553b0154236fe855d75ba9d85e6e0607.ppt
- Количество слайдов: 62

Electrochemistry Chapter 19

산화환원 반응과 전기화학 1. 2. 3. 4. 5. 6. 7. 8. 9. 산화환원 반응과 산화수 갈바니 전지 표준환원전위 산화환원 반응의 자발성. 전위차와 자유에너지 전위차와 농도, Nernst 방정식 전지 부식 전기분해 전기야금
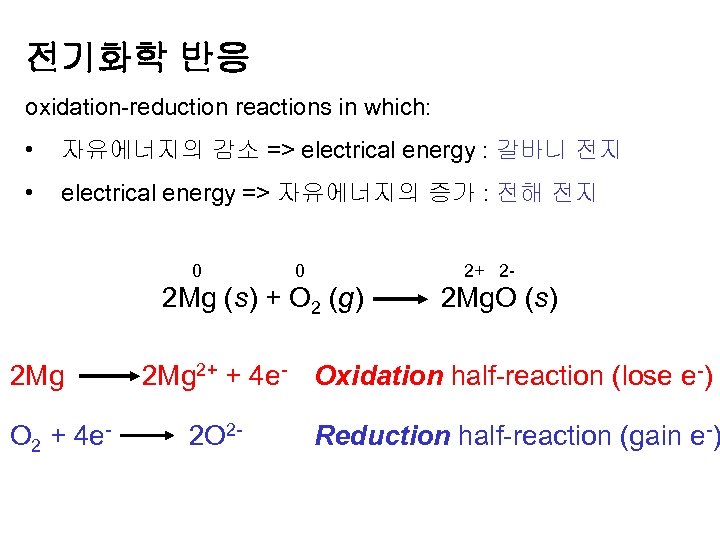
전기화학 반응 oxidation-reduction reactions in which: • 자유에너지의 감소 => electrical energy : 갈바니 전지 • electrical energy => 자유에너지의 증가 : 전해 전지 0 0 2+ 2 - 2 Mg (s) + O 2 (g) 2 Mg. O (s) 2 Mg 2+ + 4 e- Oxidation half-reaction (lose e-) O 2 + 4 e- 2 O 2 - Reduction half-reaction (gain e-)
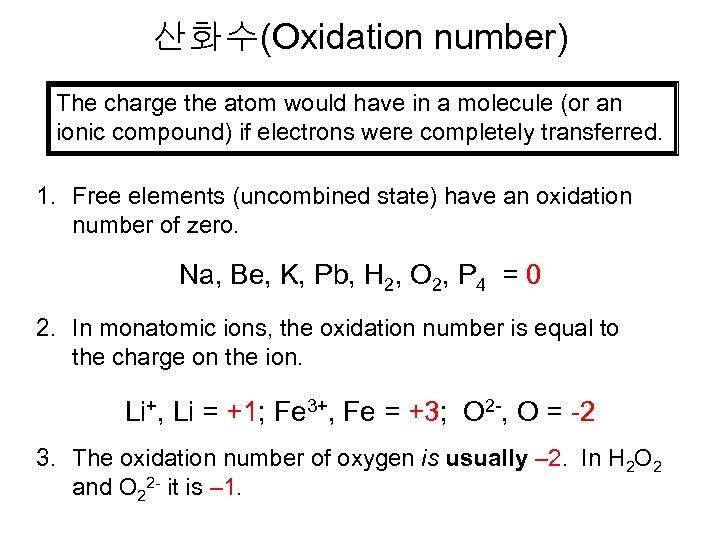
산화수(Oxidation number) The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na, Be, K, Pb, H 2, O 2, P 4 = 0 2. In monatomic ions, the oxidation number is equal to the charge on the ion. Li+, Li = +1; Fe 3+, Fe = +3; O 2 -, O = -2 3. The oxidation number of oxygen is usually – 2. In H 2 O 2 and O 22 - it is – 1.
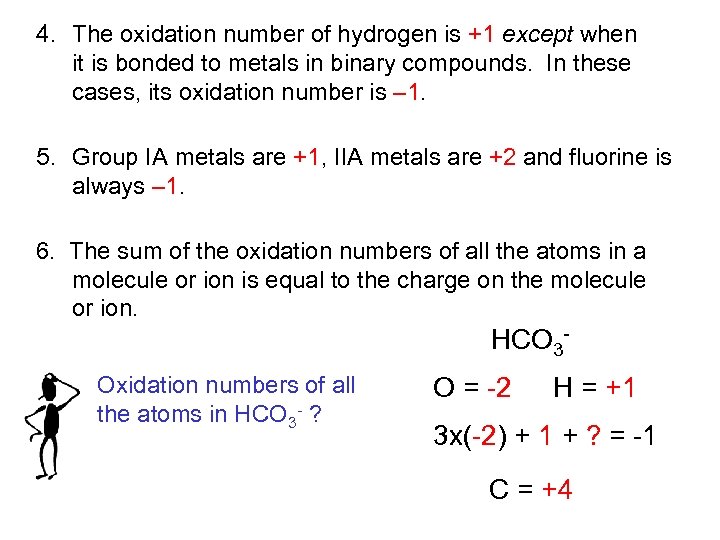
4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is – 1. 5. Group IA metals are +1, IIA metals are +2 and fluorine is always – 1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. HCO 3 Oxidation numbers of all the atoms in HCO 3 - ? O = -2 H = +1 3 x(-2) + 1 + ? = -1 C = +4
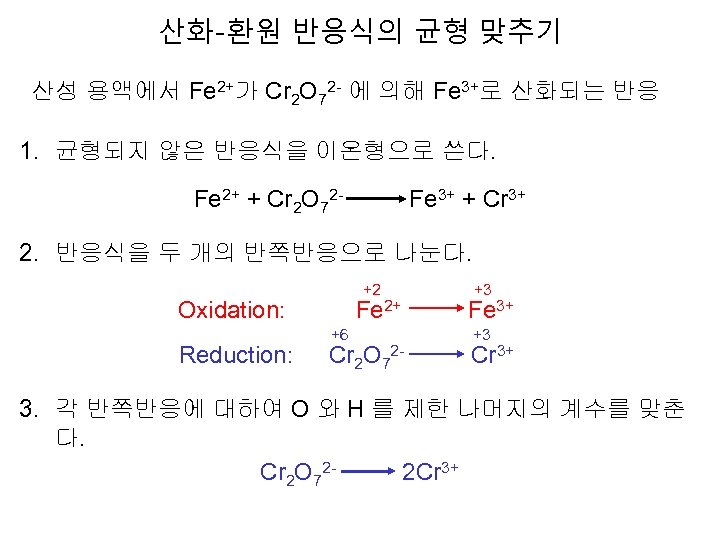
산화-환원 반응식의 균형 맞추기 산성 용액에서 Fe 2+가 Cr 2 O 72 - 에 의해 Fe 3+로 산화되는 반응 1. 균형되지 않은 반응식을 이온형으로 쓴다. Fe 2+ + Cr 2 O 72 - Fe 3+ + Cr 3+ 2. 반응식을 두 개의 반쪽반응으로 나눈다. +2 Oxidation: Reduction: +3 Fe 2+ Fe 3+ +6 Cr 2 O 7 +3 2 - Cr 3+ 3. 각 반쪽반응에 대하여 O 와 H 를 제한 나머지의 계수를 맞춘 다. Cr 2 O 72 - 2 Cr 3+
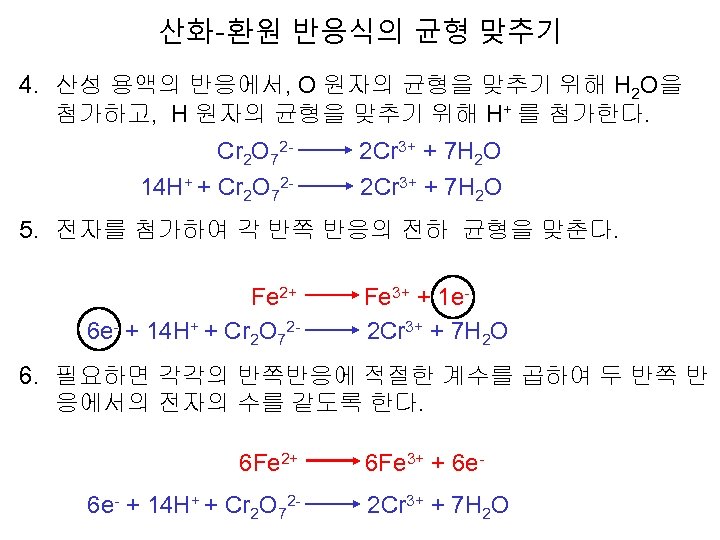
산화-환원 반응식의 균형 맞추기 4. 산성 용액의 반응에서, O 원자의 균형을 맞추기 위해 H 2 O을 첨가하고, H 원자의 균형을 맞추기 위해 H+ 를 첨가한다. Cr 2 O 72 - 2 Cr 3+ + 7 H 2 O 14 H+ + Cr 2 O 72 - 2 Cr 3+ + 7 H 2 O 5. 전자를 첨가하여 각 반쪽 반응의 전하 균형을 맞춘다. Fe 2+ Fe 3+ + 1 e 6 e- + 14 H+ + Cr 2 O 72 - 2 Cr 3+ + 7 H 2 O 6. 필요하면 각각의 반쪽반응에 적절한 계수를 곱하여 두 반쪽 반 응에서의 전자의 수를 같도록 한다. 6 Fe 2+ 6 Fe 3+ + 6 e 6 e- + 14 H+ + Cr 2 O 72 - 2 Cr 3+ + 7 H 2 O

산화-환원 반응식의 균형 맞추기 7. 두 반쪽반응을 더하여 최종적으로 균형을 맞춘다. 양변의 전 자수가 상쇄되어야 한다. Oxidation: 6 Fe 2+ 6 Fe 3+ + 6 e- Reduction: 6 e- + 14 H+ + Cr 2 O 72 - 2 Cr 3+ + 7 H 2 O 14 H+ + Cr 2 O 72 - + 6 Fe 2+ 6 Fe 3+ + 2 Cr 3+ + 7 H 2 O 8. 균형을 맞춘 반응식의 각 변의 원자와 전자의 수가 같은지 확인 한다. 14 x 1 – 2 + 6 x 2 = 24 = 6 x 3 + 2 x 3 9. 염기성 용액에 대하여는, 최종 식에 나오는 H+ 마다 OH-를 양 변에 모두 추가한다. .
