ebdb081d30c48900102feba616f187ae.ppt
- Количество слайдов: 69

Doxil® (doxorubicin HCl liposome injection) Doxil in the Treatment of Advanced, Metastatic Ovarian Cancer Oncologic Drugs Advisory Committee Meeting June 8, 1999 1

Proposed New Indication Doxil (doxorubicin HCl liposomal injection) is indicated for: The treatment of patients with metastatic carcinoma of the ovary who are refractory to both paclitaxel- and platinum-based chemotherapy regimens and who may also be refractory to topotecan. Refractory is defined as a patient having progressive disease while on treatment, or within 6 months of treatment. 2

Agenda Unmet Medical Need: STEALTH® Technology and Doxil Pharmacology: Frank Martin, Ph. D, Principal Scientist Efficacy of Doxil: Ed Schnipper, MD, VP, Clinical Research Safety of Doxil: Ken Cunningham, MD, VP, European Clinical Research Risk/Benefit: 3 Maurie Markman, MD, Director Cleveland Clinic Taussig Cancer Center Ed Schnipper, MD, VP, Clinical Research

Experts Available for Questions Consultants Alan Gordon, MD, Sammons Cancer Center, Dallas William Mc. Guire, MD, University of Mississippi Franco Muggia, MD, NYU Medical Center 4 Sponsor Representatives Martin O’Connell, Ph. D, Sr Dir, Biostatistics Randy Allred, Dr PH, Dir, Biostatistics Peter Working, Ph. D, VP, Nonclinical Research Tim Sharpington, Assoc Dir, Clinical Research Tom Tarlow, Director, Regulatory Affairs

Agenda Unmet Medical Need: 5 Maurie Markman, MD, Cleveland Clinic Taussig Cancer Center

Overview of Ovarian Cancer • 25, 200 patients diagnosed in US in 1999 • 14, 500 deaths • 70% present with advanced disease – Standard treatment is with platinum and paclitaxel • Despite improvements with combination therapy – >20% fail to respond to first-line chemotherapy – 80% ultimately relapse 6

Patient Population Definitions for “second line therapy” • Sensitive patients (those who have a durable response >6 months) likely to respond to retreatment • Refractory patients (those who progressed while on or within 6 months of treatment) unlikely to respond to retreatment 7

Chemotherapy Agents Approved for Second Line Treatment in Ovarian Cancer • Paclitaxel (Taxol) • Altretamine (Hexalen) • Topotecan (Hycamtin) 8
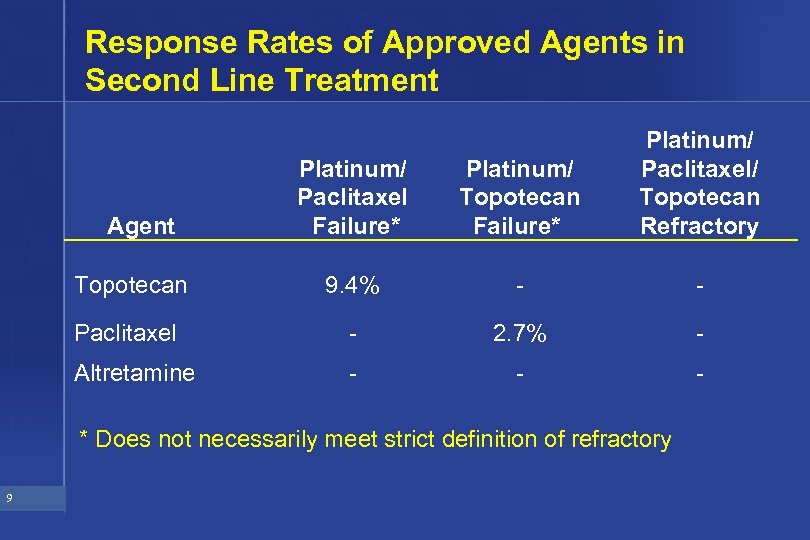
Response Rates of Approved Agents in Second Line Treatment Platinum/ Paclitaxel Failure* Platinum/ Topotecan Failure* Platinum/ Paclitaxel/ Topotecan Refractory Topotecan 9. 4% - - Paclitaxel - 2. 7% - Altretamine - - - Agent * Does not necessarily meet strict definition of refractory 9

Factors Affecting Response • Patients are less likely to respond if they: – Progress while receiving platinum therapy – Progress after receiving multiple regimens 10

Patients with Ovarian Cancer • Relapse common • Long survival • Good Performance Status 11

Medical Need • Need for options and alternative treatments for patients who experienced toxicity on prior therapy: – Neurotoxicity – Bone marrow toxicity – Nausea/Vomiting 12

Quality of Life • Patients treated for long periods • Need for agents which are well tolerated and convenient for patients 13

