52fe9335379b29f3b0d70ef47febb8b4.ppt
- Количество слайдов: 47
 Electrochemical Techniques to Study Solid Materials Fritz Scholz Universität Greifswald Institut für Biochemie Lehrstuhl für Analytische Chemie und Umweltchemie (Editor-in-Chief Journal of Solid State Electrochemistry) Felix-Hausdorff-Straße 4, 17487 Greifswald, Germany Tel. : +49 -(0)3834 -864450, Fax: +49 -(0)3834 -864451 e-mail: fscholz@uni-greifswald. de http: //www. chemie. uni-greifswald. de/~analytik/ Copyright statement: Most of the figures and part of the text are subject to copyright restrictions, and should not be copied without permission of the copyright holders.
Electrochemical Techniques to Study Solid Materials Fritz Scholz Universität Greifswald Institut für Biochemie Lehrstuhl für Analytische Chemie und Umweltchemie (Editor-in-Chief Journal of Solid State Electrochemistry) Felix-Hausdorff-Straße 4, 17487 Greifswald, Germany Tel. : +49 -(0)3834 -864450, Fax: +49 -(0)3834 -864451 e-mail: fscholz@uni-greifswald. de http: //www. chemie. uni-greifswald. de/~analytik/ Copyright statement: Most of the figures and part of the text are subject to copyright restrictions, and should not be copied without permission of the copyright holders.
 The Electrochemistry of Microparticles -1 -
The Electrochemistry of Microparticles -1 -
 Previous techniques to study the electrochemistry of solid materials/compounds (material that is capable of faradaic reactions)* • • Compact electrodes Composite electrodes (powder + binder + ‘carbon’) Electrography Paste electrodes Voltammetry of suspended particles Film electrodes; surface modified electrodes Cells with solid electrolytes (classic SSEC)* References to the historic development: 1. 2. 3. Electrochemical solid state analysis - state of the art, F. Scholz, B. Meyer, Chem. Soc. Rev. 23 (1994) 341 -347 Abrasive Stripping Voltammetry - an Electrochemical Solid State Spectroscopy of Wide Applicability, F. Scholz, B. Lange, Trends Anal. Chem. 11 (1992) 359 - 367 Electrochemistry of Immobilized Particles and Droplets, F. Scholz, U. Schröder, R. Gulaboski, Springer, Berlin 2005, XIII, 290 p. , ISBN: 3 -54022005 -4 * Solid ionic conductors (solid electrolytes) are not considered here.
Previous techniques to study the electrochemistry of solid materials/compounds (material that is capable of faradaic reactions)* • • Compact electrodes Composite electrodes (powder + binder + ‘carbon’) Electrography Paste electrodes Voltammetry of suspended particles Film electrodes; surface modified electrodes Cells with solid electrolytes (classic SSEC)* References to the historic development: 1. 2. 3. Electrochemical solid state analysis - state of the art, F. Scholz, B. Meyer, Chem. Soc. Rev. 23 (1994) 341 -347 Abrasive Stripping Voltammetry - an Electrochemical Solid State Spectroscopy of Wide Applicability, F. Scholz, B. Lange, Trends Anal. Chem. 11 (1992) 359 - 367 Electrochemistry of Immobilized Particles and Droplets, F. Scholz, U. Schröder, R. Gulaboski, Springer, Berlin 2005, XIII, 290 p. , ISBN: 3 -54022005 -4 * Solid ionic conductors (solid electrolytes) are not considered here.
 Compact electrodes • Very suitable (even necessary!!) for corrosion studies, plating etc. However: • Often difficult to prepare • High currents • Bad signal resolution, especially when multicomponent systems are studied • Very sensitive to surface coverage by oxidized layers • Impossible to make from (a) non-conducting materials, from (b) powder materials, (c) from precious material that is only available in very tiny amounts
Compact electrodes • Very suitable (even necessary!!) for corrosion studies, plating etc. However: • Often difficult to prepare • High currents • Bad signal resolution, especially when multicomponent systems are studied • Very sensitive to surface coverage by oxidized layers • Impossible to make from (a) non-conducting materials, from (b) powder materials, (c) from precious material that is only available in very tiny amounts
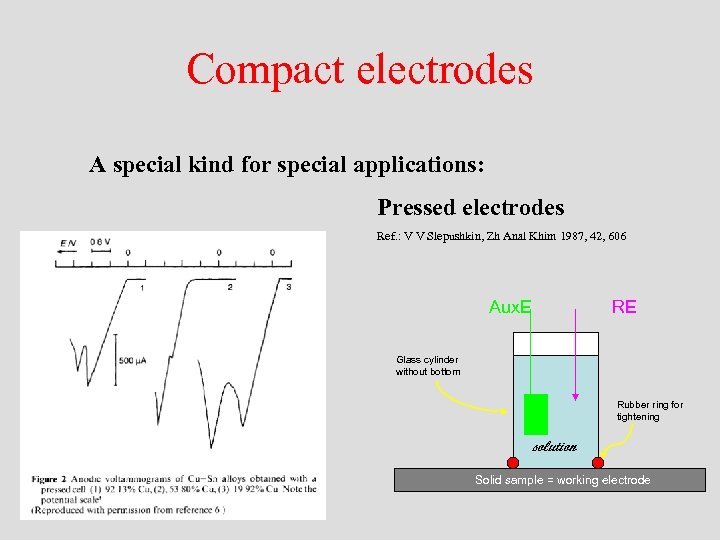 Compact electrodes A special kind for special applications: Pressed electrodes Ref. : V V Slepushkin, Zh Anal Khim 1987, 42, 606 Aux. E RE Glass cylinder without bottom Rubber ring for tightening solution Solid sample = working electrode
Compact electrodes A special kind for special applications: Pressed electrodes Ref. : V V Slepushkin, Zh Anal Khim 1987, 42, 606 Aux. E RE Glass cylinder without bottom Rubber ring for tightening solution Solid sample = working electrode
 Composite electrodes • Frequently: powdered electroactive compound + binder + e-conductor (Carbon) • Standard technique to prepare electrodes for batteries • Advantages: Almost universally applicable • Disadvantages: - tedious preparation, - results depend strongly on preparation (pressure, binder, etc. ), - needs rather larger amounts of compounds*, - needs special holders for electrodes or conductive gluing, • large amounts = large charges for complete reactions, i. e. , slow scan rates and long times for experiments!
Composite electrodes • Frequently: powdered electroactive compound + binder + e-conductor (Carbon) • Standard technique to prepare electrodes for batteries • Advantages: Almost universally applicable • Disadvantages: - tedious preparation, - results depend strongly on preparation (pressure, binder, etc. ), - needs rather larger amounts of compounds*, - needs special holders for electrodes or conductive gluing, • large amounts = large charges for complete reactions, i. e. , slow scan rates and long times for experiments!
