59c62f47f3d3b43cfe5735255e142c09.ppt
- Количество слайдов: 29

Early studies: from organic light harvesting assemblies to light powered nanoreactors Jean M. J. Fréchet Material Science Division, LBNL and Department of Chemistry, University of California Berkeley, CA 94720 -160
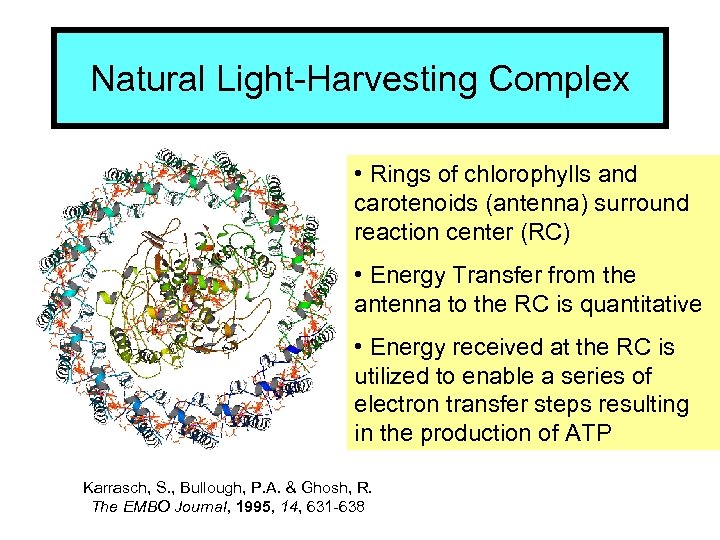
Natural Light-Harvesting Complex • Rings of chlorophylls and carotenoids (antenna) surround reaction center (RC) • Energy Transfer from the antenna to the RC is quantitative • Energy received at the RC is utilized to enable a series of electron transfer steps resulting in the production of ATP Karrasch, S. , Bullough, P. A. & Ghosh, R. The EMBO Journal, 1995, 14, 631 -638
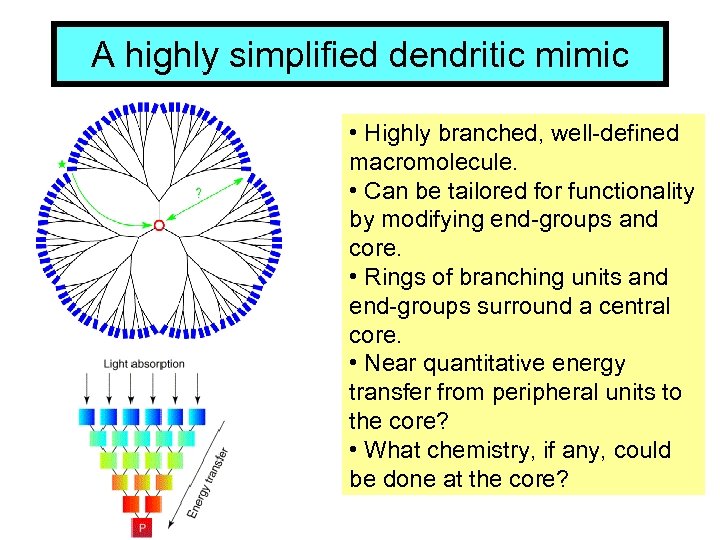
A highly simplified dendritic mimic • Highly branched, well-defined macromolecule. • Can be tailored for functionality by modifying end-groups and core. • Rings of branching units and end-groups surround a central core. • Near quantitative energy transfer from peripheral units to the core? • What chemistry, if any, could be done at the core?
