7c6857cdc1cd18b6214b65b013dc6a3d.ppt
- Количество слайдов: 67
 Efficacy and Safety Data from the Advair Diskus 500/50 Clinical Program Katharine Knobil, MD Vice President Respiratory Clinical Development A 1
Efficacy and Safety Data from the Advair Diskus 500/50 Clinical Program Katharine Knobil, MD Vice President Respiratory Clinical Development A 1
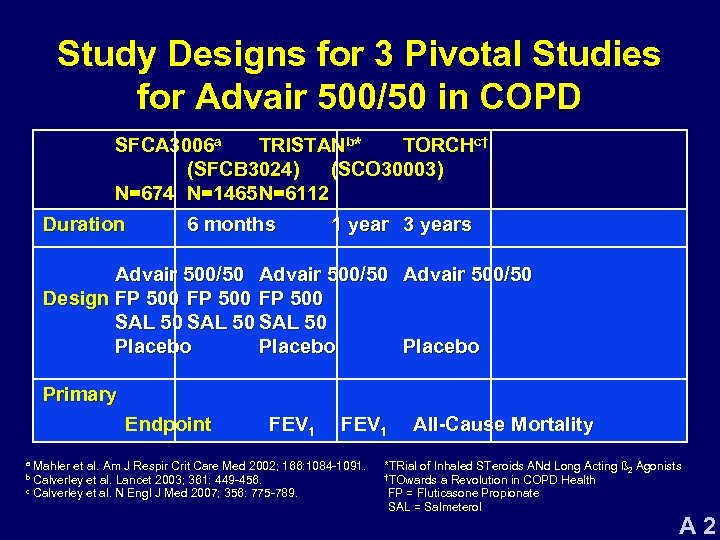 Study Designs for 3 Pivotal Studies for Advair 500/50 in COPD SFCA 3006 a TRISTANb* TORCHc† (SFCB 3024) (SCO 30003) N=674 N=1465 N=6112 Duration 6 months 1 year 3 years Advair 500/50 Design FP 500 SAL 50 Placebo Primary Endpoint FEV 1 Mahler et al. Am J Respir Crit Care Med 2002; 166: 1084 -1091. Calverley et al. Lancet 2003; 361: 449 -456. c Calverley et al. N Engl J Med 2007; 356: 775 -789. a b All-Cause Mortality *TRial of Inhaled STeroids ANd Long Acting ß 2 Agonists †TOwards a Revolution in COPD Health FP = Fluticasone Propionate SAL = Salmeterol A 2
Study Designs for 3 Pivotal Studies for Advair 500/50 in COPD SFCA 3006 a TRISTANb* TORCHc† (SFCB 3024) (SCO 30003) N=674 N=1465 N=6112 Duration 6 months 1 year 3 years Advair 500/50 Design FP 500 SAL 50 Placebo Primary Endpoint FEV 1 Mahler et al. Am J Respir Crit Care Med 2002; 166: 1084 -1091. Calverley et al. Lancet 2003; 361: 449 -456. c Calverley et al. N Engl J Med 2007; 356: 775 -789. a b All-Cause Mortality *TRial of Inhaled STeroids ANd Long Acting ß 2 Agonists †TOwards a Revolution in COPD Health FP = Fluticasone Propionate SAL = Salmeterol A 2
 Order of the Presentation • Efficacy – Forced Expiratory Volume in 1 second (FEV 1) – Health Related Quality of Life – Exacerbations – Mortality • Safety – Adverse Events of Special Interest – TORCH Safety Sub-study – Cardiovascular and HPA-axis assessments from pivotal studies • Benefit : Risk A 3
Order of the Presentation • Efficacy – Forced Expiratory Volume in 1 second (FEV 1) – Health Related Quality of Life – Exacerbations – Mortality • Safety – Adverse Events of Special Interest – TORCH Safety Sub-study – Cardiovascular and HPA-axis assessments from pivotal studies • Benefit : Risk A 3
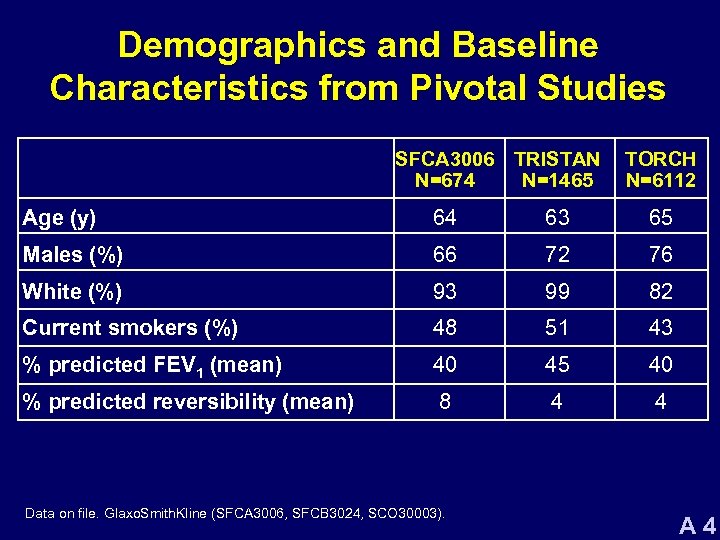 Demographics and Baseline Characteristics from Pivotal Studies SFCA 3006 TRISTAN N=674 N=1465 TORCH N=6112 Age (y) 64 63 65 Males (%) 66 72 76 White (%) 93 99 82 Current smokers (%) 48 51 43 % predicted FEV 1 (mean) 40 45 40 % predicted reversibility (mean) 8 4 4 Data on file. Glaxo. Smith. Kline (SFCA 3006, SFCB 3024, SCO 30003). A 4
Demographics and Baseline Characteristics from Pivotal Studies SFCA 3006 TRISTAN N=674 N=1465 TORCH N=6112 Age (y) 64 63 65 Males (%) 66 72 76 White (%) 93 99 82 Current smokers (%) 48 51 43 % predicted FEV 1 (mean) 40 45 40 % predicted reversibility (mean) 8 4 4 Data on file. Glaxo. Smith. Kline (SFCA 3006, SFCB 3024, SCO 30003). A 4
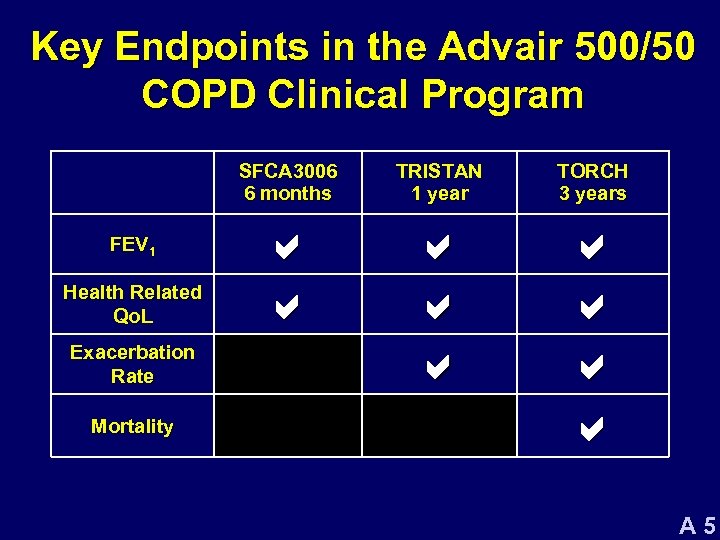 Key Endpoints in the Advair 500/50 COPD Clinical Program SFCA 3006 6 months FEV 1 Health Related Qo. L Exacerbation Rate Mortality TRISTAN 1 year TORCH 3 years a a a a a A 5
Key Endpoints in the Advair 500/50 COPD Clinical Program SFCA 3006 6 months FEV 1 Health Related Qo. L Exacerbation Rate Mortality TRISTAN 1 year TORCH 3 years a a a a a A 5
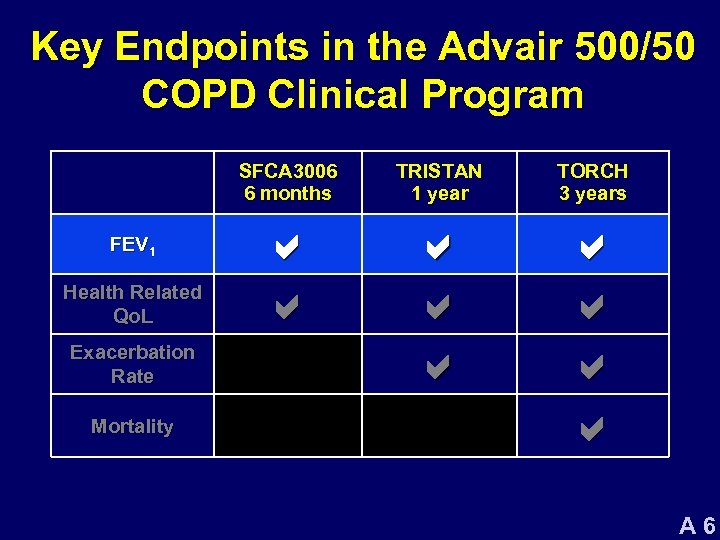 Key Endpoints in the Advair 500/50 COPD Clinical Program SFCA 3006 6 months FEV 1 Health Related Qo. L Exacerbation Rate Mortality TRISTAN 1 year TORCH 3 years a a a a a A 6
Key Endpoints in the Advair 500/50 COPD Clinical Program SFCA 3006 6 months FEV 1 Health Related Qo. L Exacerbation Rate Mortality TRISTAN 1 year TORCH 3 years a a a a a A 6
 Relevance of Measuring FEV 1 in Patients with COPD • In COPD, FEV 1 declines over time • Lower lung function is associated with poor outcomes, including mortality • FEV 1 as an endpoint in COPD – Accepted, important, reproducible – Forms the basis of the indication of all drugs approved for COPD A 7
Relevance of Measuring FEV 1 in Patients with COPD • In COPD, FEV 1 declines over time • Lower lung function is associated with poor outcomes, including mortality • FEV 1 as an endpoint in COPD – Accepted, important, reproducible – Forms the basis of the indication of all drugs approved for COPD A 7
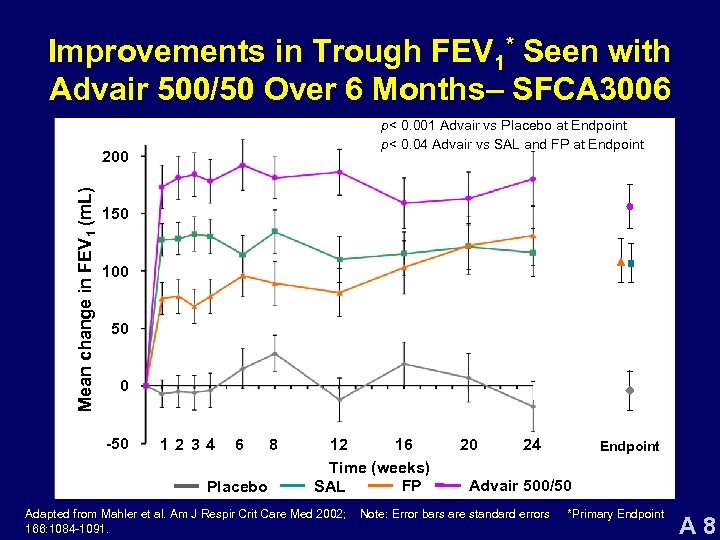 Improvements in Trough FEV 1* Seen with Advair 500/50 Over 6 Months– SFCA 3006 p< 0. 001 Advair vs Placebo at Endpoint p< 0. 04 Advair vs SAL and FP at Endpoint Mean change in FEV 1 (m. L) 200 150 100 50 0 -50 12 34 6 Placebo 8 12 16 Time (weeks) FP SAL Adapted from Mahler et al. Am J Respir Crit Care Med 2002; 166: 1084 -1091. 20 24 Endpoint Advair 500/50 Note: Error bars are standard errors *Primary Endpoint A 8
Improvements in Trough FEV 1* Seen with Advair 500/50 Over 6 Months– SFCA 3006 p< 0. 001 Advair vs Placebo at Endpoint p< 0. 04 Advair vs SAL and FP at Endpoint Mean change in FEV 1 (m. L) 200 150 100 50 0 -50 12 34 6 Placebo 8 12 16 Time (weeks) FP SAL Adapted from Mahler et al. Am J Respir Crit Care Med 2002; 166: 1084 -1091. 20 24 Endpoint Advair 500/50 Note: Error bars are standard errors *Primary Endpoint A 8
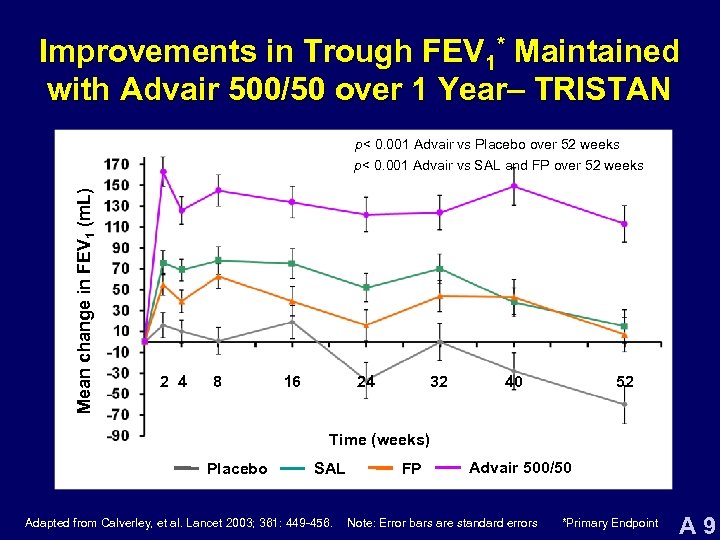 Improvements in Trough FEV 1* Maintained with Advair 500/50 over 1 Year– TRISTAN p< 0. 001 Advair vs Placebo over 52 weeks Mean change in FEV 1 (m. L) p< 0. 001 Advair vs SAL and FP over 52 weeks 2 4 8 16 24 32 40 52 Time (weeks) Placebo SAL Adapted from Calverley, et al. Lancet 2003; 361: 449 -456. FP Advair 500/50 Note: Error bars are standard errors *Primary Endpoint A 9