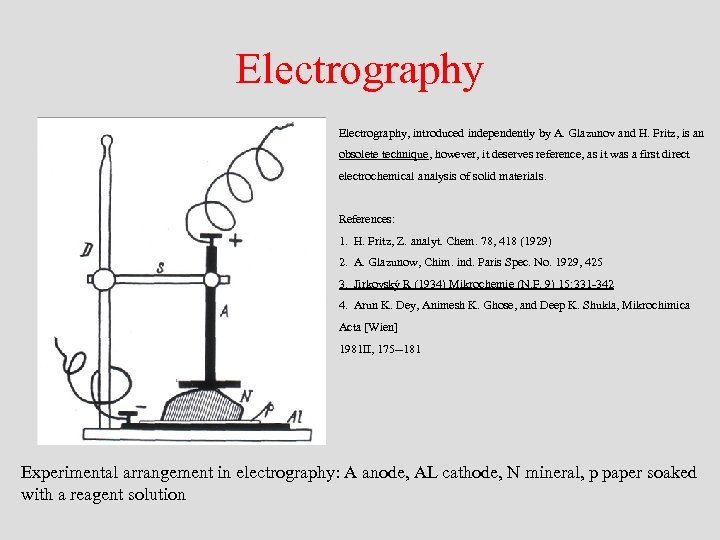 Electrography, introduced independently by A. Glazunov and H. Fritz, is an obsolete technique, however, it deserves reference, as it was a first direct electrochemical analysis of solid materials. References: 1. H. Fritz, Z. analyt. Chem. 78, 418 (1929) 2. A. Glazunow, Chim. ind. Paris Spec. No. 1929, 425 3. Jirkovský R (1934) Mikrochemie (N. F. 9) 15: 331 -342 4. Arun K. Dey, Animesh K. Ghose, and Deep K. Shukla, Mikrochimica Acta [Wien] 1981 II, 175 --181 Experimental arrangement in electrography: A anode, AL cathode, N mineral, p paper soaked with a reagent solution
Electrography, introduced independently by A. Glazunov and H. Fritz, is an obsolete technique, however, it deserves reference, as it was a first direct electrochemical analysis of solid materials. References: 1. H. Fritz, Z. analyt. Chem. 78, 418 (1929) 2. A. Glazunow, Chim. ind. Paris Spec. No. 1929, 425 3. Jirkovský R (1934) Mikrochemie (N. F. 9) 15: 331 -342 4. Arun K. Dey, Animesh K. Ghose, and Deep K. Shukla, Mikrochimica Acta [Wien] 1981 II, 175 --181 Experimental arrangement in electrography: A anode, AL cathode, N mineral, p paper soaked with a reagent solution
 Electrographic print of a nickel ore. The black parts represent the originally red spots of nickel dimethylglyoxime complex that have been formed where nickel ions were released into the reagent paper by oxidation of the nickel mineral Ref: Jirkovský R (1934) Mikrochemie (N. F. 9) 15: 331 -342
Electrographic print of a nickel ore. The black parts represent the originally red spots of nickel dimethylglyoxime complex that have been formed where nickel ions were released into the reagent paper by oxidation of the nickel mineral Ref: Jirkovský R (1934) Mikrochemie (N. F. 9) 15: 331 -342
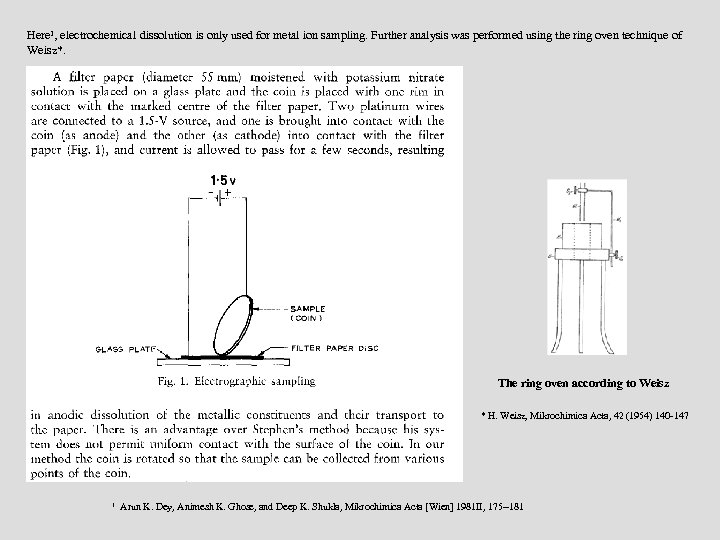 Here 1, electrochemical dissolution is only used for metal ion sampling. Further analysis was performed using the ring oven technique of Weisz*. The ring oven according to Weisz * H. Weisz, Mikrochimica Acta, 42 (1954) 140 -147 1 Arun K. Dey, Animesh K. Ghose, and Deep K. Shukla, Mikrochimica Acta [Wien] 1981 II, 175 --181
Here 1, electrochemical dissolution is only used for metal ion sampling. Further analysis was performed using the ring oven technique of Weisz*. The ring oven according to Weisz * H. Weisz, Mikrochimica Acta, 42 (1954) 140 -147 1 Arun K. Dey, Animesh K. Ghose, and Deep K. Shukla, Mikrochimica Acta [Wien] 1981 II, 175 --181
 Carbon paste electrodes with organic binder • Powdered solid compound +graphite powder + organic binder (e. g. nujol) • First publication: T. Kuwana and W. G. French, Anal Chem, 1964, 36, 241 • Soviet Union / Russia: Ol’ga Al’fredovna Songina, Anna Zalmanovna Brainina, Nina Fyodorovna Zakharchuk
Carbon paste electrodes with organic binder • Powdered solid compound +graphite powder + organic binder (e. g. nujol) • First publication: T. Kuwana and W. G. French, Anal Chem, 1964, 36, 241 • Soviet Union / Russia: Ol’ga Al’fredovna Songina, Anna Zalmanovna Brainina, Nina Fyodorovna Zakharchuk
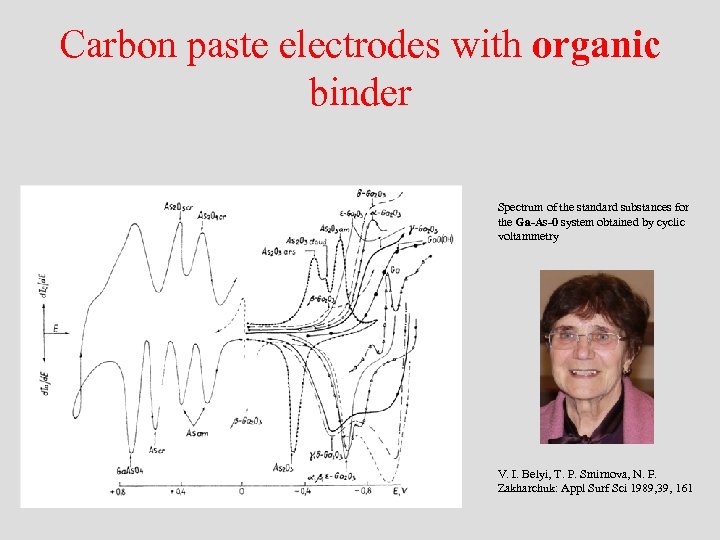 Carbon paste electrodes with organic binder Spectrum of the standard substances for the Ga-As-0 system obtained by cyclic voltammetry V. I. Belyi, T. P. Smirnova, N. F. Zakharchuk: Appl Surf Sci 1989, 39, 161
Carbon paste electrodes with organic binder Spectrum of the standard substances for the Ga-As-0 system obtained by cyclic voltammetry V. I. Belyi, T. P. Smirnova, N. F. Zakharchuk: Appl Surf Sci 1989, 39, 161
 Carbon paste electrodes with organic binder • General properties: - Easy to prepare, but usually the pastes do not keep well and c hange their properties with time - Organic binder* may form a film on the sample particles (possible inhibition of electrode reactions; sometimes also beneficial) * Typical binders are Nujol (as used in infrared spectroscopy), and other paraffin oils
Carbon paste electrodes with organic binder • General properties: - Easy to prepare, but usually the pastes do not keep well and c hange their properties with time - Organic binder* may form a film on the sample particles (possible inhibition of electrode reactions; sometimes also beneficial) * Typical binders are Nujol (as used in infrared spectroscopy), and other paraffin oils
