64419a3809f91a30dc1c4b22d362895f.ppt
- Количество слайдов: 51
 Electroanalytical chemistry Potentiometry, Voltammetry and Polarography
Electroanalytical chemistry Potentiometry, Voltammetry and Polarography
 Electroanalysis • measure the variation of an electrical parameter (potential, current, charge, conductivity) and relate this to a chemical parameter (the analyte concentration) • Conductimetry, potentiometry (p. H, ISE), coulometry, voltammetry
Electroanalysis • measure the variation of an electrical parameter (potential, current, charge, conductivity) and relate this to a chemical parameter (the analyte concentration) • Conductimetry, potentiometry (p. H, ISE), coulometry, voltammetry
 Potentiometry the measure of the cell potential to yield chemical information (conc. , activity, charge) Measure difference in potential between two electrodes: reference electrode (E constant) indicator electrode (signal α analyte)
Potentiometry the measure of the cell potential to yield chemical information (conc. , activity, charge) Measure difference in potential between two electrodes: reference electrode (E constant) indicator electrode (signal α analyte)
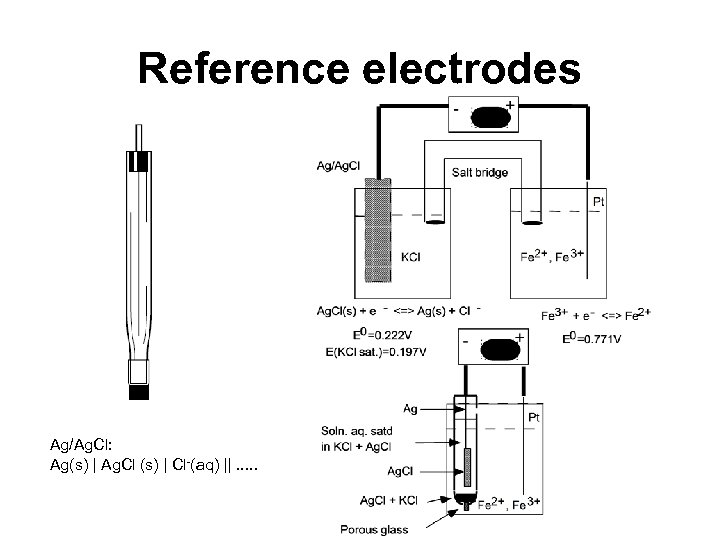 Reference electrodes Ag/Ag. Cl: Ag(s) | Ag. Cl (s) | Cl-(aq) ||. . .
Reference electrodes Ag/Ag. Cl: Ag(s) | Ag. Cl (s) | Cl-(aq) ||. . .
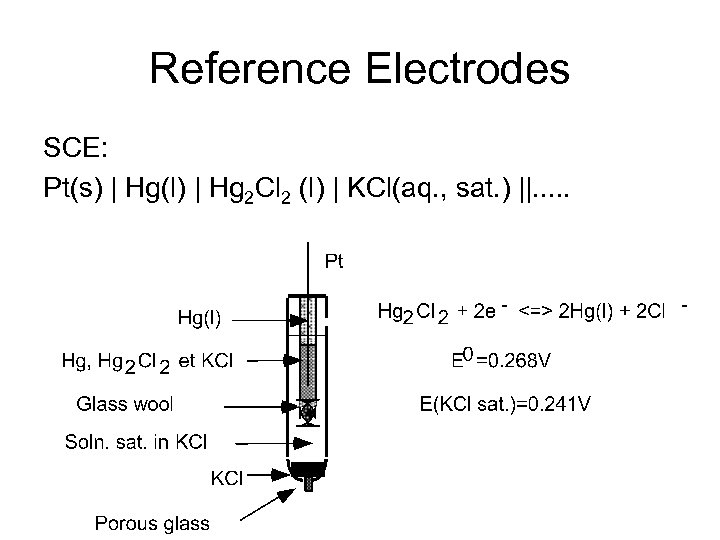 Reference Electrodes SCE: Pt(s) | Hg(l) | Hg 2 Cl 2 (l) | KCl(aq. , sat. ) ||. . .
Reference Electrodes SCE: Pt(s) | Hg(l) | Hg 2 Cl 2 (l) | KCl(aq. , sat. ) ||. . .
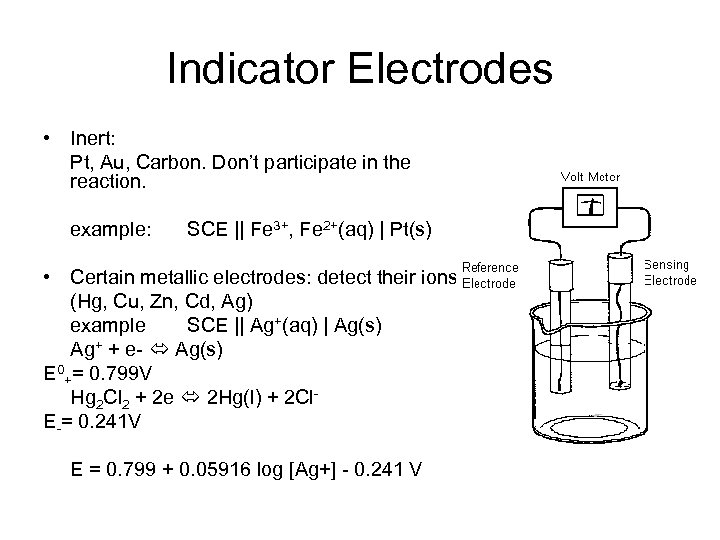 Indicator Electrodes • Inert: Pt, Au, Carbon. Don’t participate in the reaction. example: SCE || Fe 3+, Fe 2+(aq) | Pt(s) • Certain metallic electrodes: detect their ions (Hg, Cu, Zn, Cd, Ag) example SCE || Ag+(aq) | Ag(s) Ag+ + e- Ag(s) E 0+= 0. 799 V Hg 2 Cl 2 + 2 e 2 Hg(l) + 2 Cl. E-= 0. 241 V E = 0. 799 + 0. 05916 log [Ag+] - 0. 241 V
Indicator Electrodes • Inert: Pt, Au, Carbon. Don’t participate in the reaction. example: SCE || Fe 3+, Fe 2+(aq) | Pt(s) • Certain metallic electrodes: detect their ions (Hg, Cu, Zn, Cd, Ag) example SCE || Ag+(aq) | Ag(s) Ag+ + e- Ag(s) E 0+= 0. 799 V Hg 2 Cl 2 + 2 e 2 Hg(l) + 2 Cl. E-= 0. 241 V E = 0. 799 + 0. 05916 log [Ag+] - 0. 241 V
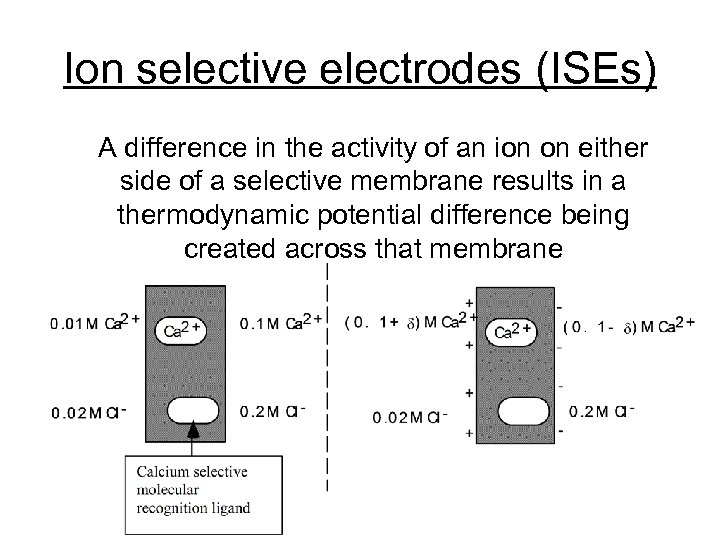 Ion selective electrodes (ISEs) A difference in the activity of an ion on either side of a selective membrane results in a thermodynamic potential difference being created across that membrane
Ion selective electrodes (ISEs) A difference in the activity of an ion on either side of a selective membrane results in a thermodynamic potential difference being created across that membrane
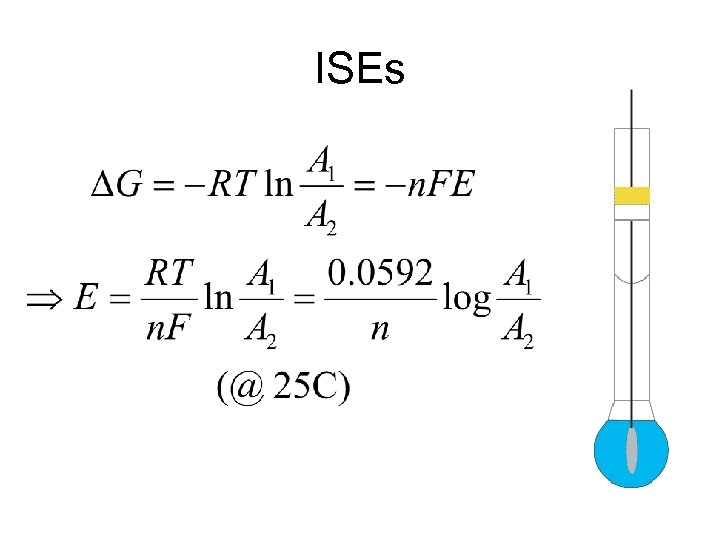 ISEs
ISEs
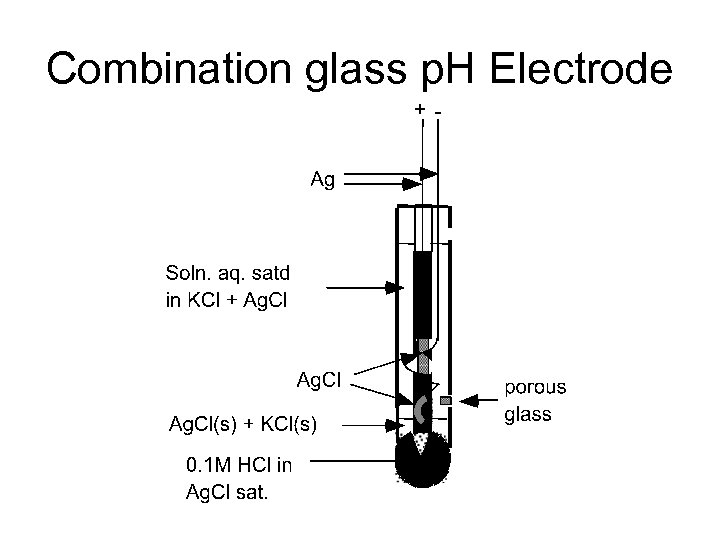 Combination glass p. H Electrode
Combination glass p. H Electrode
