3f0b3bd9279f0f1039d152349f9069dc.ppt
- Количество слайдов: 26
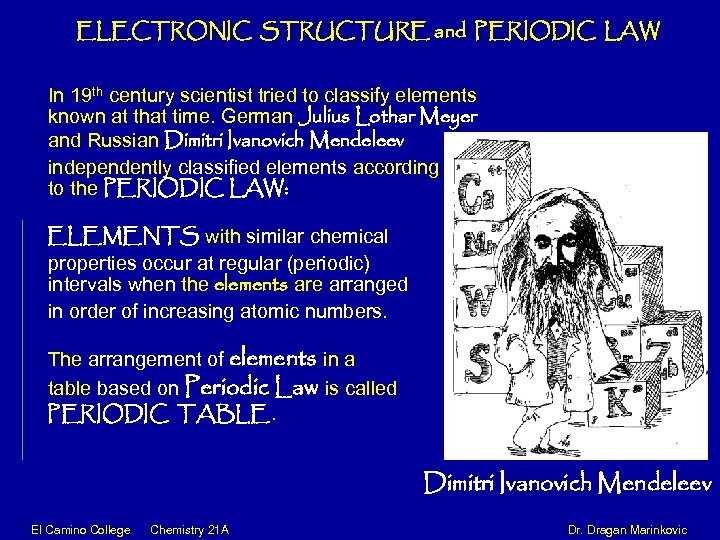 ELECTRONIC STRUCTURE and PERIODIC LAW In 19 th century scientist tried to classify elements known at that time. German Julius Lothar Meyer and Russian Dimitri Ivanovich Mendeleev independently classified elements according to the PERIODIC LAW: ELEMENTS with similar chemical properties occur at regular (periodic) intervals when the elements are arranged in order of increasing atomic numbers. The arrangement of elements in a table based on Periodic Law is called PERIODIC TABLE. Dimitri Ivanovich Mendeleev El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW In 19 th century scientist tried to classify elements known at that time. German Julius Lothar Meyer and Russian Dimitri Ivanovich Mendeleev independently classified elements according to the PERIODIC LAW: ELEMENTS with similar chemical properties occur at regular (periodic) intervals when the elements are arranged in order of increasing atomic numbers. The arrangement of elements in a table based on Periodic Law is called PERIODIC TABLE. Dimitri Ivanovich Mendeleev El Camino College Chemistry 21 A Dr. Dragan Marinkovic
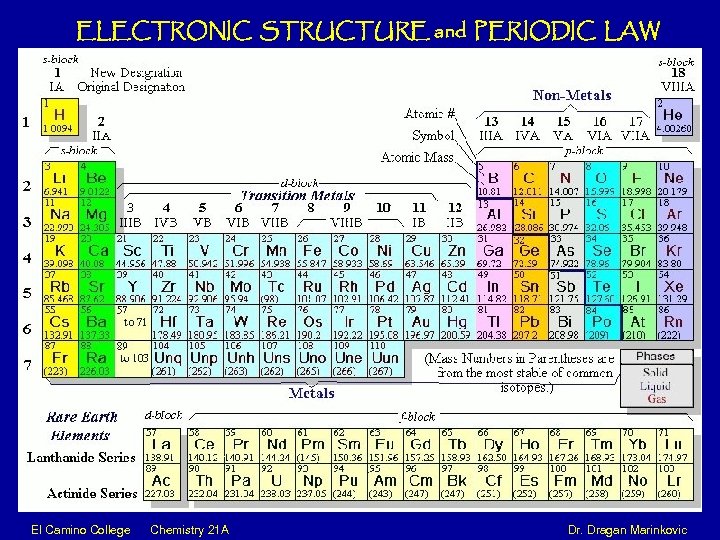 ELECTRONIC STRUCTURE and PERIODIC LAW El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW El Camino College Chemistry 21 A Dr. Dragan Marinkovic
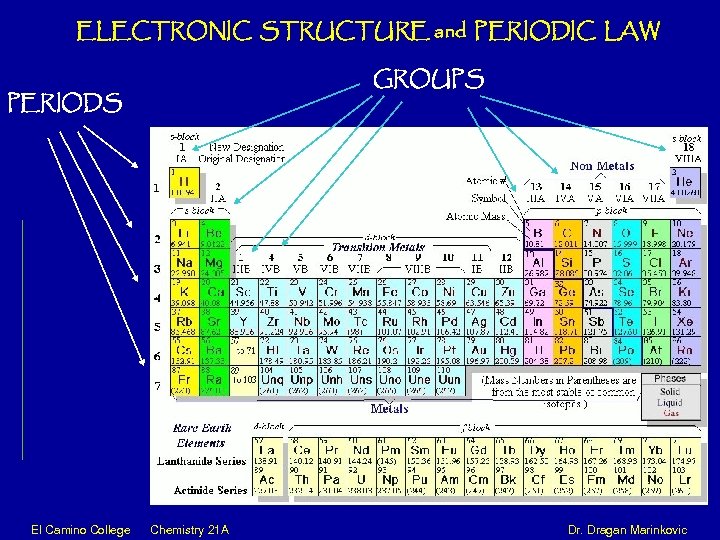 ELECTRONIC STRUCTURE and PERIODIC LAW PERIODS GROUPS El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW PERIODS GROUPS El Camino College Chemistry 21 A Dr. Dragan Marinkovic
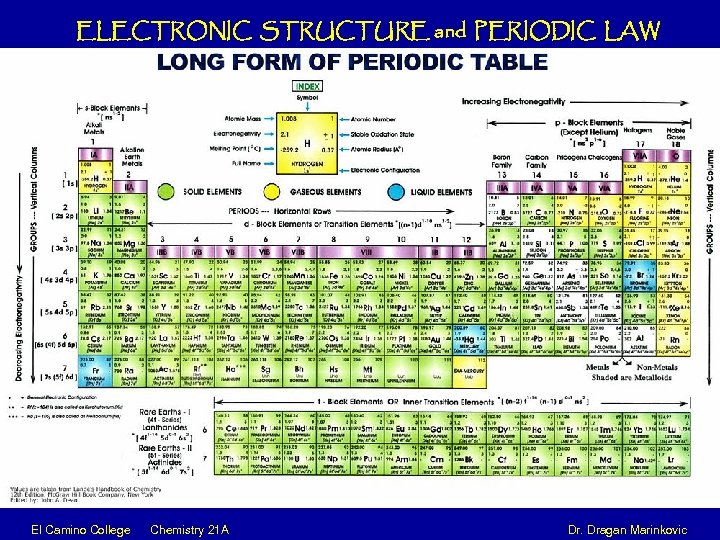 ELECTRONIC STRUCTURE and PERIODIC LAW El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW El Camino College Chemistry 21 A Dr. Dragan Marinkovic
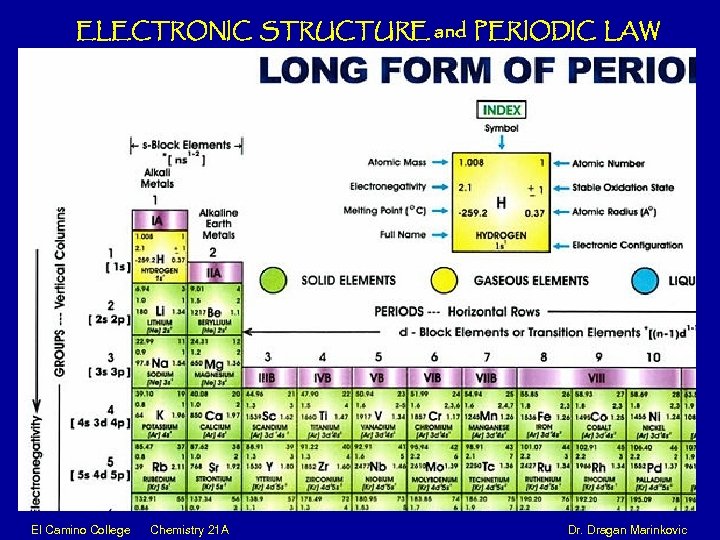 ELECTRONIC STRUCTURE and PERIODIC LAW El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW El Camino College Chemistry 21 A Dr. Dragan Marinkovic
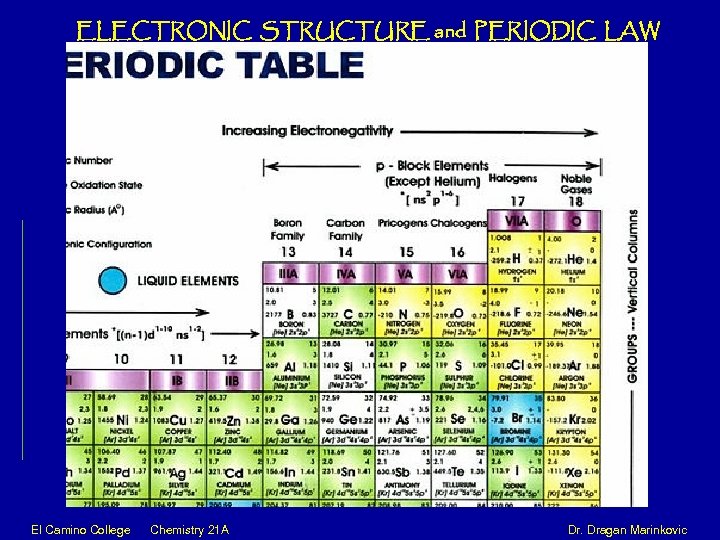 ELECTRONIC STRUCTURE and PERIODIC LAW El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW El Camino College Chemistry 21 A Dr. Dragan Marinkovic