 Carbon paste electrodes with an aqueous electrolyte binder • Powdered solid sample + graphite powder + aqueous electrolyte binder • General properties: - Easy to prepare - Aqueous binder may react with solid particles (mostly undesirable, however, this may even enhance the electrochemical response) (Many contributions from P. Sánchez-Batanero, Valladolid, Spain)
Carbon paste electrodes with an aqueous electrolyte binder • Powdered solid sample + graphite powder + aqueous electrolyte binder • General properties: - Easy to prepare - Aqueous binder may react with solid particles (mostly undesirable, however, this may even enhance the electrochemical response) (Many contributions from P. Sánchez-Batanero, Valladolid, Spain)
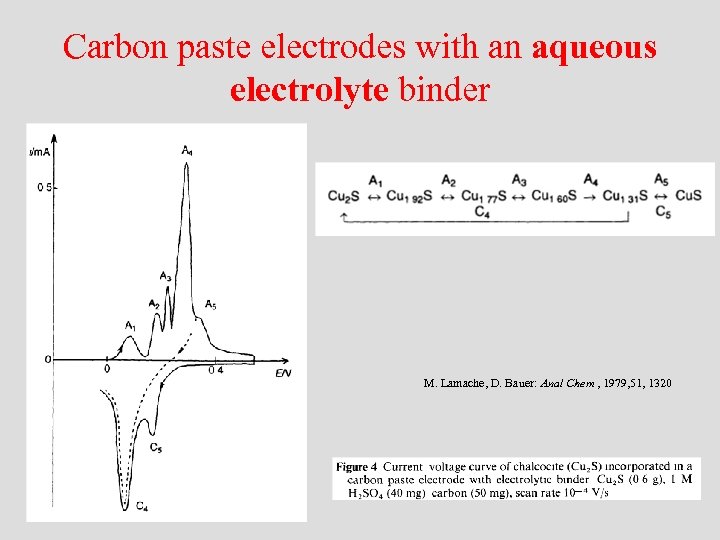 Carbon paste electrodes with an aqueous electrolyte binder M. Lamache, D. Bauer: Anal Chem , 1979, 51, 1320
Carbon paste electrodes with an aqueous electrolyte binder M. Lamache, D. Bauer: Anal Chem , 1979, 51, 1320
 Voltammetry of suspended particles • Suspensions of micro-particles • Colloidal suspensions • General properties: - Strongly affected by surface charge of particles and electrode charge - No theory, no clear understanding, lack of experimental data
Voltammetry of suspended particles • Suspensions of micro-particles • Colloidal suspensions • General properties: - Strongly affected by surface charge of particles and electrode charge - No theory, no clear understanding, lack of experimental data
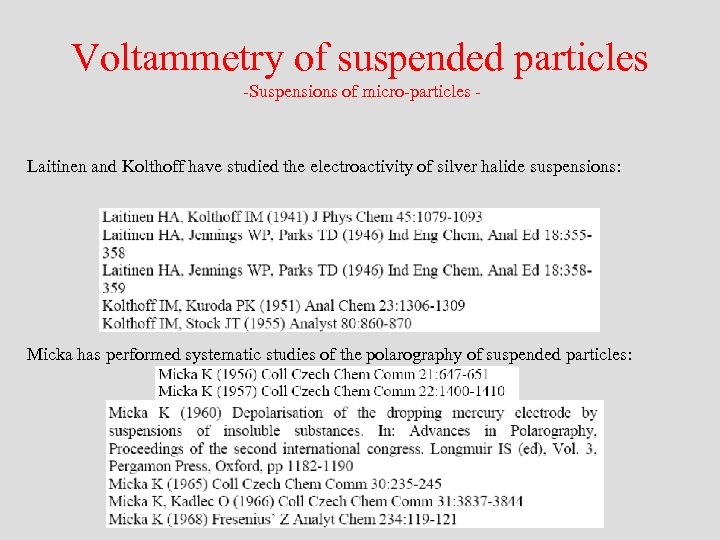 Voltammetry of suspended particles -Suspensions of micro-particles - Laitinen and Kolthoff have studied the electroactivity of silver halide suspensions: Micka has performed systematic studies of the polarography of suspended particles:
Voltammetry of suspended particles -Suspensions of micro-particles - Laitinen and Kolthoff have studied the electroactivity of silver halide suspensions: Micka has performed systematic studies of the polarography of suspended particles:
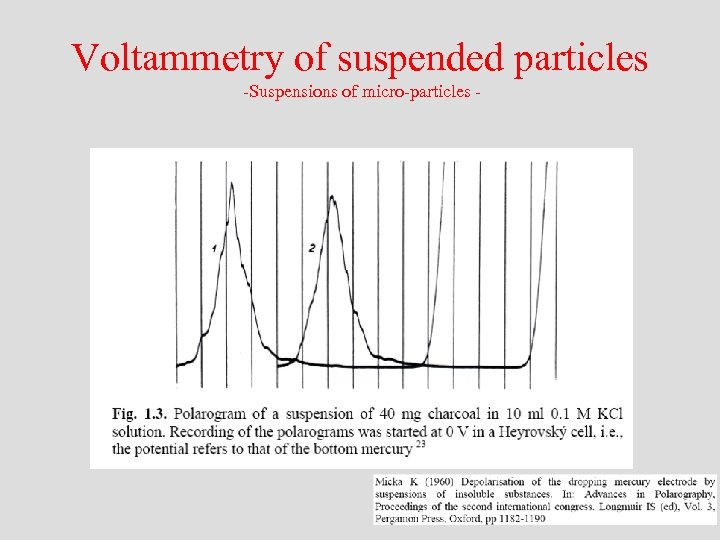 Voltammetry of suspended particles -Suspensions of micro-particles -
Voltammetry of suspended particles -Suspensions of micro-particles -
 Voltammetry of suspended particles -Suspensions of micro-particles - Dausheva and Songina have discussed the stability of the electrolyte film at the electrode in dependence on the electrode potential. They have assumed that only around the pzc this film is fragile enough to allow impinging particles penetrating it and contacting the metal surface allowing electron transfer. The same authors have experimentally shown that mercury(I) iodide particles strongly adhere at a mercury electrode only in the vicinity of the pzc.
Voltammetry of suspended particles -Suspensions of micro-particles - Dausheva and Songina have discussed the stability of the electrolyte film at the electrode in dependence on the electrode potential. They have assumed that only around the pzc this film is fragile enough to allow impinging particles penetrating it and contacting the metal surface allowing electron transfer. The same authors have experimentally shown that mercury(I) iodide particles strongly adhere at a mercury electrode only in the vicinity of the pzc.
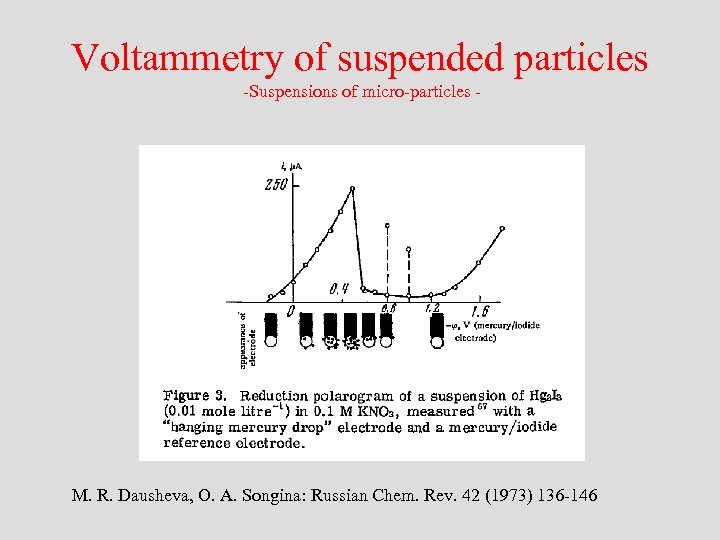 Voltammetry of suspended particles -Suspensions of micro-particles - M. R. Dausheva, O. A. Songina: Russian Chem. Rev. 42 (1973) 136 -146
Voltammetry of suspended particles -Suspensions of micro-particles - M. R. Dausheva, O. A. Songina: Russian Chem. Rev. 42 (1973) 136 -146
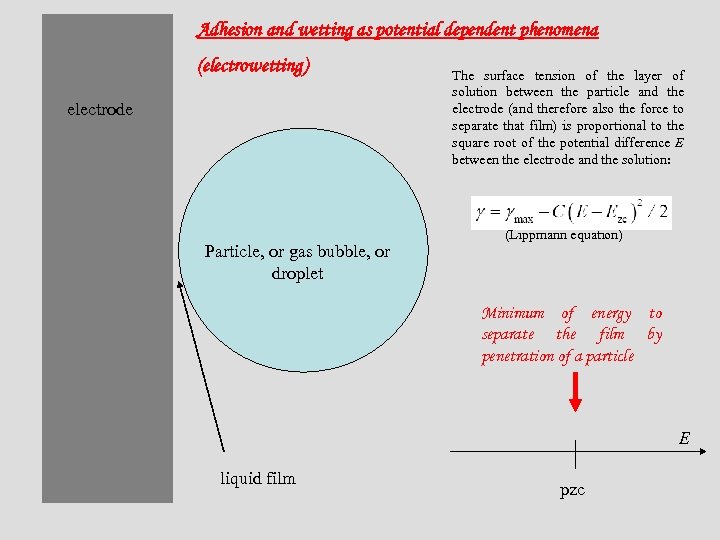 Adhesion and wetting as potential dependent phenomena (electrowetting) electrode Particle, or gas bubble, or droplet The surface tension of the layer of solution between the particle and the electrode (and therefore also the force to separate that film) is proportional to the square root of the potential difference E between the electrode and the solution: (Lippmann equation) Minimum of energy to separate the film by penetration of a particle E liquid film pzc