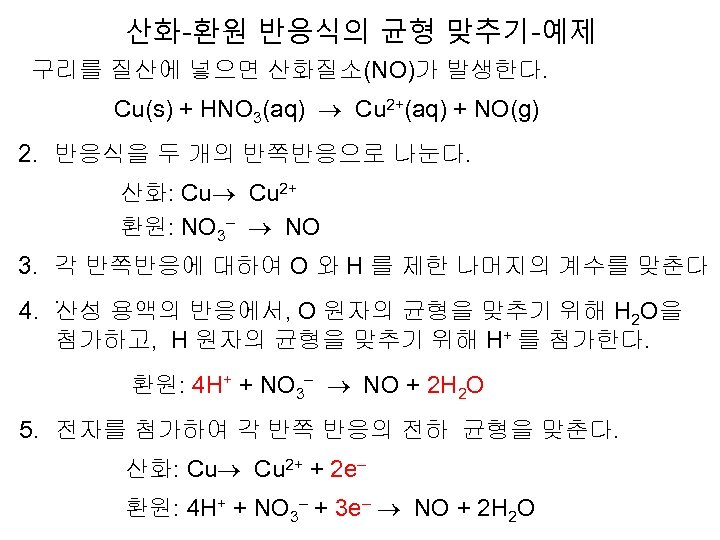
산화-환원 반응식의 균형 맞추기-예제 구리를 질산에 넣으면 산화질소(NO)가 발생한다. Cu(s) + HNO 3(aq) Cu 2+(aq) + NO(g) 2. 반응식을 두 개의 반쪽반응으로 나눈다. 산화: Cu 2+ 환원: NO 3– NO 3. 각 반쪽반응에 대하여 O 와 H 를 제한 나머지의 계수를 맞춘다. 4. 산성 용액의 반응에서, O 원자의 균형을 맞추기 위해 H 2 O을 첨가하고, H 원자의 균형을 맞추기 위해 H+ 를 첨가한다. 환원: 4 H+ + NO 3– NO + 2 H 2 O 5. 전자를 첨가하여 각 반쪽 반응의 전하 균형을 맞춘다. 산화: Cu 2+ + 2 e– 환원: 4 H+ + NO 3– + 3 e– NO + 2 H 2 O
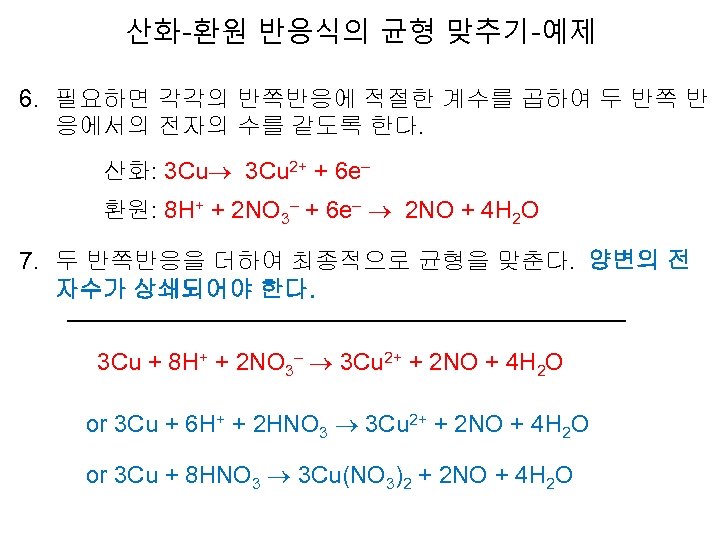
산화-환원 반응식의 균형 맞추기-예제 6. 필요하면 각각의 반쪽반응에 적절한 계수를 곱하여 두 반쪽 반 응에서의 전자의 수를 같도록 한다. 산화: 3 Cu 2+ + 6 e– 환원: 8 H+ + 2 NO 3– + 6 e– 2 NO + 4 H 2 O 7. 두 반쪽반응을 더하여 최종적으로 균형을 맞춘다. 양변의 전 자수가 상쇄되어야 한다. 3 Cu + 8 H+ + 2 NO 3– 3 Cu 2+ + 2 NO + 4 H 2 O or 3 Cu + 6 H+ + 2 HNO 3 3 Cu 2+ + 2 NO + 4 H 2 O or 3 Cu + 8 HNO 3 3 Cu(NO 3)2 + 2 NO + 4 H 2 O

산화-환원 반응식의 균형 맞추기-예제 1) H 2 O 2 + Fe 2+ → Fe 3+ + H 2 O (acidic) 2) Cu + HNO 3 → Cu 2+ + NO + H 2 O (acidic) 3) CN- + Mn. O 4 - → CNO- + Mn. O 2 (basic, 힌트 C의 산화수는 양쪽에서 모두 +2) 4) Br 2 → Br. O 3 - + Br- (basic, 힌트 Br 이 산화와 환원이 함께 일어난다) 2 5) Cr(OH)3(s) + Cl. O 3−(aq) → Cr. O 42−(aq) + Cl−(aq) (basic)
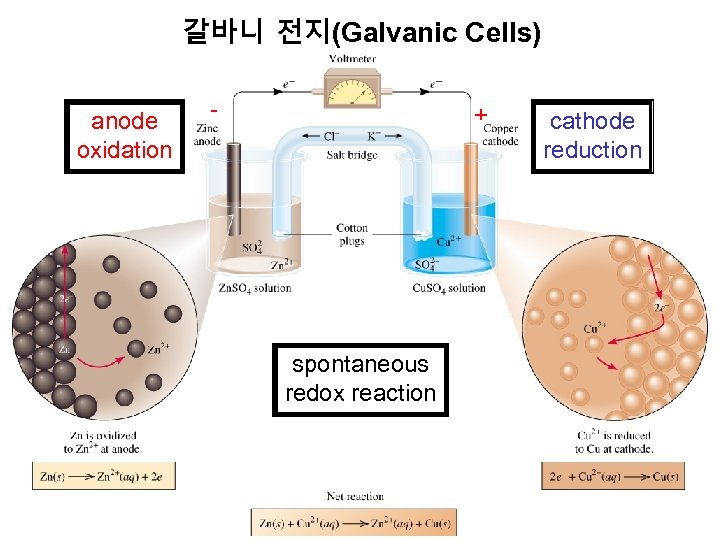
갈바니 전지(Galvanic Cells) anode oxidation - + spontaneous redox reaction cathode reduction
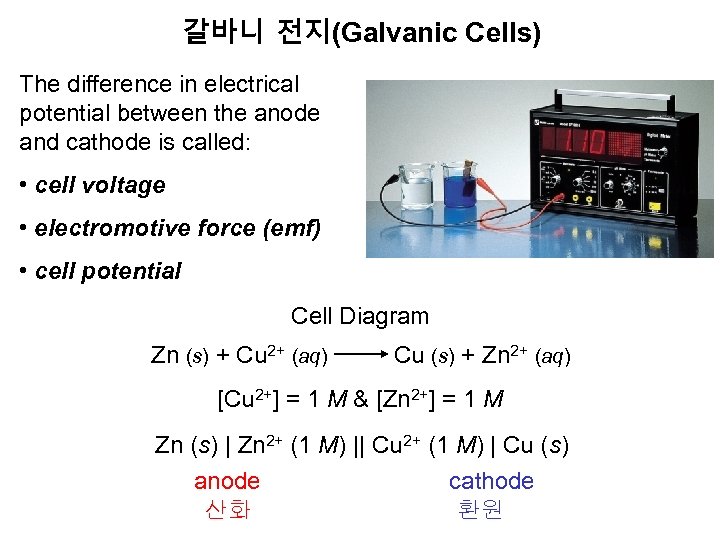
갈바니 전지(Galvanic Cells) The difference in electrical potential between the anode and cathode is called: • cell voltage • electromotive force (emf) • cell potential Cell Diagram Zn (s) + Cu 2+ (aq) Cu (s) + Zn 2+ (aq) [Cu 2+] = 1 M & [Zn 2+] = 1 M Zn (s) | Zn 2+ (1 M) || Cu 2+ (1 M) | Cu (s) anode cathode 산화 환원
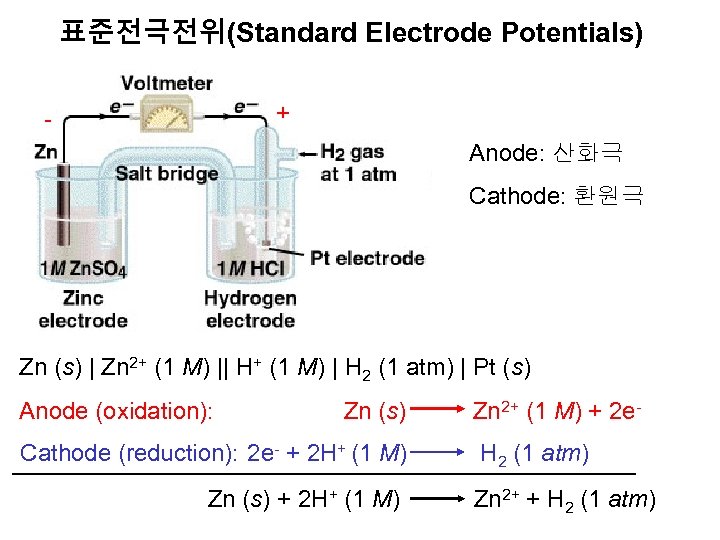
표준전극전위(Standard Electrode Potentials) + - Anode: 산화극 Cathode: 환원극 Zn (s) | Zn 2+ (1 M) || H+ (1 M) | H 2 (1 atm) | Pt (s) Anode (oxidation): Zn (s) Zn 2+ (1 M) + 2 e- Cathode (reduction): 2 e- + 2 H+ (1 M) H 2 (1 atm) Zn (s) + 2 H+ (1 M) Zn 2+ + H 2 (1 atm)
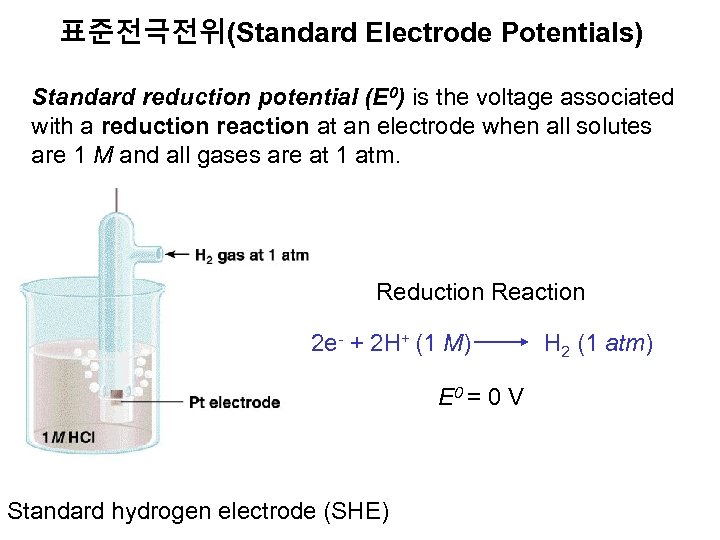
표준전극전위(Standard Electrode Potentials) Standard reduction potential (E 0) is the voltage associated with a reduction reaction at an electrode when all solutes are 1 M and all gases are at 1 atm. Reduction Reaction 2 e- + 2 H+ (1 M) H 2 (1 atm) E 0 = 0 V Standard hydrogen electrode (SHE)
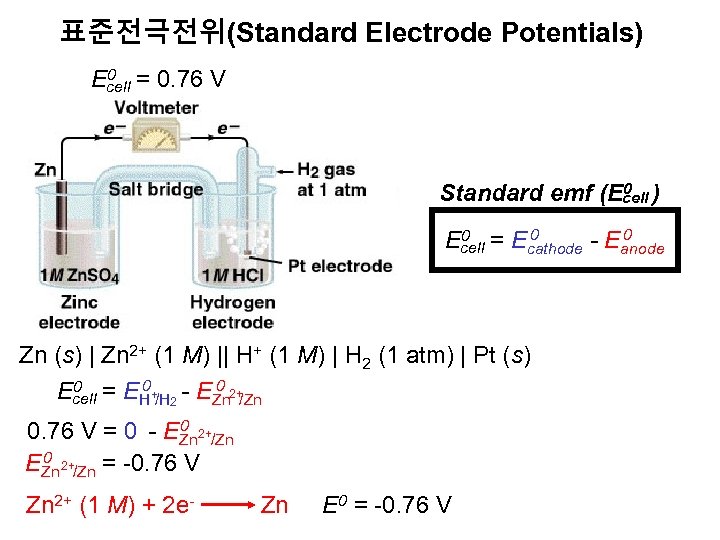
표준전극전위(Standard Electrode Potentials) 0 = 0. 76 V Ecell 0 Standard emf (Ecell ) 0 0 = E 0 Ecell cathode - Eanode Zn (s) | Zn 2+ (1 M) || H+ (1 M) | H 2 (1 atm) | Pt (s) 0 = E 0 + - E 0 2+ Ecell H /H Zn /Zn 2 0 2+ 0. 76 V = 0 - EZn /Zn 0 2+ EZn /Zn = -0. 76 V Zn 2+ (1 M) + 2 e- Zn E 0 = -0. 76 V
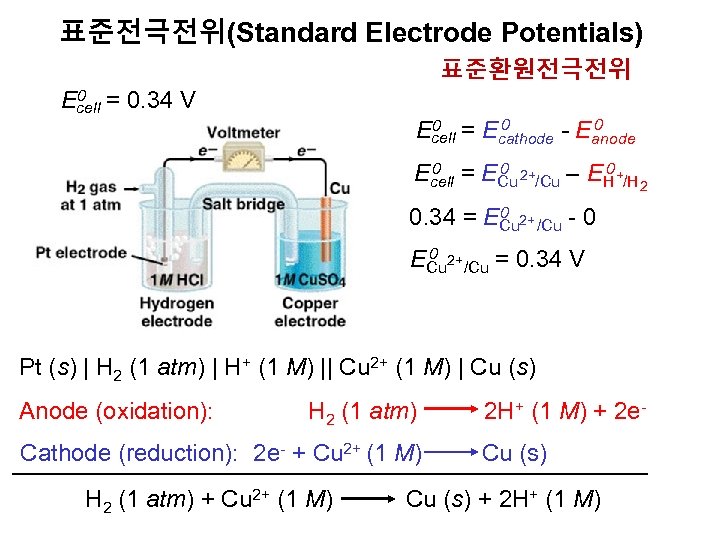
표준전극전위(Standard Electrode Potentials) 표준환원전극전위 0 = 0. 34 V Ecell 0 0 = E 0 Ecell cathode - Eanode 0 0 2+ 0+ Ecell = ECu /Cu – EH /H 2 0 2+ 0. 34 = ECu /Cu - 0 0 2+ ECu /Cu = 0. 34 V Pt (s) | H 2 (1 atm) | H+ (1 M) || Cu 2+ (1 M) | Cu (s) Anode (oxidation): H 2 (1 atm) 2 H+ (1 M) + 2 e- Cathode (reduction): 2 e- + Cu 2+ (1 M) Cu (s) H 2 (1 atm) + Cu 2+ (1 M) Cu (s) + 2 H+ (1 M)
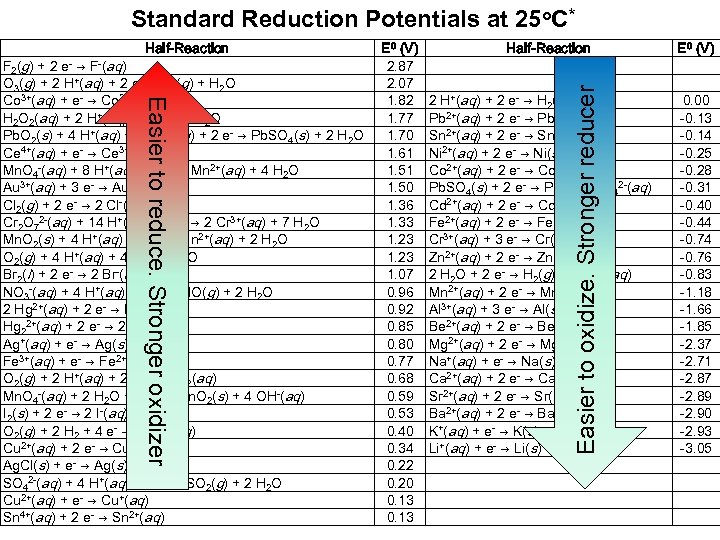
