c6b1c8928efa58f24e5ffe3580aec7bc.ppt
- Количество слайдов: 51
 Electrons What causes what you are seeing?
Electrons What causes what you are seeing?
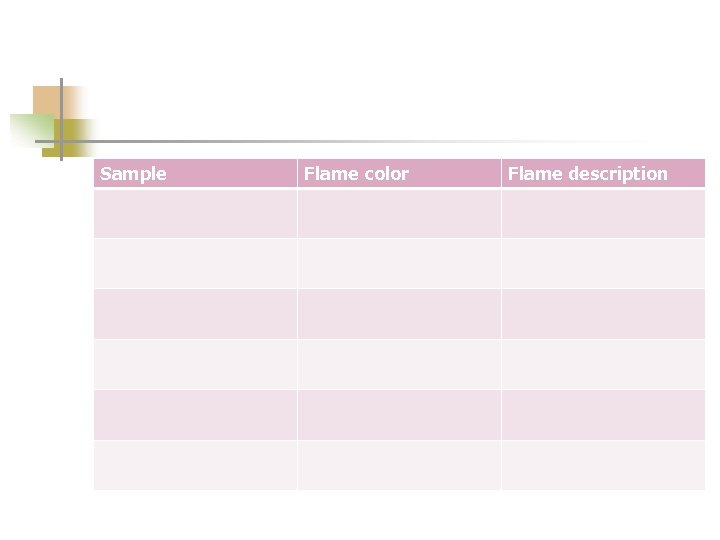 Sample Flame color Flame description
Sample Flame color Flame description
 Electron Configuration Mapping the electrons
Electron Configuration Mapping the electrons
 Electron Configuration n The way electrons are arranged around the nucleus.
Electron Configuration n The way electrons are arranged around the nucleus.
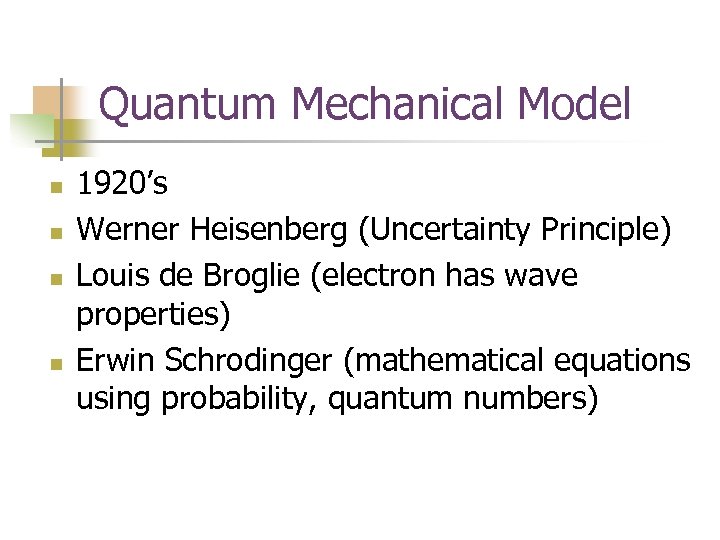 Quantum Mechanical Model n n 1920’s Werner Heisenberg (Uncertainty Principle) Louis de Broglie (electron has wave properties) Erwin Schrodinger (mathematical equations using probability, quantum numbers)
Quantum Mechanical Model n n 1920’s Werner Heisenberg (Uncertainty Principle) Louis de Broglie (electron has wave properties) Erwin Schrodinger (mathematical equations using probability, quantum numbers)
 Heisenberg uncertainty principle n it is impossible to determine simultaneously both the position and velocity of an electron or any other particle with any great degree of accuracy or certainty.
Heisenberg uncertainty principle n it is impossible to determine simultaneously both the position and velocity of an electron or any other particle with any great degree of accuracy or certainty.