Adhesion and wetting as potential dependent phenomena (electrowetting) electrode Particle, or gas bubble, or droplet The surface tension of the layer of solution between the particle and the electrode (and therefore also the force to separate that film) is proportional to the square root of the potential difference E between the electrode and the solution: (Lippmann equation) Minimum of energy to separate the film by penetration of a particle E liquid film pzc
 Ольга Альфредовна Сонгина (17. 04. 1901, Санкт. Петербург – 07. 05. 1989, Алма-Ата) Ol‘ga Alfredovna Songina (April 17, 1901 Saint Petersburg, Russia – May 5, 1989, Alma-Ata (now Almaty, Kazakhstan) “Songina –– A pioneer of electrochemical solid state analysis, and Yevgeniya Nikolayevna Varasova –– A pioneer of polarography” F. Scholz: J. Solid State Electrochem. 17 (2013) 1493– 1504
Ольга Альфредовна Сонгина (17. 04. 1901, Санкт. Петербург – 07. 05. 1989, Алма-Ата) Ol‘ga Alfredovna Songina (April 17, 1901 Saint Petersburg, Russia – May 5, 1989, Alma-Ata (now Almaty, Kazakhstan) “Songina –– A pioneer of electrochemical solid state analysis, and Yevgeniya Nikolayevna Varasova –– A pioneer of polarography” F. Scholz: J. Solid State Electrochem. 17 (2013) 1493– 1504
 1928 1952(? )
1928 1952(? )
 The expulsion of O. A. Songina from Leningrad was part of the repression against her husband I. V. Songin. He was born in 1894 in Rostov-on-Don as the son of Vladislav Osipovich Songin who was the director of the “Sankt Petersburg Metal Factory” (the later “Leningrad Metal Factory”) from 1911 to 1917. Iosif V. Songin was of Polish origin, which, most probably, was one of the reasons for the repression in 1937, as he was accused with two others 1 of being a member of a “counterrevolutionary Polish nationalistic group”. Ethnic Poles suffered disproportionally to their share in the overall population. According to the KGB files, I. V. Songin had declared (most likely when he was arrested in 1937) that his father V. O. Songin was a nobleman (in Russian ‘dvoryanin’). He was arrested October 4, 1937, sentenced to death on November 22, 1937 and executed on November 27, 1937! In a Iosif Vladislavovich Songin handwritten CV, dated April 12, 1959, O A. Songina mentions: “In 1957, I received the information that my husband died in January 1942 in a (Ol’ga Al’fredovna Songina’s camp; and in August of the same year (1957), my husband was husband) postmortem rehabilitated (reference 31/VIII-57, no. 6202) of the military tribunal of the Leningrad Military District. ” 1 Konstantin Stanislavovich Olevskiy (born 1901 in Tbilisi, arrested November 23 rd 1937, sentenced to death on December 15 th 1937 and executed on Dec. 20 th 1937) and Romual’dovich Czerwiński (born 1909 in Sankt Petersburg, arresed September 2 nd 1937, sentenced to death on Oct. 1 st 1937 and executed on October 6 th 1937)
The expulsion of O. A. Songina from Leningrad was part of the repression against her husband I. V. Songin. He was born in 1894 in Rostov-on-Don as the son of Vladislav Osipovich Songin who was the director of the “Sankt Petersburg Metal Factory” (the later “Leningrad Metal Factory”) from 1911 to 1917. Iosif V. Songin was of Polish origin, which, most probably, was one of the reasons for the repression in 1937, as he was accused with two others 1 of being a member of a “counterrevolutionary Polish nationalistic group”. Ethnic Poles suffered disproportionally to their share in the overall population. According to the KGB files, I. V. Songin had declared (most likely when he was arrested in 1937) that his father V. O. Songin was a nobleman (in Russian ‘dvoryanin’). He was arrested October 4, 1937, sentenced to death on November 22, 1937 and executed on November 27, 1937! In a Iosif Vladislavovich Songin handwritten CV, dated April 12, 1959, O A. Songina mentions: “In 1957, I received the information that my husband died in January 1942 in a (Ol’ga Al’fredovna Songina’s camp; and in August of the same year (1957), my husband was husband) postmortem rehabilitated (reference 31/VIII-57, no. 6202) of the military tribunal of the Leningrad Military District. ” 1 Konstantin Stanislavovich Olevskiy (born 1901 in Tbilisi, arrested November 23 rd 1937, sentenced to death on December 15 th 1937 and executed on Dec. 20 th 1937) and Romual’dovich Czerwiński (born 1909 in Sankt Petersburg, arresed September 2 nd 1937, sentenced to death on Oct. 1 st 1937 and executed on October 6 th 1937)
