50986f0452c3914f02e90bb8e3c820d4.ppt
- Количество слайдов: 34
 ELECTROCHEMISTRY CHEM 4700 CHAPTER 5 Dr. au. Gustine ofori a. Gyeman assistant professor of chemistry Department of natural sciences clayton state university
ELECTROCHEMISTRY CHEM 4700 CHAPTER 5 Dr. au. Gustine ofori a. Gyeman assistant professor of chemistry Department of natural sciences clayton state university
 CHAPTER 5 POTENTIOMETRY
CHAPTER 5 POTENTIOMETRY
 POTENTIOMETRY - Based on static (zero-current) measurements - Used to obtain information on the composition of an analyte - Potential between two electrodes is measured Applications - Environmental monitoring - Clinical diagnostics (blood testing, electrolytes in blood) - Control of reaction processes
POTENTIOMETRY - Based on static (zero-current) measurements - Used to obtain information on the composition of an analyte - Potential between two electrodes is measured Applications - Environmental monitoring - Clinical diagnostics (blood testing, electrolytes in blood) - Control of reaction processes
 ION-SELECTIVE ELECTRODES (ISE) - Also known as indicator electrodes - Respond directly to the analyte - Used for direct potentiometric measurements - Selectively binds and measures the activity of one ion (no redox chemistry) Examples p. H electrode Calcium (Ca 2+) electrode Chloride (Cl-) electrode
ION-SELECTIVE ELECTRODES (ISE) - Also known as indicator electrodes - Respond directly to the analyte - Used for direct potentiometric measurements - Selectively binds and measures the activity of one ion (no redox chemistry) Examples p. H electrode Calcium (Ca 2+) electrode Chloride (Cl-) electrode
 ION-SELECTIVE ELECTRODES (ISE) Advanteages - Exhibit wide response - Exhibit wide linear range - Low cost - Color or turbidity of analyte does not affect results - Come in different shapes and sizes
ION-SELECTIVE ELECTRODES (ISE) Advanteages - Exhibit wide response - Exhibit wide linear range - Low cost - Color or turbidity of analyte does not affect results - Come in different shapes and sizes
 ION-SELECTIVE ELECTRODES (ISE) - Made from a permselective ion-conducting membrane (ion-exchange material that allows ions of one electrical sign to pass through) - Reference electrode is inbuilt - Internal solution (solution inside electrode) contains ion of interest with constant activity - Ion of interest is also mixed with membrane - Membrane is nonporous and water insoluble
