6dac9c59630cebbfa089d3444fe418b2.ppt
- Количество слайдов: 90

 Elemental, My Dear Watson Paul Middlestead University of Ottawa, G. G. Hatch Laboratory For 19 th Continuous Flow Conference, Calgary, 2013
Elemental, My Dear Watson Paul Middlestead University of Ottawa, G. G. Hatch Laboratory For 19 th Continuous Flow Conference, Calgary, 2013
 Who/what is this talk for? This talk is for new users We will touch the basics on Elemental analysers A mix bag of tricks and advices Refer to manufacturer’s instruction manuals Only endorsed products are: Rickards White Rickards Red and
Who/what is this talk for? This talk is for new users We will touch the basics on Elemental analysers A mix bag of tricks and advices Refer to manufacturer’s instruction manuals Only endorsed products are: Rickards White Rickards Red and
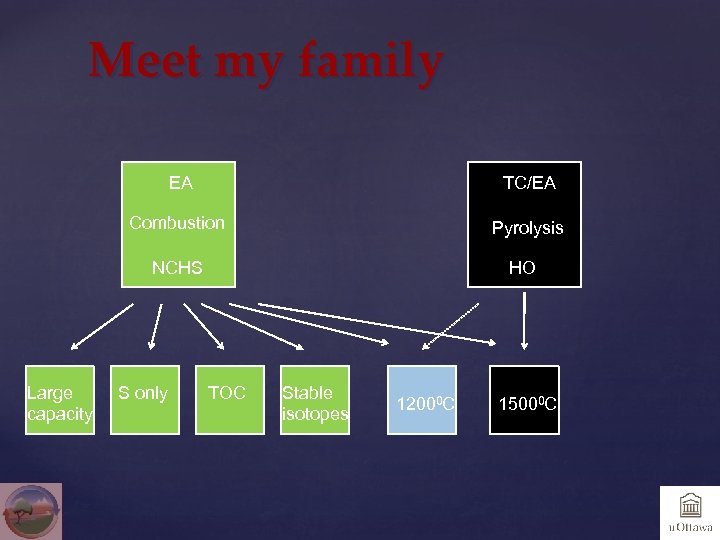 Meet my family EA TC/EA Combustion Pyrolysis NCHS Large capacity S only HO TOC Stable isotopes 12000 C 15000 C
Meet my family EA TC/EA Combustion Pyrolysis NCHS Large capacity S only HO TOC Stable isotopes 12000 C 15000 C
 Meet Grand Pa Leco is the most popular manufacturer of elemental analysers. Every department has one or two of those accumulating dust. Static combustion using oxygen: not suitable for IRMS
Meet Grand Pa Leco is the most popular manufacturer of elemental analysers. Every department has one or two of those accumulating dust. Static combustion using oxygen: not suitable for IRMS
 Let’s touch on • Elemental Analyser as an…instrument • Schematics • Autosamplers • Flash combustion • Chemicals & Configurations • Gas Chromatography • Thermal Conductivity Detector • Typical run • Common problems • EA-IRMS
Let’s touch on • Elemental Analyser as an…instrument • Schematics • Autosamplers • Flash combustion • Chemicals & Configurations • Gas Chromatography • Thermal Conductivity Detector • Typical run • Common problems • EA-IRMS
 EA as an …. instrument Simultaneous determination of Nitrogen, Carbon, Hydrogen, Sulfur, Oxygen Measuring range: 100 ppm to 100% Sample size: 0. 1 to 1000 mg / 0. 1 to 25 ul Detection limit: 10 ppm Accuracy: 0. 3% - 0. 02% absolute
EA as an …. instrument Simultaneous determination of Nitrogen, Carbon, Hydrogen, Sulfur, Oxygen Measuring range: 100 ppm to 100% Sample size: 0. 1 to 1000 mg / 0. 1 to 25 ul Detection limit: 10 ppm Accuracy: 0. 3% - 0. 02% absolute
 Manufacturers, models blablabla CE instruments (Carlo Erba) models 1108, 1110, NA 1500, NA 2100, Flash 1112 Costech Model ECS 4010 Elementar cube family Eurovector models Euro. Ea 3028 -HT, Ea 3024 IRMS, pyrolysis model Sercon-Integra TCEA Thermo …
Manufacturers, models blablabla CE instruments (Carlo Erba) models 1108, 1110, NA 1500, NA 2100, Flash 1112 Costech Model ECS 4010 Elementar cube family Eurovector models Euro. Ea 3028 -HT, Ea 3024 IRMS, pyrolysis model Sercon-Integra TCEA Thermo …
 EA : Bulk analysis of NCHS/O Organic compounds Pharmaceuticals Organometallics Petrochemicals Gasoline & fuels Graphite Carbides & nitrides Metals & alloys Polymers Explosives Hydrocarbons Soils Coal & coke Liquids In short, you can combust your grandmother!
EA : Bulk analysis of NCHS/O Organic compounds Pharmaceuticals Organometallics Petrochemicals Gasoline & fuels Graphite Carbides & nitrides Metals & alloys Polymers Explosives Hydrocarbons Soils Coal & coke Liquids In short, you can combust your grandmother!
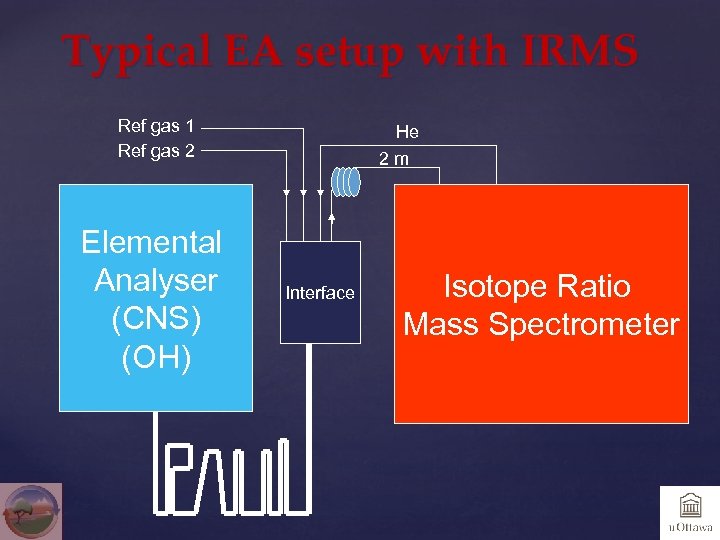 Typical EA setup with IRMS Ref gas 1 Ref gas 2 Elemental Analyser (CNS) (OH) He 2 m Interface Isotope Ratio Mass Spectrometer
Typical EA setup with IRMS Ref gas 1 Ref gas 2 Elemental Analyser (CNS) (OH) He 2 m Interface Isotope Ratio Mass Spectrometer
 EA picture
EA picture
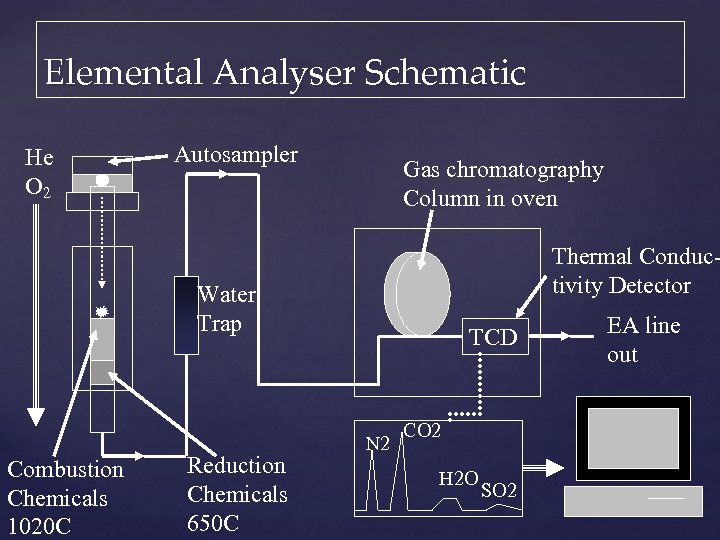 Elemental Analyser Schematic He O 2 Autosampler Gas chromatography Column in oven Thermal Conductivity Detector Water Trap Combustion Chemicals 1020 C Reduction Chemicals 650 C TCD N 2 CO 2 H 2 O SO 2 EA line out
Elemental Analyser Schematic He O 2 Autosampler Gas chromatography Column in oven Thermal Conductivity Detector Water Trap Combustion Chemicals 1020 C Reduction Chemicals 650 C TCD N 2 CO 2 H 2 O SO 2 EA line out
 Found the problem with the previous slide? A beer if you do… (5 seconds)
Found the problem with the previous slide? A beer if you do… (5 seconds)
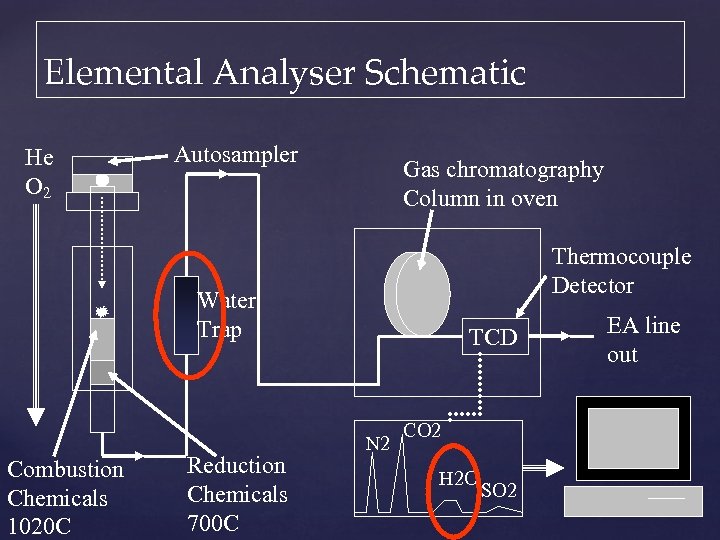 Elemental Analyser Schematic He O 2 Autosampler Gas chromatography Column in oven Thermocouple Detector Water Trap Combustion Chemicals 1020 C Reduction Chemicals 700 C TCD N 2 CO 2 H 2 O SO 2 EA line out
Elemental Analyser Schematic He O 2 Autosampler Gas chromatography Column in oven Thermocouple Detector Water Trap Combustion Chemicals 1020 C Reduction Chemicals 700 C TCD N 2 CO 2 H 2 O SO 2 EA line out
 A cold Rickard’s Red for me please.
A cold Rickard’s Red for me please.
