eaddc057d30ea8a5fcfa35da3a455226.ppt
- Количество слайдов: 104
 Dosage Form Design: Pharmaceutical and Formulation Considerations Chapter 4 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Dosage Form Design: Pharmaceutical and Formulation Considerations Chapter 4 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Objectives After reading this chapter, the student will be able to: 1. List reasons drugs are incorporated into various dosage forms. 2. Compare and contrast the advantages/disadvantages of various drug dosage forms. 3. Describe the information needed in preformulation studies to characterize a drug substance for possible inclusion into a dosage form. 4. Describe the mechanisms of drug degradation and provide examples of each. 5. Describe the five types of drug instability of concern to the practicing pharmacist. 6. Summarize approaches employed to stabilize drugs in pharmaceutical dosage forms. 7. Calculate reactions for various liquid dosage forms. 8. Categorize various pharmaceutical ingredients and excipients. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Objectives After reading this chapter, the student will be able to: 1. List reasons drugs are incorporated into various dosage forms. 2. Compare and contrast the advantages/disadvantages of various drug dosage forms. 3. Describe the information needed in preformulation studies to characterize a drug substance for possible inclusion into a dosage form. 4. Describe the mechanisms of drug degradation and provide examples of each. 5. Describe the five types of drug instability of concern to the practicing pharmacist. 6. Summarize approaches employed to stabilize drugs in pharmaceutical dosage forms. 7. Calculate reactions for various liquid dosage forms. 8. Categorize various pharmaceutical ingredients and excipients. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Dosage Form Design: Pharmaceutical and Formulation Considerations • The need for dosage forms • General considerations in dosage form design • Pharmaceutical ingredients and excipients Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Dosage Form Design: Pharmaceutical and Formulation Considerations • The need for dosage forms • General considerations in dosage form design • Pharmaceutical ingredients and excipients Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 General Considerations in Dosage Form Design • Preformulation Studies – Physical Description – Microscopic Examination – Heat of Vaporization – Melting Point Depression – The Phase Rule – Particle Size – Polymorphism Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
General Considerations in Dosage Form Design • Preformulation Studies – Physical Description – Microscopic Examination – Heat of Vaporization – Melting Point Depression – The Phase Rule – Particle Size – Polymorphism Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 General Considerations in Dosage Form Design (cont’d) • Preformulation Studies (cont’d) – Solubility and Particle Size – Solubility and p. H – Dissolution – Membrane Permeability – Partition Coefficient – p. Ka/Dissociation Constants Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
General Considerations in Dosage Form Design (cont’d) • Preformulation Studies (cont’d) – Solubility and Particle Size – Solubility and p. H – Dissolution – Membrane Permeability – Partition Coefficient – p. Ka/Dissociation Constants Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 General Considerations in Dosage Form Design (cont’d) • Drug and Drug Product Stability – Drug Stability: Mechanisms of Degradation – Drug and Drug Product Stability: Kinetics and Shelf Life – Rate Reactions – Q 10 Method of Shelf Life Estimation – Enhancing Stability of Drug Products – Stability Testing Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
General Considerations in Dosage Form Design (cont’d) • Drug and Drug Product Stability – Drug Stability: Mechanisms of Degradation – Drug and Drug Product Stability: Kinetics and Shelf Life – Rate Reactions – Q 10 Method of Shelf Life Estimation – Enhancing Stability of Drug Products – Stability Testing Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Pharmaceutical Ingredients and Excipients • Definitions and Types • Handbook of Pharmaceutical Excipients • Harmonization of Standards • Appearance and Palatability – Flavoring Pharmaceuticals – Sweetening Pharmaceuticals – Coloring Pharmaceuticals Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Pharmaceutical Ingredients and Excipients • Definitions and Types • Handbook of Pharmaceutical Excipients • Harmonization of Standards • Appearance and Palatability – Flavoring Pharmaceuticals – Sweetening Pharmaceuticals – Coloring Pharmaceuticals Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Pharmaceutical Ingredients and Excipients (cont’d) • Preservatives – Sterilization and Preservation – Preservative Selection – General Preservative Considerations – Mode of Action – Preservative Utilization Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Pharmaceutical Ingredients and Excipients (cont’d) • Preservatives – Sterilization and Preservation – Preservative Selection – General Preservative Considerations – Mode of Action – Preservative Utilization Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 The Need for Dosage Forms Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
The Need for Dosage Forms Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 The Need for Dosage Forms • Mechanism for safe and convenient delivery of accurate dosage • Protection of drug from atmosphere • Protection of drug from gastric acid (EC) • Conceal bitter, salty, or offensive taste or odor • Provide liquid preparations of insoluble drugs Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
The Need for Dosage Forms • Mechanism for safe and convenient delivery of accurate dosage • Protection of drug from atmosphere • Protection of drug from gastric acid (EC) • Conceal bitter, salty, or offensive taste or odor • Provide liquid preparations of insoluble drugs Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 The Need for Dosage Forms (cont’d) • Provide clear liquid dosage forms (solutions) • Provide rate-controlled drug action • Provide topical drug action (ointments, creams, patches, ophthalmic, otic, nasal) • Provide for insertion into body cavity • Provide for placement into bloodstream • Provide for inhalation therapy Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
The Need for Dosage Forms (cont’d) • Provide clear liquid dosage forms (solutions) • Provide rate-controlled drug action • Provide topical drug action (ointments, creams, patches, ophthalmic, otic, nasal) • Provide for insertion into body cavity • Provide for placement into bloodstream • Provide for inhalation therapy Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 General Considerations in Dosage Form Design Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
