c56dae53ddff035b67ed198ca17261c7.ppt
- Количество слайдов: 18
 Efficacy and Safety of the PCSK 9 Monoclonal Antibody Alirocumab versus Placebo in 1257 Patients with Heterozygous Familial Hypercholesterolaemia: Analyses up to 78 Weeks from Four ODYSSEY Trials John J. P. Kastelein 1, Michel Farnier 2, G. Kees Hovingh 1, Gisle Langslet 3, Marie T. Baccara-Dinet 4, Daniel A. Gipe 5, Umesh Chaudhari 6, Jian Zhao 7, Christelle Lorenzato 8, Henry N. Ginsberg 9 1 Department of Vascular Medicine, Academic Medical Center, University of Amsterdam, The Netherlands; 2 Lipid Clinic, Point Médical, Dijon, France; 3 Lipid Clinic, Oslo University Hospital, Oslo, Norway; 4 Sanofi, Montpellier, France; 5 Regeneron Pharmaceuticals, Inc. , Tarrytown, NY, USA; 6 Sanofi, Bridgewater, NJ, USA; 7 Regeneron Pharmaceuticals, Inc. , Basking Ridge, NJ, USA; 8 Sanofi, Paris, France; 9 Columbia University, New York, NY, USA This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Efficacy and Safety of the PCSK 9 Monoclonal Antibody Alirocumab versus Placebo in 1257 Patients with Heterozygous Familial Hypercholesterolaemia: Analyses up to 78 Weeks from Four ODYSSEY Trials John J. P. Kastelein 1, Michel Farnier 2, G. Kees Hovingh 1, Gisle Langslet 3, Marie T. Baccara-Dinet 4, Daniel A. Gipe 5, Umesh Chaudhari 6, Jian Zhao 7, Christelle Lorenzato 8, Henry N. Ginsberg 9 1 Department of Vascular Medicine, Academic Medical Center, University of Amsterdam, The Netherlands; 2 Lipid Clinic, Point Médical, Dijon, France; 3 Lipid Clinic, Oslo University Hospital, Oslo, Norway; 4 Sanofi, Montpellier, France; 5 Regeneron Pharmaceuticals, Inc. , Tarrytown, NY, USA; 6 Sanofi, Bridgewater, NJ, USA; 7 Regeneron Pharmaceuticals, Inc. , Basking Ridge, NJ, USA; 8 Sanofi, Paris, France; 9 Columbia University, New York, NY, USA This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
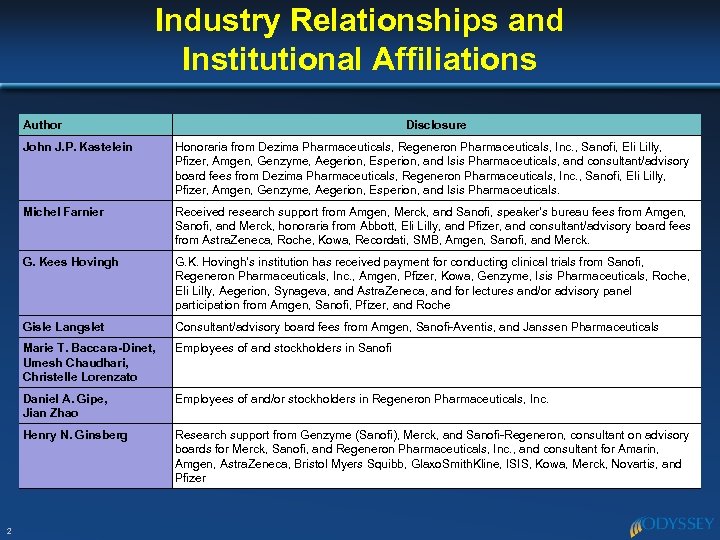 Industry Relationships and Institutional Affiliations Author Disclosure John J. P. Kastelein Michel Farnier Received research support from Amgen, Merck, and Sanofi, speaker’s bureau fees from Amgen, Sanofi, and Merck, honoraria from Abbott, Eli Lilly, and Pfizer, and consultant/advisory board fees from Astra. Zeneca, Roche, Kowa, Recordati, SMB, Amgen, Sanofi, and Merck. G. Kees Hovingh G. K. Hovingh’s institution has received payment for conducting clinical trials from Sanofi, Regeneron Pharmaceuticals, Inc. , Amgen, Pfizer, Kowa, Genzyme, Isis Pharmaceuticals, Roche, Eli Lilly, Aegerion, Synageva, and Astra. Zeneca, and for lectures and/or advisory panel participation from Amgen, Sanofi, Pfizer, and Roche Gisle Langslet Consultant/advisory board fees from Amgen, Sanofi-Aventis, and Janssen Pharmaceuticals Marie T. Baccara-Dinet, Umesh Chaudhari, Christelle Lorenzato Employees of and stockholders in Sanofi Daniel A. Gipe, Jian Zhao Employees of and/or stockholders in Regeneron Pharmaceuticals, Inc. Henry N. Ginsberg 2 Honoraria from Dezima Pharmaceuticals, Regeneron Pharmaceuticals, Inc. , Sanofi, Eli Lilly, Pfizer, Amgen, Genzyme, Aegerion, Esperion, and Isis Pharmaceuticals, and consultant/advisory board fees from Dezima Pharmaceuticals, Regeneron Pharmaceuticals, Inc. , Sanofi, Eli Lilly, Pfizer, Amgen, Genzyme, Aegerion, Esperion, and Isis Pharmaceuticals. Research support from Genzyme (Sanofi), Merck, and Sanofi-Regeneron, consultant on advisory boards for Merck, Sanofi, and Regeneron Pharmaceuticals, Inc. , and consultant for Amarin, Amgen, Astra. Zeneca, Bristol Myers Squibb, Glaxo. Smith. Kline, ISIS, Kowa, Merck, Novartis, and Pfizer
Industry Relationships and Institutional Affiliations Author Disclosure John J. P. Kastelein Michel Farnier Received research support from Amgen, Merck, and Sanofi, speaker’s bureau fees from Amgen, Sanofi, and Merck, honoraria from Abbott, Eli Lilly, and Pfizer, and consultant/advisory board fees from Astra. Zeneca, Roche, Kowa, Recordati, SMB, Amgen, Sanofi, and Merck. G. Kees Hovingh G. K. Hovingh’s institution has received payment for conducting clinical trials from Sanofi, Regeneron Pharmaceuticals, Inc. , Amgen, Pfizer, Kowa, Genzyme, Isis Pharmaceuticals, Roche, Eli Lilly, Aegerion, Synageva, and Astra. Zeneca, and for lectures and/or advisory panel participation from Amgen, Sanofi, Pfizer, and Roche Gisle Langslet Consultant/advisory board fees from Amgen, Sanofi-Aventis, and Janssen Pharmaceuticals Marie T. Baccara-Dinet, Umesh Chaudhari, Christelle Lorenzato Employees of and stockholders in Sanofi Daniel A. Gipe, Jian Zhao Employees of and/or stockholders in Regeneron Pharmaceuticals, Inc. Henry N. Ginsberg 2 Honoraria from Dezima Pharmaceuticals, Regeneron Pharmaceuticals, Inc. , Sanofi, Eli Lilly, Pfizer, Amgen, Genzyme, Aegerion, Esperion, and Isis Pharmaceuticals, and consultant/advisory board fees from Dezima Pharmaceuticals, Regeneron Pharmaceuticals, Inc. , Sanofi, Eli Lilly, Pfizer, Amgen, Genzyme, Aegerion, Esperion, and Isis Pharmaceuticals. Research support from Genzyme (Sanofi), Merck, and Sanofi-Regeneron, consultant on advisory boards for Merck, Sanofi, and Regeneron Pharmaceuticals, Inc. , and consultant for Amarin, Amgen, Astra. Zeneca, Bristol Myers Squibb, Glaxo. Smith. Kline, ISIS, Kowa, Merck, Novartis, and Pfizer