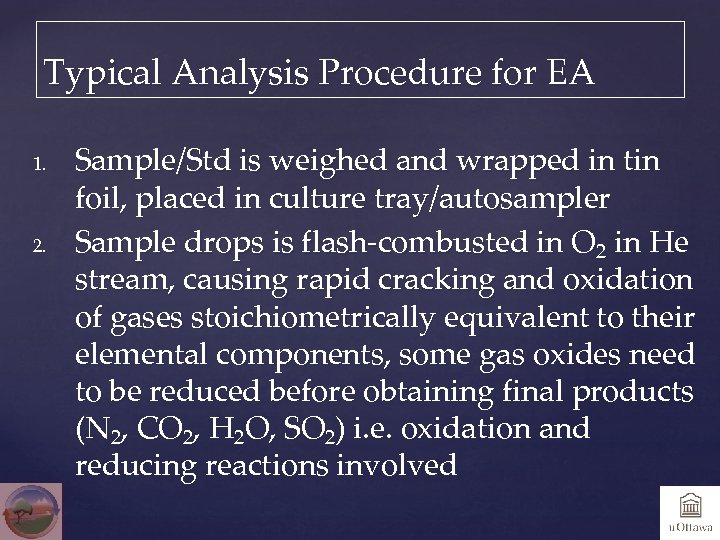 Typical Analysis Procedure for EA 1. 2. Sample/Std is weighed and wrapped in tin foil, placed in culture tray/autosampler Sample drops is flash-combusted in O 2 in He stream, causing rapid cracking and oxidation of gases stoichiometrically equivalent to their elemental components, some gas oxides need to be reduced before obtaining final products (N 2, CO 2, H 2 O, SO 2) i. e. oxidation and reducing reactions involved
Typical Analysis Procedure for EA 1. 2. Sample/Std is weighed and wrapped in tin foil, placed in culture tray/autosampler Sample drops is flash-combusted in O 2 in He stream, causing rapid cracking and oxidation of gases stoichiometrically equivalent to their elemental components, some gas oxides need to be reduced before obtaining final products (N 2, CO 2, H 2 O, SO 2) i. e. oxidation and reducing reactions involved
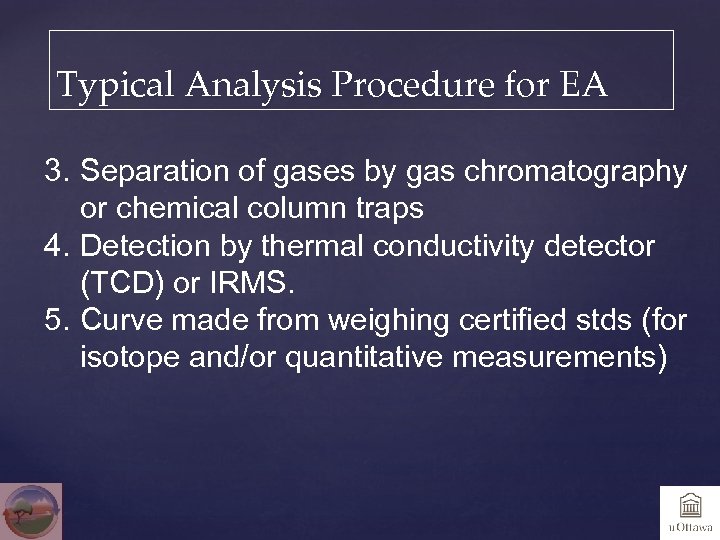 Typical Analysis Procedure for EA 3. Separation of gases by gas chromatography or chemical column traps 4. Detection by thermal conductivity detector (TCD) or IRMS. 5. Curve made from weighing certified stds (for isotope and/or quantitative measurements)
Typical Analysis Procedure for EA 3. Separation of gases by gas chromatography or chemical column traps 4. Detection by thermal conductivity detector (TCD) or IRMS. 5. Curve made from weighing certified stds (for isotope and/or quantitative measurements)
 Some torture tools…
Some torture tools…
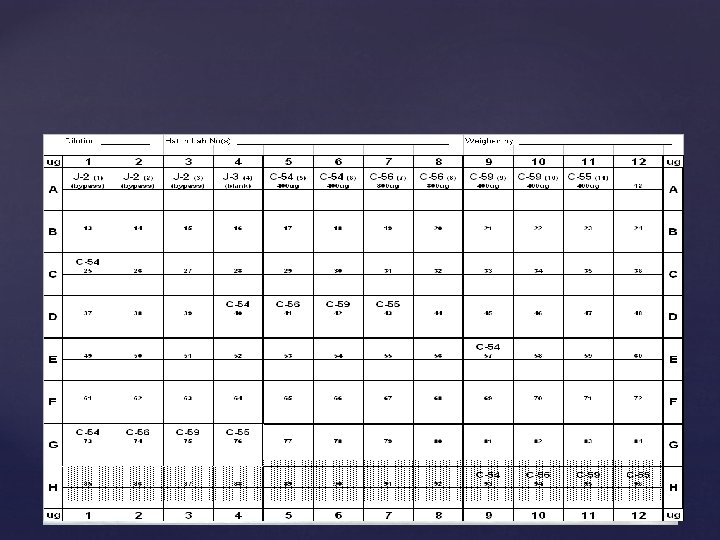 Well well, got culture?
Well well, got culture?
 Super size me
Super size me
 MAIS C’EST IMPOSSIBLE!
MAIS C’EST IMPOSSIBLE!
 C’EST FANTASTIQUE! Filters? Careful!
C’EST FANTASTIQUE! Filters? Careful!
 Not flat please
Not flat please

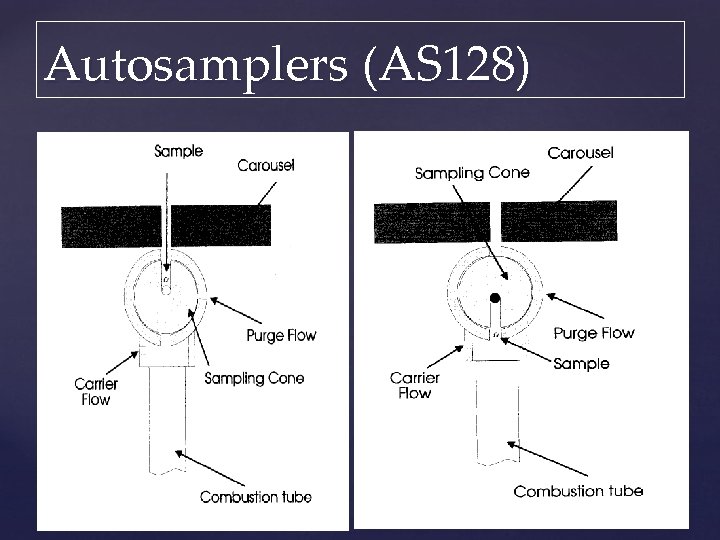 Autosamplers (AS 128)
Autosamplers (AS 128)
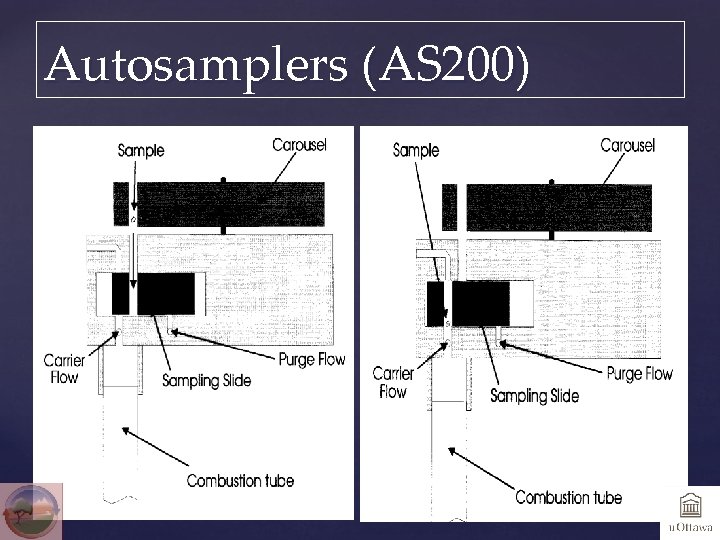 Autosamplers (AS 200)
Autosamplers (AS 200)
 Zero Blank
Zero Blank
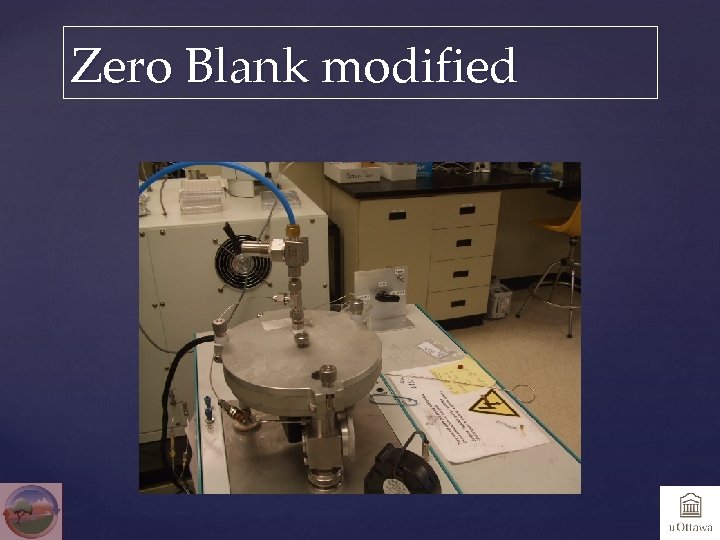 Zero Blank modified
Zero Blank modified
 Special tools required ECS 4010
Special tools required ECS 4010
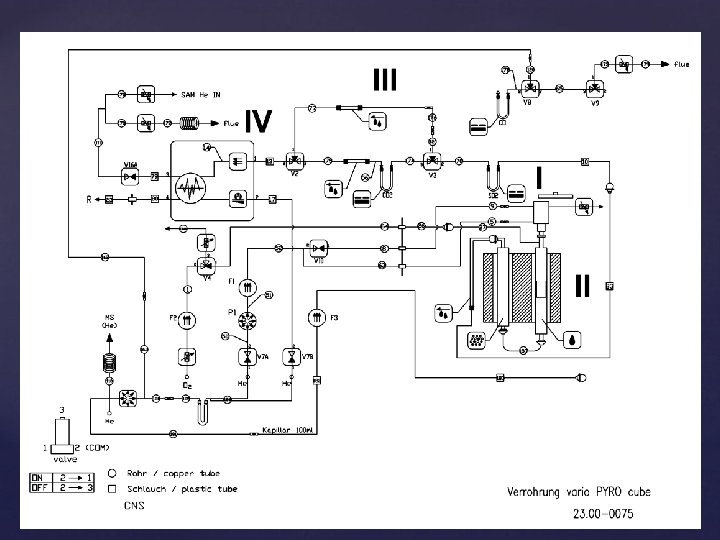
 Be good to yourself Take 20 minutes to study the gas schematics of your instrument
Be good to yourself Take 20 minutes to study the gas schematics of your instrument
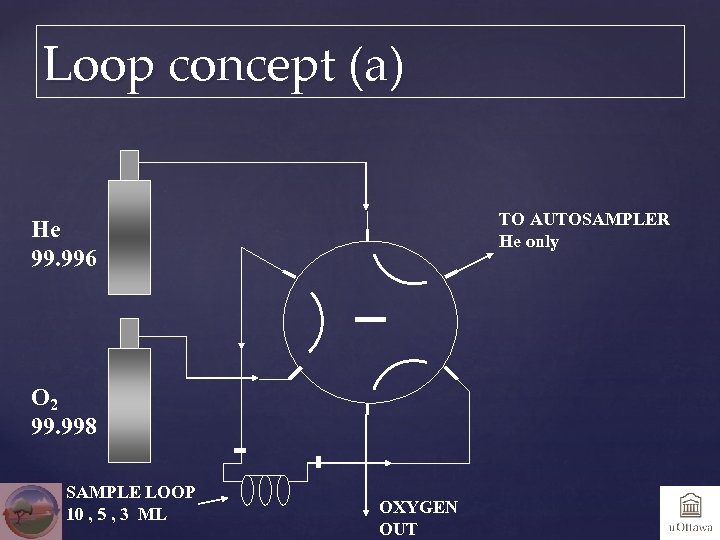 Loop concept (a) TO AUTOSAMPLER He only He 99. 996 O 2 99. 998 SAMPLE LOOP 10 , 5 , 3 ML OXYGEN OUT
Loop concept (a) TO AUTOSAMPLER He only He 99. 996 O 2 99. 998 SAMPLE LOOP 10 , 5 , 3 ML OXYGEN OUT
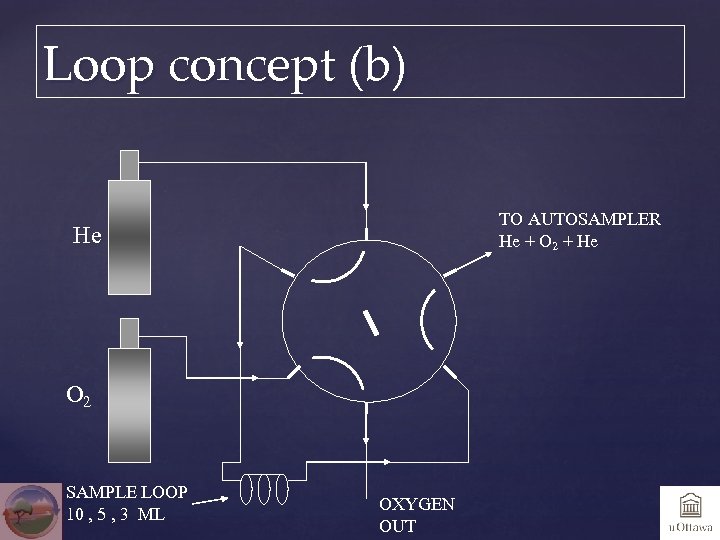 Loop concept (b) TO AUTOSAMPLER He + O 2 + He He O 2 SAMPLE LOOP 10 , 5 , 3 ML OXYGEN OUT
Loop concept (b) TO AUTOSAMPLER He + O 2 + He He O 2 SAMPLE LOOP 10 , 5 , 3 ML OXYGEN OUT
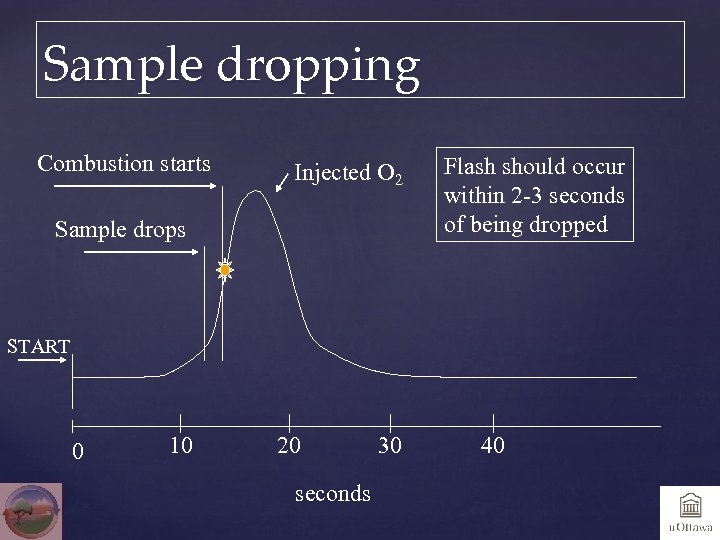 Sample dropping Combustion starts Injected O 2 Sample drops Flash should occur within 2 -3 seconds of being dropped START 0 10 20 seconds 30 40
Sample dropping Combustion starts Injected O 2 Sample drops Flash should occur within 2 -3 seconds of being dropped START 0 10 20 seconds 30 40