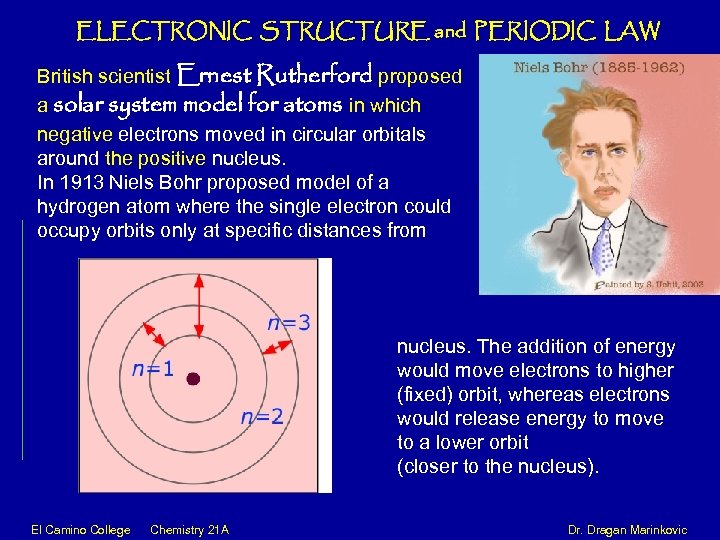 ELECTRONIC STRUCTURE and PERIODIC LAW British scientist Ernest Rutherford proposed a solar system model for atoms in which negative electrons moved in circular orbitals around the positive nucleus. In 1913 Niels Bohr proposed model of a hydrogen atom where the single electron could occupy orbits only at specific distances from Nitrogen Atom, 14 7 N nucleus. The addition of energy would move electrons to higher (fixed) orbit, whereas electrons would release energy to move to a lower orbit (closer to the nucleus). El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW British scientist Ernest Rutherford proposed a solar system model for atoms in which negative electrons moved in circular orbitals around the positive nucleus. In 1913 Niels Bohr proposed model of a hydrogen atom where the single electron could occupy orbits only at specific distances from Nitrogen Atom, 14 7 N nucleus. The addition of energy would move electrons to higher (fixed) orbit, whereas electrons would release energy to move to a lower orbit (closer to the nucleus). El Camino College Chemistry 21 A Dr. Dragan Marinkovic
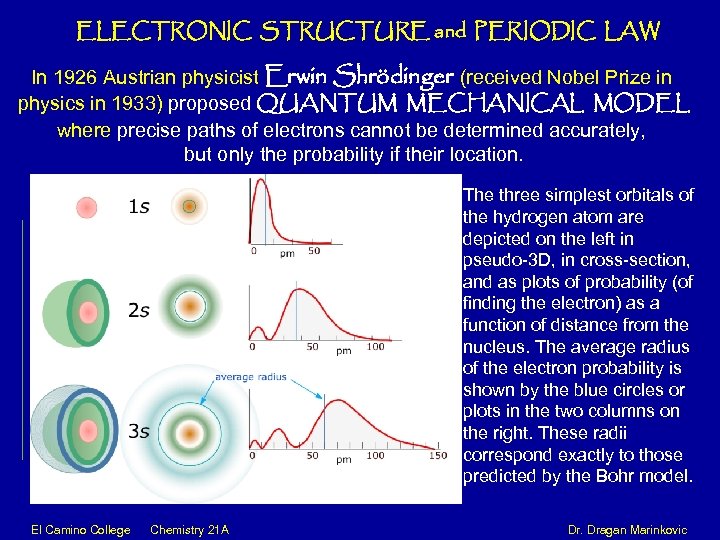 ELECTRONIC STRUCTURE and PERIODIC LAW In 1926 Austrian physicist Erwin Shrödinger (received Nobel Prize in physics in 1933) proposed QUANTUM MECHANICAL MODEL where precise paths of electrons cannot be determined accurately, but only the probability if their location. The three simplest orbitals of the hydrogen atom are depicted on the left in pseudo-3 D, in cross-section, and as plots of probability (of finding the electron) as a function of distance from the nucleus. The average radius of the electron probability is shown by the blue circles or plots in the two columns on the right. These radii correspond exactly to those predicted by the Bohr model. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW In 1926 Austrian physicist Erwin Shrödinger (received Nobel Prize in physics in 1933) proposed QUANTUM MECHANICAL MODEL where precise paths of electrons cannot be determined accurately, but only the probability if their location. The three simplest orbitals of the hydrogen atom are depicted on the left in pseudo-3 D, in cross-section, and as plots of probability (of finding the electron) as a function of distance from the nucleus. The average radius of the electron probability is shown by the blue circles or plots in the two columns on the right. These radii correspond exactly to those predicted by the Bohr model. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