Improvements in Trough FEV 1* Maintained with Advair 500/50 over 1 Year– TRISTAN p< 0. 001 Advair vs Placebo over 52 weeks Mean change in FEV 1 (m. L) p< 0. 001 Advair vs SAL and FP over 52 weeks 2 4 8 16 24 32 40 52 Time (weeks) Placebo SAL Adapted from Calverley, et al. Lancet 2003; 361: 449 -456. FP Advair 500/50 Note: Error bars are standard errors *Primary Endpoint A 9
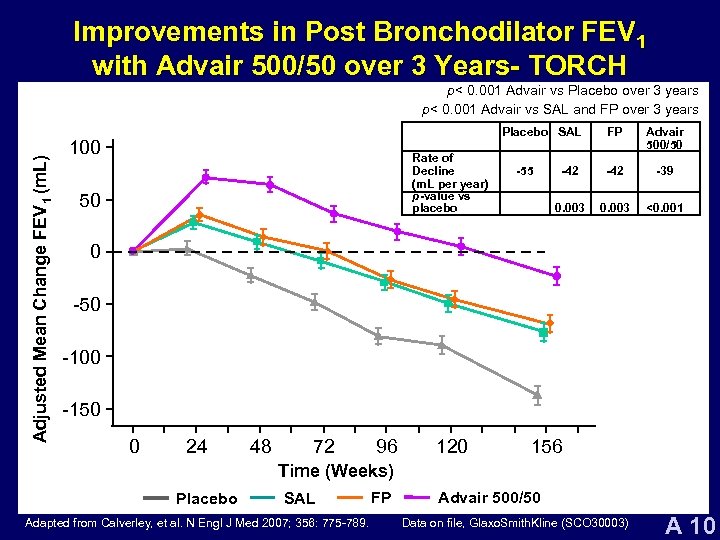 Improvements in Post Bronchodilator FEV 1 with Advair 500/50 over 3 Years- TORCH Adjusted Mean Change FEV 1 (m. L) p< 0. 001 Advair vs Placebo over 3 years p< 0. 001 Advair vs SAL and FP over 3 years Placebo SAL 100 Rate of Decline (m. L per year) p-value vs placebo 50 Advair 500/50 -42 -39 0. 003 -55 FP 0. 003 <0. 001 0 -50 -100 -150 0 24 48 72 96 120 156 Time (Weeks) Placebo SAL Adapted from Calverley, et al. N Engl J Med 2007; 356: 775 -789. FP Advair 500/50 Data on file, Glaxo. Smith. Kline (SCO 30003) A 10
Improvements in Post Bronchodilator FEV 1 with Advair 500/50 over 3 Years- TORCH Adjusted Mean Change FEV 1 (m. L) p< 0. 001 Advair vs Placebo over 3 years p< 0. 001 Advair vs SAL and FP over 3 years Placebo SAL 100 Rate of Decline (m. L per year) p-value vs placebo 50 Advair 500/50 -42 -39 0. 003 -55 FP 0. 003 <0. 001 0 -50 -100 -150 0 24 48 72 96 120 156 Time (Weeks) Placebo SAL Adapted from Calverley, et al. N Engl J Med 2007; 356: 775 -789. FP Advair 500/50 Data on file, Glaxo. Smith. Kline (SCO 30003) A 10
 Advair 500/50 Improves Lung Function • Consistent improvements in FEV 1 seen in all 3 pivotal studies for Advair 500/50 • Advair 500/50 significantly improved FEV 1 compared with SAL, FP and placebo in all three studies • First time Advair 500/50 has shown an effect on rate of decline of FEV 1 A 11
Advair 500/50 Improves Lung Function • Consistent improvements in FEV 1 seen in all 3 pivotal studies for Advair 500/50 • Advair 500/50 significantly improved FEV 1 compared with SAL, FP and placebo in all three studies • First time Advair 500/50 has shown an effect on rate of decline of FEV 1 A 11
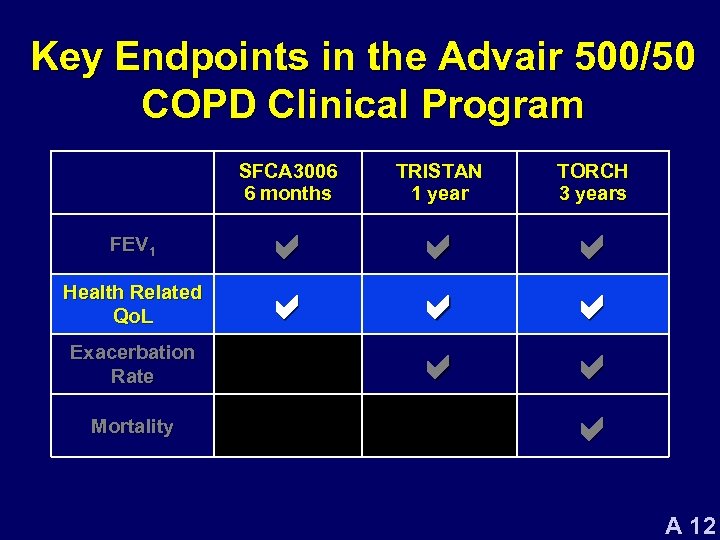 Key Endpoints in the Advair 500/50 COPD Clinical Program SFCA 3006 6 months FEV 1 Health Related Qo. L Exacerbation Rate Mortality TRISTAN 1 year TORCH 3 years a a a a a A 12
Key Endpoints in the Advair 500/50 COPD Clinical Program SFCA 3006 6 months FEV 1 Health Related Qo. L Exacerbation Rate Mortality TRISTAN 1 year TORCH 3 years a a a a a A 12
 Importance of Measuring Health Related QOL in Patients with COPD • Important to measure impact of the disease and the effect of treatment • Health-related quality of life declines over time • Associated with poor outcomes – Exacerbation frequency and mortality A 13
Importance of Measuring Health Related QOL in Patients with COPD • Important to measure impact of the disease and the effect of treatment • Health-related quality of life declines over time • Associated with poor outcomes – Exacerbation frequency and mortality A 13
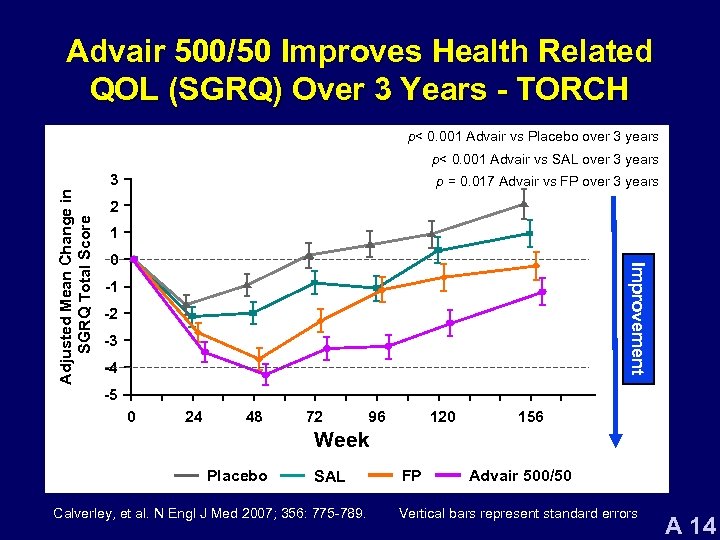 Advair 500/50 Improves Health Related QOL (SGRQ) Over 3 Years - TORCH p< 0. 001 Advair vs Placebo over 3 years p< 0. 001 Advair vs SAL over 3 years p = 0. 017 Advair vs FP over 3 years 2 1 0 Improvement Adjusted Mean Change in SGRQ Total Score 3 -1 -2 -3 -4 -5 0 24 48 72 96 120 156 Week Placebo SAL Calverley, et al. N Engl J Med 2007; 356: 775 -789. FP Advair 500/50 Vertical bars represent standard errors A 14
Advair 500/50 Improves Health Related QOL (SGRQ) Over 3 Years - TORCH p< 0. 001 Advair vs Placebo over 3 years p< 0. 001 Advair vs SAL over 3 years p = 0. 017 Advair vs FP over 3 years 2 1 0 Improvement Adjusted Mean Change in SGRQ Total Score 3 -1 -2 -3 -4 -5 0 24 48 72 96 120 156 Week Placebo SAL Calverley, et al. N Engl J Med 2007; 356: 775 -789. FP Advair 500/50 Vertical bars represent standard errors A 14
 Advair 500/50 Improves Health Related Qo. L • Consistent improvements seen in all 3 pivotal studies for Advair 500/50 • Advair 500/50 significantly improved HRQo. L compared with SAL, FP and placebo in all three studies • HRQo. L maintained for 3 years A 15
Advair 500/50 Improves Health Related Qo. L • Consistent improvements seen in all 3 pivotal studies for Advair 500/50 • Advair 500/50 significantly improved HRQo. L compared with SAL, FP and placebo in all three studies • HRQo. L maintained for 3 years A 15
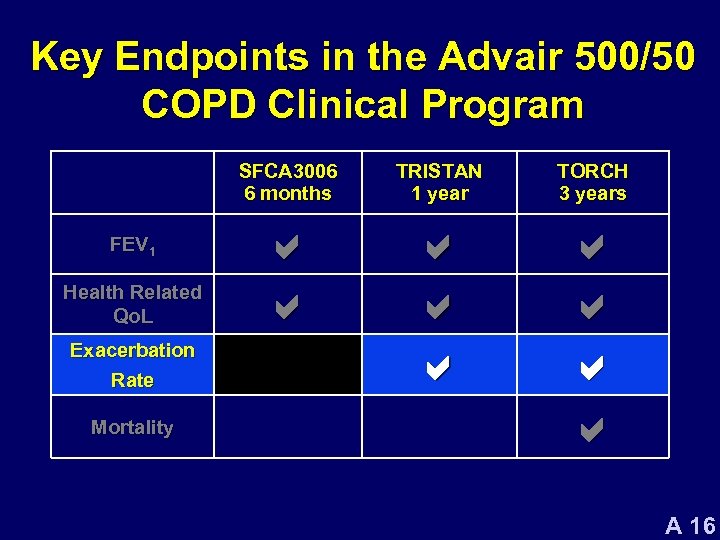 Key Endpoints in the Advair 500/50 COPD Clinical Program SFCA 3006 6 months FEV 1 Health Related Qo. L Exacerbation Rate Mortality TRISTAN 1 year TORCH 3 years a a a a a A 16
Key Endpoints in the Advair 500/50 COPD Clinical Program SFCA 3006 6 months FEV 1 Health Related Qo. L Exacerbation Rate Mortality TRISTAN 1 year TORCH 3 years a a a a a A 16
 Importance of Reducing Exacerbations • COPD exacerbations are closely associated with symptomatic and physiological deterioration and impaired health status 1, 2 • Following a COPD exacerbation, the likelihood of further exacerbations increases 3 • High rate of COPD exacerbations is associated with a rapid decline in lung function, increased risk of hospitalization 4, 5 and death 6 1. Osman et al. Thorax 1997 3. Seemungal et al. Am J Respir Crit Care Med 2000 5. Garcia-Aymerich et al. Am J Respir Crit Care Med 2001 2. Seemungal et al. Am J Respir Crit Care Med 1998 4. Donaldson et al. Thorax 2002 6. Soler-Cataluna et al. Thorax 2005 A 17
Importance of Reducing Exacerbations • COPD exacerbations are closely associated with symptomatic and physiological deterioration and impaired health status 1, 2 • Following a COPD exacerbation, the likelihood of further exacerbations increases 3 • High rate of COPD exacerbations is associated with a rapid decline in lung function, increased risk of hospitalization 4, 5 and death 6 1. Osman et al. Thorax 1997 3. Seemungal et al. Am J Respir Crit Care Med 2000 5. Garcia-Aymerich et al. Am J Respir Crit Care Med 2001 2. Seemungal et al. Am J Respir Crit Care Med 1998 4. Donaldson et al. Thorax 2002 6. Soler-Cataluna et al. Thorax 2005 A 17