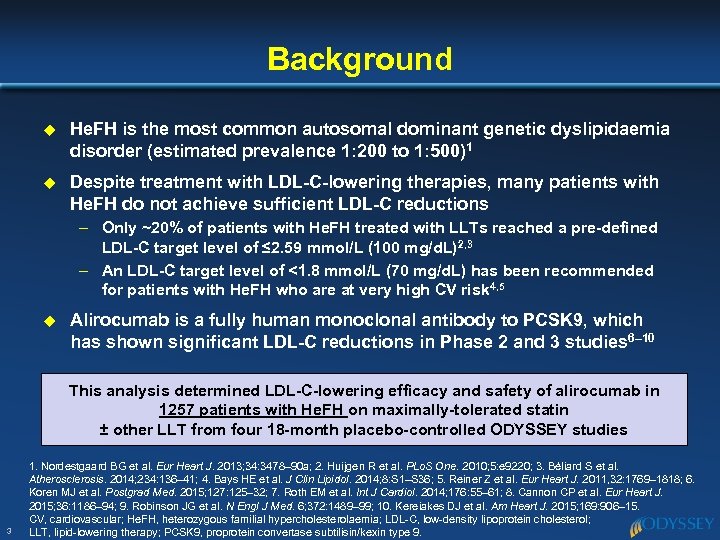 Background u He. FH is the most common autosomal dominant genetic dyslipidaemia disorder (estimated prevalence 1: 200 to 1: 500)1 u Despite treatment with LDL-C-lowering therapies, many patients with He. FH do not achieve sufficient LDL-C reductions – Only ~20% of patients with He. FH treated with LLTs reached a pre-defined LDL-C target level of ≤ 2. 59 mmol/L (100 mg/d. L)2, 3 – An LDL-C target level of <1. 8 mmol/L (70 mg/d. L) has been recommended for patients with He. FH who are at very high CV risk 4, 5 u Alirocumab is a fully human monoclonal antibody to PCSK 9, which has shown significant LDL-C reductions in Phase 2 and 3 studies 6– 10 This analysis determined LDL-C-lowering efficacy and safety of alirocumab in 1257 patients with He. FH on maximally-tolerated statin ± other LLT from four 18 -month placebo-controlled ODYSSEY studies 3 1. Nordestgaard BG et al. Eur Heart J. 2013; 34: 3478– 90 a; 2. Huijgen R et al. PLo. S One. 2010; 5: e 9220; 3. Béliard S et al. Atherosclerosis. 2014; 234: 136– 41; 4. Bays HE et al. J Clin Lipidol. 2014; 8: S 1–S 36; 5. Reiner Z et al. Eur Heart J. 2011, 32: 1769– 1818; 6. Koren MJ et al. Postgrad Med. 2015; 127: 125– 32; 7. Roth EM et al. Int J Cardiol. 2014; 176: 55– 61; 8. Cannon CP et al. Eur Heart J. 2015; 36: 1186– 94; 9. Robinson JG et al. N Engl J Med. 6; 372: 1489– 99; 10. Kereiakes DJ et al. Am Heart J. 2015; 169: 906– 15. CV, cardiovascular; He. FH, heterozygous familial hypercholesterolaemia; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; PCSK 9, proprotein convertase subtilisin/kexin type 9.
Background u He. FH is the most common autosomal dominant genetic dyslipidaemia disorder (estimated prevalence 1: 200 to 1: 500)1 u Despite treatment with LDL-C-lowering therapies, many patients with He. FH do not achieve sufficient LDL-C reductions – Only ~20% of patients with He. FH treated with LLTs reached a pre-defined LDL-C target level of ≤ 2. 59 mmol/L (100 mg/d. L)2, 3 – An LDL-C target level of <1. 8 mmol/L (70 mg/d. L) has been recommended for patients with He. FH who are at very high CV risk 4, 5 u Alirocumab is a fully human monoclonal antibody to PCSK 9, which has shown significant LDL-C reductions in Phase 2 and 3 studies 6– 10 This analysis determined LDL-C-lowering efficacy and safety of alirocumab in 1257 patients with He. FH on maximally-tolerated statin ± other LLT from four 18 -month placebo-controlled ODYSSEY studies 3 1. Nordestgaard BG et al. Eur Heart J. 2013; 34: 3478– 90 a; 2. Huijgen R et al. PLo. S One. 2010; 5: e 9220; 3. Béliard S et al. Atherosclerosis. 2014; 234: 136– 41; 4. Bays HE et al. J Clin Lipidol. 2014; 8: S 1–S 36; 5. Reiner Z et al. Eur Heart J. 2011, 32: 1769– 1818; 6. Koren MJ et al. Postgrad Med. 2015; 127: 125– 32; 7. Roth EM et al. Int J Cardiol. 2014; 176: 55– 61; 8. Cannon CP et al. Eur Heart J. 2015; 36: 1186– 94; 9. Robinson JG et al. N Engl J Med. 6; 372: 1489– 99; 10. Kereiakes DJ et al. Am Heart J. 2015; 169: 906– 15. CV, cardiovascular; He. FH, heterozygous familial hypercholesterolaemia; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; PCSK 9, proprotein convertase subtilisin/kexin type 9.
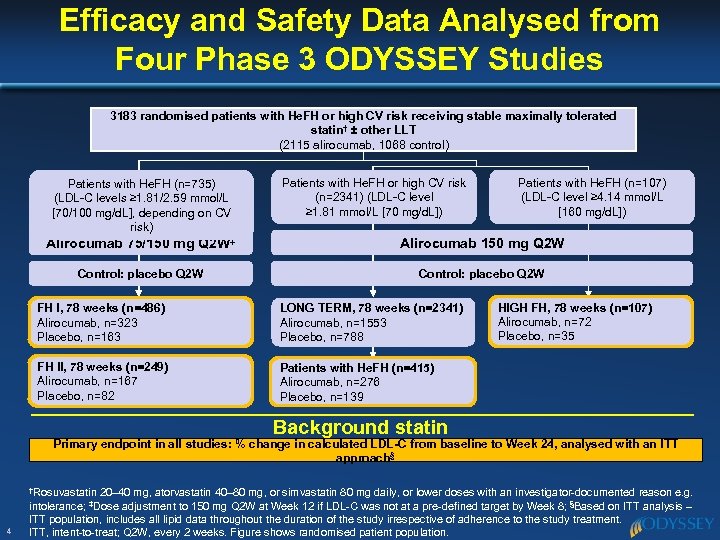 Efficacy and Safety Data Analysed from Four Phase 3 ODYSSEY Studies 3183 randomised patients with He. FH or high CV risk receiving stable maximally tolerated statin† ± other LLT (2115 alirocumab, 1068 control) Patients with He. FH (n=735) (LDL-C levels ≥ 1. 81/2. 59 mmol/L [70/100 mg/d. L], depending on CV risk) Patients with He. FH or high CV risk (n=2341) (LDL-C level ≥ 1. 81 mmol/L [70 mg/d. L]) Patients with He. FH (n=107) (LDL-C level ≥ 4. 14 mmol/L [160 mg/d. L]) Alirocumab 75/150 mg Q 2 W‡ Alirocumab 150 mg Q 2 W Control: placebo Q 2 W FH I, 78 weeks (n=486) Alirocumab, n=323 Placebo, n=163 LONG TERM, 78 weeks (n=2341) Alirocumab, n=1553 Placebo, n=788 FH II, 78 weeks (n=249) Alirocumab, n=167 Placebo, n=82 HIGH FH, 78 weeks (n=107) Alirocumab, n=72 Placebo, n=35 Patients with He. FH (n=415) Alirocumab, n=276 Placebo, n=139 Background statin Primary endpoint in all studies: % change in calculated LDL-C from baseline to Week 24, analysed with an ITT approach§ †Rosuvastatin 4 20– 40 mg, atorvastatin 40– 80 mg, or simvastatin 80 mg daily, or lower doses with an investigator-documented reason e. g. intolerance; ‡Dose adjustment to 150 mg Q 2 W at Week 12 if LDL-C was not at a pre-defined target by Week 8; §Based on ITT analysis – ITT population, includes all lipid data throughout the duration of the study irrespective of adherence to the study treatment. ITT, intent-to-treat; Q 2 W, every 2 weeks. Figure shows randomised patient population.