 ELECTRONIC STRUCTURE and PERIODIC LAW The location and energy of electrons around a nucleus can be specified Using three terms: SHELL, SUBSHELL and ORBITAL. SHELL : a location and energy of electrons around a nucleus that is designated by a value for n, where n = 1, 2, 3, etc. SUBSHELL : a component of a SHELL that is designated by a letter from the group s, p, d and f. ORBITAL : a volume of space around atomic nuclei in which electrons of the same energy move. Groups of orbitals with the same n value form subshells. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW The location and energy of electrons around a nucleus can be specified Using three terms: SHELL, SUBSHELL and ORBITAL. SHELL : a location and energy of electrons around a nucleus that is designated by a value for n, where n = 1, 2, 3, etc. SUBSHELL : a component of a SHELL that is designated by a letter from the group s, p, d and f. ORBITAL : a volume of space around atomic nuclei in which electrons of the same energy move. Groups of orbitals with the same n value form subshells. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
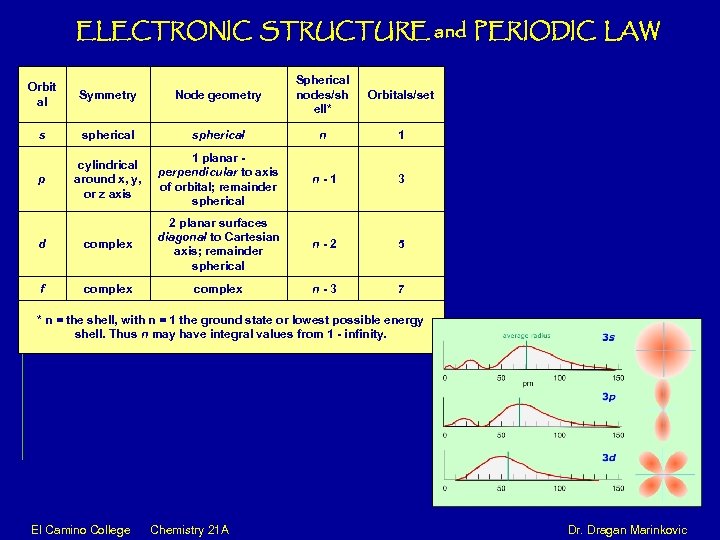 ELECTRONIC STRUCTURE and PERIODIC LAW Orbit al Symmetry Node geometry Spherical nodes/sh ell* Orbitals/set s spherical n 1 p cylindrical around x, y, or z axis 1 planar - perpendicular to axis of orbital; remainder spherical n - 1 3 d complex 2 planar surfaces diagonal to Cartesian axis; remainder spherical n - 2 5 f complex n - 3 7 * n = the shell, with n = 1 the ground state or lowest possible energy shell. Thus n may have integral values from 1 - infinity. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW Orbit al Symmetry Node geometry Spherical nodes/sh ell* Orbitals/set s spherical n 1 p cylindrical around x, y, or z axis 1 planar - perpendicular to axis of orbital; remainder spherical n - 1 3 d complex 2 planar surfaces diagonal to Cartesian axis; remainder spherical n - 2 5 f complex n - 3 7 * n = the shell, with n = 1 the ground state or lowest possible energy shell. Thus n may have integral values from 1 - infinity. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
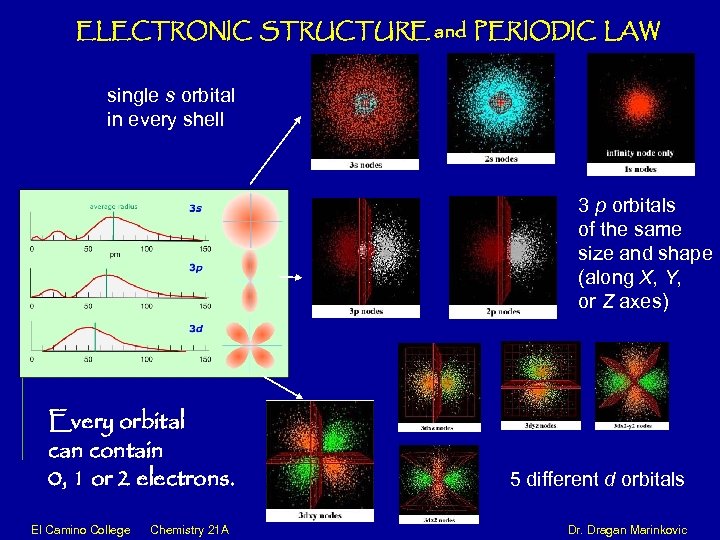 ELECTRONIC STRUCTURE and PERIODIC LAW single s orbital in every shell 3 p orbitals of the same size and shape (along X, Y, or Z axes) Every orbital can contain 0, 1 or 2 electrons. 5 different d orbitals El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW single s orbital in every shell 3 p orbitals of the same size and shape (along X, Y, or Z axes) Every orbital can contain 0, 1 or 2 electrons. 5 different d orbitals El Camino College Chemistry 21 A Dr. Dragan Marinkovic