Standard Reduction Potentials at 25 o. C* Easier to reduce. Stronger oxidizer F 2(g) + 2 e- → F-(aq) O 3(g) + 2 H+(aq) + 2 e- → O 2(g) + H 2 O Co 3+(aq) + e- → Co 2+(aq) H 2 O 2(aq) + 2 H+(aq) + 2 e- → 2 H 2 O Pb. O 2(s) + 4 H+(aq) + SO 42 -(aq) + 2 e- → Pb. SO 4(s) + 2 H 2 O Ce 4+(aq) + e- → Ce 3+(aq) Mn. O 4 -(aq) + 8 H+(aq) + 5 e- → Mn 2+(aq) + 4 H 2 O Au 3+(aq) + 3 e- → Au(s) Cl 2(g) + 2 e- → 2 Cl-(aq) Cr 2 O 72 -(aq) + 14 H+(aq) + 6 e- → 2 Cr 3+(aq) + 7 H 2 O Mn. O 2(s) + 4 H+(aq) + 2 e- → Mn 2+(aq) + 2 H 2 O O 2(g) + 4 H+(aq) + 4 e- → 2 H 2 O Br 2(l) + 2 e- → 2 Br-(aq) NO 3 -(aq) + 4 H+(aq) + 3 e- → NO(g) + 2 H 2 O 2 Hg 2+(aq) + 2 e- → Hg 22+(aq) + 2 e- → 2 Hg(l) Ag+(aq) + e- → Ag(s) Fe 3+(aq) + e- → Fe 2+(aq) O 2(g) + 2 H+(aq) + 2 e- → H 2 O 2(aq) Mn. O 4 -(aq) + 2 H 2 O + 3 e- → Mn. O 2(s) + 4 OH-(aq) I 2(s) + 2 e- → 2 I-(aq) O 2(g) + 2 H 2 + 4 e- → 4 OH-(aq) Cu 2+(aq) + 2 e- → Cu(s) Ag. Cl(s) + e- → Ag(s) + Cl-(aq) SO 42 -(aq) + 4 H+(aq) + 2 e- → SO 2(g) + 2 H 2 O Cu 2+(aq) + e- → Cu+(aq) Sn 4+(aq) + 2 e- → Sn 2+(aq) E 0 (V) 2. 87 2. 07 1. 82 1. 77 1. 70 1. 61 1. 50 1. 36 1. 33 1. 23 1. 07 0. 96 0. 92 0. 85 0. 80 0. 77 0. 68 0. 59 0. 53 0. 40 0. 34 0. 22 0. 20 0. 13 Half-Reaction 2 H+(aq) + 2 e- → H 2(g) Pb 2+(aq) + 2 e- → Pb(s) Sn 2+(aq) + 2 e- → Sn(s) Ni 2+(aq) + 2 e- → Ni(s) Co 2+(aq) + 2 e- → Co(s) Pb. SO 4(s) + 2 e- → Pb(s) + SO 42 -(aq) Cd 2+(aq) + 2 e- → Cd(s) Fe 2+(aq) + 2 e- → Fe(s) Cr 3+(aq) + 3 e- → Cr(s) Zn 2+(aq) + 2 e- → Zn(s) 2 H 2 O + 2 e- → H 2(g) + 2 OH-(aq) Mn 2+(aq) + 2 e- → Mn(s) Al 3+(aq) + 3 e- → Al(s) Be 2+(aq) + 2 e- → Be(s) Mg 2+(aq) + 2 e- → Mg(s) Na+(aq) + e- → Na(s) Ca 2+(aq) + 2 e- → Ca(s) Sr 2+(aq) + 2 e- → Sr(s) Ba 2+(aq) + 2 e- → Ba(s) K+(aq) + e- → K(s) Li+(aq) + e- → Li(s) Easier to oxidize. Stronger reducer Half-Reaction E 0 (V) 0. 00 -0. 13 -0. 14 -0. 25 -0. 28 -0. 31 -0. 40 -0. 44 -0. 76 -0. 83 -1. 18 -1. 66 -1. 85 -2. 37 -2. 71 -2. 87 -2. 89 -2. 90 -2. 93 -3. 05

갈바니 전지에 대한 전위 계산 1. 0 0 = E 0 Ecell - Eanode cathode 2. 열역학 방법, using DG = -n. FE
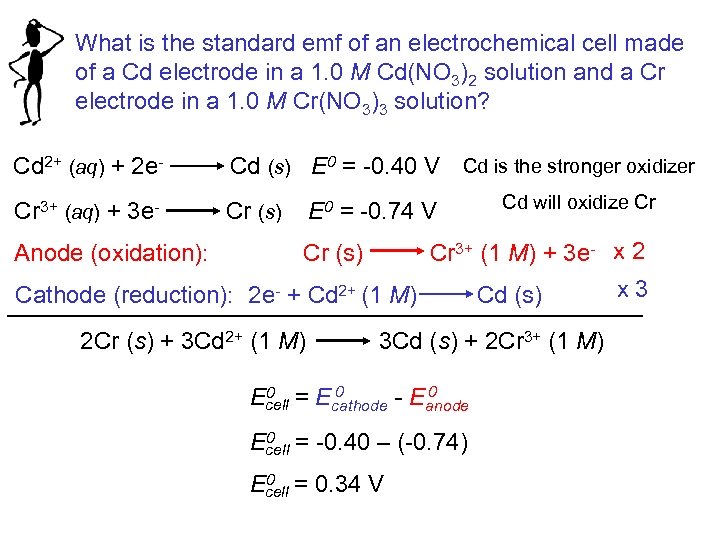
What is the standard emf of an electrochemical cell made of a Cd electrode in a 1. 0 M Cd(NO 3)2 solution and a Cr electrode in a 1. 0 M Cr(NO 3)3 solution? Cd 2+ (aq) + 2 e- Cd (s) E 0 = -0. 40 V Cd is the stronger oxidizer Cr 3+ (aq) + 3 e- Cr (s) E 0 = -0. 74 V Cd will oxidize Cr Cr (s) Cr 3+ (1 M) + 3 e- x 2 x 3 Cathode (reduction): 2 e- + Cd 2+ (1 M) Cd (s) Anode (oxidation): 2 Cr (s) + 3 Cd 2+ (1 M) 3 Cd (s) + 2 Cr 3+ (1 M) 0 0 = E 0 Ecell cathode - Eanode 0 = -0. 40 – (-0. 74) Ecell 0 = 0. 34 V Ecell
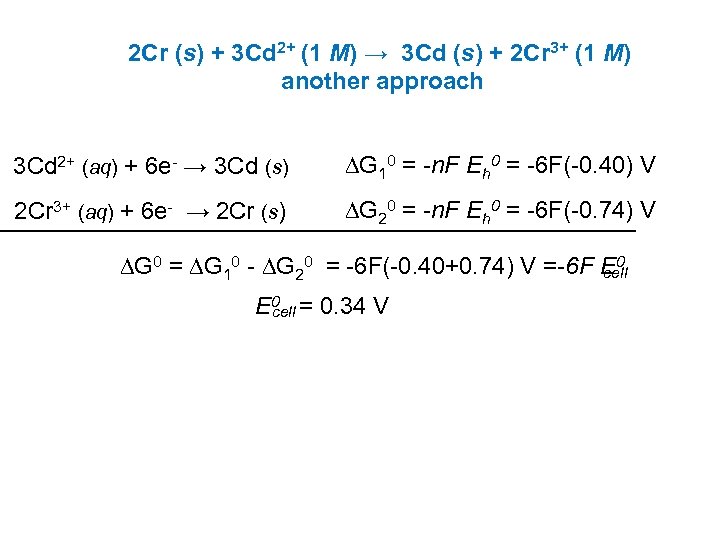
2 Cr (s) + 3 Cd 2+ (1 M) → 3 Cd (s) + 2 Cr 3+ (1 M) another approach 3 Cd 2+ (aq) + 6 e- → 3 Cd (s) DG 10 = -n. F Eh 0 = -6 F(-0. 40) V 2 Cr 3+ (aq) + 6 e- → 2 Cr (s) DG 20 = -n. F Eh 0 = -6 F(-0. 74) V DG 0 = DG 10 - DG 20 = -6 F(-0. 40+0. 74) V =-6 F E 0 cell E 0 = 0. 34 V cell
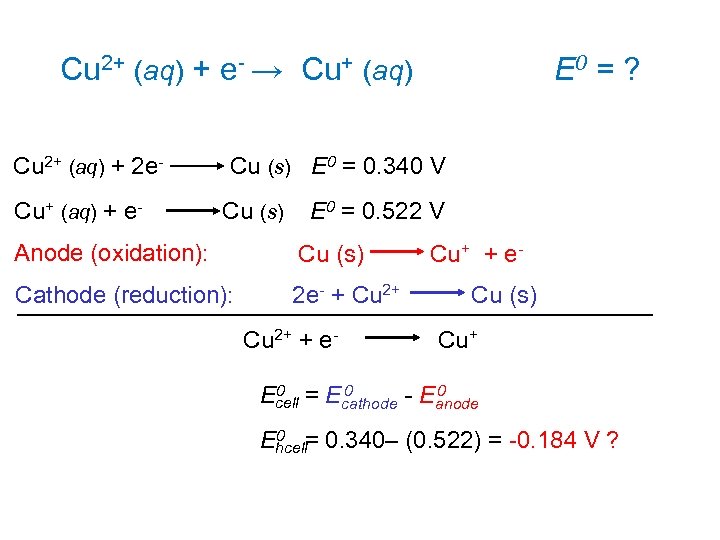
Cu 2+ (aq) + e- → Cu+ (aq) E 0 = ? Cu 2+ (aq) + 2 e- Cu (s) E 0 = 0. 340 V Cu+ (aq) + e- Cu (s) E 0 = 0. 522 V Anode (oxidation): Cu (s) Cu+ + e- Cathode (reduction): 2 e- + Cu 2+ Cu (s) Cu 2+ + e- Cu+ 0 0 = E 0 Ecell - Eanode cathode 0 = 0. 340– (0. 522) = -0. 184 V ? Ehcell
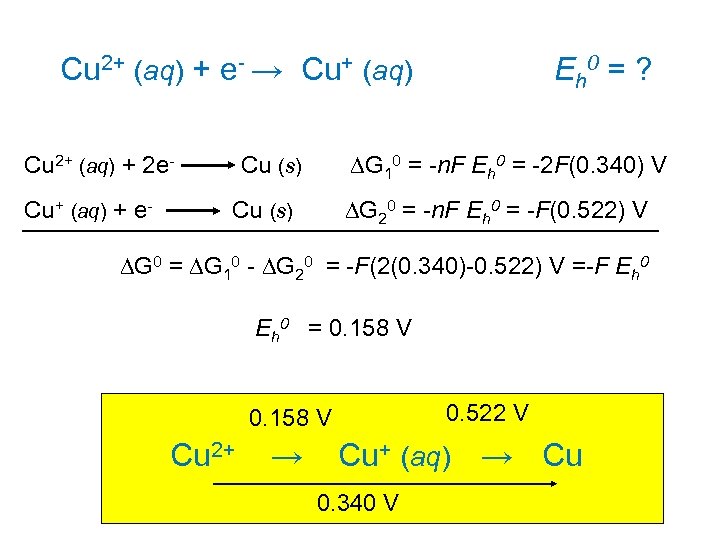
Cu 2+ (aq) + e- → Cu+ (aq) Eh 0 = ? Cu 2+ (aq) + 2 e- Cu (s) DG 10 = -n. F Eh 0 = -2 F(0. 340) V Cu+ (aq) + e- Cu (s) DG 20 = -n. F Eh 0 = -F(0. 522) V DG 0 = DG 10 - DG 20 = -F(2(0. 340)-0. 522) V =-F Eh 0 = 0. 158 V 0. 522 V Cu 2+ → Cu+ (aq) → Cu 0. 340 V
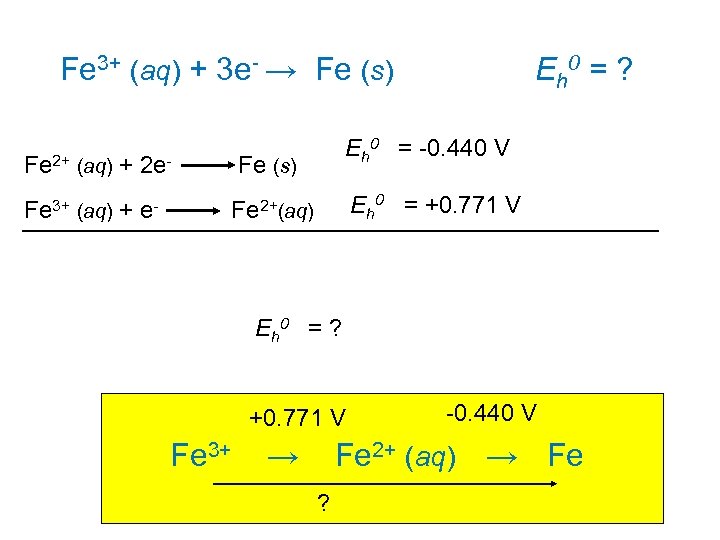
Fe 3+ (aq) + 3 e- → Fe (s) Eh 0 = ? Eh 0 = -0. 440 V Fe 2+ (aq) + 2 e- Fe (s) Fe 3+ (aq) + e- Fe 2+(aq) Eh 0 = +0. 771 V Eh 0 = ? +0. 771 V -0. 440 V Fe 3+ → Fe 2+ (aq) → Fe ?
