c3718a0355ec2fc8eb348a4a61713a1c.ppt
- Количество слайдов: 15
 Dura. Seal Dural Sealant System TM PMA P 040034
Dura. Seal Dural Sealant System TM PMA P 040034
 Presenters Eric Ankerud, J. D. – Confluent Surgical, Inc. Vice President, Clinical, Regulatory, and Quality Patrick Campbell, Ph. D. – Confluent Surgical, Inc. Vice President, Research & Development John Tew, M. D. – Mayfield Clinic Professor, Department of Neurosurgery, University of Cincinnati G. Rees Cosgrove, M. D. – Massachusetts General Hospital Associate Professor of Surgery, Harvard Medical School Harry van Loveren, M. D. –Tampa General Hospital Professor and Chairman, Director of Skull Base and Cerebrovascular Surgery, University of South Florida
Presenters Eric Ankerud, J. D. – Confluent Surgical, Inc. Vice President, Clinical, Regulatory, and Quality Patrick Campbell, Ph. D. – Confluent Surgical, Inc. Vice President, Research & Development John Tew, M. D. – Mayfield Clinic Professor, Department of Neurosurgery, University of Cincinnati G. Rees Cosgrove, M. D. – Massachusetts General Hospital Associate Professor of Surgery, Harvard Medical School Harry van Loveren, M. D. –Tampa General Hospital Professor and Chairman, Director of Skull Base and Cerebrovascular Surgery, University of South Florida
 Presentation Overview Project Overview – Eric Ankerud, J. D. Technology Overview – Pat Campbell, Ph. D. Project Rationale – John Tew, M. D. US Pivotal Trial Results – G. Rees Cosgrove, M. D. Safety Evaluation – Harry van Loveren, M. D.
Presentation Overview Project Overview – Eric Ankerud, J. D. Technology Overview – Pat Campbell, Ph. D. Project Rationale – John Tew, M. D. US Pivotal Trial Results – G. Rees Cosgrove, M. D. Safety Evaluation – Harry van Loveren, M. D.
 Company Overview Mission: Development of in-situ polymerized biomaterials to address the unmet needs of surgical wound healing including surgical tissue sealing, post surgical adhesion prevention, and hemostasis Founded: 1998 People: 30 employees Facility: Waltham, Massachusetts Products: Commercially available OUS
Company Overview Mission: Development of in-situ polymerized biomaterials to address the unmet needs of surgical wound healing including surgical tissue sealing, post surgical adhesion prevention, and hemostasis Founded: 1998 People: 30 employees Facility: Waltham, Massachusetts Products: Commercially available OUS
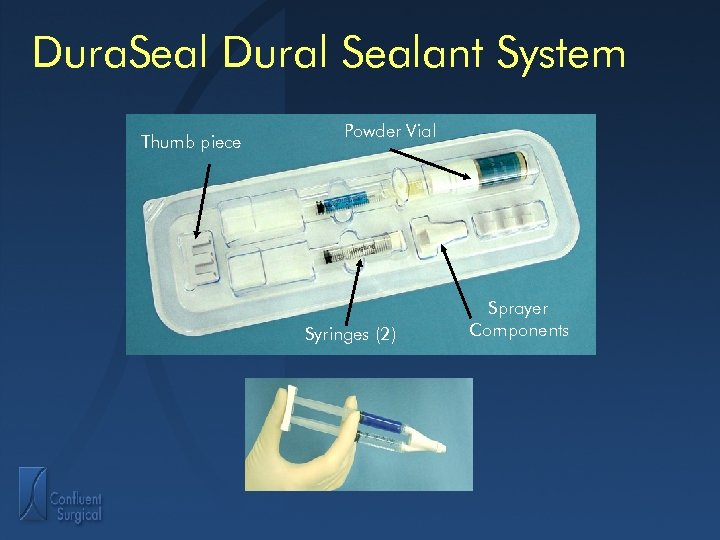 Dura. Seal Dural Sealant System Thumb piece Powder Vial Syringes (2) Sprayer Components
Dura. Seal Dural Sealant System Thumb piece Powder Vial Syringes (2) Sprayer Components
