EARTH SCIENCES. GEOLOGY SCIENCE. ELEMENTS. Presented by: AZAMAT





































science.pptx
- Размер: 6.7 Мб
- Автор:
- Количество слайдов: 45
Описание презентации EARTH SCIENCES. GEOLOGY SCIENCE. ELEMENTS. Presented by: AZAMAT по слайдам
 EARTH SCIENCES. GEOLOGY SCIENCE. ELEMENTS. Presented by: AZAMAT ABAY ZARINA ABDIKAMAL ABDIKARIMOV ADILBEK NAZIRA ABDIMANAP
EARTH SCIENCES. GEOLOGY SCIENCE. ELEMENTS. Presented by: AZAMAT ABAY ZARINA ABDIKAMAL ABDIKARIMOV ADILBEK NAZIRA ABDIMANAP
 WHAT IS EARTH SCIENCE? The study of Earth’s interior, rocks and soil, atmosphere, oceans and outer space.
WHAT IS EARTH SCIENCE? The study of Earth’s interior, rocks and soil, atmosphere, oceans and outer space.
 SEARTH SCIENCE IS ABOUT CONNECTIONS Today scientists look more for the connections among the different parts of the Earth- its oceans, atmosphere, living things and rocks and soil.
SEARTH SCIENCE IS ABOUT CONNECTIONS Today scientists look more for the connections among the different parts of the Earth- its oceans, atmosphere, living things and rocks and soil.
 HEAT ENERGY FROM INSIDE THE EARTH AND RADIATION FROM THE SUN PROVIDE ENERGY FOR EARTH’S PROCESSES. • EARTH SCIENCE VOCABULARY • Energy — the ability to cause change. • Evaporation — the process by which liquid changes into gas. • Convection — hot material rises, cools, then sinks until it is heated enough to rise again. • Radiation — energy that moves in certain types of waves.
HEAT ENERGY FROM INSIDE THE EARTH AND RADIATION FROM THE SUN PROVIDE ENERGY FOR EARTH’S PROCESSES. • EARTH SCIENCE VOCABULARY • Energy — the ability to cause change. • Evaporation — the process by which liquid changes into gas. • Convection — hot material rises, cools, then sinks until it is heated enough to rise again. • Radiation — energy that moves in certain types of waves.
 WHY IS EARTH SCIENCE IMPORTANT? Understanding Earth’s processes makes it possible to: • Know what types of crops to plant and when to plant them • Know when to watch for dangerous weather • Predict a volcano’s eruption in time to save people
WHY IS EARTH SCIENCE IMPORTANT? Understanding Earth’s processes makes it possible to: • Know what types of crops to plant and when to plant them • Know when to watch for dangerous weather • Predict a volcano’s eruption in time to save people
 CHAPTER 1: VIEWS OF EARTH TODAYBIG IDEA: Modern technology has changed the way we view and map the Earth. KEY CONCEPT 1. 1: Technology is used to explore the Earth system.
CHAPTER 1: VIEWS OF EARTH TODAYBIG IDEA: Modern technology has changed the way we view and map the Earth. KEY CONCEPT 1. 1: Technology is used to explore the Earth system.