Agenda Unmet Medical Need: STEALTH® Technology and Doxil Pharmacology: 14 Maurie Markman, MD, Cleveland Clinic Taussig Cancer Center Frank Martin, Ph. D, Principal Scientist
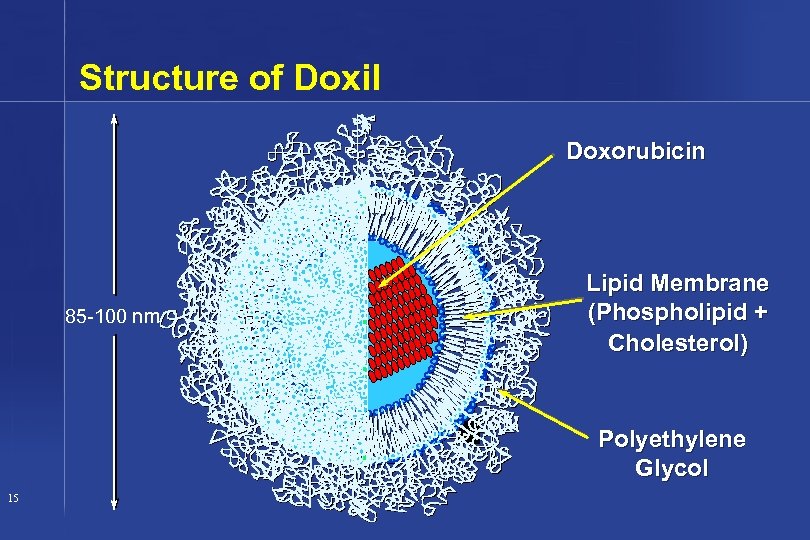
Structure of Doxil Doxorubicin 85 -100 nm Lipid Membrane (Phospholipid + Cholesterol) Polyethylene Glycol 15
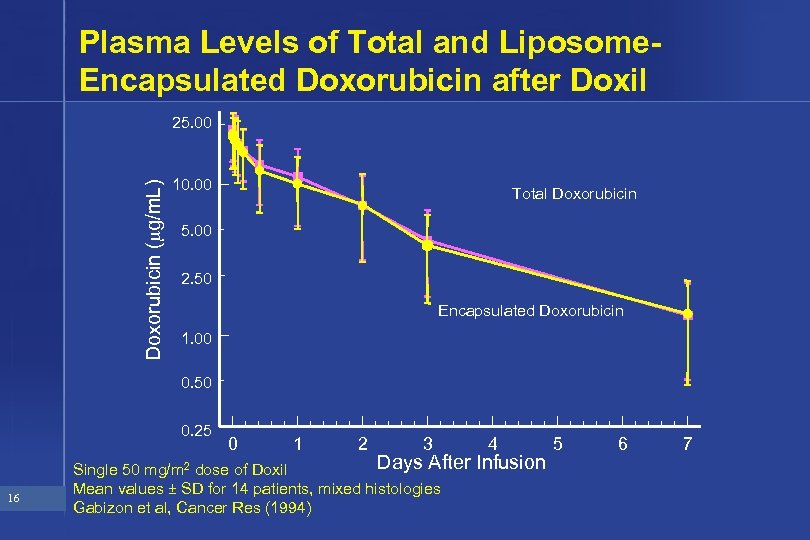
Plasma Levels of Total and Liposome. Encapsulated Doxorubicin after Doxil Doxorubicin ( g/m. L) 25. 00 10. 00 Total Doxorubicin 5. 00 2. 50 Encapsulated Doxorubicin 1. 00 0. 50 0. 25 16 0 1 2 3 Days After Single 50 mg/m 2 dose of Doxil Mean values ± SD for 14 patients, mixed histologies Gabizon et al, Cancer Res (1994) 4 Infusion 5 6 7
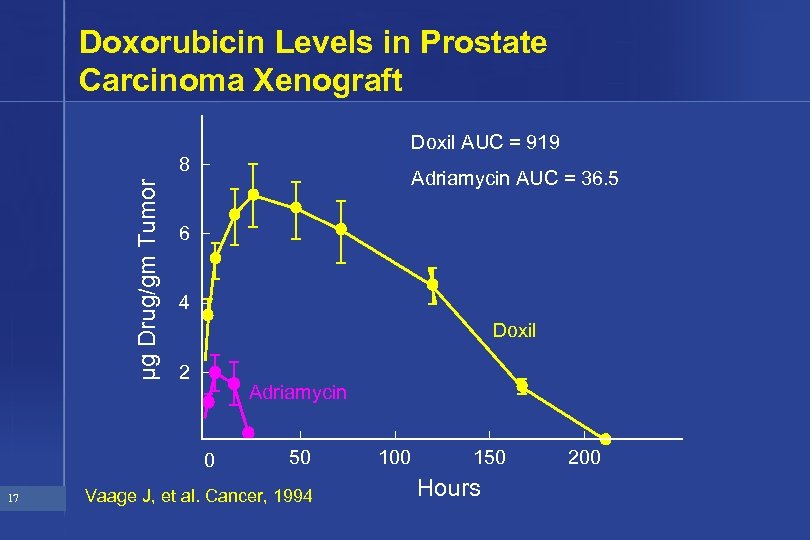
Doxorubicin Levels in Prostate Carcinoma Xenograft Doxil AUC = 919 µg Drug/gm Tumor 8 Adriamycin AUC = 36. 5 6 4 Doxil 2 Adriamycin 0 17 50 Vaage J, et al. Cancer, 1994 100 150 Hours 200
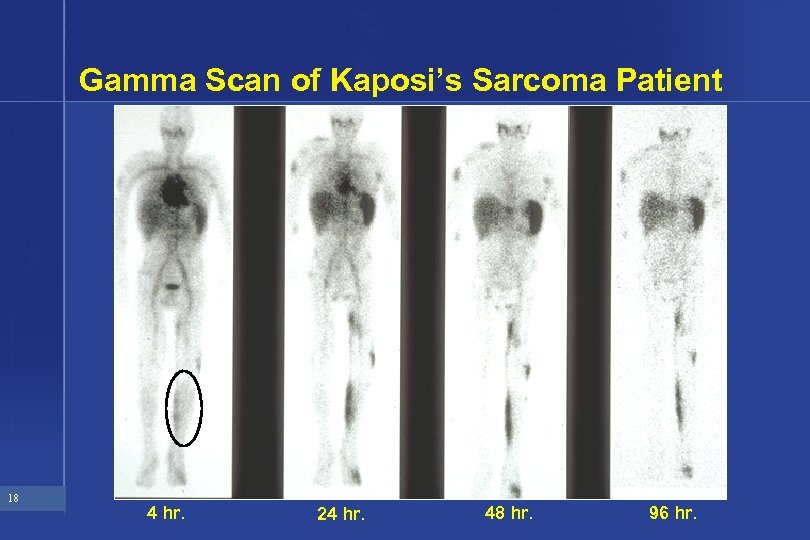
Gamma Scan of Kaposi’s Sarcoma Patient 18 4 hr. 24 hr. 48 hr. 96 hr.
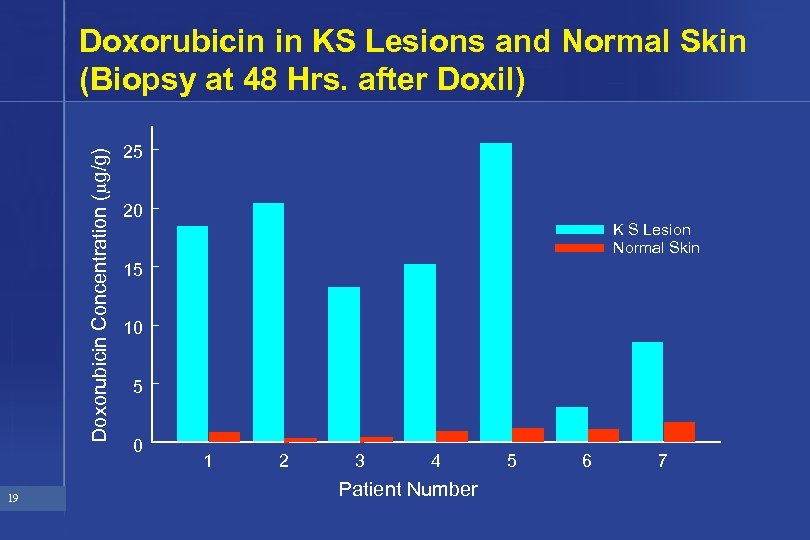
Doxorubicin Concentration ( g/g) Doxorubicin in KS Lesions and Normal Skin (Biopsy at 48 Hrs. after Doxil) 19 25 20 K S Lesion Normal Skin 15 10 5 0 1 2 3 4 Patient Number 5 6 7
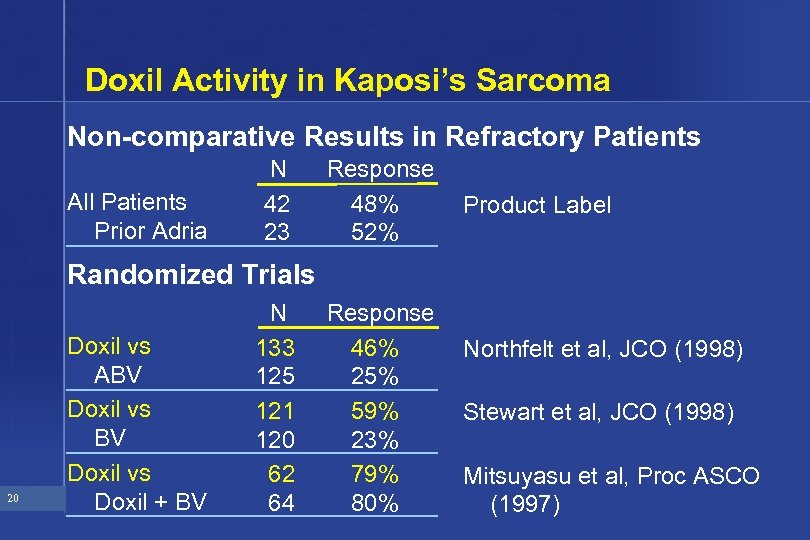
Doxil Activity in Kaposi’s Sarcoma Non-comparative Results in Refractory Patients All Patients Prior Adria N 42 23 Response 48% 52% Product Label Randomized Trials 20 Doxil vs ABV Doxil vs Doxil + BV N 133 125 121 120 62 64 Response 46% 25% 59% 23% 79% 80% Northfelt et al, JCO (1998) Stewart et al, JCO (1998) Mitsuyasu et al, Proc ASCO (1997)
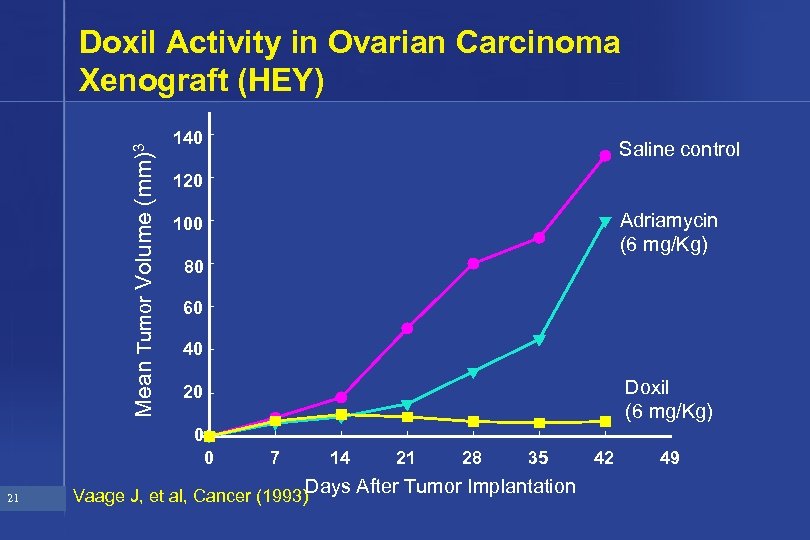
Mean Tumor Volume (mm)3 Doxil Activity in Ovarian Carcinoma Xenograft (HEY) 140 120 Adriamycin (6 mg/Kg) 100 80 60 40 Doxil (6 mg/Kg) 20 0 0 21 Saline control 7 14 21 28 35 Vaage J, et al, Cancer (1993)Days After Tumor Implantation 42 49