 Pre-Clinical Summary Dura. Seal is non-toxic and safe, up to 40 X human dose Dura. Seal is not neurotoxic, and allows complete neodura formation Intraoperative and postoperative sealing efficacy demonstrated in relevant canine model Ability to image Dura. Seal has been demonstrated, and has complete absorption over 8 weeks
Pre-Clinical Summary Dura. Seal is non-toxic and safe, up to 40 X human dose Dura. Seal is not neurotoxic, and allows complete neodura formation Intraoperative and postoperative sealing efficacy demonstrated in relevant canine model Ability to image Dura. Seal has been demonstrated, and has complete absorption over 8 weeks
 “Water Tight” Closure Remains Elusive Achieving “water tight” closure is basic tenet of neurosurgical practice Controlling intraoperative leakage is important to prevent CSF leakage and development of postoperative complications: Suture pinholes and space between sutures act as “one-way” valve for CSF leakage Subdural collections of CSF fluid may develop into pseudomeningoceles and overt incisional leaks with potential for serious post-operative sequelae
“Water Tight” Closure Remains Elusive Achieving “water tight” closure is basic tenet of neurosurgical practice Controlling intraoperative leakage is important to prevent CSF leakage and development of postoperative complications: Suture pinholes and space between sutures act as “one-way” valve for CSF leakage Subdural collections of CSF fluid may develop into pseudomeningoceles and overt incisional leaks with potential for serious post-operative sequelae
 CSF Leak Causes Postoperative Morbidity Meningitis – may lead to delayed neurologic complications Prolonged hospitalization and increased patient cost Interference with wound healing (dehiscence) Abscess formation Additional surgical intervention often required
CSF Leak Causes Postoperative Morbidity Meningitis – may lead to delayed neurologic complications Prolonged hospitalization and increased patient cost Interference with wound healing (dehiscence) Abscess formation Additional surgical intervention often required
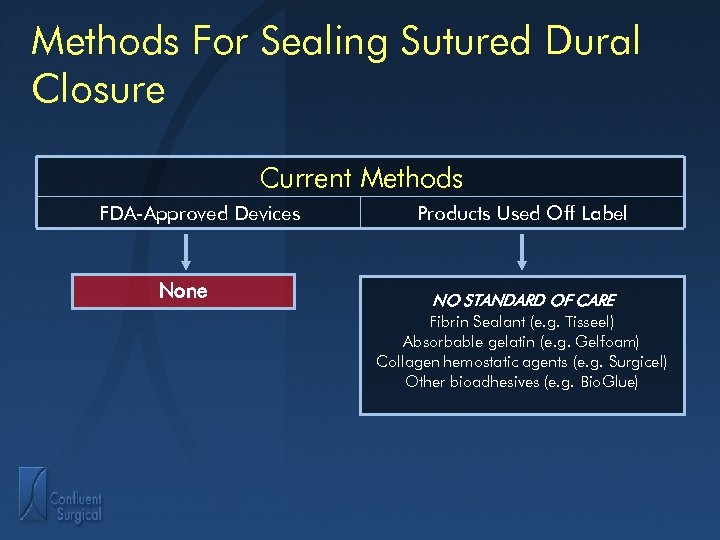 Methods For Sealing Sutured Dural Closure Current Methods FDA-Approved Devices None Products Used Off Label NO STANDARD OF CARE Fibrin Sealant (e. g. Tisseel) Absorbable gelatin (e. g. Gelfoam) Collagen hemostatic agents (e. g. Surgicel) Other bioadhesives (e. g. Bio. Glue)
Methods For Sealing Sutured Dural Closure Current Methods FDA-Approved Devices None Products Used Off Label NO STANDARD OF CARE Fibrin Sealant (e. g. Tisseel) Absorbable gelatin (e. g. Gelfoam) Collagen hemostatic agents (e. g. Surgicel) Other bioadhesives (e. g. Bio. Glue)
 US Pivotal Study Objective To evaluate the safety and effectiveness of the Dura. Seal Dural Sealant System as an adjunct to sutured dural repair during cranial surgery to provide watertight closure