 THE EARTH SYSTEM Has four major parts: Biosphere Hydrosphere Geosphere Atmosphere
THE EARTH SYSTEM Has four major parts: Biosphere Hydrosphere Geosphere Atmosphere
 The Four Parts of the Earth System — an organized group of parts that work together to form a whole. ATMOSPHERE : THE MIXTURE OF GASES THAT SURROUNDS AND PROTECTS THE EARTH COMPOSITION: • NITROGEN (78%) • OXYGEN (21%) • CARBON DIOXIDE • WATER VAPOR • OTHER GASES STUDIED WITH: • WEATHER BALLONS • PLANES • SATELLITES
The Four Parts of the Earth System — an organized group of parts that work together to form a whole. ATMOSPHERE : THE MIXTURE OF GASES THAT SURROUNDS AND PROTECTS THE EARTH COMPOSITION: • NITROGEN (78%) • OXYGEN (21%) • CARBON DIOXIDE • WATER VAPOR • OTHER GASES STUDIED WITH: • WEATHER BALLONS • PLANES • SATELLITES
 The Four Parts of the Earth System HYDROSPHERE : MADE UP OF ALL THE WATER ON THE EARTH (OCEANS, LAKES, GLACIERS, RIVERS, UNDERGROUND ) WATER COVERS ¾ OF THE EARTH’S SURFACE • ONLY 3% IS FRESH WATER • NEARLY 70% IS IN GLACIERS AND POLAR ICE CAPS STUDIED WITH: • DEAP –SEA VEHICLES • BUOYS • SATELLITE IMAGES • DIVING SUITS AFFECTS THE ATMOSPHERE WHEN WARM/COLD OCEAN CURRENTS INTERACT WITH WIND CURRENTS
The Four Parts of the Earth System HYDROSPHERE : MADE UP OF ALL THE WATER ON THE EARTH (OCEANS, LAKES, GLACIERS, RIVERS, UNDERGROUND ) WATER COVERS ¾ OF THE EARTH’S SURFACE • ONLY 3% IS FRESH WATER • NEARLY 70% IS IN GLACIERS AND POLAR ICE CAPS STUDIED WITH: • DEAP –SEA VEHICLES • BUOYS • SATELLITE IMAGES • DIVING SUITS AFFECTS THE ATMOSPHERE WHEN WARM/COLD OCEAN CURRENTS INTERACT WITH WIND CURRENTS
 The Four Parts of the Earth System BIOSPHERE: INCLUDES ALL LIFE ON EARTH (IN THE AIR, WATER, AND ON LAND) STUDIED WITH: • SATELLITE PHOTOS TRACK YEARLY CHANGES IN PLANT AND ANIMAL LIFE • SPECIAL EQUIPMENT ALLOWS SCIENTISTS TO STUDY PLACES WITHOUT HARMING THEM WHEN TREES EXCHANGE GASES THE BIOSPHERE INTERACTS WITH THE ATMOSPHER
The Four Parts of the Earth System BIOSPHERE: INCLUDES ALL LIFE ON EARTH (IN THE AIR, WATER, AND ON LAND) STUDIED WITH: • SATELLITE PHOTOS TRACK YEARLY CHANGES IN PLANT AND ANIMAL LIFE • SPECIAL EQUIPMENT ALLOWS SCIENTISTS TO STUDY PLACES WITHOUT HARMING THEM WHEN TREES EXCHANGE GASES THE BIOSPHERE INTERACTS WITH THE ATMOSPHER
 The Four Parts of the Earth System GEOSPHERE: ALL FEATURES ON THE EARTH’S SURFACE (CRUST) ALONG WITH THE MANTLE, INNER AND OUTER CORES. STUDIED WITH: • SATELLITE IMAGES • SOUND WAVES • COMPUTER MODELING MATTER MOVES FROM THE GEOSPHERE TO THE ATMOSPHERE WHEN VOLCANOES EXPLO
The Four Parts of the Earth System GEOSPHERE: ALL FEATURES ON THE EARTH’S SURFACE (CRUST) ALONG WITH THE MANTLE, INNER AND OUTER CORES. STUDIED WITH: • SATELLITE IMAGES • SOUND WAVES • COMPUTER MODELING MATTER MOVES FROM THE GEOSPHERE TO THE ATMOSPHERE WHEN VOLCANOES EXPLO
 ALL FOUR PARTS OF THE SYSTEM SHAPE THE PLANET’S SURFACE • Atmosphere and Hydrosphere- Not even the hardest stone can withstand the forces of wind and water. Over millions of years rain, wind, and moving water carved the Grand Canyon.
ALL FOUR PARTS OF THE SYSTEM SHAPE THE PLANET’S SURFACE • Atmosphere and Hydrosphere- Not even the hardest stone can withstand the forces of wind and water. Over millions of years rain, wind, and moving water carved the Grand Canyon.
