0ece5a81a6390d65ec0630df4c7b0631.ppt
- Количество слайдов: 31

Drug discovery and development • Leeds UK Objectives of next 5 lectures: you will: • be aware of why/how new drugs are discovered • know the processes involved in drug discovery and development • see where pharmacologists/bioscientists may contribute • know about the difficulties and dangers inherent in the drug development process.

What is a drug? • Any chemical compound - sugar ? ? ? • Anything which produces a change in the body - an axe ? ? ? • Define by characteristics: 1. use or potential use in diagnosis or treatment of disease 2. selective in their actions

What costs what in Leeds? (GPs; 98/99) • • • Omeprazole (anti-gastric acid) £ 3. 5 m Simvastatin (cholesterol lowering) £ 2. 4 m Beclomethasone (asthma) £ 1. 8 m Fluoxetine (antidepressant) £ 1. 5 m Lansoprazole (anti-gastric acid) £ 1. 4 m Ranitidine (anti-gastric acid) £ 1. 3 m Paroxetine (antidepressant) £ 1. 2 m TOP 7 TOTAL >£ 13 m Total GP drugs for Leeds >£ 67 m

Why are new drugs needed? • unmet medical need; new diseases (BSE; AIDS, Alzheimer’s; obesity); low efficacy (dementia, cancer); side effects (antidepressants, antipsychotics) • • downstream health costs; (Alzheimer’s; spinal injury) cost of therapy; (Viagra, Interleukins) costs to individual/country; (depression) sustain industrial activity; pharmaceutical industry employs thousands and makes a massive contribution to overseas earnings); patent expiry

The changed context of drug discovery and development The 1800 s: natural sources; limited possibilities; prepared by individuals; small scale; not purified, standardised or tested; limited administration; no controls; no idea of mechanisms. The 1990 s: synthetic source; unlimited possibilities; prepared by companies; massive scale; highly purified, standardised and tested; world-wide administration; tight legislative control; mechanisms partly understood.
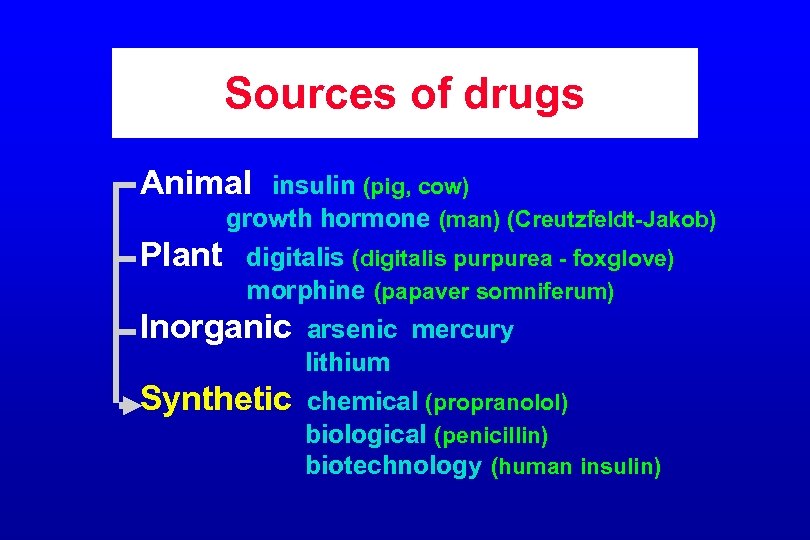
Sources of drugs Animal insulin (pig, cow) growth hormone (man) (Creutzfeldt-Jakob) Plant digitalis (digitalis purpurea - foxglove) morphine (papaver somniferum) Inorganic arsenic mercury lithium Synthetic chemical (propranolol) biological (penicillin) biotechnology (human insulin)
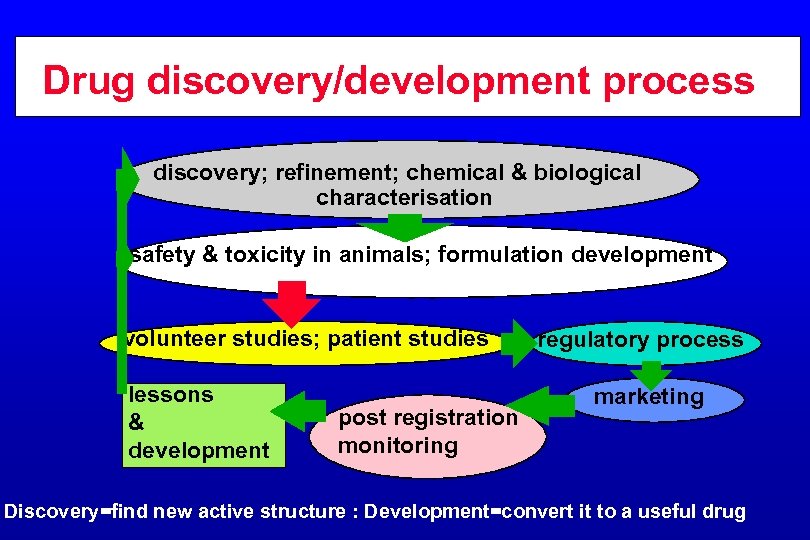
Drug discovery/development process discovery; refinement; chemical & biological characterisation safety & toxicity in animals; formulation development volunteer studies; patient studies lessons & development post registration monitoring regulatory process marketing Discovery=find new active structure : Development=convert it to a useful drug

