30df419f0ea32360137fbfbe0c8dc0af.ppt
- Количество слайдов: 22
 EFPIA IT Proposals EFPIA Proposals for IT Support to the European Regulatory Procedures Mr S. Hasler EFPIA PAT Regulation 2000 EFPIA IT Proposals 200401. ppt EFPIA 1
EFPIA IT Proposals EFPIA Proposals for IT Support to the European Regulatory Procedures Mr S. Hasler EFPIA PAT Regulation 2000 EFPIA IT Proposals 200401. ppt EFPIA 1
 EFPIA IT Proposals Key Benefits • • More rapid processes More efficient processes Remove duplication Increase transparency EFPIA IT Proposals 200401. ppt EFPIA 2
EFPIA IT Proposals Key Benefits • • More rapid processes More efficient processes Remove duplication Increase transparency EFPIA IT Proposals 200401. ppt EFPIA 2
 EFPIA IT Proposals General Principles (1) • • • Intent to remove need for paper copy Sufficient IT and business resource committed by agencies and industry Collaborative industry/agency approach to agreed initiatives Clear benefits for industry and agencies Agreed priority order for progression of initiatives EFPIA IT Proposals 200401. ppt EFPIA 3
EFPIA IT Proposals General Principles (1) • • • Intent to remove need for paper copy Sufficient IT and business resource committed by agencies and industry Collaborative industry/agency approach to agreed initiatives Clear benefits for industry and agencies Agreed priority order for progression of initiatives EFPIA IT Proposals 200401. ppt EFPIA 3
 EFPIA IT Proposals General Principles (2) • • • Single standards, in line with ICH, implemented across Europe One system across all agencies, wherever possible Integrated systems across all agencies, otherwise EFPIA IT Proposals 200401. ppt EFPIA 4
EFPIA IT Proposals General Principles (2) • • • Single standards, in line with ICH, implemented across Europe One system across all agencies, wherever possible Integrated systems across all agencies, otherwise EFPIA IT Proposals 200401. ppt EFPIA 4
 EFPIA IT Proposals The Future for Europe • Dossiers and data submitted to a single EU • repository e-submissions implemented in support of full lifecycle » e. CTD implemented » e. ADR submission implemented » management of static and dynamic data • Transparent process tracking systems • Secure email and document exchange systems EFPIA IT Proposals 200401. ppt EFPIA 5
EFPIA IT Proposals The Future for Europe • Dossiers and data submitted to a single EU • repository e-submissions implemented in support of full lifecycle » e. CTD implemented » e. ADR submission implemented » management of static and dynamic data • Transparent process tracking systems • Secure email and document exchange systems EFPIA IT Proposals 200401. ppt EFPIA 5
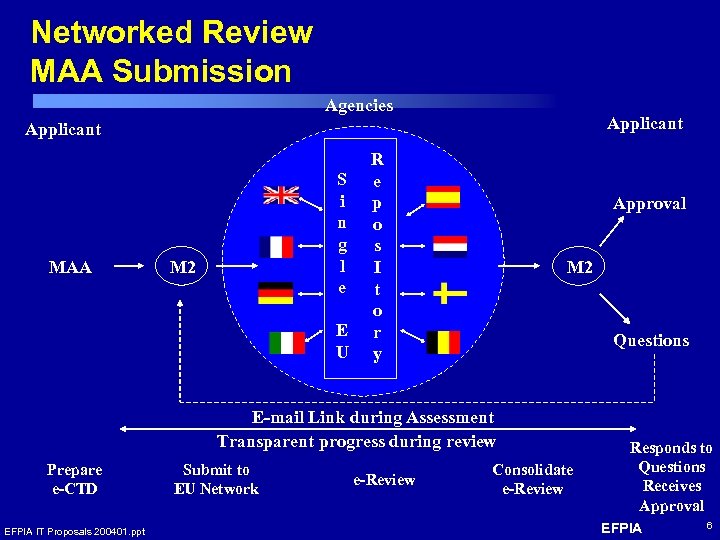 Networked Review MAA Submission Agencies Applicant MAA S i n g l e M 2 E U R e p o s I t o r y Approval M 2 Questions E-mail Link during Assessment Transparent progress during review Prepare e-CTD EFPIA IT Proposals 200401. ppt Submit to EU Network e-Review Consolidate e-Review Responds to Questions Receives Approval EFPIA 6
Networked Review MAA Submission Agencies Applicant MAA S i n g l e M 2 E U R e p o s I t o r y Approval M 2 Questions E-mail Link during Assessment Transparent progress during review Prepare e-CTD EFPIA IT Proposals 200401. ppt Submit to EU Network e-Review Consolidate e-Review Responds to Questions Receives Approval EFPIA 6
 EFPIA IT Proposals The Benefits • • • Single set of information for EU decision making Supports EU expansion Improves Pharmaco-vigilance system Bring products more quickly to patients Supports single review for Europe Improved filing and archiving Data integration across product life-cycle Improved information sharing and exchange Improved tracking and project management EFPIA IT Proposals 200401. ppt EFPIA 7