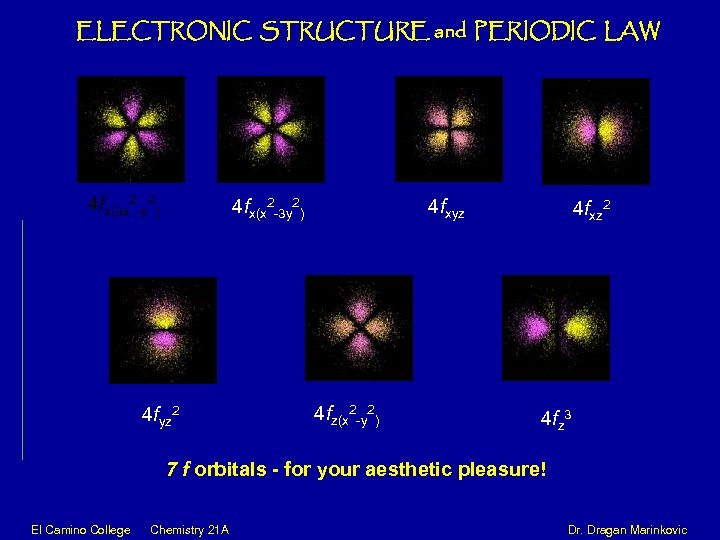 ELECTRONIC STRUCTURE and PERIODIC LAW 4 fx(3 x 2 -y 2) 4 fx(x 2 -3 y 2) 4 fyz 2 4 fz(x 2 -y 2) 4 fxyz 4 fxz 2 4 fz 3 7 f orbitals - for your aesthetic pleasure! El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW 4 fx(3 x 2 -y 2) 4 fx(x 2 -3 y 2) 4 fyz 2 4 fz(x 2 -y 2) 4 fxyz 4 fxz 2 4 fz 3 7 f orbitals - for your aesthetic pleasure! El Camino College Chemistry 21 A Dr. Dragan Marinkovic
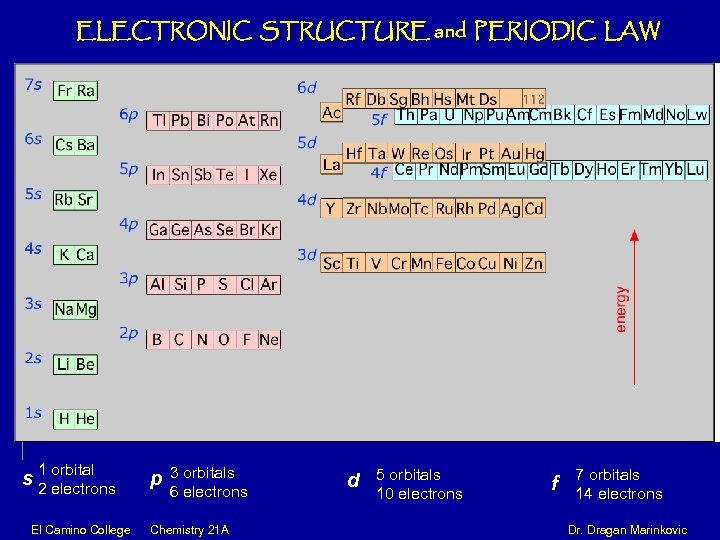 ELECTRONIC STRUCTURE and PERIODIC LAW s 1 orbital 2 electrons p 3 orbitals 6 electrons d 5 orbitals 10 electrons f 7 orbitals 14 electrons El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW s 1 orbital 2 electrons p 3 orbitals 6 electrons d 5 orbitals 10 electrons f 7 orbitals 14 electrons El Camino College Chemistry 21 A Dr. Dragan Marinkovic
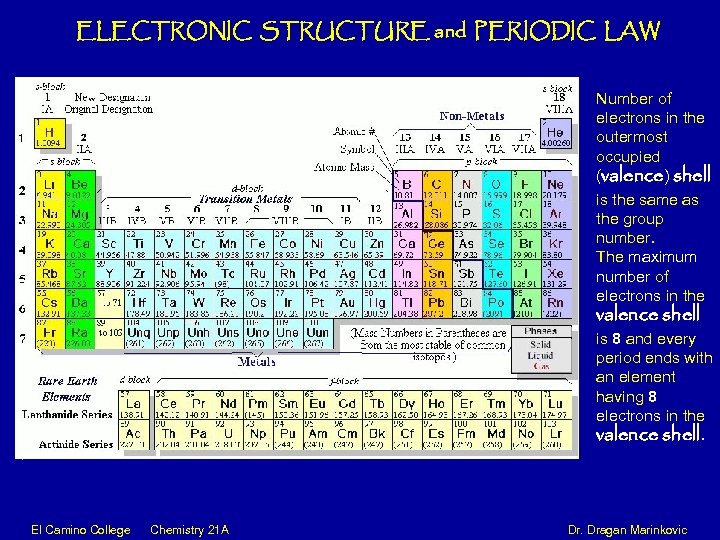 ELECTRONIC STRUCTURE and PERIODIC LAW Number of electrons in the outermost occupied (valence) shell is the same as the group number. The maximum number of electrons in the valence shell is 8 and every period ends with an element having 8 electrons in the valence shell. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW Number of electrons in the outermost occupied (valence) shell is the same as the group number. The maximum number of electrons in the valence shell is 8 and every period ends with an element having 8 electrons in the valence shell. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
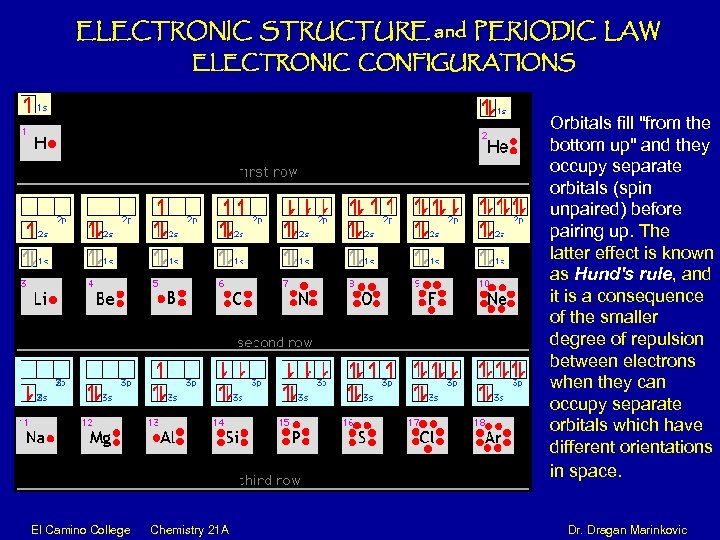 ELECTRONIC STRUCTURE and PERIODIC LAW ELECTRONIC CONFIGURATIONS Orbitals fill "from the bottom up" and they occupy separate orbitals (spin unpaired) before pairing up. The latter effect is known as Hund's rule, and it is a consequence of the smaller degree of repulsion between electrons when they can occupy separate orbitals which have different orientations in space. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW ELECTRONIC CONFIGURATIONS Orbitals fill "from the bottom up" and they occupy separate orbitals (spin unpaired) before pairing up. The latter effect is known as Hund's rule, and it is a consequence of the smaller degree of repulsion between electrons when they can occupy separate orbitals which have different orientations in space. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
