick & Holt, 1983 J. Immunol. Methods
DETECTION OF CYTOKINESECRETING CELLS BY ELISPOT ASSAY Czerkinsky et al, 1983; Sedgwick & Holt, 1983 J. Immunol. Methods
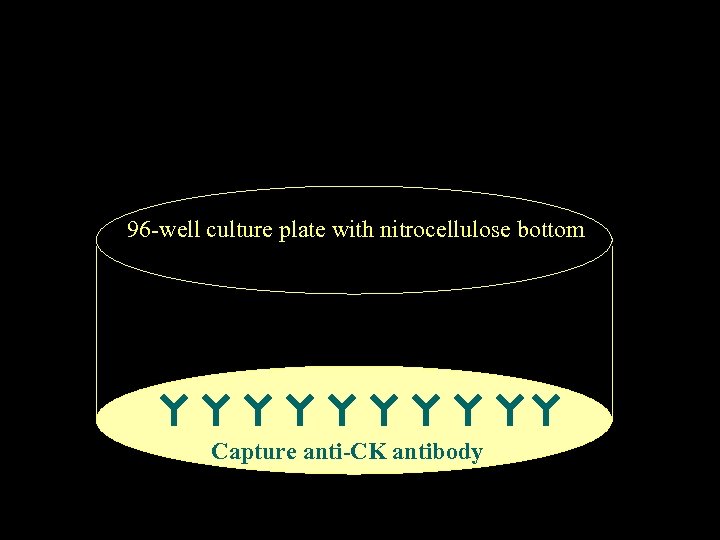 96 -well culture plate with nitrocellulose bottom Capture anti-CK antibody
96 -well culture plate with nitrocellulose bottom Capture anti-CK antibody
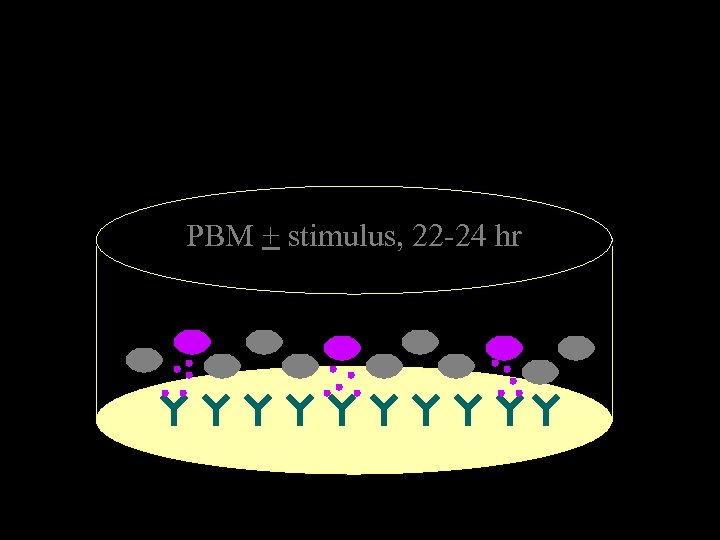 PBM + stimulus, 22 -24 hr
PBM + stimulus, 22 -24 hr
 WASH
WASH
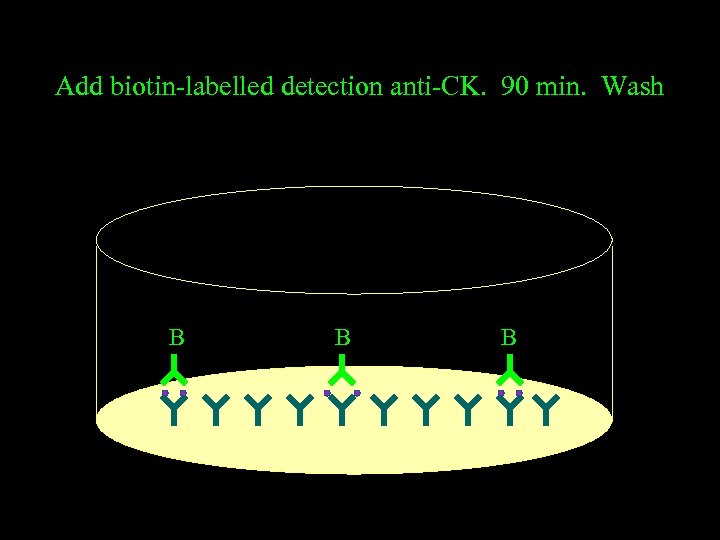 Add biotin-labelled detection anti-CK. 90 min. Wash B B B
Add biotin-labelled detection anti-CK. 90 min. Wash B B B
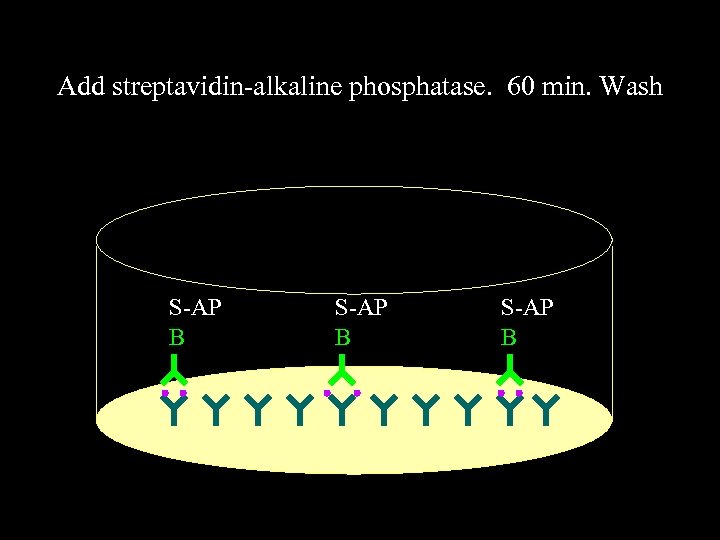 Add streptavidin-alkaline phosphatase. 60 min. Wash S-AP B
Add streptavidin-alkaline phosphatase. 60 min. Wash S-AP B
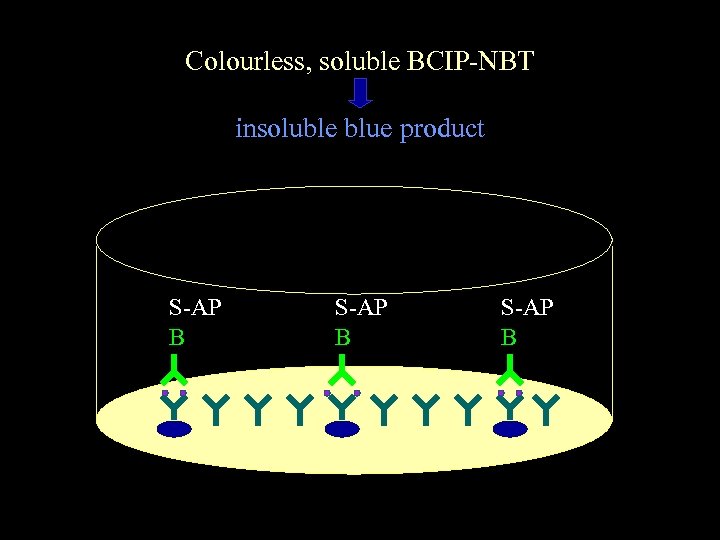 Colourless, soluble BCIP-NBT insoluble blue product S-AP B
Colourless, soluble BCIP-NBT insoluble blue product S-AP B
 Unstimulated PBM PHA, Con A, anti-CD 3, antigen, alloantigen (T-cells) LPS, SAC (monocytes) Type 1 cytokines (CMI, proinflammatory) - IFN , IL 2, IL 12, TNF Type 2 cytokines (antibody, anti-inflammatory) - IL 4, IL 6, IL 10
Unstimulated PBM PHA, Con A, anti-CD 3, antigen, alloantigen (T-cells) LPS, SAC (monocytes) Type 1 cytokines (CMI, proinflammatory) - IFN , IL 2, IL 12, TNF Type 2 cytokines (antibody, anti-inflammatory) - IL 4, IL 6, IL 10