Rationale for Exploring Ovarian Indication • Preclinical activity superior to Adriamycin • Activity in heavily pretreated ovarian patients in Phase I trial (Muggia et al, JCO 1995) – 1 PR – 3 minor responses (CA 125 , tumor shrinkage <50%) – 1 disease stabilization 22

Agenda Unmet Medical Need: STEALTH® Technology and Doxil Pharmacology: Frank Martin, Ph. D, Principal Scientist Efficacy of Doxil: 23 Maurie Markman, MD, Cleveland Clinic Taussig Cancer Center Ed Schnipper, MD, VP, Clinical Research
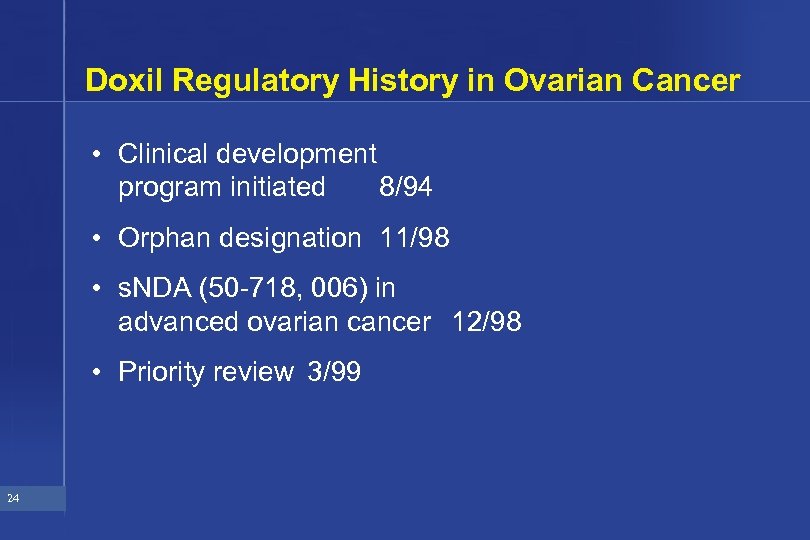
Doxil Regulatory History in Ovarian Cancer • Clinical development program initiated 8/94 • Orphan designation 11/98 • s. NDA (50 -718, 006) in advanced ovarian cancer 12/98 • Priority review 3/99 24

Doxil in Refractory Ovarian Cancer • Doxil is active • Doxil is generally well tolerated • Doxil is convenient 25

Program Overview • Phase II non-comparative studies – 3 studies in relapsed or refractory ovarian cancer • 30 -22, 30 -47, and 30 -47 E • Phase III randomized comparative trial (study 30 -49) – Doxil vs Topotecan – Second line following failure of platinum containing regimen 26
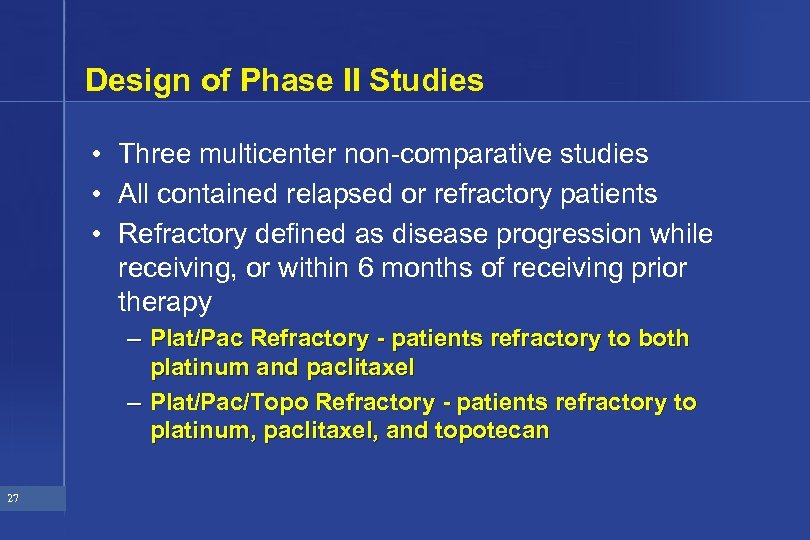
Design of Phase II Studies • Three multicenter non-comparative studies • All contained relapsed or refractory patients • Refractory defined as disease progression while receiving, or within 6 months of receiving prior therapy – Plat/Pac Refractory - patients refractory to both platinum and paclitaxel – Plat/Pac/Topo Refractory - patients refractory to platinum, paclitaxel, and topotecan 27

Endpoints of Phase II Studies • Primary endpoint was response rate – All responses based on measurable disease (SWOG criteria) – All responses confirmed – Independent radiological review • Secondary endpoints – – 28 Time to progression Duration of response Survival Safety