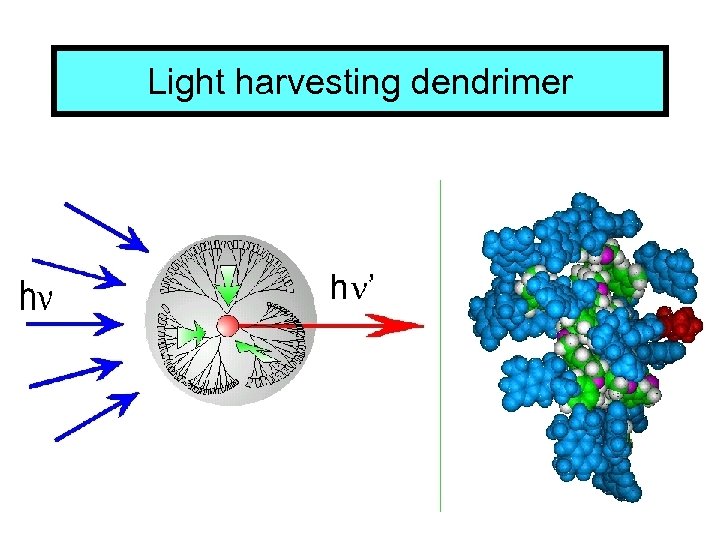
Light harvesting dendrimer
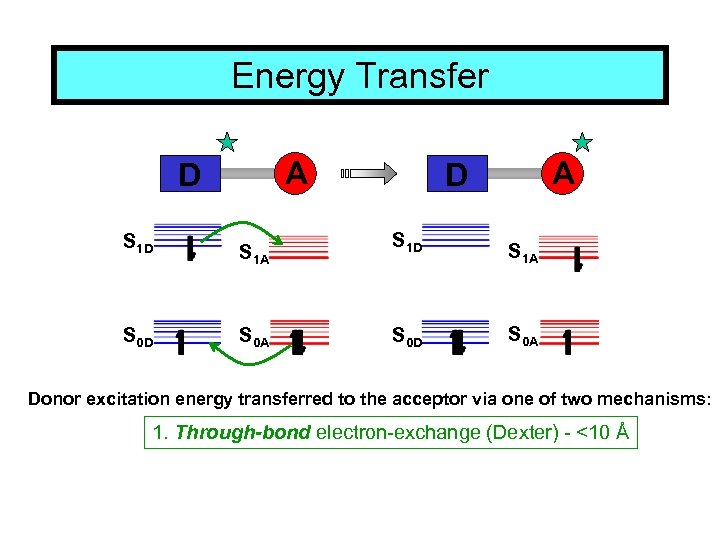
Energy Transfer A D S 1 D S 0 D S 1 A S 0 A Donor excitation energy transferred to the acceptor via one of two mechanisms: 1. Through-bond electron-exchange (Dexter) - <10 Å
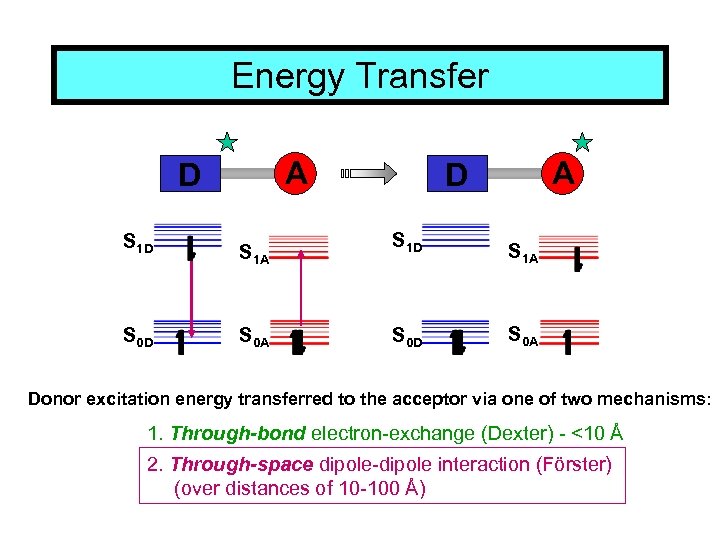
Energy Transfer A D S 1 D S 0 D S 1 A S 0 A Donor excitation energy transferred to the acceptor via one of two mechanisms: 1. Through-bond electron-exchange (Dexter) - <10 Å 2. Through-space dipole-dipole interaction (Förster) (over distances of 10 -100 Å)
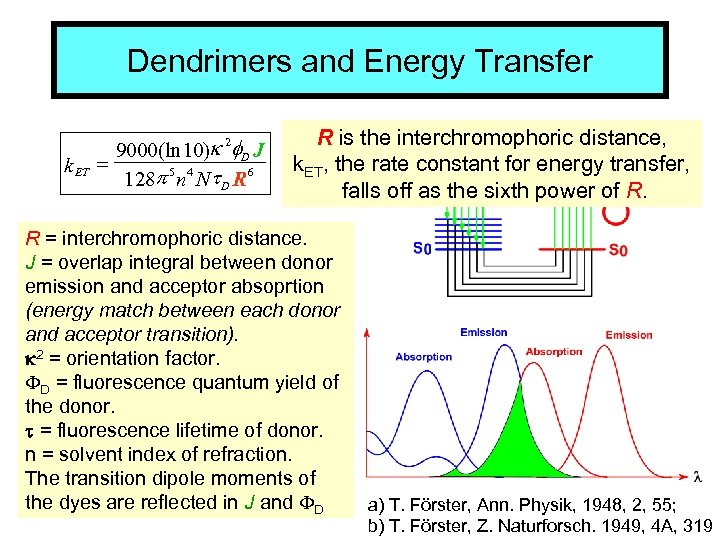
Dendrimers and Energy Transfer k ET 9000(ln 10)k 2 f. D J = 128 p 5 n 4 Nt D R 6 R is the interchromophoric distance, k. ET, the rate constant for energy transfer, falls off as the sixth power of R. R = interchromophoric distance. J = overlap integral between donor emission and acceptor absoprtion (energy match between each donor and acceptor transition). R k 2 = orientation factor. D = fluorescence quantum yield of the donor. t = fluorescence