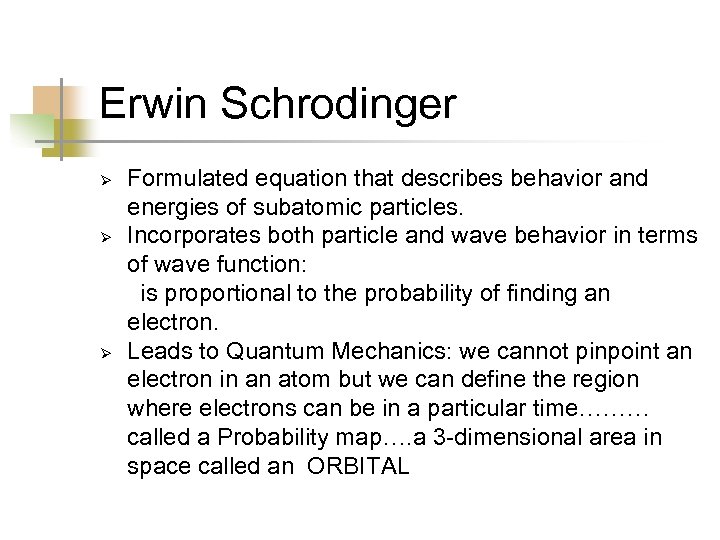 Erwin Schrodinger Ø Ø Ø Formulated equation that describes behavior and energies of subatomic particles. Incorporates both particle and wave behavior in terms of wave function: is proportional to the probability of finding an electron. Leads to Quantum Mechanics: we cannot pinpoint an electron in an atom but we can define the region where electrons can be in a particular time……… called a Probability map…. a 3 -dimensional area in space called an ORBITAL
Erwin Schrodinger Ø Ø Ø Formulated equation that describes behavior and energies of subatomic particles. Incorporates both particle and wave behavior in terms of wave function: is proportional to the probability of finding an electron. Leads to Quantum Mechanics: we cannot pinpoint an electron in an atom but we can define the region where electrons can be in a particular time……… called a Probability map…. a 3 -dimensional area in space called an ORBITAL
 Like building a house…. . n Orbitals are like a house. There are floors, rooms, closets and balconies. n Keep that in mind…… n
Like building a house…. . n Orbitals are like a house. There are floors, rooms, closets and balconies. n Keep that in mind…… n
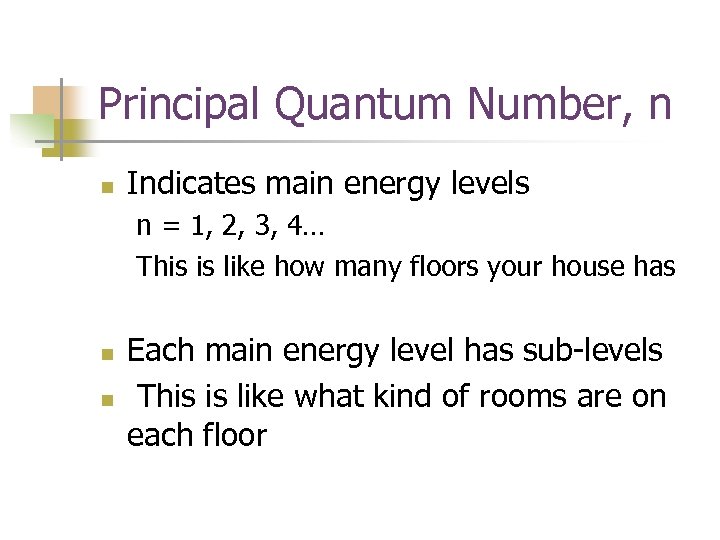 Principal Quantum Number, n n Indicates main energy levels n = 1, 2, 3, 4… This is like how many floors your house has n n Each main energy level has sub-levels This is like what kind of rooms are on each floor
Principal Quantum Number, n n Indicates main energy levels n = 1, 2, 3, 4… This is like how many floors your house has n n Each main energy level has sub-levels This is like what kind of rooms are on each floor
 Energy Sublevels s p d f
Energy Sublevels s p d f
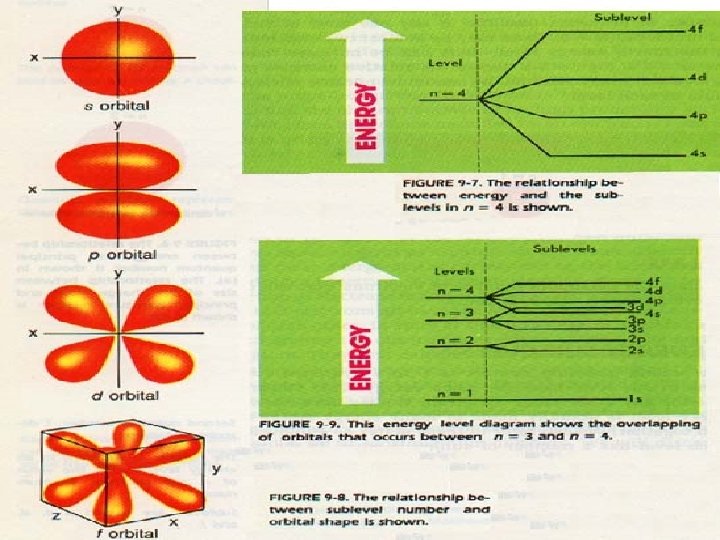
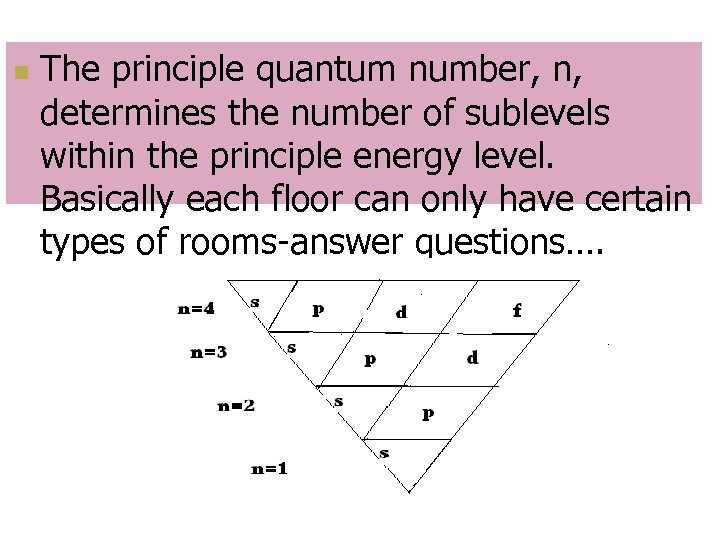 n The principle quantum number, n, determines the number of sublevels within the principle energy level. Basically each floor can only have certain types of rooms-answer questions….
n The principle quantum number, n, determines the number of sublevels within the principle energy level. Basically each floor can only have certain types of rooms-answer questions….