Approaches to drug discovery • Historical; cinchona (quinine) & willow barks (aspirin); chinese medicine currently. • Study disease process; breast cancer (tamoxifen); Parkinson’s disease (L-dopa) • Study biochem/physiological pathway; renin/angiotensin • Develop SAR to natural compound; beta-adrenoceptors (propranolol), H 2 -receptors (cimetidine) • Design to fit known structurally identified biological site; angiotensin-converting enzyme inhibitors • By chance (serendipidy); random screening (HTS); penicillin; dimenhydramate; pethidine • Genomics; identification of receptors; gene therapy; recombinant materials; DRIVER IS UNMET MEDICAL NEED IN A VIABLE MARKET
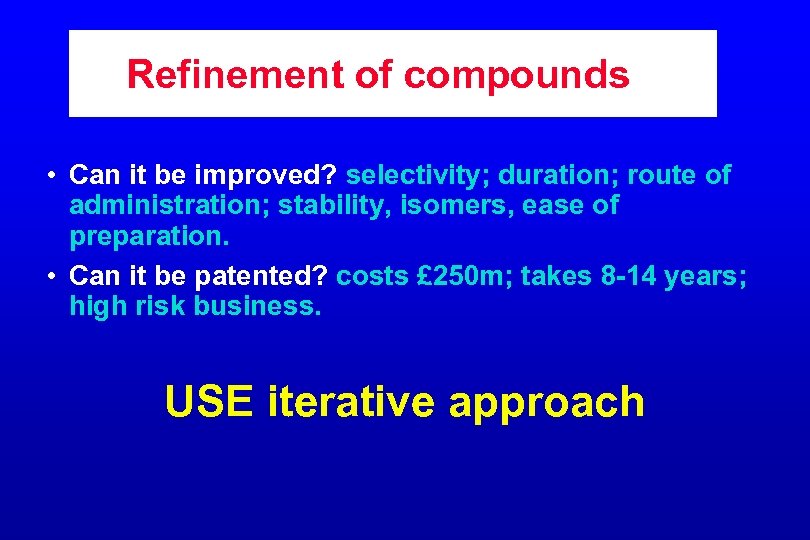
Refinement of compounds • Can it be improved? selectivity; duration; route of administration; stability, isomers, ease of preparation. • Can it be patented? costs £ 250 m; takes 8 -14 years; high risk business. USE iterative approach
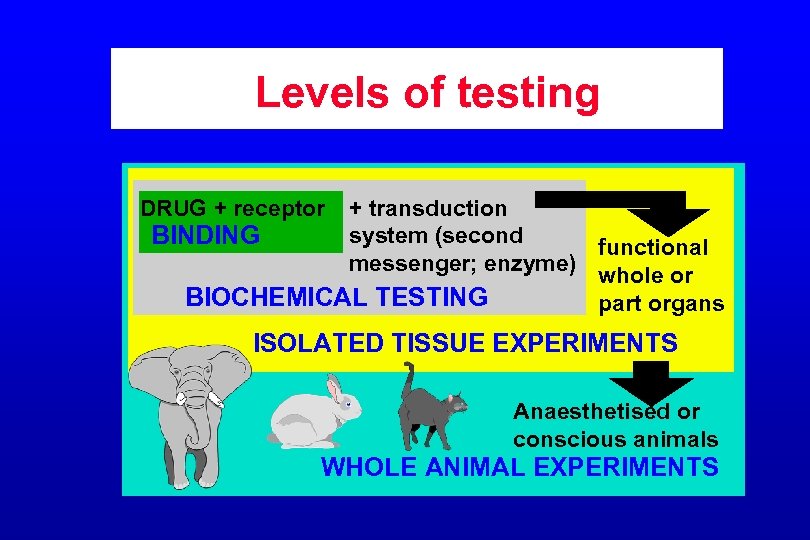
Levels of testing DRUG + receptor + transduction system (second BINDING functional messenger; enzyme) whole or BIOCHEMICAL TESTING part organs ISOLATED TISSUE EXPERIMENTS Anaesthetised or conscious animals WHOLE ANIMAL EXPERIMENTS
Animal models of efficacy • Existing normal behaviours/effects (anaesthesia; contraception; paralysis) • Create behaviours (fat rats; hypertensive rats; anxious rats; epileptic rats) • Find unrelated behaviour affected by existing drugs (Straub tail for narcotic analgesics; learned helplessness for antidepressants) How predictive is the model? exact replica = 100% predictor mechanism same = good predictor mechanisms different = poor predictor