lifetime of donor. n = solvent index of refraction. The transition dipole moments of the dyes are reflected in J and D a) T. Förster, Ann. Physik, 1948, 2, 55; b) T. Förster, Z. Naturforsch. 1949, 4 A, 319
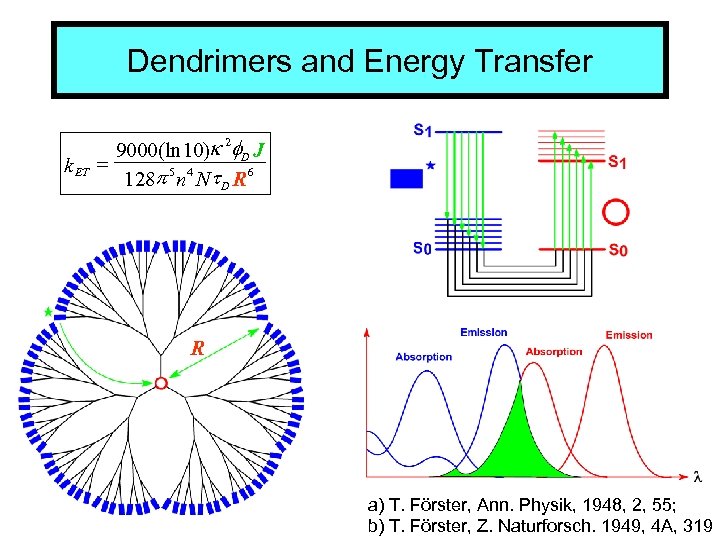
Dendrimers and Energy Transfer k ET 9000(ln 10)k 2 f. D J = 128 p 5 n 4 Nt D R 6 R a) T. Förster, Ann. Physik, 1948, 2, 55; b) T. Förster, Z. Naturforsch. 1949, 4 A, 319
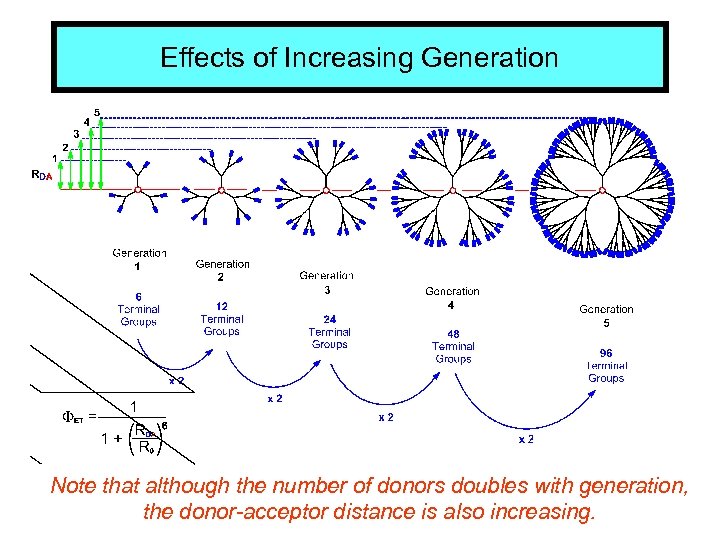
Effects of Increasing Generation Note that although the number of donors doubles with generation, the donor-acceptor distance is also increasing.
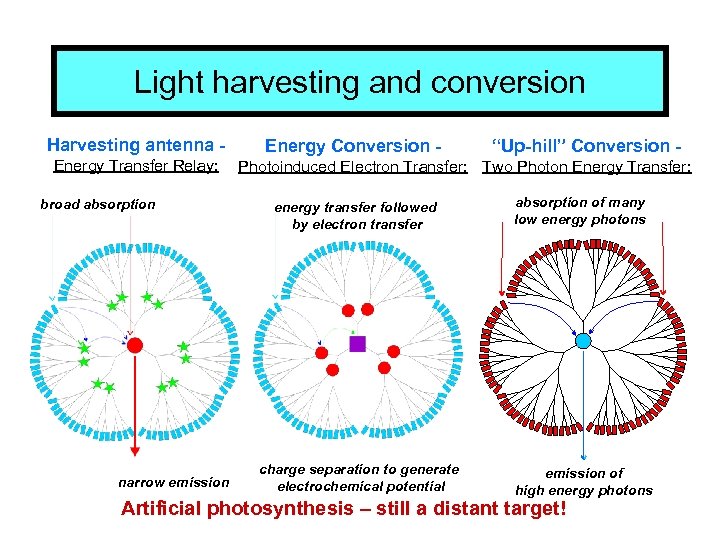
Light harvesting and conversion Harvesting antenna Energy Transfer Relay: broad absorption narrow emission Energy Conversion - “Up-hill” Conversion - Photoinduced Electron Transfer: Two Photon Energy Transfer: energy transfer followed by electron transfer absorption of many low energy photons charge separation to generate electrochemical potential emission of high energy photons Artificial photosynthesis – still a distant target!