ION-SELECTIVE ELECTRODES (ISE) - Made from a permselective ion-conducting membrane (ion-exchange material that allows ions of one electrical sign to pass through) - Reference electrode is inbuilt - Internal solution (solution inside electrode) contains ion of interest with constant activity - Ion of interest is also mixed with membrane - Membrane is nonporous and water insoluble
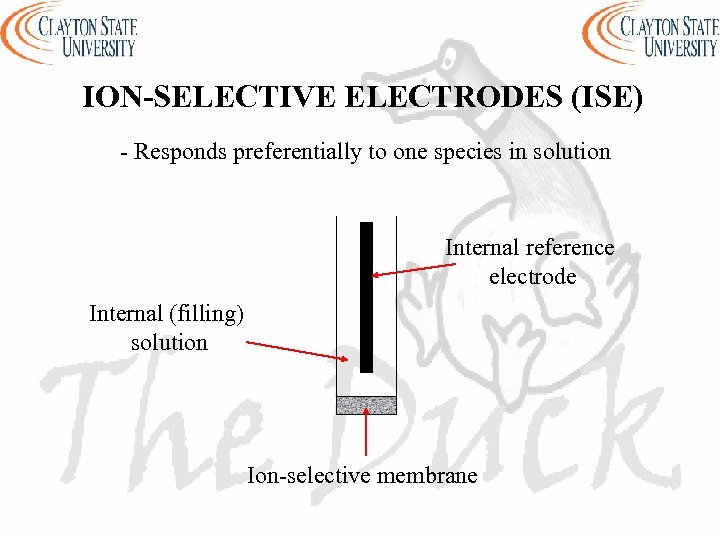 ION-SELECTIVE ELECTRODES (ISE) - Responds preferentially to one species in solution Internal reference electrode Internal (filling) solution Ion-selective membrane
ION-SELECTIVE ELECTRODES (ISE) - Responds preferentially to one species in solution Internal reference electrode Internal (filling) solution Ion-selective membrane
 ION-SELECTIVE ELECTRODES (ISE) - Selective (preferential) ion is C+ - Membrane is made of poly(vinyl chloride) (PVC) - Membrane is impregnated with nonpolar liquid - Membrane contains ligand L (ion-selective ionophore) - Membrane contains the complex LC+ - Membrane contains hydrophobic anion R- (ion exchanger)
ION-SELECTIVE ELECTRODES (ISE) - Selective (preferential) ion is C+ - Membrane is made of poly(vinyl chloride) (PVC) - Membrane is impregnated with nonpolar liquid - Membrane contains ligand L (ion-selective ionophore) - Membrane contains the complex LC+ - Membrane contains hydrophobic anion R- (ion exchanger)
![ION-SELECTIVE ELECTRODES (ISE) - [C+] inside the electrode ≠ [C+] outside the electrode - ION-SELECTIVE ELECTRODES (ISE) - [C+] inside the electrode ≠ [C+] outside the electrode -](https://present5.com/presentation/50986f0452c3914f02e90bb8e3c820d4/image-9.jpg) ION-SELECTIVE ELECTRODES (ISE) - [C+] inside the electrode ≠ [C+] outside the electrode - Results in a potential difference across the membrane Generally (at 25 o. C) - 10 -fold change in activity implies 59/zi m. V change in E - zi is the charge on the selective ion (negative for anions) - zi = +1 for K+, zi = +2 for Ca 2+, zi = -2 for CO 32 -
ION-SELECTIVE ELECTRODES (ISE) - [C+] inside the electrode ≠ [C+] outside the electrode - Results in a potential difference across the membrane Generally (at 25 o. C) - 10 -fold change in activity implies 59/zi m. V change in E - zi is the charge on the selective ion (negative for anions) - zi = +1 for K+, zi = +2 for Ca 2+, zi = -2 for CO 32 -