Animal models • predictive for efficacy AND toxicity? • expensive; time consuming; variable; uncertain; troublesome; ethical questions; skilled workers • legislative control Animal (Scientific Procedures) Act (1986) • PERSONAL LICENCE - competent, trained, procedures specified • PROJECT LICENCE - allows a personal licence holder to carry out specified procedures for a specified project that cannot be done without animals and where severity justifies likely gain. • GET INTO MAN EARLY

RRR

Reducing animal usage • About 2. 6 m animals/y used in procedures in UK (11. 6 m in Europe) • Likely to increase; more research, more targets, genetic capability • 3 Rs -- 3 Rs • REPLACEMENT: use non-animal tests if possible (cheaper, less trouble, less variable but not possible for everything at this time) • REDUCTION: get the statistics right, don’t replicate work unnecessarily, don’t overbreed • REFINEMENT: reduce suffering and severity of procedure, pay attention to housing, stress, husbandry and rich environments, proper analgesia and pre- and post- operative care
Chemical and biological characterisation • CHEMICAL; structure, synthesis, purity, isomers, p. Ka, stability, solubility, salts, assay • BIOLOGICAL; acute pharmacological profile LD 50, ED 50, binding data for many receptors, dose-effect relationships, open field tests, particular tests for different activities (e. g. CVS, CNS, GI tract) Both positive and negative information is useful.

Safety & toxicity in animals • Acute toxicity profile • Chronic toxicity profile -- 14 day toxicity test in one rodent and one non-rodent species before use in man. -- 3 month study read out at 28 days -- longer studies (12 & 24 month) Three dose levels (below, about, well above human dose). It is insufficient to to use doses which are not toxic; the doses producing toxic effects and the nature of these effects MUST be established.

Formulation studies • DRUG + Additive: filler, lubricant, coating, stabiliser, colour, binder, disintegrator Dosage form: capsule, tablet, injection, other? Manipulate duration/profile: e. g. sustained release Bioequivalence Bioavailability Ease of use
Clinical testing • {Phase 0 (non-clinical)} • Phase 1 (volunteers) • Phase 2 (patients) • Phase 3 (large scale multi-centre) • Phase 4 (post registration monitoring) phases can also be defined by the information you are trying to get out of the testing

Volunteer studies (phase I trials) • • pharmacologists & employees (15 -30 in number) ethical approval healthy informed consent full rescussitation + medical backup monitor single and repeat doses increase dose levels