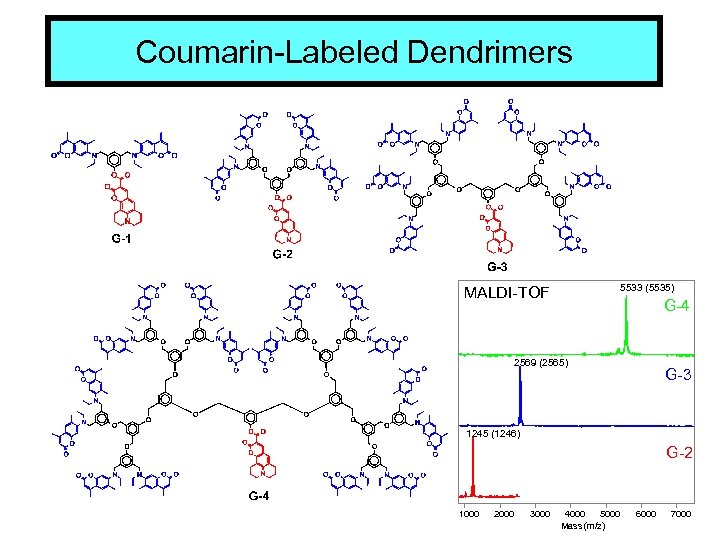
Coumarin-Labeled Dendrimers 5533 (5535) MALDI-TOF G-4 2569 (2565) G-3 1245 (1246) G-2 1000 2000 3000 4000 5000 Mass (m/z) 6000 7000
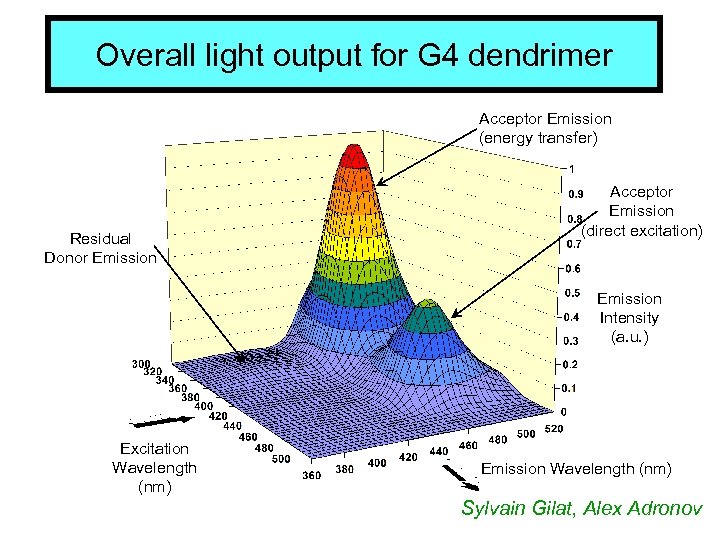
Overall light output for G 4 dendrimer Acceptor Emission (energy transfer) Residual Donor Emission Acceptor Emission (direct excitation) Emission Intensity (a. u. ) Excitation Wavelength (nm) Emission Wavelength (nm) Sylvain Gilat, Alex Adronov
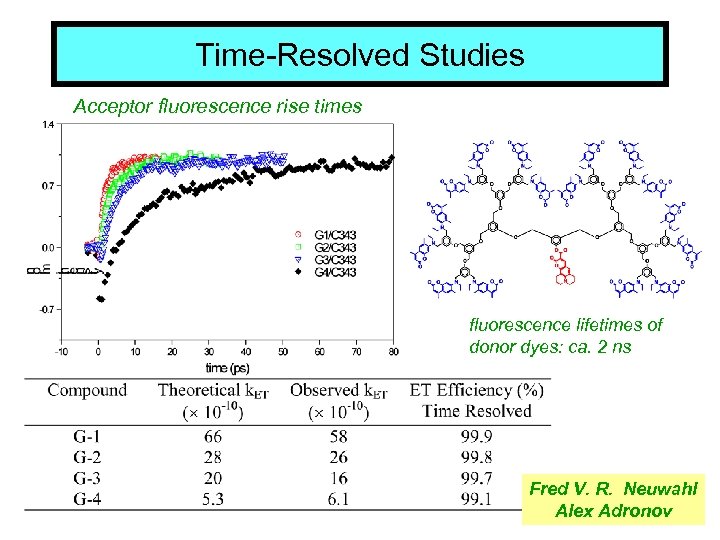
Time-Resolved Studies Acceptor fluorescence rise times fluorescence lifetimes of donor dyes: ca. 2 ns Fred V. R. Neuwahl Alex Adronov
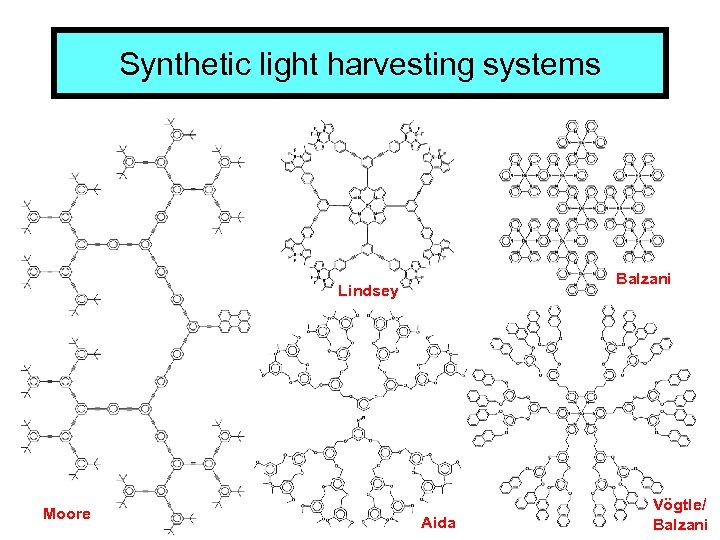
Synthetic light harvesting systems Balzani Lindsey Moore Aida Vögtle/ Balzani