Dosing in Phase II Studies Study Initial Dosing Regimen 30 -22 30 -47 E 50 mg/m 2 q 3 weeks 50 mg/m 2 q 4 weeks Median dose received in all 3 studies was 50 mg/m 2 q 4 weeks 29
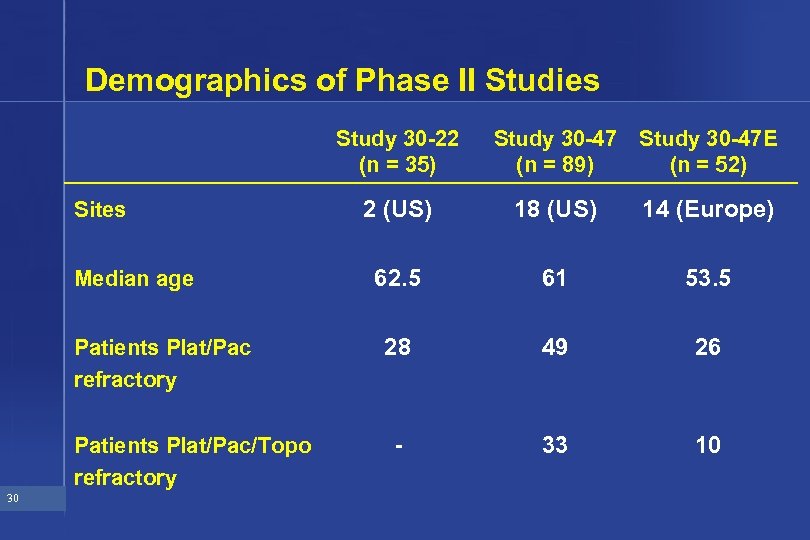
Demographics of Phase II Studies Study 30 -22 (n = 35) Sites Median age Patients Plat/Pac refractory Patients Plat/Pac/Topo refractory 30 Study 30 -47 (n = 89) Study 30 -47 E (n = 52) 2 (US) 18 (US) 14 (Europe) 62. 5 61 53. 5 28 49 26 - 33 10
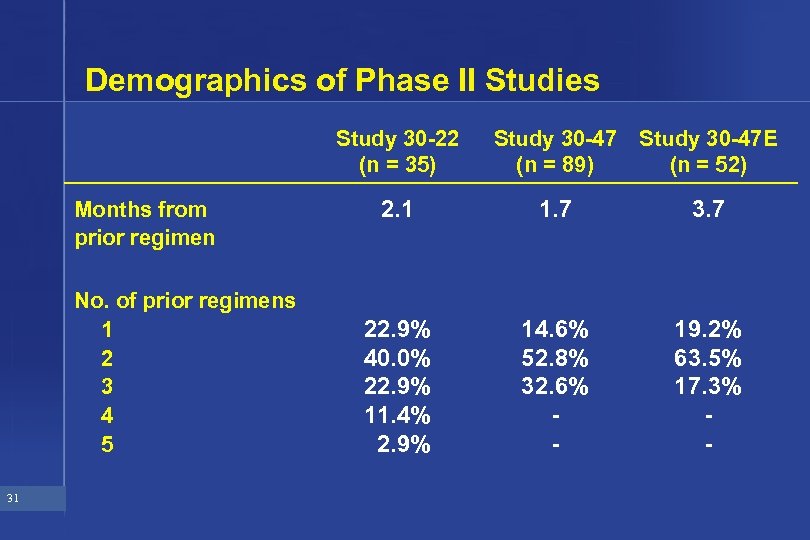
Demographics of Phase II Studies Study 30 -22 (n = 35) Months from prior regimen No. of prior regimens 1 2 3 4 5 31 Study 30 -47 (n = 89) Study 30 -47 E (n = 52) 2. 1 1. 7 3. 7 22. 9% 40. 0% 22. 9% 11. 4% 2. 9% 14. 6% 52. 8% 32. 6% - 19. 2% 63. 5% 17. 3% -
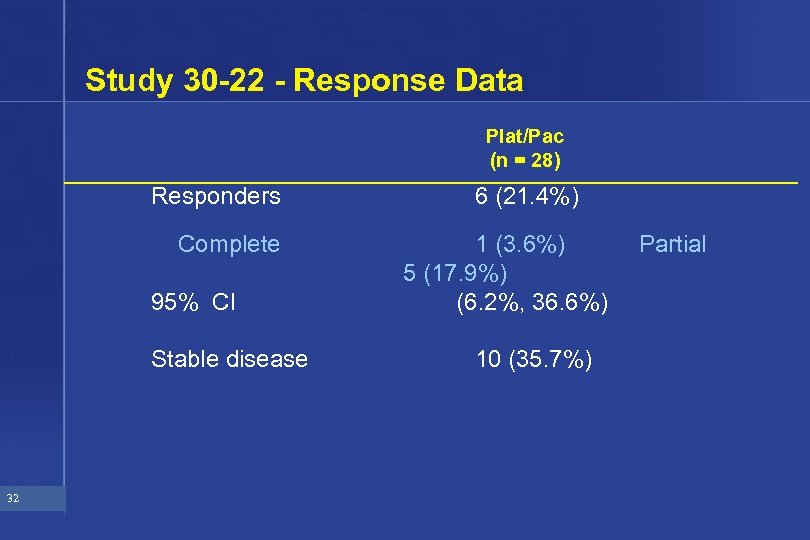
Study 30 -22 - Response Data Plat/Pac (n = 28) Responders Complete 95% CI Stable disease 32 6 (21. 4%) 1 (3. 6%) 5 (17. 9%) (6. 2%, 36. 6%) 10 (35. 7%) Partial
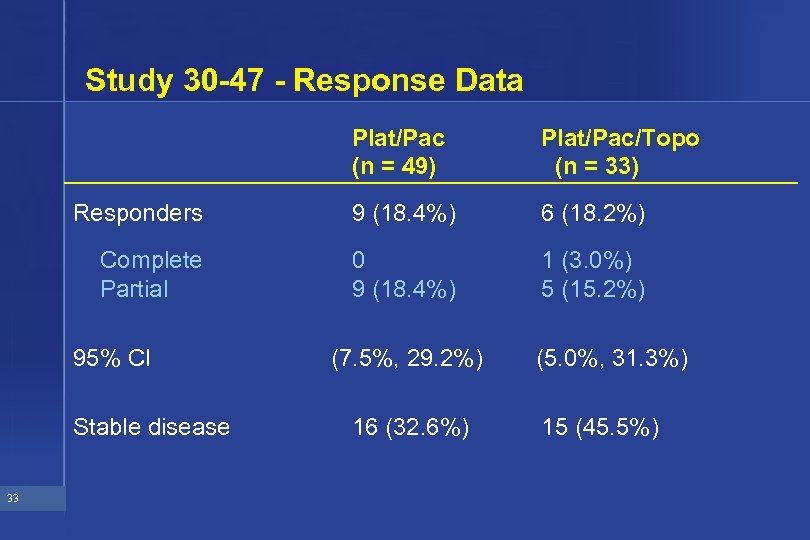
Study 30 -47 - Response Data Plat/Pac (n = 49) Plat/Pac/Topo (n = 33) Responders 9 (18. 4%) 6 (18. 2%) Complete Partial 0 9 (18. 4%) 1 (3. 0%) 5 (15. 2%) 95% CI Stable disease 33 (7. 5%, 29. 2%) 16 (32. 6%) (5. 0%, 31. 3%) 15 (45. 5%)