 • Geosphere: Landmasses pushing together have set off earthquakes and formed volcanoes and mountain ranges like the Andes around the world.
• Geosphere: Landmasses pushing together have set off earthquakes and formed volcanoes and mountain ranges like the Andes around the world.
 • Biosphere: Plants, animals and humans have also changed the Earth. Walking or riding a bike over open land changes the surface of the planet.
• Biosphere: Plants, animals and humans have also changed the Earth. Walking or riding a bike over open land changes the surface of the planet.
 GEOLOGY SCIEN
GEOLOGY SCIEN
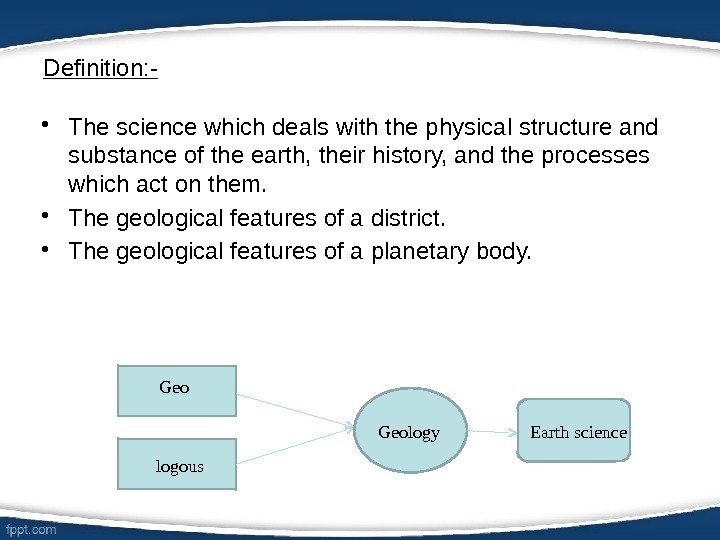
 Definition: — • The science which deals with the physical structure and substance of the earth, their history, and the processes which act on them. • The geological features of a district. • The geological features of a planetary body. Geo logous Geology Earth science
Definition: — • The science which deals with the physical structure and substance of the earth, their history, and the processes which act on them. • The geological features of a district. • The geological features of a planetary body. Geo logous Geology Earth science
 The importance of geology in civil engineering may briefly as follows: • Geology provides a systematic knowledge of construction material, its occurrence, composition, durability and other properties. Example of such construction materials is building stones, road metal, clay, limestones and laterite.
The importance of geology in civil engineering may briefly as follows: • Geology provides a systematic knowledge of construction material, its occurrence, composition, durability and other properties. Example of such construction materials is building stones, road metal, clay, limestones and laterite.
 • The knowledge of the geological work of natural agencies such as water, wind, ice and earthquakes helps in planning and carrying out major civil engineering works. For example the knowledge of erosion, transportation and deposition helps greatly in solving the expensive problems of river control, coastal and soil conservation.
• The knowledge of the geological work of natural agencies such as water, wind, ice and earthquakes helps in planning and carrying out major civil engineering works. For example the knowledge of erosion, transportation and deposition helps greatly in solving the expensive problems of river control, coastal and soil conservation.
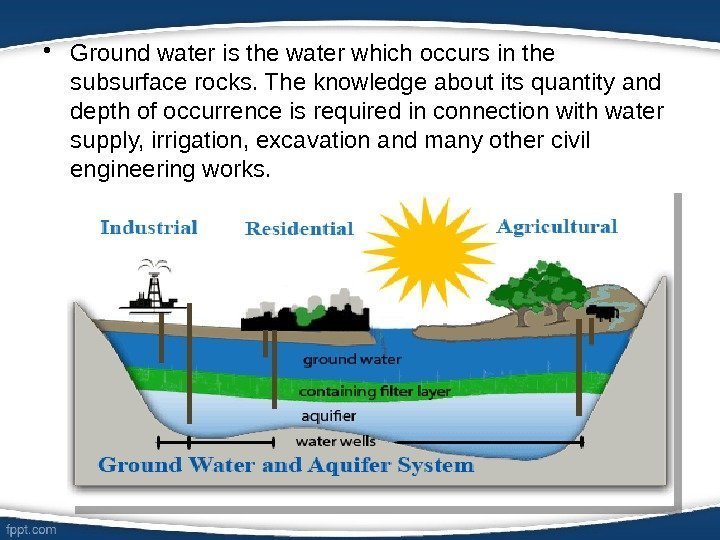
 • Ground water is the water which occurs in the subsurface rocks. The knowledge about its quantity and depth of occurrence is required in connection with water supply, irrigation, excavation and many other civil engineering works.
