63c2a7a0df22b3c16265d15ca7d70c00.ppt
- Количество слайдов: 21
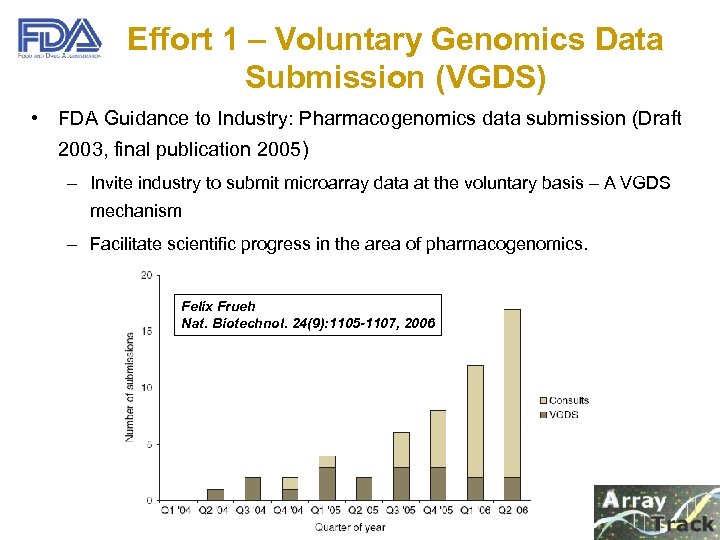
Effort 1 – Voluntary Genomics Data Submission (VGDS) • FDA Guidance to Industry: Pharmacogenomics data submission (Draft 2003, final publication 2005) – Invite industry to submit microarray data at the voluntary basis – A VGDS mechanism – Facilitate scientific progress in the area of pharmacogenomics. Felix Frueh Nat. Biotechnol. 24(9): 1105 -1107, 2006
Effort 2 - Array. Track • Need a bioinformatics tool to accomplish: – Objective 1: Data repository – Objective 2: Reproduce the sponsor’s results – Objective 3: Conduct alternative analysis • Array. Track – A FDA genomic tool – AT version 1 (2001): Filter array; data management tool – AT version 2 (2002): in-house microarray core facility – AT version 2. 2 (late 2003): Open to public – AT version 3. 1 (2004): VGDS – AT version 3. 2 (2005): MAQC – AT version 4 (2006 – present): VGDS VXDS
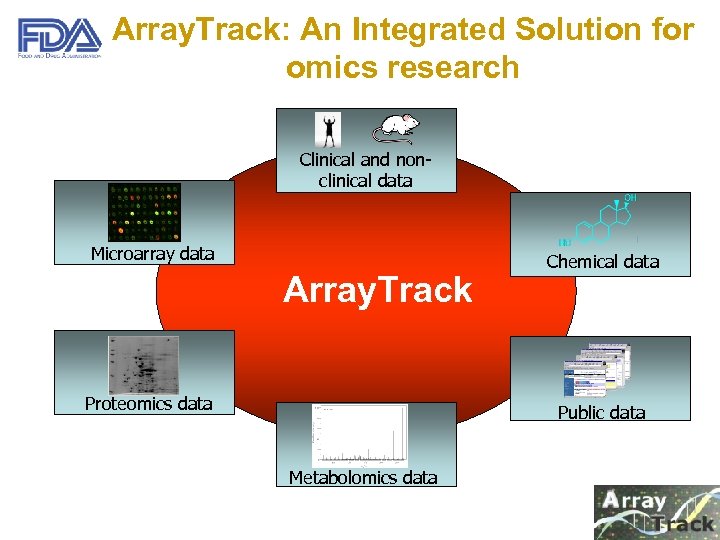
Array. Track: An Integrated Solution for omics research Clinical and nonclinical data Microarray data Array. Track Proteomics data Chemical data Public data Metabolomics data
Protein Gene Metabolite
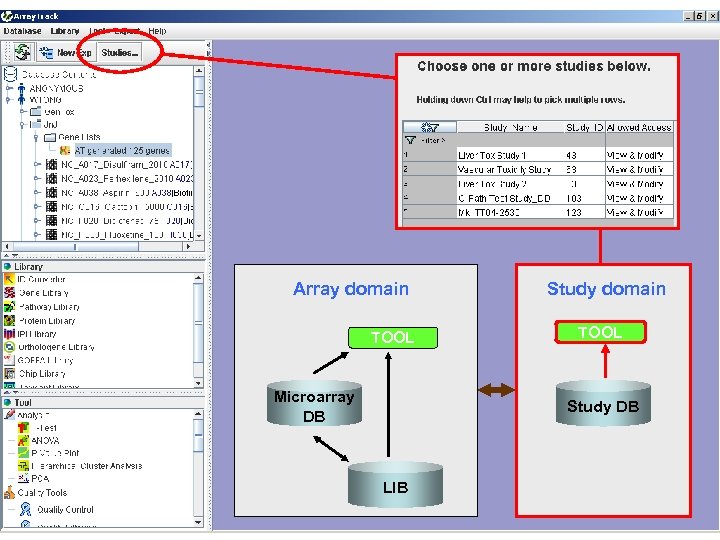
Array domain TOOL Microarray DB Study domain TOOL Study DB LIB