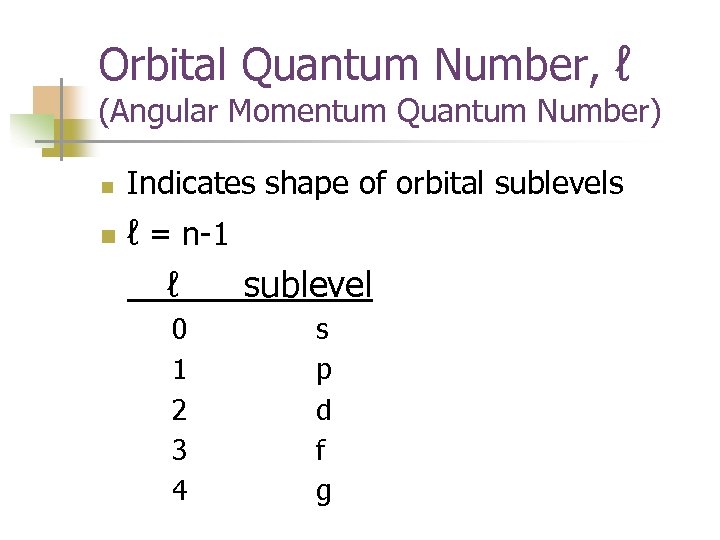 Orbital Quantum Number, ℓ (Angular Momentum Quantum Number) n n Indicates shape of orbital sublevels ℓ = n-1 ℓ sublevel 0 1 2 3 4 s p d f g
Orbital Quantum Number, ℓ (Angular Momentum Quantum Number) n n Indicates shape of orbital sublevels ℓ = n-1 ℓ sublevel 0 1 2 3 4 s p d f g
 Orbital n n The space where there is a high probability that it is occupied by a pair of electrons. How many in a pair? Orbitals are solutions of Schrodinger’s equations.
Orbital n n The space where there is a high probability that it is occupied by a pair of electrons. How many in a pair? Orbitals are solutions of Schrodinger’s equations.
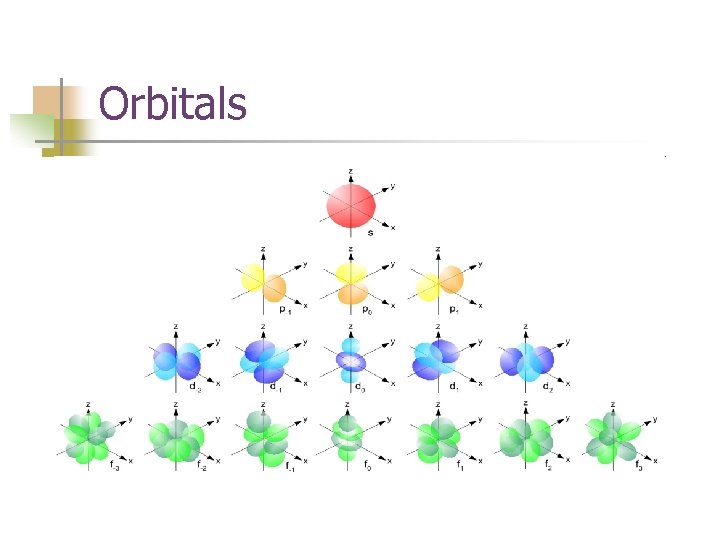 Orbitals
Orbitals
 Visualizing the orbitals n n Back to the house…… Orbitals are the rooms that hold 2 people per room.
Visualizing the orbitals n n Back to the house…… Orbitals are the rooms that hold 2 people per room.
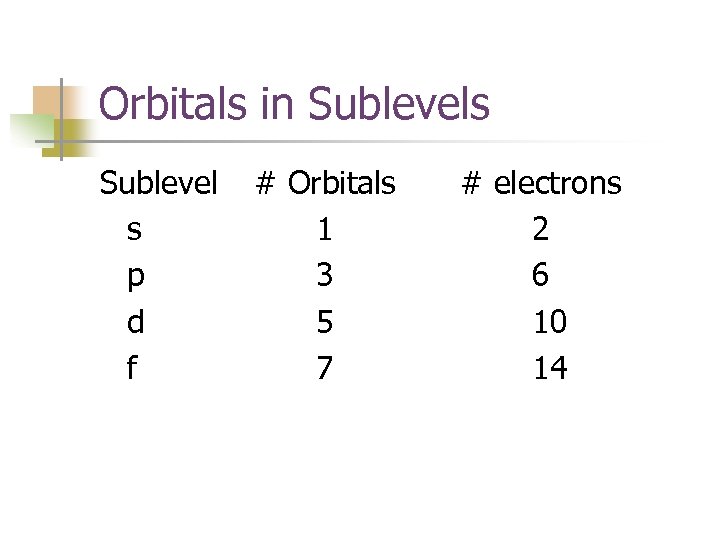 Orbitals in Sublevels Sublevel # Orbitals s 1 p 3 d 5 f 7 # electrons 2 6 10 14
Orbitals in Sublevels Sublevel # Orbitals s 1 p 3 d 5 f 7 # electrons 2 6 10 14
 Three rules are used to build the electron configuration: Aufbau principle n Pauli Exclusion Principle n Hund’s Rule n
Three rules are used to build the electron configuration: Aufbau principle n Pauli Exclusion Principle n Hund’s Rule n
 Aufbau Principle Electrons occupy orbitals of lower energy first. n Like building a house- lower floors fill up first n