 O. A. Songina in 1961 together with her students. First row from left to right: Gena Makarov, Sonya Sinickaya, Ol’ga Al’fredovna Songina, Anya Yermenko and Nadiya Sharipova. Second row from left to right: Vadim Grigor’evich Barikov, Zoya Borisovna Rozhdestvenskaya, Olya Enyutina, Mariyam Rakhimovna Dausheva and Andrey Brandt (Courtesy of M. R. Dausheva)
O. A. Songina in 1961 together with her students. First row from left to right: Gena Makarov, Sonya Sinickaya, Ol’ga Al’fredovna Songina, Anya Yermenko and Nadiya Sharipova. Second row from left to right: Vadim Grigor’evich Barikov, Zoya Borisovna Rozhdestvenskaya, Olya Enyutina, Mariyam Rakhimovna Dausheva and Andrey Brandt (Courtesy of M. R. Dausheva)
 Voltammetry of suspended particles - colloidal suspensions - First observation of a voltammetric signal of a colloidal solution: Majer V. (1943) Chem listy 37: 202 -204: colloidal iron(III) hydoxide Voltammetric signals of colloidal suspensions of Sn, Ti, and Ti-Fe hydroxides have been reported by M. Heyrovský, however, that are all indirect reductions caused by hydrogen:
Voltammetry of suspended particles - colloidal suspensions - First observation of a voltammetric signal of a colloidal solution: Majer V. (1943) Chem listy 37: 202 -204: colloidal iron(III) hydoxide Voltammetric signals of colloidal suspensions of Sn, Ti, and Ti-Fe hydroxides have been reported by M. Heyrovský, however, that are all indirect reductions caused by hydrogen:
 Detection of adhesion and conversion signals of liposomes on mercury electrodes Publications of the Scholz group on vesicle adhesion Electrochem. Commun. 4 (2002) 305 -309 J. Phys. Chem. B 109 (2005) 14715 -14726 Langmuir 22 (2006) 10723 -10731 Langmuir 23 (2007) 8650 Bioelectrochem. 74 (2008) 210 -216 Bioelectrochem. 74 (2008) 149 -156 Israel J. Chem. 48 (2008) 169 -184 J. Solid State Electrochem. 13 (2009) 639 -649 J. Solid State Electrochem. 13 (2009) 1111 -1114 J. Solid State Electrochem. 15 (2011) 1699– 1702 Angew. Chem. Int. Ed. 50 (2011) 6872 – 6875 J. Electroanal. Chem. 671 (2012) 33– 37 J. Solid State Electrochem. 16 (2012) 2391 -2397 “Electrochemistry of adhesion and spreading of lipid vesicles on electrodes” V. Agmo Hernández, U. Lendeckel, F. Scholz, Series "Modern Aspects of Electrochemistry”, Vol. 56, Editors C. G. Vayenas, R. E. White; Volume “Applications of Electrochemistry in Medicine“ Editor M. Schlesinger, Springer, New York, 2013, pp. 189 -247
Detection of adhesion and conversion signals of liposomes on mercury electrodes Publications of the Scholz group on vesicle adhesion Electrochem. Commun. 4 (2002) 305 -309 J. Phys. Chem. B 109 (2005) 14715 -14726 Langmuir 22 (2006) 10723 -10731 Langmuir 23 (2007) 8650 Bioelectrochem. 74 (2008) 210 -216 Bioelectrochem. 74 (2008) 149 -156 Israel J. Chem. 48 (2008) 169 -184 J. Solid State Electrochem. 13 (2009) 639 -649 J. Solid State Electrochem. 13 (2009) 1111 -1114 J. Solid State Electrochem. 15 (2011) 1699– 1702 Angew. Chem. Int. Ed. 50 (2011) 6872 – 6875 J. Electroanal. Chem. 671 (2012) 33– 37 J. Solid State Electrochem. 16 (2012) 2391 -2397 “Electrochemistry of adhesion and spreading of lipid vesicles on electrodes” V. Agmo Hernández, U. Lendeckel, F. Scholz, Series "Modern Aspects of Electrochemistry”, Vol. 56, Editors C. G. Vayenas, R. E. White; Volume “Applications of Electrochemistry in Medicine“ Editor M. Schlesinger, Springer, New York, 2013, pp. 189 -247
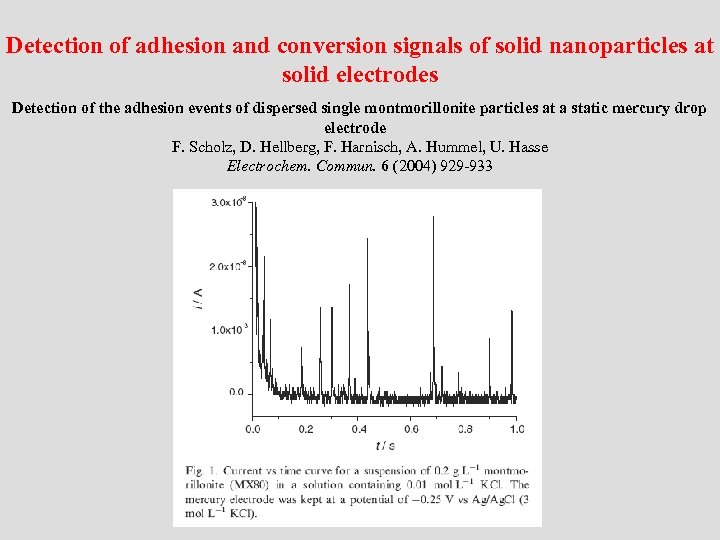 Detection of adhesion and conversion signals of solid nanoparticles at solid electrodes Detection of the adhesion events of dispersed single montmorillonite particles at a static mercury drop electrode F. Scholz, D. Hellberg, F. Harnisch, A. Hummel, U. Hasse Electrochem. Commun. 6 (2004) 929 -933
Detection of adhesion and conversion signals of solid nanoparticles at solid electrodes Detection of the adhesion events of dispersed single montmorillonite particles at a static mercury drop electrode F. Scholz, D. Hellberg, F. Harnisch, A. Hummel, U. Hasse Electrochem. Commun. 6 (2004) 929 -933