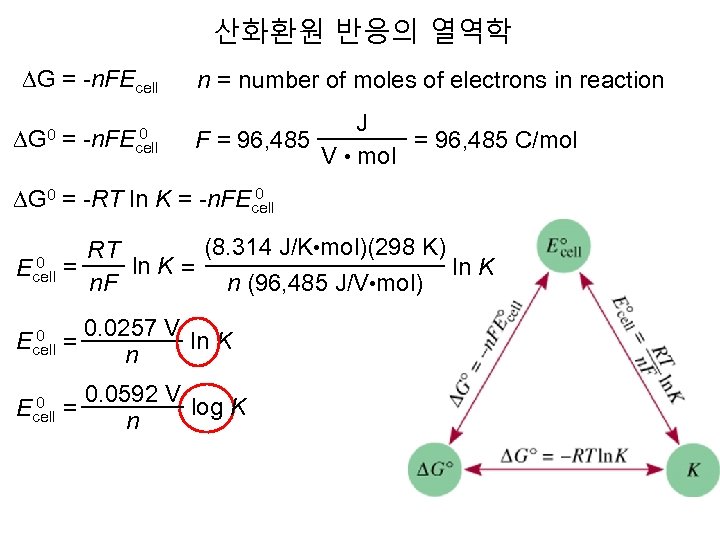
산화환원 반응의 열역학 DG = -n. FEcell 0 DG 0 = -n. FEcell n = number of moles of electrons in reaction J F = 96, 485 C/mol V • mol 0 DG 0 = -RT ln K = -n. FEcell (8. 314 J/K • mol)(298 K) RT 0 ln K = ln K Ecell = n. F n (96, 485 J/V • mol) 0 Ecell = 0 Ecell 0. 0257 V ln K n 0. 0592 V log K = n
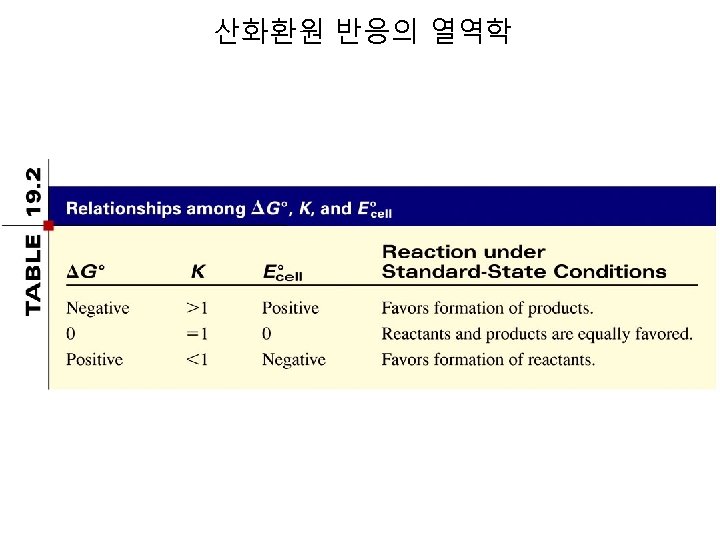
산화환원 반응의 열역학
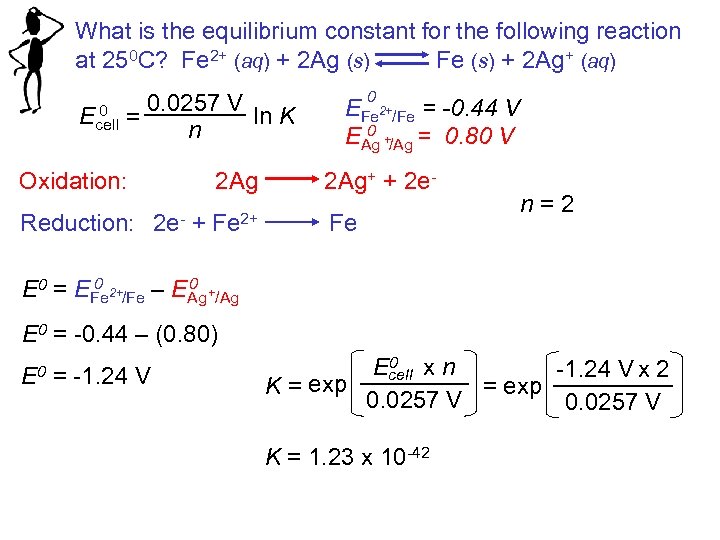
What is the equilibrium constant for the following reaction at 250 C? Fe 2+ (aq) + 2 Ag (s) Fe (s) + 2 Ag+ (aq) 0 Ecell 0. 0257 V ln K = n Oxidation: Reduction: 0 2+ EFe /Fe = -0. 44 V 0 + EAg /Ag = 0. 80 V 2 Ag 2 Ag+ + 2 e 2 e- + Fe 2+ Fe n = 2 0 0 E 0 = EFe /Fe – EAg /Ag 2+ + E 0 = -0. 44 – (0. 80) E 0 = -1. 24 V 0 Ecell x n -1. 24 V x 2 exp = exp K = 0. 0257 V K = 1. 23 x 10 -42
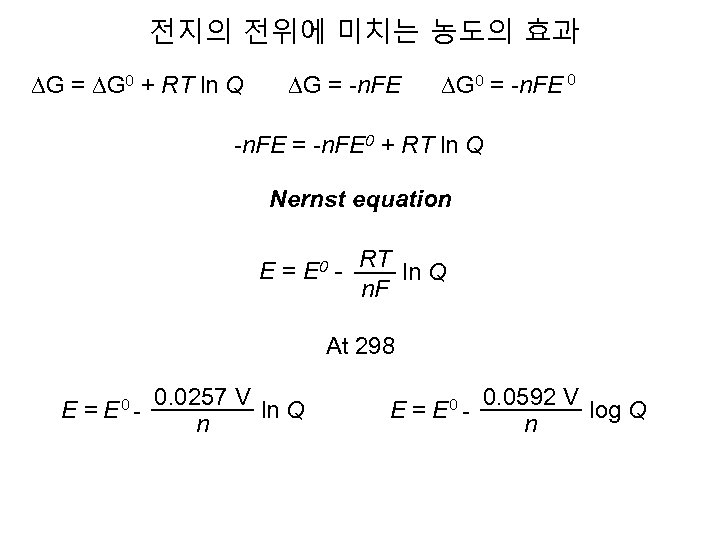
전지의 전위에 미치는 농도의 효과 DG = DG 0 + RT ln Q DG = -n. FE DG 0 = -n. FE 0 -n. FE = -n. FE 0 + RT ln Q Nernst equation E = E 0 - RT ln Q n. F At 298 E = E 0 - 0. 0257 V ln Q n E = E 0 - 0. 0592 V log Q n
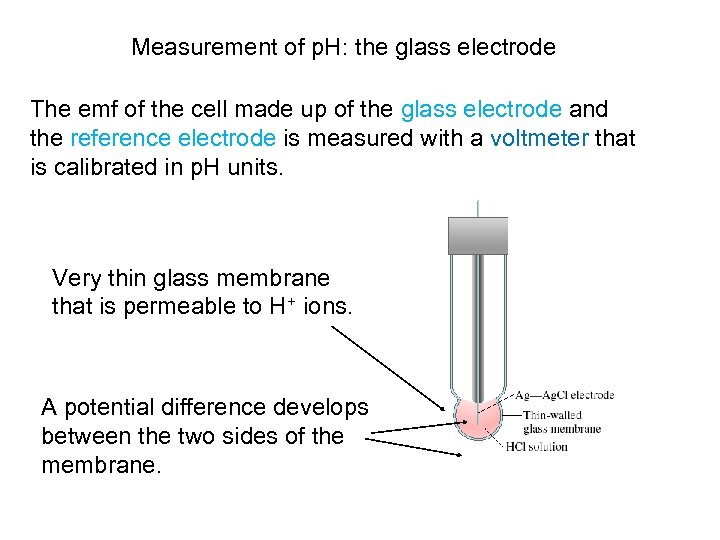
Measurement of p. H: the glass electrode The emf of the cell made up of the glass electrode and the reference electrode is measured with a voltmeter that is calibrated in p. H units. Very thin glass membrane that is permeable to H+ ions. A potential difference develops between the two sides of the membrane.
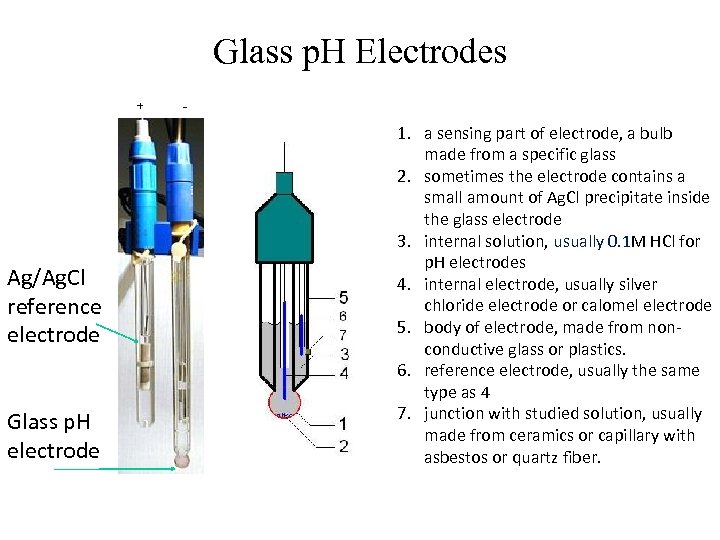
Glass p. H Electrodes + - Ag/Ag. Cl reference electrode Glass p. H electrode 1. a sensing part of electrode, a bulb made from a specific glass 2. sometimes the electrode contains a small amount of Ag. Cl precipitate inside the glass electrode 3. internal solution, usually 0. 1 M HCl for p. H electrodes 4. internal electrode, usually silver chloride electrode or calomel electrode 5. body of electrode, made from nonconductive glass or plastics. 6. reference electrode, usually the same type as 4 7. junction with studied solution, usually made from ceramics or capillary with asbestos or quartz fiber.