• Ground water is the water which occurs in the subsurface rocks. The knowledge about its quantity and depth of occurrence is required in connection with water supply, irrigation, excavation and many other civil engineering works.
 • The foundation problems of dams, bridges and buildings are directly concerned with the geology of the area where they are to be built. In these works drilling is commonly undertaken to explore the ground conditions. Geology helps greatly in interpreting the drilling data. • In tunneling, constructing roads, canals, docks and in determining the stability of cuts and slopes, the knowledge about the nature and structure of rocks is very necessary. • Before staring a major engineering project at a place, a detailed geological report which is accompanied by geological maps and sections, is prepared. Such a report helps in planning and constructing the projects.
• The foundation problems of dams, bridges and buildings are directly concerned with the geology of the area where they are to be built. In these works drilling is commonly undertaken to explore the ground conditions. Geology helps greatly in interpreting the drilling data. • In tunneling, constructing roads, canals, docks and in determining the stability of cuts and slopes, the knowledge about the nature and structure of rocks is very necessary. • Before staring a major engineering project at a place, a detailed geological report which is accompanied by geological maps and sections, is prepared. Such a report helps in planning and constructing the projects.
 Physical Geology: • Physical Geology uses the scientific method to explain natural aspects of the Earth — for example, how mountains form or why oil resources are concentrated in some rocks and not in others. • This chapter briefly explains how and why Earth’s surface, and its interior, are constantly changing. It relates this constant change to the major geological topics of interaction of the atmosphere, water and rock.
Physical Geology: • Physical Geology uses the scientific method to explain natural aspects of the Earth — for example, how mountains form or why oil resources are concentrated in some rocks and not in others. • This chapter briefly explains how and why Earth’s surface, and its interior, are constantly changing. It relates this constant change to the major geological topics of interaction of the atmosphere, water and rock.

 Petrology: — • Petrology is the branch of geology that studies the origin, composition, distribution and structure of rocks. • (from the Greek language : petra — «rock» and logos — «study») • “ Lithology” was once approximately synonymous with petrography, but in current usage, lithology focuses on macroscopic hand-sample or outcrop-scale description of rocks while petrography is the specialty that deals with microscopic details.
Petrology: — • Petrology is the branch of geology that studies the origin, composition, distribution and structure of rocks. • (from the Greek language : petra — «rock» and logos — «study») • “ Lithology” was once approximately synonymous with petrography, but in current usage, lithology focuses on macroscopic hand-sample or outcrop-scale description of rocks while petrography is the specialty that deals with microscopic details.
 Branches: • There are three branches of petrology, corresponding to the three types of rocks: Igneous, metamorphic, and sedimentary. • Igneous petrology focuses on the composition and texture of igneous rocks (rocks such as granite or basalt which have crystallized from molten rock or magma). Igneous rocks include volcanic and plutonic rocks.
Branches: • There are three branches of petrology, corresponding to the three types of rocks: Igneous, metamorphic, and sedimentary. • Igneous petrology focuses on the composition and texture of igneous rocks (rocks such as granite or basalt which have crystallized from molten rock or magma). Igneous rocks include volcanic and plutonic rocks.