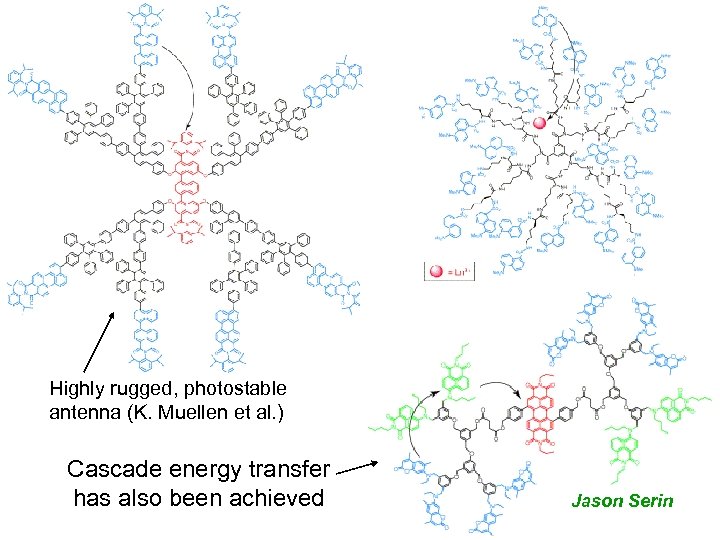
Highly rugged, photostable antenna (K. Muellen et al. ) Cascade energy transfer has also been achieved Jason Serin
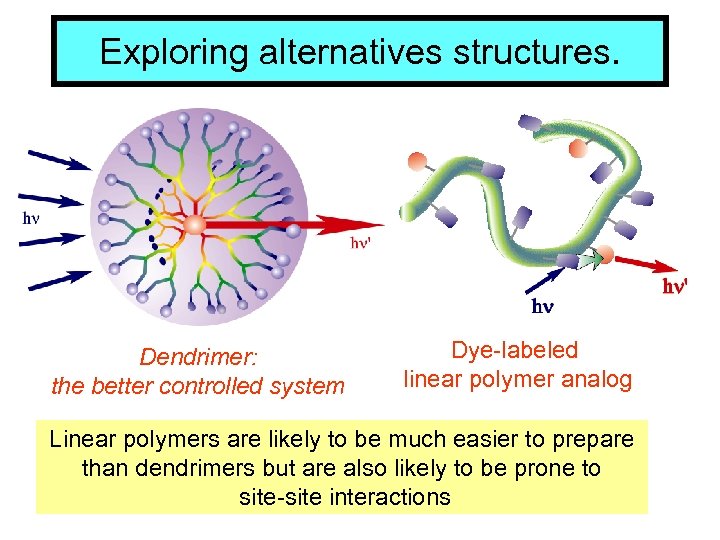
Exploring alternatives structures. Dendrimer: the better controlled system Dye-labeled linear polymer analog Linear polymers are likely to be much easier to prepare than dendrimers but are also likely to be prone to site-site interactions
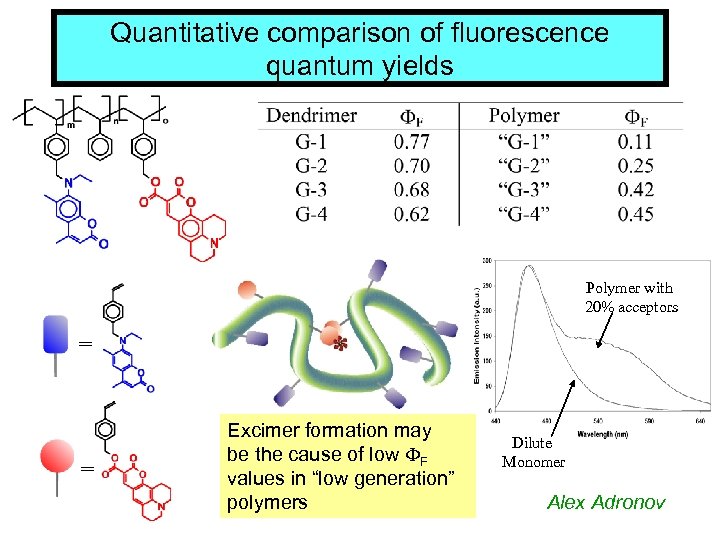
Quantitative comparison of fluorescence quantum yields Polymer with 20% acceptors = = * Excimer formation may be the cause of low F values in “low generation” polymers Dilute Monomer Alex Adronov
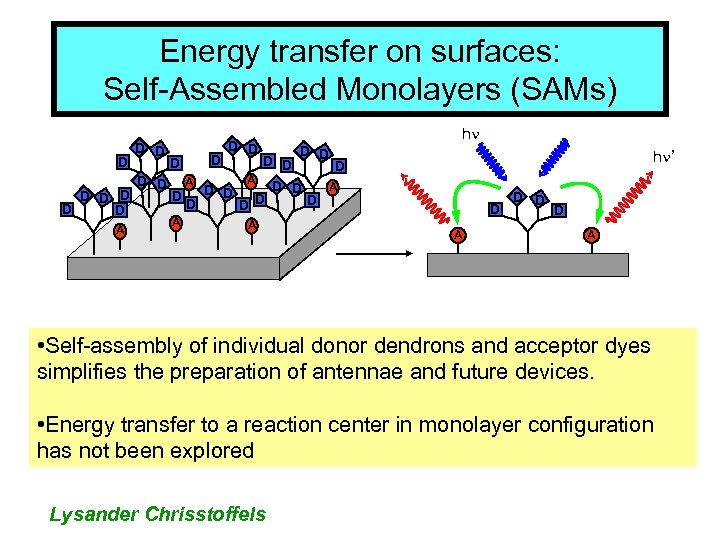