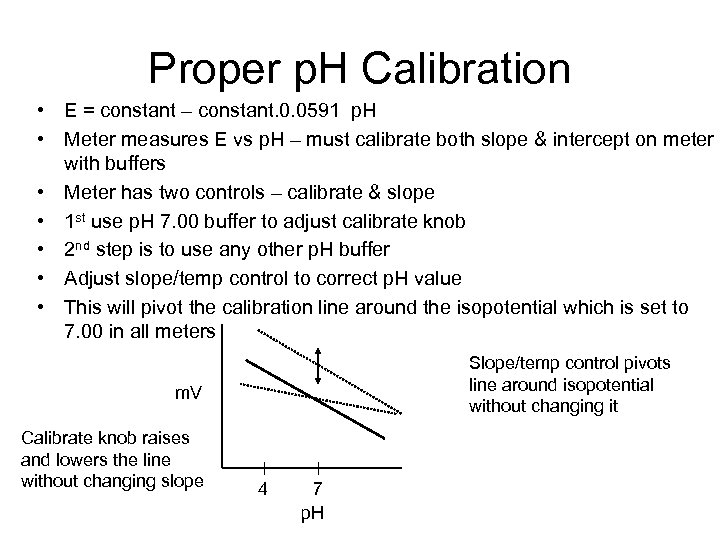 Proper p. H Calibration • E = constant – constant. 0. 0591 p. H • Meter measures E vs p. H – must calibrate both slope & intercept on meter with buffers • Meter has two controls – calibrate & slope • 1 st use p. H 7. 00 buffer to adjust calibrate knob • 2 nd step is to use any other p. H buffer • Adjust slope/temp control to correct p. H value • This will pivot the calibration line around the isopotential which is set to 7. 00 in all meters Slope/temp control pivots line around isopotential without changing it m. V Calibrate knob raises and lowers the line without changing slope 4 7 p. H
Proper p. H Calibration • E = constant – constant. 0. 0591 p. H • Meter measures E vs p. H – must calibrate both slope & intercept on meter with buffers • Meter has two controls – calibrate & slope • 1 st use p. H 7. 00 buffer to adjust calibrate knob • 2 nd step is to use any other p. H buffer • Adjust slope/temp control to correct p. H value • This will pivot the calibration line around the isopotential which is set to 7. 00 in all meters Slope/temp control pivots line around isopotential without changing it m. V Calibrate knob raises and lowers the line without changing slope 4 7 p. H
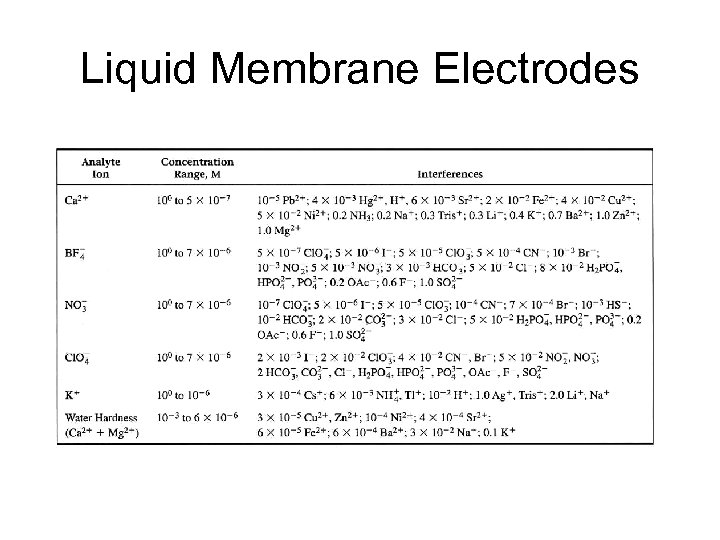 Liquid Membrane Electrodes
Liquid Membrane Electrodes
![Solid State Membrane Electrodes Ag wire Filling solution with fixed [Cl-] and cation that Solid State Membrane Electrodes Ag wire Filling solution with fixed [Cl-] and cation that](https://present5.com/presentation/64419a3809f91a30dc1c4b22d362895f/image-12.jpg) Solid State Membrane Electrodes Ag wire Filling solution with fixed [Cl-] and cation that electrode responds to Ag/Ag. Cl Solid state membrane (must be ionic conductor) Solid State Membrane Chemistry Membrane Ion Determined La. F 3 F-, La 3+ Ag. Cl Ag+, Cl. Ag. Br Ag+, Br. Ag. I Ag+, IAg 2 S Ag+, S 2 Ag 2 S + Cu. S Cu 2+ Ag 2 S + Cd. S Cd 2+ Ag 2 S + Pb. S Pb 2+
Solid State Membrane Electrodes Ag wire Filling solution with fixed [Cl-] and cation that electrode responds to Ag/Ag. Cl Solid state membrane (must be ionic conductor) Solid State Membrane Chemistry Membrane Ion Determined La. F 3 F-, La 3+ Ag. Cl Ag+, Cl. Ag. Br Ag+, Br. Ag. I Ag+, IAg 2 S Ag+, S 2 Ag 2 S + Cu. S Cu 2+ Ag 2 S + Cd. S Cd 2+ Ag 2 S + Pb. S Pb 2+
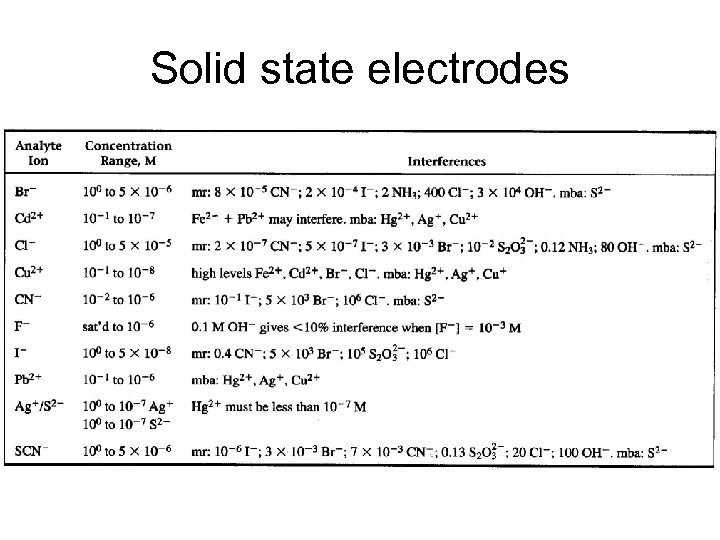 Solid state electrodes
Solid state electrodes
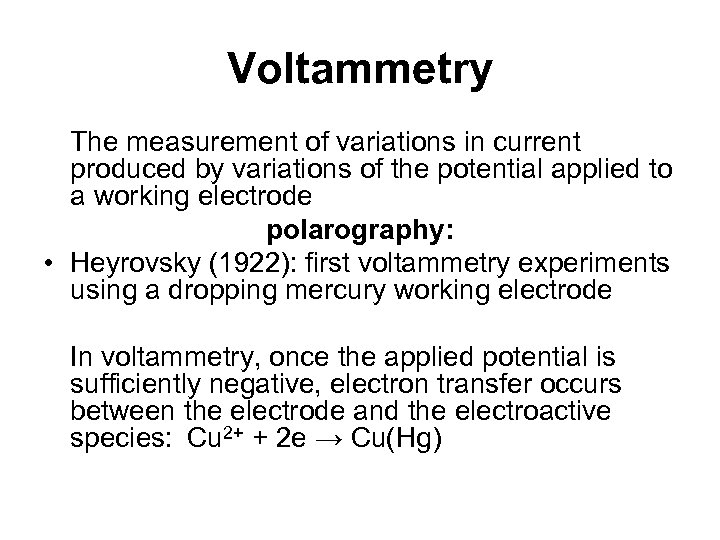 Voltammetry The measurement of variations in current produced by variations of the potential applied to a working electrode polarography: • Heyrovsky (1922): first voltammetry experiments using a dropping mercury working electrode In voltammetry, once the applied potential is sufficiently negative, electron transfer occurs between the electrode and the electroactive species: Cu 2+ + 2 e → Cu(Hg)