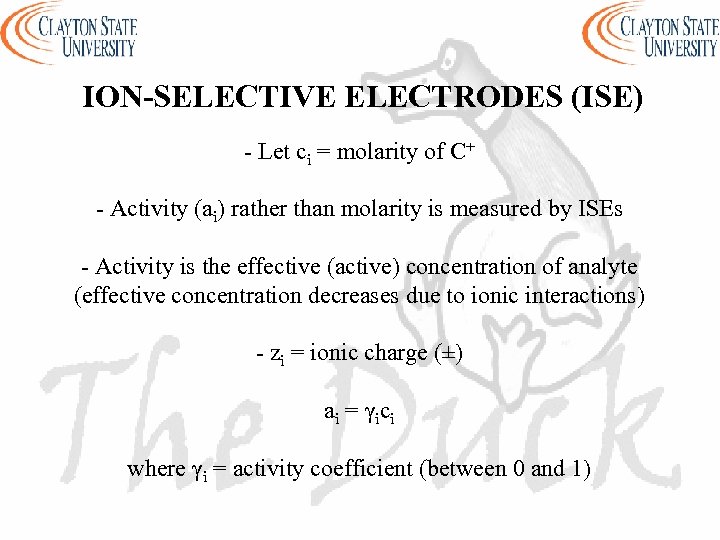 ION-SELECTIVE ELECTRODES (ISE) - Let ci = molarity of C+ - Activity (ai) rather than molarity is measured by ISEs - Activity is the effective (active) concentration of analyte (effective concentration decreases due to ionic interactions) - zi = ionic charge (±) a i = γ ic i where γi = activity coefficient (between 0 and 1)
ION-SELECTIVE ELECTRODES (ISE) - Let ci = molarity of C+ - Activity (ai) rather than molarity is measured by ISEs - Activity is the effective (active) concentration of analyte (effective concentration decreases due to ionic interactions) - zi = ionic charge (±) a i = γ ic i where γi = activity coefficient (between 0 and 1)
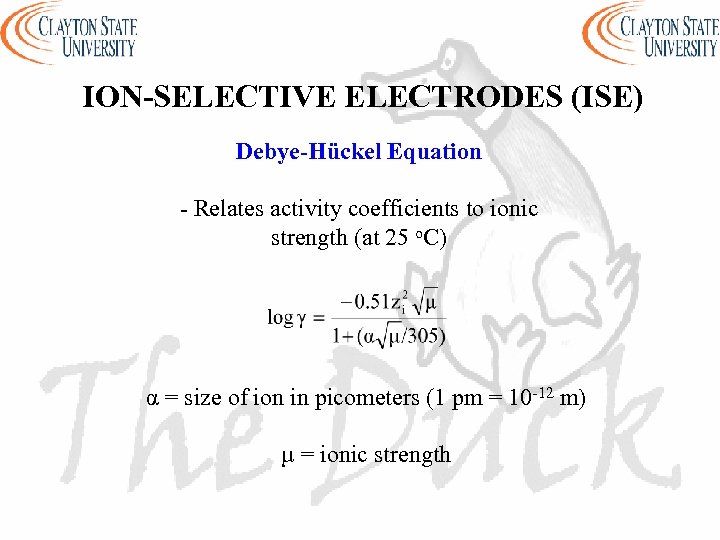 ION-SELECTIVE ELECTRODES (ISE) Debye-Hückel Equation - Relates activity coefficients to ionic strength (at 25 o. C) α = size of ion in picometers (1 pm = 10 -12 m) µ = ionic strength
ION-SELECTIVE ELECTRODES (ISE) Debye-Hückel Equation - Relates activity coefficients to ionic strength (at 25 o. C) α = size of ion in picometers (1 pm = 10 -12 m) µ = ionic strength
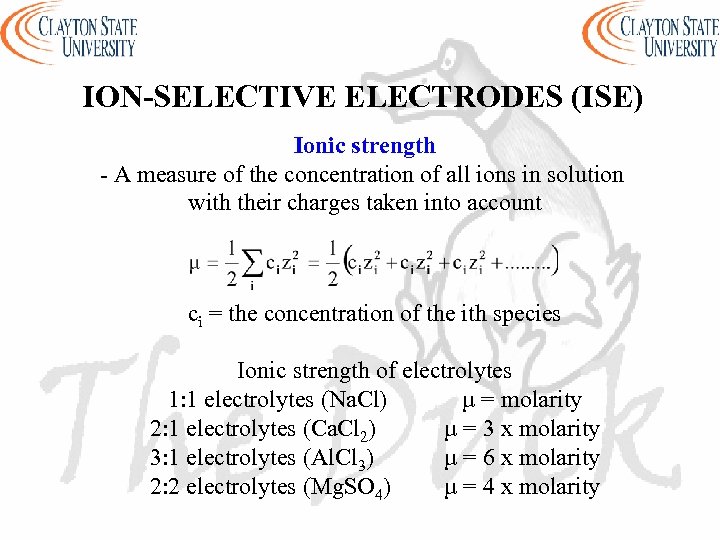 ION-SELECTIVE ELECTRODES (ISE) Ionic strength - A measure of the concentration of all ions in solution with their charges taken into account ci = the concentration of the ith species Ionic strength of electrolytes 1: 1 electrolytes (Na. Cl) µ = molarity 2: 1 electrolytes (Ca. Cl 2) µ = 3 x molarity 3: 1 electrolytes (Al. Cl 3) µ = 6 x molarity 2: 2 electrolytes (Mg. SO 4) µ = 4 x molarity
ION-SELECTIVE ELECTRODES (ISE) Ionic strength - A measure of the concentration of all ions in solution with their charges taken into account ci = the concentration of the ith species Ionic strength of electrolytes 1: 1 electrolytes (Na. Cl) µ = molarity 2: 1 electrolytes (Ca. Cl 2) µ = 3 x molarity 3: 1 electrolytes (Al. Cl 3) µ = 6 x molarity 2: 2 electrolytes (Mg. SO 4) µ = 4 x molarity
 ION-SELECTIVE ELECTRODES (ISE) - For very dilute solutions ai ≈ ci - Activity coefficient decreases as ionic strength increases For zi = 1 - 1 m. V change in potential implies 4% change in activity For zi = 2 - 1 m. V change in potential implies 8% change in activity - This is known as Nernstian behavior
ION-SELECTIVE ELECTRODES (ISE) - For very dilute solutions ai ≈ ci - Activity coefficient decreases as ionic strength increases For zi = 1 - 1 m. V change in potential implies 4% change in activity For zi = 2 - 1 m. V change in potential implies 8% change in activity - This is known as Nernstian behavior