Aufbau Principle Electrons occupy orbitals of lower energy first. n Like building a house- lower floors fill up first n
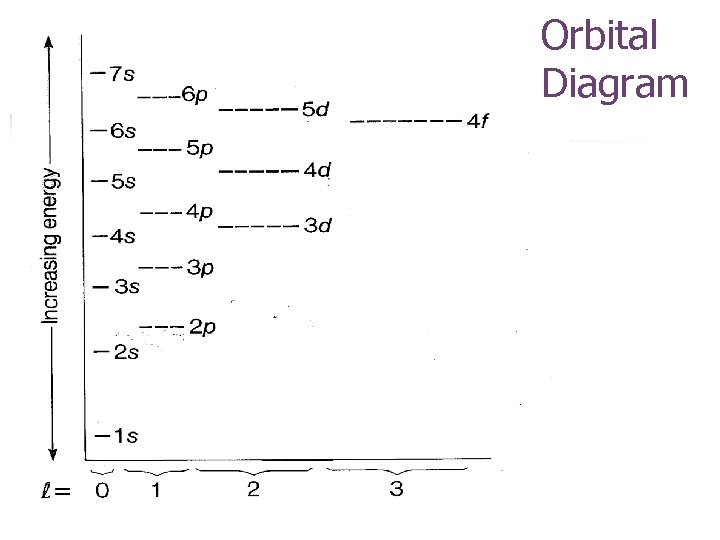 Orbital Diagram
Orbital Diagram
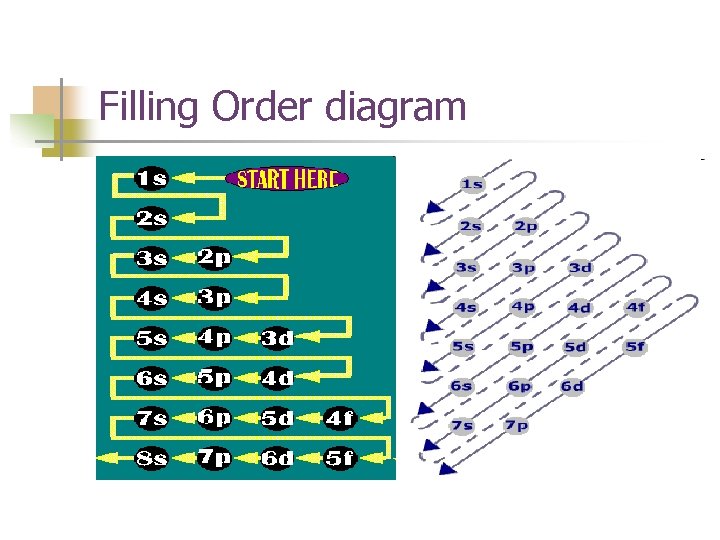 Filling Order diagram
Filling Order diagram
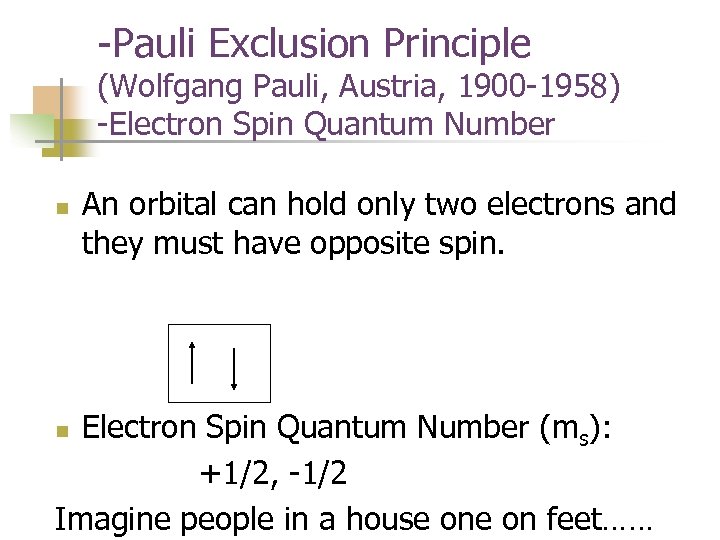 -Pauli Exclusion Principle (Wolfgang Pauli, Austria, 1900 -1958) -Electron Spin Quantum Number n An orbital can hold only two electrons and they must have opposite spin. Electron Spin Quantum Number (ms): +1/2, -1/2 Imagine people in a house on feet…… n
-Pauli Exclusion Principle (Wolfgang Pauli, Austria, 1900 -1958) -Electron Spin Quantum Number n An orbital can hold only two electrons and they must have opposite spin. Electron Spin Quantum Number (ms): +1/2, -1/2 Imagine people in a house on feet…… n
 Hund’s Rule In a set of orbitals, the electrons will fill the orbitals in a way that would give the maximum number of parallel spins (maximum number of unpaired electrons). Analogy: Students could fill each seat of a school bus, one person at a time, before doubling up.
Hund’s Rule In a set of orbitals, the electrons will fill the orbitals in a way that would give the maximum number of parallel spins (maximum number of unpaired electrons). Analogy: Students could fill each seat of a school bus, one person at a time, before doubling up.