Study 30 -47 E - Response Data • Refractory patients - no responders 34
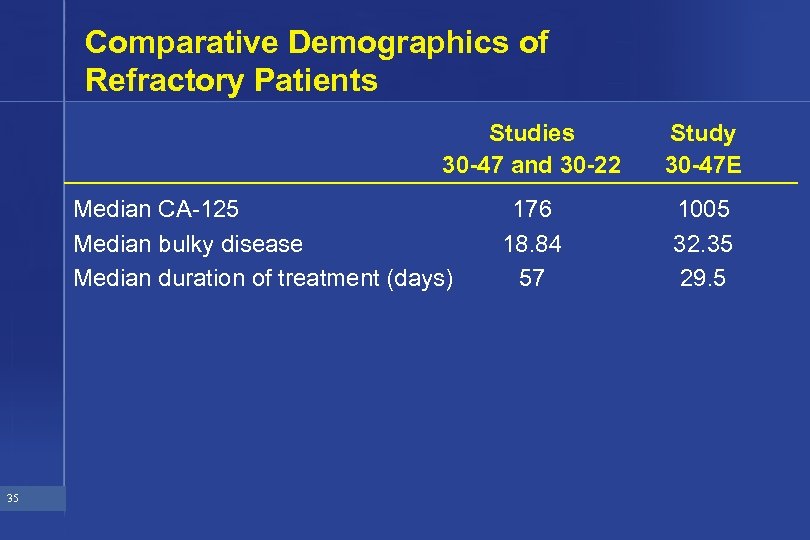
Comparative Demographics of Refractory Patients Studies 30 -47 and 30 -22 Median CA-125 Median bulky disease Median duration of treatment (days) 35 176 18. 84 57 Study 30 -47 E 1005 32. 35 29. 5
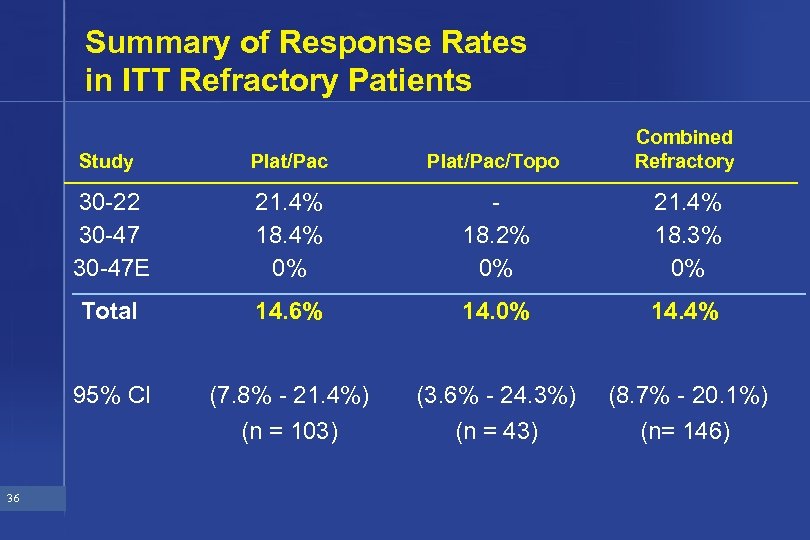
Summary of Response Rates in ITT Refractory Patients Study Plat/Pac/Topo 30 -22 30 -47 E 21. 4% 18. 4% 0% 18. 2% 0% 21. 4% 18. 3% 0% Total 14. 6% 14. 0% 14. 4% 95% CI (7. 8% - 21. 4%) (3. 6% - 24. 3%) (8. 7% - 20. 1%) (n = 103) 36 Plat/Pac Combined Refractory (n = 43) (n= 146)
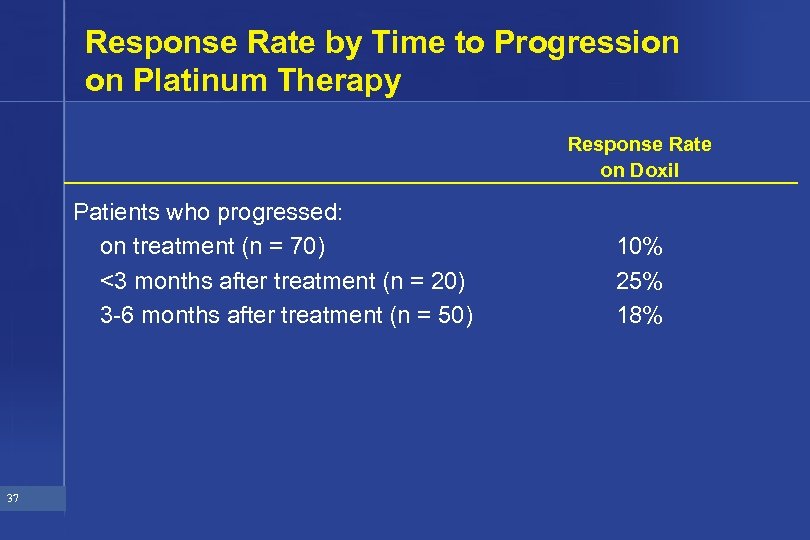
Response Rate by Time to Progression on Platinum Therapy Response Rate on Doxil Patients who progressed: on treatment (n = 70) <3 months after treatment (n = 20) 3 -6 months after treatment (n = 50) 37 10% 25% 18%
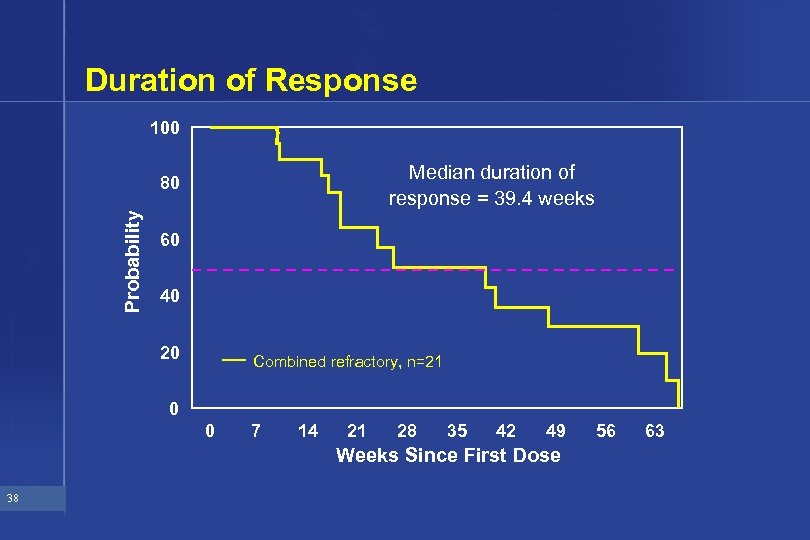
Duration of Response 100 Median duration of response = 39. 4 weeks Probability 80 60 40 20 Combined refractory, n=21 0 0 7 14 21 28 35 42 49 Weeks Since First Dose 38 56 63
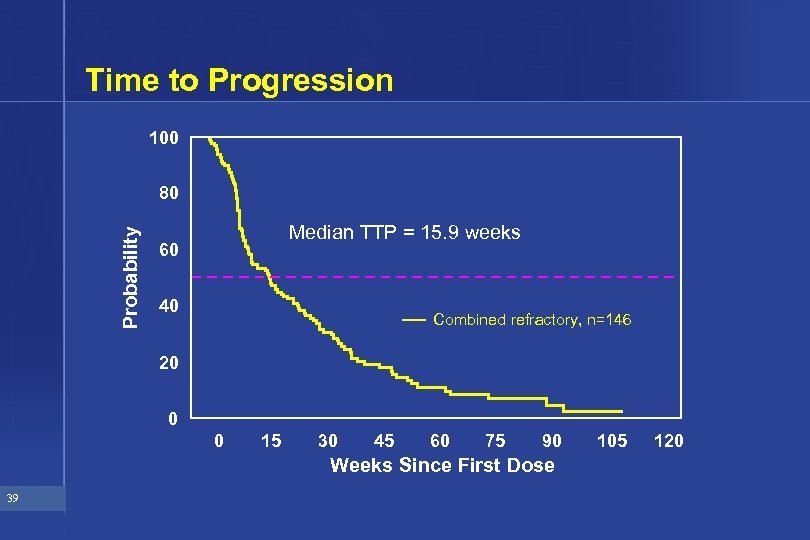
Time to Progression 100 Probability 80 Median TTP = 15. 9 weeks 60 40 Combined refractory, n=146 20 0 0 15 30 45 60 75 90 Weeks Since First Dose 39 105 120
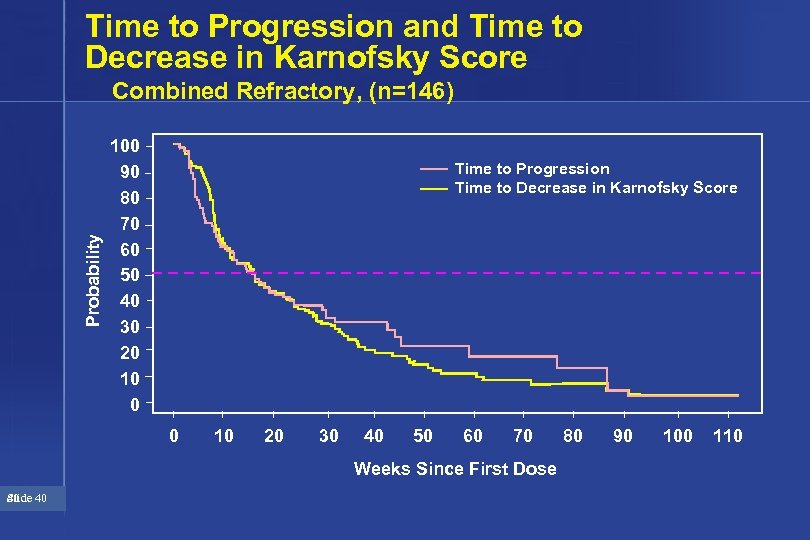
Time to Progression and Time to Decrease in Karnofsky Score Probability Combined Refractory, (n=146) 100 90 80 70 60 50 40 30 20 10 0 Time to Progression Time to Decrease in Karnofsky Score 0 10 20 30 40 50 60 70 Weeks Since First Dose 40 Slide 40 80 90 100 110

Phase III Randomized Comparative Trial (Study 30 -49) • Study design – Second line study following failure of platinum-containing regimen – Patients randomized to receive • Doxil 50 mg/m 2 q 4 weeks or • Topotecan 1. 5 mg/m 2 x 5 d q 3 weeks – Patients with measurable disease – Primary endpoint — time to progression – Secondary endpoints — response rate, duration of response, survival, and safety 41