Volunteer studies (phase I trials) OBJECTIVES • metabolic and excretory pathways (impinges on toxicity testing in animals) • variability between individuals; effect of route; bioavailability • tolerated dose range • indication of therapeutic effects • indication of side effects
Patient studies (phase 2 trials) • 150 -350 ill people; informed consent • needs licence • maximum monitoring; full rescussitation • often patients where other treatment failed • OBJECTIVES: indication for use; type of patient; severity of disease; dose range, schedule and increment; pharmacokinetic studies in ill people; nature of side effects and severity; effects in special groups.
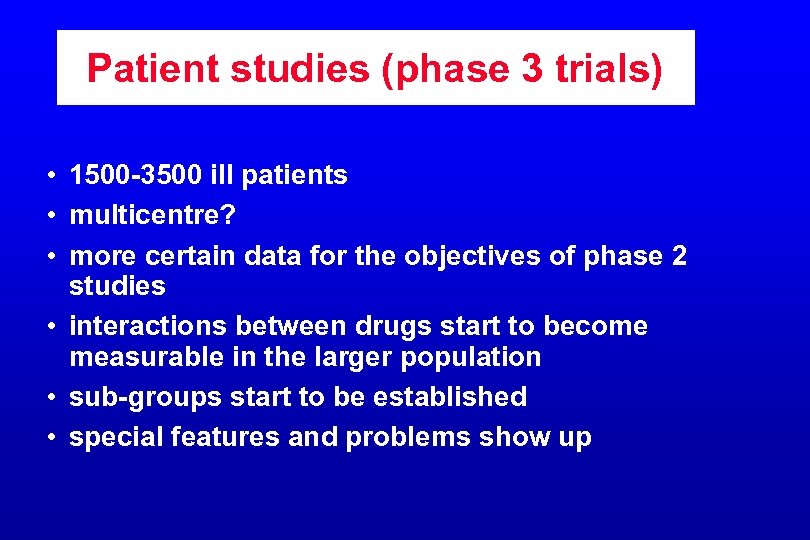
Patient studies (phase 3 trials) • 1500 -3500 ill patients • multicentre? • more certain data for the objectives of phase 2 studies • interactions between drugs start to become measurable in the larger population • sub-groups start to be established • special features and problems show up

Clinical trials Drug action depends on: • pharmacodynamics • pharmacokinetics and dose regimen • drug interactions • receptor sensitivity of patient • mood/personality of patient & doctor • patients expectations and past experience • social environment of patient • clinical state of patient Clinical trial controls these variables and examines action of drug in defined set of circumstances
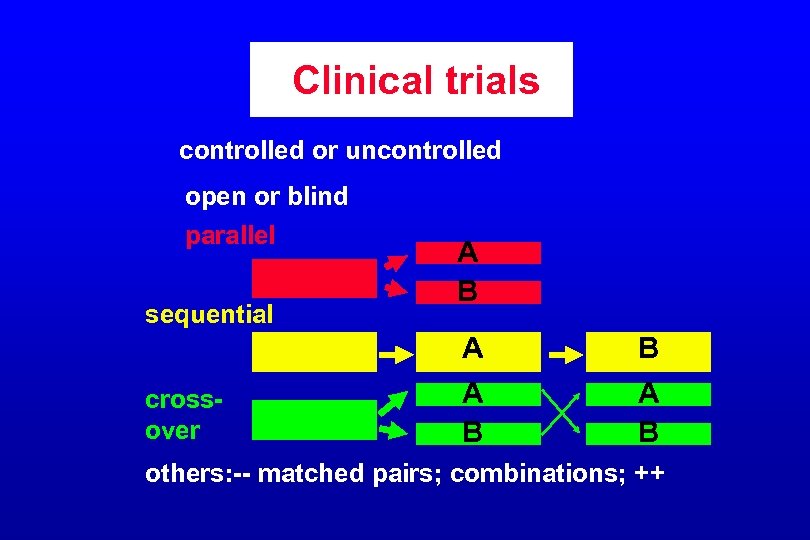
Clinical trials controlled or uncontrolled open or blind parallel sequential A B A crossover B A B others: -- matched pairs; combinations; ++

The Regulatory process • • differs from country to country demands safety and quality of product encourages efficacy and need for product grants clinical trials certificate if volunteer and animal data OK approves protocols and examines data 50 -400 volumes (30, 000 -150, 000 pages) original data available two way process; authority and company trying to produce a safe effective product • release for a specific purpose and use