Voltammetry The measurement of variations in current produced by variations of the potential applied to a working electrode polarography: • Heyrovsky (1922): first voltammetry experiments using a dropping mercury working electrode In voltammetry, once the applied potential is sufficiently negative, electron transfer occurs between the electrode and the electroactive species: Cu 2+ + 2 e → Cu(Hg)
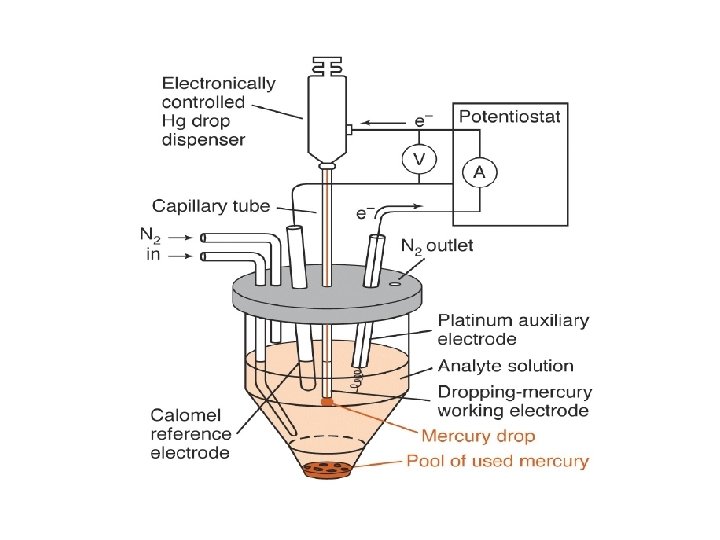
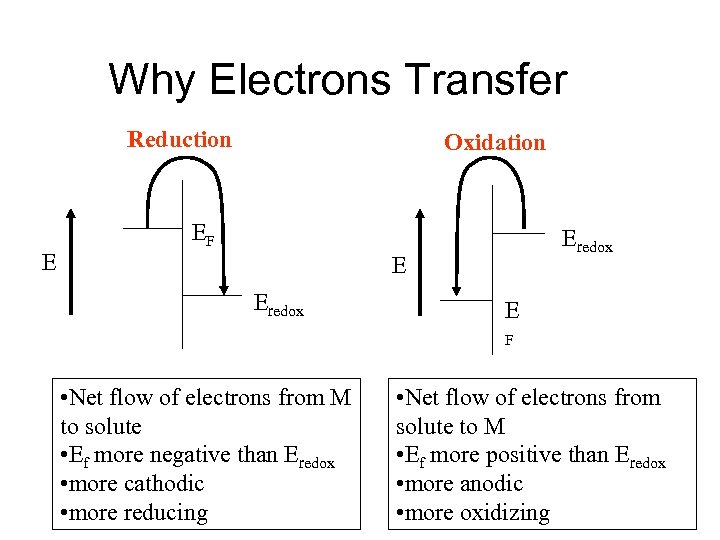 Why Electrons Transfer Reduction E Oxidation EF Eredox E F • Net flow of electrons from M to solute • Ef more negative than Eredox • more cathodic • more reducing • Net flow of electrons from solute to M • Ef more positive than Eredox • more anodic • more oxidizing
Why Electrons Transfer Reduction E Oxidation EF Eredox E F • Net flow of electrons from M to solute • Ef more negative than Eredox • more cathodic • more reducing • Net flow of electrons from solute to M • Ef more positive than Eredox • more anodic • more oxidizing
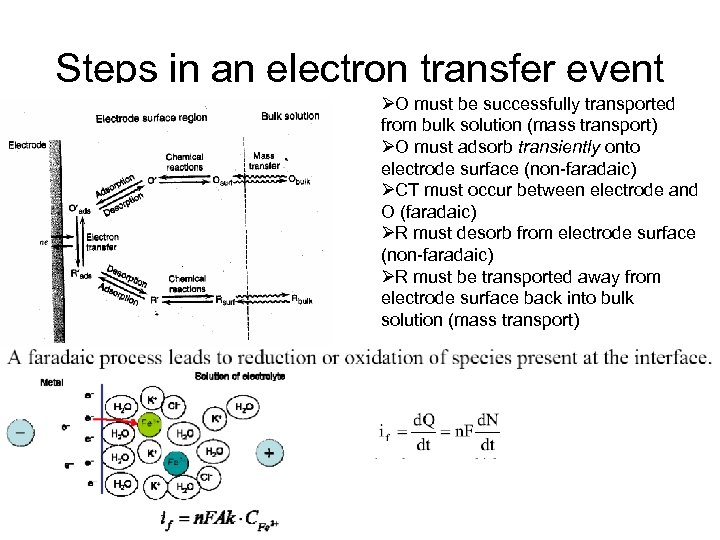 Steps in an electron transfer event ØO must be successfully transported from bulk solution (mass transport) ØO must adsorb transiently onto electrode surface (non-faradaic) ØCT must occur between electrode and O (faradaic) ØR must desorb from electrode surface (non-faradaic) ØR must be transported away from electrode surface back into bulk solution (mass transport)
Steps in an electron transfer event ØO must be successfully transported from bulk solution (mass transport) ØO must adsorb transiently onto electrode surface (non-faradaic) ØCT must occur between electrode and O (faradaic) ØR must desorb from electrode surface (non-faradaic) ØR must be transported away from electrode surface back into bulk solution (mass transport)
 Mass Transport or Mass Transfer • • • Migration – movement of a charged particle in a potential field Diffusion – movement due to a concentration gradient. If electrochemical reaction depletes (or produces) some species at the electrode surface, then a concentration gradient develops and the electroactive species will tend to diffuse from the bulk solution to the electrode (or from the electrode out into the bulk solution) Convection – mass transfer due to stirring. Achieved by some form of mechanical movement of the solution or the electrode i. e. , stir solution, rotate or vibrate electrode Difficult to get perfect reproducibility with stirring, better to move the electrode Convection is considerably more efficient than diffusion or migration = higher currents for a given concentration = greater analytical sensitivity