Study Data Management and Analysis • FDA e. Submission efforts – Clinical data: Clinical Data Interchanges Standards Consortium (CDISC) – Non-clinical data: Standard for Exchange of Nonclinical Data (SEND) • Subject, treatment, Clinical pathology, histopathology, … • Conforming to SDTM used for CDISC/SEND • Microarray data management and analysis are processed in Array Domain and the findings are available to correlate with data in Study Domain
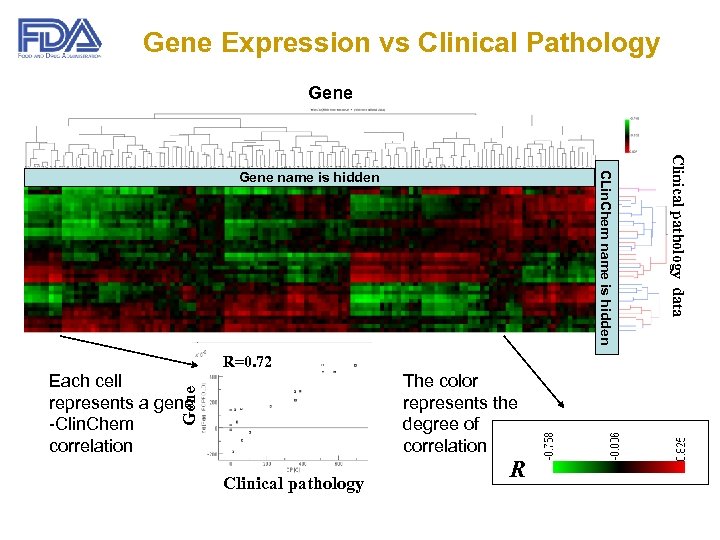
Gene Expression vs Clinical Pathology Gene R=0. 72 The color represents the degree of correlation Gene Each cell represents a gene -Clin. Chem correlation Clinical pathology R Clinical pathology data CLin. Chem name is hidden Gene name is hidden
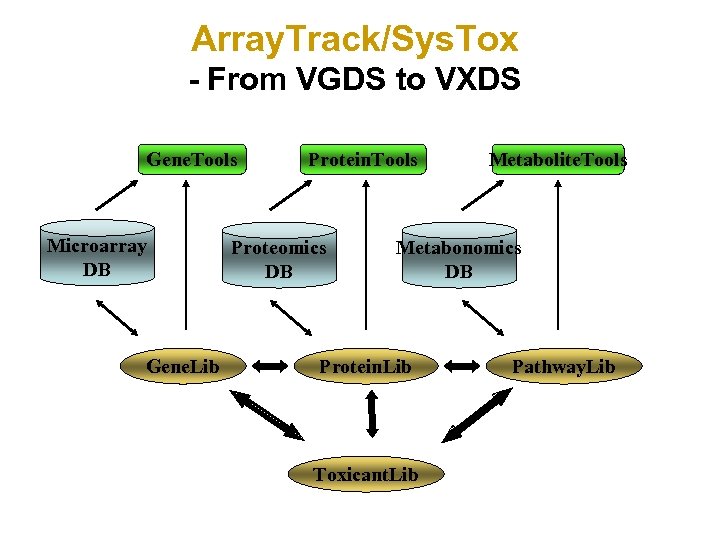
Array. Track/Sys. Tox - From VGDS to VXDS Gene. Tools Microarray DB Gene. Lib Protein. Tools Proteomics DB Metabolite. Tools Metabonomics DB Protein. Lib Toxicant. Lib Pathway. Lib
Storing Protein and Metabolite Lists Examining common pathways and functions shard by expression data from genomics, proteomics and metabolomics
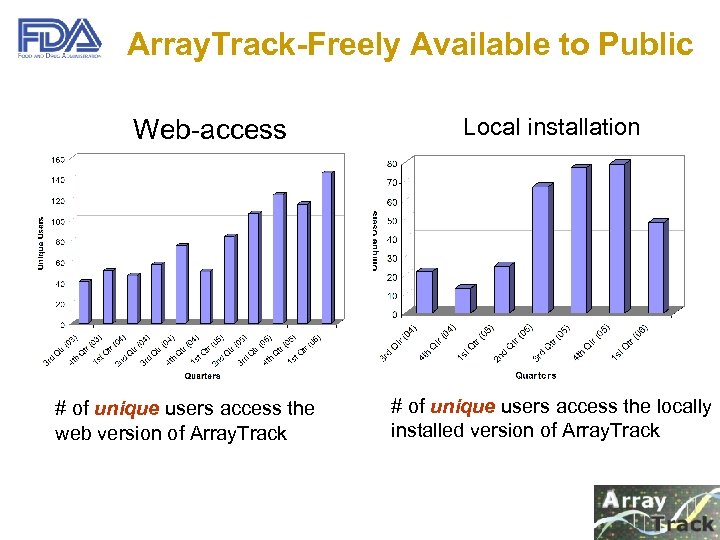
Array. Track-Freely Available to Public Web-access # of unique users access the web version of Array. Track Local installation # of unique users access the locally installed version of Array. Track
Knowledge Base 1. 2. 3. 4. Toxicant. Lib Liver Tox Knowledge Base (LTKB) Sex Determined Toxicity in Gene Expression …