EFPIA IT Proposals The Benefits • • • Single set of information for EU decision making Supports EU expansion Improves Pharmaco-vigilance system Bring products more quickly to patients Supports single review for Europe Improved filing and archiving Data integration across product life-cycle Improved information sharing and exchange Improved tracking and project management EFPIA IT Proposals 200401. ppt EFPIA 7
 EFPIA IT Proposals Short Term Initiatives (Before 2002) • Secure electronic transmission » document exchange » e-mail • e-Submission of small dossiers • Management of Dynamic Data - PIM • e-Submission of ADRs • Transparent process tracking systems Support with Appropriate Guidelines and Directives EFPIA IT Proposals 200401. ppt EFPIA 8
EFPIA IT Proposals Short Term Initiatives (Before 2002) • Secure electronic transmission » document exchange » e-mail • e-Submission of small dossiers • Management of Dynamic Data - PIM • e-Submission of ADRs • Transparent process tracking systems Support with Appropriate Guidelines and Directives EFPIA IT Proposals 200401. ppt EFPIA 8
 Secure Electronic Transmission between Industry and Agencies • Implement system supporting » Secure 2 -way transmission of small volumes of information, e. g. – variations – responses • • • » Secure 2 -way e-mail Platform underpinning all e-interactions Eudrasafe? One system for EU EFPIA IT Proposals 200401. ppt EFPIA 9
Secure Electronic Transmission between Industry and Agencies • Implement system supporting » Secure 2 -way transmission of small volumes of information, e. g. – variations – responses • • • » Secure 2 -way e-mail Platform underpinning all e-interactions Eudrasafe? One system for EU EFPIA IT Proposals 200401. ppt EFPIA 9
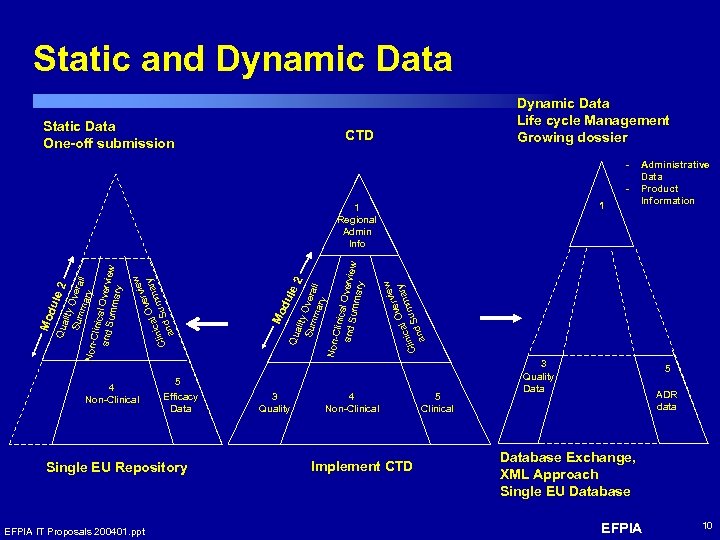 Static and Dynamic Data Static Data One-off submission Dynamic Data Life cycle Management Growing dossier CTD 1 4 Non-Clinical 5 Efficacy Data Single EU Repository EFPIA IT Proposals 200401. ppt 3 Quality w rvie Ove ary m ical Clin d Sum n a y Sum Overa mar ll y Non-C linica and S l Overview umma ry Mo du le Qua 2 lit w vie v er ry O ma ical Clin d Sum an Mo du le 2 Qua lity Sum Overa mar ll y Non-C linica l Ove and S umma rview ry 1 Regional Admin Info Administrative Data Product Information 4 Non-Clinical Implement CTD 5 Clinical 3 Quality Data 5 ADR data Database Exchange, XML Approach Single EU Database EFPIA 10
Static and Dynamic Data Static Data One-off submission Dynamic Data Life cycle Management Growing dossier CTD 1 4 Non-Clinical 5 Efficacy Data Single EU Repository EFPIA IT Proposals 200401. ppt 3 Quality w rvie Ove ary m ical Clin d Sum n a y Sum Overa mar ll y Non-C linica and S l Overview umma ry Mo du le Qua 2 lit w vie v er ry O ma ical Clin d Sum an Mo du le 2 Qua lity Sum Overa mar ll y Non-C linica l Ove and S umma rview ry 1 Regional Admin Info Administrative Data Product Information 4 Non-Clinical Implement CTD 5 Clinical 3 Quality Data 5 ADR data Database Exchange, XML Approach Single EU Database EFPIA 10
 Implement e-submissions Stepwise approach using e. CTD standards: • Small dossiers submitted as pdf, e. g. variations • Establish dynamic data exchange and management • » continue to support PIM Implement e. CTD for new products EFPIA IT Proposals 200401. ppt EFPIA 11
Implement e-submissions Stepwise approach using e. CTD standards: • Small dossiers submitted as pdf, e. g. variations • Establish dynamic data exchange and management • » continue to support PIM Implement e. CTD for new products EFPIA IT Proposals 200401. ppt EFPIA 11
 Establish Dynamic Data Exchange and Management • • Dynamic data in the dossier to be exchanged » Using defined exchange standard » Database to database Potential areas for collaborative initiatives » Management and submission of product information (PIM) » Management and submission of CMC (Module 3) – Cumulative table of contents – CMC data, starting with small element of data, e. g. specification • » Administrative data (Module 1) Provides EU input to evolving e. CTD » PIM included in module 1 of e. CTD EFPIA IT Proposals 200401. ppt EFPIA 12