Mass Transport or Mass Transfer • • • Migration – movement of a charged particle in a potential field Diffusion – movement due to a concentration gradient. If electrochemical reaction depletes (or produces) some species at the electrode surface, then a concentration gradient develops and the electroactive species will tend to diffuse from the bulk solution to the electrode (or from the electrode out into the bulk solution) Convection – mass transfer due to stirring. Achieved by some form of mechanical movement of the solution or the electrode i. e. , stir solution, rotate or vibrate electrode Difficult to get perfect reproducibility with stirring, better to move the electrode Convection is considerably more efficient than diffusion or migration = higher currents for a given concentration = greater analytical sensitivity
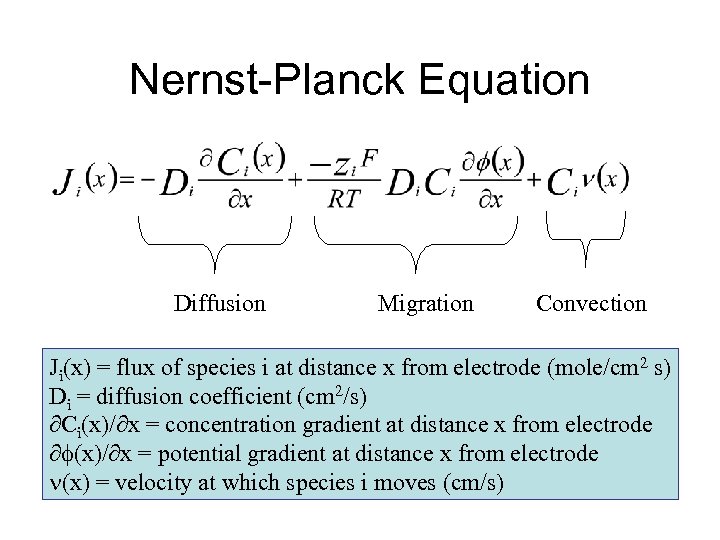 Nernst-Planck Equation Diffusion Migration Convection Ji(x) = flux of species i at distance x from electrode (mole/cm 2 s) Di = diffusion coefficient (cm 2/s) Ci(x)/ x = concentration gradient at distance x from electrode (x)/ x = potential gradient at distance x from electrode (x) = velocity at which species i moves (cm/s)
Nernst-Planck Equation Diffusion Migration Convection Ji(x) = flux of species i at distance x from electrode (mole/cm 2 s) Di = diffusion coefficient (cm 2/s) Ci(x)/ x = concentration gradient at distance x from electrode (x)/ x = potential gradient at distance x from electrode (x) = velocity at which species i moves (cm/s)
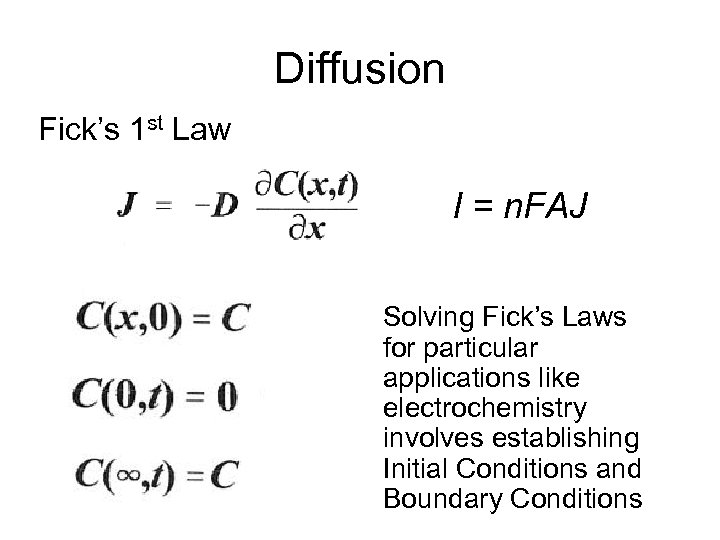 Diffusion Fick’s 1 st Law I = n. FAJ Solving Fick’s Laws for particular applications like electrochemistry involves establishing Initial Conditions and Boundary Conditions
Diffusion Fick’s 1 st Law I = n. FAJ Solving Fick’s Laws for particular applications like electrochemistry involves establishing Initial Conditions and Boundary Conditions
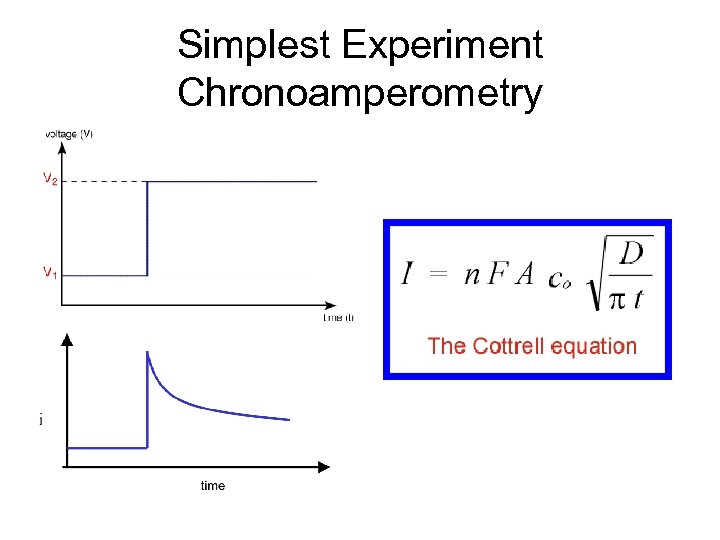 Simplest Experiment Chronoamperometry
Simplest Experiment Chronoamperometry
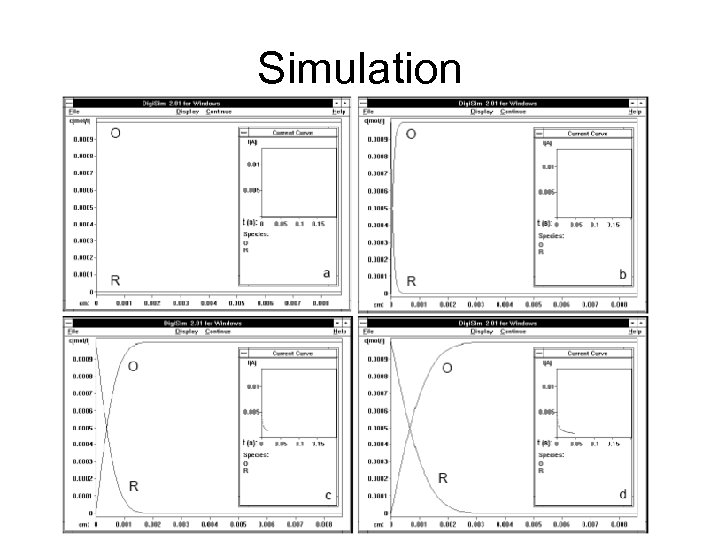 Simulation
Simulation
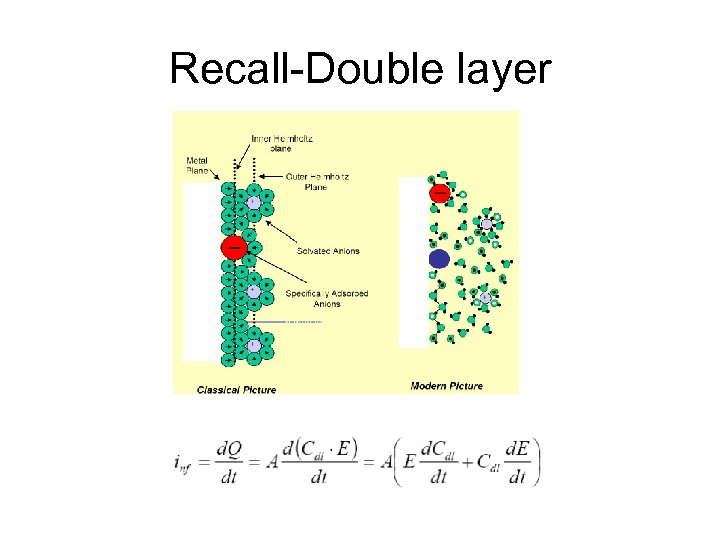 Recall-Double layer
Recall-Double layer
 Double-Layer charging • Charging/discharging a capacitor upon application of a potential step Itotal = Ic + IF
Double-Layer charging • Charging/discharging a capacitor upon application of a potential step Itotal = Ic + IF
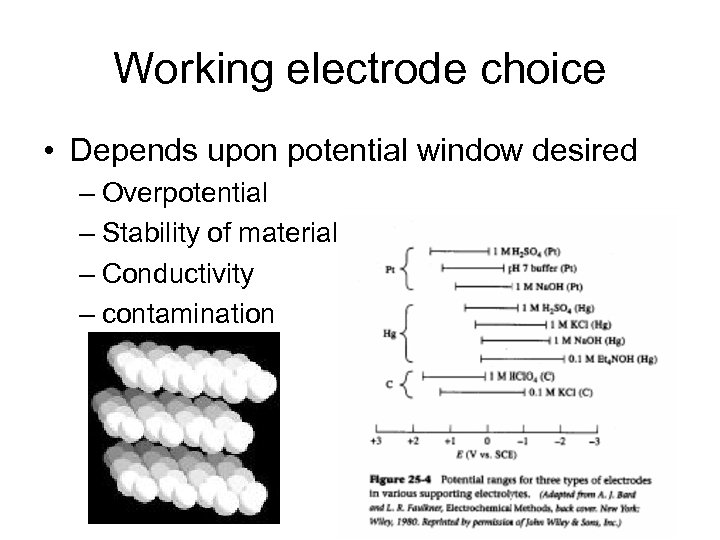 Working electrode choice • Depends upon potential window desired – Overpotential – Stability of material – Conductivity – contamination
Working electrode choice • Depends upon potential window desired – Overpotential – Stability of material – Conductivity – contamination
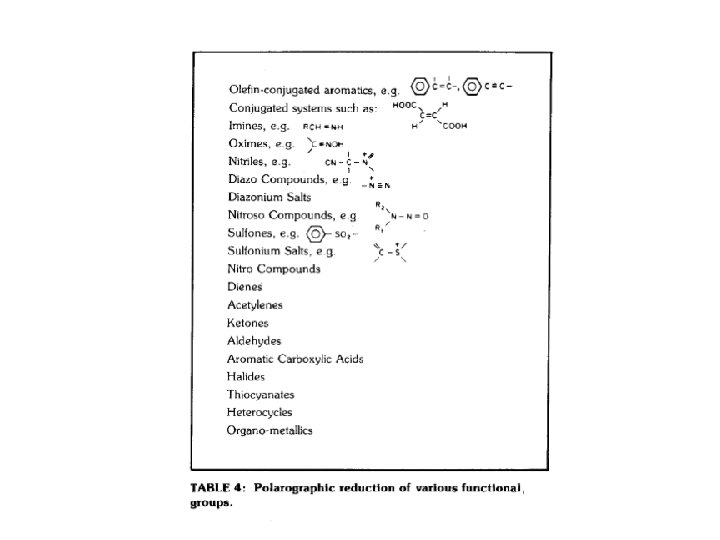
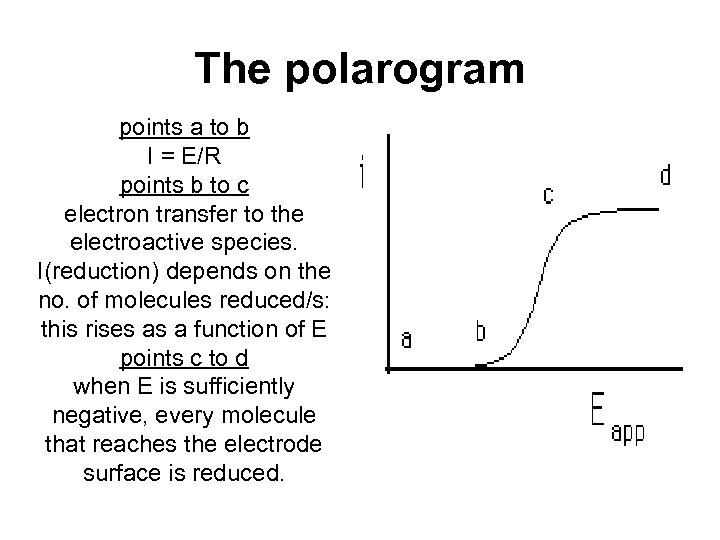 The polarogram points a to b I = E/R points b to c electron transfer to the electroactive species. I(reduction) depends on the no. of molecules reduced/s: this rises as a function of E points c to d when E is sufficiently negative, every molecule that reaches the electrode surface is reduced.