 ION-SELECTIVE ELECTRODES (ISE) Selectivity Coefficient (k) - A measure of the ability of ISE to discriminate against an interfering ion - It is assumed that ISEs respond only to ion of interest - In practice, no electrode responds to only one specific ion - The lower the value of k the more selective is the electrode - k = 0 for an ideal electrode (implies no interference)
ION-SELECTIVE ELECTRODES (ISE) Selectivity Coefficient (k) - A measure of the ability of ISE to discriminate against an interfering ion - It is assumed that ISEs respond only to ion of interest - In practice, no electrode responds to only one specific ion - The lower the value of k the more selective is the electrode - k = 0 for an ideal electrode (implies no interference)
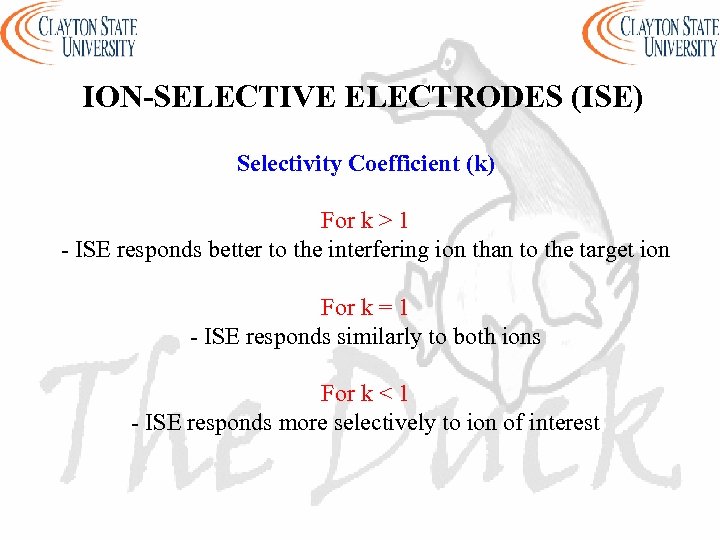 ION-SELECTIVE ELECTRODES (ISE) Selectivity Coefficient (k) For k > 1 - ISE responds better to the interfering ion than to the target ion For k = 1 - ISE responds similarly to both ions For k < 1 - ISE responds more selectively to ion of interest
ION-SELECTIVE ELECTRODES (ISE) Selectivity Coefficient (k) For k > 1 - ISE responds better to the interfering ion than to the target ion For k = 1 - ISE responds similarly to both ions For k < 1 - ISE responds more selectively to ion of interest
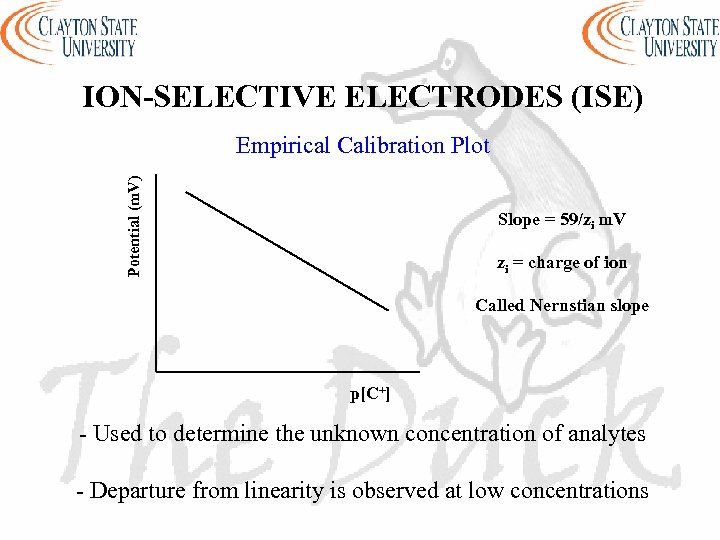 ION-SELECTIVE ELECTRODES (ISE) Potential (m. V) Empirical Calibration Plot Slope = 59/zi m. V zi = charge of ion Called Nernstian slope p[C+] - Used to determine the unknown concentration of analytes - Departure from linearity is observed at low concentrations
ION-SELECTIVE ELECTRODES (ISE) Potential (m. V) Empirical Calibration Plot Slope = 59/zi m. V zi = charge of ion Called Nernstian slope p[C+] - Used to determine the unknown concentration of analytes - Departure from linearity is observed at low concentrations
 ION-SELECTIVE ELECTRODES (ISE) Three groups of ISEs - Glass electrodes - Liquid electrodes - Solid electrodes
ION-SELECTIVE ELECTRODES (ISE) Three groups of ISEs - Glass electrodes - Liquid electrodes - Solid electrodes
 GLASS ELECTRODES - Responsive to univalent cations - Employs thin ion-selective glass membrane
GLASS ELECTRODES - Responsive to univalent cations - Employs thin ion-selective glass membrane