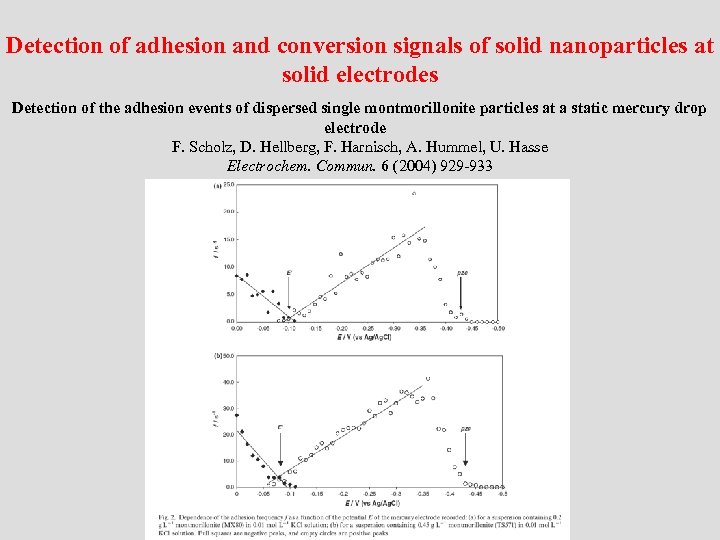 Detection of adhesion and conversion signals of solid nanoparticles at solid electrodes Detection of the adhesion events of dispersed single montmorillonite particles at a static mercury drop electrode F. Scholz, D. Hellberg, F. Harnisch, A. Hummel, U. Hasse Electrochem. Commun. 6 (2004) 929 -933
Detection of adhesion and conversion signals of solid nanoparticles at solid electrodes Detection of the adhesion events of dispersed single montmorillonite particles at a static mercury drop electrode F. Scholz, D. Hellberg, F. Harnisch, A. Hummel, U. Hasse Electrochem. Commun. 6 (2004) 929 -933
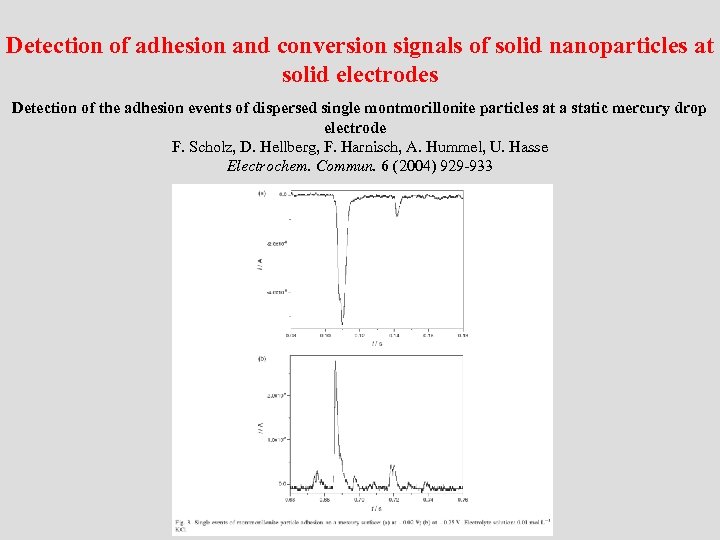 Detection of adhesion and conversion signals of solid nanoparticles at solid electrodes Detection of the adhesion events of dispersed single montmorillonite particles at a static mercury drop electrode F. Scholz, D. Hellberg, F. Harnisch, A. Hummel, U. Hasse Electrochem. Commun. 6 (2004) 929 -933
Detection of adhesion and conversion signals of solid nanoparticles at solid electrodes Detection of the adhesion events of dispersed single montmorillonite particles at a static mercury drop electrode F. Scholz, D. Hellberg, F. Harnisch, A. Hummel, U. Hasse Electrochem. Commun. 6 (2004) 929 -933
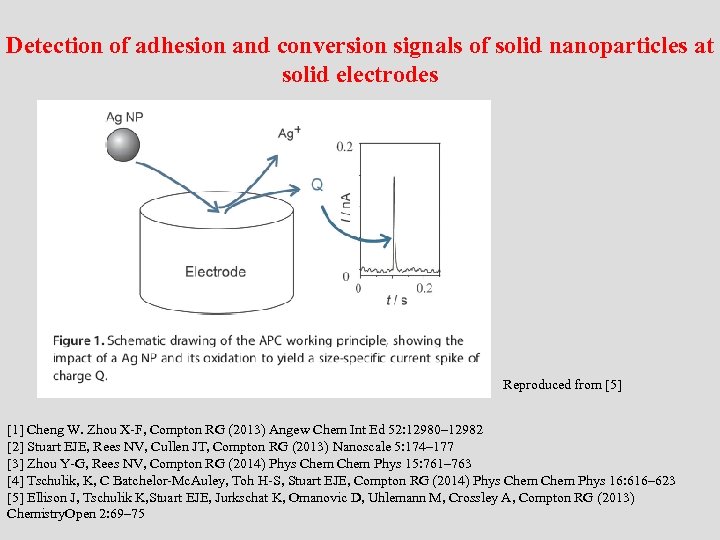 Detection of adhesion and conversion signals of solid nanoparticles at solid electrodes Reproduced from [5] [1] Cheng W. Zhou X-F, Compton RG (2013) Angew Chem Int Ed 52: 12980– 12982 [2] Stuart EJE, Rees NV, Cullen JT, Compton RG (2013) Nanoscale 5: 174– 177 [3] Zhou Y-G, Rees NV, Compton RG (2014) Phys Chem Phys 15: 761– 763 [4] Tschulik, K, C Batchelor-Mc. Auley, Toh H-S, Stuart EJE, Compton RG (2014) Phys Chem Phys 16: 616– 623 [5] Ellison J, Tschulik K, Stuart EJE, Jurkschat K, Omanovic D, Uhlemann M, Crossley A, Compton RG (2013) Chemistry. Open 2: 69– 75
Detection of adhesion and conversion signals of solid nanoparticles at solid electrodes Reproduced from [5] [1] Cheng W. Zhou X-F, Compton RG (2013) Angew Chem Int Ed 52: 12980– 12982 [2] Stuart EJE, Rees NV, Cullen JT, Compton RG (2013) Nanoscale 5: 174– 177 [3] Zhou Y-G, Rees NV, Compton RG (2014) Phys Chem Phys 15: 761– 763 [4] Tschulik, K, C Batchelor-Mc. Auley, Toh H-S, Stuart EJE, Compton RG (2014) Phys Chem Phys 16: 616– 623 [5] Ellison J, Tschulik K, Stuart EJE, Jurkschat K, Omanovic D, Uhlemann M, Crossley A, Compton RG (2013) Chemistry. Open 2: 69– 75
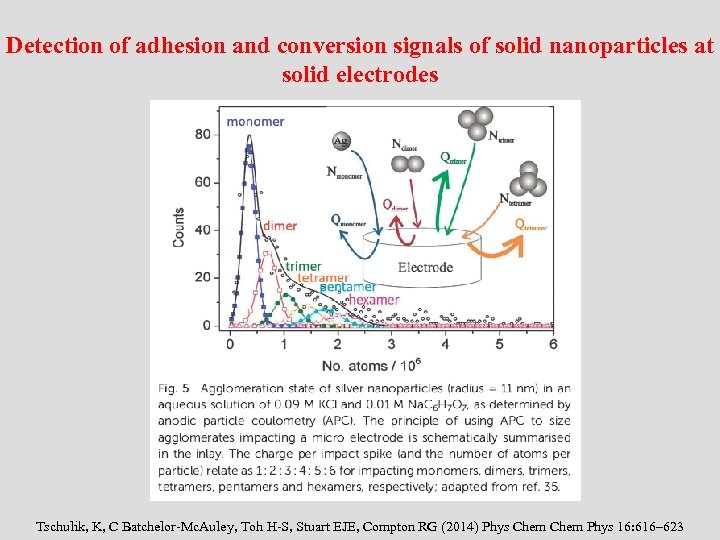 Detection of adhesion and conversion signals of solid nanoparticles at solid electrodes Tschulik, K, C Batchelor-Mc. Auley, Toh H-S, Stuart EJE, Compton RG (2014) Phys Chem Phys 16: 616– 623
Detection of adhesion and conversion signals of solid nanoparticles at solid electrodes Tschulik, K, C Batchelor-Mc. Auley, Toh H-S, Stuart EJE, Compton RG (2014) Phys Chem Phys 16: 616– 623
 Voltammetry of Immobilized Microparticles (Abrasive Stripping Voltammetry) • Mechanical immobilization of microparticles on the surface of suitable electrodes • Introduced: A New Procedure for Fast Electrochemical Analysis of Solid Materials F. Scholz, L. Nitschke, G. Henrion: Naturwissenschaften 76 (1989) 71 A New Technique to Study the Electrochemistry of Minerals F. Scholz, L. Nitschke, G. Henrion, F. Damaschun: Naturwissenschaften 76 (1989) 167 -168
Voltammetry of Immobilized Microparticles (Abrasive Stripping Voltammetry) • Mechanical immobilization of microparticles on the surface of suitable electrodes • Introduced: A New Procedure for Fast Electrochemical Analysis of Solid Materials F. Scholz, L. Nitschke, G. Henrion: Naturwissenschaften 76 (1989) 71 A New Technique to Study the Electrochemistry of Minerals F. Scholz, L. Nitschke, G. Henrion, F. Damaschun: Naturwissenschaften 76 (1989) 167 -168
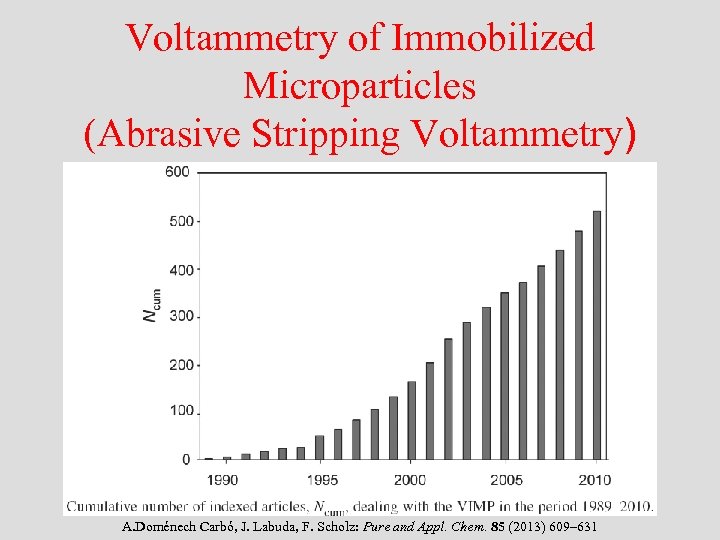 Voltammetry of Immobilized Microparticles (Abrasive Stripping Voltammetry) A. Doménech Carbó, J. Labuda, F. Scholz: Pure and Appl. Chem. 85 (2013) 609– 631
Voltammetry of Immobilized Microparticles (Abrasive Stripping Voltammetry) A. Doménech Carbó, J. Labuda, F. Scholz: Pure and Appl. Chem. 85 (2013) 609– 631