Energy transfer on surfaces: Self-Assembled Monolayers (SAMs) D D hn D D D A D D D D A D A hn’ D A D D D A • Self-assembly of individual donor dendrons and acceptor dyes simplifies the preparation of antennae and future devices. • Energy transfer to a reaction center in monolayer configuration has not been explored Lysander Chrisstoffels
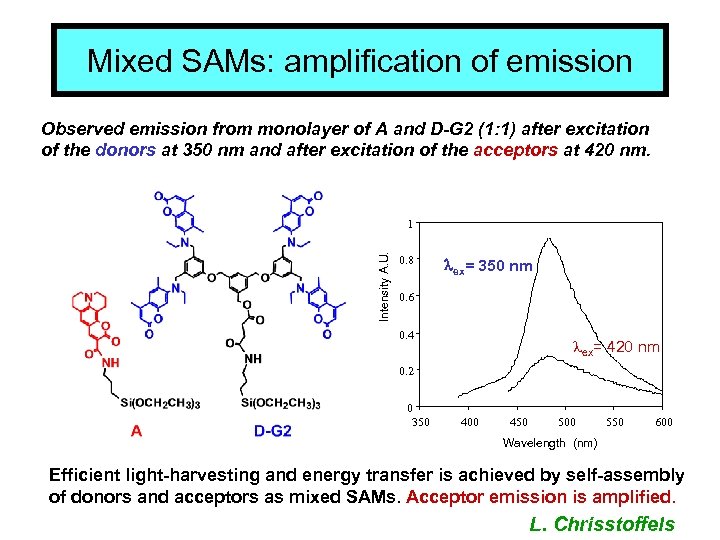
Mixed SAMs: amplification of emission Observed emission from monolayer of A and D-G 2 (1: 1) after excitation of the donors at 350 nm and after excitation of the acceptors at 420 nm. Intensity A. U. 1 0. 8 lex= 350 nm 0. 6 0. 4 lex= 420 nm 0. 2 0 350 400 450 500 550 600 Wavelength (nm) Efficient light-harvesting and energy transfer is achieved by self-assembly of donors and acceptors as mixed SAMs. Acceptor emission is amplified. L. Chrisstoffels