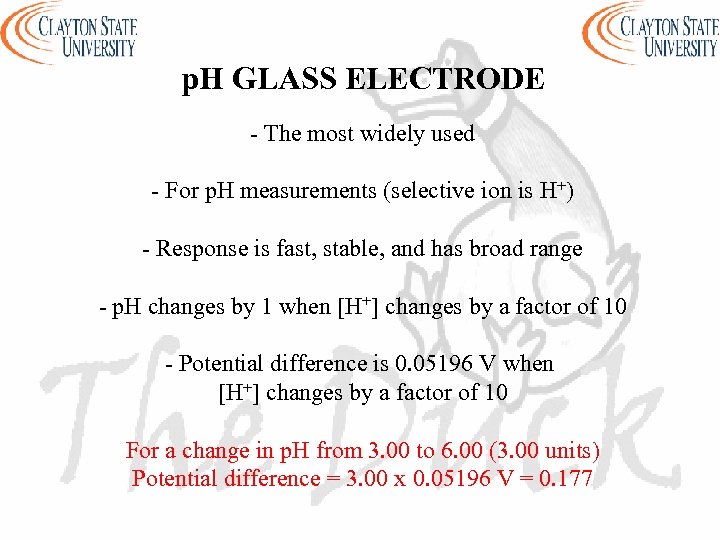 p. H GLASS ELECTRODE - The most widely used - For p. H measurements (selective ion is H+) - Response is fast, stable, and has broad range - p. H changes by 1 when [H+] changes by a factor of 10 - Potential difference is 0. 05196 V when [H+] changes by a factor of 10 For a change in p. H from 3. 00 to 6. 00 (3. 00 units) Potential difference = 3. 00 x 0. 05196 V = 0. 177
p. H GLASS ELECTRODE - The most widely used - For p. H measurements (selective ion is H+) - Response is fast, stable, and has broad range - p. H changes by 1 when [H+] changes by a factor of 10 - Potential difference is 0. 05196 V when [H+] changes by a factor of 10 For a change in p. H from 3. 00 to 6. 00 (3. 00 units) Potential difference = 3. 00 x 0. 05196 V = 0. 177
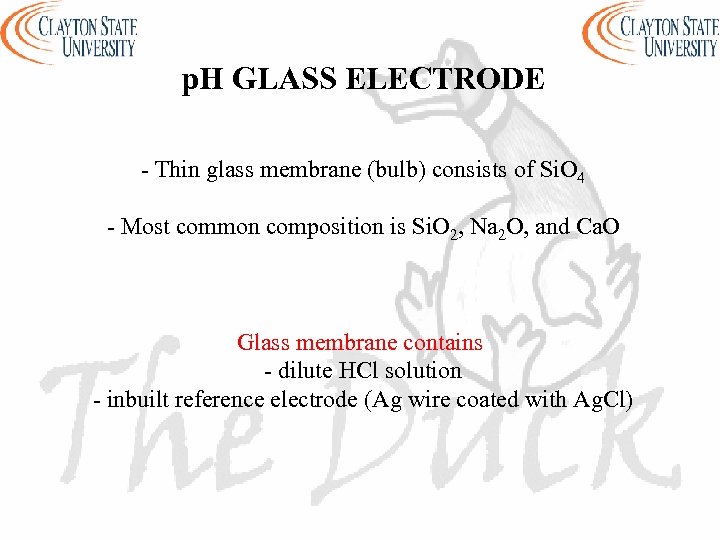 p. H GLASS ELECTRODE - Thin glass membrane (bulb) consists of Si. O 4 - Most common composition is Si. O 2, Na 2 O, and Ca. O Glass membrane contains - dilute HCl solution - inbuilt reference electrode (Ag wire coated with Ag. Cl)
p. H GLASS ELECTRODE - Thin glass membrane (bulb) consists of Si. O 4 - Most common composition is Si. O 2, Na 2 O, and Ca. O Glass membrane contains - dilute HCl solution - inbuilt reference electrode (Ag wire coated with Ag. Cl)
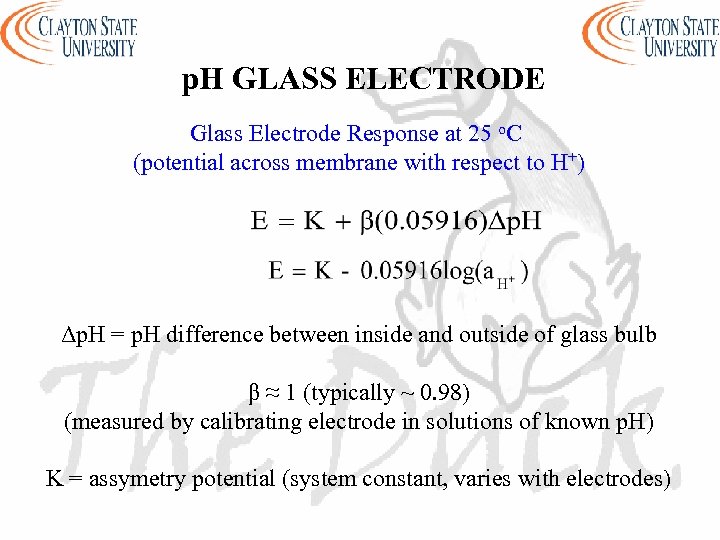 p. H GLASS ELECTRODE Glass Electrode Response at 25 o. C (potential across membrane with respect to H+) Δp. H = p. H difference between inside and outside of glass bulb β ≈ 1 (typically ~ 0. 98) (measured by calibrating electrode in solutions of known p. H) K = assymetry potential (system constant, varies with electrodes)
p. H GLASS ELECTRODE Glass Electrode Response at 25 o. C (potential across membrane with respect to H+) Δp. H = p. H difference between inside and outside of glass bulb β ≈ 1 (typically ~ 0. 98) (measured by calibrating electrode in solutions of known p. H) K = assymetry potential (system constant, varies with electrodes)
 p. H GLASS ELECTRODE - Equilibrium establishes across the glass membrane with respect to H+ in inner and outer solutions - This produces the potential, E - Linearity between p. H and potential - Calibration plot yields slope = 59 m. V/p. H units - Electrode is prevented from drying out by storing in aqueous solution when not in use