Establish Dynamic Data Exchange and Management • • Dynamic data in the dossier to be exchanged » Using defined exchange standard » Database to database Potential areas for collaborative initiatives » Management and submission of product information (PIM) » Management and submission of CMC (Module 3) – Cumulative table of contents – CMC data, starting with small element of data, e. g. specification • » Administrative data (Module 1) Provides EU input to evolving e. CTD » PIM included in module 1 of e. CTD EFPIA IT Proposals 200401. ppt EFPIA 12
 Submit Updated Parts only Overdose_s Overdose_p Duration Life XML V 1. 0 XML V 1. 1 EFPIA IT Proposals 200401. ppt EFPIA 13
Submit Updated Parts only Overdose_s Overdose_p Duration Life XML V 1. 0 XML V 1. 1 EFPIA IT Proposals 200401. ppt EFPIA 13
 Benefits from PIM • • • Removes duplication of work across a product » Creation of documents by applicant » Review of documents by agency Brings resource efficiencies Reduces number of translations Faster notification of changes proposed by agencies or industry Capability to support administrative aspects of Decision Making Process and reduce time Capability to support MR and National processes EFPIA IT Proposals 200401. ppt EFPIA 14
Benefits from PIM • • • Removes duplication of work across a product » Creation of documents by applicant » Review of documents by agency Brings resource efficiencies Reduces number of translations Faster notification of changes proposed by agencies or industry Capability to support administrative aspects of Decision Making Process and reduce time Capability to support MR and National processes EFPIA IT Proposals 200401. ppt EFPIA 14
 e-Submission of ADRs • • Implement full production system » Engage more agencies » Engage more of industry Ensure ICSR activity supported by secure electronic data exchange EFPIA IT Proposals 200401. ppt EFPIA 15
e-Submission of ADRs • • Implement full production system » Engage more agencies » Engage more of industry Ensure ICSR activity supported by secure electronic data exchange EFPIA IT Proposals 200401. ppt EFPIA 15
 Process tracking system • • Implement process tracking system in EU Key benefits » Transparent European information » Instant access for industry and regulators to key information about specific regulatory tasks » Automated process measurement and prompting / alerting of key dates and milestones » Instant access to the tracking system and information about own applications » Availability both for the centralised and the mutual recognition procedures » Different tracking systems implemented at present at a national level (e. g. MCA's RAMA) made compatible EFPIA IT Proposals 200401. ppt EFPIA 16
Process tracking system • • Implement process tracking system in EU Key benefits » Transparent European information » Instant access for industry and regulators to key information about specific regulatory tasks » Automated process measurement and prompting / alerting of key dates and milestones » Instant access to the tracking system and information about own applications » Availability both for the centralised and the mutual recognition procedures » Different tracking systems implemented at present at a national level (e. g. MCA's RAMA) made compatible EFPIA IT Proposals 200401. ppt EFPIA 16
 EFPIA IT Proposals Longer Term Initiatives (Before 2004) • Implement e. CTD • Single EU Submission Repository • Management of Dynamic Data - other dossier components » CMC » Administrative data • Single EU ADR database Support with Appropriate Guidelines and Directives EFPIA IT Proposals 200401. ppt EFPIA 17
EFPIA IT Proposals Longer Term Initiatives (Before 2004) • Implement e. CTD • Single EU Submission Repository • Management of Dynamic Data - other dossier components » CMC » Administrative data • Single EU ADR database Support with Appropriate Guidelines and Directives EFPIA IT Proposals 200401. ppt EFPIA 17