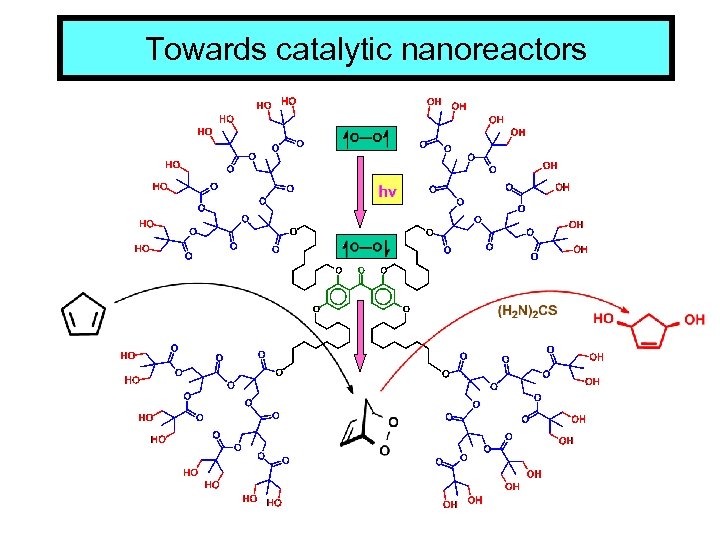
Towards catalytic nanoreactors
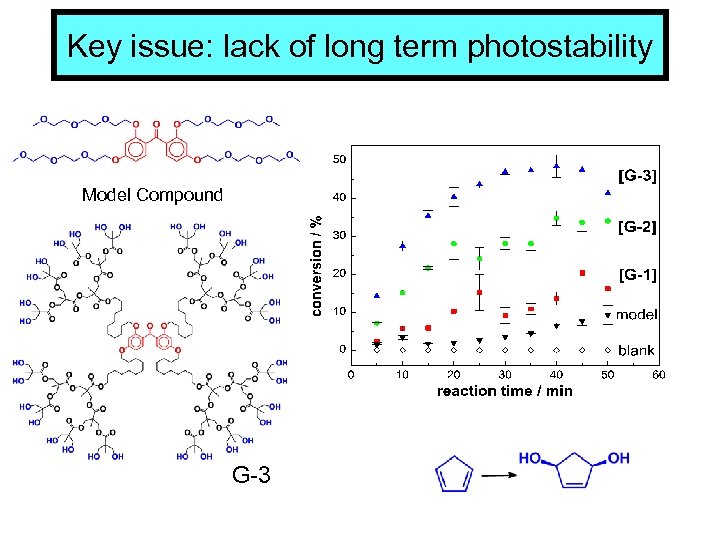
Key issue: lack of long term photostability Model Compound G-3
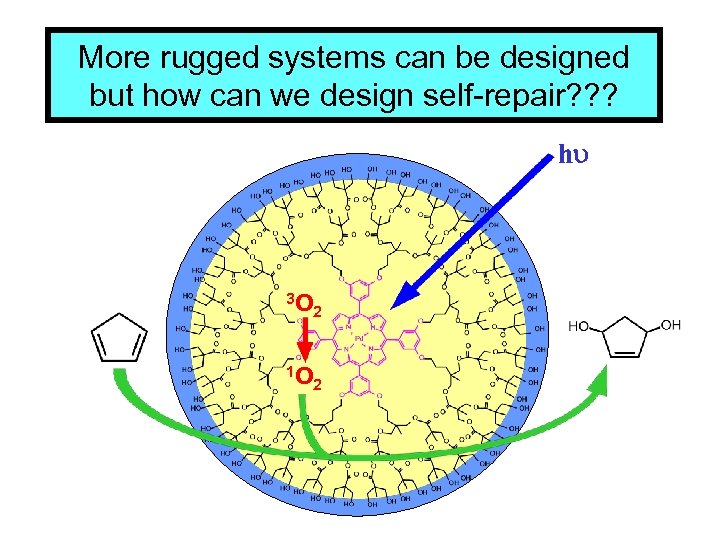
More rugged systems can be designed but how can we design self-repair? ? ? hu 3 O 2 1 O 2
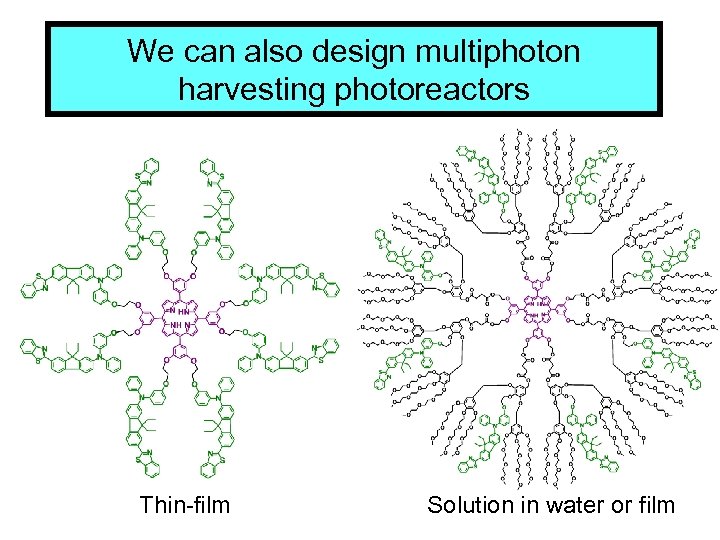
We can also design multiphoton harvesting photoreactors Thin-film Solution in water or film
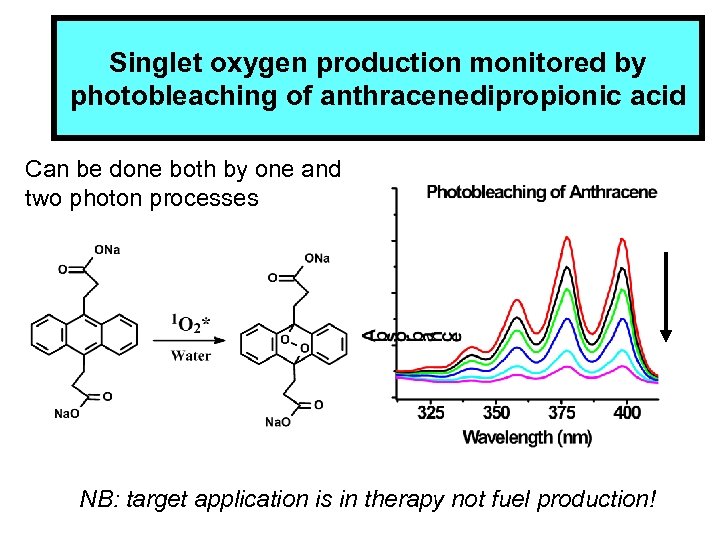
Singlet oxygen production monitored by photobleaching of anthracenedipropionic acid Can be done both by one and two photon processes NB: target application is in therapy not fuel production!