The polarogram points a to b I = E/R points b to c electron transfer to the electroactive species. I(reduction) depends on the no. of molecules reduced/s: this rises as a function of E points c to d when E is sufficiently negative, every molecule that reaches the electrode surface is reduced.
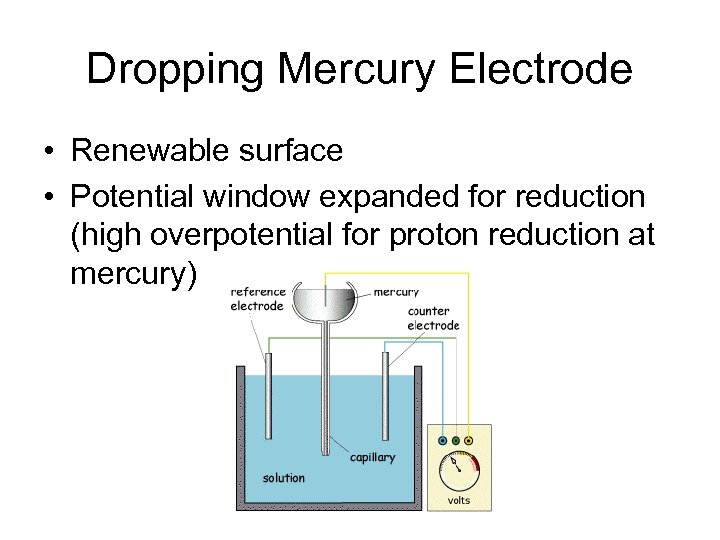 Dropping Mercury Electrode • Renewable surface • Potential window expanded for reduction (high overpotential for proton reduction at mercury)
Dropping Mercury Electrode • Renewable surface • Potential window expanded for reduction (high overpotential for proton reduction at mercury)
 Polarography A = 4 (3 mt/4 d)2/3 = 0. 85(mt)2/3 Density of drop Mass flow rate of drop We can substitute this into Cottrell Equation i(t) = n. FACD 1/2/ 1/2 t 1/2 We also replace D by 7/3 D to account for the compression of the diffusion layer by the expanding drop Giving the Ilkovich Equation: id = 708 n. D 1/2 m 2/3 t 1/6 C I has units of Amps when D is in cm 2 s-1, m is in g/s and t is in seconds. C is in mol/cm 3 This expression gives the current at the end of the drop life. The average current is obtained by integrating the current over this time period iav = 607 n. D 1/2 m 2/3 t 1/6 C
Polarography A = 4 (3 mt/4 d)2/3 = 0. 85(mt)2/3 Density of drop Mass flow rate of drop We can substitute this into Cottrell Equation i(t) = n. FACD 1/2/ 1/2 t 1/2 We also replace D by 7/3 D to account for the compression of the diffusion layer by the expanding drop Giving the Ilkovich Equation: id = 708 n. D 1/2 m 2/3 t 1/6 C I has units of Amps when D is in cm 2 s-1, m is in g/s and t is in seconds. C is in mol/cm 3 This expression gives the current at the end of the drop life. The average current is obtained by integrating the current over this time period iav = 607 n. D 1/2 m 2/3 t 1/6 C
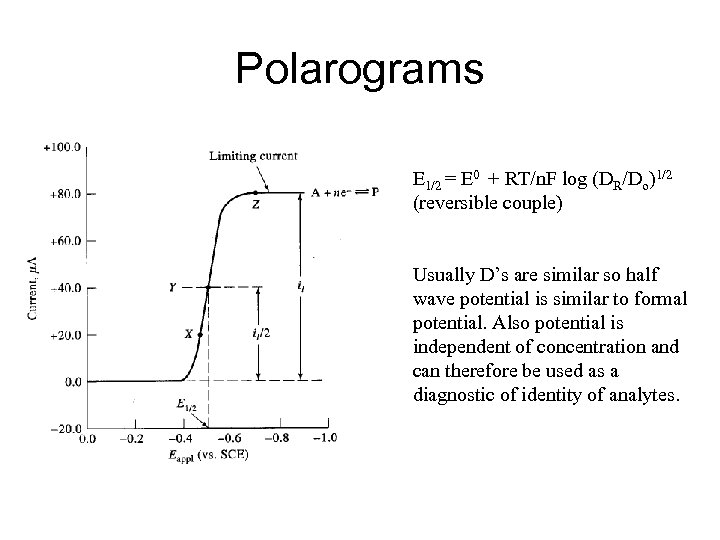 Polarograms E 1/2 = E 0 + RT/n. F log (DR/Do)1/2 (reversible couple) Usually D’s are similar so half wave potential is similar to formal potential. Also potential is independent of concentration and can therefore be used as a diagnostic of identity of analytes.
Polarograms E 1/2 = E 0 + RT/n. F log (DR/Do)1/2 (reversible couple) Usually D’s are similar so half wave potential is similar to formal potential. Also potential is independent of concentration and can therefore be used as a diagnostic of identity of analytes.