 Voltammetry of Immobilized Microparticles (Abrasive Stripping Voltammetry) Reviews: • F. Scholz, B. Lange: Trends Anal. Chem. 11 (1992) 359 - 367 • F. Scholz, B. Meyer: Chem. Soc. Rev. 23 (1994) 341 -347 • T. Grygar, F. Marken, U. Schröder, F. Scholz: Coll. Czech. Chem. Commun. 67 (2002) 163 -208 • A. Doménech Carbó, J. Labuda, F. Scholz: Pure and Appl. Chem. 85 (2013) 609– 631 Book chapters: • “Voltammetry of Solid Microparticles Immobilized on Electrode Surfaces” F. Scholz, B. Meyer: Electroanalytical Chemistry, A Series of Advances, A. J. Bard, I. Rubinstein (Edts. ), Vol. 20, 1998, pp. 1, Marcel Dekker, Inc. , New York • “Electrochemical studies of solid compounds and materials” D. A. Fiedler, F. Scholz, Chapter II. 8, in “Electroanalytical Methods – Guide to Experiments and Applications”; F. Scholz (Ed. ); Springer, Berlin, 2005, 2 nd printing, ISBN 3 -540 -42229 -3 Book: • “Electrochemistry of Immobilized Particles and Droplets” F. Scholz, U. Schröder, R. Gulaboski, Springer, Heidelberg, Berlin 2005, XIII, 290 p. , ISBN: 3 -54022005 -4
Voltammetry of Immobilized Microparticles (Abrasive Stripping Voltammetry) Reviews: • F. Scholz, B. Lange: Trends Anal. Chem. 11 (1992) 359 - 367 • F. Scholz, B. Meyer: Chem. Soc. Rev. 23 (1994) 341 -347 • T. Grygar, F. Marken, U. Schröder, F. Scholz: Coll. Czech. Chem. Commun. 67 (2002) 163 -208 • A. Doménech Carbó, J. Labuda, F. Scholz: Pure and Appl. Chem. 85 (2013) 609– 631 Book chapters: • “Voltammetry of Solid Microparticles Immobilized on Electrode Surfaces” F. Scholz, B. Meyer: Electroanalytical Chemistry, A Series of Advances, A. J. Bard, I. Rubinstein (Edts. ), Vol. 20, 1998, pp. 1, Marcel Dekker, Inc. , New York • “Electrochemical studies of solid compounds and materials” D. A. Fiedler, F. Scholz, Chapter II. 8, in “Electroanalytical Methods – Guide to Experiments and Applications”; F. Scholz (Ed. ); Springer, Berlin, 2005, 2 nd printing, ISBN 3 -540 -42229 -3 Book: • “Electrochemistry of Immobilized Particles and Droplets” F. Scholz, U. Schröder, R. Gulaboski, Springer, Heidelberg, Berlin 2005, XIII, 290 p. , ISBN: 3 -54022005 -4
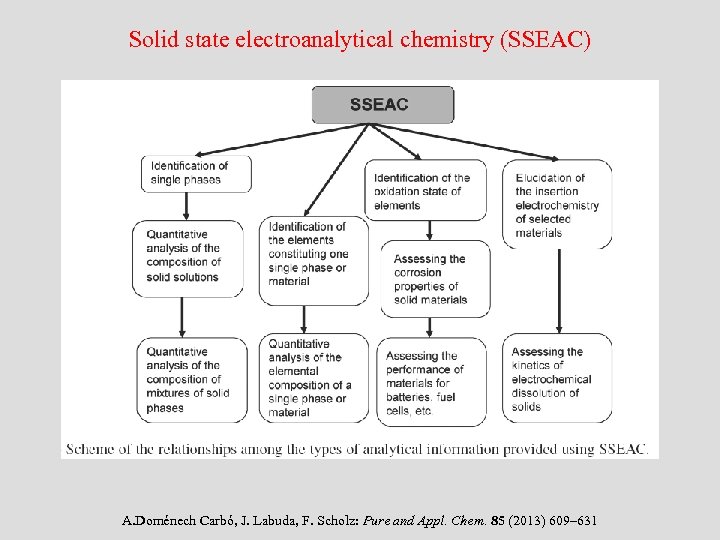 Solid state electroanalytical chemistry (SSEAC) A. Doménech Carbó, J. Labuda, F. Scholz: Pure and Appl. Chem. 85 (2013) 609– 631
Solid state electroanalytical chemistry (SSEAC) A. Doménech Carbó, J. Labuda, F. Scholz: Pure and Appl. Chem. 85 (2013) 609– 631
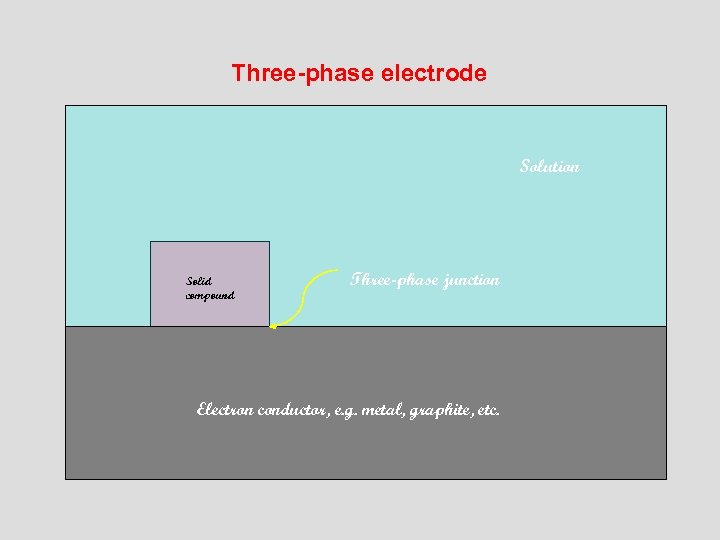 Three-phase electrode Solution Solid compound Three-phase junction Electron conductor, e. g. metal, graphite, etc.
Three-phase electrode Solution Solid compound Three-phase junction Electron conductor, e. g. metal, graphite, etc.
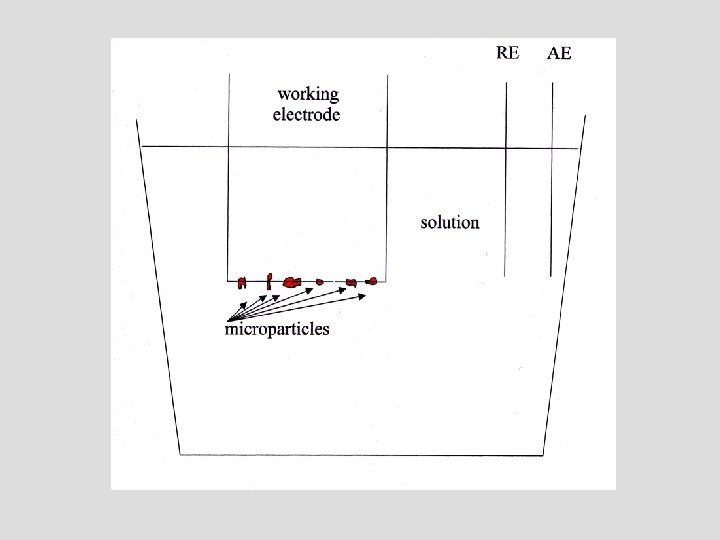
 Immobolization of Microparticles
Immobolization of Microparticles
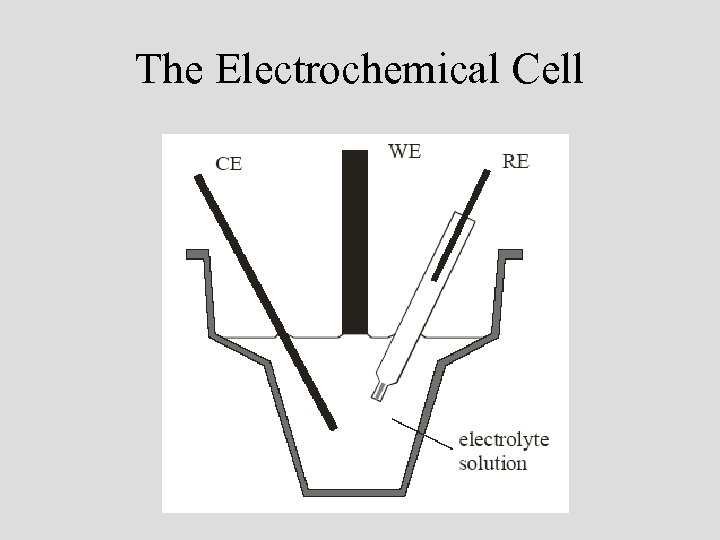 The Electrochemical Cell
The Electrochemical Cell
 Cleaning the Electrode
Cleaning the Electrode
 The Electrode • • Paraffin Impregnated Graphite Electrode (PIGE) Pyrolytic Graphite Electrode Highly Oriented Pyrolytic Greaphite Electrode Glassy Carbon Electrode Metal (Pt, Au) Electrode Pencil Leads Boron Doped Diamond Electrodes Composite Electrodes
The Electrode • • Paraffin Impregnated Graphite Electrode (PIGE) Pyrolytic Graphite Electrode Highly Oriented Pyrolytic Greaphite Electrode Glassy Carbon Electrode Metal (Pt, Au) Electrode Pencil Leads Boron Doped Diamond Electrodes Composite Electrodes
 Paraffin Impregnated Graphite Electrodes These electrodes consist of high purity graphite rods whose pores are filled with paraffin in order to prevent high background currents and the spoiling of the electrode by ingression of solution. To prepare PIGE‘s, paraffin (melting point between 56 and 70 °C or higher) is melted in a closed vessel in a water bath. The graphite rods are added to the melt and the vessel is carefully evacuated. The melt remains under vacuum until no more bubbles evolve from the rods. It is then returned to ambient pressure which leads to a complete penetration of the rod by the paraffin. The rods are removed from the melt before the Paraffin solidifies and are placed onto filter paper in order to remove excess paraffin.