 Why you Tin man? • • PLATINUM: Absorbs much of the heat of the reactor before passing it on to the sample, slowing down reaction ALUMINIUM: Supplies good oxidation flash and prime sample ignition but does not melt or mix with the sample thus does not promote inner oxidation SILVER: Melts at 9600 C, does not take part in the combustion, retains trace of carbon when in molten state TIN: Inexpensive & takes active part in process. Melts at 2350 C with very low enthalpy, intermixes with organic and inorganic substances, expedites the final oxidation reaction
Why you Tin man? • • PLATINUM: Absorbs much of the heat of the reactor before passing it on to the sample, slowing down reaction ALUMINIUM: Supplies good oxidation flash and prime sample ignition but does not melt or mix with the sample thus does not promote inner oxidation SILVER: Melts at 9600 C, does not take part in the combustion, retains trace of carbon when in molten state TIN: Inexpensive & takes active part in process. Melts at 2350 C with very low enthalpy, intermixes with organic and inorganic substances, expedites the final oxidation reaction
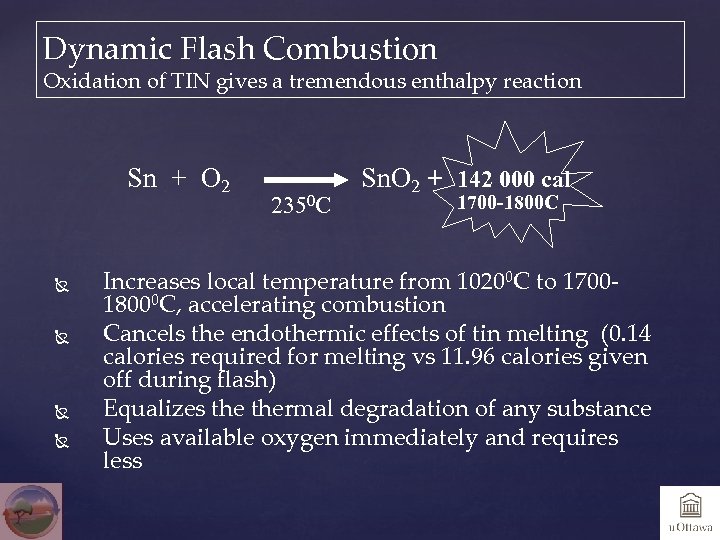 Dynamic Flash Combustion Oxidation of TIN gives a tremendous enthalpy reaction Sn + O 2 2350 C Sn. O 2 + 142 000 cal 1700 -1800 C Increases local temperature from 10200 C to 170018000 C, accelerating combustion Cancels the endothermic effects of tin melting (0. 14 calories required for melting vs 11. 96 calories given off during flash) Equalizes thermal degradation of any substance Uses available oxygen immediately and requires less
Dynamic Flash Combustion Oxidation of TIN gives a tremendous enthalpy reaction Sn + O 2 2350 C Sn. O 2 + 142 000 cal 1700 -1800 C Increases local temperature from 10200 C to 170018000 C, accelerating combustion Cancels the endothermic effects of tin melting (0. 14 calories required for melting vs 11. 96 calories given off during flash) Equalizes thermal degradation of any substance Uses available oxygen immediately and requires less
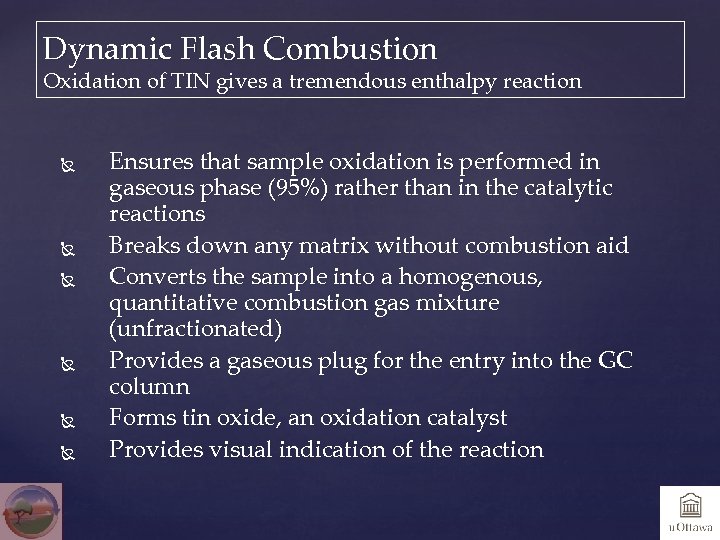 Dynamic Flash Combustion Oxidation of TIN gives a tremendous enthalpy reaction Ensures that sample oxidation is performed in gaseous phase (95%) rather than in the catalytic reactions Breaks down any matrix without combustion aid Converts the sample into a homogenous, quantitative combustion gas mixture (unfractionated) Provides a gaseous plug for the entry into the GC column Forms tin oxide, an oxidation catalyst Provides visual indication of the reaction
Dynamic Flash Combustion Oxidation of TIN gives a tremendous enthalpy reaction Ensures that sample oxidation is performed in gaseous phase (95%) rather than in the catalytic reactions Breaks down any matrix without combustion aid Converts the sample into a homogenous, quantitative combustion gas mixture (unfractionated) Provides a gaseous plug for the entry into the GC column Forms tin oxide, an oxidation catalyst Provides visual indication of the reaction
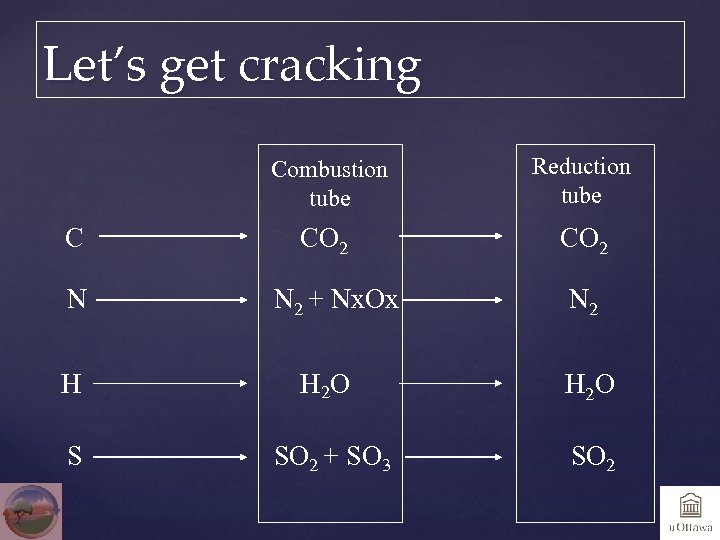 Let’s get cracking Combustion tube C N Reduction tube CO 2 N 2 + Nx. Ox N 2 H H 2 O S SO 2 + SO 3 SO 2
Let’s get cracking Combustion tube C N Reduction tube CO 2 N 2 + Nx. Ox N 2 H H 2 O S SO 2 + SO 3 SO 2
 Chemicals and Configurations • System should be optimized for element(s) to be analyzed • One-tube system: both oxidant catalyst and reducing chemicals in one tube; usually used if S is to be analyzed • Two-tube system: one tube for oxidant, one tube for reducing chemicals; usually used for N, NC or NCH • Chemical traps: Mg Perchlorate or Anhydrone will trap H 2 O, and Carbosorb will trap CO 2 (and SO 2)