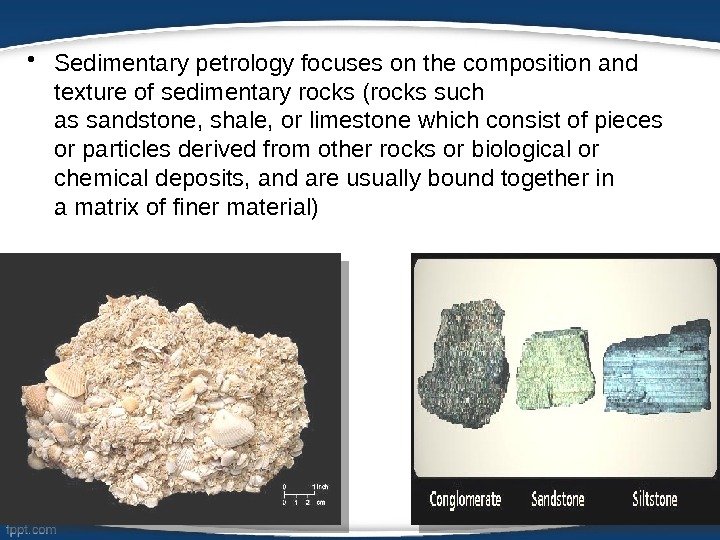
 • Sedimentary petrology focuses on the composition and texture of sedimentary rocks (rocks such as sandstone, shale, or limestone which consist of pieces or particles derived from other rocks or biological or chemical deposits, and are usually bound together in a matrix of finer material)
• Sedimentary petrology focuses on the composition and texture of sedimentary rocks (rocks such as sandstone, shale, or limestone which consist of pieces or particles derived from other rocks or biological or chemical deposits, and are usually bound together in a matrix of finer material)
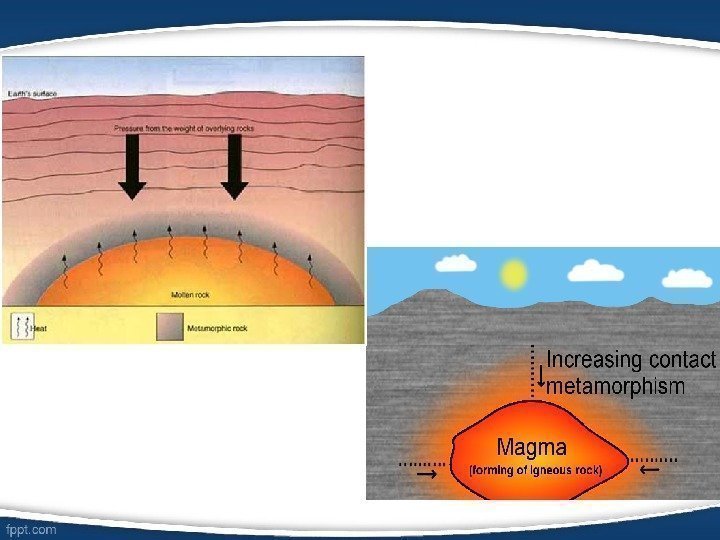
 • Metamorphic petrology focuses on the composition and texture of metamorphic rocks (rocks such as slate, marble, gneiss, or schist which started out as sedimentary or igneous rocks but which have undergone chemical, mineralogical or textural changes due to extremes of pressure, temperature or both). • Metamorphic rocks arise from the transformation of existing rock types, in a process called metamorphism, which means «change in form». The original rock (protolith) is subjected to heat (temperatures greater than 150 to 200 °C) causing profound physical and/or chemical change.
• Metamorphic petrology focuses on the composition and texture of metamorphic rocks (rocks such as slate, marble, gneiss, or schist which started out as sedimentary or igneous rocks but which have undergone chemical, mineralogical or textural changes due to extremes of pressure, temperature or both). • Metamorphic rocks arise from the transformation of existing rock types, in a process called metamorphism, which means «change in form». The original rock (protolith) is subjected to heat (temperatures greater than 150 to 200 °C) causing profound physical and/or chemical change.