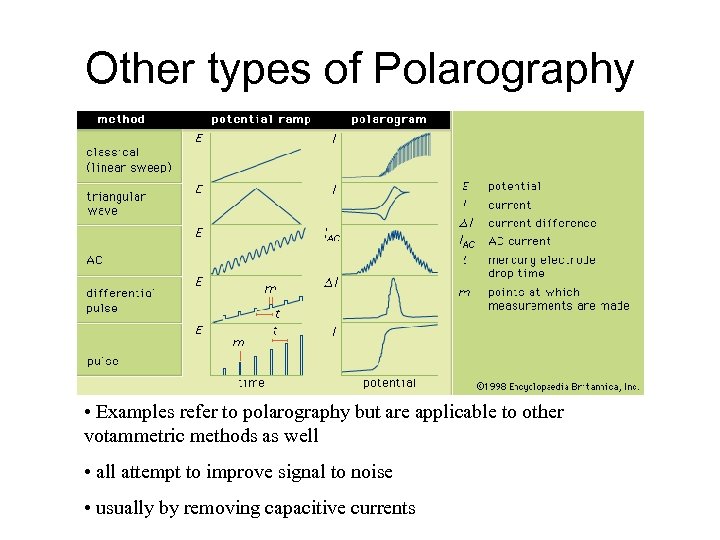 Other types of Polarography • Examples refer to polarography but are applicable to other votammetric methods as well • all attempt to improve signal to noise • usually by removing capacitive currents
Other types of Polarography • Examples refer to polarography but are applicable to other votammetric methods as well • all attempt to improve signal to noise • usually by removing capacitive currents
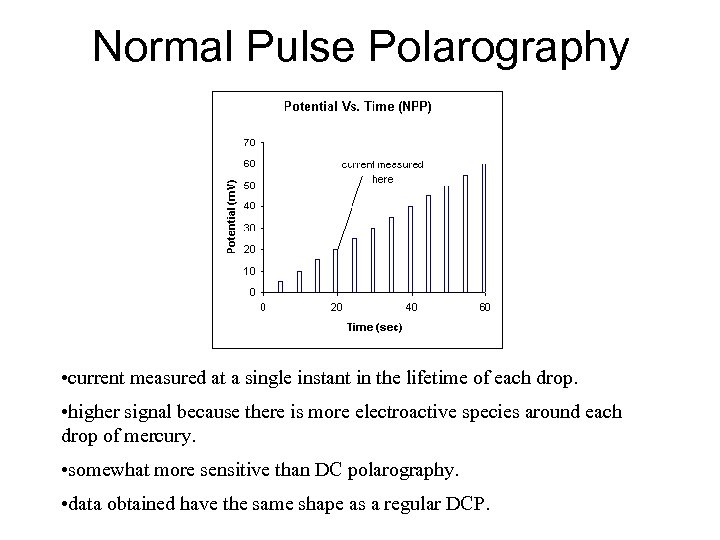 Normal Pulse Polarography • current measured at a single instant in the lifetime of each drop. • higher signal because there is more electroactive species around each drop of mercury. • somewhat more sensitive than DC polarography. • data obtained have the same shape as a regular DCP.
Normal Pulse Polarography • current measured at a single instant in the lifetime of each drop. • higher signal because there is more electroactive species around each drop of mercury. • somewhat more sensitive than DC polarography. • data obtained have the same shape as a regular DCP.
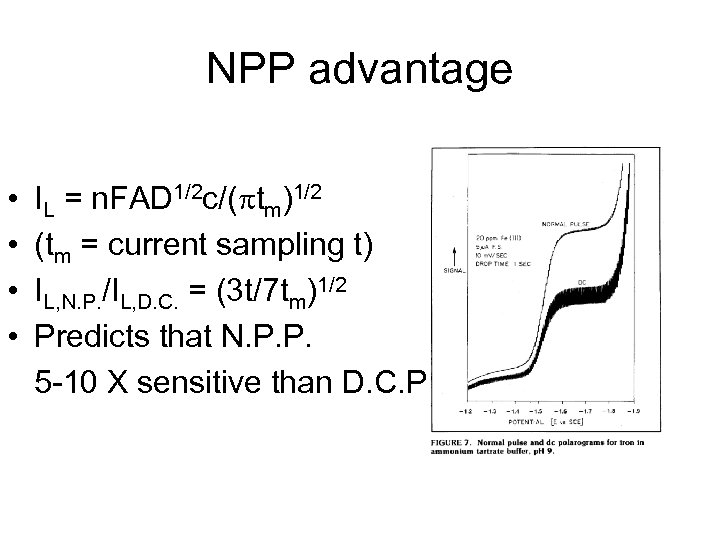 NPP advantage • • IL = n. FAD 1/2 c/( tm)1/2 (tm = current sampling t) IL, N. P. /IL, D. C. = (3 t/7 tm)1/2 Predicts that N. P. P. 5 -10 X sensitive than D. C. P.
NPP advantage • • IL = n. FAD 1/2 c/( tm)1/2 (tm = current sampling t) IL, N. P. /IL, D. C. = (3 t/7 tm)1/2 Predicts that N. P. P. 5 -10 X sensitive than D. C. P.
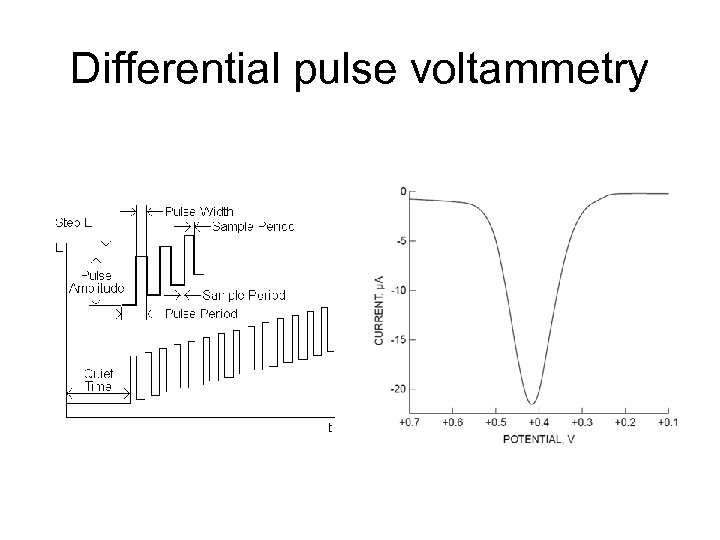 Differential pulse voltammetry
Differential pulse voltammetry
 DPP • current measured twice during the lifetime of each drop difference in current is plotted. • Results in a peak-shaped feature, where the top of the peak corresponds to E 1/2, and the height gives concentration • This shape is the derivative of the regular DC data. • DPP has the advantage of sensitive detection limits and discrimination against background currents. Traditionally, metals in the ppm range can be determined with DPP. • Derivative improves contrast (resolution) between overlapping waves
DPP • current measured twice during the lifetime of each drop difference in current is plotted. • Results in a peak-shaped feature, where the top of the peak corresponds to E 1/2, and the height gives concentration • This shape is the derivative of the regular DC data. • DPP has the advantage of sensitive detection limits and discrimination against background currents. Traditionally, metals in the ppm range can be determined with DPP. • Derivative improves contrast (resolution) between overlapping waves