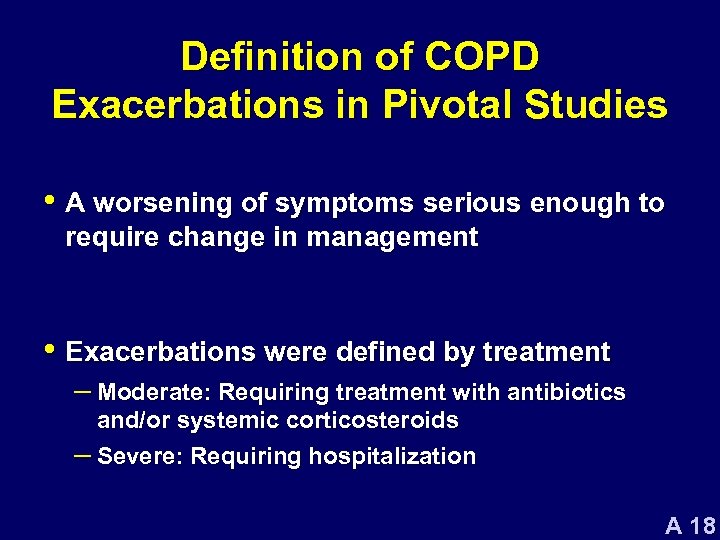 Definition of COPD Exacerbations in Pivotal Studies • A worsening of symptoms serious enough to require change in management • Exacerbations were defined by treatment – Moderate: Requiring treatment with antibiotics and/or systemic corticosteroids – Severe: Requiring hospitalization A 18
Definition of COPD Exacerbations in Pivotal Studies • A worsening of symptoms serious enough to require change in management • Exacerbations were defined by treatment – Moderate: Requiring treatment with antibiotics and/or systemic corticosteroids – Severe: Requiring hospitalization A 18
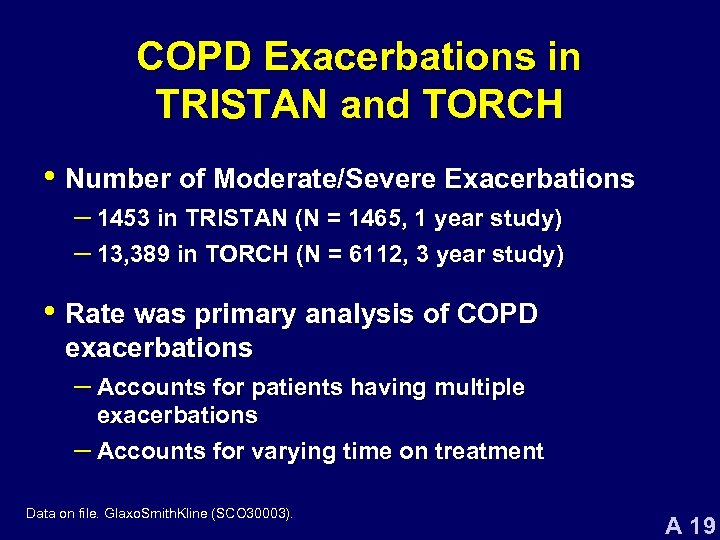 COPD Exacerbations in TRISTAN and TORCH • Number of Moderate/Severe Exacerbations – 1453 in TRISTAN (N = 1465, 1 year study) – 13, 389 in TORCH (N = 6112, 3 year study) • Rate was primary analysis of COPD exacerbations – Accounts for patients having multiple exacerbations – Accounts for varying time on treatment Data on file. Glaxo. Smith. Kline (SCO 30003). A 19
COPD Exacerbations in TRISTAN and TORCH • Number of Moderate/Severe Exacerbations – 1453 in TRISTAN (N = 1465, 1 year study) – 13, 389 in TORCH (N = 6112, 3 year study) • Rate was primary analysis of COPD exacerbations – Accounts for patients having multiple exacerbations – Accounts for varying time on treatment Data on file. Glaxo. Smith. Kline (SCO 30003). A 19
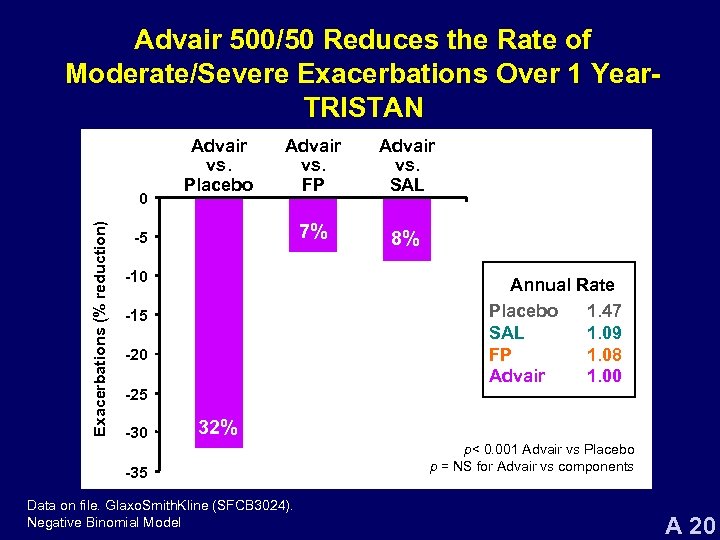 Advair 500/50 Reduces the Rate of Moderate/Severe Exacerbations Over 1 Year. TRISTAN Exacerbations (% reduction) 0 Advair vs. Placebo Advair vs. FP Advair vs. SAL 7% 8% -5 -10 Annual Rate Placebo 1. 47 SAL 1. 09 FP 1. 08 Advair 1. 00 -15 -20 -25 -30 32% -35 Data on file. Glaxo. Smith. Kline (SFCB 3024). Negative Binomial Model p< 0. 001 Advair vs Placebo p = NS for Advair vs components A 20
Advair 500/50 Reduces the Rate of Moderate/Severe Exacerbations Over 1 Year. TRISTAN Exacerbations (% reduction) 0 Advair vs. Placebo Advair vs. FP Advair vs. SAL 7% 8% -5 -10 Annual Rate Placebo 1. 47 SAL 1. 09 FP 1. 08 Advair 1. 00 -15 -20 -25 -30 32% -35 Data on file. Glaxo. Smith. Kline (SFCB 3024). Negative Binomial Model p< 0. 001 Advair vs Placebo p = NS for Advair vs components A 20
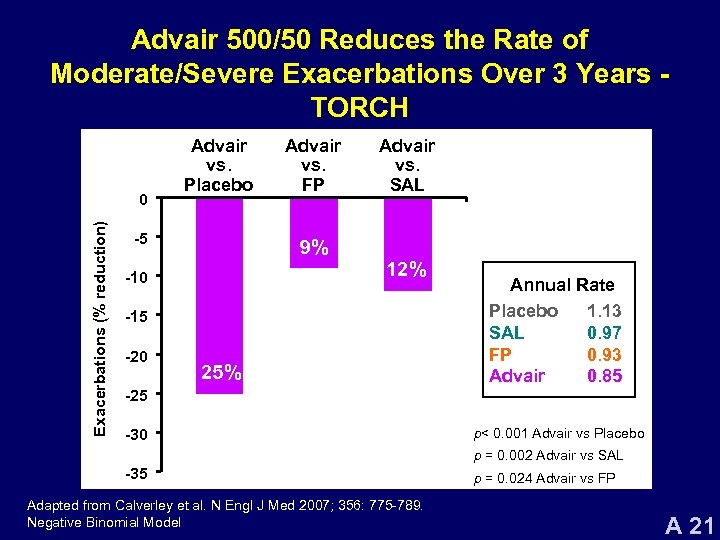 Advair 500/50 Reduces the Rate of Moderate/Severe Exacerbations Over 3 Years TORCH Exacerbations (% reduction) 0 Advair vs. Placebo -5 Advair vs. FP Advair vs. SAL 9% 12% -10 -15 -20 25% Annual Rate Placebo 1. 13 SAL 0. 97 FP 0. 93 Advair 0. 85 -25 -30 p< 0. 001 Advair vs Placebo p = 0. 002 Advair vs SAL -35 Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. Negative Binomial Model p = 0. 024 Advair vs FP A 21
Advair 500/50 Reduces the Rate of Moderate/Severe Exacerbations Over 3 Years TORCH Exacerbations (% reduction) 0 Advair vs. Placebo -5 Advair vs. FP Advair vs. SAL 9% 12% -10 -15 -20 25% Annual Rate Placebo 1. 13 SAL 0. 97 FP 0. 93 Advair 0. 85 -25 -30 p< 0. 001 Advair vs Placebo p = 0. 002 Advair vs SAL -35 Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. Negative Binomial Model p = 0. 024 Advair vs FP A 21
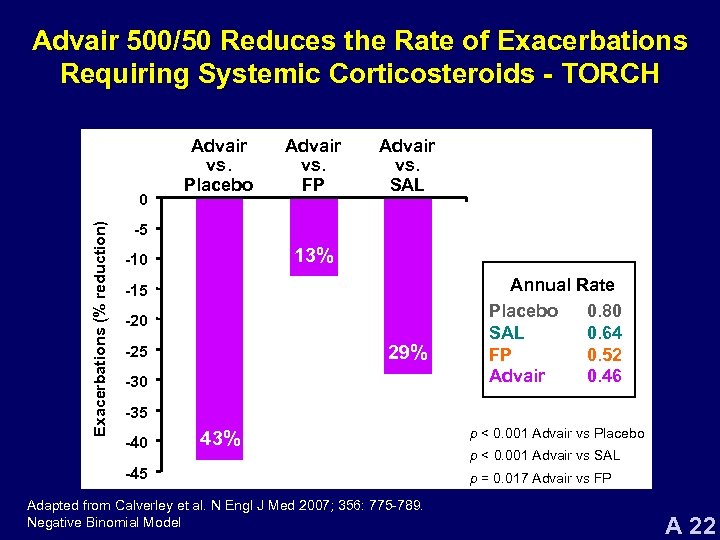 Advair 500/50 Reduces the Rate of Exacerbations Requiring Systemic Corticosteroids - TORCH Exacerbations (% reduction) 0 Advair vs. Placebo Advair vs. FP Advair vs. SAL -5 13% -10 -15 -20 29% -25 -30 Annual Rate Placebo 0. 80 SAL 0. 64 FP 0. 52 Advair 0. 46 -35 -40 43% -45 Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. Negative Binomial Model p < 0. 001 Advair vs Placebo p < 0. 001 Advair vs SAL p = 0. 017 Advair vs FP A 22
Advair 500/50 Reduces the Rate of Exacerbations Requiring Systemic Corticosteroids - TORCH Exacerbations (% reduction) 0 Advair vs. Placebo Advair vs. FP Advair vs. SAL -5 13% -10 -15 -20 29% -25 -30 Annual Rate Placebo 0. 80 SAL 0. 64 FP 0. 52 Advair 0. 46 -35 -40 43% -45 Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. Negative Binomial Model p < 0. 001 Advair vs Placebo p < 0. 001 Advair vs SAL p = 0. 017 Advair vs FP A 22
 Advair 500/50 Reduced Rate of COPD Exacerbations • Consistently reduced the rate of moderate and/or severe exacerbations – Significant vs SAL and FP in TORCH • Consistently reduced rate of exacerbations requiring systemic corticosteroids – Significant vs SAL and FP in TORCH • Advair 500/50 always provided the greatest benefit A 23
Advair 500/50 Reduced Rate of COPD Exacerbations • Consistently reduced the rate of moderate and/or severe exacerbations – Significant vs SAL and FP in TORCH • Consistently reduced rate of exacerbations requiring systemic corticosteroids – Significant vs SAL and FP in TORCH • Advair 500/50 always provided the greatest benefit A 23
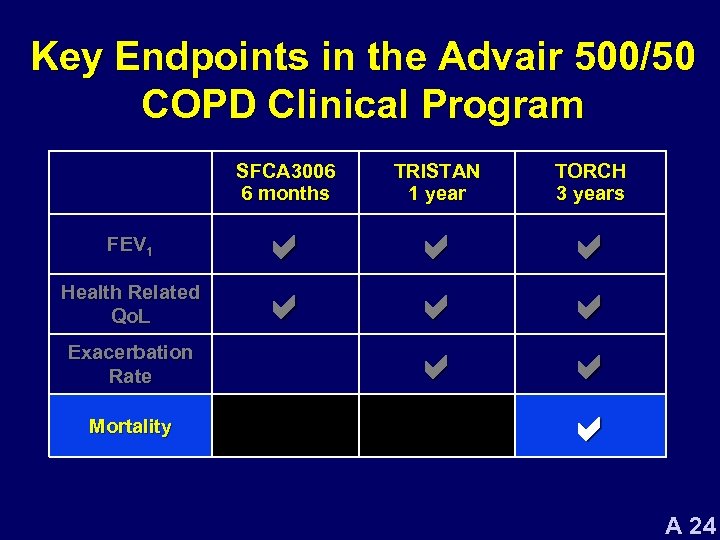 Key Endpoints in the Advair 500/50 COPD Clinical Program SFCA 3006 6 months FEV 1 Health Related Qo. L Exacerbation Rate Mortality TRISTAN 1 year TORCH 3 years a a a a a A 24
Key Endpoints in the Advair 500/50 COPD Clinical Program SFCA 3006 6 months FEV 1 Health Related Qo. L Exacerbation Rate Mortality TRISTAN 1 year TORCH 3 years a a a a a A 24
 External Committees Involved in Conduct of TORCH • Steering Committee (SC) – Worked with sponsor to design, implement and manage the study • Clinical Endpoint Committee (CEC) – Independently reviewed all deaths, blinded to treatment – Assigned a primary cause of death and provided assessment of whether death was COPD-related • Safety and Efficacy Data Monitoring Committee – Conducted 2 planned interim analyses and 6 -monthly safety reviews A 25