![Will the following reaction occur spontaneously at 250 C if [Fe 2+] = 0. Will the following reaction occur spontaneously at 250 C if [Fe 2+] = 0.](https://present5.com/presentation/553b0154236fe855d75ba9d85e6e0607/image-31.jpg)
Will the following reaction occur spontaneously at 250 C if [Fe 2+] = 0. 60 M and [Cd 2+] = 0. 010 M? 0 2+ Fe 2+ (aq) + Cd (s) Fe (s) + Cd 2+ (aq) EFe /Fe = -0. 44 V 0 2+ ECd /Cd = -0. 40 V Oxidation: Cd 2+ + 2 e- Reduction: 2 e- + Fe 2+ 2 Fe 0 0 E 0 = EFe /Fe – ECd /Cd 2+ 2+ E 0 = -0. 44 – (-0. 40) E 0 = -0. 04 V n = 2 0. 0257 V ln Q n 0. 010 0. 0257 V ln E = -0. 04 V 2 0. 60 E = 0. 013 E = E 0 - E > 0 Spontaneous
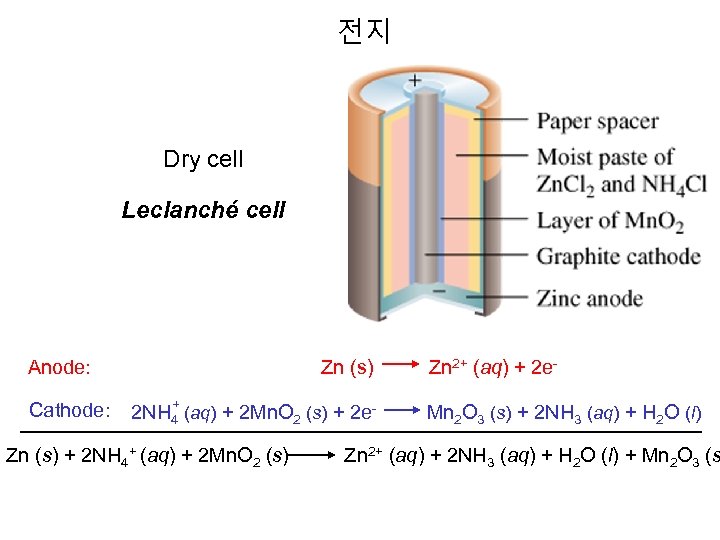
전지 Dry cell Leclanché cell Anode: Cathode: Zn (s) Zn 2+ (aq) + 2 e+ 2 NH 4 (aq) + 2 Mn. O 2 (s) + 2 e- Mn 2 O 3 (s) + 2 NH 3 (aq) + H 2 O (l) Zn (s) + 2 NH 4+ (aq) + 2 Mn. O 2 (s) Zn 2+ (aq) + 2 NH 3 (aq) + H 2 O (l) + Mn 2 O 3 (s
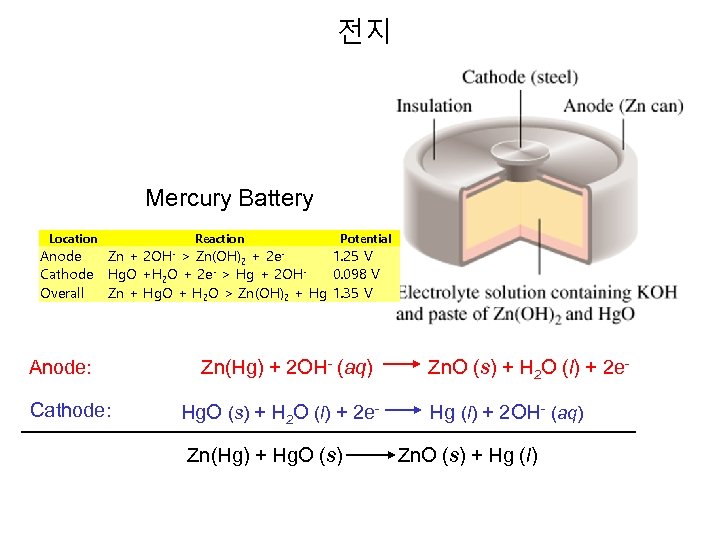
전지 Mercury Battery Location Anode Cathode Overall Reaction Potential Zn + 2 OH- > Zn(OH)2 + 2 e 1. 25 V - > Hg + 2 OHHg. O +H 2 O + 2 e 0. 098 V Zn + Hg. O + H 2 O > Zn(OH)2 + Hg 1. 35 V Anode: Cathode: Zn(Hg) + 2 OH- (aq) Zn. O (s) + H 2 O (l) + 2 e. Hg. O (s) + H 2 O (l) + 2 e- Hg (l) + 2 OH- (aq) Zn(Hg) + Hg. O (s) Zn. O (s) + Hg (l)
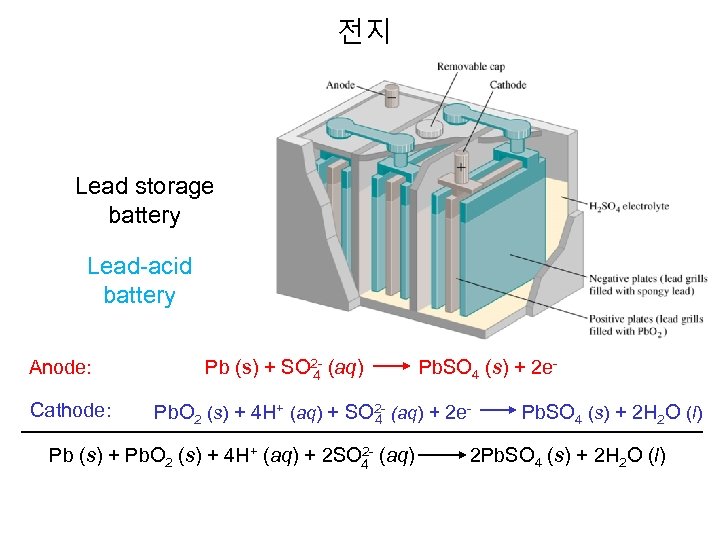
전지 Lead storage battery Lead-acid battery Anode: Cathode: Pb (s) + SO 2 - (aq) Pb. SO 4 (s) + 2 e 4 Pb. O 2 (s) + 4 H+ (aq) + SO 2 - (aq) + 2 e- Pb. SO 4 (s) + 2 H 2 O (l) 4 2 Pb (s) + Pb. O 2 (s) + 4 H+ (aq) + 2 SO 4 (aq) 2 Pb. SO 4 (s) + 2 H 2 O (l)
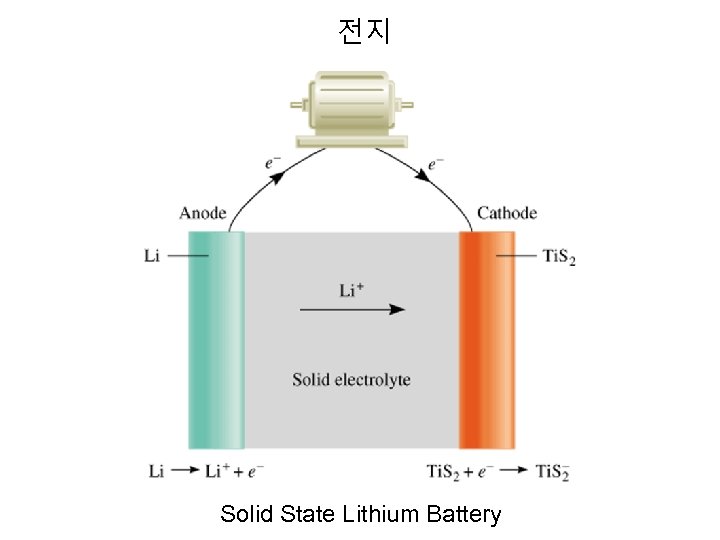
전지 Solid State Lithium Battery
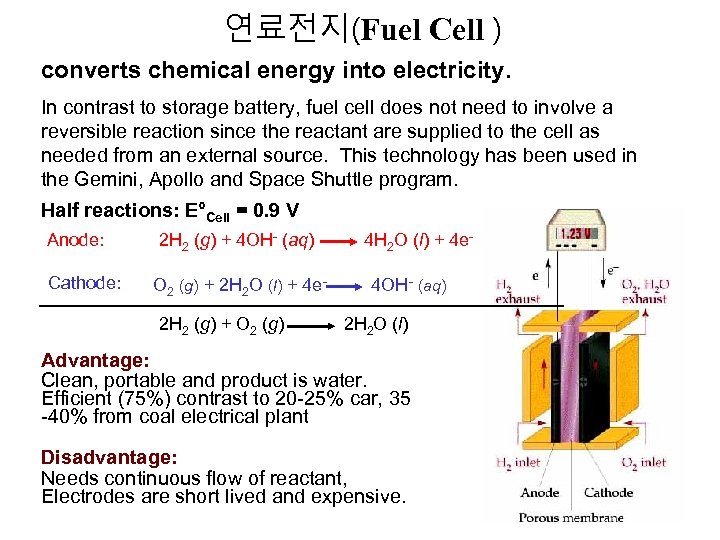
연료전지(Fuel Cell ) converts chemical energy into electricity. In contrast to storage battery, fuel cell does not need to involve a reversible reaction since the reactant are supplied to the cell as needed from an external source. This technology has been used in the Gemini, Apollo and Space Shuttle program. Half reactions: E°Cell = 0. 9 V Anode: Cathode: 2 H 2 (g) + 4 OH- (aq) 4 H 2 O (l) + 4 e. O 2 (g) + 2 H 2 O (l) + 4 e- 4 OH- (aq) 2 H 2 (g) + O 2 (g) 2 H 2 O (l) Advantage: Clean, portable and product is water. Efficient (75%) contrast to 20 -25% car, 35 -40% from coal electrical plant Disadvantage: Needs continuous flow of reactant, Electrodes are short lived and expensive.