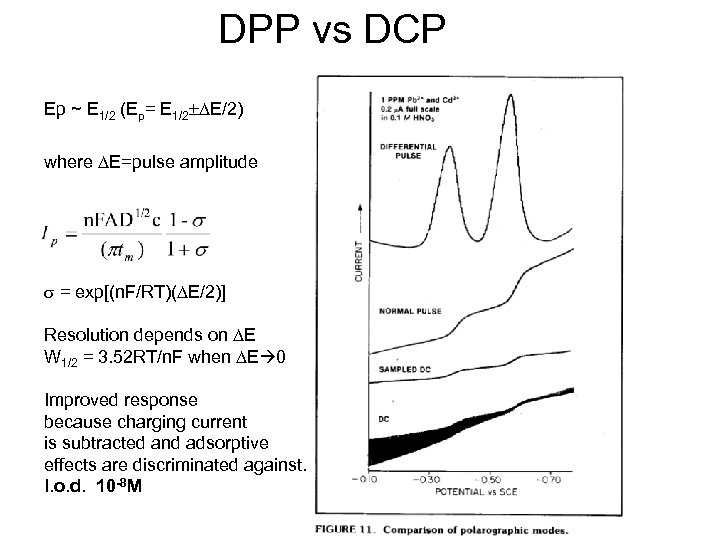 DPP vs DCP Ep ~ E 1/2 (Ep= E 1/2±DE/2) where DE=pulse amplitude s = exp[(n. F/RT)(DE/2)] Resolution depends on DE W 1/2 = 3. 52 RT/n. F when DE 0 Improved response because charging current is subtracted and adsorptive effects are discriminated against. l. o. d. 10 -8 M
DPP vs DCP Ep ~ E 1/2 (Ep= E 1/2±DE/2) where DE=pulse amplitude s = exp[(n. F/RT)(DE/2)] Resolution depends on DE W 1/2 = 3. 52 RT/n. F when DE 0 Improved response because charging current is subtracted and adsorptive effects are discriminated against. l. o. d. 10 -8 M
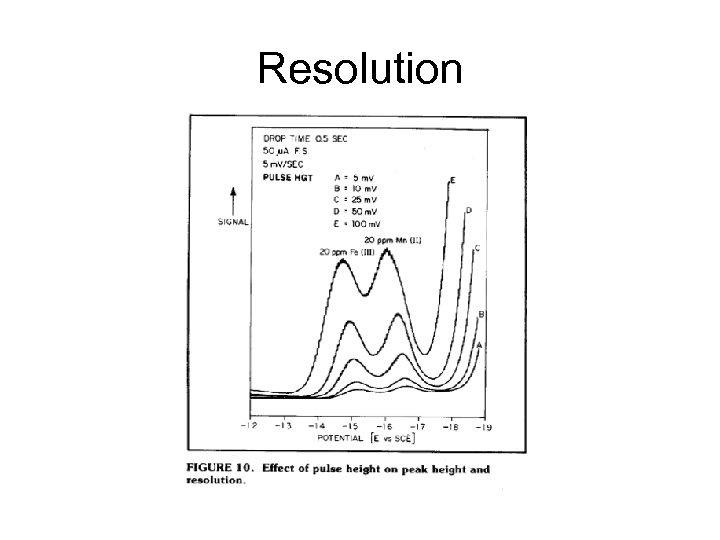 Resolution
Resolution
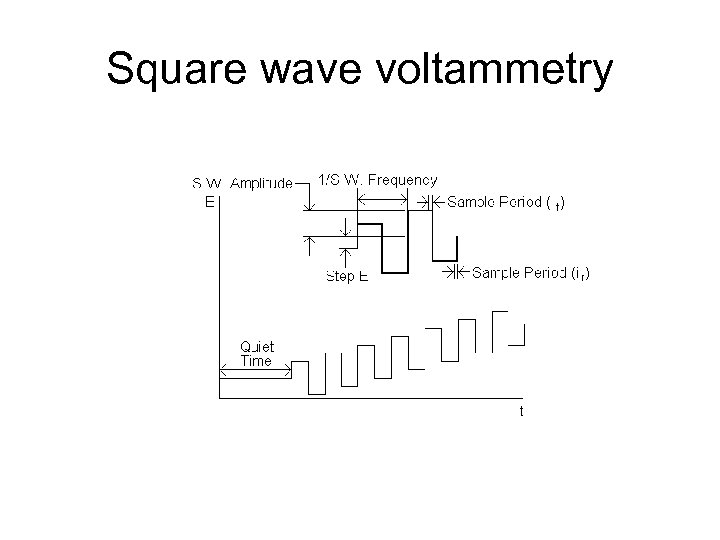 Square wave voltammetry
Square wave voltammetry
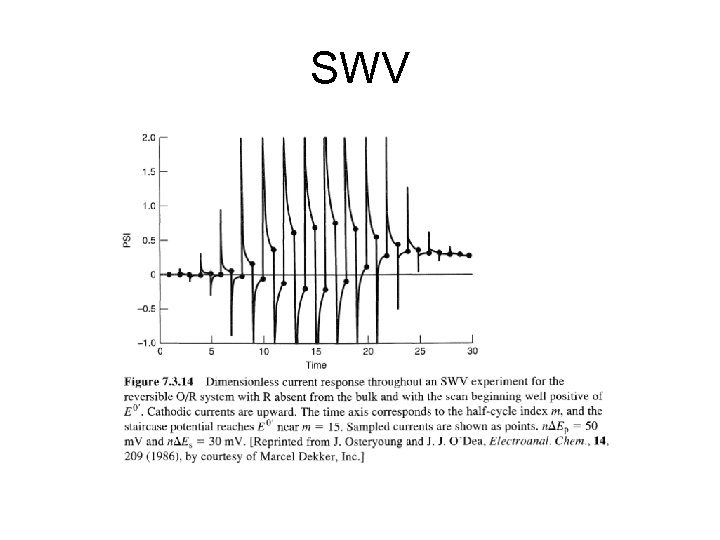 SWV
SWV
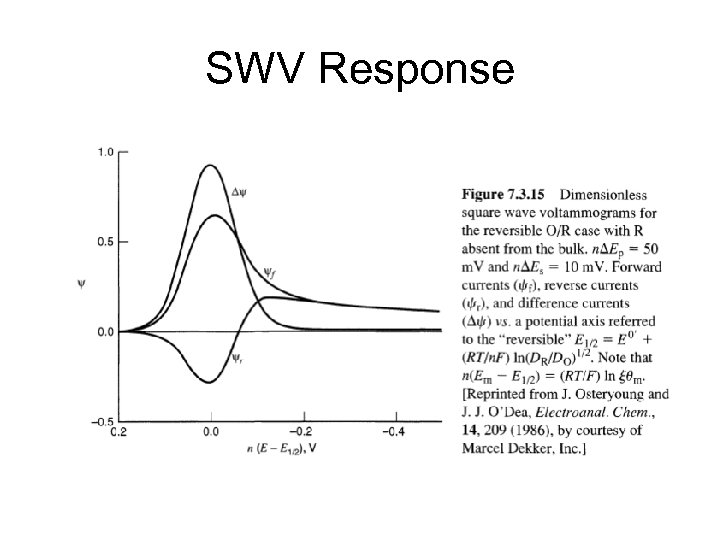 SWV Response
SWV Response
 SWV • advantage of square wave voltammetry is that the entire scan be performed on a single mercury drop in about 10 seconds, as opposed to about 5 minutes for the techniques described previously. SWV saves time, reduces the amount of mercury used per scan by a factor of 100. If used with a prereduction step, detection limits of 1 -10 ppb can be achieved, which rivals graphite furnace AA in sensitivity. • data for SWV similar to DPP • height and width of the wave depends on the exact combination of experimental parameters (i. e. scan rate and pulse height
SWV • advantage of square wave voltammetry is that the entire scan be performed on a single mercury drop in about 10 seconds, as opposed to about 5 minutes for the techniques described previously. SWV saves time, reduces the amount of mercury used per scan by a factor of 100. If used with a prereduction step, detection limits of 1 -10 ppb can be achieved, which rivals graphite furnace AA in sensitivity. • data for SWV similar to DPP • height and width of the wave depends on the exact combination of experimental parameters (i. e. scan rate and pulse height
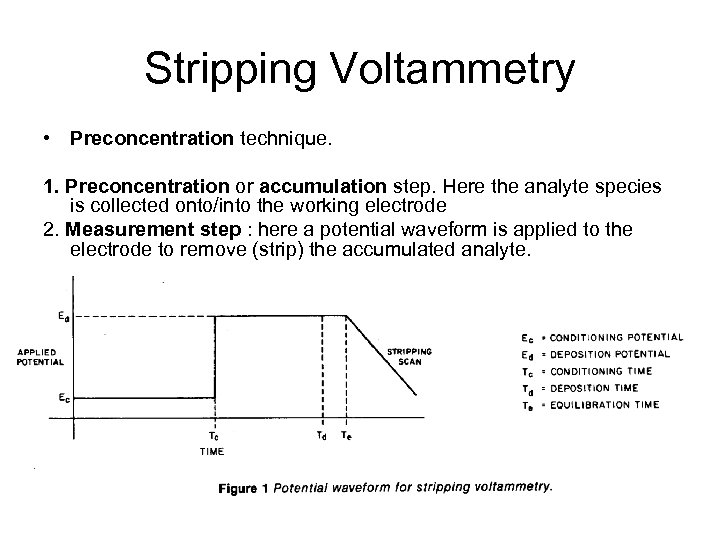 Stripping Voltammetry • Preconcentration technique. 1. Preconcentration or accumulation step. Here the analyte species is collected onto/into the working electrode 2. Measurement step : here a potential waveform is applied to the electrode to remove (strip) the accumulated analyte.
Stripping Voltammetry • Preconcentration technique. 1. Preconcentration or accumulation step. Here the analyte species is collected onto/into the working electrode 2. Measurement step : here a potential waveform is applied to the electrode to remove (strip) the accumulated analyte.