Chemicals and Configurations • System should be optimized for element(s) to be analyzed • One-tube system: both oxidant catalyst and reducing chemicals in one tube; usually used if S is to be analyzed • Two-tube system: one tube for oxidant, one tube for reducing chemicals; usually used for N, NC or NCH • Chemical traps: Mg Perchlorate or Anhydrone will trap H 2 O, and Carbosorb will trap CO 2 (and SO 2)
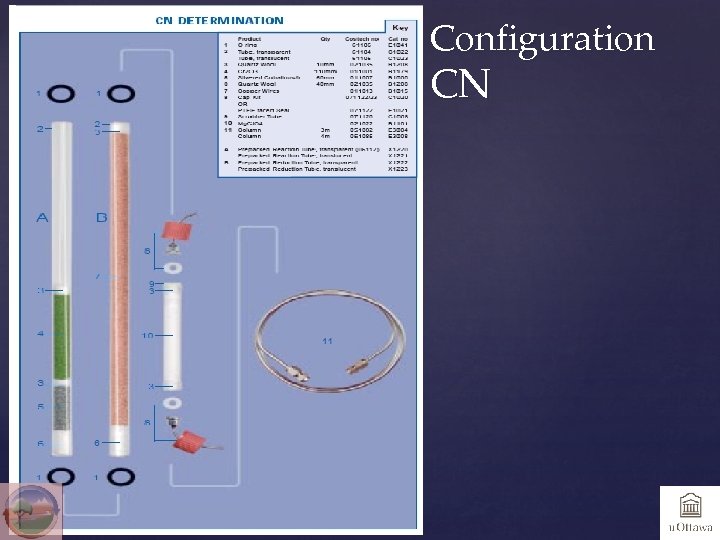 Configuration CN
Configuration CN
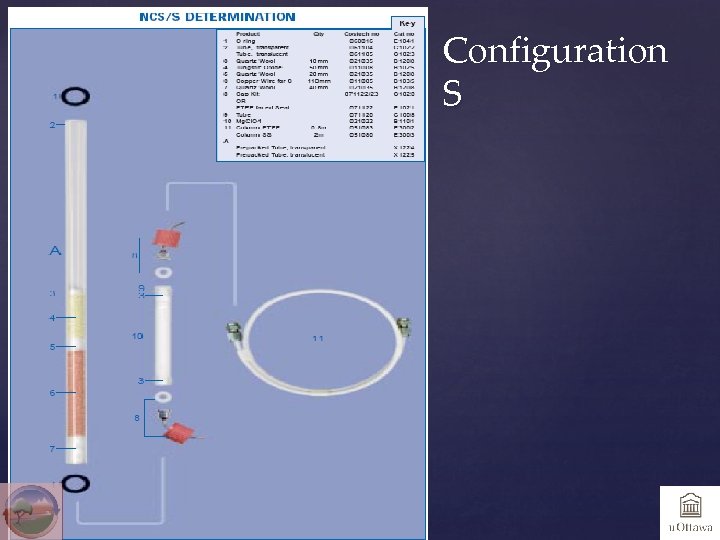 Configuration S
Configuration S
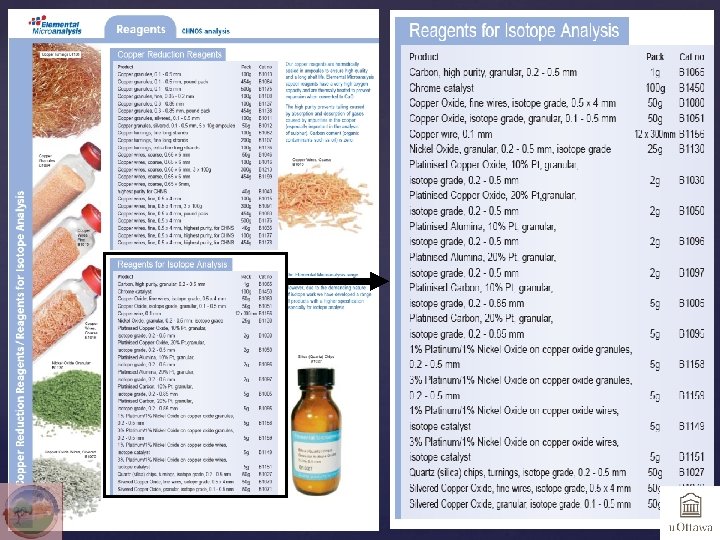
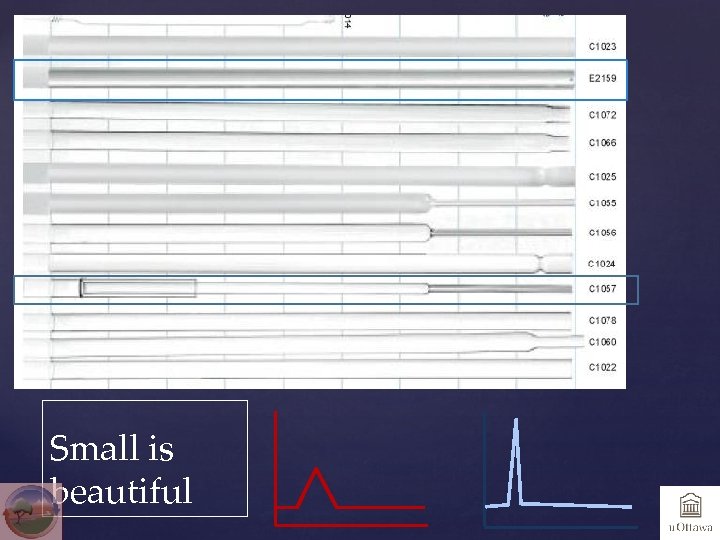 Small is beautiful
Small is beautiful
 More torture tools Using grinding tools and diluted Nitric acid, one can re-use combustion and reduction tubes
More torture tools Using grinding tools and diluted Nitric acid, one can re-use combustion and reduction tubes
 Gas Chromatography Gas chromatographic columns will separate different components according to their polarity and molecular size. Factors influencing the quality of the chromatography: column length, size of packing, tube diameter, stationary phase type, flow rate, temperature. Packed column: packed polymer beads, different sizes available. High capacity , low resolution. Capillary column: small capillary with polymeric film on inner wall. High resolution, low capacity. Deactivated fused silica is free of adsorption problems encountered with most packings or capillaries columns.
Gas Chromatography Gas chromatographic columns will separate different components according to their polarity and molecular size. Factors influencing the quality of the chromatography: column length, size of packing, tube diameter, stationary phase type, flow rate, temperature. Packed column: packed polymer beads, different sizes available. High capacity , low resolution. Capillary column: small capillary with polymeric film on inner wall. High resolution, low capacity. Deactivated fused silica is free of adsorption problems encountered with most packings or capillaries columns.
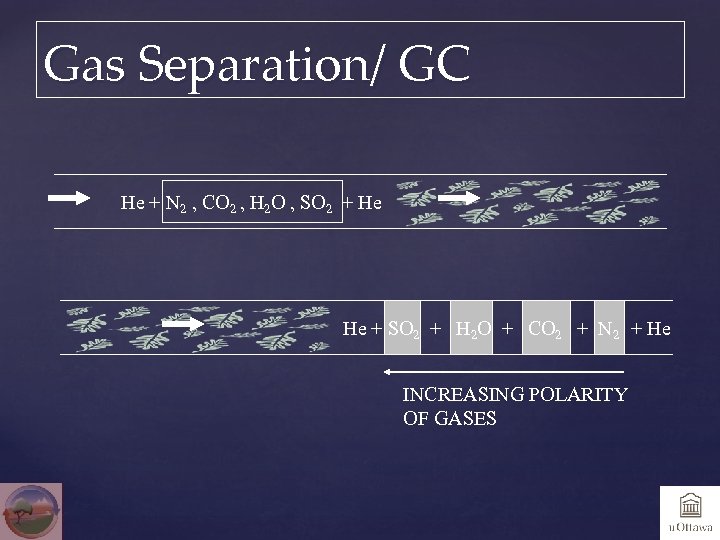 Gas Separation/ GC He + N 2 , CO 2 , H 2 O , SO 2 + He He + SO 2 + H 2 O + CO 2 + N 2 + He INCREASING POLARITY OF GASES
Gas Separation/ GC He + N 2 , CO 2 , H 2 O , SO 2 + He He + SO 2 + H 2 O + CO 2 + N 2 + He INCREASING POLARITY OF GASES
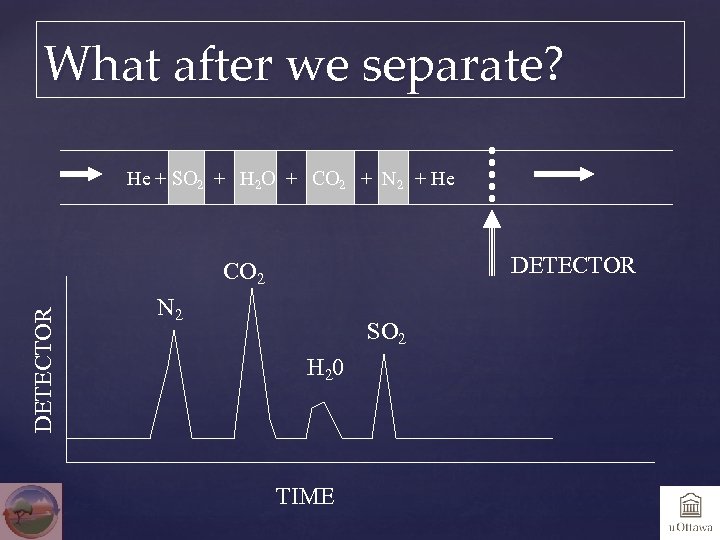 What after we separate? He + SO 2 + H 2 O + CO 2 + N 2 + He DETECTOR CO 2 N 2 SO 2 H 20 TIME
What after we separate? He + SO 2 + H 2 O + CO 2 + N 2 + He DETECTOR CO 2 N 2 SO 2 H 20 TIME
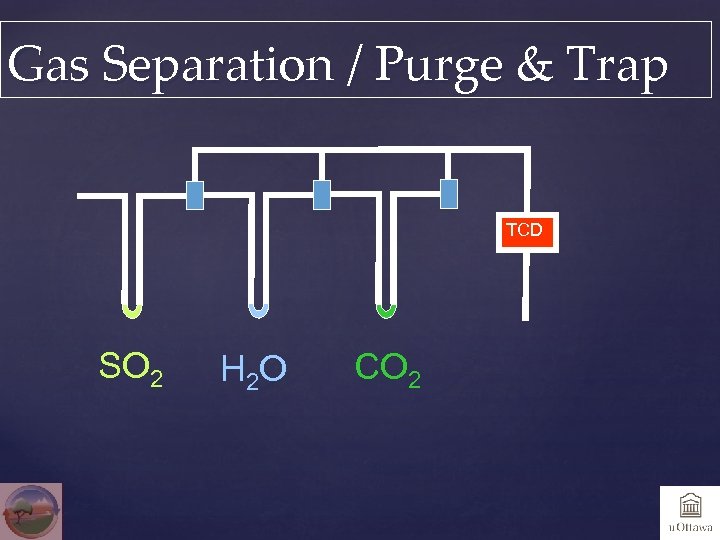 Gas Separation / Purge & Trap TCD SO 2 H 2 O CO 2
Gas Separation / Purge & Trap TCD SO 2 H 2 O CO 2
 Detectors Thermal conductivity detector (TCD) Heated filament from which heat is removed at a constant rate by He gas stream. Change in heat transfer is caused by presence of analyte molecules with different thermal conductivities than He. Relatively low sensitivity, excellent range and linearity. Non-destructive. Electron capture detector (ECD) Electrons are captured by organic species in ionized carrier; used for trace sulfur determination. Isotope Ratio Mass Spectrometer (IRMS)