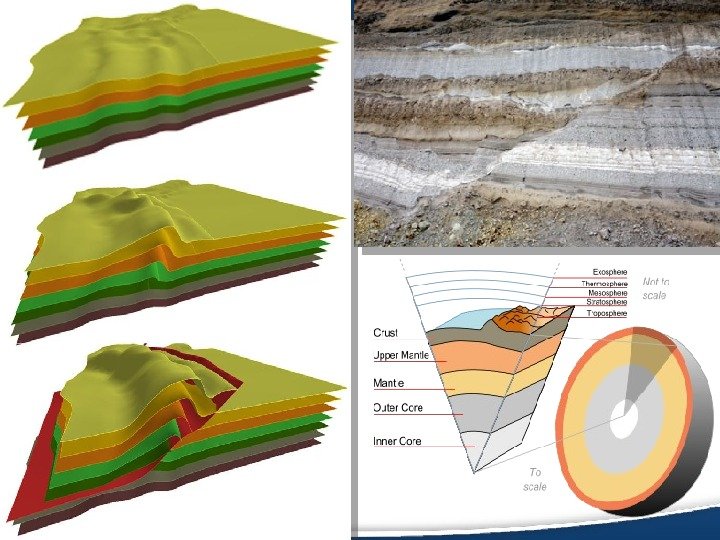
 Structural geology: • Structural geology is the study of the three-dimensional distribution of rock units with respect to their deformational histories. • The primary goal of structural geology is to use measurements of present-day rock geometries to uncover information about the history of deformation (strain) in the rocks, and ultimately, to understand the stress field that resulted in the observed strain and geometries.
Structural geology: • Structural geology is the study of the three-dimensional distribution of rock units with respect to their deformational histories. • The primary goal of structural geology is to use measurements of present-day rock geometries to uncover information about the history of deformation (strain) in the rocks, and ultimately, to understand the stress field that resulted in the observed strain and geometries.

 Weathering of Rocks: • Weathering breaks down and loosens the surface minerals of rock so they can be transported away by agents of erosion such as water, wind and ice. • There are two types of weathering: mechanical and chemical. • Mechanical or physical weathering involves the breakdown of rocks and soils through direct contact with atmospheric conditions, such as heat, water, ice and pressure. • The second classification, chemical weathering involves the direct effect of atmospheric chemicals or biologically produced chemicals also known as biological weathering in the breakdown of rocks, soils and minerals.
Weathering of Rocks: • Weathering breaks down and loosens the surface minerals of rock so they can be transported away by agents of erosion such as water, wind and ice. • There are two types of weathering: mechanical and chemical. • Mechanical or physical weathering involves the breakdown of rocks and soils through direct contact with atmospheric conditions, such as heat, water, ice and pressure. • The second classification, chemical weathering involves the direct effect of atmospheric chemicals or biologically produced chemicals also known as biological weathering in the breakdown of rocks, soils and minerals.

 Elements found in the Earth’s Crust
Elements found in the Earth’s Crust
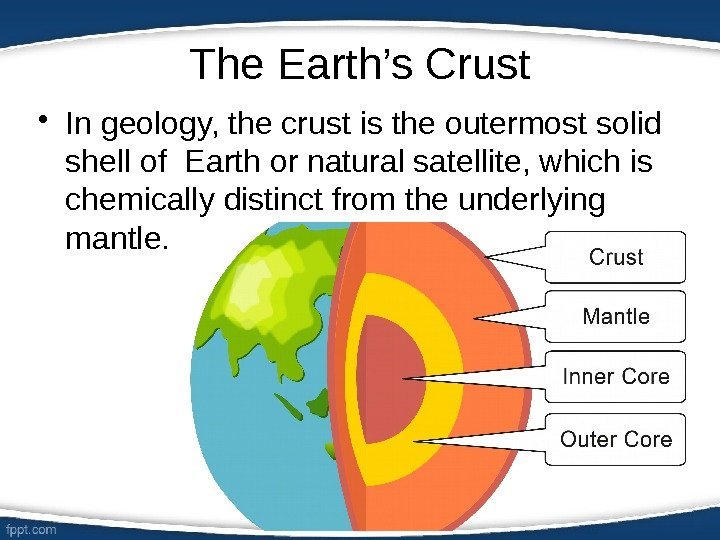
 The Earth’s Crust • In geology, the crust is the outermost solid shell of Earth or natural satellite, which is chemically distinct from the underlying mantle.