p. H GLASS ELECTRODE - Equilibrium establishes across the glass membrane with respect to H+ in inner and outer solutions - This produces the potential, E - Linearity between p. H and potential - Calibration plot yields slope = 59 m. V/p. H units - Electrode is prevented from drying out by storing in aqueous solution when not in use
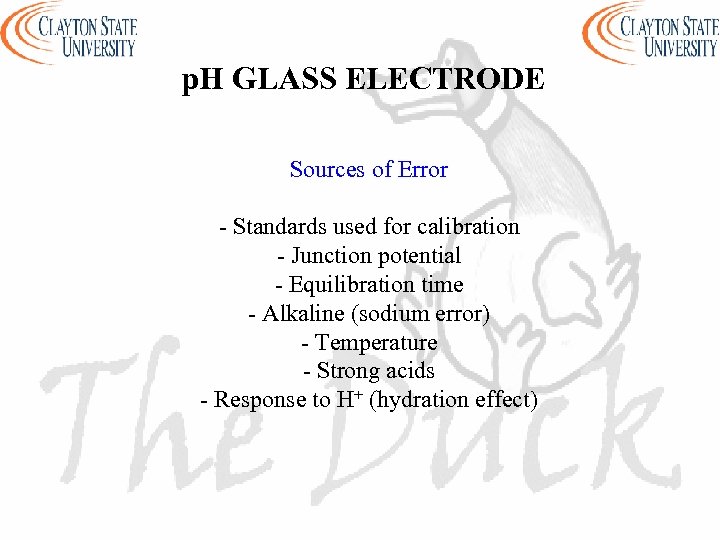 p. H GLASS ELECTRODE Sources of Error - Standards used for calibration - Junction potential - Equilibration time - Alkaline (sodium error) - Temperature - Strong acids - Response to H+ (hydration effect)
p. H GLASS ELECTRODE Sources of Error - Standards used for calibration - Junction potential - Equilibration time - Alkaline (sodium error) - Temperature - Strong acids - Response to H+ (hydration effect)
 OTHEER GLASS ELECTRODES Glass Electrodes For Other Cations K+ -, NH 4+-, Na+-selective electrodes - Mechanism is complex - Employs aluminosilicate glasses (Na 2 O, Al 2 O 3, Si. O 2) - Minimizes interference from H+ when solution p. H > 5 p. H Nonglass Electrodes - Quinhydrone electrode (quinone – hydroquinone couple) - Antimony electrode
OTHEER GLASS ELECTRODES Glass Electrodes For Other Cations K+ -, NH 4+-, Na+-selective electrodes - Mechanism is complex - Employs aluminosilicate glasses (Na 2 O, Al 2 O 3, Si. O 2) - Minimizes interference from H+ when solution p. H > 5 p. H Nonglass Electrodes - Quinhydrone electrode (quinone – hydroquinone couple) - Antimony electrode
 LIQUID MEMBRANE ELECTRODES - Employs water-immiscible substances impregnated in a polymeric membrane (PVC) - For direct measurement of polyvalent cations and some anions - The inner solution is a saturated solution of the target ion - Hydrophilic complexing agents (e. g. EDTA) are added to inner solutions to improve detection limits - Inner wire is Ag/Ag. Cl
LIQUID MEMBRANE ELECTRODES - Employs water-immiscible substances impregnated in a polymeric membrane (PVC) - For direct measurement of polyvalent cations and some anions - The inner solution is a saturated solution of the target ion - Hydrophilic complexing agents (e. g. EDTA) are added to inner solutions to improve detection limits - Inner wire is Ag/Ag. Cl
 LIQUID MEMBRANE ELECTRODES Ion-Exchange Electrodes - The basis is the ability of phosphate ions to form stable complexes with calcium ions - Selective towards calcium - Employs cation-exchanger that has high affinity for calcium ions (diester of phosphoric acid) - Inner solution is a saturated solution of calcium chloride - Cell potential is given by