Detectors Thermal conductivity detector (TCD) Heated filament from which heat is removed at a constant rate by He gas stream. Change in heat transfer is caused by presence of analyte molecules with different thermal conductivities than He. Relatively low sensitivity, excellent range and linearity. Non-destructive. Electron capture detector (ECD) Electrons are captured by organic species in ionized carrier; used for trace sulfur determination. Isotope Ratio Mass Spectrometer (IRMS)
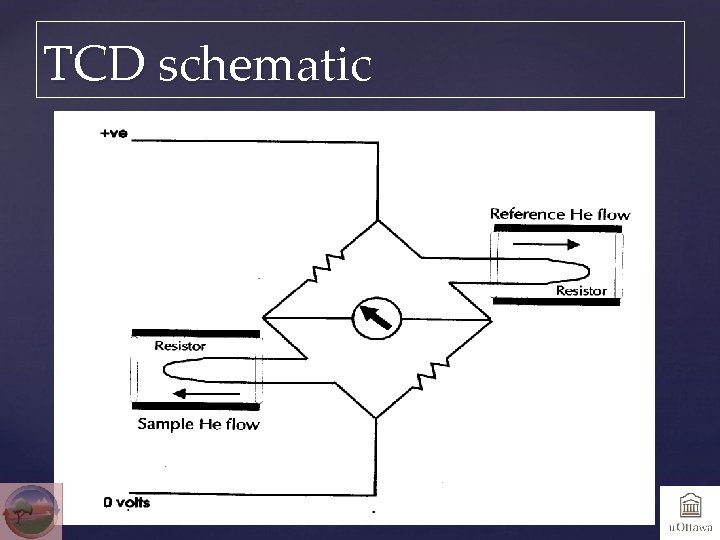 TCD schematic
TCD schematic
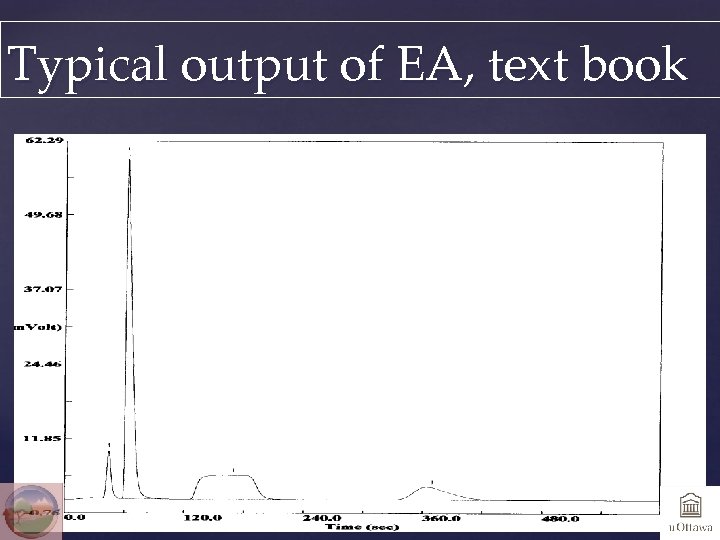 Typical output of EA, text book CO 2 N 2 H 2 O SO 2
Typical output of EA, text book CO 2 N 2 H 2 O SO 2
 Balance: Get the best A 0. 01 mg readability translates in an error of 0. 25% on a 2 mg sample (say on 100% carbon)
Balance: Get the best A 0. 01 mg readability translates in an error of 0. 25% on a 2 mg sample (say on 100% carbon)
 IAEA (and NIST) standards Links available on Isogeochem
IAEA (and NIST) standards Links available on Isogeochem
 STD: Making it on your own… Best : %N, 15 N, %C, 13 C Test different materials and pray Make your own: Caffeine L-glutamic acid Mixtures such as sucrose + potassium nitrate Mix in solution of natural + enriched/depleted material then dried, powdered, sieved
STD: Making it on your own… Best : %N, 15 N, %C, 13 C Test different materials and pray Make your own: Caffeine L-glutamic acid Mixtures such as sucrose + potassium nitrate Mix in solution of natural + enriched/depleted material then dried, powdered, sieved
 STD: Not making it on your own…
STD: Not making it on your own…
 STD: Not making it on your own… Can also contact other university labs to buy or obtain their internal standards.
STD: Not making it on your own… Can also contact other university labs to buy or obtain their internal standards.
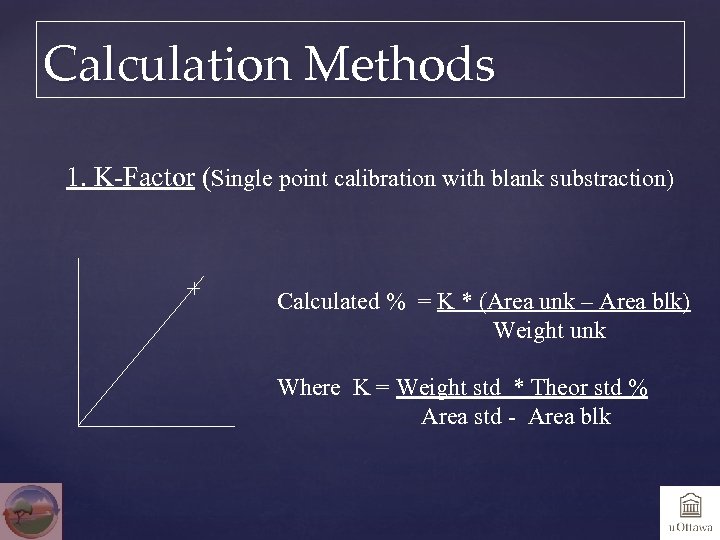 Calculation Methods 1. K-Factor (Single point calibration with blank substraction) Calculated % = K * (Area unk – Area blk) Weight unk Where K = Weight std * Theor std % Area std - Area blk
Calculation Methods 1. K-Factor (Single point calibration with blank substraction) Calculated % = K * (Area unk – Area blk) Weight unk Where K = Weight std * Theor std % Area std - Area blk
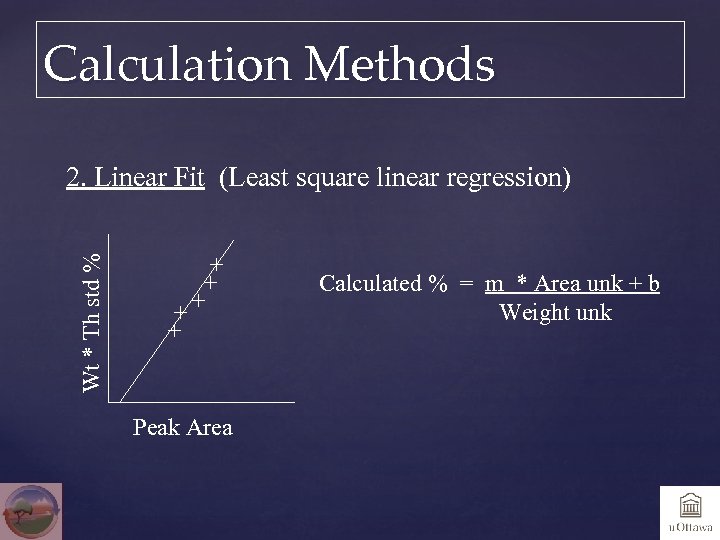 Calculation Methods Wt * Th std % 2. Linear Fit (Least square linear regression) Calculated % = m * Area unk + b Weight unk Peak Area
Calculation Methods Wt * Th std % 2. Linear Fit (Least square linear regression) Calculated % = m * Area unk + b Weight unk Peak Area
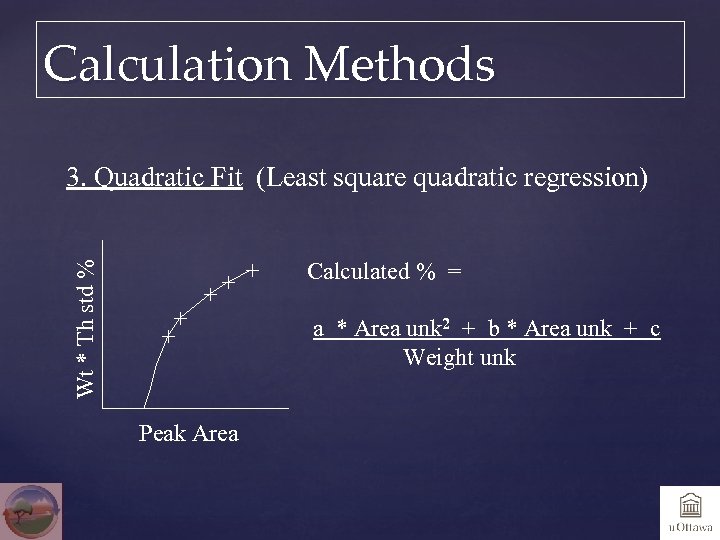 Calculation Methods 3. Quadratic Fit (Least square quadratic regression) Wt * Th std % Calculated % = a * Area unk 2 + b * Area unk + c Weight unk Peak Area
Calculation Methods 3. Quadratic Fit (Least square quadratic regression) Wt * Th std % Calculated % = a * Area unk 2 + b * Area unk + c Weight unk Peak Area
 An update… Most elemental analysers manufacturers have considerably refined their calculation methods, with more complex algorithms, low-high ranges and statistical tools.
An update… Most elemental analysers manufacturers have considerably refined their calculation methods, with more complex algorithms, low-high ranges and statistical tools.