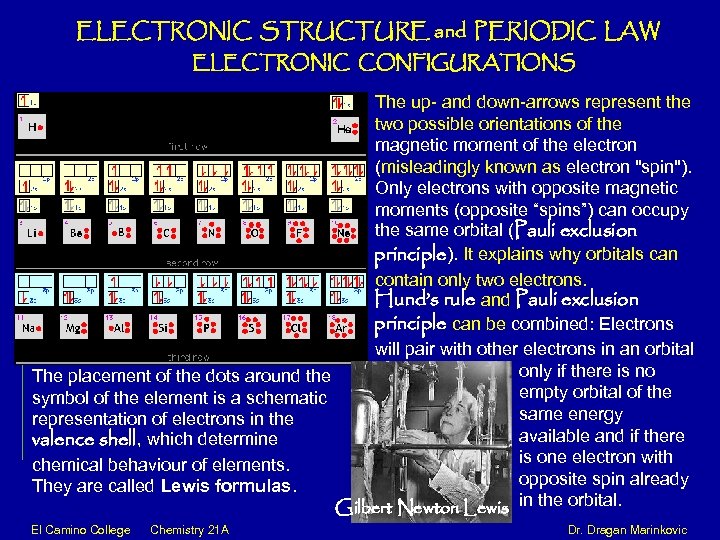 ELECTRONIC STRUCTURE and PERIODIC LAW ELECTRONIC CONFIGURATIONS The up- and down-arrows represent the two possible orientations of the magnetic moment of the electron (misleadingly known as electron "spin"). Only electrons with opposite magnetic moments (opposite “spins”) can occupy the same orbital (Pauli exclusion principle). It explains why orbitals can contain only two electrons. Hund’s rule and Pauli exclusion principle can be combined: Electrons will pair with other electrons in an orbital only if there is no The placement of the dots around the empty orbital of the symbol of the element is a schematic same energy representation of electrons in the available and if there valence shell, which determine is one electron with chemical behaviour of elements. opposite spin already They are called Lewis formulas. Gilbert Newton Lewis in the orbital. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW ELECTRONIC CONFIGURATIONS The up- and down-arrows represent the two possible orientations of the magnetic moment of the electron (misleadingly known as electron "spin"). Only electrons with opposite magnetic moments (opposite “spins”) can occupy the same orbital (Pauli exclusion principle). It explains why orbitals can contain only two electrons. Hund’s rule and Pauli exclusion principle can be combined: Electrons will pair with other electrons in an orbital only if there is no The placement of the dots around the empty orbital of the symbol of the element is a schematic same energy representation of electrons in the available and if there valence shell, which determine is one electron with chemical behaviour of elements. opposite spin already They are called Lewis formulas. Gilbert Newton Lewis in the orbital. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
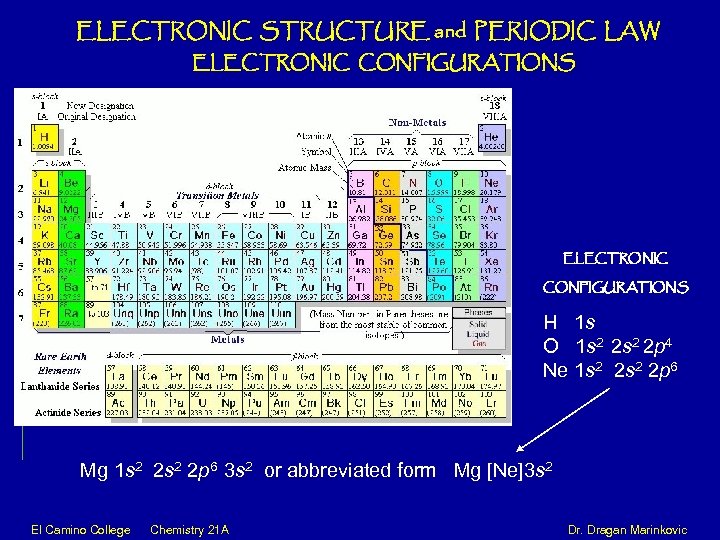 ELECTRONIC STRUCTURE and PERIODIC LAW ELECTRONIC CONFIGURATIONS H 1 s O 1 s 2 2 p 4 Ne 1 s 2 2 p 6 Mg 1 s 2 2 p 6 3 s 2 or abbreviated form Mg [Ne]3 s 2 El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW ELECTRONIC CONFIGURATIONS H 1 s O 1 s 2 2 p 4 Ne 1 s 2 2 p 6 Mg 1 s 2 2 p 6 3 s 2 or abbreviated form Mg [Ne]3 s 2 El Camino College Chemistry 21 A Dr. Dragan Marinkovic
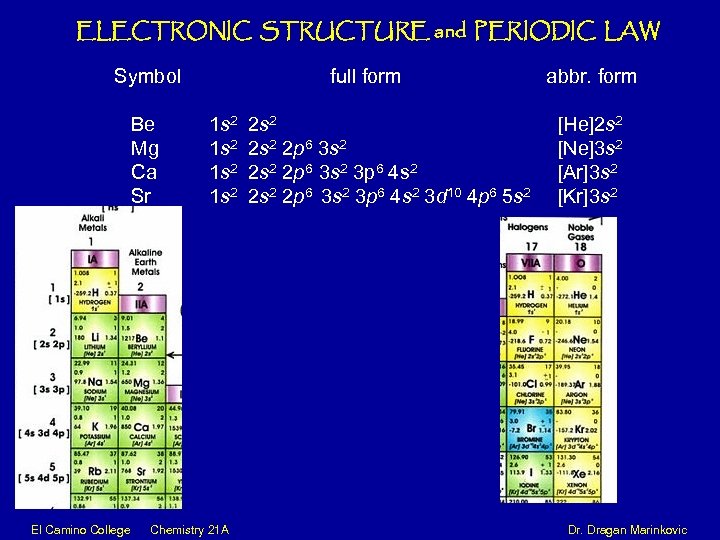 ELECTRONIC STRUCTURE and PERIODIC LAW Symbol Be Mg Ca Sr full form 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 abbr. form [He]2 s 2 [Ne]3 s 2 [Ar]3 s 2 [Kr]3 s 2 El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW Symbol Be Mg Ca Sr full form 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 abbr. form [He]2 s 2 [Ne]3 s 2 [Ar]3 s 2 [Kr]3 s 2 El Camino College Chemistry 21 A Dr. Dragan Marinkovic