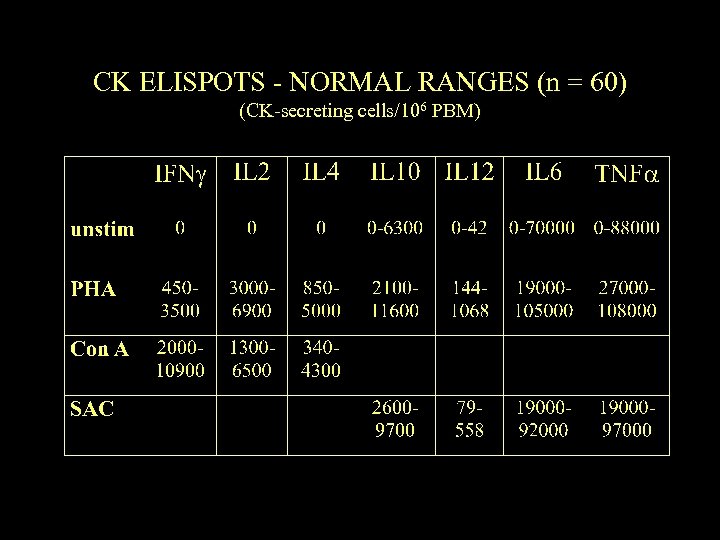 CK ELISPOTS - NORMAL RANGES (n = 60) (CK-secreting cells/106 PBM)
CK ELISPOTS - NORMAL RANGES (n = 60) (CK-secreting cells/106 PBM)
 CASE STUDY: POTENTIAL OF CYTOKINE PROFILING IN CLINICAL PRACTICE • 48 year old woman with frequent life-long respiratory and intestinal infections • Cytokine profile : IL 6, TNF , IL 10, IFN , IL 12, normal IL 4 • r. IL 12 in vitro normalized IFN • r. IFN in vitro normalized IL 12 • Anti-IL 10 in vitro normalized IFN and IL 12 • Treatment with r. IFN , r. IL 12, anti-IL 10 not possible • Thymosin- 1 normal IL 6, IFN , IL 4; near-normal IL 12, TNF ; IL 10
CASE STUDY: POTENTIAL OF CYTOKINE PROFILING IN CLINICAL PRACTICE • 48 year old woman with frequent life-long respiratory and intestinal infections • Cytokine profile : IL 6, TNF , IL 10, IFN , IL 12, normal IL 4 • r. IL 12 in vitro normalized IFN • r. IFN in vitro normalized IL 12 • Anti-IL 10 in vitro normalized IFN and IL 12 • Treatment with r. IFN , r. IL 12, anti-IL 10 not possible • Thymosin- 1 normal IL 6, IFN , IL 4; near-normal IL 12, TNF ; IL 10
 DEMONSTRATION Room 508/511, Clinical Pathology Building ELISA washer, reader UNICAP system Ax. SYM ELISPOTS
DEMONSTRATION Room 508/511, Clinical Pathology Building ELISA washer, reader UNICAP system Ax. SYM ELISPOTS