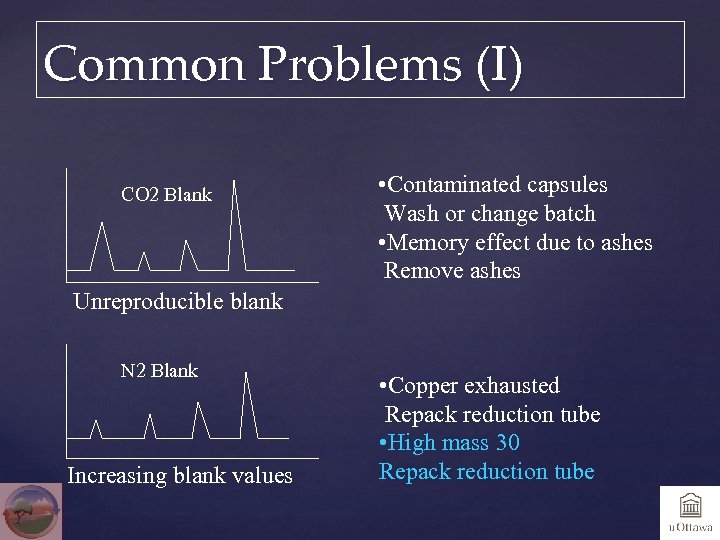 Common Problems (I) CO 2 Blank • Contaminated capsules Wash or change batch • Memory effect due to ashes Remove ashes Unreproducible blank N 2 Blank Increasing blank values • Copper exhausted Repack reduction tube • High mass 30 Repack reduction tube
Common Problems (I) CO 2 Blank • Contaminated capsules Wash or change batch • Memory effect due to ashes Remove ashes Unreproducible blank N 2 Blank Increasing blank values • Copper exhausted Repack reduction tube • High mass 30 Repack reduction tube
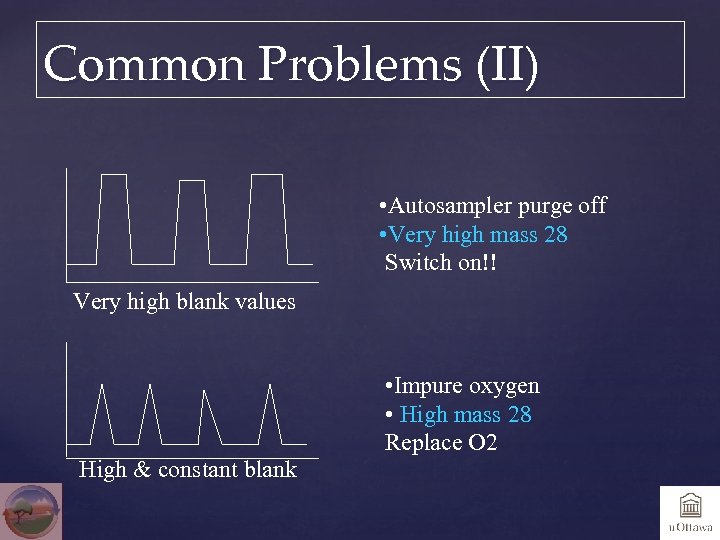 Common Problems (II) • Autosampler purge off • Very high mass 28 Switch on!! Very high blank values High & constant blank • Impure oxygen • High mass 28 Replace O 2
Common Problems (II) • Autosampler purge off • Very high mass 28 Switch on!! Very high blank values High & constant blank • Impure oxygen • High mass 28 Replace O 2
 Common Problems (III) No signal N 2 ? CO 2? Ghost/double peaks • Detector off Switch on! • Broken filament Call engineer • Low carrier flow Check flow rate & blockage • MS valve closed, open split problem • Leak Find and fix • CO 2 or H 2 O trap exhausted Replace packing of trap
Common Problems (III) No signal N 2 ? CO 2? Ghost/double peaks • Detector off Switch on! • Broken filament Call engineer • Low carrier flow Check flow rate & blockage • MS valve closed, open split problem • Leak Find and fix • CO 2 or H 2 O trap exhausted Replace packing of trap
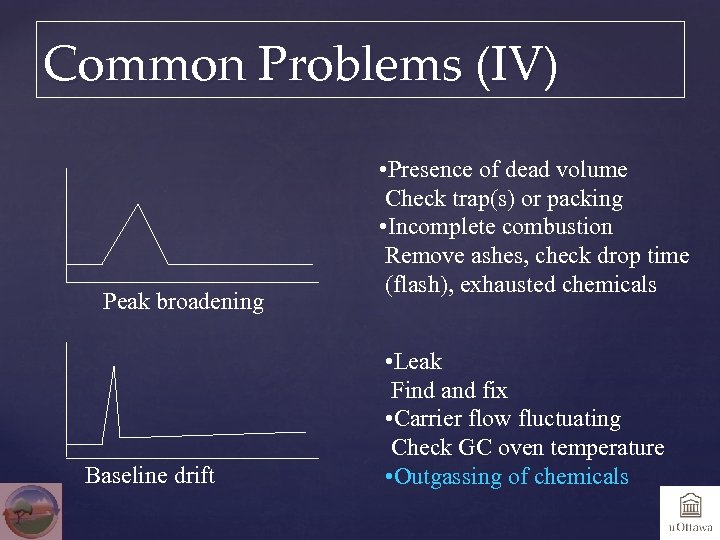 Common Problems (IV) Peak broadening Baseline drift • Presence of dead volume Check trap(s) or packing • Incomplete combustion Remove ashes, check drop time (flash), exhausted chemicals • Leak Find and fix • Carrier flow fluctuating Check GC oven temperature • Outgassing of chemicals
Common Problems (IV) Peak broadening Baseline drift • Presence of dead volume Check trap(s) or packing • Incomplete combustion Remove ashes, check drop time (flash), exhausted chemicals • Leak Find and fix • Carrier flow fluctuating Check GC oven temperature • Outgassing of chemicals
 Leak check or check leaks (1) Pressure check EA • Cap exit port • Increase P of He to 1. 3 bar, wait 3 minutes • Close regulator • Pressure gauge should not move for 5 minutes Flow check (requires electronic mass flow controller) EA • Cap exit port • Monitor flow for 3 minutes • Flow should drop to 0 ml/min
Leak check or check leaks (1) Pressure check EA • Cap exit port • Increase P of He to 1. 3 bar, wait 3 minutes • Close regulator • Pressure gauge should not move for 5 minutes Flow check (requires electronic mass flow controller) EA • Cap exit port • Monitor flow for 3 minutes • Flow should drop to 0 ml/min
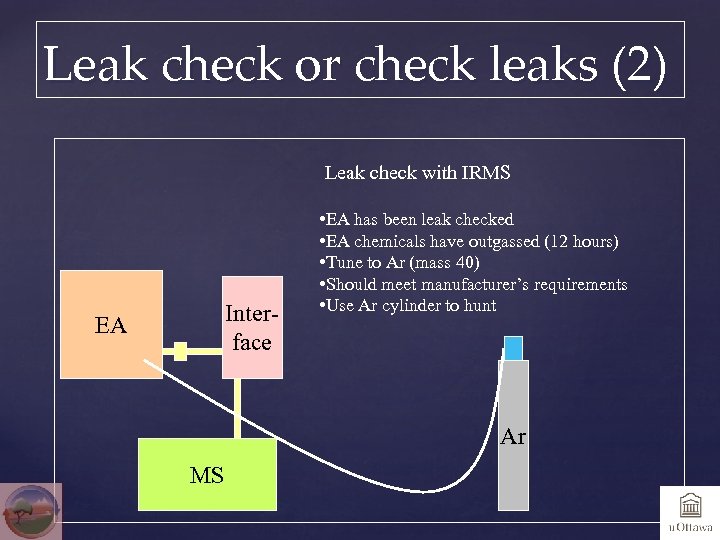 Leak check or check leaks (2) Leak check with IRMS Interface EA • EA has been leak checked • EA chemicals have outgassed (12 hours) • Tune to Ar (mass 40) • Should meet manufacturer’s requirements • Use Ar cylinder to hunt Ar MS
Leak check or check leaks (2) Leak check with IRMS Interface EA • EA has been leak checked • EA chemicals have outgassed (12 hours) • Tune to Ar (mass 40) • Should meet manufacturer’s requirements • Use Ar cylinder to hunt Ar MS
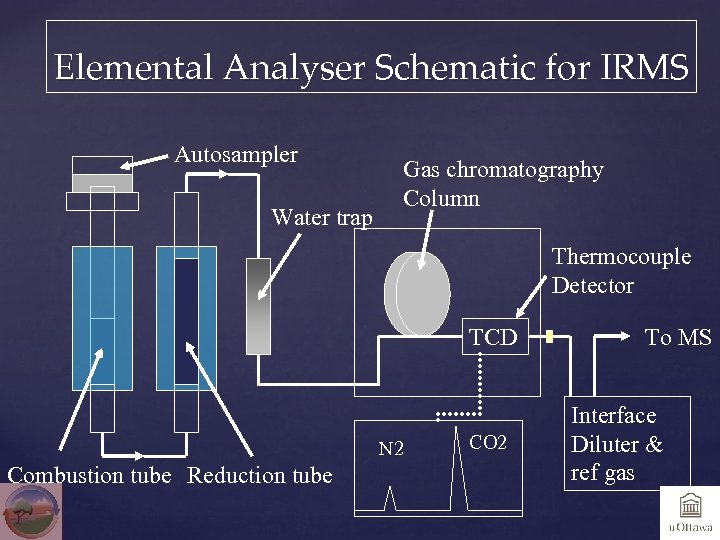 Elemental Analyser Schematic for IRMS Autosampler Water trap Gas chromatography Column Thermocouple Detector TCD N 2 Combustion tube Reduction tube CO 2 To MS Interface Diluter & ref gas
Elemental Analyser Schematic for IRMS Autosampler Water trap Gas chromatography Column Thermocouple Detector TCD N 2 Combustion tube Reduction tube CO 2 To MS Interface Diluter & ref gas
 WAKE UP! Did you find the small error in the last slide?
WAKE UP! Did you find the small error in the last slide?