Efficacy and Safety Data Analysed from Four Phase 3 ODYSSEY Studies 3183 randomised patients with He. FH or high CV risk receiving stable maximally tolerated statin† ± other LLT (2115 alirocumab, 1068 control) Patients with He. FH (n=735) (LDL-C levels ≥ 1. 81/2. 59 mmol/L [70/100 mg/d. L], depending on CV risk) Patients with He. FH or high CV risk (n=2341) (LDL-C level ≥ 1. 81 mmol/L [70 mg/d. L]) Patients with He. FH (n=107) (LDL-C level ≥ 4. 14 mmol/L [160 mg/d. L]) Alirocumab 75/150 mg Q 2 W‡ Alirocumab 150 mg Q 2 W Control: placebo Q 2 W FH I, 78 weeks (n=486) Alirocumab, n=323 Placebo, n=163 LONG TERM, 78 weeks (n=2341) Alirocumab, n=1553 Placebo, n=788 FH II, 78 weeks (n=249) Alirocumab, n=167 Placebo, n=82 HIGH FH, 78 weeks (n=107) Alirocumab, n=72 Placebo, n=35 Patients with He. FH (n=415) Alirocumab, n=276 Placebo, n=139 Background statin Primary endpoint in all studies: % change in calculated LDL-C from baseline to Week 24, analysed with an ITT approach§ †Rosuvastatin 4 20– 40 mg, atorvastatin 40– 80 mg, or simvastatin 80 mg daily, or lower doses with an investigator-documented reason e. g. intolerance; ‡Dose adjustment to 150 mg Q 2 W at Week 12 if LDL-C was not at a pre-defined target by Week 8; §Based on ITT analysis – ITT population, includes all lipid data throughout the duration of the study irrespective of adherence to the study treatment. ITT, intent-to-treat; Q 2 W, every 2 weeks. Figure shows randomised patient population.
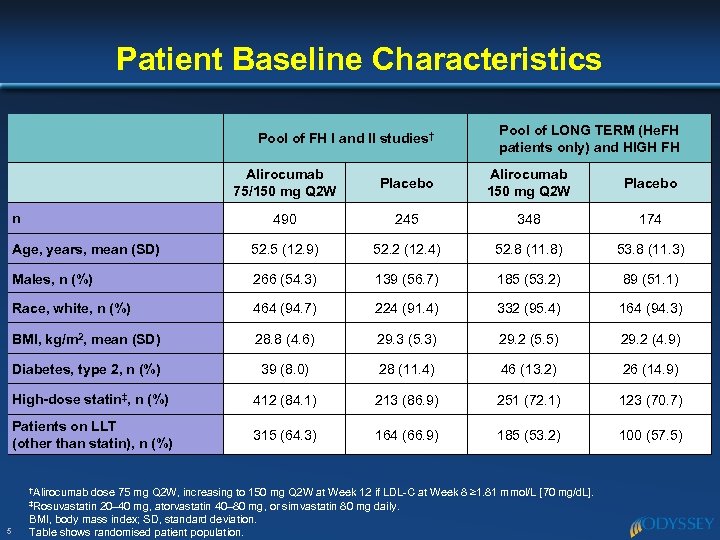 Patient Baseline Characteristics Pool of FH I and II studies† Pool of LONG TERM (He. FH patients only) and HIGH FH Alirocumab 75/150 mg Q 2 W Placebo Alirocumab 150 mg Q 2 W Placebo 490 245 348 174 Age, years, mean (SD) 52. 5 (12. 9) 52. 2 (12. 4) 52. 8 (11. 8) 53. 8 (11. 3) Males, n (%) 266 (54. 3) 139 (56. 7) 185 (53. 2) 89 (51. 1) Race, white, n (%) 464 (94. 7) 224 (91. 4) 332 (95. 4) 164 (94. 3) BMI, kg/m 2, mean (SD) 28. 8 (4. 6) 29. 3 (5. 3) 29. 2 (5. 5) 29. 2 (4. 9) Diabetes, type 2, n (%) 39 (8. 0) 28 (11. 4) 46 (13. 2) 26 (14. 9) High-dose statin‡, n (%) 412 (84. 1) 213 (86. 9) 251 (72. 1) 123 (70. 7) Patients on LLT (other than statin), n (%) 315 (64. 3) 164 (66. 9) 185 (53. 2) 100 (57. 5) n †Alirocumab dose 75 mg Q 2 W, increasing to 150 mg Q 2 W at Week 12 if LDL-C at Week 8 ≥ 1. 81 mmol/L [70 mg/d. L]. 20– 40 mg, atorvastatin 40– 80 mg, or simvastatin 80 mg daily. BMI, body mass index; SD, standard deviation. Table shows randomised patient population. ‡Rosuvastatin 5
Patient Baseline Characteristics Pool of FH I and II studies† Pool of LONG TERM (He. FH patients only) and HIGH FH Alirocumab 75/150 mg Q 2 W Placebo Alirocumab 150 mg Q 2 W Placebo 490 245 348 174 Age, years, mean (SD) 52. 5 (12. 9) 52. 2 (12. 4) 52. 8 (11. 8) 53. 8 (11. 3) Males, n (%) 266 (54. 3) 139 (56. 7) 185 (53. 2) 89 (51. 1) Race, white, n (%) 464 (94. 7) 224 (91. 4) 332 (95. 4) 164 (94. 3) BMI, kg/m 2, mean (SD) 28. 8 (4. 6) 29. 3 (5. 3) 29. 2 (5. 5) 29. 2 (4. 9) Diabetes, type 2, n (%) 39 (8. 0) 28 (11. 4) 46 (13. 2) 26 (14. 9) High-dose statin‡, n (%) 412 (84. 1) 213 (86. 9) 251 (72. 1) 123 (70. 7) Patients on LLT (other than statin), n (%) 315 (64. 3) 164 (66. 9) 185 (53. 2) 100 (57. 5) n †Alirocumab dose 75 mg Q 2 W, increasing to 150 mg Q 2 W at Week 12 if LDL-C at Week 8 ≥ 1. 81 mmol/L [70 mg/d. L]. 20– 40 mg, atorvastatin 40– 80 mg, or simvastatin 80 mg daily. BMI, body mass index; SD, standard deviation. Table shows randomised patient population. ‡Rosuvastatin 5