Study 30 -49 • • 90 sites in US and Europe Target of 460 patients accrued First planned interim analysis 200 evaluable patients (237 ITT) with at least 6 months follow-up • Patients stratified as platinum refractory or sensitive • Subset of 81 patients met the definition of Plat/Pac refractory 42
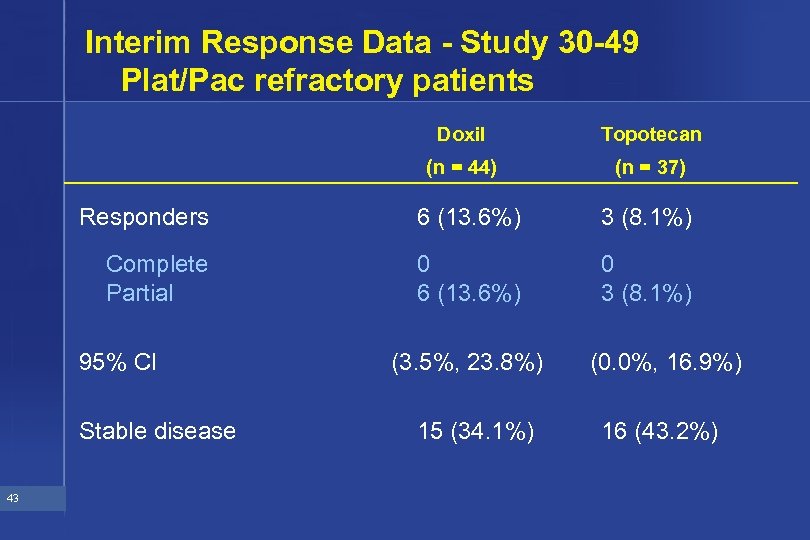
Interim Response Data - Study 30 -49 Plat/Pac refractory patients Doxil Topotecan (n = 44) (n = 37) Responders 6 (13. 6%) 3 (8. 1%) Complete Partial 0 6 (13. 6%) 0 3 (8. 1%) 95% CI Stable disease 43 (3. 5%, 23. 8%) 15 (34. 1%) (0. 0%, 16. 9%) 16 (43. 2%)
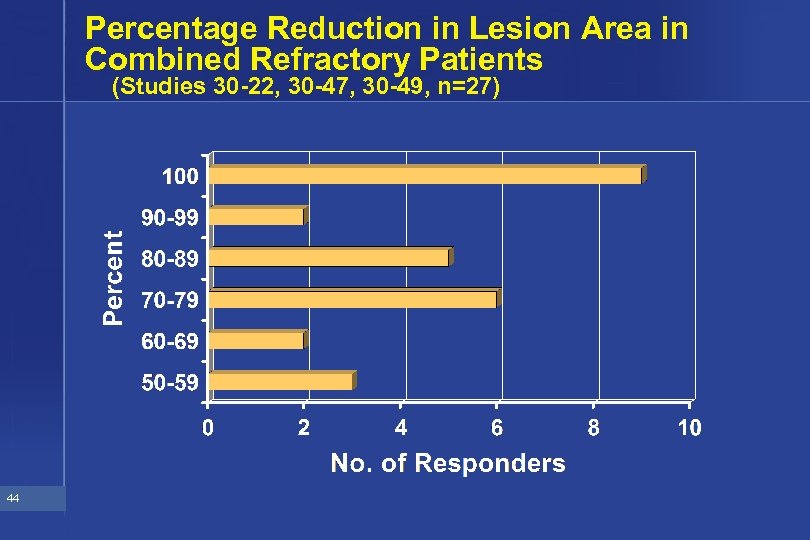
Percentage Reduction in Lesion Area in Combined Refractory Patients (Studies 30 -22, 30 -47, 30 -49, n=27) 44

Efficacy Summary from Phase II Studies • Response 14. 4% in refractory patients • Duration of response 39. 5 weeks • TTP 15. 9 weeks 45

Agenda Unmet Medical Need: STEALTH® Technology and Doxil Pharmacology: Frank Martin, Ph. D, Principal Scientist Efficacy of Doxil: Ed Schnipper, MD, VP, Clinical Research Safety of Doxil: 46 Maurie Markman, MD, Cleveland Clinic Taussig Cancer Center Ken Cunningham, MD, VP, European Clinical Research

Safety Population • 5 studies in ovarian cancer – Total patients 408 • 16 studies in a variety of solid tumors – Total patients 772 • Kaposi’s sarcoma – Total patients 1721 47

Drug Exposure Slide 48
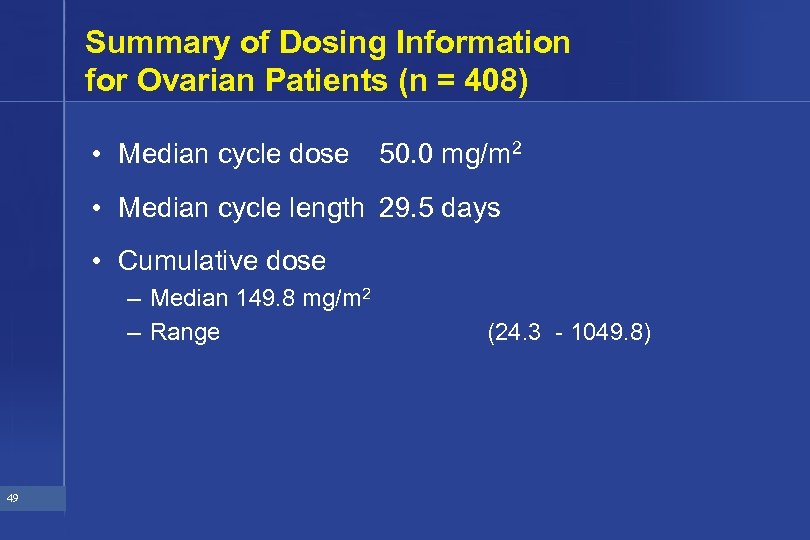
Summary of Dosing Information for Ovarian Patients (n = 408) • Median cycle dose 50. 0 mg/m 2 • Median cycle length 29. 5 days • Cumulative dose – Median 149. 8 mg/m 2 – Range 49 (24. 3 - 1049. 8)
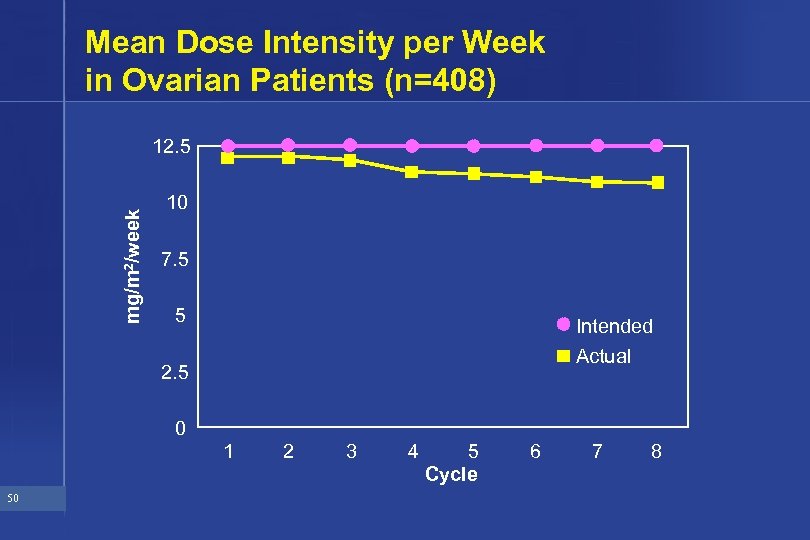
Mean Dose Intensity per Week in Ovarian Patients (n=408) mg/m 2/week 12. 5 10 7. 5 5 Intended Actual 2. 5 0 1 50 2 3 4 5 Cycle 6 7 8