LIQUID MEMBRANE ELECTRODES Ion-Exchange Electrodes - The basis is the ability of phosphate ions to form stable complexes with calcium ions - Selective towards calcium - Employs cation-exchanger that has high affinity for calcium ions (diester of phosphoric acid) - Inner solution is a saturated solution of calcium chloride - Cell potential is given by
 LIQUID MEMBRANE ELECTRODES Other Ion-Exchange Electrodes - Have poor selectivity and are limited to pharmaceutical formulations Examples - IEE for polycationic species (polyarginine, protamine) - IEE for polyanionic species (DNA) - IEE for detection of commonly abused drugs (large organic species)
LIQUID MEMBRANE ELECTRODES Other Ion-Exchange Electrodes - Have poor selectivity and are limited to pharmaceutical formulations Examples - IEE for polycationic species (polyarginine, protamine) - IEE for polyanionic species (DNA) - IEE for detection of commonly abused drugs (large organic species)
 LIQUID MEMBRANE ELECTRODES Neutral Carrier Electrodes - Employs neutral carriers such as crown ethers and cyclic polyesters - Carriers envelope target ions in their pockets Used for clinical analysis - detection of blood electrolytes - detection alkali and alkaline earth metal cations
LIQUID MEMBRANE ELECTRODES Neutral Carrier Electrodes - Employs neutral carriers such as crown ethers and cyclic polyesters - Carriers envelope target ions in their pockets Used for clinical analysis - detection of blood electrolytes - detection alkali and alkaline earth metal cations
 LIQUID MEMBRANE ELECTRODES Neutral Carrier Electrodes Examples of Carriers - Monensin for sodium - Macrocyclic thioethers for Hg and Ag - Valinomycin for potassium ions - Calixarene derivatives for lead - 14 -crown-4 -ether for lithium
LIQUID MEMBRANE ELECTRODES Neutral Carrier Electrodes Examples of Carriers - Monensin for sodium - Macrocyclic thioethers for Hg and Ag - Valinomycin for potassium ions - Calixarene derivatives for lead - 14 -crown-4 -ether for lithium
 LIQUID MEMBRANE ELECTRODES Anion-Selective Electrodes - For sensing organic and inorganic anions Examples of Anions - Phosphate - Salicylate - Thiocyanate - Carbonate
LIQUID MEMBRANE ELECTRODES Anion-Selective Electrodes - For sensing organic and inorganic anions Examples of Anions - Phosphate - Salicylate - Thiocyanate - Carbonate