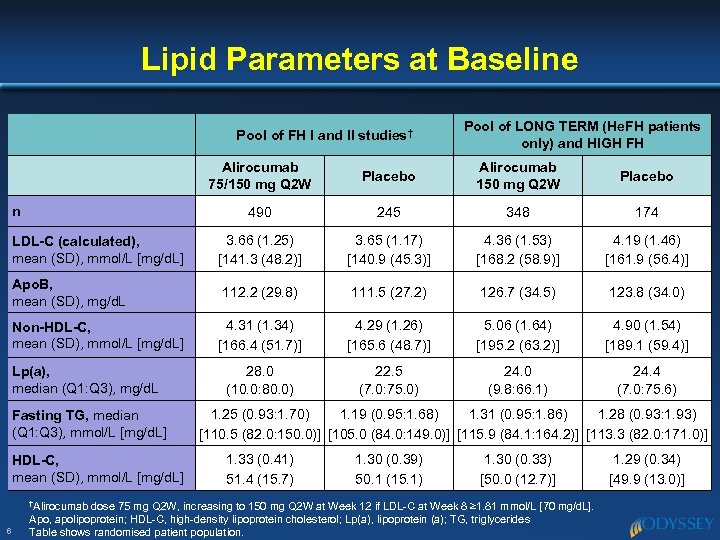 Lipid Parameters at Baseline Pool of FH I and II studies† Pool of LONG TERM (He. FH patients only) and HIGH FH Alirocumab 75/150 mg Q 2 W Placebo Alirocumab 150 mg Q 2 W Placebo 490 245 348 174 LDL-C (calculated), mean (SD), mmol/L [mg/d. L] 3. 66 (1. 25) [141. 3 (48. 2)] 3. 65 (1. 17) [140. 9 (45. 3)] 4. 36 (1. 53) [168. 2 (58. 9)] 4. 19 (1. 46) [161. 9 (56. 4)] Apo. B, mean (SD), mg/d. L 112. 2 (29. 8) 111. 5 (27. 2) 126. 7 (34. 5) 123. 8 (34. 0) Non-HDL-C, mean (SD), mmol/L [mg/d. L] 4. 31 (1. 34) [166. 4 (51. 7)] 4. 29 (1. 26) [165. 6 (48. 7)] 5. 06 (1. 64) [195. 2 (63. 2)] 4. 90 (1. 54) [189. 1 (59. 4)] 28. 0 (10. 0: 80. 0) 22. 5 (7. 0: 75. 0) 24. 0 (9. 8: 66. 1) 24. 4 (7. 0: 75. 6) n Lp(a), median (Q 1: Q 3), mg/d. L Fasting TG, median (Q 1: Q 3), mmol/L [mg/d. L] HDL-C, mean (SD), mmol/L [mg/d. L] †Alirocumab 6 1. 25 (0. 93: 1. 70) 1. 19 (0. 95: 1. 68) 1. 31 (0. 95: 1. 86) 1. 28 (0. 93: 1. 93) [110. 5 (82. 0: 150. 0)] [105. 0 (84. 0: 149. 0)] [115. 9 (84. 1: 164. 2)] [113. 3 (82. 0: 171. 0)] 1. 33 (0. 41) 51. 4 (15. 7) 1. 30 (0. 39) 50. 1 (15. 1) 1. 30 (0. 33) [50. 0 (12. 7)] dose 75 mg Q 2 W, increasing to 150 mg Q 2 W at Week 12 if LDL-C at Week 8 ≥ 1. 81 mmol/L [70 mg/d. L]. Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; Lp(a), lipoprotein (a); TG, triglycerides Table shows randomised patient population. 1. 29 (0. 34) [49. 9 (13. 0)]
Lipid Parameters at Baseline Pool of FH I and II studies† Pool of LONG TERM (He. FH patients only) and HIGH FH Alirocumab 75/150 mg Q 2 W Placebo Alirocumab 150 mg Q 2 W Placebo 490 245 348 174 LDL-C (calculated), mean (SD), mmol/L [mg/d. L] 3. 66 (1. 25) [141. 3 (48. 2)] 3. 65 (1. 17) [140. 9 (45. 3)] 4. 36 (1. 53) [168. 2 (58. 9)] 4. 19 (1. 46) [161. 9 (56. 4)] Apo. B, mean (SD), mg/d. L 112. 2 (29. 8) 111. 5 (27. 2) 126. 7 (34. 5) 123. 8 (34. 0) Non-HDL-C, mean (SD), mmol/L [mg/d. L] 4. 31 (1. 34) [166. 4 (51. 7)] 4. 29 (1. 26) [165. 6 (48. 7)] 5. 06 (1. 64) [195. 2 (63. 2)] 4. 90 (1. 54) [189. 1 (59. 4)] 28. 0 (10. 0: 80. 0) 22. 5 (7. 0: 75. 0) 24. 0 (9. 8: 66. 1) 24. 4 (7. 0: 75. 6) n Lp(a), median (Q 1: Q 3), mg/d. L Fasting TG, median (Q 1: Q 3), mmol/L [mg/d. L] HDL-C, mean (SD), mmol/L [mg/d. L] †Alirocumab 6 1. 25 (0. 93: 1. 70) 1. 19 (0. 95: 1. 68) 1. 31 (0. 95: 1. 86) 1. 28 (0. 93: 1. 93) [110. 5 (82. 0: 150. 0)] [105. 0 (84. 0: 149. 0)] [115. 9 (84. 1: 164. 2)] [113. 3 (82. 0: 171. 0)] 1. 33 (0. 41) 51. 4 (15. 7) 1. 30 (0. 39) 50. 1 (15. 1) 1. 30 (0. 33) [50. 0 (12. 7)] dose 75 mg Q 2 W, increasing to 150 mg Q 2 W at Week 12 if LDL-C at Week 8 ≥ 1. 81 mmol/L [70 mg/d. L]. Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; Lp(a), lipoprotein (a); TG, triglycerides Table shows randomised patient population. 1. 29 (0. 34) [49. 9 (13. 0)]
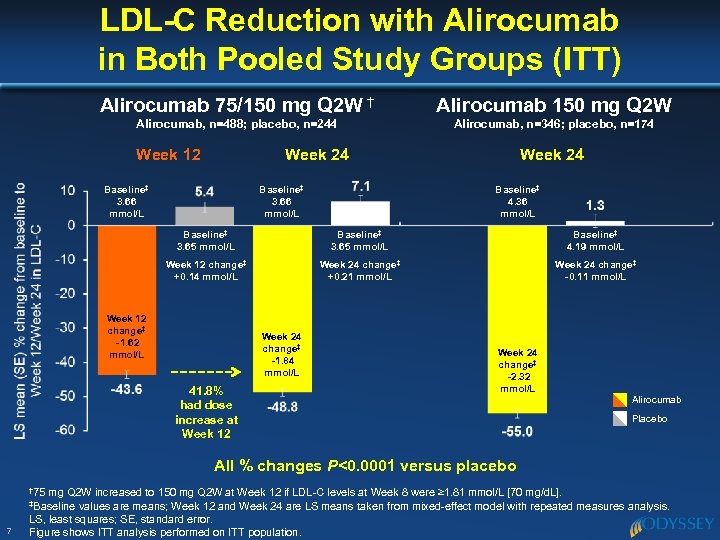 LDL-C Reduction with Alirocumab in Both Pooled Study Groups (ITT) Alirocumab 75/150 mg Q 2 W † Alirocumab 150 mg Q 2 W Alirocumab, n=488; placebo, n=244 Alirocumab, n=346; placebo, n=174 Week 12 Week 24 Baseline‡ 3. 66 mmol/L Week 24 Baseline‡ 4. 36 mmol/L Baseline‡ 3. 65 mmol/L Baseline‡ 4. 19 mmol/L Week 12 change‡ +0. 14 mmol/L Week 24 change‡ +0. 21 mmol/L Week 24 change‡ -0. 11 mmol/L Week 12 change‡ -1. 62 mmol/L Week 24 change‡ -1. 84 mmol/L 41. 8% had dose increase at Week 12 Week 24 change‡ -2. 32 mmol/L Alirocumab Placebo All % changes P<0. 0001 versus placebo † 75 mg Q 2 W increased to 150 mg Q 2 W at Week 12 if LDL-C levels at Week 8 were ≥ 1. 81 mmol/L [70 mg/d. L]. values are means; Week 12 and Week 24 are LS means taken from mixed-effect model with repeated measures analysis. LS, least squares; SE, standard error. Figure shows ITT analysis performed on ITT population. ‡Baseline 7