External Committees Involved in Conduct of TORCH • Steering Committee (SC) – Worked with sponsor to design, implement and manage the study • Clinical Endpoint Committee (CEC) – Independently reviewed all deaths, blinded to treatment – Assigned a primary cause of death and provided assessment of whether death was COPD-related • Safety and Efficacy Data Monitoring Committee – Conducted 2 planned interim analyses and 6 -monthly safety reviews A 25
 TORCH: Study Design • Ambitious study design – Over 400 centers, 42 countries, 6112 patients • All-cause mortality was the primary endpoint – COPD-related mortality assessed • 3 years of follow up – Allowed COPD medications except long-acting bronchodilators, ICS, long-term systemic corticosteroids A 26
TORCH: Study Design • Ambitious study design – Over 400 centers, 42 countries, 6112 patients • All-cause mortality was the primary endpoint – COPD-related mortality assessed • 3 years of follow up – Allowed COPD medications except long-acting bronchodilators, ICS, long-term systemic corticosteroids A 26
 Robust Assessment of Mortality • Conservative analysis of mortality – All patients were included in the primary analysis regardless of time spent on study treatment – Patients withdrawn early could take any treatment for COPD; analyzed by original treatment arm • Rigorous follow-up for 3 years, even if withdrawn from study medication – Only 1 patient of 6112 had unknown survival status at 3 years A 27
Robust Assessment of Mortality • Conservative analysis of mortality – All patients were included in the primary analysis regardless of time spent on study treatment – Patients withdrawn early could take any treatment for COPD; analyzed by original treatment arm • Rigorous follow-up for 3 years, even if withdrawn from study medication – Only 1 patient of 6112 had unknown survival status at 3 years A 27
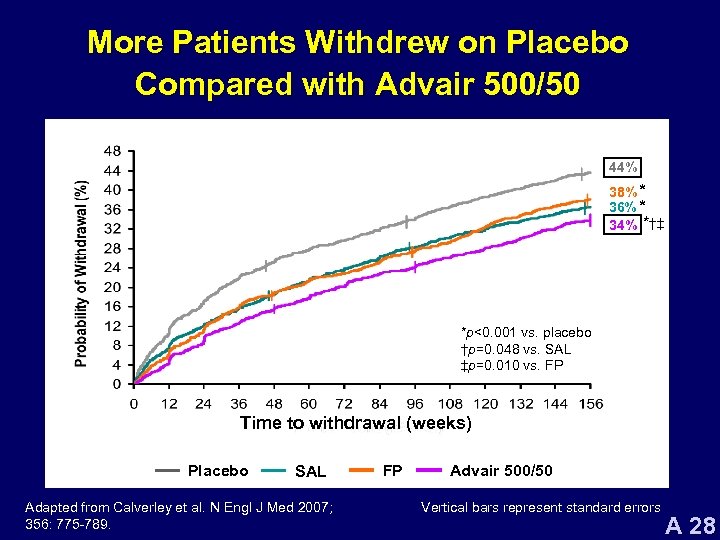 More Patients Withdrew on Placebo Compared with Advair 500/50 44% 38% * 36% * 34% *†‡ *p<0. 001 vs. placebo †p=0. 048 vs. SAL ‡p=0. 010 vs. FP Time to withdrawal (weeks) Placebo SAL Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. FP Advair 500/50 Vertical bars represent standard errors A 28
More Patients Withdrew on Placebo Compared with Advair 500/50 44% 38% * 36% * 34% *†‡ *p<0. 001 vs. placebo †p=0. 048 vs. SAL ‡p=0. 010 vs. FP Time to withdrawal (weeks) Placebo SAL Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. FP Advair 500/50 Vertical bars represent standard errors A 28
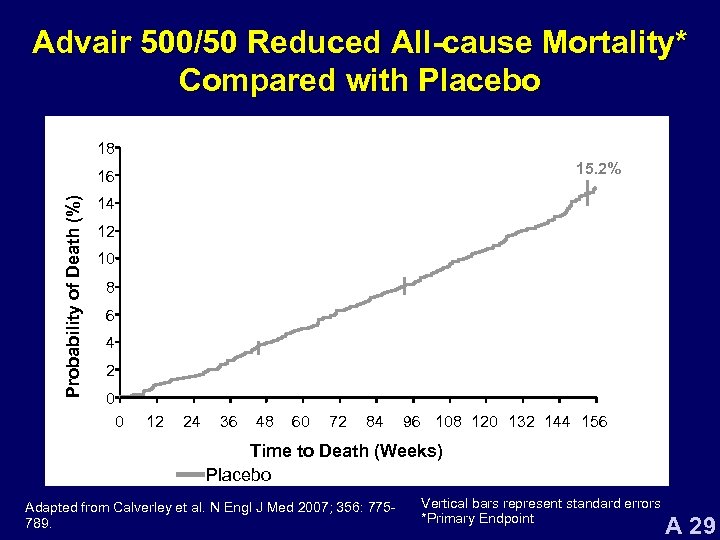 Advair 500/50 Reduced All-cause Mortality* Compared with Placebo 18 15. 2% Probability of Death (%) 16 14 12 10 8 6 4 2 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 Time to Death (Weeks) Placebo Adapted from Calverley et al. N Engl J Med 2007; 356: 775789. Vertical bars represent standard errors *Primary Endpoint A 29
Advair 500/50 Reduced All-cause Mortality* Compared with Placebo 18 15. 2% Probability of Death (%) 16 14 12 10 8 6 4 2 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 Time to Death (Weeks) Placebo Adapted from Calverley et al. N Engl J Med 2007; 356: 775789. Vertical bars represent standard errors *Primary Endpoint A 29
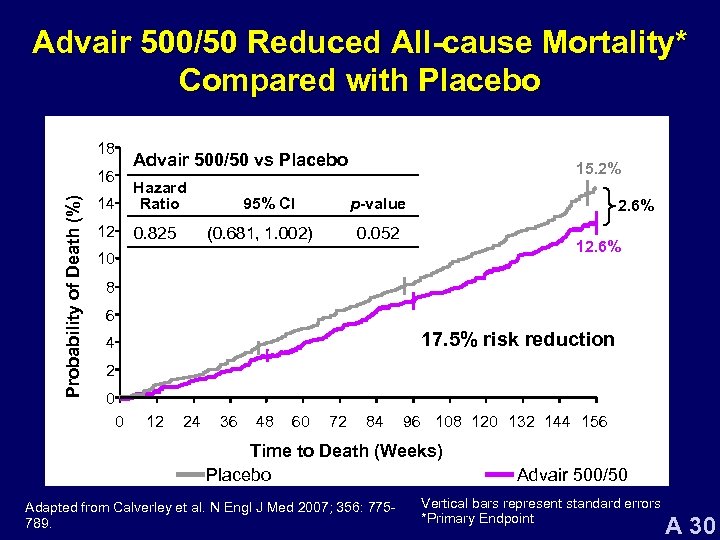 Advair 500/50 Reduced All-cause Mortality* Compared with Placebo 18 Probability of Death (%) 16 Advair 500/50 vs Placebo 14 Hazard Ratio 12 0. 825 95% CI 15. 2% p-value (0. 681, 1. 002) 2. 6% 0. 052 12. 6% 10 8 6 17. 5% risk reduction 4 2 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 Time to Death (Weeks) Placebo Adapted from Calverley et al. N Engl J Med 2007; 356: 775789. Advair 500/50 Vertical bars represent standard errors *Primary Endpoint A 30
Advair 500/50 Reduced All-cause Mortality* Compared with Placebo 18 Probability of Death (%) 16 Advair 500/50 vs Placebo 14 Hazard Ratio 12 0. 825 95% CI 15. 2% p-value (0. 681, 1. 002) 2. 6% 0. 052 12. 6% 10 8 6 17. 5% risk reduction 4 2 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 Time to Death (Weeks) Placebo Adapted from Calverley et al. N Engl J Med 2007; 356: 775789. Advair 500/50 Vertical bars represent standard errors *Primary Endpoint A 30
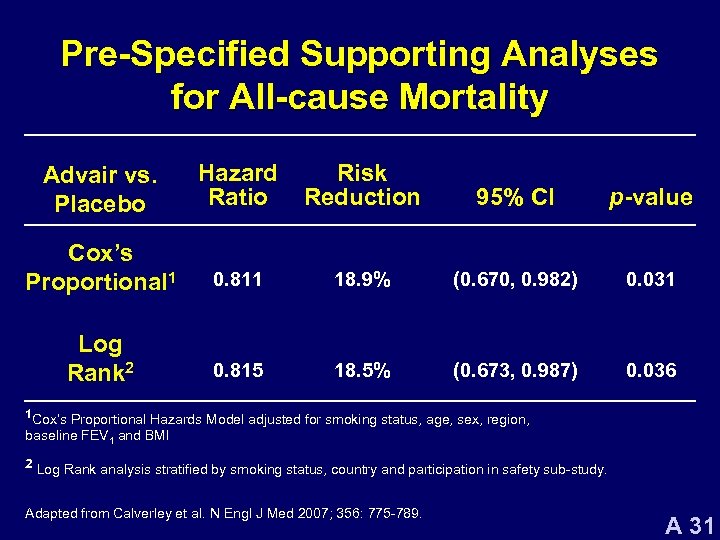 Pre-Specified Supporting Analyses for All-cause Mortality Advair vs. Placebo Hazard Ratio Risk Reduction 95% CI p-value Cox’s Proportional 1 0. 811 18. 9% (0. 670, 0. 982) 0. 031 Log Rank 2 0. 815 18. 5% (0. 673, 0. 987) 0. 036 1 Cox’s Proportional Hazards Model adjusted for smoking status, age, sex, region, baseline FEV 1 and BMI 2 Log Rank analysis stratified by smoking status, country and participation in safety sub-study. Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. A 31
Pre-Specified Supporting Analyses for All-cause Mortality Advair vs. Placebo Hazard Ratio Risk Reduction 95% CI p-value Cox’s Proportional 1 0. 811 18. 9% (0. 670, 0. 982) 0. 031 Log Rank 2 0. 815 18. 5% (0. 673, 0. 987) 0. 036 1 Cox’s Proportional Hazards Model adjusted for smoking status, age, sex, region, baseline FEV 1 and BMI 2 Log Rank analysis stratified by smoking status, country and participation in safety sub-study. Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. A 31
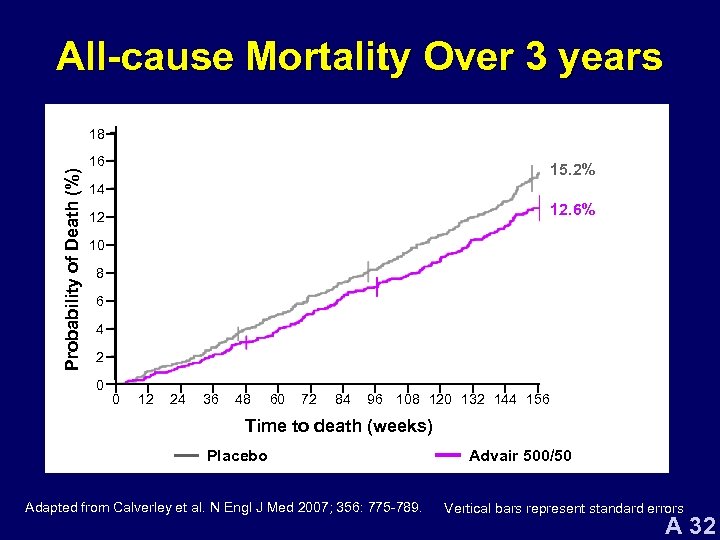 All-cause Mortality Over 3 years Probability of Death (%) 18 16 15. 2% 14 12. 6% 12 10 8 6 4 2 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 Time to death (weeks) Placebo SAL FP Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. Advair 500/50 Vertical bars represent standard errors A 32
All-cause Mortality Over 3 years Probability of Death (%) 18 16 15. 2% 14 12. 6% 12 10 8 6 4 2 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 Time to death (weeks) Placebo SAL FP Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. Advair 500/50 Vertical bars represent standard errors A 32