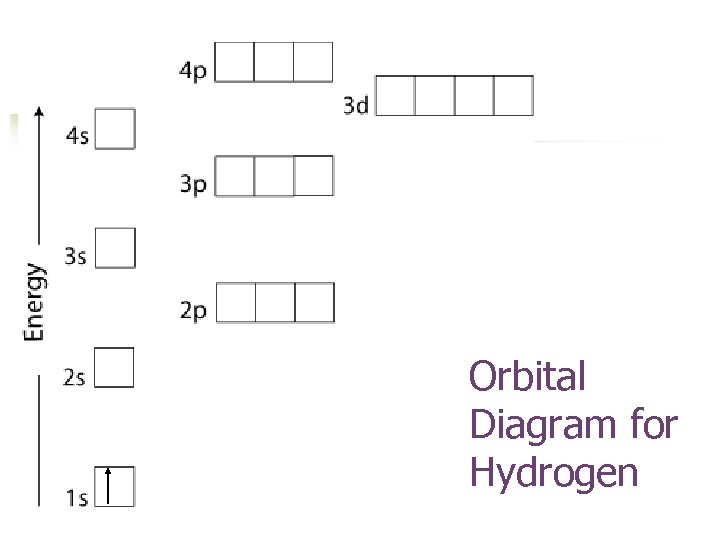 Orbital Diagram for Hydrogen
Orbital Diagram for Hydrogen
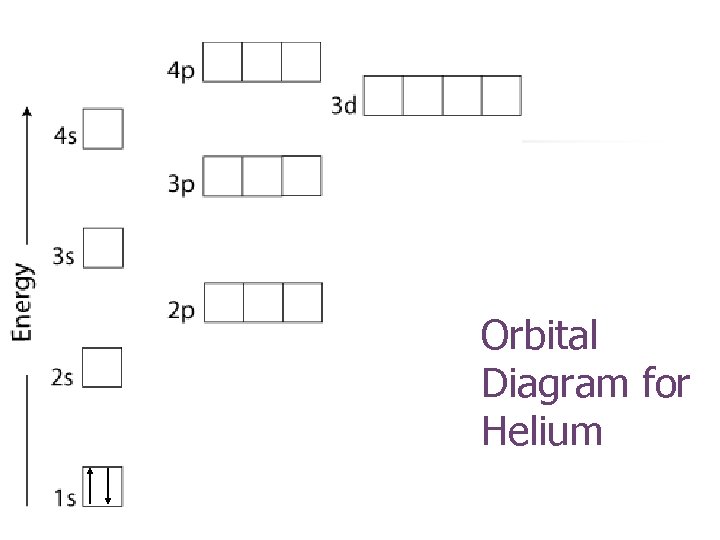 Orbital Diagram for Helium
Orbital Diagram for Helium
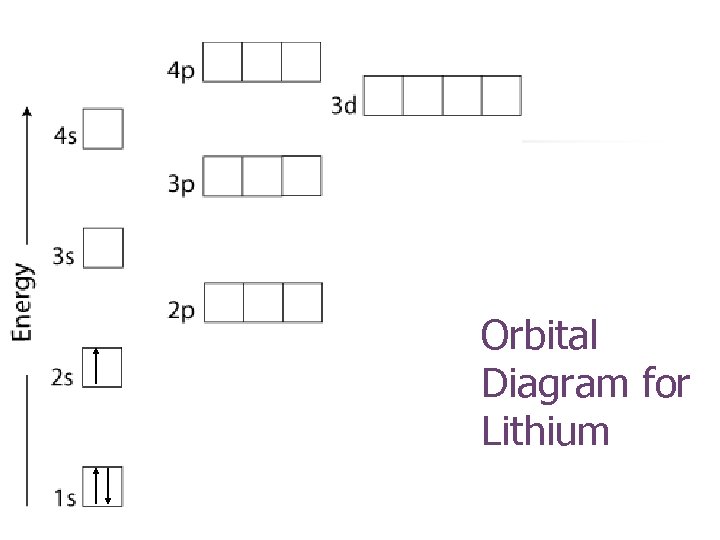 Orbital Diagram for Lithium
Orbital Diagram for Lithium
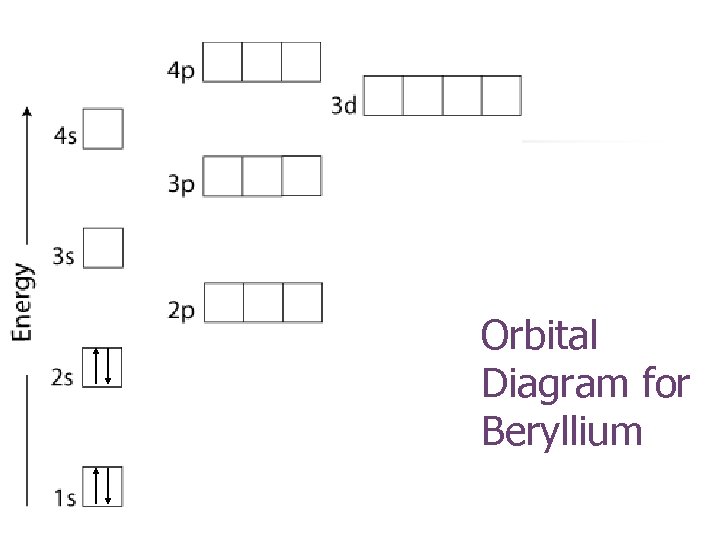 Orbital Diagram for Beryllium
Orbital Diagram for Beryllium
 Orbital Diagram for Boron
Orbital Diagram for Boron
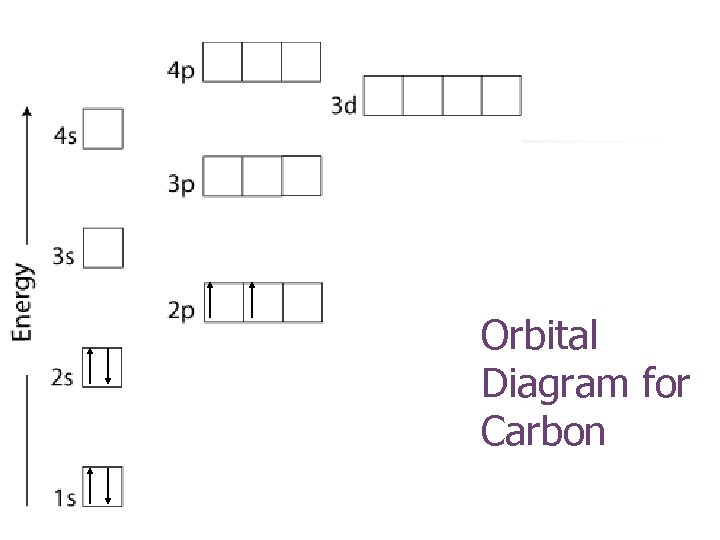 Orbital Diagram for Carbon
Orbital Diagram for Carbon
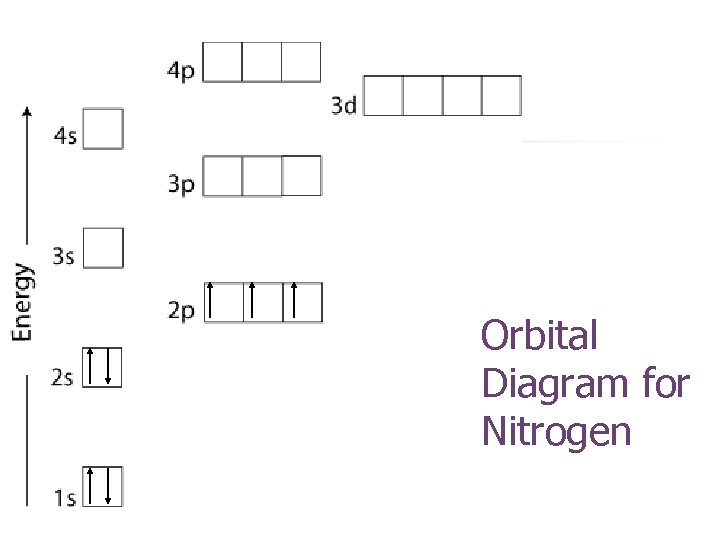 Orbital Diagram for Nitrogen
Orbital Diagram for Nitrogen
 Rappers Resort
Rappers Resort
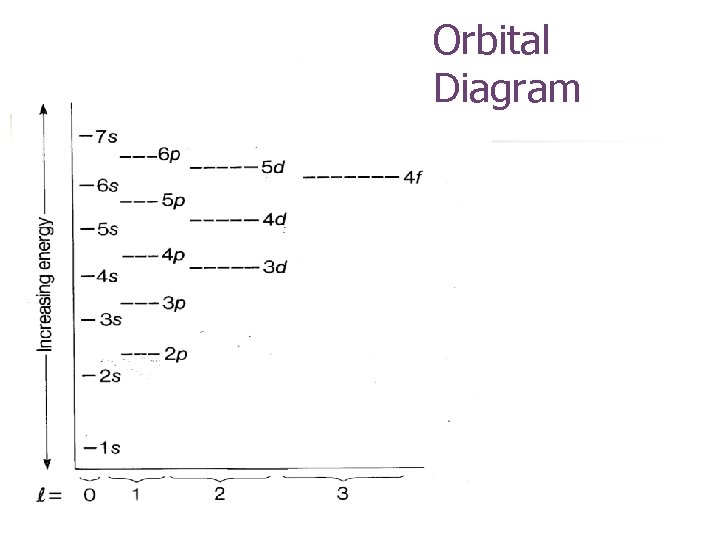 Orbital Diagram
Orbital Diagram