The Earth’s Crust • In geology, the crust is the outermost solid shell of Earth or natural satellite, which is chemically distinct from the underlying mantle.
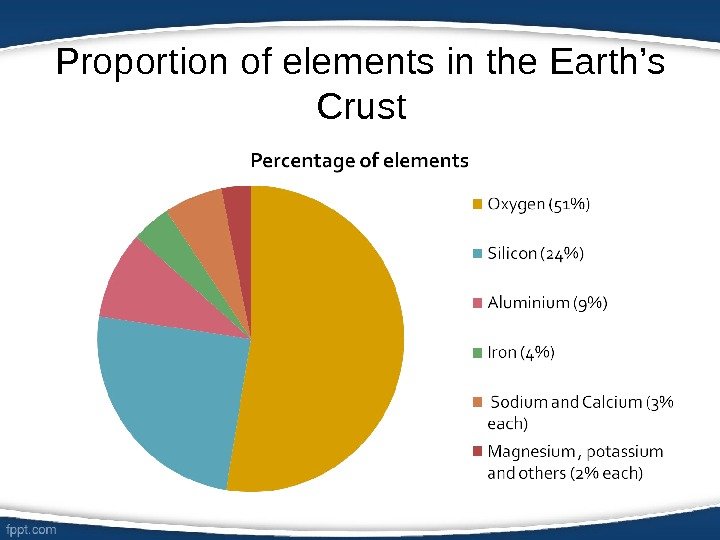
 Proportion of elements in the Earth’s Crust
Proportion of elements in the Earth’s Crust
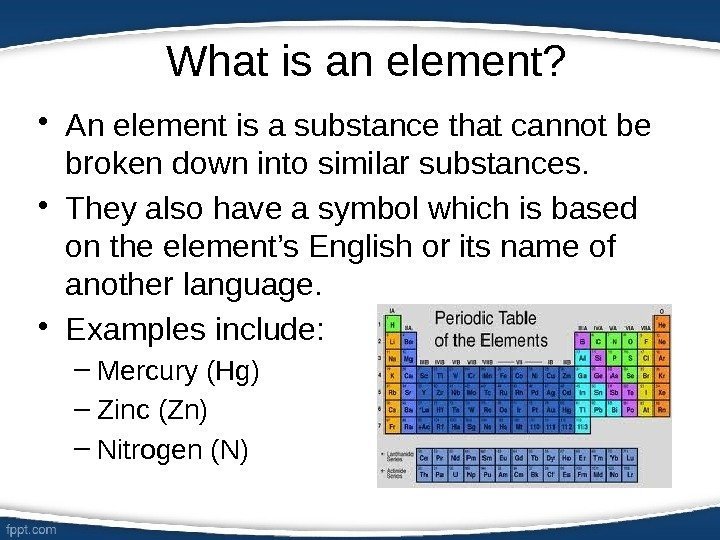
 What is an element? • An element is a substance that cannot be broken down into similar substances. • They also have a symbol which is based on the element’s English or its name of another language. • Examples include: – Mercury (Hg) – Zinc (Zn) – Nitrogen (N)
What is an element? • An element is a substance that cannot be broken down into similar substances. • They also have a symbol which is based on the element’s English or its name of another language. • Examples include: – Mercury (Hg) – Zinc (Zn) – Nitrogen (N)
 Oxygen • Chemical symbol: O • Physical state: Gas • Colour: Colourless • Uses- Combustion and needed for respiration • Interesting facts: – First discovered by Carl Wilhelm Scheele in Sweden in 1771 – Constitutes 21 % of the atmosphere – Found in water, in most rocks and minerals
Oxygen • Chemical symbol: O • Physical state: Gas • Colour: Colourless • Uses- Combustion and needed for respiration • Interesting facts: – First discovered by Carl Wilhelm Scheele in Sweden in 1771 – Constitutes 21 % of the atmosphere – Found in water, in most rocks and minerals
 Silicon • Chemical symbol: Si • Physical state: Solid • Colour: Blackish- silverish • Uses- Used in glass, ceramics, computers, calculators and cement • Interesting facts: – First discovered by Antoine Lavoisier in 1787 – Found in sand – The name originates from the Latin word ‘silex‘.