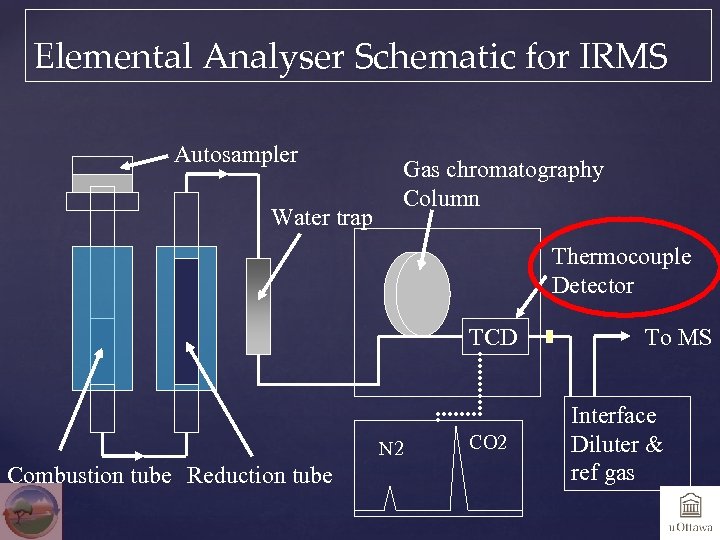 Elemental Analyser Schematic for IRMS Autosampler Water trap Gas chromatography Column Thermocouple Detector TCD N 2 Combustion tube Reduction tube CO 2 To MS Interface Diluter & ref gas
Elemental Analyser Schematic for IRMS Autosampler Water trap Gas chromatography Column Thermocouple Detector TCD N 2 Combustion tube Reduction tube CO 2 To MS Interface Diluter & ref gas
 Considerations for EA-IRMS Water is removed via Mg Perchlorate/sicapent trap ---water and mass spectrometer do not mix---Configuration should be optimized for gas of interest Leak free (mass 28 & 40, use Argon as leak probe) Low and stable background (mass 28, 18, 40, 44) Dynamic range must be respected, do use target beam Best sequence is carefully planned ie known concentration of samples Garbage in……Garbage out
Considerations for EA-IRMS Water is removed via Mg Perchlorate/sicapent trap ---water and mass spectrometer do not mix---Configuration should be optimized for gas of interest Leak free (mass 28 & 40, use Argon as leak probe) Low and stable background (mass 28, 18, 40, 44) Dynamic range must be respected, do use target beam Best sequence is carefully planned ie known concentration of samples Garbage in……Garbage out
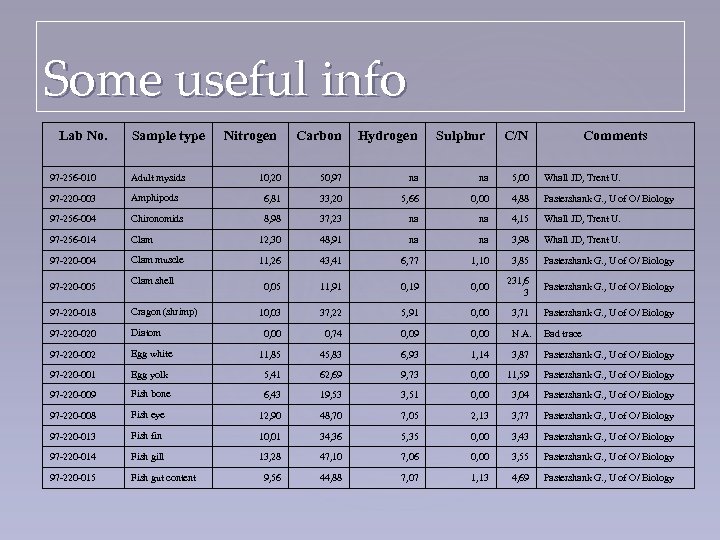 Some useful info Lab No. Sample type Nitrogen Carbon Hydrogen Sulphur C/N Comments 10, 20 50, 97 na na 5, 00 Whall JD, Trent U. 97 -256 -010 Adult mysids 97 -220 -003 Amphipods 6, 81 33, 20 5, 66 0, 00 4, 88 Pastershank G. , U of O/ Biology 97 -256 -004 Chironomids 8, 98 37, 23 na na 4, 15 Whall JD, Trent U. 97 -256 -014 Clam 12, 30 48, 91 na na 3, 98 Whall JD, Trent U. 97 -220 -004 Clam muscle 11, 26 43, 41 6, 77 1, 10 3, 85 Pastershank G. , U of O/ Biology 0, 05 11, 91 0, 19 0, 00 231, 6 3 Pastershank G. , U of O/ Biology 10, 03 37, 22 5, 91 0, 00 3, 71 Pastershank G. , U of O/ Biology 0, 00 0, 74 0, 09 0, 00 N. A. Bad trace 97 -220 -005 Clam shell 97 -220 -018 Cragon (shrimp) 97 -220 -020 Diatom 97 -220 -002 Egg white 11, 85 45, 83 6, 93 1, 14 3, 87 Pastershank G. , U of O/ Biology 97 -220 -001 Egg yolk 5, 41 62, 69 9, 73 0, 00 11, 59 Pastershank G. , U of O/ Biology 97 -220 -009 Fish bone 6, 43 19, 53 3, 51 0, 00 3, 04 Pastershank G. , U of O/ Biology 97 -220 -008 Fish eye 12, 90 48, 70 7, 05 2, 13 3, 77 Pastershank G. , U of O/ Biology 97 -220 -013 Fish fin 10, 01 34, 36 5, 35 0, 00 3, 43 Pastershank G. , U of O/ Biology 97 -220 -014 Fish gill 13, 28 47, 10 7, 06 0, 00 3, 55 Pastershank G. , U of O/ Biology 97 -220 -015 Fish gut content 9, 56 44, 88 7, 07 1, 13 4, 69 Pastershank G. , U of O/ Biology
Some useful info Lab No. Sample type Nitrogen Carbon Hydrogen Sulphur C/N Comments 10, 20 50, 97 na na 5, 00 Whall JD, Trent U. 97 -256 -010 Adult mysids 97 -220 -003 Amphipods 6, 81 33, 20 5, 66 0, 00 4, 88 Pastershank G. , U of O/ Biology 97 -256 -004 Chironomids 8, 98 37, 23 na na 4, 15 Whall JD, Trent U. 97 -256 -014 Clam 12, 30 48, 91 na na 3, 98 Whall JD, Trent U. 97 -220 -004 Clam muscle 11, 26 43, 41 6, 77 1, 10 3, 85 Pastershank G. , U of O/ Biology 0, 05 11, 91 0, 19 0, 00 231, 6 3 Pastershank G. , U of O/ Biology 10, 03 37, 22 5, 91 0, 00 3, 71 Pastershank G. , U of O/ Biology 0, 00 0, 74 0, 09 0, 00 N. A. Bad trace 97 -220 -005 Clam shell 97 -220 -018 Cragon (shrimp) 97 -220 -020 Diatom 97 -220 -002 Egg white 11, 85 45, 83 6, 93 1, 14 3, 87 Pastershank G. , U of O/ Biology 97 -220 -001 Egg yolk 5, 41 62, 69 9, 73 0, 00 11, 59 Pastershank G. , U of O/ Biology 97 -220 -009 Fish bone 6, 43 19, 53 3, 51 0, 00 3, 04 Pastershank G. , U of O/ Biology 97 -220 -008 Fish eye 12, 90 48, 70 7, 05 2, 13 3, 77 Pastershank G. , U of O/ Biology 97 -220 -013 Fish fin 10, 01 34, 36 5, 35 0, 00 3, 43 Pastershank G. , U of O/ Biology 97 -220 -014 Fish gill 13, 28 47, 10 7, 06 0, 00 3, 55 Pastershank G. , U of O/ Biology 97 -220 -015 Fish gut content 9, 56 44, 88 7, 07 1, 13 4, 69 Pastershank G. , U of O/ Biology
 Filters, we can do that! Best are quartz filters; they are stable. However they are more expensive and offer less choice. Silver filters are great too. Mostly glass filters, cheap, huge choice. How much to use?
Filters, we can do that! Best are quartz filters; they are stable. However they are more expensive and offer less choice. Silver filters are great too. Mostly glass filters, cheap, huge choice. How much to use?