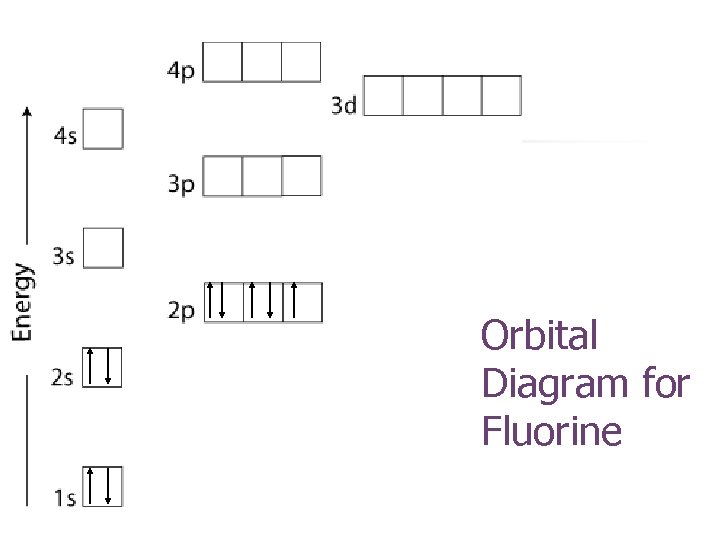 Orbital Diagram for Fluorine
Orbital Diagram for Fluorine
 Notations of Electron Configurations n n Standard Shorthand
Notations of Electron Configurations n n Standard Shorthand
 Standard Notation – electron configurations n n n If I had to send mail to someone living in the house you just built how would I address the envelope? I would have to do the following. Floors I have to climb to get to you Type of rooms I pass How many people are living in the rooms
Standard Notation – electron configurations n n n If I had to send mail to someone living in the house you just built how would I address the envelope? I would have to do the following. Floors I have to climb to get to you Type of rooms I pass How many people are living in the rooms