LDL-C Reduction with Alirocumab in Both Pooled Study Groups (ITT) Alirocumab 75/150 mg Q 2 W † Alirocumab 150 mg Q 2 W Alirocumab, n=488; placebo, n=244 Alirocumab, n=346; placebo, n=174 Week 12 Week 24 Baseline‡ 3. 66 mmol/L Week 24 Baseline‡ 4. 36 mmol/L Baseline‡ 3. 65 mmol/L Baseline‡ 4. 19 mmol/L Week 12 change‡ +0. 14 mmol/L Week 24 change‡ +0. 21 mmol/L Week 24 change‡ -0. 11 mmol/L Week 12 change‡ -1. 62 mmol/L Week 24 change‡ -1. 84 mmol/L 41. 8% had dose increase at Week 12 Week 24 change‡ -2. 32 mmol/L Alirocumab Placebo All % changes P<0. 0001 versus placebo † 75 mg Q 2 W increased to 150 mg Q 2 W at Week 12 if LDL-C levels at Week 8 were ≥ 1. 81 mmol/L [70 mg/d. L]. values are means; Week 12 and Week 24 are LS means taken from mixed-effect model with repeated measures analysis. LS, least squares; SE, standard error. Figure shows ITT analysis performed on ITT population. ‡Baseline 7
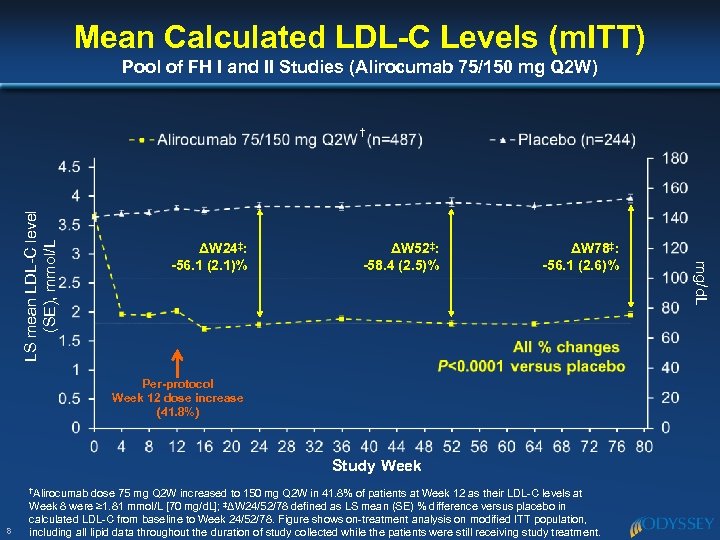 Mean Calculated LDL-C Levels (m. ITT) Pool of FH I and II Studies (Alirocumab 75/150 mg Q 2 W) ΔW 24‡: -56. 1 (2. 1)% ΔW 52‡: -58. 4 (2. 5)% ΔW 78‡: -56. 1 (2. 6)% Per-protocol Week 12 dose increase (41. 8%) Study Week †Alirocumab 8 dose 75 mg Q 2 W increased to 150 mg Q 2 W in 41. 8% of patients at Week 12 as their LDL-C levels at Week 8 were ≥ 1. 81 mmol/L [70 mg/d. L]; ‡ΔW 24/52/78 defined as LS mean (SE) % difference versus placebo in calculated LDL-C from baseline to Week 24/52/78. Figure shows on-treatment analysis on modified ITT population, including all lipid data throughout the duration of study collected while the patients were still receiving study treatment. mg/d. L LS mean LDL-C level (SE), mmol/L †
Mean Calculated LDL-C Levels (m. ITT) Pool of FH I and II Studies (Alirocumab 75/150 mg Q 2 W) ΔW 24‡: -56. 1 (2. 1)% ΔW 52‡: -58. 4 (2. 5)% ΔW 78‡: -56. 1 (2. 6)% Per-protocol Week 12 dose increase (41. 8%) Study Week †Alirocumab 8 dose 75 mg Q 2 W increased to 150 mg Q 2 W in 41. 8% of patients at Week 12 as their LDL-C levels at Week 8 were ≥ 1. 81 mmol/L [70 mg/d. L]; ‡ΔW 24/52/78 defined as LS mean (SE) % difference versus placebo in calculated LDL-C from baseline to Week 24/52/78. Figure shows on-treatment analysis on modified ITT population, including all lipid data throughout the duration of study collected while the patients were still receiving study treatment. mg/d. L LS mean LDL-C level (SE), mmol/L †
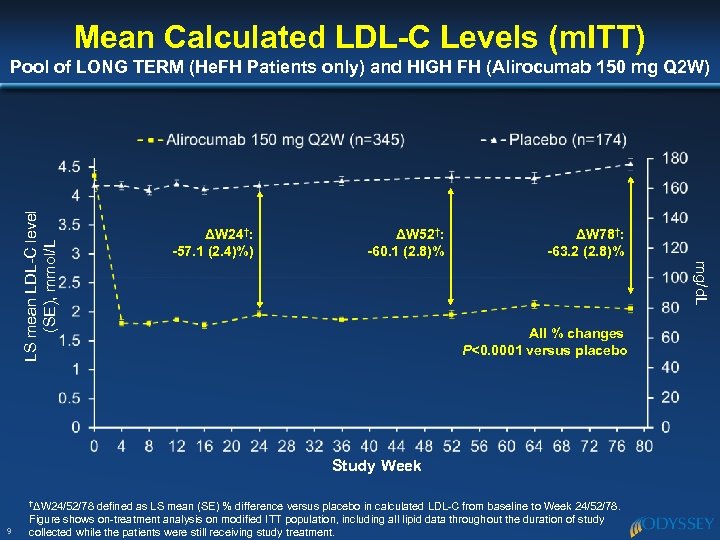 Mean Calculated LDL-C Levels (m. ITT) ΔW 24†: -57. 1 (2. 4)%) ΔW 52†: -60. 1 (2. 8)% ΔW 78†: -63. 2 (2. 8)% mg/d. L LS mean LDL-C level (SE), mmol/L Pool of LONG TERM (He. FH Patients only) and HIGH FH (Alirocumab 150 mg Q 2 W) All % changes P<0. 0001 versus placebo Study Week †ΔW 24/52/78 9 defined as LS mean (SE) % difference versus placebo in calculated LDL-C from baseline to Week 24/52/78. Figure shows on-treatment analysis on modified ITT population, including all lipid data throughout the duration of study collected while the patients were still receiving study treatment.
Mean Calculated LDL-C Levels (m. ITT) ΔW 24†: -57. 1 (2. 4)%) ΔW 52†: -60. 1 (2. 8)% ΔW 78†: -63. 2 (2. 8)% mg/d. L LS mean LDL-C level (SE), mmol/L Pool of LONG TERM (He. FH Patients only) and HIGH FH (Alirocumab 150 mg Q 2 W) All % changes P<0. 0001 versus placebo Study Week †ΔW 24/52/78 9 defined as LS mean (SE) % difference versus placebo in calculated LDL-C from baseline to Week 24/52/78. Figure shows on-treatment analysis on modified ITT population, including all lipid data throughout the duration of study collected while the patients were still receiving study treatment.