 SOLID-STATE ELECTRODES - Solid membranes that are selective primarily to anions Solid-state membrane may be - single crystals - polycrystalline pellets or - mixed crystals
SOLID-STATE ELECTRODES - Solid membranes that are selective primarily to anions Solid-state membrane may be - single crystals - polycrystalline pellets or - mixed crystals
 SOLID-STATE ELECTRODES Examples - Most common is fluoride-ion-selective electrode (limited p. H range of 0 -8. 5) (OH- is the only interfering ion due to similar size and charge) - Iodide electrode (high selectivity over Br- and Cl-) Chloride electrode (suffers interference from Br- and I-) Thiocynate (SCN-) and cyanide (CN-) electrodes
SOLID-STATE ELECTRODES Examples - Most common is fluoride-ion-selective electrode (limited p. H range of 0 -8. 5) (OH- is the only interfering ion due to similar size and charge) - Iodide electrode (high selectivity over Br- and Cl-) Chloride electrode (suffers interference from Br- and I-) Thiocynate (SCN-) and cyanide (CN-) electrodes
 OTHER ELECTRODES - Coated-wire electrodes (CWE) - Solid-state electrodes without inner solutions - Made up of metallic wire or disk conductor (Cu, Ag, Pt) - Mechanism is not well understood due to lack of internal reference - Usually not reproducible For detection of amino acids, cocaine, methadone, sodium
OTHER ELECTRODES - Coated-wire electrodes (CWE) - Solid-state electrodes without inner solutions - Made up of metallic wire or disk conductor (Cu, Ag, Pt) - Mechanism is not well understood due to lack of internal reference - Usually not reproducible For detection of amino acids, cocaine, methadone, sodium
 APPLICATIONS OF ISEs - Used as detectors for automated flow analyzers (flow injection systems) - High-speed determination of blood electrolytes in hospitals (H+, K+, Cl-, Ca 2+, Na+) - For measuring soil samples (NO 3 -, Cl-, Li+, Ca 2+, Mg 2+) - Coupling ion chromatography with potentiometric detection - Micro ISEs as probe tips for SECM - Column detectors for capillary-zone electrophoresis
APPLICATIONS OF ISEs - Used as detectors for automated flow analyzers (flow injection systems) - High-speed determination of blood electrolytes in hospitals (H+, K+, Cl-, Ca 2+, Na+) - For measuring soil samples (NO 3 -, Cl-, Li+, Ca 2+, Mg 2+) - Coupling ion chromatography with potentiometric detection - Micro ISEs as probe tips for SECM - Column detectors for capillary-zone electrophoresis