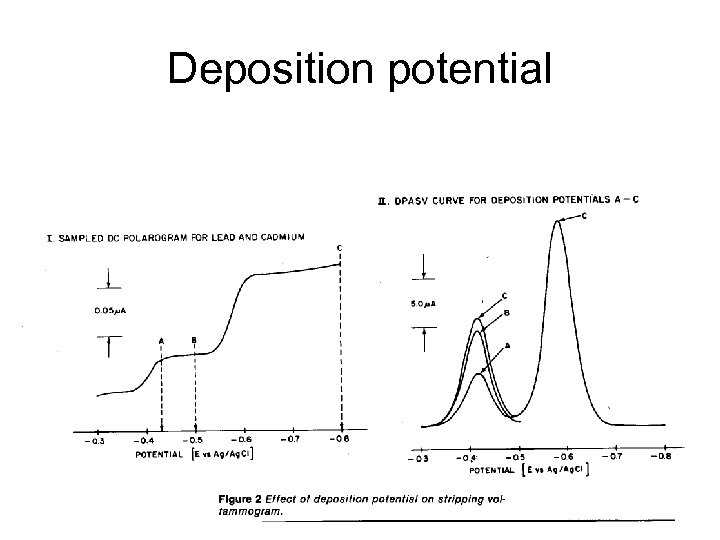 Deposition potential
Deposition potential
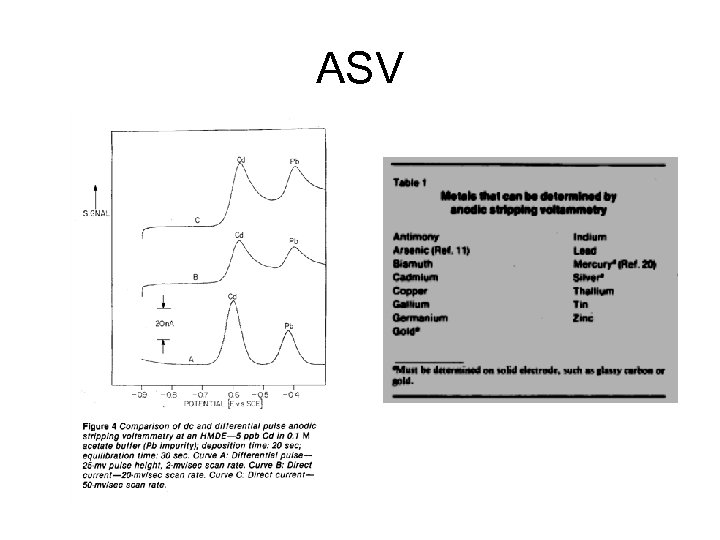 ASV
ASV
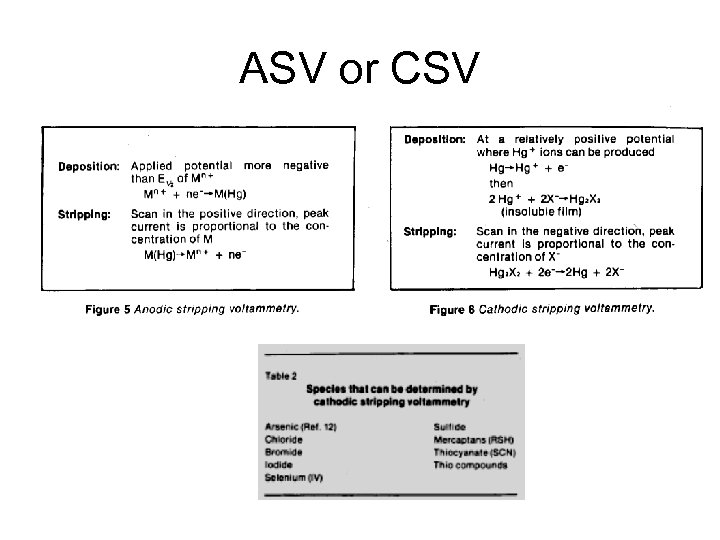 ASV or CSV
ASV or CSV
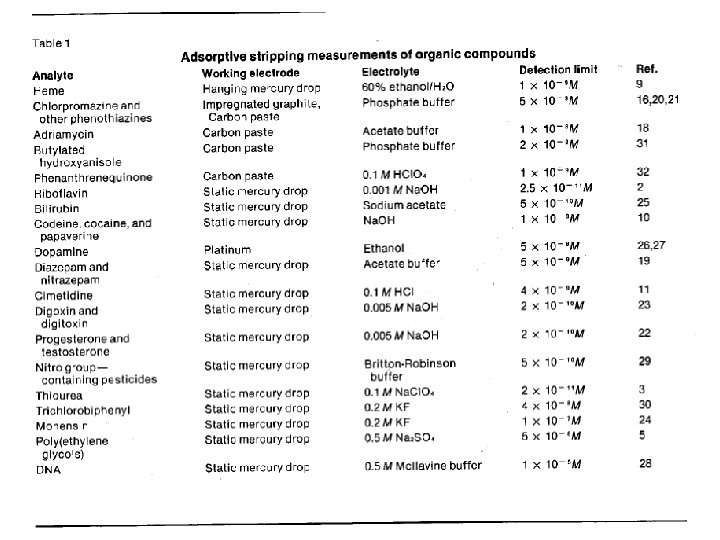
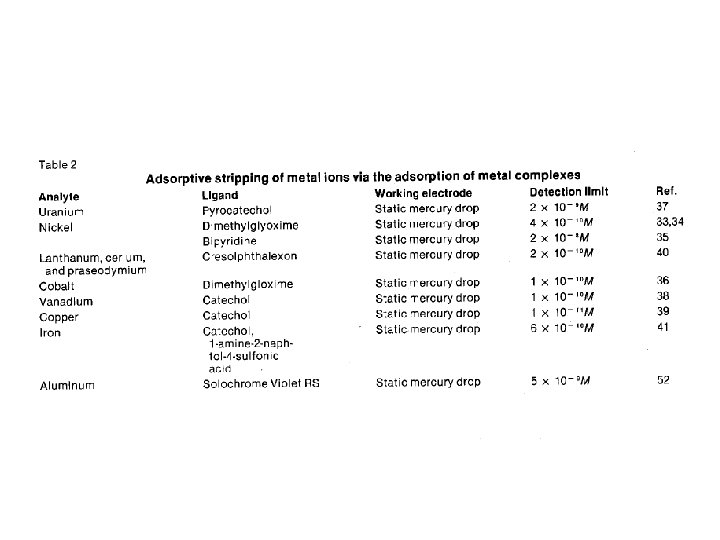
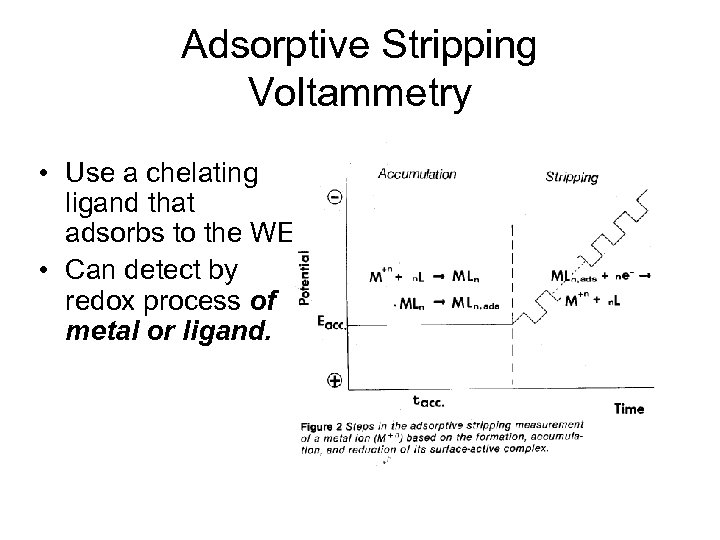 Adsorptive Stripping Voltammetry • Use a chelating ligand that adsorbs to the WE. • Can detect by redox process of metal or ligand.
Adsorptive Stripping Voltammetry • Use a chelating ligand that adsorbs to the WE. • Can detect by redox process of metal or ligand.
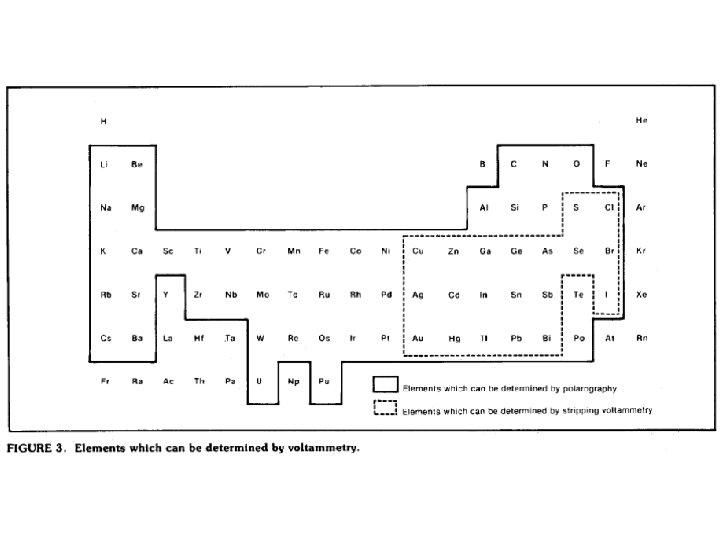
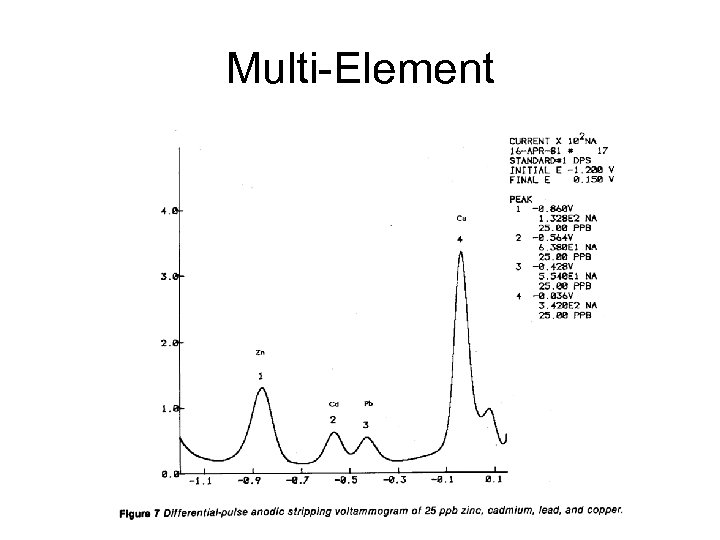 Multi-Element
Multi-Element
 Standard Addition
Standard Addition


