68527ce4aad7fec0f3f838e8c7fb4111.ppt
- Количество слайдов: 22
 Drug Research Unit Maastricht The Early Phase (0 -I-II) Clinical Drug Research Unit of Maastricht University Medical Center (MUMC) prof dr L. Van Bortel
Drug Research Unit Maastricht The Early Phase (0 -I-II) Clinical Drug Research Unit of Maastricht University Medical Center (MUMC) prof dr L. Van Bortel
 Mission Statement To contribute to the development of new drugs by conducting early phase clinical trials (phase 0 -I-II) in compliance with (inter)national legislation and quality standards
Mission Statement To contribute to the development of new drugs by conducting early phase clinical trials (phase 0 -I-II) in compliance with (inter)national legislation and quality standards
 Position in MUMC-holding/valorisation chain • essential part in chain of drug development preclinical → early phase → phase 3&4 • by filling the gap in the drug research chain • facilitates translational research
Position in MUMC-holding/valorisation chain • essential part in chain of drug development preclinical → early phase → phase 3&4 • by filling the gap in the drug research chain • facilitates translational research
 Strategic alliance Close collaboration with Drug Research Unit Ghent (DRUG): 1 CEO for 2 units • ↑ possibilities of conducting larger trials or trials with difficult recruitment • DRUM can take advantage of the elaborated quality management system and training at DRUG • Joint PR for internal and external sponsors • Cross-fertilisation of know-how/methods …
Strategic alliance Close collaboration with Drug Research Unit Ghent (DRUG): 1 CEO for 2 units • ↑ possibilities of conducting larger trials or trials with difficult recruitment • DRUM can take advantage of the elaborated quality management system and training at DRUG • Joint PR for internal and external sponsors • Cross-fertilisation of know-how/methods …
 sponsors • Pharmaceutical industry (+ nutriceuticals) • Non for profit organisations • MUMC • Other University departments
sponsors • Pharmaceutical industry (+ nutriceuticals) • Non for profit organisations • MUMC • Other University departments
 What can DRUM offer sponsors State of the art conduct of early phase clinical studies • • • High quality Large expertise Short timelines High flexibility Fair cost
What can DRUM offer sponsors State of the art conduct of early phase clinical studies • • • High quality Large expertise Short timelines High flexibility Fair cost
 What can DRUM offer sponsors State of the art conduct of early phase clinical studies • • • High quality Large expertise Short timelines High flexibility Fair cost
What can DRUM offer sponsors State of the art conduct of early phase clinical studies • • • High quality Large expertise Short timelines High flexibility Fair cost
 Quality quality control first party (internal) audits • scheduled on main procedures • ad hoc for (near) errors – training in SOP (all SOP’s revised within 2 yrs) – close feedback on the spot / approval system – quality assurance 1 GCP-ICH third party audit/yr – ISO 9001: 2008 → 1 ISO audit/yr (within 1 year? ) –
Quality quality control first party (internal) audits • scheduled on main procedures • ad hoc for (near) errors – training in SOP (all SOP’s revised within 2 yrs) – close feedback on the spot / approval system – quality assurance 1 GCP-ICH third party audit/yr – ISO 9001: 2008 → 1 ISO audit/yr (within 1 year? ) –
 What can DRUM offer sponsors State of the art conduct of early phase clinical studies • • • High quality Large expertise Short timelines High flexibility Fair cost
What can DRUM offer sponsors State of the art conduct of early phase clinical studies • • • High quality Large expertise Short timelines High flexibility Fair cost
 Large expertise takes advantage of experts in 2 knowledge centers: MUMC - U(Z)Gent → many therapeutic areas → high tec investigations experience in early phase clinical drug research – principal investigator: 24 years – investigator: 4 years
Large expertise takes advantage of experts in 2 knowledge centers: MUMC - U(Z)Gent → many therapeutic areas → high tec investigations experience in early phase clinical drug research – principal investigator: 24 years – investigator: 4 years
 What can DRUM offer sponsors State of the art conduct of early phase clinical studies • • • High quality Large expertise Short timelines High flexibility Fair cost
What can DRUM offer sponsors State of the art conduct of early phase clinical studies • • • High quality Large expertise Short timelines High flexibility Fair cost
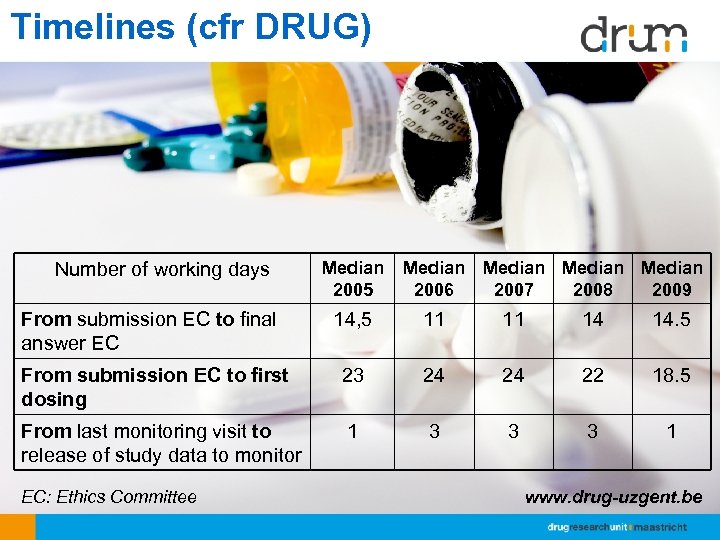 Timelines (cfr DRUG) Number of working days From submission EC to final answer EC Median 2005 Median 2006 2007 2008 2009 14, 5 11 11 14 14. 5 From submission EC to first dosing 23 24 24 22 18. 5 From last monitoring visit to release of study data to monitor 1 3 3 3 1 EC: Ethics Committee www. drug-uzgent. be