 Match up n Who is who in this situation? Floors I have to climb electrons Type of rooms I pass Principle energy levels How many people are living orbitals in the rooms
Match up n Who is who in this situation? Floors I have to climb electrons Type of rooms I pass Principle energy levels How many people are living orbitals in the rooms
 ALWAYS!!! ROW BLOCK # OF BLOCKS
ALWAYS!!! ROW BLOCK # OF BLOCKS
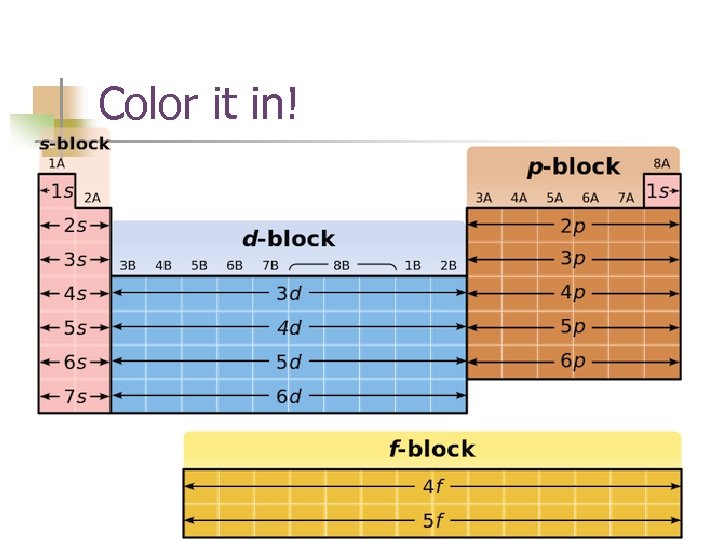 Color it in!
Color it in!
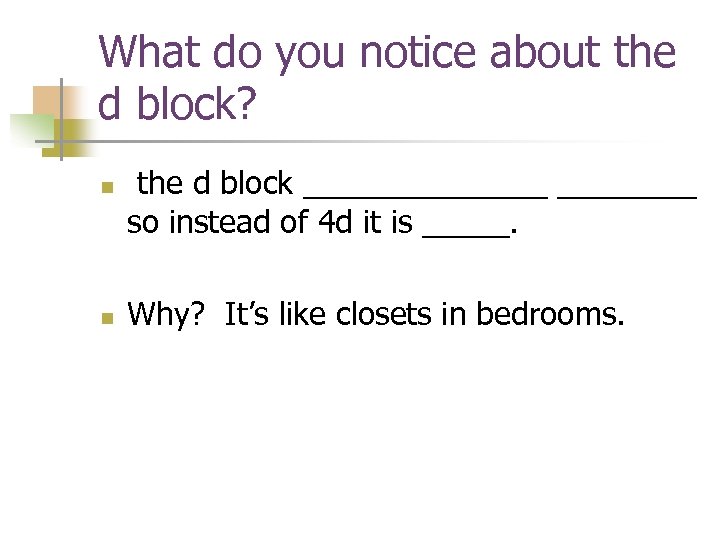 What do you notice about the d block? n n the d block _______ so instead of 4 d it is _____. Why? It’s like closets in bedrooms.
What do you notice about the d block? n n the d block _______ so instead of 4 d it is _____. Why? It’s like closets in bedrooms.