 Implement e. CTD • • Implement e. CTD as standard for e-submission across product lifecycle: » When signed off at step 4 » Collaboration between agencies and industry » Agencies to develop internal review environments Support e. CTD with EU legislation » Encouraging e-submission » Removing need for paper copy of submission Develop single EU submission repository Develop new aspects of e. CTD in collaboration with industry (e. g. manage CMC as dynamic data) EFPIA IT Proposals 200401. ppt EFPIA 18
Implement e. CTD • • Implement e. CTD as standard for e-submission across product lifecycle: » When signed off at step 4 » Collaboration between agencies and industry » Agencies to develop internal review environments Support e. CTD with EU legislation » Encouraging e-submission » Removing need for paper copy of submission Develop single EU submission repository Develop new aspects of e. CTD in collaboration with industry (e. g. manage CMC as dynamic data) EFPIA IT Proposals 200401. ppt EFPIA 18
 Other Benefits of Dynamic Data Exchange and Management • Dossier content always up-to-date • Provides transparency of information • Provides resource savings and efficiencies • Up-to-date information for better decision making, e. g. » Manufacturing inspections • Eliminates or speeds up administrative steps • Tackles common business problem for industry and agencies EFPIA IT Proposals 200401. ppt EFPIA 19
Other Benefits of Dynamic Data Exchange and Management • Dossier content always up-to-date • Provides transparency of information • Provides resource savings and efficiencies • Up-to-date information for better decision making, e. g. » Manufacturing inspections • Eliminates or speeds up administrative steps • Tackles common business problem for industry and agencies EFPIA IT Proposals 200401. ppt EFPIA 19
 Single EU ADR Database • • • Single EU repository for product AE data » Single point of submission » Single data set for safety profile Up-to-date information for better decision making, e. g. » Protection of public health Provides resource savings and efficiencies Tackles common business problem for industry and agencies Facilitates PSUR creation and submission EFPIA IT Proposals 200401. ppt EFPIA 20
Single EU ADR Database • • • Single EU repository for product AE data » Single point of submission » Single data set for safety profile Up-to-date information for better decision making, e. g. » Protection of public health Provides resource savings and efficiencies Tackles common business problem for industry and agencies Facilitates PSUR creation and submission EFPIA IT Proposals 200401. ppt EFPIA 20
 EFPIA IT Proposals Conclusions (1) • Progress towards future IT implementation • Realise benefits » » Support EU Expansion Improve Pharmaco-vigilance system Bring products more quickly to patients Enhance competitiveness of EU Regulatory Procedures EFPIA IT Proposals 200401. ppt EFPIA 21
EFPIA IT Proposals Conclusions (1) • Progress towards future IT implementation • Realise benefits » » Support EU Expansion Improve Pharmaco-vigilance system Bring products more quickly to patients Enhance competitiveness of EU Regulatory Procedures EFPIA IT Proposals 200401. ppt EFPIA 21
 EFPIA IT Proposals Conclusions (2) • Progress towards future IT implementation • Industry and Agencies work together • • » shared benefits » shared steering committees and task forces Require clear commitment to ongoing and future projects » agency business and IT resources Support changes with legislation » remove the need for paper INDUSTRY WILL PLAY OUR PART EFPIA IT Proposals 200401. ppt EFPIA 22
EFPIA IT Proposals Conclusions (2) • Progress towards future IT implementation • Industry and Agencies work together • • » shared benefits » shared steering committees and task forces Require clear commitment to ongoing and future projects » agency business and IT resources Support changes with legislation » remove the need for paper INDUSTRY WILL PLAY OUR PART EFPIA IT Proposals 200401. ppt EFPIA 22