Timelines (cfr DRUG) Number of working days From submission EC to final answer EC Median 2005 Median 2006 2007 2008 2009 14, 5 11 11 14 14. 5 From submission EC to first dosing 23 24 24 22 18. 5 From last monitoring visit to release of study data to monitor 1 3 3 3 1 EC: Ethics Committee www. drug-uzgent. be
 What can DRUM offer sponsors State of the art conduct of early phase clinical studies • • • High quality Large expertise Short timelines High flexibility Fair cost
What can DRUM offer sponsors State of the art conduct of early phase clinical studies • • • High quality Large expertise Short timelines High flexibility Fair cost
 High flexibility • Dose adjustments • Pharmacy with GMPz • Pharmacell bv with full GMP • Additional safety measures • Puts pressure on working hours/social environment of staff, particularly in the early years
High flexibility • Dose adjustments • Pharmacy with GMPz • Pharmacell bv with full GMP • Additional safety measures • Puts pressure on working hours/social environment of staff, particularly in the early years
 What can DRUM offer the MUMC • Service center for early phase drug investigations • Take advantage of knowledge center (experts in MUMC) • Facilitates education (training) in Medicine/ Health Sciences/clinical pharmacologist
What can DRUM offer the MUMC • Service center for early phase drug investigations • Take advantage of knowledge center (experts in MUMC) • Facilitates education (training) in Medicine/ Health Sciences/clinical pharmacologist
 staff • Principal investigator - quality coordinator managing coordinator - clinical research coordinator - trial nurse office manager • investigator/principal investigator experienced in early phase studies • All are well-trained training at DRUG • Highly motivated • Bank personnel: trial nurses / co-investigators
staff • Principal investigator - quality coordinator managing coordinator - clinical research coordinator - trial nurse office manager • investigator/principal investigator experienced in early phase studies • All are well-trained training at DRUG • Highly motivated • Bank personnel: trial nurses / co-investigators
 facilities • 16 beds for clinical research and overnight stay • 2 sample handling laboratories (temperature controlled) • 1 drug storage room (temperature controlled / alarm) • 1 freezer (-18°C) + 1 (-70°C) temperature controlled / alarm) • 2 refrigerators (temperature controlled / alarm) • offices, archive, meeting room, reception, kitchen, storage room
facilities • 16 beds for clinical research and overnight stay • 2 sample handling laboratories (temperature controlled) • 1 drug storage room (temperature controlled / alarm) • 1 freezer (-18°C) + 1 (-70°C) temperature controlled / alarm) • 2 refrigerators (temperature controlled / alarm) • offices, archive, meeting room, reception, kitchen, storage room
 facilities • 16 beds for clinical research and overnight stay • 2 sample handling laboratories (temperature controlled) • 1 drug storage room (temperature controlled / alarm) • 1 freezer (-18°C) + 1 (-70°C) temperature controlled / alarm) • 2 refrigerators (temperature controlled / alarm) • offices, archive, meeting room, reception, kitchen, storage room
facilities • 16 beds for clinical research and overnight stay • 2 sample handling laboratories (temperature controlled) • 1 drug storage room (temperature controlled / alarm) • 1 freezer (-18°C) + 1 (-70°C) temperature controlled / alarm) • 2 refrigerators (temperature controlled / alarm) • offices, archive, meeting room, reception, kitchen, storage room
 facilities • 16 beds for clinical research and overnight stay • 2 sample handling laboratories (temperature controlled) • 1 drug storage room (temperature controlled / alarm) • 1 freezer (-18°C) + 1 (-70°C) temperature controlled / alarm) • 2 refrigerators (temperature controlled / alarm) • offices, archive, meeting room, reception, kitchen, storage room
facilities • 16 beds for clinical research and overnight stay • 2 sample handling laboratories (temperature controlled) • 1 drug storage room (temperature controlled / alarm) • 1 freezer (-18°C) + 1 (-70°C) temperature controlled / alarm) • 2 refrigerators (temperature controlled / alarm) • offices, archive, meeting room, reception, kitchen, storage room
 Ready to start!
Ready to start!
 Summary • Centre of Excellence (qualified staff / approval / external audits / ISO 9001: 2008) • short timelines, high flexibility • takes advantage of university knowledge centre
Summary • Centre of Excellence (qualified staff / approval / external audits / ISO 9001: 2008) • short timelines, high flexibility • takes advantage of university knowledge centre
 YES, we can!
YES, we can!