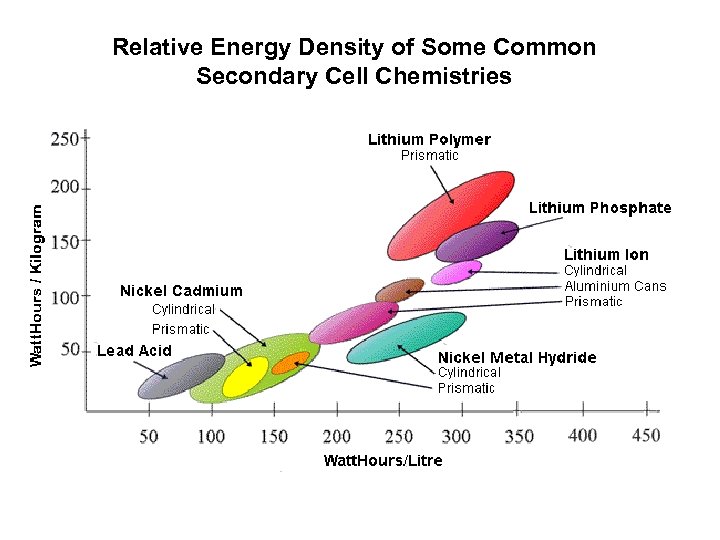
Relative Energy Density of Some Common Secondary Cell Chemistries
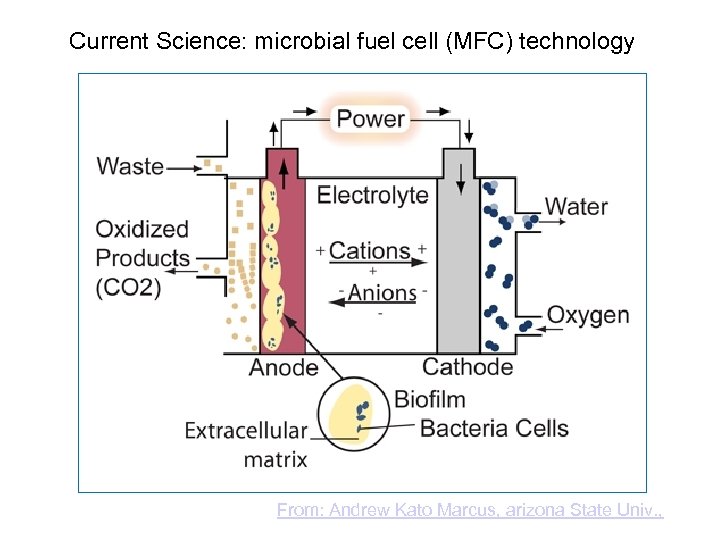
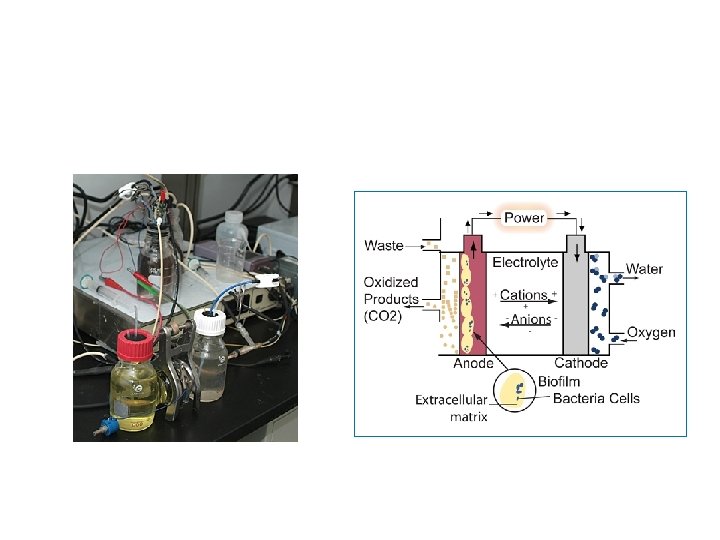
Current Science: microbial fuel cell (MFC) technology From: Andrew Kato Marcus, arizona State Univ. ,
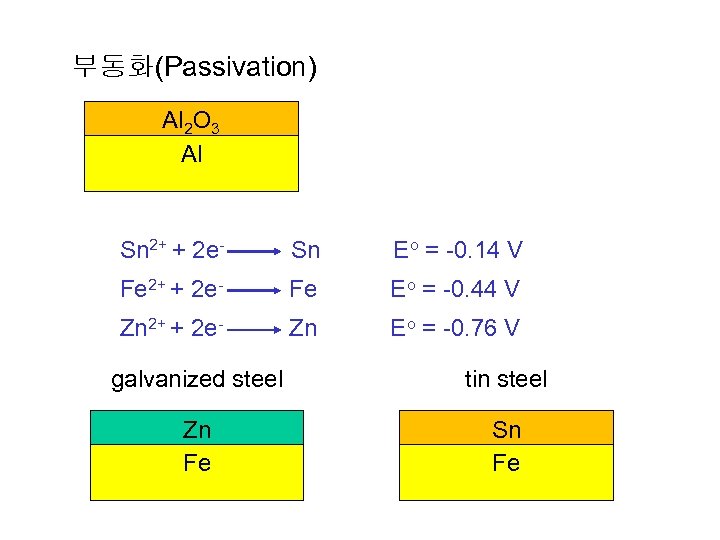
부동화(Passivation) Al 2 O 3 Al Sn 2+ + 2 e- Sn Eo = -0. 14 V Fe 2+ + 2 e- Fe Eo = -0. 44 V Zn 2+ + 2 e- Zn Eo = -0. 76 V galvanized steel tin steel Zn Fe Sn Fe
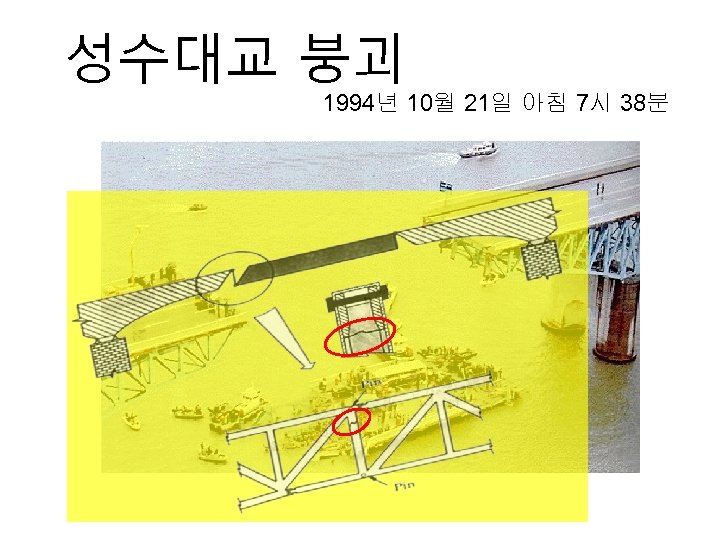
성수대교 붕괴 1994년 10월 21일 아침 7시 38분
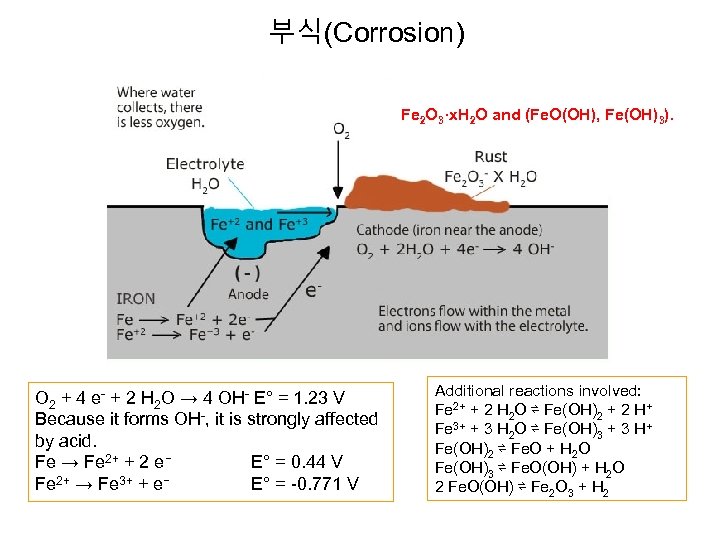
부식(Corrosion) Fe 2 O 3·x. H 2 O and (Fe. O(OH), Fe(OH)3). O 2 + 4 e- + 2 H 2 O → 4 OH- E° = 1. 23 V Because it forms OH-, it is strongly affected by acid. Fe → Fe 2+ + 2 e− E° = 0. 44 V Fe 2+ → Fe 3+ + e− E° = -0. 771 V Additional reactions involved: Fe 2+ + 2 H 2 O ⇌ Fe(OH)2 + 2 H+ Fe 3+ + 3 H 2 O ⇌ Fe(OH)3 + 3 H+ Fe(OH)2 ⇌ Fe. O + H 2 O Fe(OH)3 ⇌ Fe. O(OH) + H 2 O 2 Fe. O(OH) ⇌ Fe 2 O 3 + H 2
Conditions for Corrosion Conditions for Iron Oxidation: Iron will oxidize in acidic medium SO 2 g H 2 SO 4 g H+ + HSO 4+ Anions improve conductivity for oxidation. Cl- from seawater or Na. Cl (snow melting) enhances rusting Conditions for Prevention: Iron will not rust in dry air; moisture must be present Iron will not rust in air-free water; oxygen must be present Iron rusts most rapidly in ionic solution and low p. H (high H+) The loss of iron and deposit of rust occur at different placm on object Iron rust faster in contact with a less active metal (Cu) Iron rust slower in contact with a more active metal (Zn)
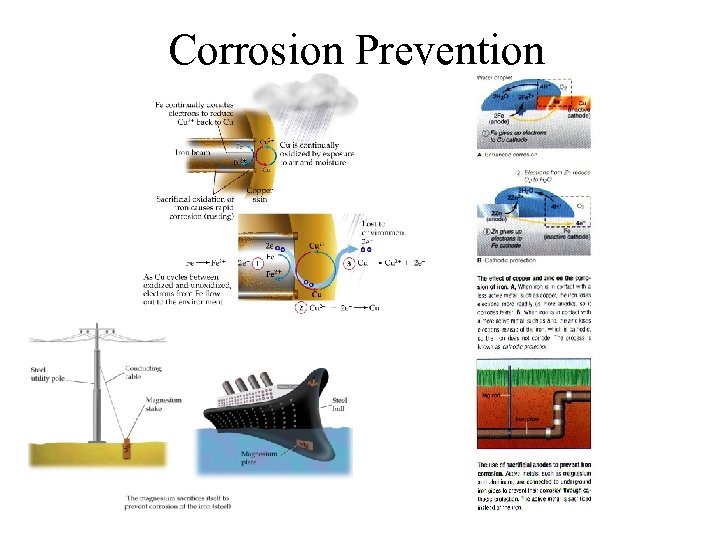
Corrosion Prevention
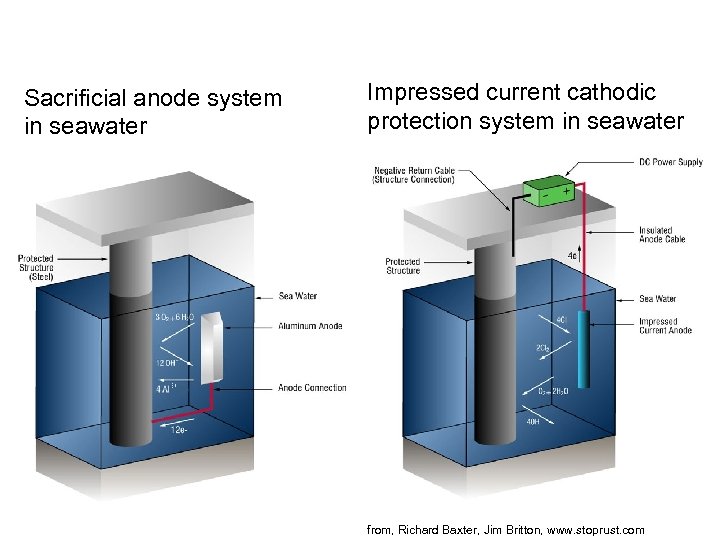
Sacrificial anode system in seawater Impressed current cathodic protection system in seawater from, Richard Baxter, Jim Britton, www. stoprust. com
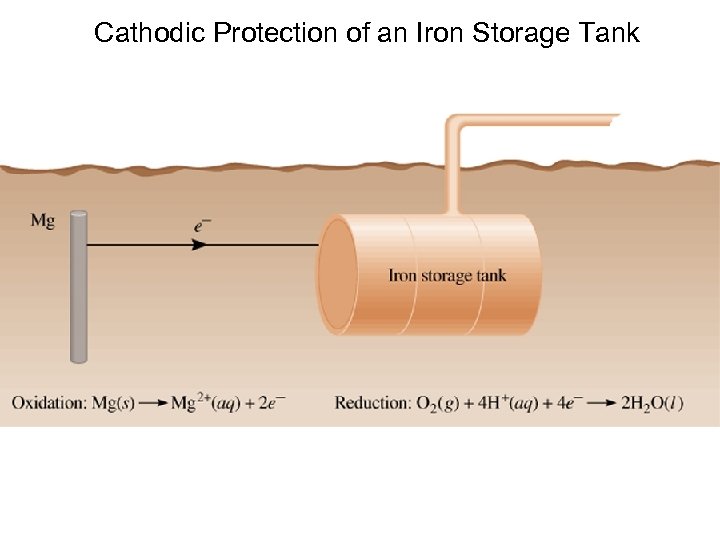
Cathodic Protection of an Iron Storage Tank
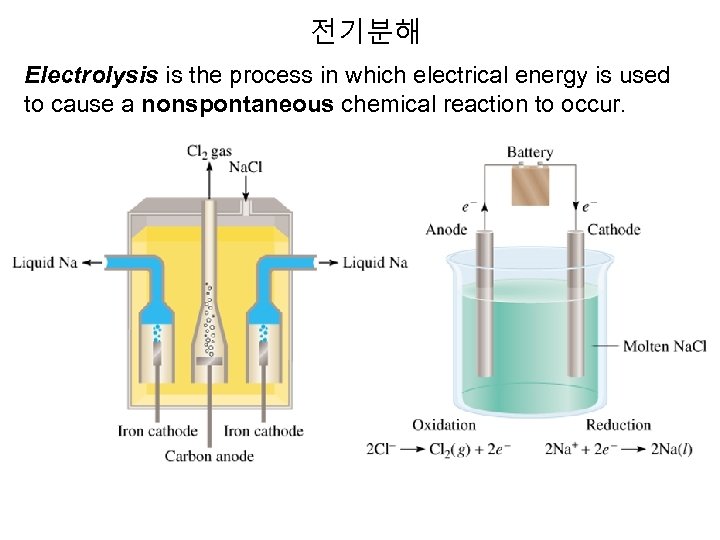
전기분해 Electrolysis is the process in which electrical energy is used to cause a nonspontaneous chemical reaction to occur.