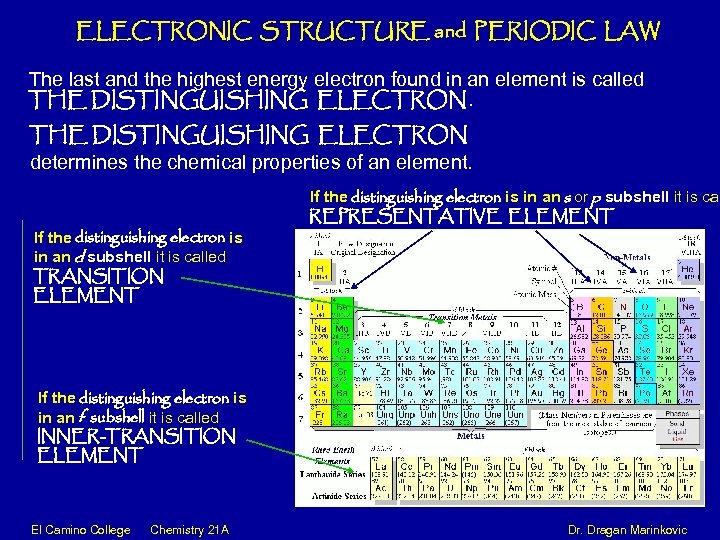 ELECTRONIC STRUCTURE and PERIODIC LAW The last and the highest energy electron found in an element is called THE DISTINGUISHING ELECTRON determines the chemical properties of an element. If the distinguishing electron is in an d subshell it is called TRANSITION ELEMENT If the distinguishing electron is in an s or p subshell it is cal REPRESENTATIVE ELEMENT If the distinguishing electron is in an f subshell it is called INNER-TRANSITION ELEMENT El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW The last and the highest energy electron found in an element is called THE DISTINGUISHING ELECTRON determines the chemical properties of an element. If the distinguishing electron is in an d subshell it is called TRANSITION ELEMENT If the distinguishing electron is in an s or p subshell it is cal REPRESENTATIVE ELEMENT If the distinguishing electron is in an f subshell it is called INNER-TRANSITION ELEMENT El Camino College Chemistry 21 A Dr. Dragan Marinkovic
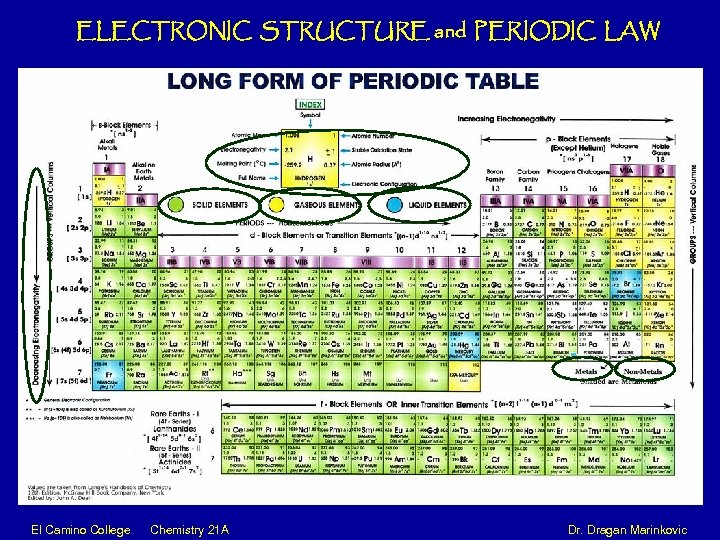 ELECTRONIC STRUCTURE and PERIODIC LAW El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW El Camino College Chemistry 21 A Dr. Dragan Marinkovic
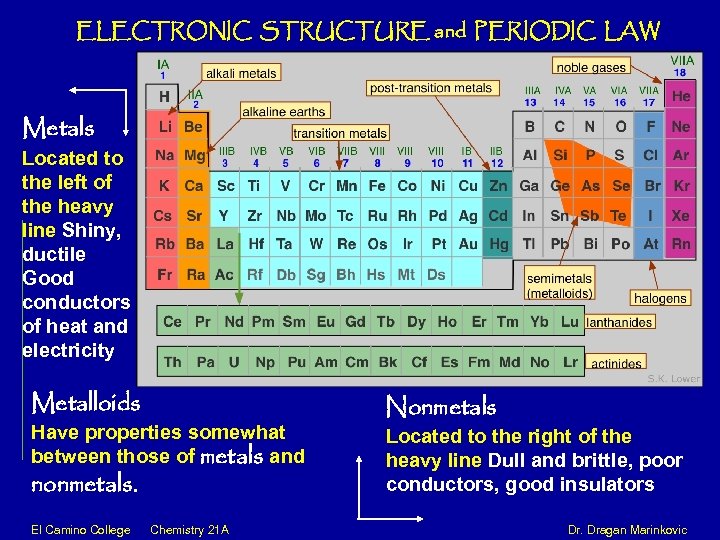 ELECTRONIC STRUCTURE and PERIODIC LAW Metals Located to the left of the heavy line Shiny, ductile Good conductors of heat and electricity Metalloids Have properties somewhat between those of metals and nonmetals. Nonmetals Located to the right of the heavy line Dull and brittle, poor conductors, good insulators El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW Metals Located to the left of the heavy line Shiny, ductile Good conductors of heat and electricity Metalloids Have properties somewhat between those of metals and nonmetals. Nonmetals Located to the right of the heavy line Dull and brittle, poor conductors, good insulators El Camino College Chemistry 21 A Dr. Dragan Marinkovic
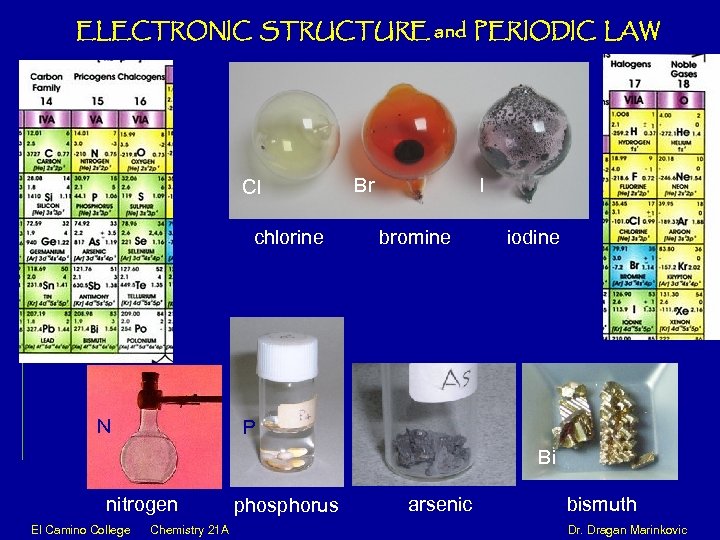 ELECTRONIC STRUCTURE and PERIODIC LAW Cl Br I chlorine bromine iodine N P Bi nitrogen phosphorus arsenic bismuth El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW Cl Br I chlorine bromine iodine N P Bi nitrogen phosphorus arsenic bismuth El Camino College Chemistry 21 A Dr. Dragan Marinkovic