Effort 3 - Best Practice Document • One of the VGDS objectives is to communicate with the private industry and gain experience on – How to exchange genomic data (data submission) – How to analyze genomic data – How to interpret genomic data • Lessons Learned from VGDS has led to development of Best Practice Document (Led by Federico Goodsaid) – Recommendations for the Generation and Submission of Genomic Data (Nov 2006) (http: //www. fda. gov/cder/genomics/conceptpaper_20061107. pdf) • Array. Track translates “Best Practice” into real practice
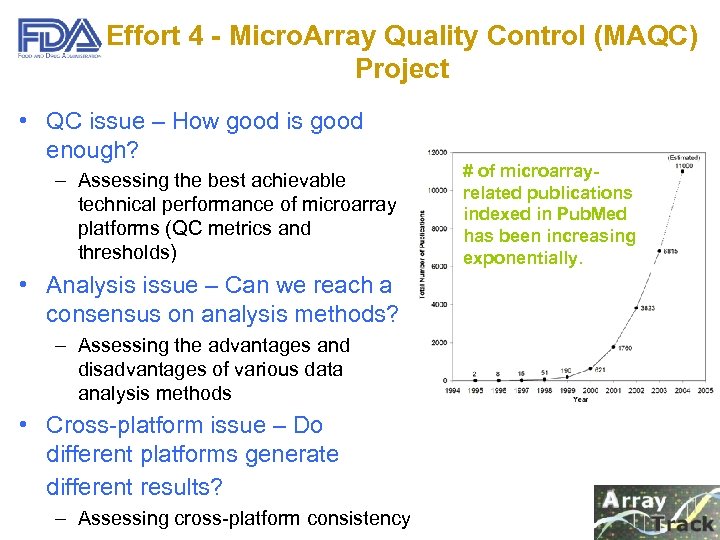
Effort 4 - Micro. Array Quality Control (MAQC) Project • QC issue – How good is good enough? – Assessing the best achievable technical performance of microarray platforms (QC metrics and thresholds) • Analysis issue – Can we reach a consensus on analysis methods? – Assessing the advantages and disadvantages of various data analysis methods • Cross-platform issue – Do different platforms generate different results? – Assessing cross-platform consistency # of microarrayrelated publications indexed in Pub. Med has been increasing exponentially.

Results from the MAQC Study Published in Nature Biotechnology on Sept and Oct 2006 Six research papers: • MAQC Main Paper • Validation of Microarray Results • RNA Sample Titrations • One-color vs. Two-color Microarrays • External RNA Controls • Rat Toxicogenomics Validation Plus: Editorial Foreword Stanford Commentary FDA Commentary EPA Commentary Nat. Biotechnol. 24(9) and 24(10 s), 2006 Nature Biotechnology Casciano DA and Woodcock J Ji H and Davis RW Frueh FW Dix DJ et al.