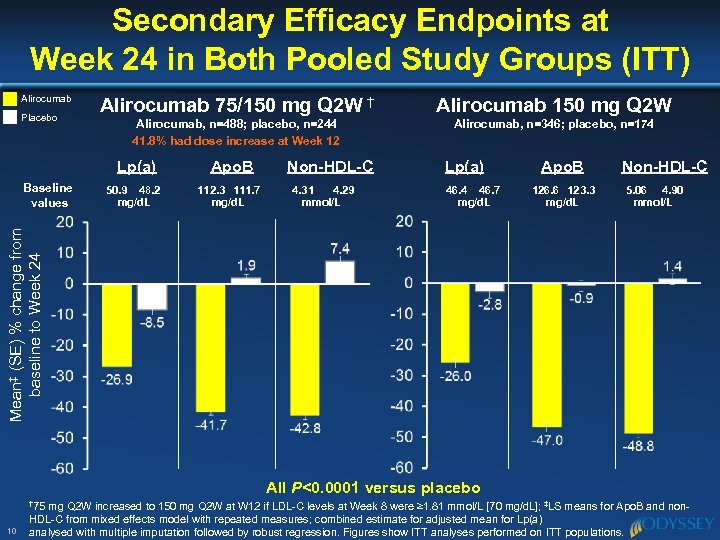 Secondary Efficacy Endpoints at Week 24 in Both Pooled Study Groups (ITT) Alirocumab Placebo Alirocumab 75/150 mg Q 2 W † Alirocumab 150 mg Q 2 W Alirocumab, n=488; placebo, n=244 41. 8% had dose increase at Week 12 Alirocumab, n=346; placebo, n=174 Lp(a) 50. 9 48. 2 mg/d. L 112. 3 111. 7 mg/d. L Non-HDL-C 4. 31 4. 29 mmol/L Lp(a) 46. 4 46. 7 mg/d. L Apo. B 126. 6 123. 3 mg/d. L Non-HDL-C 5. 06 4. 90 mmol/L Mean‡ (SE) % change from baseline to Week 24 Baseline values Apo. B All P<0. 0001 versus placebo † 75 10 mg Q 2 W increased to 150 mg Q 2 W at W 12 if LDL-C levels at Week 8 were ≥ 1. 81 mmol/L [70 mg/d. L]; ‡LS means for Apo. B and non. HDL-C from mixed effects model with repeated measures; combined estimate for adjusted mean for Lp(a) analysed with multiple imputation followed by robust regression. Figures show ITT analyses performed on ITT populations.
Secondary Efficacy Endpoints at Week 24 in Both Pooled Study Groups (ITT) Alirocumab Placebo Alirocumab 75/150 mg Q 2 W † Alirocumab 150 mg Q 2 W Alirocumab, n=488; placebo, n=244 41. 8% had dose increase at Week 12 Alirocumab, n=346; placebo, n=174 Lp(a) 50. 9 48. 2 mg/d. L 112. 3 111. 7 mg/d. L Non-HDL-C 4. 31 4. 29 mmol/L Lp(a) 46. 4 46. 7 mg/d. L Apo. B 126. 6 123. 3 mg/d. L Non-HDL-C 5. 06 4. 90 mmol/L Mean‡ (SE) % change from baseline to Week 24 Baseline values Apo. B All P<0. 0001 versus placebo † 75 10 mg Q 2 W increased to 150 mg Q 2 W at W 12 if LDL-C levels at Week 8 were ≥ 1. 81 mmol/L [70 mg/d. L]; ‡LS means for Apo. B and non. HDL-C from mixed effects model with repeated measures; combined estimate for adjusted mean for Lp(a) analysed with multiple imputation followed by robust regression. Figures show ITT analyses performed on ITT populations.
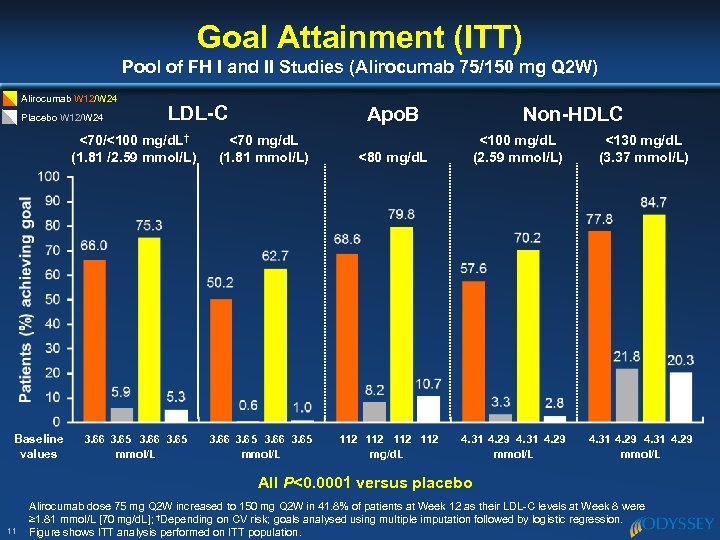 Goal Attainment (ITT) Pool of FH I and II Studies (Alirocumab 75/150 mg Q 2 W) Alirocumab W 12/W 24 Placebo W 12/W 24 LDL-C <70/<100 mg/d. L† (1. 81 /2. 59 mmol/L) Baseline values 3. 66 3. 65 mmol/L Apo. B <70 mg/d. L (1. 81 mmol/L) 3. 66 3. 65 mmol/L Non-HDLC <100 mg/d. L (2. 59 mmol/L) <130 mg/d. L (3. 37 mmol/L) 4. 31 4. 29 mmol/L <80 mg/d. L 112 112 mg/d. L All P<0. 0001 versus placebo 11 Alirocumab dose 75 mg Q 2 W increased to 150 mg Q 2 W in 41. 8% of patients at Week 12 as their LDL-C levels at Week 8 were ≥ 1. 81 mmol/L [70 mg/d. L]; †Depending on CV risk; goals analysed using multiple imputation followed by logistic regression. Figure shows ITT analysis performed on ITT population.
Goal Attainment (ITT) Pool of FH I and II Studies (Alirocumab 75/150 mg Q 2 W) Alirocumab W 12/W 24 Placebo W 12/W 24 LDL-C <70/<100 mg/d. L† (1. 81 /2. 59 mmol/L) Baseline values 3. 66 3. 65 mmol/L Apo. B <70 mg/d. L (1. 81 mmol/L) 3. 66 3. 65 mmol/L Non-HDLC <100 mg/d. L (2. 59 mmol/L) <130 mg/d. L (3. 37 mmol/L) 4. 31 4. 29 mmol/L <80 mg/d. L 112 112 mg/d. L All P<0. 0001 versus placebo 11 Alirocumab dose 75 mg Q 2 W increased to 150 mg Q 2 W in 41. 8% of patients at Week 12 as their LDL-C levels at Week 8 were ≥ 1. 81 mmol/L [70 mg/d. L]; †Depending on CV risk; goals analysed using multiple imputation followed by logistic regression. Figure shows ITT analysis performed on ITT population.