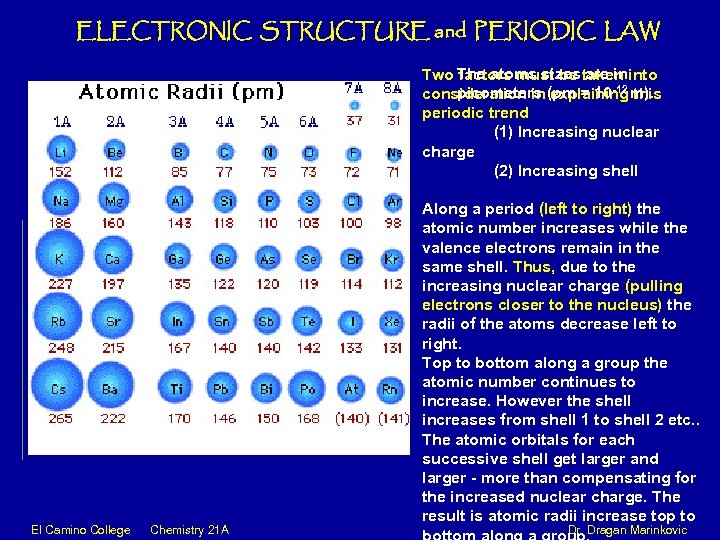 ELECTRONIC STRUCTURE and PERIODIC LAW The atoms sizes are in Two factors must be taken into picometers (pm = 10 -12 m). consideration in explaining this periodic trend (1) Increasing nuclear charge (2) Increasing shell Along a period (left to right) the atomic number increases while the valence electrons remain in the same shell. Thus, due to the increasing nuclear charge (pulling electrons closer to the nucleus) the radii of the atoms decrease left to right. Top to bottom along a group the atomic number continues to increase. However the shell increases from shell 1 to shell 2 etc. . The atomic orbitals for each successive shell get larger and larger - more than compensating for the increased nuclear charge. The result is atomic radii increase top to El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW The atoms sizes are in Two factors must be taken into picometers (pm = 10 -12 m). consideration in explaining this periodic trend (1) Increasing nuclear charge (2) Increasing shell Along a period (left to right) the atomic number increases while the valence electrons remain in the same shell. Thus, due to the increasing nuclear charge (pulling electrons closer to the nucleus) the radii of the atoms decrease left to right. Top to bottom along a group the atomic number continues to increase. However the shell increases from shell 1 to shell 2 etc. . The atomic orbitals for each successive shell get larger and larger - more than compensating for the increased nuclear charge. The result is atomic radii increase top to El Camino College Chemistry 21 A Dr. Dragan Marinkovic
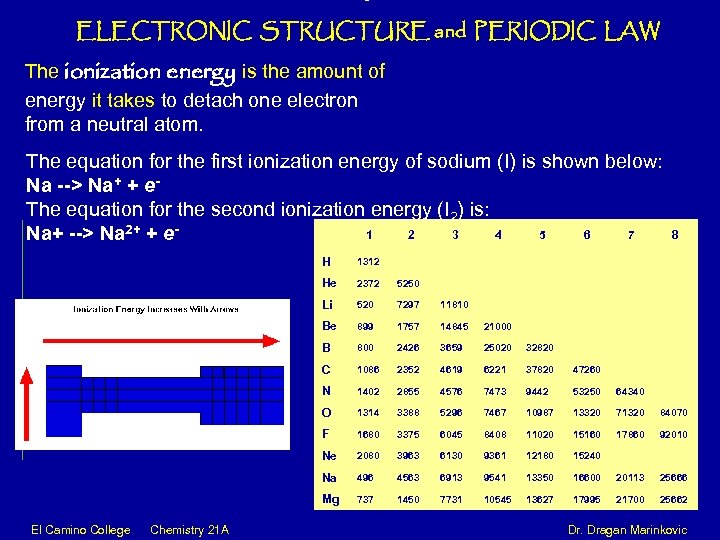 ELECTRONIC STRUCTURE and PERIODIC LAW The ionization energy is the amount of energy it takes to detach one electron from a neutral atom. The equation for the first ionization energy of sodium (I) is shown below: Na --> Na+ + e. The equation for the second ionization energy (I 2) is: 1 2 3 4 5 6 7 Na+ --> Na 2+ + e- 8 H 1312 He 2372 5250 Li 520 7297 11810 Be 899 1757 14845 21000 B 800 2426 3659 25020 32820 C 1086 2352 4619 6221 37820 47260 N 1402 2855 4576 7473 9442 53250 64340 O 1314 3388 5296 7467 10987 13320 71320 84070 F 1680 3375 6045 8408 11020 15160 17860 92010 Ne 2080 3963 6130 9361 12180 15240 Na 496 4563 6913 9541 13350 16600 20113 25666 Mg 737 1450 7731 10545 13627 17995 21700 25662 El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW The ionization energy is the amount of energy it takes to detach one electron from a neutral atom. The equation for the first ionization energy of sodium (I) is shown below: Na --> Na+ + e. The equation for the second ionization energy (I 2) is: 1 2 3 4 5 6 7 Na+ --> Na 2+ + e- 8 H 1312 He 2372 5250 Li 520 7297 11810 