Adverse Events Slide 51
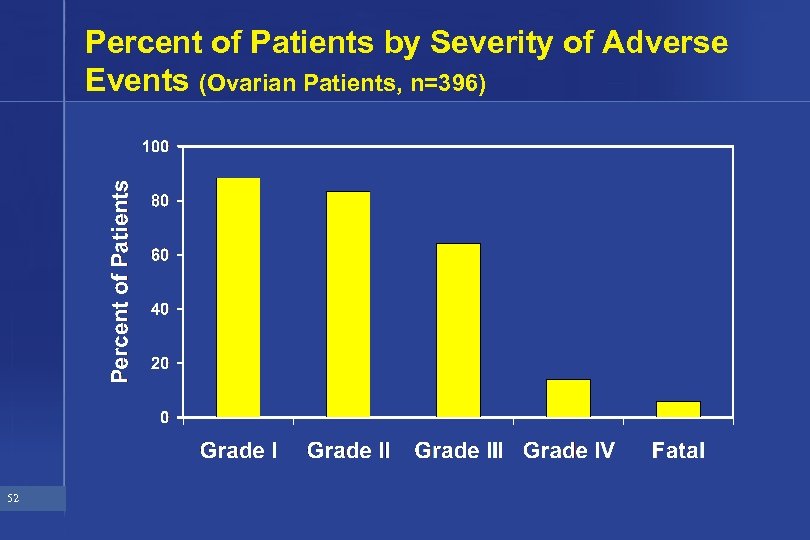
Percent of Patients by Severity of Adverse Events (Ovarian Patients, n=396) 52
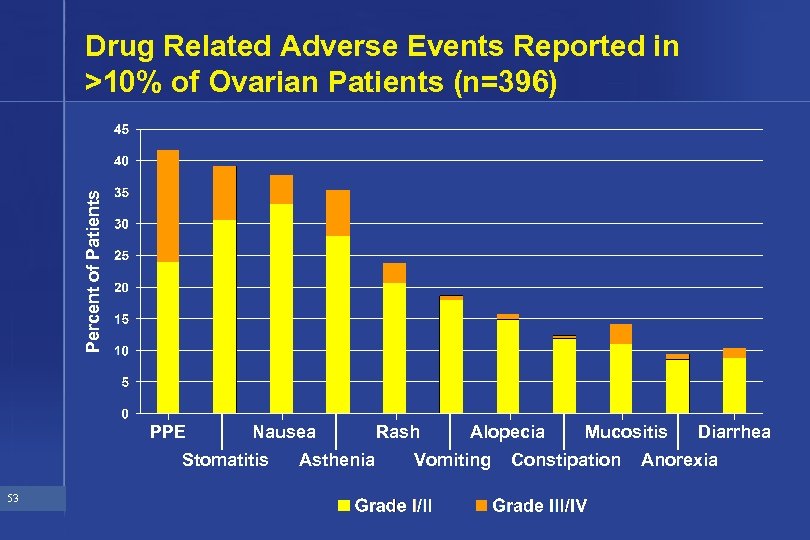
Percent of Patients Drug Related Adverse Events Reported in >10% of Ovarian Patients (n=396) PPE Nausea Stomatitis 53 Asthenia Rash Alopecia Vomiting Mucositis Constipation Diarrhea Anorexia
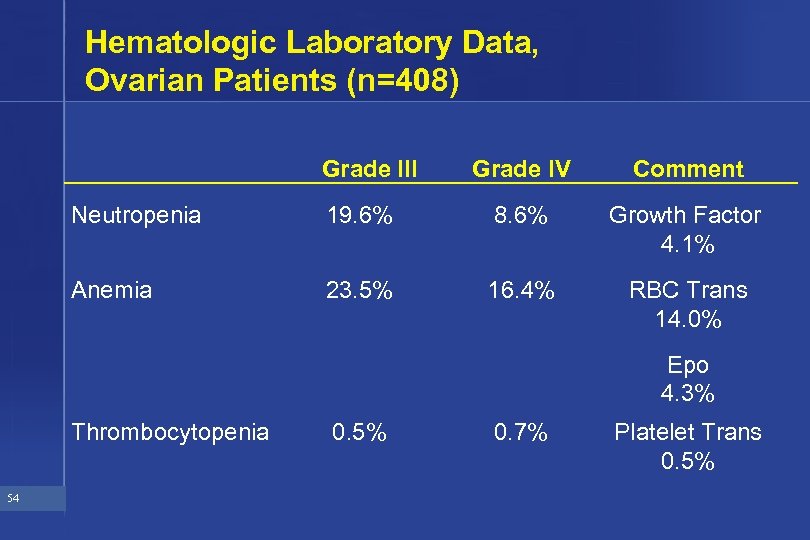
Hematologic Laboratory Data, Ovarian Patients (n=408) Grade III Grade IV Comment Neutropenia 19. 6% 8. 6% Growth Factor 4. 1% Anemia 23. 5% 16. 4% RBC Trans 14. 0% Epo 4. 3% Thrombocytopenia 54 0. 5% 0. 7% Platelet Trans 0. 5%
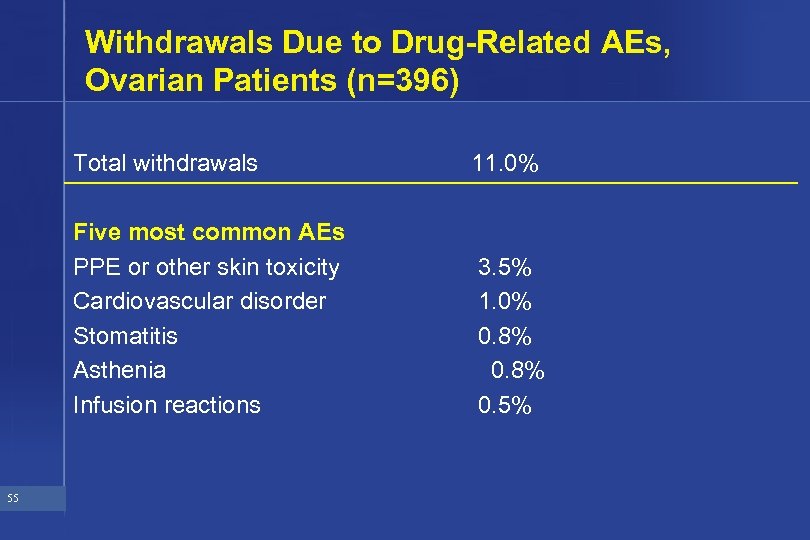
Withdrawals Due to Drug-Related AEs, Ovarian Patients (n=396) Total withdrawals Five most common AEs PPE or other skin toxicity Cardiovascular disorder Stomatitis Asthenia Infusion reactions 55 11. 0% 3. 5% 1. 0% 0. 8% 0. 5%

Palmar-Plantar Erythrodysesthesia (PPE) Management Slide 56
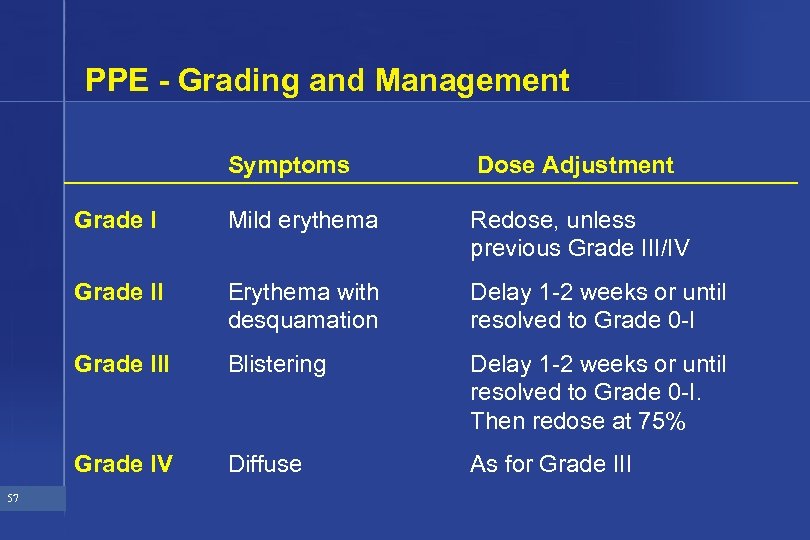
PPE - Grading and Management Symptoms Grade I Mild erythema Redose, unless previous Grade III/IV Grade II Erythema with desquamation Delay 1 -2 weeks or until resolved to Grade 0 -I Grade III Blistering Delay 1 -2 weeks or until resolved to Grade 0 -I. Then redose at 75% Grade IV 57 Dose Adjustment Diffuse As for Grade III

Cardiotoxicity Slide 58

Cardiotoxicity • 0. 8% (6/772) withdrew due to cardiotoxicity related to Doxil – 5 asymptomatic LVEF declines – 1 CHF (after 22 cycles — 944. 3 mg/m 2) • 5 additional drug related cardiac events, all Grade I 59

Cardiac Safety • Doxil PK mimics continuous infusion of doxorubicin • Preclinical - Less cardiotoxic 1 • Biopsies in 10 KS patients (469 to 860 mg/m 2) show minimal cardiotoxicity (Billingham scores 0 -1. 5; median 0. 3)2 • Biopsies in 4 solid tumor patients (675 to 1680 mg/m 2) show minimal cardiotoxicity (Billingham scores 0 -1. 5) 1 Working 60 et al, JPET (1999) 2 Berry, et al, Ann Oncol (1998)