Marketing • getting the product right (packaging; formulation) • right therapeutic slot • information on new drug • information for honest comparison • reporting problems • reporting new indications • therapeutic trends
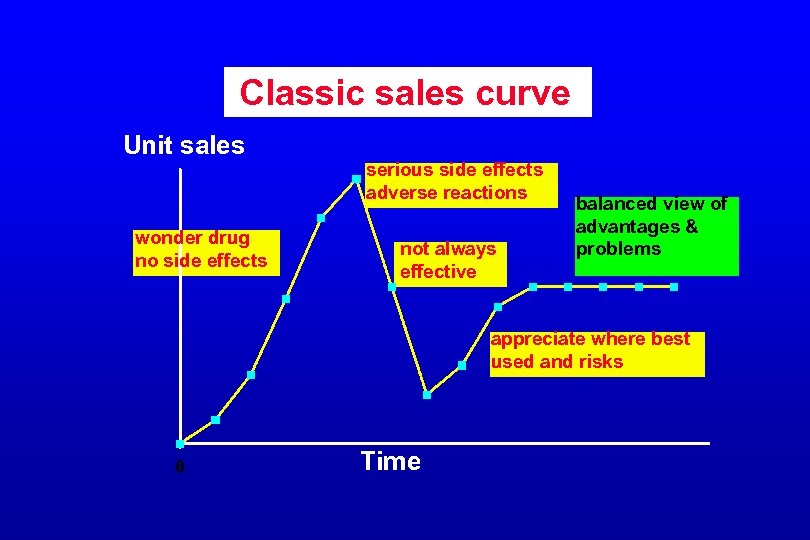
Classic sales curve Unit sales wonder drug no side effects serious side effects adverse reactions not always effective balanced view of advantages & problems appreciate where best used and risks Time

Post-registration monitoring • YELLOW CARD SYSTEM: voluntary reporting of adverse effects by GP to Committee on Safety of Medicines; easy; effective? • INTENSIVE MONITORING OF DEFINED GROUP: first 10, 000; administrative nightmare as patients move/die; costly; time-consuming. • RESTRICTED RELEASE: only available to small group of GPs; monitor their patients; elitist • MONITOR INCIDENCE OF DISEASE PROBLEM: difficult to identify cause of change.
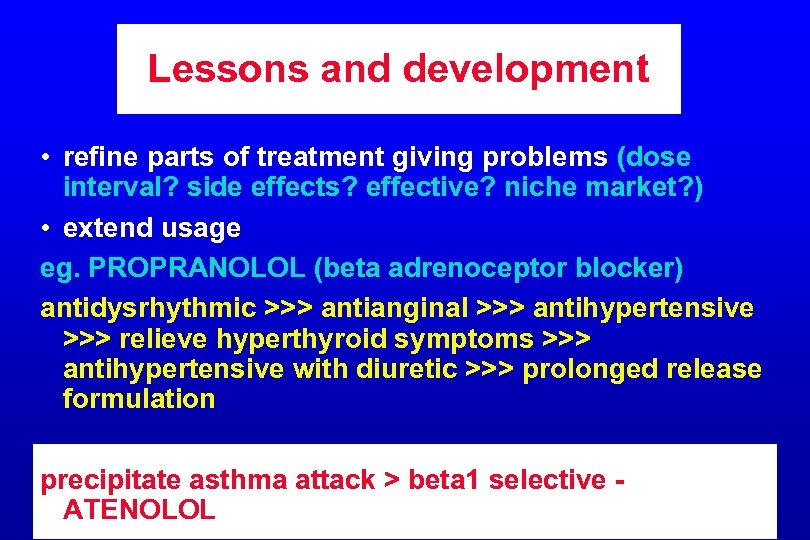
Lessons and development • refine parts of treatment giving problems (dose interval? side effects? effective? niche market? ) • extend usage eg. PROPRANOLOL (beta adrenoceptor blocker) antidysrhythmic >>> antianginal >>> antihypertensive >>> relieve hyperthyroid symptoms >>> antihypertensive with diuretic >>> prolonged release formulation precipitate asthma attack > beta 1 selective ATENOLOL

The future? • • 3 rd world diseases? orphan drugs with few users? improve safety and efficacy records reduce animal utilisation (cell lines; early human volunteers, ) • new diseases (AIDS; Alzheimer’s; CJ disease; human BSE variant; obesity; cancer) • new biology - (clone human receptors; disease model by gene changes) • patent times and increasing cost

Me-too drugs Similar to drugs already on market • parallel co-incident development • not identical - differences emerge with time • allergy to one only • unsuspected side effect causes discontinuation • particular indication in sub-group of patients • sometimes too many
0ece5a81a6390d65ec0630df4c7b0631.ppt