Be 899 1757 14845 21000 B 800 2426 3659 25020 32820 C 1086 2352 4619 6221 37820 47260 N 1402 2855 4576 7473 9442 53250 64340 O 1314 3388 5296 7467 10987 13320 71320 84070 F 1680 3375 6045 8408 11020 15160 17860 92010 Ne 2080 3963 6130 9361 12180 15240 Na 496 4563 6913 9541 13350 16600 20113 25666 Mg 737 1450 7731 10545 13627 17995 21700 25662 El Camino College Chemistry 21 A Dr. Dragan Marinkovic
 ELECTRONIC STRUCTURE and PERIODIC LAW 2 M + 2 C 2 H 5 OH = 2 C 2 H 5 OM + H 2 2 M + 2 H 2 O = 2 MOH + H 2 2 Rb + 2 H 2 O = 2 Rb. OH + H 2 El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW 2 M + 2 C 2 H 5 OH = 2 C 2 H 5 OM + H 2 2 M + 2 H 2 O = 2 MOH + H 2 2 Rb + 2 H 2 O = 2 Rb. OH + H 2 El Camino College Chemistry 21 A Dr. Dragan Marinkovic
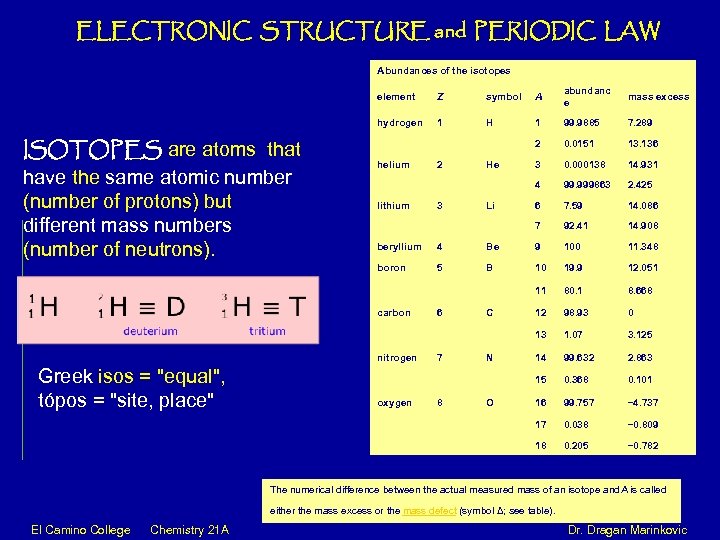 ELECTRONIC STRUCTURE and PERIODIC LAW Abundances of the isotopes element symbol A abundanc e mass excess hydrogen ISOTOPES are atoms that have the same atomic number (number of protons) but different mass numbers (number of neutrons). Z 1 H 1 99. 9885 7. 289 2 0. 0151 13. 136 3 0. 000138 14. 931 4 99. 999863 2. 425 6 7. 59 14. 086 7 92. 41 14. 908 helium lithium 2 3 He Li beryllium 4 Be 9 100 11. 348 boron 5 B 10 19. 9 12. 051 11 80. 1 8. 668 12 98. 93 0 13 1. 07 3. 125 14 99. 632 2. 863 15 0. 368 0. 101 16 99. 757 − 4. 737 17 0. 038 − 0. 809 18 0. 205 − 0. 782 carbon nitrogen Greek isos = "equal", tópos = "site, place" oxygen 6 7 8 C N O The numerical difference between the actual measured mass of an isotope and is called A either the mass excess or the mass defect (symbol Δ; see table). El Camino College Chemistry 21 A Dr. Dragan Marinkovic
ELECTRONIC STRUCTURE and PERIODIC LAW Abundances of the isotopes element symbol A abundanc e mass excess hydrogen ISOTOPES are atoms that have the same atomic number (number of protons) but different mass numbers (number of neutrons). Z 1 H 1 99. 9885 7. 289 2 0. 0151 13. 136 3 0. 000138 14. 931 4 99. 999863 2. 425 6 7. 59 14. 086 7 92. 41 14. 908 helium lithium 2 3 He Li beryllium 4 Be 9 100 11. 348 boron 5 B 10 19. 9 12. 051 11 80. 1 8. 668 12 98. 93 0 13 1. 07 3. 125 14 99. 632 2. 863 15 0. 368 0. 101 16 99. 757 − 4. 737 17 0. 038 − 0. 809 18 0. 205 − 0. 782 carbon nitrogen Greek isos = "equal", tópos = "site, place" oxygen 6 7 8 C N O The numerical difference between the actual measured mass of an isotope and is called A either the mass excess or the mass defect (symbol Δ; see table). El Camino College Chemistry 21 A Dr. Dragan Marinkovic