Phase III Randomized Comparative Trial (Study 30 -49) • Doxil - 50 mg/m 2 every 4 weeks • Topotecan - 1. 5 mg/m 2 x 5 days every 3 weeks 61
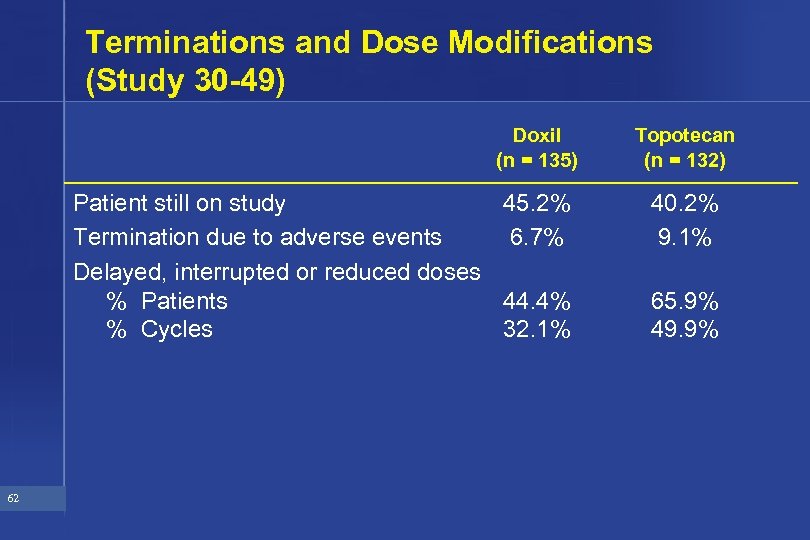
Terminations and Dose Modifications (Study 30 -49) Doxil (n = 135) Patient still on study 45. 2% Termination due to adverse events 6. 7% Delayed, interrupted or reduced doses % Patients 44. 4% % Cycles 32. 1% 62 Topotecan (n = 132) 40. 2% 9. 1% 65. 9% 49. 9%
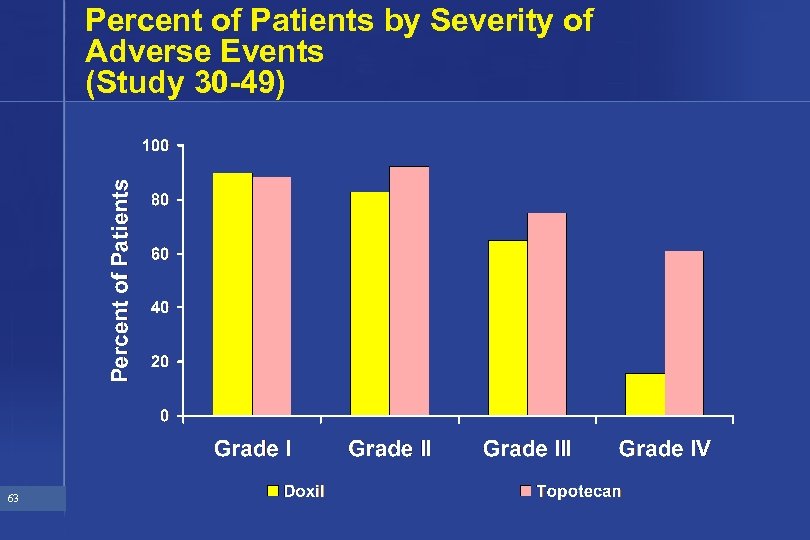
Percent of Patients by Severity of Adverse Events (Study 30 -49) 63
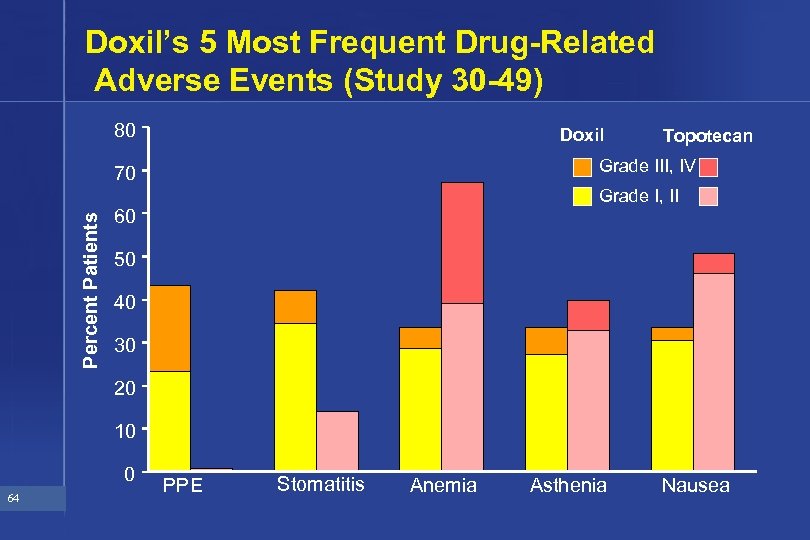
Doxil’s 5 Most Frequent Drug-Related Adverse Events (Study 30 -49) 80 Doxil Grade III, IV 70 Percent Patients Topotecan Grade I, II 60 50 40 30 20 10 0 64 PPE Stomatitis Anemia Asthenia Nausea
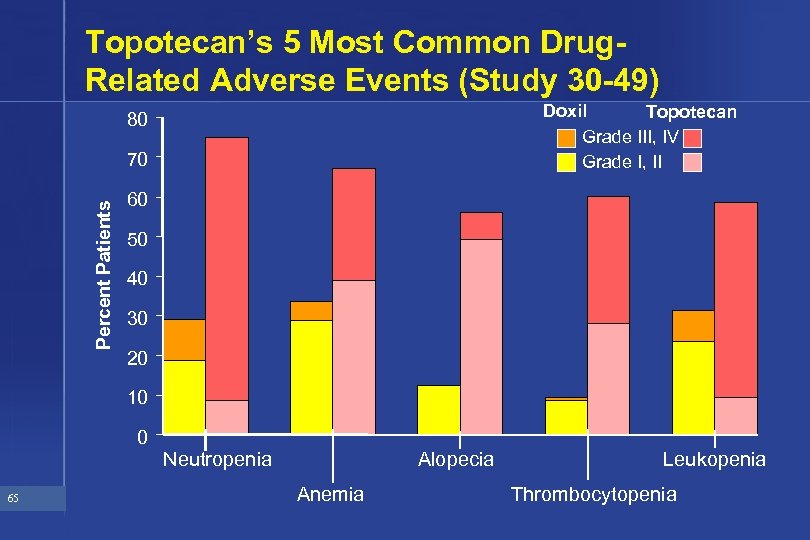
Topotecan’s 5 Most Common Drug. Related Adverse Events (Study 30 -49) Doxil Topotecan Grade III, IV Grade I, II 80 Percent Patients 70 60 50 40 30 20 10 0 65 Neutropenia Alopecia Anemia Leukopenia Thrombocytopenia

Safety Summary • Generally well tolerated • PPE is the most common adverse event and is manageable • Adverse event profile is predictable 66

Agenda Unmet Medical Need: STEALTH® Technology and Doxil Pharmacology: Frank Martin, Ph. D, Principal Scientist Efficacy of Doxil: Ed Schnipper, MD, VP, Clinical Research Safety of Doxil: Ken Cunningham, MD, VP, European Clinical Research Risk/Benefit: 67 Maurie Markman, MD, Cleveland Clinic Taussig Cancer Center Ed Schnipper, MD, VP, Clinical Research

Summary • • • 68 No approved therapy Objective response rate of 14. 4% Duration of response of 39. 4 weeks Generally well tolerated Convenient monthly dosing

Conclusions • Doxil is active in patients with ovarian cancer who are refractory to platinum and paclitaxel and who may also be refractory to Topotecan • Doxil represents a valuable addition to the treatment options for these patients 69
ebdb081d30c48900102feba616f187ae.ppt