Silicon • Chemical symbol: Si • Physical state: Solid • Colour: Blackish- silverish • Uses- Used in glass, ceramics, computers, calculators and cement • Interesting facts: – First discovered by Antoine Lavoisier in 1787 – Found in sand – The name originates from the Latin word ‘silex‘.
 Aluminium • Chemical symbol: Al • Physical state: Solid • Colour: Silver • Uses- Used in airplanes, packaging and cooking equipment. • Interesting facts: – First discovered by Hans Christian Oersted in 1825. – The name originates from the Latin word ‘alumen‘
Aluminium • Chemical symbol: Al • Physical state: Solid • Colour: Silver • Uses- Used in airplanes, packaging and cooking equipment. • Interesting facts: – First discovered by Hans Christian Oersted in 1825. – The name originates from the Latin word ‘alumen‘
 Iron • Chemical symbol: Fe • Physical state: Solid • Colour: Silver • Uses- Used in automobiles, machines the ships and buildings. • Interesting facts: – First discovered by the Egyptians in 4000 BC. – One of the most commonly used metals. – Found in meteorites and in most igneous rocks.
Iron • Chemical symbol: Fe • Physical state: Solid • Colour: Silver • Uses- Used in automobiles, machines the ships and buildings. • Interesting facts: – First discovered by the Egyptians in 4000 BC. – One of the most commonly used metals. – Found in meteorites and in most igneous rocks.
 Sodium • Chemical Symbol: Na • Physical state: Solid • Colour: Silvery-white • Uses- Used in common salt, soap and fizzy drinks. • Interesting facts: – Reacts explosively to water. – Found in many compounds and stars.
Sodium • Chemical Symbol: Na • Physical state: Solid • Colour: Silvery-white • Uses- Used in common salt, soap and fizzy drinks. • Interesting facts: – Reacts explosively to water. – Found in many compounds and stars.
 • Chemical Symbol: Ca • Physical state: Solid • Colour: Silver • Uses- Used in plaster, quicklime, cement, and metallurgic and electronic materials. • Interesting facts: – Found naturally in limestone, gypsum, and fluorite. – The name originates from the Latin word ‘calcis‘. Calcium
• Chemical Symbol: Ca • Physical state: Solid • Colour: Silver • Uses- Used in plaster, quicklime, cement, and metallurgic and electronic materials. • Interesting facts: – Found naturally in limestone, gypsum, and fluorite. – The name originates from the Latin word ‘calcis‘. Calcium
 Magnesium • Chemical Symbol: Mg • Physical state: Solid • Colour: Silvery-white • Uses- Used in structural alloys, pyrotechnics, flash photography, and incendiary bombs. • Interesting facts: – It is widely distributed in rock structures and highly reactive. – The name originates from a Greek district called Magnesia.
Magnesium • Chemical Symbol: Mg • Physical state: Solid • Colour: Silvery-white • Uses- Used in structural alloys, pyrotechnics, flash photography, and incendiary bombs. • Interesting facts: – It is widely distributed in rock structures and highly reactive. – The name originates from a Greek district called Magnesia.
 Potassium • Chemical Symbol: K • Physical state: Solid • Colour: Silvery-white • Uses- Used in fertilizers and soaps • Interesting facts: – It occurs in nature only in compoundsand can only be obtained by electrolysis of its common hydroxide. – It was first discovered by Sir Humphrey Davy in 1807.
Potassium • Chemical Symbol: K • Physical state: Solid • Colour: Silvery-white • Uses- Used in fertilizers and soaps • Interesting facts: – It occurs in nature only in compoundsand can only be obtained by electrolysis of its common hydroxide. – It was first discovered by Sir Humphrey Davy in 1807.
 References • Google search engine • Wikipedia • http: // www. periodic-table. org. uk/element-map. h tm
References • Google search engine • Wikipedia • http: // www. periodic-table. org. uk/element-map. h tm
 THE END THANK YOU FOR ATTENTION
THE END THANK YOU FOR ATTENTION