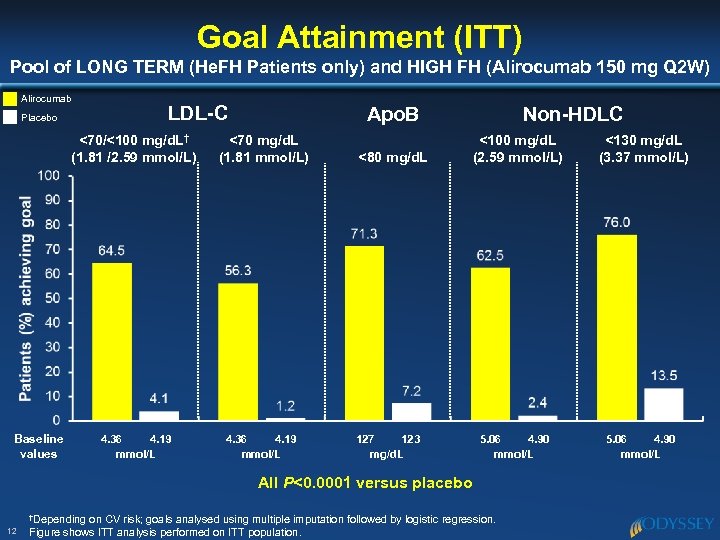 Goal Attainment (ITT) Pool of LONG TERM (He. FH Patients only) and HIGH FH (Alirocumab 150 mg Q 2 W) Alirocumab Placebo LDL-C Apo. B <70/<100 mg/d. L† (1. 81 /2. 59 mmol/L) <70 mg/d. L (1. 81 mmol/L) 4. 36 4. 19 mmol/L Baseline values <80 mg/d. L 127 123 mg/d. L Non-HDLC <100 mg/d. L (2. 59 mmol/L) 5. 06 4. 90 mmol/L All P<0. 0001 versus placebo †Depending 12 on CV risk; goals analysed using multiple imputation followed by logistic regression. Figure shows ITT analysis performed on ITT population. <130 mg/d. L (3. 37 mmol/L) 5. 06 4. 90 mmol/L
Goal Attainment (ITT) Pool of LONG TERM (He. FH Patients only) and HIGH FH (Alirocumab 150 mg Q 2 W) Alirocumab Placebo LDL-C Apo. B <70/<100 mg/d. L† (1. 81 /2. 59 mmol/L) <70 mg/d. L (1. 81 mmol/L) 4. 36 4. 19 mmol/L Baseline values <80 mg/d. L 127 123 mg/d. L Non-HDLC <100 mg/d. L (2. 59 mmol/L) 5. 06 4. 90 mmol/L All P<0. 0001 versus placebo †Depending 12 on CV risk; goals analysed using multiple imputation followed by logistic regression. Figure shows ITT analysis performed on ITT population. <130 mg/d. L (3. 37 mmol/L) 5. 06 4. 90 mmol/L
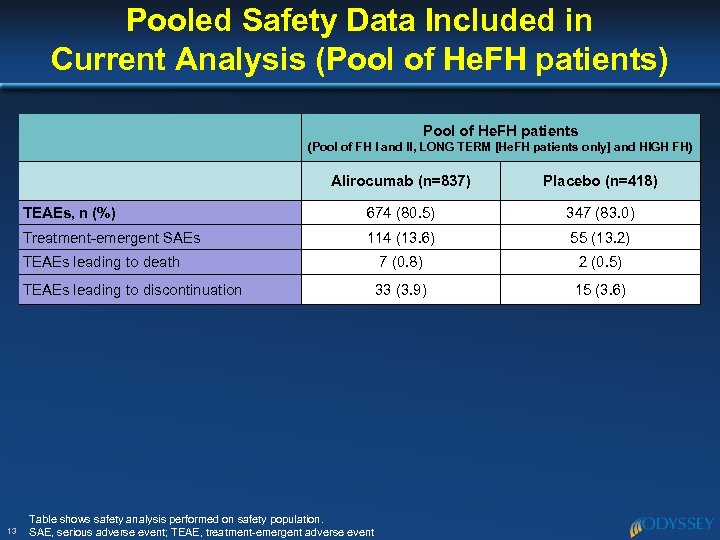 Pooled Safety Data Included in Current Analysis (Pool of He. FH patients) Pool of He. FH patients (Pool of FH I and II, LONG TERM [He. FH patients only] and HIGH FH) Alirocumab (n=837) TEAEs, n (%) 674 (80. 5) 347 (83. 0) Treatment-emergent SAEs 114 (13. 6) 55 (13. 2) TEAEs leading to death 7 (0. 8) 2 (0. 5) TEAEs leading to discontinuation 13 Placebo (n=418) 33 (3. 9) 15 (3. 6) Table shows safety analysis performed on safety population. SAE, serious adverse event; TEAE, treatment-emergent adverse event
Pooled Safety Data Included in Current Analysis (Pool of He. FH patients) Pool of He. FH patients (Pool of FH I and II, LONG TERM [He. FH patients only] and HIGH FH) Alirocumab (n=837) TEAEs, n (%) 674 (80. 5) 347 (83. 0) Treatment-emergent SAEs 114 (13. 6) 55 (13. 2) TEAEs leading to death 7 (0. 8) 2 (0. 5) TEAEs leading to discontinuation 13 Placebo (n=418) 33 (3. 9) 15 (3. 6) Table shows safety analysis performed on safety population. SAE, serious adverse event; TEAE, treatment-emergent adverse event
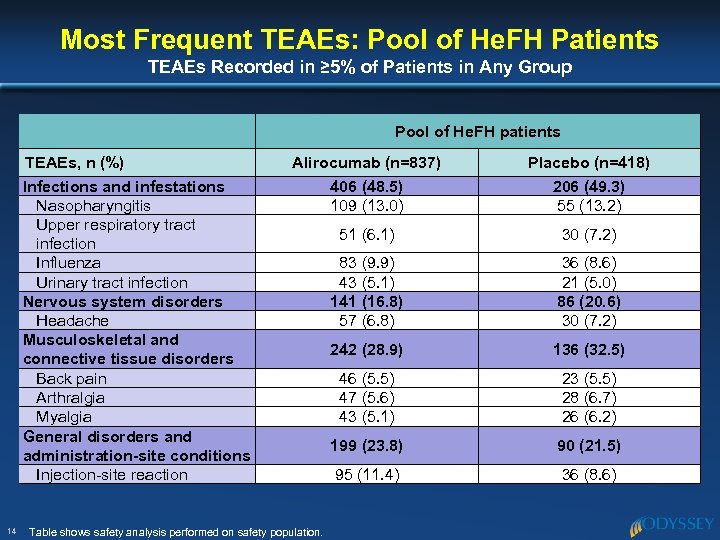 Most Frequent TEAEs: Pool of He. FH Patients TEAEs Recorded in ≥ 5% of Patients in Any Group Pool of He. FH patients TEAEs, n (%) Infections and infestations Nasopharyngitis Upper respiratory tract infection Influenza Urinary tract infection Nervous system disorders Headache Musculoskeletal and connective tissue disorders Back pain Arthralgia Myalgia General disorders and administration-site conditions Injection-site reaction 14 Alirocumab (n=837) 406 (48. 5) 109 (13. 0) Placebo (n=418) 206 (49. 3) 55 (13. 2) 51 (6. 1) 30 (7. 2) 83 (9. 9) 43 (5. 1) 141 (16. 8) 57 (6. 8) 36 (8. 6) 21 (5. 0) 86 (20. 6) 30 (7. 2) 242 (28. 9) 136 (32. 5) 46 (5. 5) 47 (5. 6) 43 (5. 1) 23 (5. 5) 28 (6. 7) 26 (6. 2) 199 (23. 8) 90 (21. 5) 95 (11. 4) 36 (8. 6) Table shows safety analysis performed on safety population.
Most Frequent TEAEs: Pool of He. FH Patients TEAEs Recorded in ≥ 5% of Patients in Any Group Pool of He. FH patients TEAEs, n (%) Infections and infestations Nasopharyngitis Upper respiratory tract infection Influenza Urinary tract infection Nervous system disorders Headache Musculoskeletal and connective tissue disorders Back pain Arthralgia Myalgia General disorders and administration-site conditions Injection-site reaction 14 Alirocumab (n=837) 406 (48. 5) 109 (13. 0) Placebo (n=418) 206 (49. 3) 55 (13. 2) 51 (6. 1) 30 (7. 2) 83 (9. 9) 43 (5. 1) 141 (16. 8) 57 (6. 8) 36 (8. 6) 21 (5. 0) 86 (20. 6) 30 (7. 2) 242 (28. 9) 136 (32. 5) 46 (5. 5) 47 (5. 6) 43 (5. 1) 23 (5. 5) 28 (6. 7) 26 (6. 2) 199 (23. 8) 90 (21. 5) 95 (11. 4) 36 (8. 6) Table shows safety analysis performed on safety population.