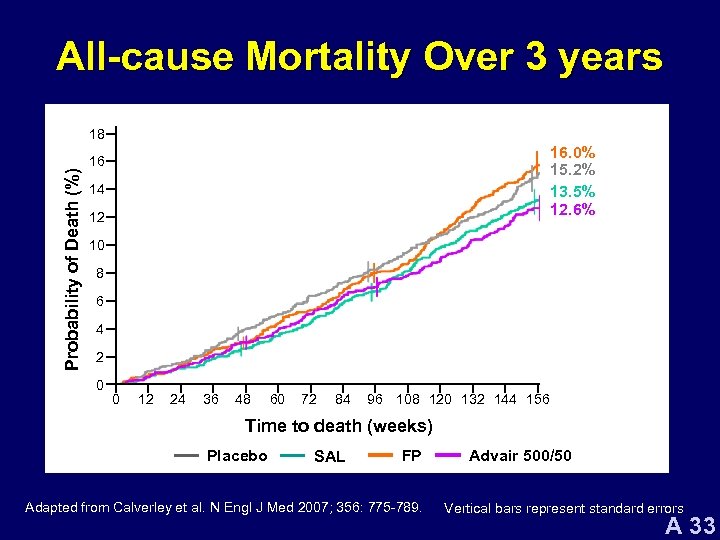 All-cause Mortality Over 3 years Probability of Death (%) 18 16. 0% 15. 2% 13. 5% 12. 6% 16 14 12 10 8 6 4 2 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 Time to death (weeks) Placebo SAL FP Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. Advair 500/50 Vertical bars represent standard errors A 33
All-cause Mortality Over 3 years Probability of Death (%) 18 16. 0% 15. 2% 13. 5% 12. 6% 16 14 12 10 8 6 4 2 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 Time to death (weeks) Placebo SAL FP Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. Advair 500/50 Vertical bars represent standard errors A 33
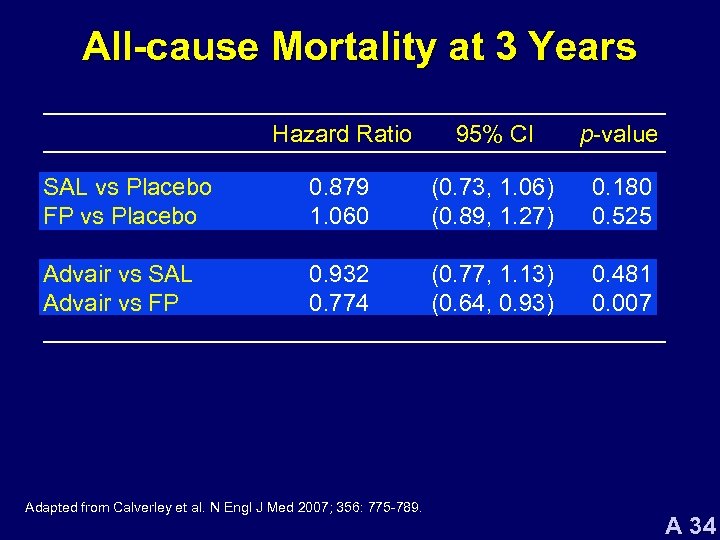 All-cause Mortality at 3 Years Hazard Ratio 95% CI p-value SAL vs Placebo FP vs Placebo 0. 879 1. 060 (0. 73, 1. 06) (0. 89, 1. 27) 0. 180 0. 525 Advair vs SAL Advair vs FP 0. 932 0. 774 (0. 77, 1. 13) (0. 64, 0. 93) 0. 481 0. 007 Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. A 34
All-cause Mortality at 3 Years Hazard Ratio 95% CI p-value SAL vs Placebo FP vs Placebo 0. 879 1. 060 (0. 73, 1. 06) (0. 89, 1. 27) 0. 180 0. 525 Advair vs SAL Advair vs FP 0. 932 0. 774 (0. 77, 1. 13) (0. 64, 0. 93) 0. 481 0. 007 Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. A 34
 All-cause Mortality: Interactions with Treatment • No statistically significant interactions of treatment with – Age – Smoking status – Region – Baseline %predicted FEV 1 (Gold stages) – Sex – Ethnic Origin – BMI i. e. no evidence of difference in treatment effect Calverley et al. N Engl J Med 2007; 356: 775 -789. Data on file. Glaxo. Smith. Kline (SCO 30003). A 35
All-cause Mortality: Interactions with Treatment • No statistically significant interactions of treatment with – Age – Smoking status – Region – Baseline %predicted FEV 1 (Gold stages) – Sex – Ethnic Origin – BMI i. e. no evidence of difference in treatment effect Calverley et al. N Engl J Med 2007; 356: 775 -789. Data on file. Glaxo. Smith. Kline (SCO 30003). A 35
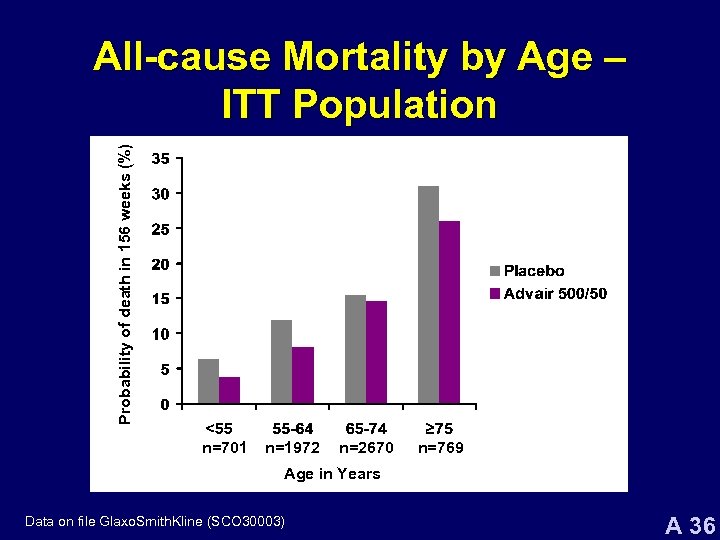 Probability of death in 156 weeks (%) All-cause Mortality by Age – ITT Population n=701 n=1972 n=2670 n=769 Age in Years Data on file Glaxo. Smith. Kline (SCO 30003) A 36
Probability of death in 156 weeks (%) All-cause Mortality by Age – ITT Population n=701 n=1972 n=2670 n=769 Age in Years Data on file Glaxo. Smith. Kline (SCO 30003) A 36
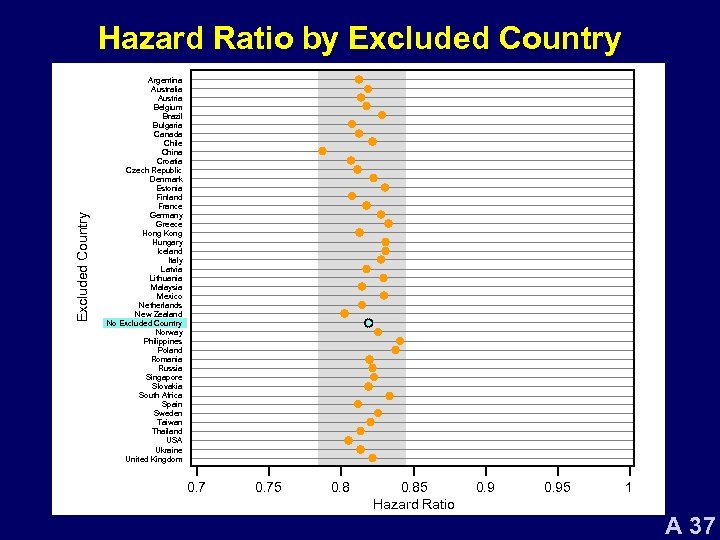 Excluded Country Hazard Ratio by Excluded Country Argentina Australia Austria Belgium Brazil Bulgaria Canada Chile China Croatia Czech Republic Denmark Estonia Finland France Germany Greece Hong Kong Hungary Iceland Italy Latvia Lithuania Malaysia Mexico Netherlands New Zealand No Excluded Country Norway Philippines Poland Romania Russia Singapore Slovakia South Africa Spain Sweden Taiwan Thailand USA Ukraine United Kingdom 0. 75 0. 85 Hazard Ratio 0. 95 1 A 37
Excluded Country Hazard Ratio by Excluded Country Argentina Australia Austria Belgium Brazil Bulgaria Canada Chile China Croatia Czech Republic Denmark Estonia Finland France Germany Greece Hong Kong Hungary Iceland Italy Latvia Lithuania Malaysia Mexico Netherlands New Zealand No Excluded Country Norway Philippines Poland Romania Russia Singapore Slovakia South Africa Spain Sweden Taiwan Thailand USA Ukraine United Kingdom 0. 75 0. 85 Hazard Ratio 0. 95 1 A 37
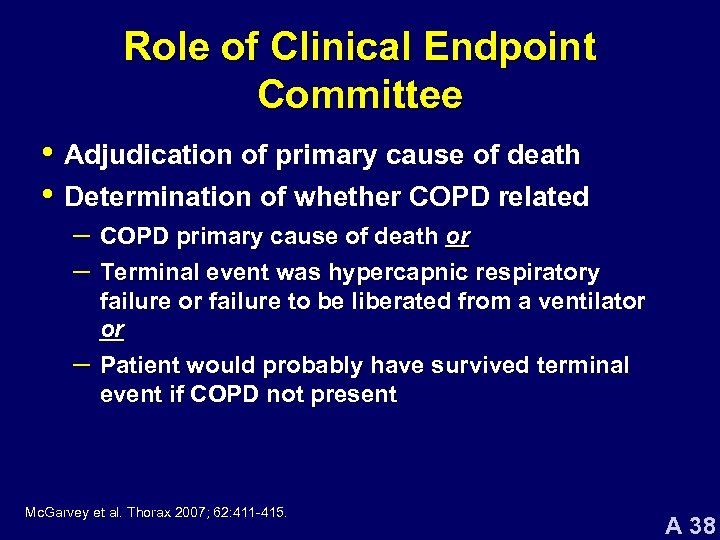 Role of Clinical Endpoint Committee • Adjudication of primary cause of death • Determination of whether COPD related – COPD primary cause of death or – Terminal event was hypercapnic respiratory – failure or failure to be liberated from a ventilator or Patient would probably have survived terminal event if COPD not present Mc. Garvey et al. Thorax 2007; 62: 411 -415. A 38
Role of Clinical Endpoint Committee • Adjudication of primary cause of death • Determination of whether COPD related – COPD primary cause of death or – Terminal event was hypercapnic respiratory – failure or failure to be liberated from a ventilator or Patient would probably have survived terminal event if COPD not present Mc. Garvey et al. Thorax 2007; 62: 411 -415. A 38
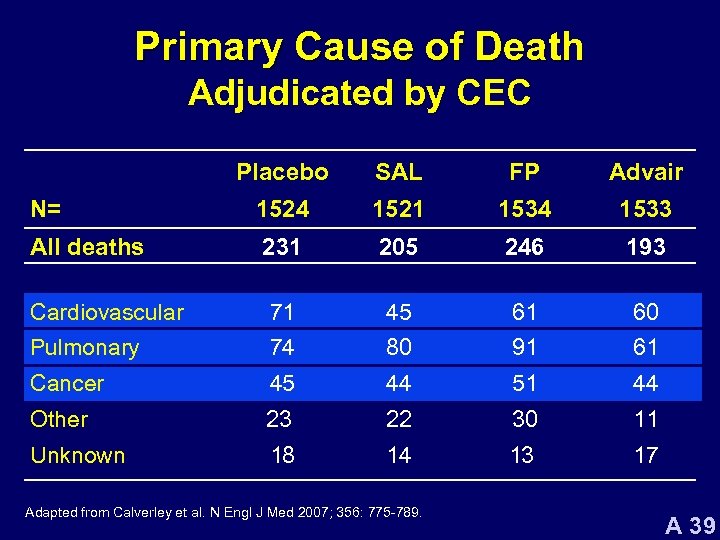 Primary Cause of Death Adjudicated by CEC Placebo SAL FP Advair N= 1524 1521 1534 1533 All deaths 231 205 246 193 Cardiovascular 71 45 61 60 Pulmonary 74 80 91 61 Cancer 45 44 51 44 Other 23 22 30 11 Unknown 18 14 13 17 Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. A 39
Primary Cause of Death Adjudicated by CEC Placebo SAL FP Advair N= 1524 1521 1534 1533 All deaths 231 205 246 193 Cardiovascular 71 45 61 60 Pulmonary 74 80 91 61 Cancer 45 44 51 44 Other 23 22 30 11 Unknown 18 14 13 17 Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. A 39
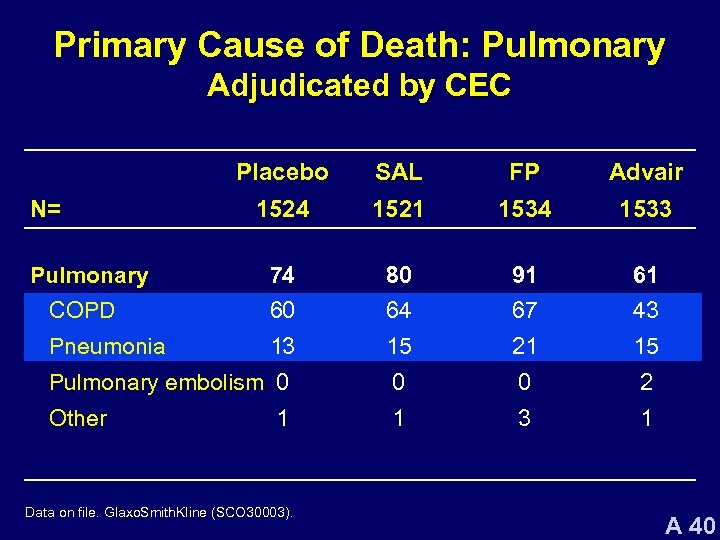 Primary Cause of Death: Pulmonary Adjudicated by CEC Placebo SAL FP Advair 1524 1521 1534 1533 74 80 91 61 COPD 60 64 67 43 Pneumonia 13 15 21 15 Pulmonary embolism 0 0 0 2 Other 1 3 1 N= Pulmonary 1 Data on file. Glaxo. Smith. Kline (SCO 30003). A 40
Primary Cause of Death: Pulmonary Adjudicated by CEC Placebo SAL FP Advair 1524 1521 1534 1533 74 80 91 61 COPD 60 64 67 43 Pneumonia 13 15 21 15 Pulmonary embolism 0 0 0 2 Other 1 3 1 N= Pulmonary 1 Data on file. Glaxo. Smith. Kline (SCO 30003). A 40
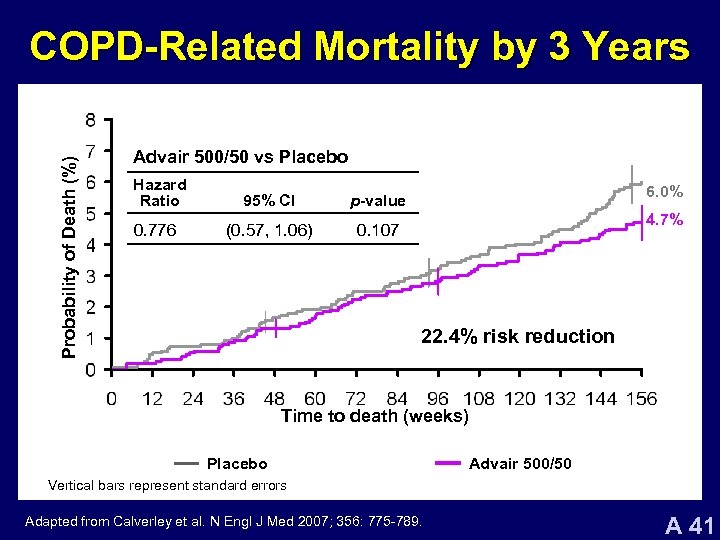 Probability of Death (%) COPD-Related Mortality by 3 Years Advair 500/50 vs Placebo Hazard Ratio 95% CI p-value 0. 776 (0. 57, 1. 06) 0. 107 6. 0% 4. 7% 22. 4% risk reduction Time to death (weeks) Placebo SAL FP Advair 500/50 Vertical bars represent standard errors Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. A 41