US Pivotal Study Objective To evaluate the safety and effectiveness of the Dura. Seal Dural Sealant System as an adjunct to sutured dural repair during cranial surgery to provide watertight closure
 Study Design Prospective Multi-center Non-randomized, single arm study 11 participating sites 10 United States 1 Europe
Study Design Prospective Multi-center Non-randomized, single arm study 11 participating sites 10 United States 1 Europe
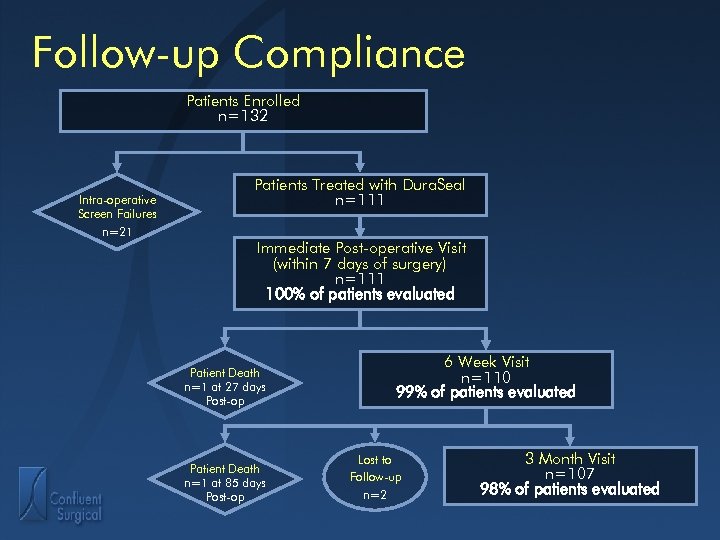 Follow-up Compliance Patients Enrolled n=132 Intra-operative Screen Failures n=21 Patients Treated with Dura. Seal n=111 Immediate Post-operative Visit (within 7 days of surgery) n=111 100% of patients evaluated Patient Death n=1 at 27 days Post-op Patient Death n=1 at 85 days Post-op 6 Week Visit n=110 99% of patients evaluated Lost to Follow-up n=2 3 Month Visit n=107 98% of patients evaluated
Follow-up Compliance Patients Enrolled n=132 Intra-operative Screen Failures n=21 Patients Treated with Dura. Seal n=111 Immediate Post-operative Visit (within 7 days of surgery) n=111 100% of patients evaluated Patient Death n=1 at 27 days Post-op Patient Death n=1 at 85 days Post-op 6 Week Visit n=110 99% of patients evaluated Lost to Follow-up n=2 3 Month Visit n=107 98% of patients evaluated
 Primary Efficacy Analysis. Intraoperative Sealing Success Analysis Results Intent To Treat* 98. 2% (109*/111) [95% CI: 93. 6 -99. 8] (includes 2 non-evaluable patients) Per Protocol (includes all evaluable patients) 100% (109/109) [95% CI: 96. 7 -100] * Two patients had no leak after Valsalva maneuver, but pressure reached was only to 10 cm H 2 O and, therefore, were not evaluable
Primary Efficacy Analysis. Intraoperative Sealing Success Analysis Results Intent To Treat* 98. 2% (109*/111) [95% CI: 93. 6 -99. 8] (includes 2 non-evaluable patients) Per Protocol (includes all evaluable patients) 100% (109/109) [95% CI: 96. 7 -100] * Two patients had no leak after Valsalva maneuver, but pressure reached was only to 10 cm H 2 O and, therefore, were not evaluable
 Summary Primary efficacy endpoint was met Adverse events consistent in nature, frequency and severity for patients undergoing cranial surgery
Summary Primary efficacy endpoint was met Adverse events consistent in nature, frequency and severity for patients undergoing cranial surgery
 Conclusion In the patient population evaluated, the benefits associated with the use of the Dura. Sealant outweigh any potential risks associated with the use of the device
Conclusion In the patient population evaluated, the benefits associated with the use of the Dura. Sealant outweigh any potential risks associated with the use of the device