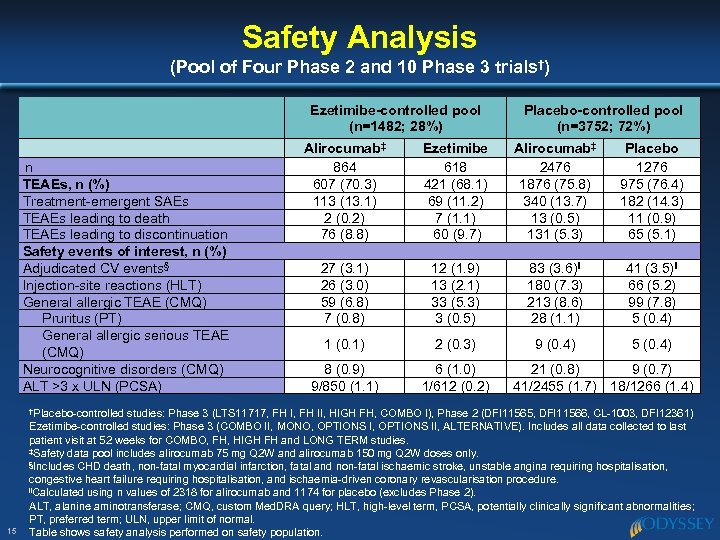 Safety Analysis (Pool of Four Phase 2 and 10 Phase 3 trials†) Ezetimibe-controlled pool (n=1482; 28%) n TEAEs, n (%) Treatment-emergent SAEs TEAEs leading to death TEAEs leading to discontinuation Safety events of interest, n (%) Adjudicated CV events§ Injection-site reactions (HLT) General allergic TEAE (CMQ) Pruritus (PT) General allergic serious TEAE (CMQ) Neurocognitive disorders (CMQ) ALT >3 x ULN (PCSA) †Placebo-controlled 15 Placebo-controlled pool (n=3752; 72%) Alirocumab‡ 864 607 (70. 3) 113 (13. 1) 2 (0. 2) 76 (8. 8) Ezetimibe 618 421 (68. 1) 69 (11. 2) 7 (1. 1) 60 (9. 7) Alirocumab‡ 2476 1876 (75. 8) 340 (13. 7) 13 (0. 5) 131 (5. 3) Placebo 1276 975 (76. 4) 182 (14. 3) 11 (0. 9) 65 (5. 1) 27 (3. 1) 26 (3. 0) 59 (6. 8) 7 (0. 8) 12 (1. 9) 13 (2. 1) 33 (5. 3) 3 (0. 5) 83 (3. 6)‖ 180 (7. 3) 213 (8. 6) 28 (1. 1) 41 (3. 5)‖ 66 (5. 2) 99 (7. 8) 5 (0. 4) 1 (0. 1) 2 (0. 3) 9 (0. 4) 5 (0. 4) 8 (0. 9) 9/850 (1. 1) 6 (1. 0) 1/612 (0. 2) 21 (0. 8) 9 (0. 7) 41/2455 (1. 7) 18/1266 (1. 4) studies: Phase 3 (LTS 11717, FH II, HIGH FH, COMBO I), Phase 2 (DFI 11565, DFI 11566, CL-1003, DFI 12361) Ezetimibe-controlled studies: Phase 3 (COMBO II, MONO, OPTIONS II, ALTERNATIVE). Includes all data collected to last patient visit at 52 weeks for COMBO, FH, HIGH FH and LONG TERM studies. ‡Safety data pool includes alirocumab 75 mg Q 2 W and alirocumab 150 mg Q 2 W doses only. §Includes CHD death, non-fatal myocardial infarction, fatal and non-fatal ischaemic stroke, unstable angina requiring hospitalisation, congestive heart failure requiring hospitalisation, and ischaemia-driven coronary revascularisation procedure. ‖Calculated using n values of 2318 for alirocumab and 1174 for placebo (excludes Phase 2). ALT, alanine aminotransferase; CMQ, custom Med. DRA query; HLT, high-level term, PCSA, potentially clinically significant abnormalities; PT, preferred term; ULN, upper limit of normal. Table shows safety analysis performed on safety population.
Safety Analysis (Pool of Four Phase 2 and 10 Phase 3 trials†) Ezetimibe-controlled pool (n=1482; 28%) n TEAEs, n (%) Treatment-emergent SAEs TEAEs leading to death TEAEs leading to discontinuation Safety events of interest, n (%) Adjudicated CV events§ Injection-site reactions (HLT) General allergic TEAE (CMQ) Pruritus (PT) General allergic serious TEAE (CMQ) Neurocognitive disorders (CMQ) ALT >3 x ULN (PCSA) †Placebo-controlled 15 Placebo-controlled pool (n=3752; 72%) Alirocumab‡ 864 607 (70. 3) 113 (13. 1) 2 (0. 2) 76 (8. 8) Ezetimibe 618 421 (68. 1) 69 (11. 2) 7 (1. 1) 60 (9. 7) Alirocumab‡ 2476 1876 (75. 8) 340 (13. 7) 13 (0. 5) 131 (5. 3) Placebo 1276 975 (76. 4) 182 (14. 3) 11 (0. 9) 65 (5. 1) 27 (3. 1) 26 (3. 0) 59 (6. 8) 7 (0. 8) 12 (1. 9) 13 (2. 1) 33 (5. 3) 3 (0. 5) 83 (3. 6)‖ 180 (7. 3) 213 (8. 6) 28 (1. 1) 41 (3. 5)‖ 66 (5. 2) 99 (7. 8) 5 (0. 4) 1 (0. 1) 2 (0. 3) 9 (0. 4) 5 (0. 4) 8 (0. 9) 9/850 (1. 1) 6 (1. 0) 1/612 (0. 2) 21 (0. 8) 9 (0. 7) 41/2455 (1. 7) 18/1266 (1. 4) studies: Phase 3 (LTS 11717, FH II, HIGH FH, COMBO I), Phase 2 (DFI 11565, DFI 11566, CL-1003, DFI 12361) Ezetimibe-controlled studies: Phase 3 (COMBO II, MONO, OPTIONS II, ALTERNATIVE). Includes all data collected to last patient visit at 52 weeks for COMBO, FH, HIGH FH and LONG TERM studies. ‡Safety data pool includes alirocumab 75 mg Q 2 W and alirocumab 150 mg Q 2 W doses only. §Includes CHD death, non-fatal myocardial infarction, fatal and non-fatal ischaemic stroke, unstable angina requiring hospitalisation, congestive heart failure requiring hospitalisation, and ischaemia-driven coronary revascularisation procedure. ‖Calculated using n values of 2318 for alirocumab and 1174 for placebo (excludes Phase 2). ALT, alanine aminotransferase; CMQ, custom Med. DRA query; HLT, high-level term, PCSA, potentially clinically significant abnormalities; PT, preferred term; ULN, upper limit of normal. Table shows safety analysis performed on safety population.
 Conclusions u This analysis represents the single largest collection of patients with He. FH (n=1257) included in a Phase 3 clinical study programme – In on-treatment analyses (using measurements that were collected while patients were still receiving treatment), alirocumab reduced mean LDL-C levels to <2. 2 mmol/L (85 mg/d. L) at Weeks 24– 78 of treatment, levels hitherto unobtainable with maximum doses of statin and addition of other LLTs in patients with He. FH – The incidence of TEAEs was generally similar between alirocumab and control group patients u 16 These findings hold potential for reducing LDL-C to levels that were previously unobtainable with existing currently standard-of-care therapy in patients with He. FH
Conclusions u This analysis represents the single largest collection of patients with He. FH (n=1257) included in a Phase 3 clinical study programme – In on-treatment analyses (using measurements that were collected while patients were still receiving treatment), alirocumab reduced mean LDL-C levels to <2. 2 mmol/L (85 mg/d. L) at Weeks 24– 78 of treatment, levels hitherto unobtainable with maximum doses of statin and addition of other LLTs in patients with He. FH – The incidence of TEAEs was generally similar between alirocumab and control group patients u 16 These findings hold potential for reducing LDL-C to levels that were previously unobtainable with existing currently standard-of-care therapy in patients with He. FH
 Results from ODYSSEY FH I and FH II are now Available in The European Heart Journal doi: 10. 1093/eurheartj/ehv 370 17 eurheartj. oxfordjournals. org
Results from ODYSSEY FH I and FH II are now Available in The European Heart Journal doi: 10. 1093/eurheartj/ehv 370 17 eurheartj. oxfordjournals. org
 Q&A
Q&A