Probability of Death (%) COPD-Related Mortality by 3 Years Advair 500/50 vs Placebo Hazard Ratio 95% CI p-value 0. 776 (0. 57, 1. 06) 0. 107 6. 0% 4. 7% 22. 4% risk reduction Time to death (weeks) Placebo SAL FP Advair 500/50 Vertical bars represent standard errors Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. A 41
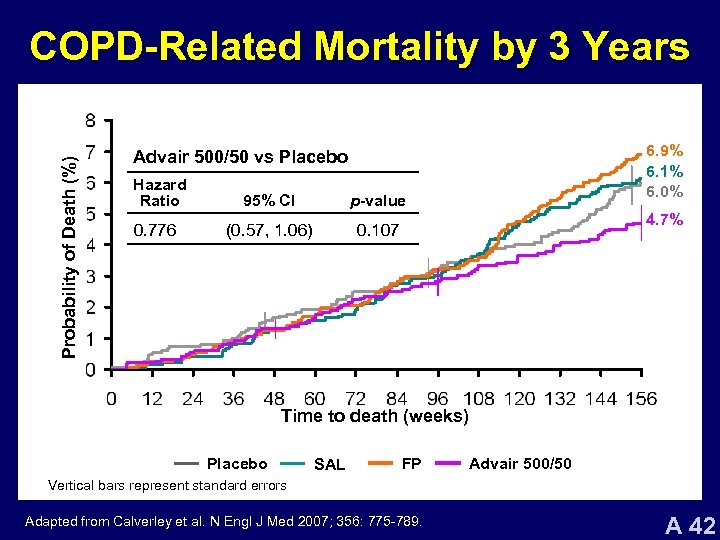 Probability of Death (%) COPD-Related Mortality by 3 Years 6. 9% 6. 1% 6. 0% Advair 500/50 vs Placebo Hazard Ratio 95% CI p-value 0. 776 (0. 57, 1. 06) 0. 107 4. 7% Time to death (weeks) Placebo SAL FP Advair 500/50 Vertical bars represent standard errors Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. A 42
Probability of Death (%) COPD-Related Mortality by 3 Years 6. 9% 6. 1% 6. 0% Advair 500/50 vs Placebo Hazard Ratio 95% CI p-value 0. 776 (0. 57, 1. 06) 0. 107 4. 7% Time to death (weeks) Placebo SAL FP Advair 500/50 Vertical bars represent standard errors Adapted from Calverley et al. N Engl J Med 2007; 356: 775 -789. A 42
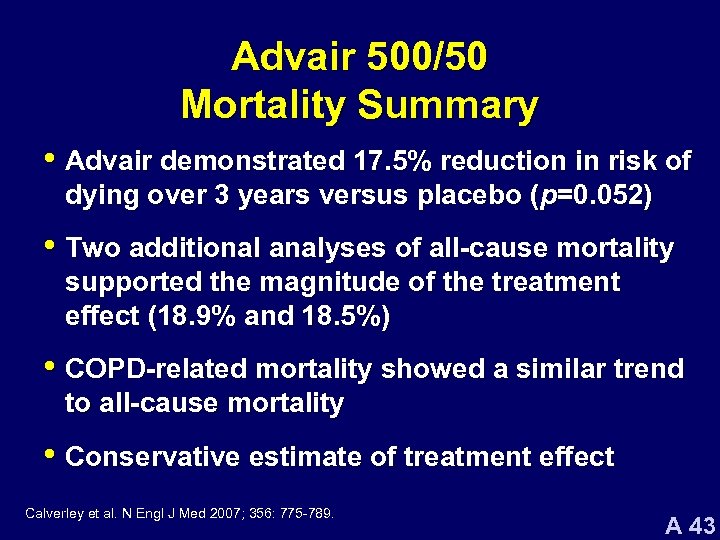 Advair 500/50 Mortality Summary • Advair demonstrated 17. 5% reduction in risk of dying over 3 years versus placebo (p=0. 052) • Two additional analyses of all-cause mortality supported the magnitude of the treatment effect (18. 9% and 18. 5%) • COPD-related mortality showed a similar trend to all-cause mortality • Conservative estimate of treatment effect Calverley et al. N Engl J Med 2007; 356: 775 -789. A 43
Advair 500/50 Mortality Summary • Advair demonstrated 17. 5% reduction in risk of dying over 3 years versus placebo (p=0. 052) • Two additional analyses of all-cause mortality supported the magnitude of the treatment effect (18. 9% and 18. 5%) • COPD-related mortality showed a similar trend to all-cause mortality • Conservative estimate of treatment effect Calverley et al. N Engl J Med 2007; 356: 775 -789. A 43
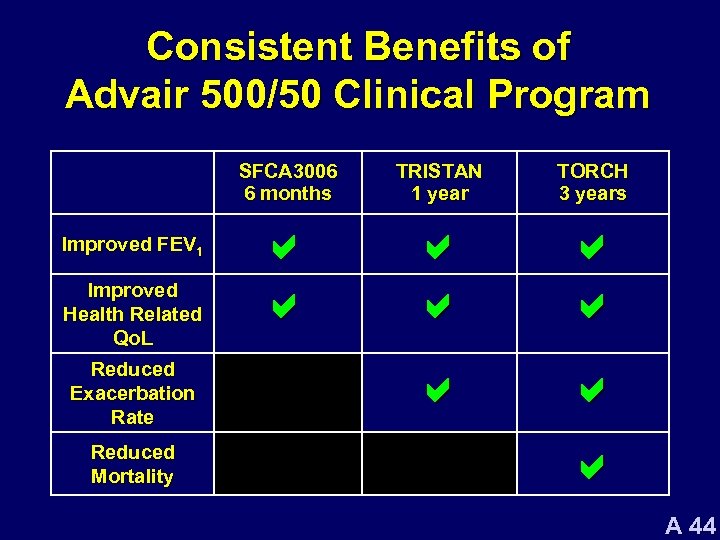 Consistent Benefits of Advair 500/50 Clinical Program SFCA 3006 6 months Improved FEV 1 Improved Health Related Qo. L Reduced Exacerbation Rate Reduced Mortality TRISTAN 1 year TORCH 3 years a a a a a A 44
Consistent Benefits of Advair 500/50 Clinical Program SFCA 3006 6 months Improved FEV 1 Improved Health Related Qo. L Reduced Exacerbation Rate Reduced Mortality TRISTAN 1 year TORCH 3 years a a a a a A 44
 Safety Profile of Advair 500/50 in Patients with COPD A 45
Safety Profile of Advair 500/50 in Patients with COPD A 45
 Safety Assessments • Adverse Event Reporting – AEs of Special Interest (for FP-containing arms) – Cardiovascular Adverse Events (for SAL-containing arms) • Prospective Safety Assessments – TORCH US Safety Sub-study – Cardiovascular and HPA-axis assessments from pivotal studies A 46
Safety Assessments • Adverse Event Reporting – AEs of Special Interest (for FP-containing arms) – Cardiovascular Adverse Events (for SAL-containing arms) • Prospective Safety Assessments – TORCH US Safety Sub-study – Cardiovascular and HPA-axis assessments from pivotal studies A 46
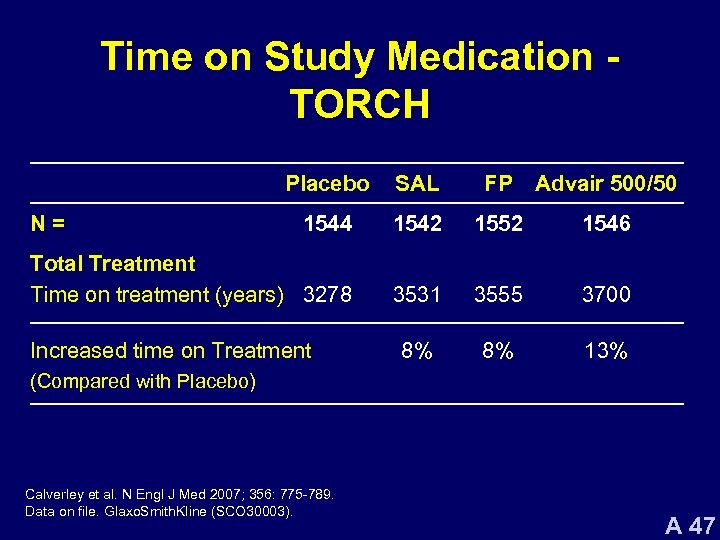 Time on Study Medication TORCH Placebo SAL FP Advair 500/50 1544 1542 1552 1546 Total Treatment Time on treatment (years) 3278 3531 3555 3700 8% 8% 13% N= Increased time on Treatment (Compared with Placebo) Calverley et al. N Engl J Med 2007; 356: 775 -789. Data on file. Glaxo. Smith. Kline (SCO 30003). A 47
Time on Study Medication TORCH Placebo SAL FP Advair 500/50 1544 1542 1552 1546 Total Treatment Time on treatment (years) 3278 3531 3555 3700 8% 8% 13% N= Increased time on Treatment (Compared with Placebo) Calverley et al. N Engl J Med 2007; 356: 775 -789. Data on file. Glaxo. Smith. Kline (SCO 30003). A 47
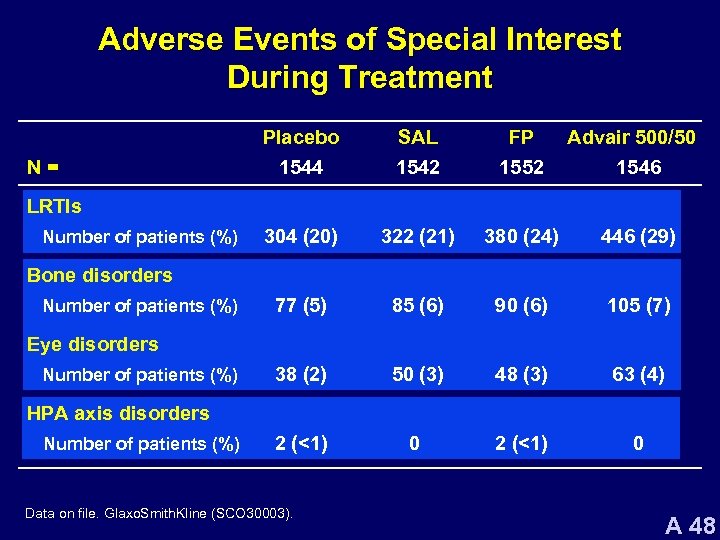 Adverse Events of Special Interest During Treatment N= Placebo 1544 SAL 1542 FP 1552 Advair 500/50 1546 304 (20) 322 (21) 380 (24) 446 (29) 77 (5) 85 (6) 90 (6) 105 (7) 38 (2) 50 (3) 48 (3) 63 (4) 2 (<1) 0 LRTIs Number of patients (%) Bone disorders Number of patients (%) Eye disorders Number of patients (%) HPA axis disorders Number of patients (%) Data on file. Glaxo. Smith. Kline (SCO 30003). A 48
Adverse Events of Special Interest During Treatment N= Placebo 1544 SAL 1542 FP 1552 Advair 500/50 1546 304 (20) 322 (21) 380 (24) 446 (29) 77 (5) 85 (6) 90 (6) 105 (7) 38 (2) 50 (3) 48 (3) 63 (4) 2 (<1) 0 LRTIs Number of patients (%) Bone disorders Number of patients (%) Eye disorders Number of patients (%) HPA axis disorders Number of patients (%) Data on file. Glaxo. Smith. Kline (SCO 30003). A 48
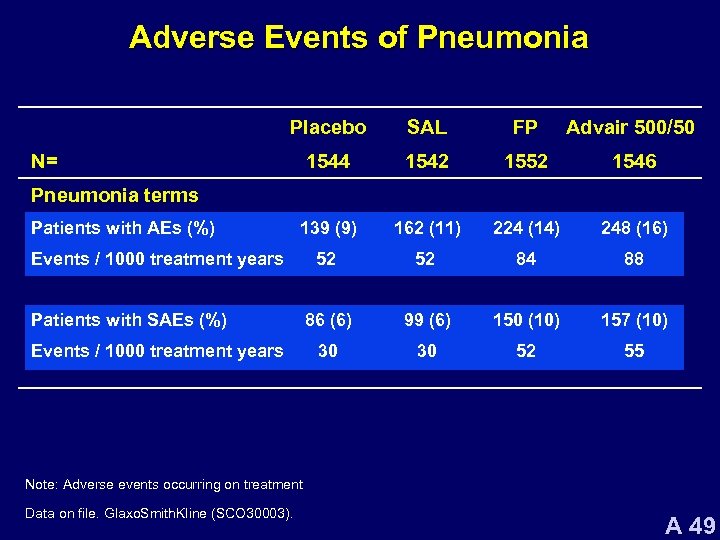 Adverse Events of Pneumonia Placebo SAL FP Advair 500/50 1544 1542 1552 1546 139 (9) 162 (11) 224 (14) 248 (16) 52 52 84 88 86 (6) 99 (6) 150 (10) 157 (10) 30 30 52 55 N= Pneumonia terms Patients with AEs (%) Events / 1000 treatment years Patients with SAEs (%) Events / 1000 treatment years Note: Adverse events occurring on treatment Data on file. Glaxo. Smith. Kline (SCO 30003). A 49
Adverse Events of Pneumonia Placebo SAL FP Advair 500/50 1544 1542 1552 1546 139 (9) 162 (11) 224 (14) 248 (16) 52 52 84 88 86 (6) 99 (6) 150 (10) 157 (10) 30 30 52 55 N= Pneumonia terms Patients with AEs (%) Events / 1000 treatment years Patients with SAEs (%) Events / 1000 treatment years Note: Adverse events occurring on treatment Data on file. Glaxo. Smith. Kline (SCO 30003). A 49
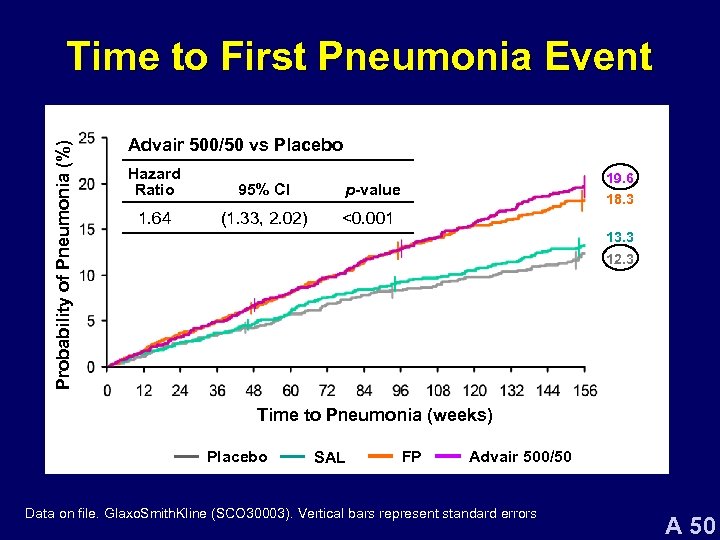 Probability of Pneumonia (%) Time to First Pneumonia Event Advair 500/50 vs Placebo Hazard Ratio 95% CI 1. 64 (1. 33, 2. 02) 19. 6 p-value 18. 3 <0. 001 13. 3 12. 3 Time to Pneumonia (weeks) Placebo SAL FP Advair 500/50 Data on file. Glaxo. Smith. Kline (SCO 30003). Vertical bars represent standard errors A 50