 Filters, we can do that! Area = P*R 2 In this case, a punch is about 16 mm 2 and our whole filter is about 1490 mm 2. Our punch hole is roughly 1% of the filter.
Filters, we can do that! Area = P*R 2 In this case, a punch is about 16 mm 2 and our whole filter is about 1490 mm 2. Our punch hole is roughly 1% of the filter.
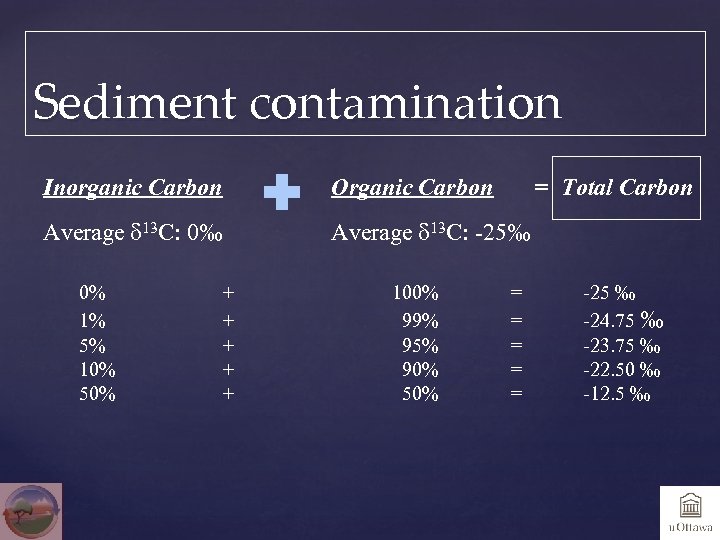 Sediment contamination Inorganic Carbon Organic Carbon Average d 13 C: 0‰ Average d 13 C: -25‰ 0% 1% 5% 10% 50% + + + 100% 99% 95% 90% 50% = Total Carbon = = = -25 ‰ -24. 75 ‰ -23. 75 ‰ -22. 50 ‰ -12. 5 ‰
Sediment contamination Inorganic Carbon Organic Carbon Average d 13 C: 0‰ Average d 13 C: -25‰ 0% 1% 5% 10% 50% + + + 100% 99% 95% 90% 50% = Total Carbon = = = -25 ‰ -24. 75 ‰ -23. 75 ‰ -22. 50 ‰ -12. 5 ‰
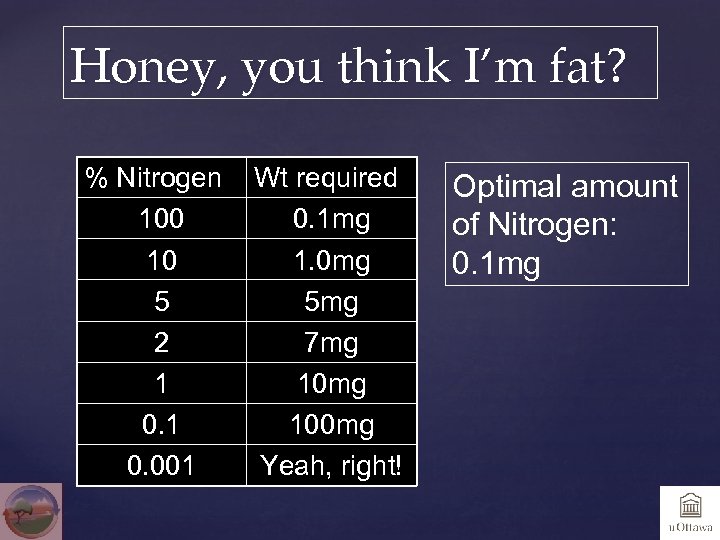 Honey, you think I’m fat? % Nitrogen 100 10 5 2 1 0. 001 Wt required 0. 1 mg 1. 0 mg 5 mg 7 mg 100 mg Yeah, right! Optimal amount of Nitrogen: 0. 1 mg
Honey, you think I’m fat? % Nitrogen 100 10 5 2 1 0. 001 Wt required 0. 1 mg 1. 0 mg 5 mg 7 mg 100 mg Yeah, right! Optimal amount of Nitrogen: 0. 1 mg
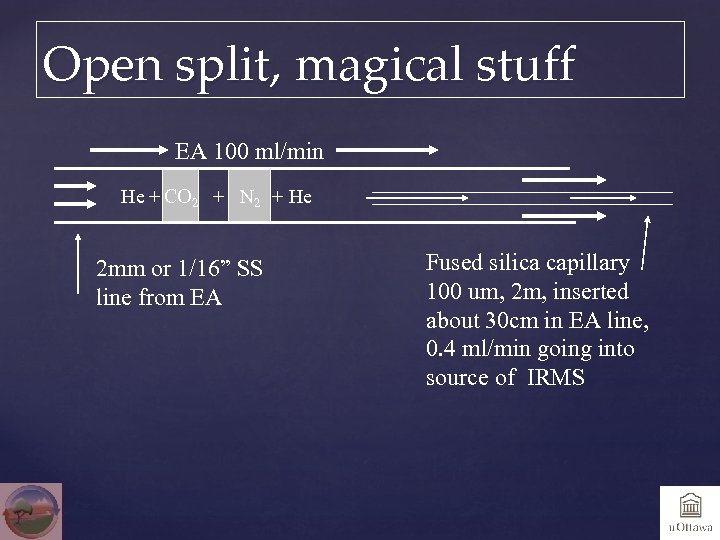 Open split, magical stuff EA 100 ml/min He + CO 2 + N 2 + He 2 mm or 1/16” SS line from EA Fused silica capillary 100 um, 2 m, inserted about 30 cm in EA line, 0. 4 ml/min going into source of IRMS
Open split, magical stuff EA 100 ml/min He + CO 2 + N 2 + He 2 mm or 1/16” SS line from EA Fused silica capillary 100 um, 2 m, inserted about 30 cm in EA line, 0. 4 ml/min going into source of IRMS
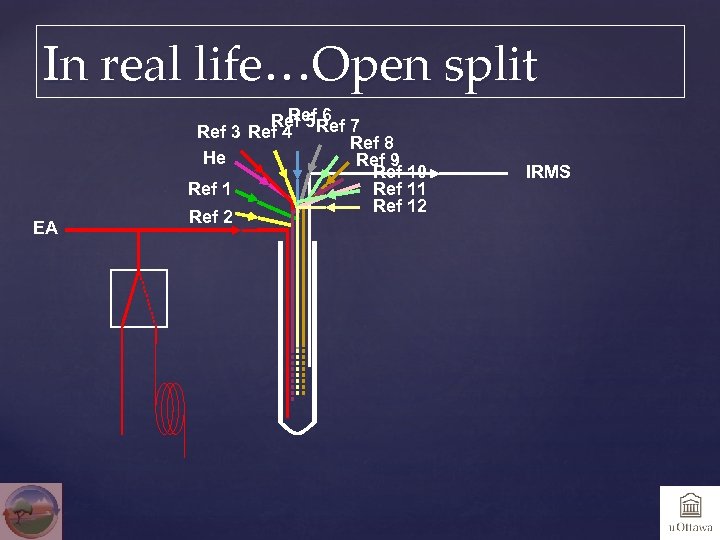 In real life…Open split EA Ref 6 Ref 5 Ref 7 Ref 3 Ref 4 Ref 8 He Ref 9 Ref 10 Ref 11 Ref 12 Ref 2 IRMS
In real life…Open split EA Ref 6 Ref 5 Ref 7 Ref 3 Ref 4 Ref 8 He Ref 9 Ref 10 Ref 11 Ref 12 Ref 2 IRMS
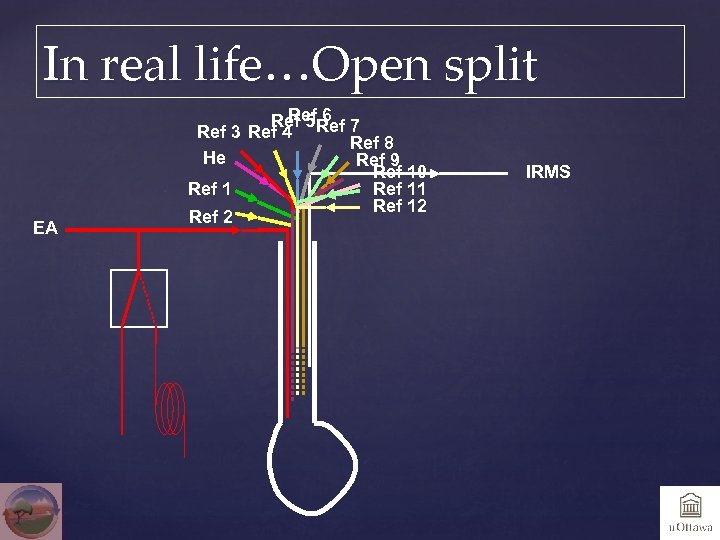 In real life…Open split EA Ref 6 Ref 5 Ref 7 Ref 3 Ref 4 Ref 8 He Ref 9 Ref 10 Ref 11 Ref 12 Ref 2 IRMS
In real life…Open split EA Ref 6 Ref 5 Ref 7 Ref 3 Ref 4 Ref 8 He Ref 9 Ref 10 Ref 11 Ref 12 Ref 2 IRMS
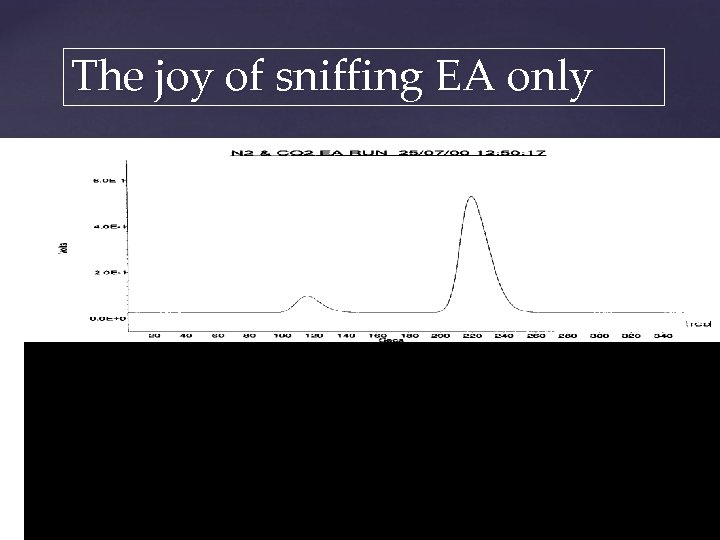 The joy of sniffing EA only CO 2 TCD output from EA N 2 Mass spectrometer output Mass 44, 45, 46 Mass 28, 29 Magnet Peakjump
The joy of sniffing EA only CO 2 TCD output from EA N 2 Mass spectrometer output Mass 44, 45, 46 Mass 28, 29 Magnet Peakjump
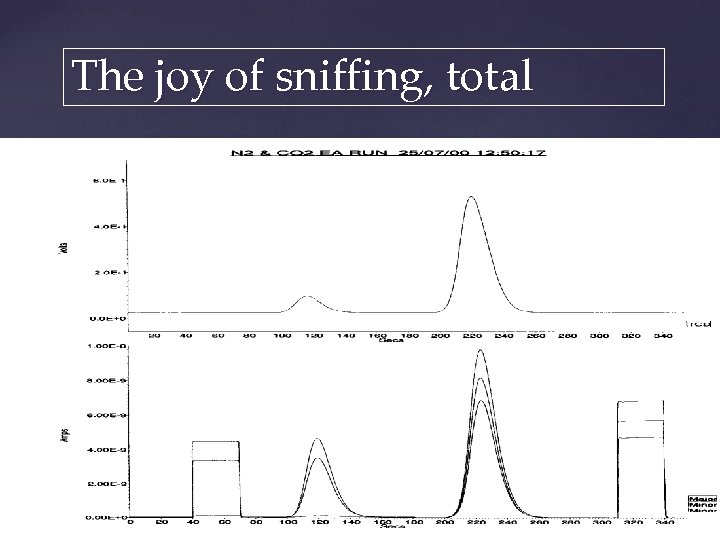 The joy of sniffing, total CO 2 TCD output from EA N 2 Mass spectrometer output Masses 28 & 29 Masses 44, 45 & 46 Mass 44, 45, 46 Mass 28, 29 Magnet Peakjump
The joy of sniffing, total CO 2 TCD output from EA N 2 Mass spectrometer output Masses 28 & 29 Masses 44, 45 & 46 Mass 44, 45, 46 Mass 28, 29 Magnet Peakjump
 To dilute or Not to dilute?
To dilute or Not to dilute?
 Second last suggestion I strongly recommend that you create a basic document for your users explaining the limits and pitfalls of EA-IRMS analysis, this will save you (and your users) an enormous amount of time.
Second last suggestion I strongly recommend that you create a basic document for your users explaining the limits and pitfalls of EA-IRMS analysis, this will save you (and your users) an enormous amount of time.



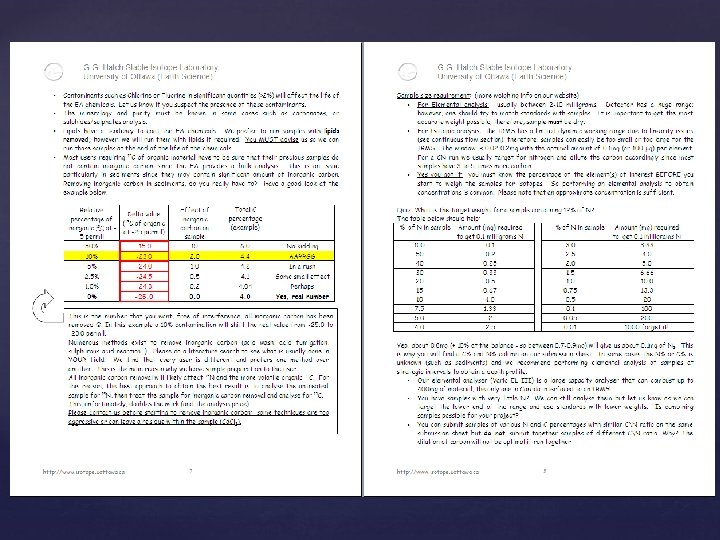

 Some unresolved issues with EA Removing inorganic carbon is not trivial Alkalies (Sodium, Potassium, Calcium) are difficult to combust, catalist is definitively required to bind and help with oxydation. Oxygen contribution to d 34 S is still a problem.
Some unresolved issues with EA Removing inorganic carbon is not trivial Alkalies (Sodium, Potassium, Calcium) are difficult to combust, catalist is definitively required to bind and help with oxydation. Oxygen contribution to d 34 S is still a problem.
 Last suggestion and only Official Endorsement The only product fully and officially endorsed by the author is: Rickard’s Red
Last suggestion and only Official Endorsement The only product fully and officially endorsed by the author is: Rickard’s Red
 Thank you for not snoring My thanks to: Wendy Abdi, Nik Binder, Fred Longstaffe, Scott Hughes, Gilles St-Jean, Peter Stow and Patricia Wickham for the use of material, brain power and time …
Thank you for not snoring My thanks to: Wendy Abdi, Nik Binder, Fred Longstaffe, Scott Hughes, Gilles St-Jean, Peter Stow and Patricia Wickham for the use of material, brain power and time …