Mimicry of photosystem II with synthetic manganese complexes. In Photosystem II, light drives the splitting of water to molecular oxygen, protons and reductive equivalents. To the plant, O 2 is a just a waste product while the protons and reductive equivalents are used in the generation of valuable carbohydrates. The primary photosynthetic processes involves absorption of light by different antenna pigments with funneling of the excitation energy to the chlorophylls of the photosynthetic reaction center, which initiate a chain of electron transfer reactions between the reaction center cofactors. An energy-rich charge-separated state is generated across the membrane, which represents the initial product of the solar energy conversion. Curr. Opin. Chem. Bio. 2003, 7, 666
Mimicry of photosystem II with synthetic manganese complexes. Target: mimic the electron donor side reactions of PSII in synthetic complexes in which manganese is linked to a photosensitizer such as a Ru(bpy)32+ complexes rather than the more chlorophyll-like porphyrins. When the Ru(bpy)32+ moiety was oxidized from Ru. II to Ru. III by a laser flash in the presence of an electron acceptor, the Ru. III complex oxidized the attached Mn. II to Mn. III by intramolecular electron transfer, with time constants of < 50 ns– 10 ms, depending on the complex [Eur J Inorg Chem 2001, 1019]. Can this reaction done at the level of single-electron transfer be used in the design of more sophisticated complexes that incorporate more than one manganese ion? Curr. Opin. Chem. Bio. 2003, 7, 666
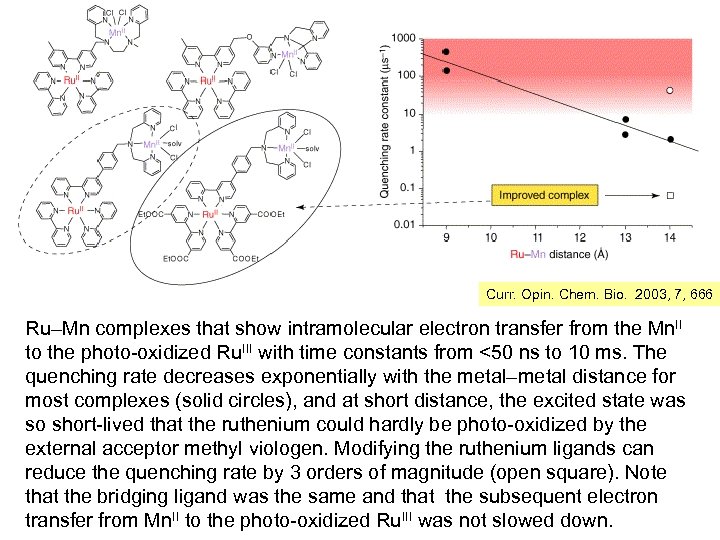
Curr. Opin. Chem. Bio. 2003, 7, 666 Ru–Mn complexes that show intramolecular electron transfer from the Mn. II to the photo-oxidized Ru. III with time constants from <50 ns to 10 ms. The quenching rate decreases exponentially with the metal–metal distance for most complexes (solid circles), and at short distance, the excited state was so short-lived that the ruthenium could hardly be photo-oxidized by the external acceptor methyl viologen. Modifying the ruthenium ligands can reduce the quenching rate by 3 orders of magnitude (open square). Note that the bridging ligand was the same and that the subsequent electron transfer from Mn. II to the photo-oxidized Ru. III was not slowed down.
Summary: we have a long way to go! hν Dendrimers Nature photoreactor eanatase Ti. O 2 ITO Photovoltaics Surface self-assembly Layer by layer assembly
Outlook Today, the most promising applications of organics are in photovoltaics. For solar to fuel, look at organic-inorganic hybrid systems. The organic portion (ligands for Mn, Ru, porphyrin centers, connectors, etc. . ) may hold the key to optimal activity of the inorganic component involved in electron transfer. The catalytic center remains a black box with much development still required. Explore self-assembly and layer-by-layer assembly.
59c62f47f3d3b43cfe5735255e142c09.ppt