Probability of Pneumonia (%) Time to First Pneumonia Event Advair 500/50 vs Placebo Hazard Ratio 95% CI 1. 64 (1. 33, 2. 02) 19. 6 p-value 18. 3 <0. 001 13. 3 12. 3 Time to Pneumonia (weeks) Placebo SAL FP Advair 500/50 Data on file. Glaxo. Smith. Kline (SCO 30003). Vertical bars represent standard errors A 50
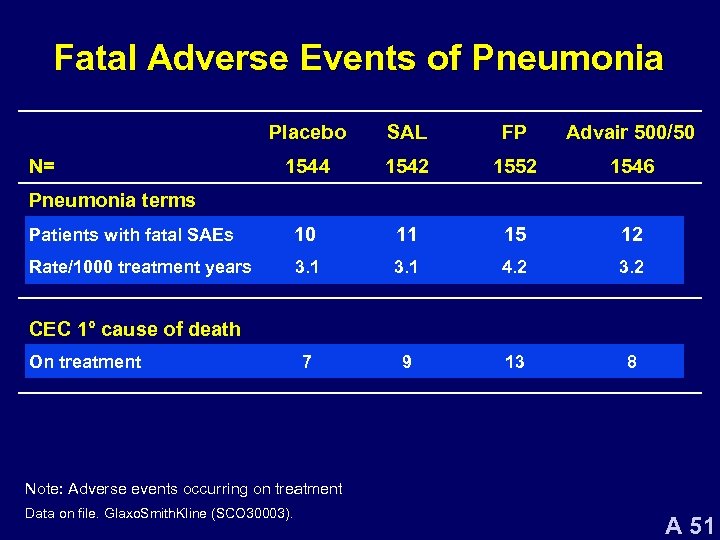 Fatal Adverse Events of Pneumonia Placebo SAL FP Advair 500/50 1544 1542 1552 1546 Patients with fatal SAEs 10 11 15 12 Rate/1000 treatment years 3. 1 4. 2 3. 2 7 9 13 8 N= Pneumonia terms CEC 1° cause of death On treatment Note: Adverse events occurring on treatment Data on file. Glaxo. Smith. Kline (SCO 30003). A 51
Fatal Adverse Events of Pneumonia Placebo SAL FP Advair 500/50 1544 1542 1552 1546 Patients with fatal SAEs 10 11 15 12 Rate/1000 treatment years 3. 1 4. 2 3. 2 7 9 13 8 N= Pneumonia terms CEC 1° cause of death On treatment Note: Adverse events occurring on treatment Data on file. Glaxo. Smith. Kline (SCO 30003). A 51
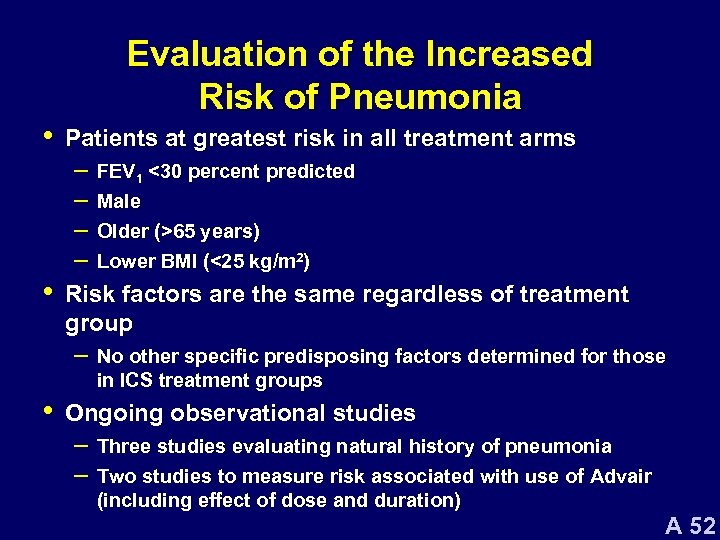 • • Evaluation of the Increased Risk of Pneumonia Patients at greatest risk in all treatment arms – FEV 1 <30 percent predicted – Male – Older (>65 years) – Lower BMI (<25 kg/m 2) Risk factors are the same regardless of treatment group – No other specific predisposing factors determined for those in ICS treatment groups • Ongoing observational studies – Three studies evaluating natural history of pneumonia – Two studies to measure risk associated with use of Advair (including effect of dose and duration) A 52
• • Evaluation of the Increased Risk of Pneumonia Patients at greatest risk in all treatment arms – FEV 1 <30 percent predicted – Male – Older (>65 years) – Lower BMI (<25 kg/m 2) Risk factors are the same regardless of treatment group – No other specific predisposing factors determined for those in ICS treatment groups • Ongoing observational studies – Three studies evaluating natural history of pneumonia – Two studies to measure risk associated with use of Advair (including effect of dose and duration) A 52
 Pneumonia Summary • • Increased risk in patients receiving Advair • No increase in pneumonia-related mortality with Advair versus placebo • • Pneumonias were a subset of exacerbations Seen in setting of reduced exacerbation rates, reduced hospitalizations and improved survival Labeling – Included in current labeling – Expanded in proposed labeling – Included in proposed medication guide A 53
Pneumonia Summary • • Increased risk in patients receiving Advair • No increase in pneumonia-related mortality with Advair versus placebo • • Pneumonias were a subset of exacerbations Seen in setting of reduced exacerbation rates, reduced hospitalizations and improved survival Labeling – Included in current labeling – Expanded in proposed labeling – Included in proposed medication guide A 53
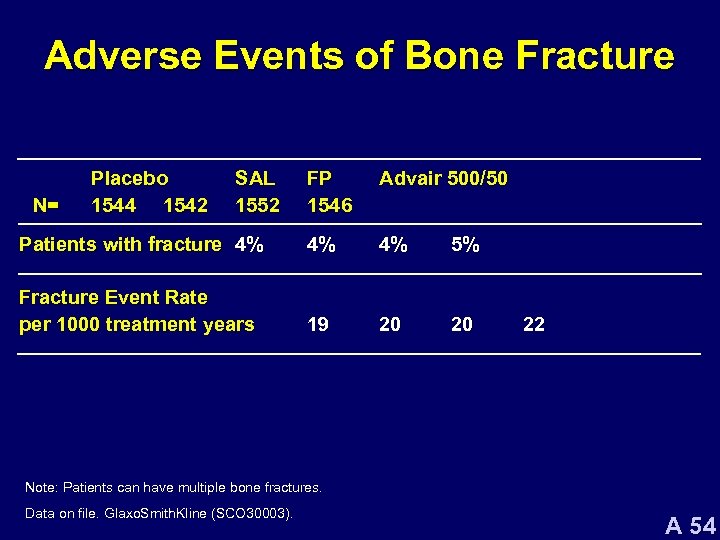 Adverse Events of Bone Fracture FP 1546 Advair 500/50 Patients with fracture 4% 4% 4% 5% Fracture Event Rate per 1000 treatment years 19 20 20 N= Placebo 1544 1542 SAL 1552 22 Note: Patients can have multiple bone fractures. Data on file. Glaxo. Smith. Kline (SCO 30003). A 54
Adverse Events of Bone Fracture FP 1546 Advair 500/50 Patients with fracture 4% 4% 4% 5% Fracture Event Rate per 1000 treatment years 19 20 20 N= Placebo 1544 1542 SAL 1552 22 Note: Patients can have multiple bone fractures. Data on file. Glaxo. Smith. Kline (SCO 30003). A 54
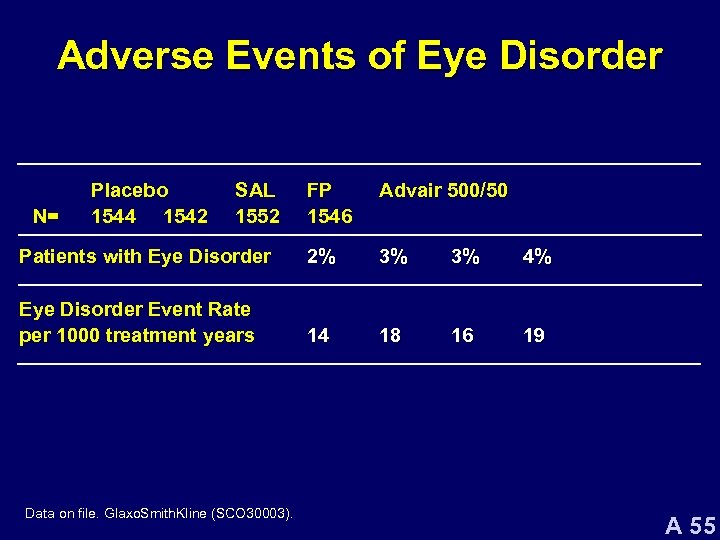 Adverse Events of Eye Disorder FP 1546 Advair 500/50 Patients with Eye Disorder 2% 3% 3% 4% Eye Disorder Event Rate per 1000 treatment years 14 18 16 19 N= Placebo 1544 1542 SAL 1552 Data on file. Glaxo. Smith. Kline (SCO 30003). A 55
Adverse Events of Eye Disorder FP 1546 Advair 500/50 Patients with Eye Disorder 2% 3% 3% 4% Eye Disorder Event Rate per 1000 treatment years 14 18 16 19 N= Placebo 1544 1542 SAL 1552 Data on file. Glaxo. Smith. Kline (SCO 30003). A 55
 Prospective Evaluation of Long-term Safety of FP – TORCH Sub-study • 88 sites in the US; 658 patients • Annual Assessments – Bone mineral density – Ophthalmic exams • All patients required to perform assessments A 56
Prospective Evaluation of Long-term Safety of FP – TORCH Sub-study • 88 sites in the US; 658 patients • Annual Assessments – Bone mineral density – Ophthalmic exams • All patients required to perform assessments A 56
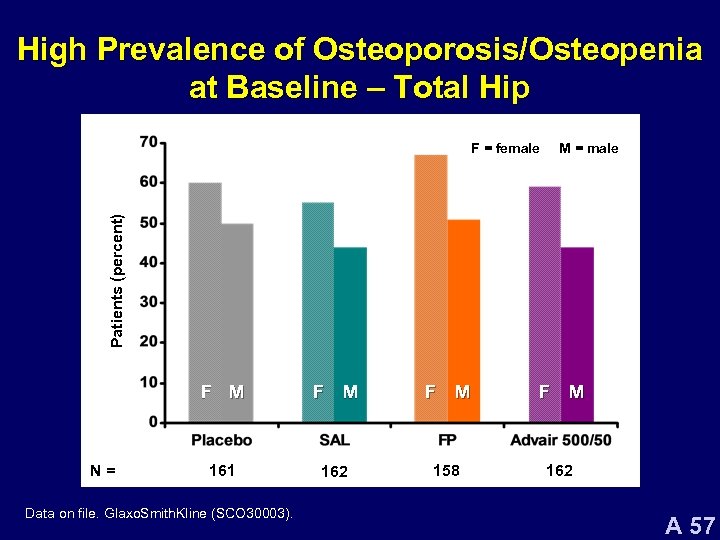 High Prevalence of Osteoporosis/Osteopenia at Baseline – Total Hip M = male Patients (percent) F = female F M N= F M 161 162 158 Data on file. Glaxo. Smith. Kline (SCO 30003). F M 162 A 57
High Prevalence of Osteoporosis/Osteopenia at Baseline – Total Hip M = male Patients (percent) F = female F M N= F M 161 162 158 Data on file. Glaxo. Smith. Kline (SCO 30003). F M 162 A 57
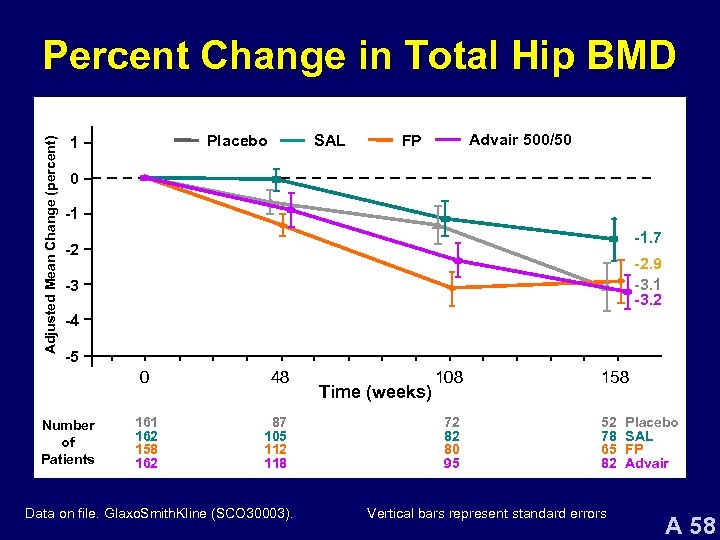 Adjusted Mean Change (percent) Percent Change in Total Hip BMD Placebo 1 SAL Advair 500/50 FP 0 -1 -1. 7 -2 -2. 9 -3. 1 -3. 2 -3 -4 -5 0 Number of Patients 161 162 158 162 48 87 105 112 118 Data on file. Glaxo. Smith. Kline (SCO 30003). Time (weeks) 108 72 82 80 95 158 52 78 65 82 Vertical bars represent standard errors Placebo SAL FP Advair A 58
Adjusted Mean Change (percent) Percent Change in Total Hip BMD Placebo 1 SAL Advair 500/50 FP 0 -1 -1. 7 -2 -2. 9 -3. 1 -3. 2 -3 -4 -5 0 Number of Patients 161 162 158 162 48 87 105 112 118 Data on file. Glaxo. Smith. Kline (SCO 30003). Time (weeks) 108 72 82 80 95 158 52 78 65 82 Vertical bars represent standard errors Placebo SAL FP Advair A 58