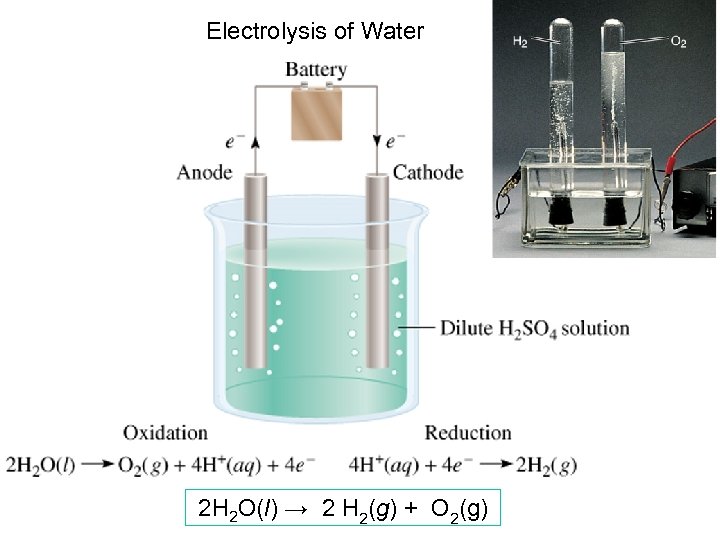
Electrolysis of Water 2 H 2 O(l) → 2 H 2(g) + O 2(g)
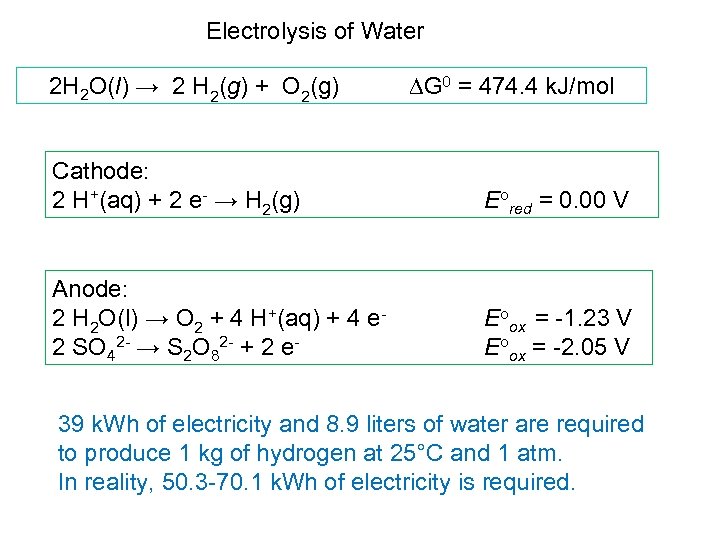
Electrolysis of Water 2 H 2 O(l) → 2 H 2(g) + O 2(g) DG 0 = 474. 4 k. J/mol Cathode: 2 H+(aq) + 2 e- → H 2(g) Eored = 0. 00 V Anode: 2 H 2 O(l) → O 2 + 4 H+(aq) + 4 e- 2 SO 42 - → S 2 O 82 - + 2 e- Eoox = -1. 23 V Eoox = -2. 05 V 39 k. Wh of electricity and 8. 9 liters of water are required to produce 1 kg of hydrogen at 25°C and 1 atm. In reality, 50. 3 -70. 1 k. Wh of electricity is required.
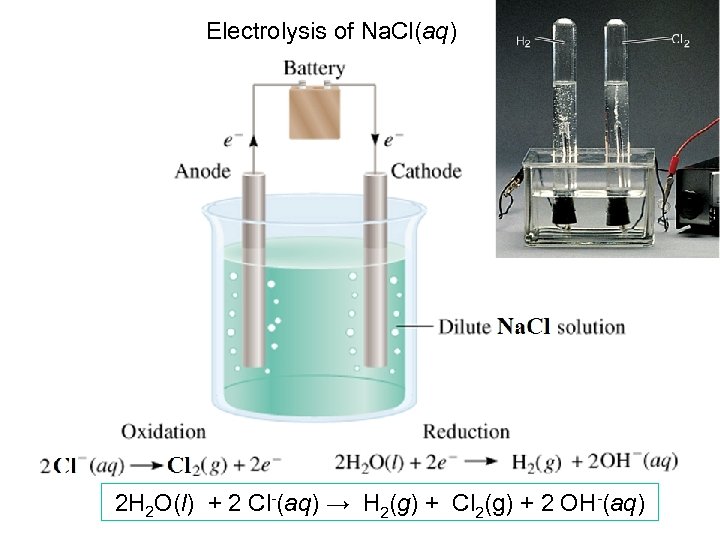
Electrolysis of Na. Cl(aq) 2 H 2 O(l) + 2 Cl-(aq) → H 2(g) + Cl 2(g) + 2 OH-(aq)
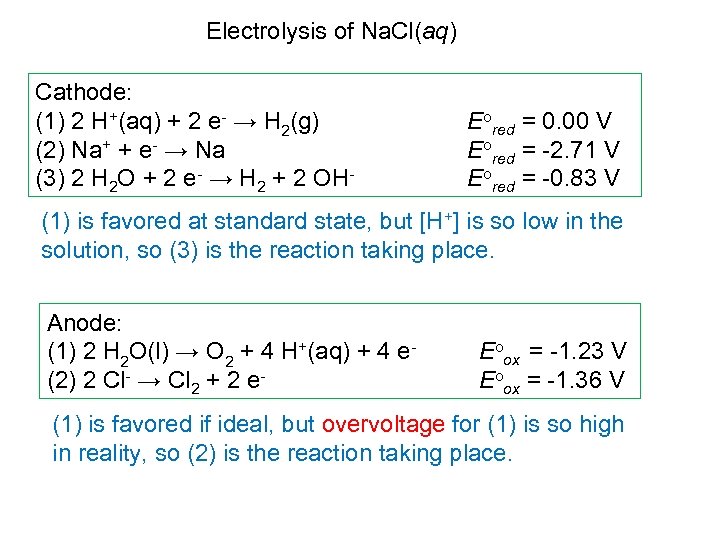
Electrolysis of Na. Cl(aq) Cathode: (1) 2 H+(aq) + 2 e- → H 2(g) (2) Na+ + e- → Na (3) 2 H 2 O + 2 e- → H 2 + 2 OH- Eored = 0. 00 V Eored = -2. 71 V Eored = -0. 83 V (1) is favored at standard state, but [H+] is so low in the solution, so (3) is the reaction taking place. Anode: (1) 2 H 2 O(l) → O 2 + 4 H+(aq) + 4 e- (2) 2 Cl- → Cl 2 + 2 e- Eoox = -1. 23 V Eoox = -1. 36 V (1) is favored if ideal, but overvoltage for (1) is so high in reality, so (2) is the reaction taking place.
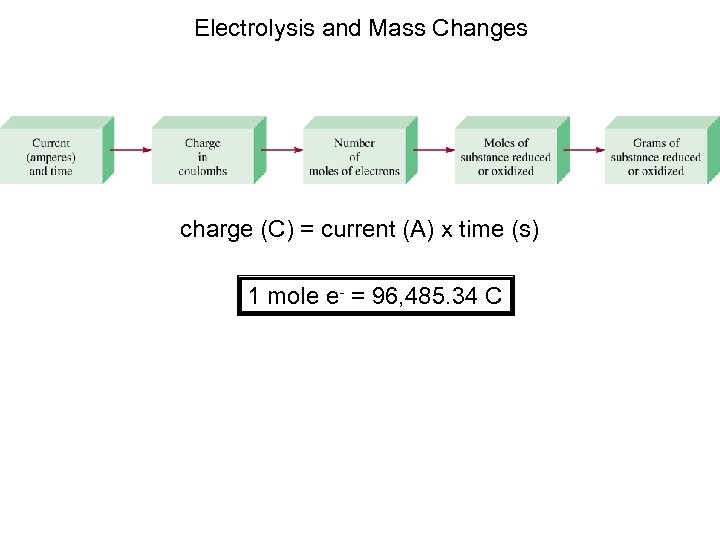
Electrolysis and Mass Changes charge (C) = current (A) x time (s) 1 mole e- = 96, 485. 34 C
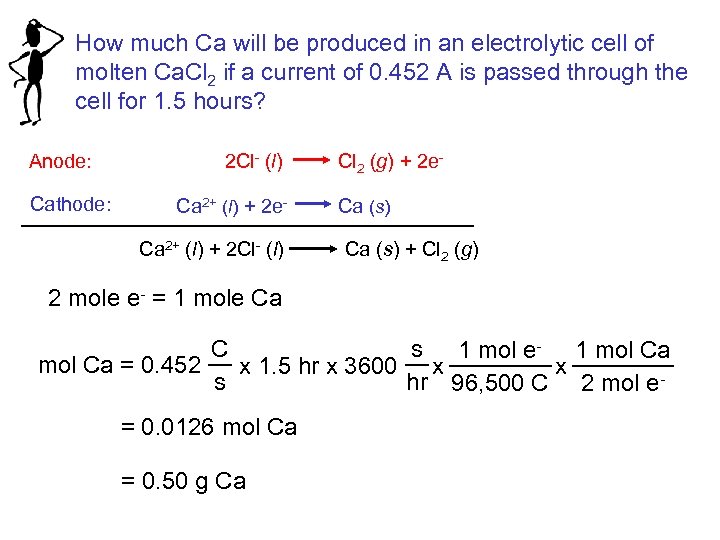
How much Ca will be produced in an electrolytic cell of molten Ca. Cl 2 if a current of 0. 452 A is passed through the cell for 1. 5 hours? Anode: Cathode: 2 Cl- (l) Cl 2 (g) + 2 e. Ca 2+ (l) + 2 e- Ca (s) Ca 2+ (l) + 2 Cl- (l) Ca (s) + Cl 2 (g) 2 mole e- = 1 mole Ca C s 1 mol e- 1 mol Ca = 0. 452 x 1. 5 hr x 3600 x x s hr 96, 500 C 2 mol e= 0. 0126 mol Ca = 0. 50 g Ca
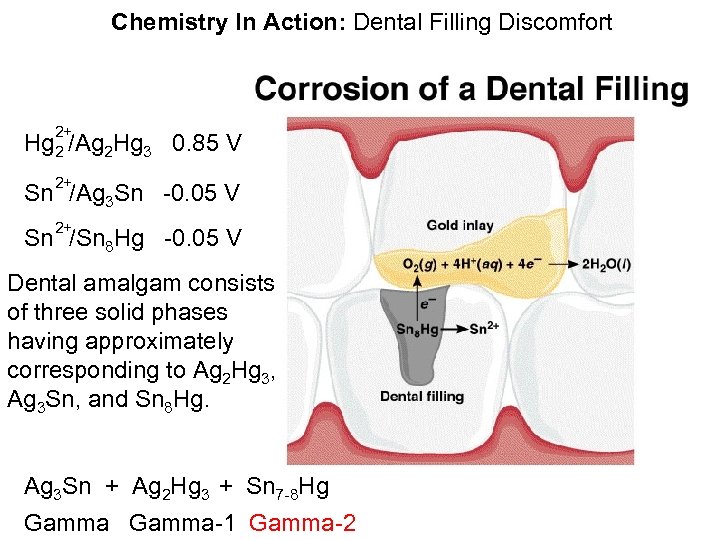
Chemistry In Action: Dental Filling Discomfort 2+ Hg 2 /Ag 2 Hg 3 0. 85 V 2+ Sn /Ag 3 Sn -0. 05 V 2+ Sn /Sn 8 Hg -0. 05 V Dental amalgam consists of three solid phases having approximately corresponding to Ag 2 Hg 3, Ag 3 Sn, and Sn 8 Hg. Ag 3 Sn + Ag 2 Hg 3 + Sn 7 -8 Hg Gamma-1 Gamma-2
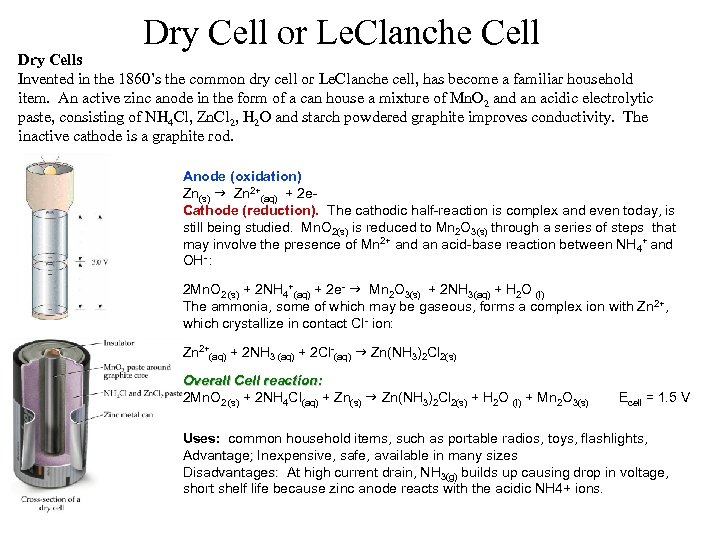
Dry Cell or Le. Clanche Cell Dry Cells Invented in the 1860’s the common dry cell or Le. Clanche cell, has become a familiar household item. An active zinc anode in the form of a can house a mixture of Mn. O 2 and an acidic electrolytic paste, consisting of NH 4 Cl, Zn. Cl 2, H 2 O and starch powdered graphite improves conductivity. The inactive cathode is a graphite rod. Anode (oxidation) Zn(s) g Zn 2+(aq) + 2 e. Cathode (reduction). The cathodic half-reaction is complex and even today, is still being studied. Mn. O 2(s) is reduced to Mn 2 O 3(s) through a series of steps that may involve the presence of Mn 2+ and an acid-base reaction between NH 4+ and OH- : 2 Mn. O 2 (s) + 2 NH 4+(aq) + 2 e- g Mn 2 O 3(s) + 2 NH 3(aq) + H 2 O (l) The ammonia, some of which may be gaseous, forms a complex ion with Zn 2+, which crystallize in contact Cl- ion: Zn 2+(aq) + 2 NH 3 (aq) + 2 Cl-(aq) g Zn(NH 3)2 Cl 2(s) Overall Cell reaction: 2 Mn. O 2 (s) + 2 NH 4 Cl(aq) + Zn(s) g Zn(NH 3)2 Cl 2(s) + H 2 O (l) + Mn 2 O 3(s) Ecell = 1. 5 V Uses: common household items, such as portable radios, toys, flashlights, Advantage; Inexpensive, safe, available in many sizes Advantage; Disadvantages: At high current drain, NH 3(g) builds up causing drop in voltage, Disadvantages: short shelf life because zinc anode reacts with the acidic NH 4+ ions.