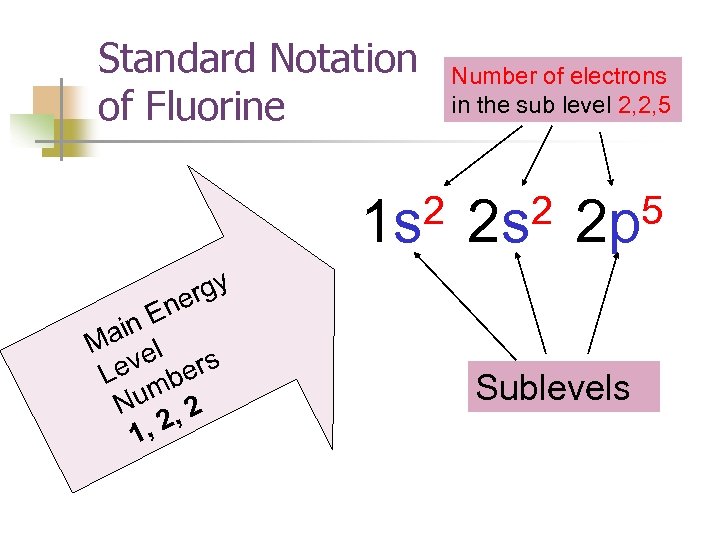 Standard Notation of Fluorine 2 1 s rgy ne in E Ma el ev bers L um 2 N , , 2 1 Number of electrons in the sub level 2, 2, 5 2 2 s 5 2 p Sublevels
Standard Notation of Fluorine 2 1 s rgy ne in E Ma el ev bers L um 2 N , , 2 1 Number of electrons in the sub level 2, 2, 5 2 2 s 5 2 p Sublevels
 Practice n He Li n P n O n Fe n
Practice n He Li n P n O n Fe n
 Shorthand Notation- Noble gas configurations n n Use the last noble gas that is located in the periodic table right before the element. Write the symbol of the noble gas in brackets. Write the remaining configuration after the brackets. Ex: Fluorine: [He] 2 s 2 2 p 5
Shorthand Notation- Noble gas configurations n n Use the last noble gas that is located in the periodic table right before the element. Write the symbol of the noble gas in brackets. Write the remaining configuration after the brackets. Ex: Fluorine: [He] 2 s 2 2 p 5
 Practice n n Ca Al Br Cu
Practice n n Ca Al Br Cu
 Lewis Dot Structures n Models to show many valence electrons are in the A group elements. This doesn’t apply to group B n Group # = Dots! n
Lewis Dot Structures n Models to show many valence electrons are in the A group elements. This doesn’t apply to group B n Group # = Dots! n
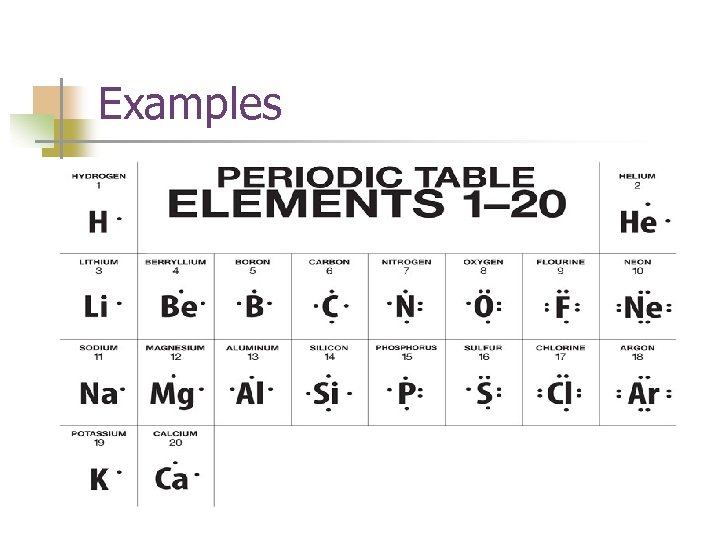 Examples
Examples
 You try! n Xe n Cs n Sr n I
You try! n Xe n Cs n Sr n I
 Electrons get excited! n n Electrons can gain energy and move to an excited state and emit energy in specific wavelengths of light we can see! How?
Electrons get excited! n n Electrons can gain energy and move to an excited state and emit energy in specific wavelengths of light we can see! How?
 Ground state n n Electrons have a ground state – or a state in which they normally exist. When energy is added to the system they jump to the next level ON the RETURN to ground level they emit light in our visible spectrum.
Ground state n n Electrons have a ground state – or a state in which they normally exist. When energy is added to the system they jump to the next level ON the RETURN to ground level they emit light in our visible spectrum.
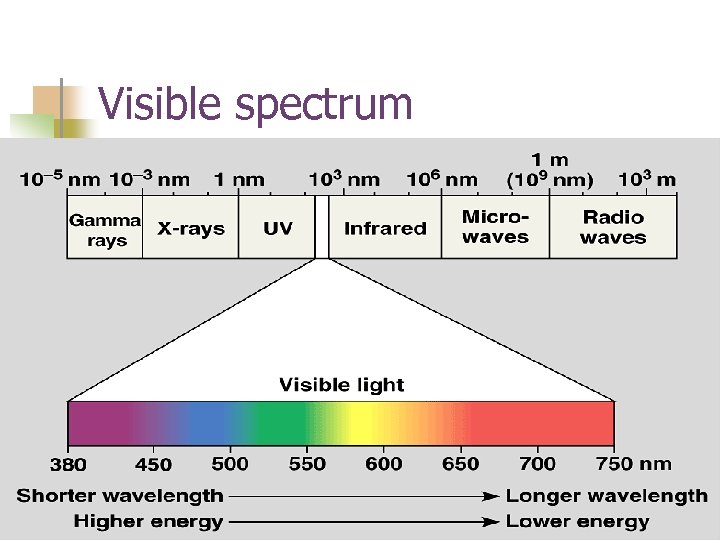 Visible spectrum
Visible spectrum
 Flame test. n n We are going to solve a crime……. See flame test worksheets
Flame test. n n We are going to solve a crime……. See flame test worksheets
 That’s it n Good luck on your test!
That’s it n Good luck on your test!