Paraffin Impregnated Graphite Electrodes These electrodes consist of high purity graphite rods whose pores are filled with paraffin in order to prevent high background currents and the spoiling of the electrode by ingression of solution. To prepare PIGE‘s, paraffin (melting point between 56 and 70 °C or higher) is melted in a closed vessel in a water bath. The graphite rods are added to the melt and the vessel is carefully evacuated. The melt remains under vacuum until no more bubbles evolve from the rods. It is then returned to ambient pressure which leads to a complete penetration of the rod by the paraffin. The rods are removed from the melt before the Paraffin solidifies and are placed onto filter paper in order to remove excess paraffin.
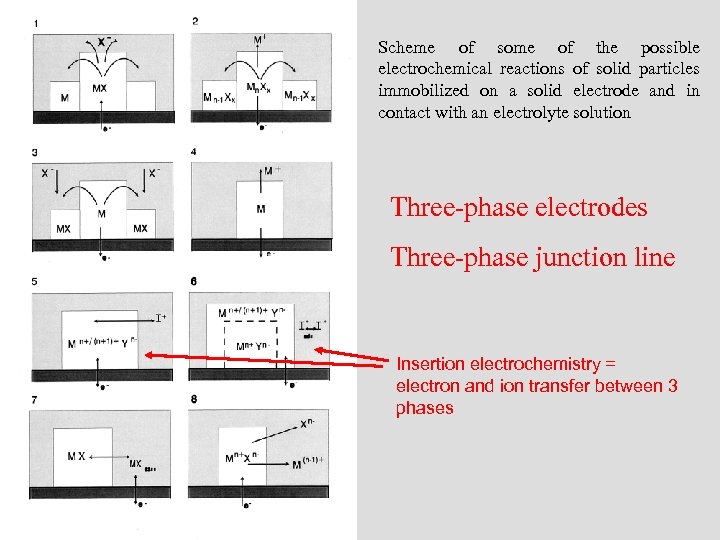 Scheme of some of the possible electrochemical reactions of solid particles immobilized on a solid electrode and in contact with an electrolyte solution Three-phase electrodes Three-phase junction line Insertion electrochemistry = electron and ion transfer between 3 phases
Scheme of some of the possible electrochemical reactions of solid particles immobilized on a solid electrode and in contact with an electrolyte solution Three-phase electrodes Three-phase junction line Insertion electrochemistry = electron and ion transfer between 3 phases
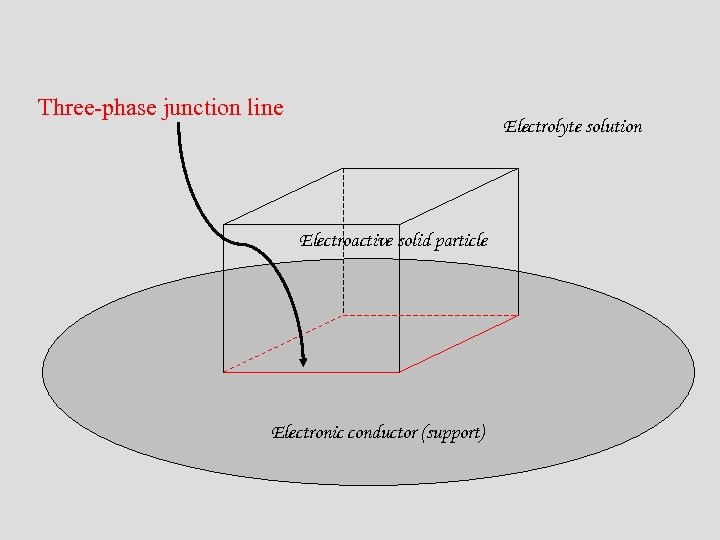 Three-phase junction line Electrolyte solution Electroactive solid particle Electronic conductor (support)
Three-phase junction line Electrolyte solution Electroactive solid particle Electronic conductor (support)
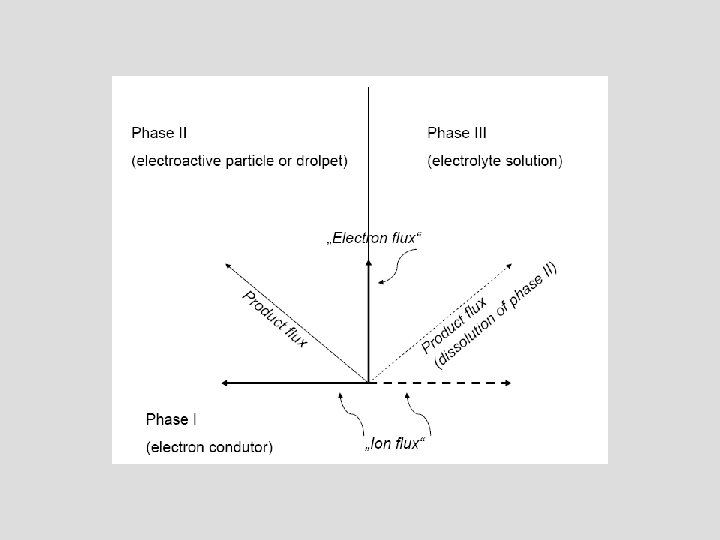
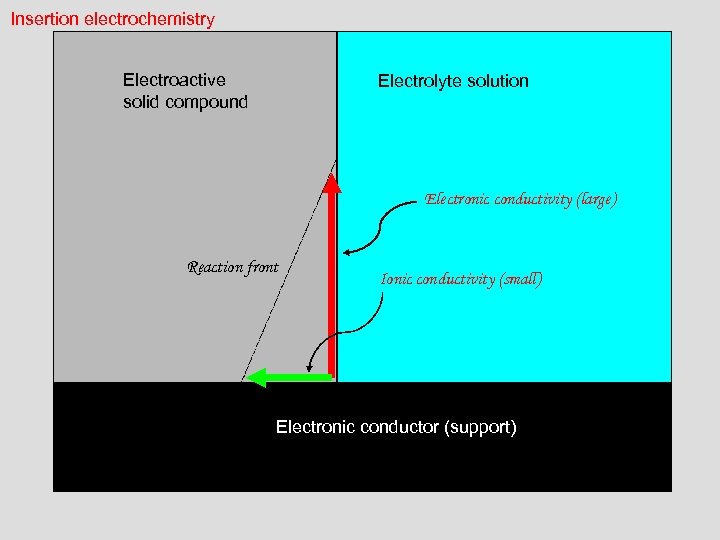 Insertion electrochemistry Electroactive solid compound Electrolyte solution Electronic conductivity (large) Reaction front Ionic conductivity (small) Electronic conductor (support)
Insertion electrochemistry Electroactive solid compound Electrolyte solution Electronic conductivity (large) Reaction front Ionic conductivity (small) Electronic conductor (support)
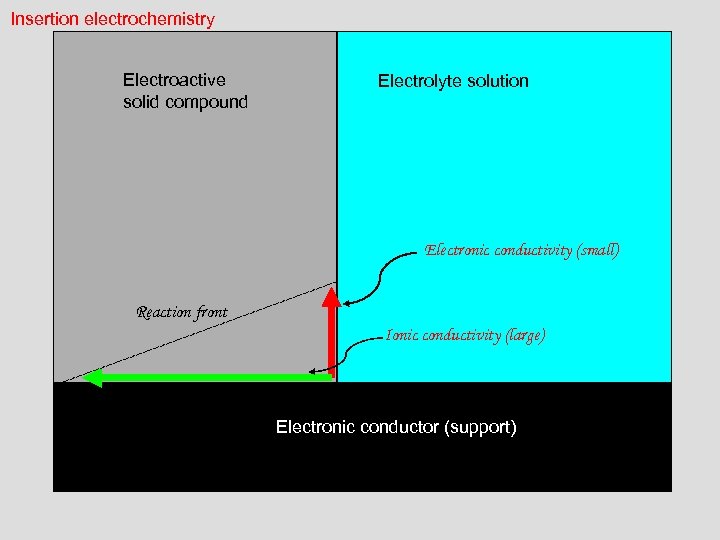 Insertion electrochemistry Electroactive solid compound Electrolyte solution Electronic conductivity (small) Reaction front Ionic conductivity (large) Electronic conductor (support)
Insertion electrochemistry Electroactive solid compound Electrolyte solution Electronic conductivity (small) Reaction front Ionic conductivity (large) Electronic conductor (support)