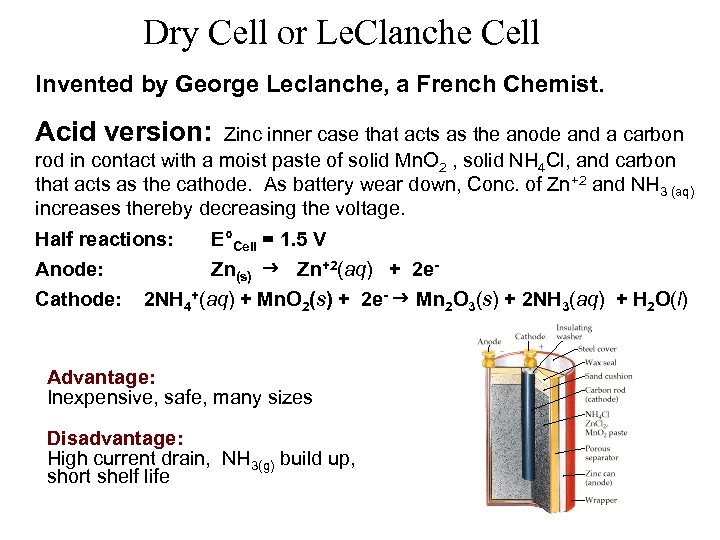
Dry Cell or Le. Clanche Cell Invented by George Leclanche, a French Chemist. Acid version: Zinc inner case that acts as the anode and a carbon rod in contact with a moist paste of solid Mn. O 2 , solid NH 4 Cl, and carbon that acts as the cathode. As battery wear down, Conc. of Zn+2 and NH 3 (aq) increases thereby decreasing the voltage. Half reactions: E°Cell = 1. 5 V Anode: Zn(s) Zn+2(aq) + 2 e. Cathode: 2 NH 4+(aq) + Mn. O 2(s) + 2 e- Mn 2 O 3(s) + 2 NH 3(aq) + H 2 O(l) Advantage: Inexpensive, safe, many sizes Disadvantage: High current drain, NH 3(g) build up, short shelf life

Alkaline Battery The alkaline battery is an improved dry cell. The half-reactions are similar, but the electrolyte is a basic KOH paste, which eliminates the buildup of gases and maintains the Zn electrode. Anode (oxidation) Zn(s) + 2 OH- (aq) g Zn. O(s) + H 2 O (l) + 2 e. Cathode (reduction). 2 Mn. O 2 (s) + 2 H 2 O (l) + 2 e- g Mn(OH)2(s) + 2 OH-(aq) Overall Cell reaction: 2 Mn. O 2 (s) + H 2 O (l) + Zn(s) g Zn. O(s) + Mn(OH)2(s) Ecell = 1. 5 V Uses: Same as for dry cell. Uses: Advantages: No voltage drop and longer shell life than dry cell Advantages: because of alkaline electrolyte; sale , amu sizes. Disadvantages; More expensive than common dry cell. Disadvantages;
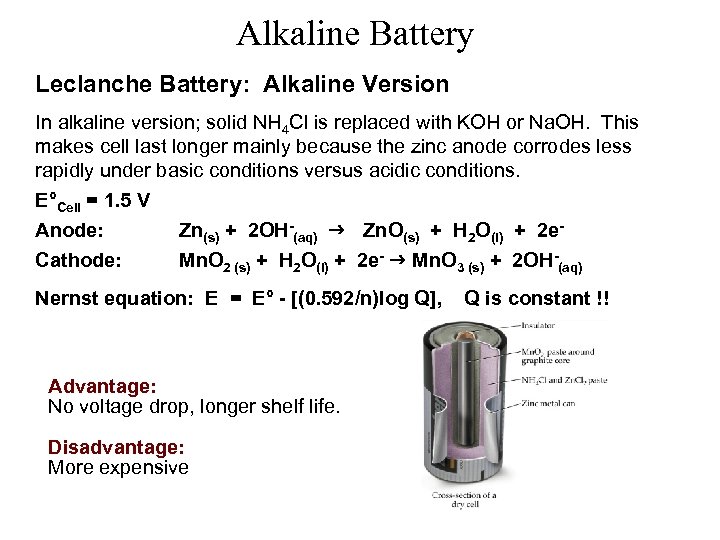
Alkaline Battery Leclanche Battery: Alkaline Version In alkaline version; solid NH 4 Cl is replaced with KOH or Na. OH. This makes cell last longer mainly because the zinc anode corrodes less rapidly under basic conditions versus acidic conditions. E°Cell = 1. 5 V Anode: Cathode: Zn(s) + 2 OH-(aq) Zn. O(s) + H 2 O(l) + 2 e. Mn. O 2 (s) + H 2 O(l) + 2 e- Mn. O 3 (s) + 2 OH-(aq) Nernst equation: E = E° - [(0. 592/n)log Q], Advantage: No voltage drop, longer shelf life. Disadvantage: More expensive Q is constant !!

Mercury Button Battery Mercury and Silver batteries are similar. Like the alkaline dry cell, both of these batteries use zinc in a basic medium as the anode. The solid reactants are each compressed with KOH, and moist paper acts as a salt bridge. E°Cell = 1. 6 V Anode: Zn(s) + 2 OH-(aq) Zn. O(s) + H 2 O(l) + 2 e. Cathode (Hg): Hg. O (s) + 2 H 2 O(l) + 2 e- Hg(s) + 2 OH-(aq) Cathode (Ag): Ag 2 O (s) + H 2 O(l) + 2 e- 2 Ag(s) + 2 OH-(aq) Advantage: Small, large potential, silver is nontoxic. Disadvantage: Mercury is toxic, silver is expensive.
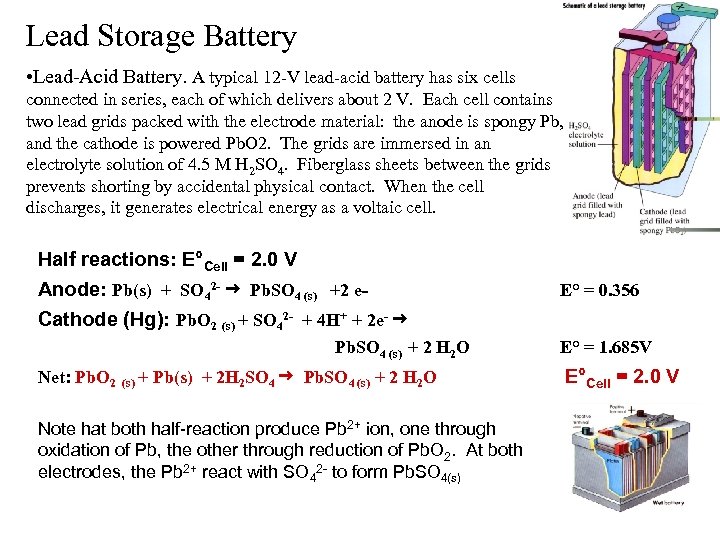
Lead Storage Battery • Lead-Acid Battery. A typical 12 -V lead-acid battery has six cells connected in series, each of which delivers about 2 V. Each cell contains two lead grids packed with the electrode material: the anode is spongy Pb, and the cathode is powered Pb. O 2. The grids are immersed in an electrolyte solution of 4. 5 M H 2 SO 4. Fiberglass sheets between the grids prevents shorting by accidental physical contact. When the cell discharges, it generates electrical energy as a voltaic cell. Half reactions: E°Cell = 2. 0 V Anode: Pb(s) + SO 42 - Pb. SO 4 (s) +2 e. Cathode (Hg): Pb. O 2 (s) + SO 42 - + 4 H+ + 2 e- Pb. SO 4 (s) + 2 H 2 O Net: Pb. O 2 (s) + Pb(s) + 2 H 2 SO 4 Pb. SO 4 (s) + 2 H 2 O Note hat both half-reaction produce Pb 2+ ion, one through oxidation of Pb, the other through reduction of Pb. O 2. At both electrodes, the Pb 2+ react with SO 42 - to form Pb. SO 4(s) E° = 0. 356 E° = 1. 685 V E°Cell = 2. 0 V

Nickel-Cadmium Battery for the Technological Age Rechargeable, lightweight “ni-cad” are used for variety of cordless appliances. Main advantage is that the oxidizing and reducing agent can be regenerated easily when recharged. These produce constant potential. Half reactions: E°Cell = 1. 4 V Anode: Cd(s) + 2 OH-(aq) Cd(OH)2 (s) + 2 e- Cathode: 2 Ni(OH) (s) + 2 H 2 O(l) + 2 e- Ni(OH)2 (s) + 2 OH-(aq)

보통 anode를 "양극", cathode를 "음극"이라고 번역한다. 우리가 흔히 사용하는 CRT (cathode ray tube)를 음극관이라고 한다. 그러나 이러한 명칭이 혼돈을 주는 경우도 있다. 우리가 일상에서 접하는 배터리의 플러스 극은 cathode이고 마이너스 극은 anode이다. 다음을 명심하면 혼돈을 피할 수 있다. 1) 갈바니 전지와 전해전지에서 모두 산화가 일어나는 전극은 "anode"이고, 환원이 일어나 는 전극은 "cathode"이다. 2) 갈바니 전지는 자발적인 반응이고, 전해전지는 비자발적인 변화를 강제로 일으키는 것이 다. 3)전류는 전위가 높은 곳에서 낮은 곳으로 흐르며, 전자의 이동 방향은 전류의 방향과는 반 대이다. 갈바니 전지에서는 산화가 일어나는 anode에서 전자가 나와서 환원이 일어나는 cathode 로 흘러 들어가게 되므로, 전류는 cathode에서 anode로 흐른다. 그래서 cathode의 전위가 더 높다. cathode는 플러스극이고 anode는 마이너스 극이 된다. 전해 전지에서도 anode에서 전자가 나오고, cathode로 전자가 들어간다. 그러나 이 때의 전자는 자발적으로 흐르는 것이 아니라 외부에 설치한 전류 공급원에서 강제로 흘려주는 것 이다. 그러므로 외부의 전류 공급원의 전위가 낮은 쪽에서 흘러나온 전자가 cathode로 들어 가고, 전위가 더 높은 쪽에서는 anode에서 전자를 강제로 빼앗아 온다. 따라서 이 경우에는 cathode의 전위가 anode의 전위보다 더 낮다. 대한화학회에서는 anode를 "산화극", cathode를 "환원극"이라고도 번역한다.
553b0154236fe855d75ba9d85e6e0607.ppt