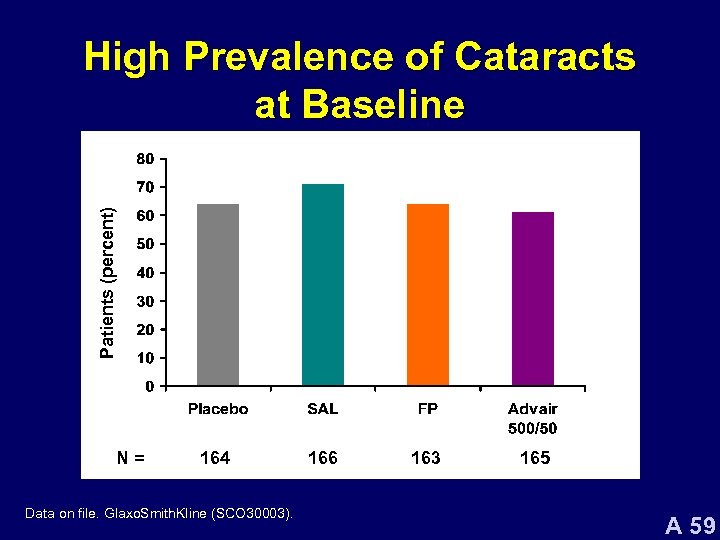 Patients (percent) High Prevalence of Cataracts at Baseline N= 164 Data on file. Glaxo. Smith. Kline (SCO 30003). 166 163 165 A 59
Patients (percent) High Prevalence of Cataracts at Baseline N= 164 Data on file. Glaxo. Smith. Kline (SCO 30003). 166 163 165 A 59
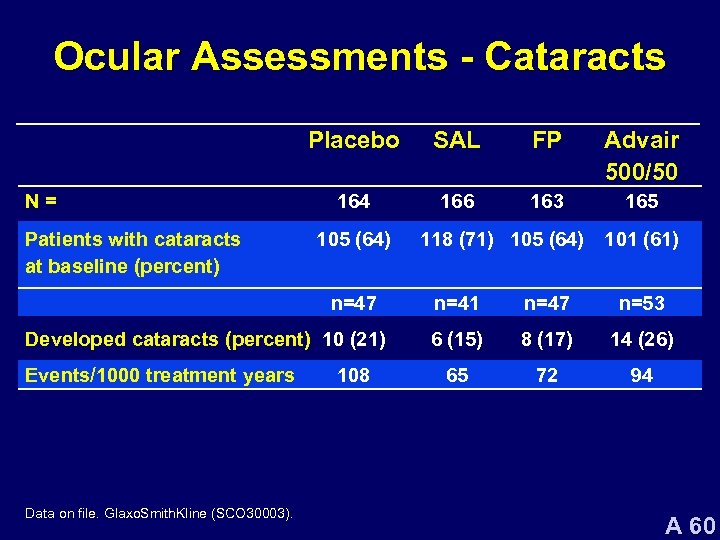 Ocular Assessments - Cataracts Placebo N= Patients with cataracts at baseline (percent) SAL FP Advair 500/50 164 166 163 165 105 (64) n=47 Developed cataracts (percent) 10 (21) Events/1000 treatment years Data on file. Glaxo. Smith. Kline (SCO 30003). 108 118 (71) 105 (64) 101 (61) n=41 n=47 n=53 6 (15) 8 (17) 14 (26) 65 72 94 A 60
Ocular Assessments - Cataracts Placebo N= Patients with cataracts at baseline (percent) SAL FP Advair 500/50 164 166 163 165 105 (64) n=47 Developed cataracts (percent) 10 (21) Events/1000 treatment years Data on file. Glaxo. Smith. Kline (SCO 30003). 108 118 (71) 105 (64) 101 (61) n=41 n=47 n=53 6 (15) 8 (17) 14 (26) 65 72 94 A 60
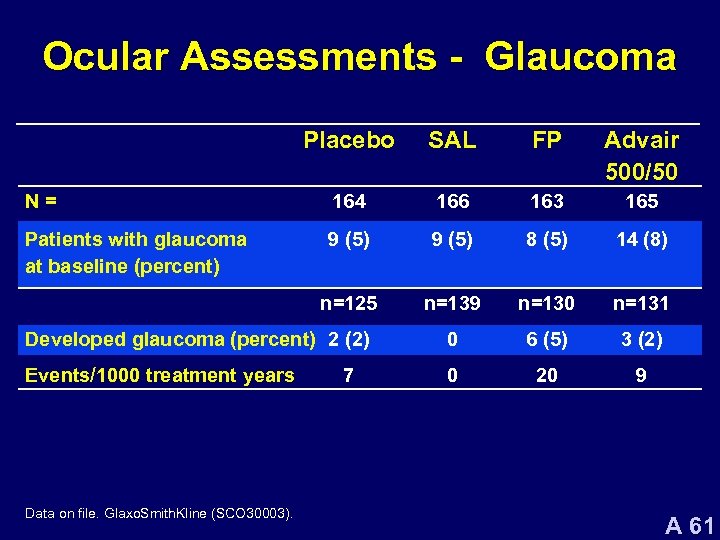 Ocular Assessments - Glaucoma Placebo SAL FP Advair 500/50 N= 164 166 163 165 Patients with glaucoma at baseline (percent) 9 (5) 8 (5) 14 (8) n=125 n=139 n=130 n=131 Developed glaucoma (percent) 2 (2) 0 6 (5) 3 (2) Events/1000 treatment years 0 20 9 Data on file. Glaxo. Smith. Kline (SCO 30003). 7 A 61
Ocular Assessments - Glaucoma Placebo SAL FP Advair 500/50 N= 164 166 163 165 Patients with glaucoma at baseline (percent) 9 (5) 8 (5) 14 (8) n=125 n=139 n=130 n=131 Developed glaucoma (percent) 2 (2) 0 6 (5) 3 (2) Events/1000 treatment years 0 20 9 Data on file. Glaxo. Smith. Kline (SCO 30003). 7 A 61
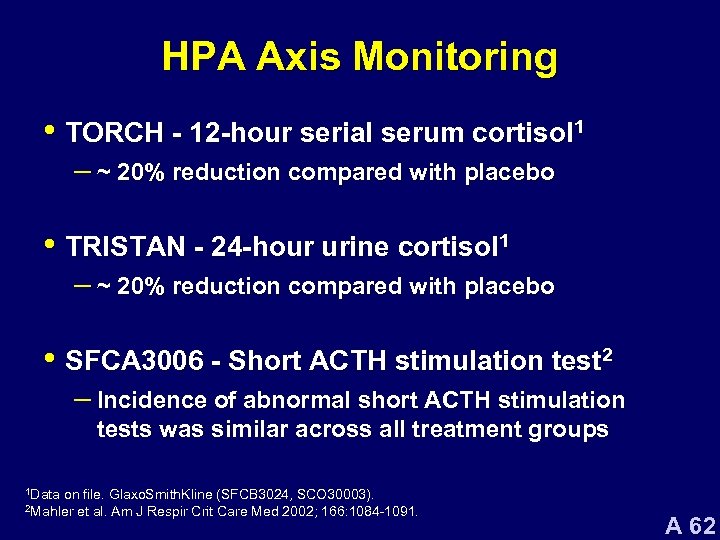 HPA Axis Monitoring • TORCH - 12 -hour serial serum cortisol 1 – ~ 20% reduction compared with placebo • TRISTAN - 24 -hour urine cortisol 1 – ~ 20% reduction compared with placebo • SFCA 3006 - Short ACTH stimulation test 2 – Incidence of abnormal short ACTH stimulation tests was similar across all treatment groups 1 Data on file. Glaxo. Smith. Kline (SFCB 3024, SCO 30003). et al. Am J Respir Crit Care Med 2002; 166: 1084 -1091. 2 Mahler A 62
HPA Axis Monitoring • TORCH - 12 -hour serial serum cortisol 1 – ~ 20% reduction compared with placebo • TRISTAN - 24 -hour urine cortisol 1 – ~ 20% reduction compared with placebo • SFCA 3006 - Short ACTH stimulation test 2 – Incidence of abnormal short ACTH stimulation tests was similar across all treatment groups 1 Data on file. Glaxo. Smith. Kline (SFCB 3024, SCO 30003). et al. Am J Respir Crit Care Med 2002; 166: 1084 -1091. 2 Mahler A 62
 Cardiac Safety • No increase of QTc prolongation or arrhythmias in SAL-containing treatment groups • No difference in incidence of ventricular ectopic events and changes in ECG rates across treatment groups • No increase in cardiac adverse event reporting Data on file. Glaxo. Smith. Kline (SFCA 3006, SFCB 3024, SCO 30003). A 63
Cardiac Safety • No increase of QTc prolongation or arrhythmias in SAL-containing treatment groups • No difference in incidence of ventricular ectopic events and changes in ECG rates across treatment groups • No increase in cardiac adverse event reporting Data on file. Glaxo. Smith. Kline (SFCA 3006, SFCB 3024, SCO 30003). A 63
 Benefit/Risk Profile of Advair 500/50 in Patients with COPD A 64
Benefit/Risk Profile of Advair 500/50 in Patients with COPD A 64
 Benefit/Risk Profile for Advair 500/50 in COPD Potential Risks • HPA - axis • Cardiac Effects • Bone and Eye Effects • Pneumonia – No clinically significant suppression – No increase in events in the clinical program – No clinically relevant differences – Increased reporting of pneumonia – No increased mortality from pneumonia – Labeling has detailed info for patients & physicians Data on file. Glaxo. Smith. Kline (SFCA 3006, SFCB 3024, SCO 30003). A 65
Benefit/Risk Profile for Advair 500/50 in COPD Potential Risks • HPA - axis • Cardiac Effects • Bone and Eye Effects • Pneumonia – No clinically significant suppression – No increase in events in the clinical program – No clinically relevant differences – Increased reporting of pneumonia – No increased mortality from pneumonia – Labeling has detailed info for patients & physicians Data on file. Glaxo. Smith. Kline (SFCA 3006, SFCB 3024, SCO 30003). A 65
 Benefit/Risk Profile for Advair 500/50 in COPD Benefits • Lung Function – Improvement vs Placebo, FP and SAL • Health-Related Quality of Life – Improvement vs Placebo, FP and SAL • Exacerbation Rate – Reduction vs Placebo, FP and SAL • Survival – 17. 5% reduction in all-cause mortality vs Placebo Data on file. Glaxo. Smith. Kline (SFCA 3006, SFCB 3024, SCO 30003). A 66
Benefit/Risk Profile for Advair 500/50 in COPD Benefits • Lung Function – Improvement vs Placebo, FP and SAL • Health-Related Quality of Life – Improvement vs Placebo, FP and SAL • Exacerbation Rate – Reduction vs Placebo, FP and SAL • Survival – 17. 5% reduction in all-cause mortality vs Placebo Data on file. Glaxo. Smith. Kline (SFCA 3006, SFCB 3024, SCO 30003). A 66
 Proposed Indication “ADVAIR DISKUS 500/50 is indicated for the twice-daily maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), which includes chronic bronchitis and emphysema, and to increase survival and reduce exacerbations in patients with forced expiratory volume in 1 second (FEV 1) <60% of predicted. ” A 67
Proposed Indication “ADVAIR DISKUS 500/50 is indicated for the twice-daily maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), which includes chronic bronchitis and emphysema, and to increase survival and reduce exacerbations in patients with forced expiratory volume in 1 second (FEV 1) <60% of predicted. ” A 67