An Array of FDA Endeavors VGDS Array. Track MAQC
Not One-Trick-Pony fo oin Bi inf or ma tic s Regulation-Oriented Projects Ch em o cs ati rm Bioinformatics Computational Toxicology statistics
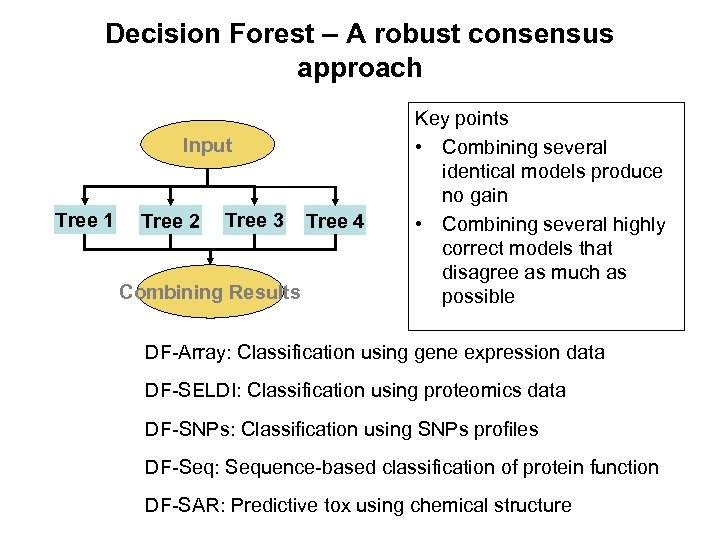
Decision Forest – A robust consensus approach Input Tree 1 Tree 2 Tree 3 Combining Results Tree 4 Key points • Combining several identical models produce no gain • Combining several highly correct models that disagree as much as possible DF-Array: Classification using gene expression data DF-SELDI: Classification using proteomics data DF-SNPs: Classification using SNPs profiles DF-Seq: Sequence-based classification of protein function DF-SAR: Predictive tox using chemical structure
Not One Trick Pony fo oin Bi inf or ma tic s Bioinformatics Ch em o cs ati rm Predictive Toxicology Computational Toxicology statistics

Endocrine Disruptors • An international issue • Two laws passed by US congress require evaluation of chemicals found in foods and water for endocrine disruption. • Similar regulation is also implemented in Europe and Asia • ~ 90, 000 commercial chemicals needs to be screened • EPA has identified ~58, 000 eligible chemicals • A minimum of 8, 000 of the 58, 000 chemicals are FDAregulated, including cosmetic ingredients, drug products …

Overview of NCTR’s Endocrine Disruptor Knowledge Base (EDKB) • Begun 1996, prior to endocrine disruptor (ED) issues • ED issues emerge - ACC and EPA collaboration & support results • Program expands: – Separately assayed over >200 chemicals for estrogen (ER), androgen (AR), serum protein (AFP and SHBG) receptor binding – Web-based relational database with in vitro and in vivo assay data, bibliography and chemical structure search – Exhaustive SAR/QSAR model development for both ER and AR binding, guided by data and crystal structures
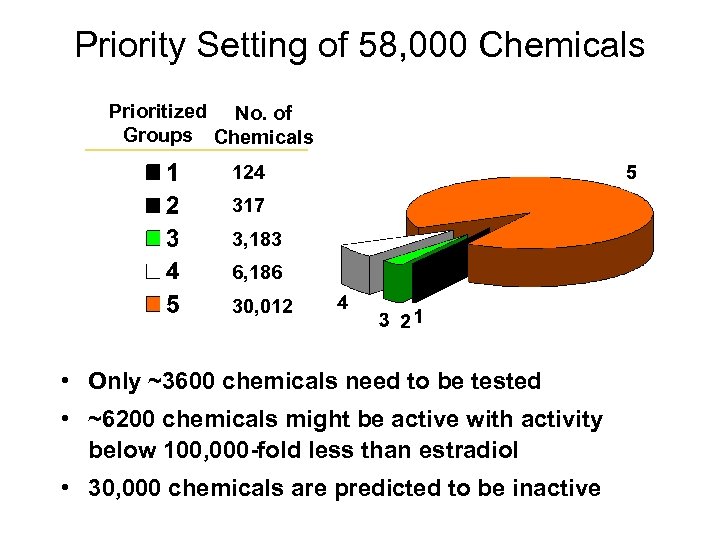
Priority Setting of 58, 000 Chemicals Prioritized No. of Groups Chemicals 124 317 3, 183 6, 186 30, 012 • Only ~3600 chemicals need to be tested • ~6200 chemicals might be active with activity below 100, 000 -fold less than estradiol • 30, 000 chemicals are predicted to be inactive
63c2a7a0df22b3c16265d15ca7d70c00.ppt