General Considerations in Dosage Form Design Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Physiological States Altering Response to Drugs • Age (infants) • Body weight • Age (elderly) • Time of administration • Diurnal variation • Tolerance • Pregnancy • Temperature • Sex • Physiological reserve • Menopause • Milieu • Race Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Physiological States Altering Response to Drugs • Age (infants) • Body weight • Age (elderly) • Time of administration • Diurnal variation • Tolerance • Pregnancy • Temperature • Sex • Physiological reserve • Menopause • Milieu • Race Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Factors Affecting Drug Presentation to the Body • Portal of drug entry into the body • Physical form of the drug product • Design and formulation of the product • Method of manufacture of the product • Physicochemical properties of the drug and excipients Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Factors Affecting Drug Presentation to the Body • Portal of drug entry into the body • Physical form of the drug product • Design and formulation of the product • Method of manufacture of the product • Physicochemical properties of the drug and excipients Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Factors Affecting Drug Presentation to the Body (cont’d) • Physicochemical properties of the drug product • Control and maintenance of location of drug at the absorption site • Control of the release rate of the drug from the drug product Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Factors Affecting Drug Presentation to the Body (cont’d) • Physicochemical properties of the drug product • Control and maintenance of location of drug at the absorption site • Control of the release rate of the drug from the drug product Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Design of Drug Products • Effectiveness • Safety • Reliability • Stability – Physical – Chemical – Microbiological Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Design of Drug Products • Effectiveness • Safety • Reliability • Stability – Physical – Chemical – Microbiological Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Design of Drug Products (cont’d) • Pharmaceutical elegance – Appearance – Organoleptic properties • Convenience – Ease of use – Dosing frequency – Consumer acceptance Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Design of Drug Products (cont’d) • Pharmaceutical elegance – Appearance – Organoleptic properties • Convenience – Ease of use – Dosing frequency – Consumer acceptance Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 General Considerations in Dosage Form Design • Preformulation Studies • Drug and Drug Product Stability Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
General Considerations in Dosage Form Design • Preformulation Studies • Drug and Drug Product Stability Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Preformulation Studies • Chemical characterization • Physical characterization Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Preformulation Studies • Chemical characterization • Physical characterization Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Physical Description • Solids, liquids, gases • Chemical Properties – Structure, form, reactivity • Physical Properties – Description, particle size, crystalline structure, melting point, solubility • Biological Properties – Ability to get to site of action and elicit a response Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Physical Description • Solids, liquids, gases • Chemical Properties – Structure, form, reactivity • Physical Properties – Description, particle size, crystalline structure, melting point, solubility • Biological Properties – Ability to get to site of action and elicit a response Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Microscopic Examination • Particle size range • Crystal structure • Particle shape Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Microscopic Examination • Particle size range • Crystal structure • Particle shape Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Heat of Vaporization • Vapor pressure • Volatile drugs can migrate within a solid dosage form • Personnel exposure Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Heat of Vaporization • Vapor pressure • Volatile drugs can migrate within a solid dosage form • Personnel exposure Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Melting Point Depression • Purity determination • Identity Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Melting Point Depression • Purity determination • Identity Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 The Phase Rule • Phase diagrams • Visual picture of presence of solid and liquid phases in binary, ternary, and other mixtures Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
The Phase Rule • Phase diagrams • Visual picture of presence of solid and liquid phases in binary, ternary, and other mixtures Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Particle Size • Dissolution rate • Bioavailability • Content uniformity • Taste • Texture • Color • Stability • Flow characteristics • Sedimentation rates Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Particle Size • Dissolution rate • Bioavailability • Content uniformity • Taste • Texture • Color • Stability • Flow characteristics • Sedimentation rates Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Polymorphism • Crystalline • Amorphous • Melting point variation • Solubility differences Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Polymorphism • Crystalline • Amorphous • Melting point variation • Solubility differences Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Solubility • Some aqueous solubility required for therapeutic efficacy • Equilibrium solubility • Solubility in different solvents Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Solubility • Some aqueous solubility required for therapeutic efficacy • Equilibrium solubility • Solubility in different solvents Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Solubility and Particle Size • Small increases in solubility can be achieved by particle size reduction. • Decreases in particle size may enhance dissolution rates. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Solubility and Particle Size • Small increases in solubility can be achieved by particle size reduction. • Decreases in particle size may enhance dissolution rates. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Solubility and p. H • p. H: solubility profiles are important. • p. H can affect solubility. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Solubility and p. H • p. H: solubility profiles are important. • p. H can affect solubility. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Dissolution • Dissolution may be rate-limiting step in the absorption of poorly soluble drugs. • Can affect onset, intensity, and duration of response and control overall bioavailability of the drug from the dosage form Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Dissolution • Dissolution may be rate-limiting step in the absorption of poorly soluble drugs. • Can affect onset, intensity, and duration of response and control overall bioavailability of the drug from the dosage form Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Membrane Permeability • p. Ka, solubility, and dissolution rate data can provide an indication of absorption. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Membrane Permeability • p. Ka, solubility, and dissolution rate data can provide an indication of absorption. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Partition Coefficient • Octanol: water partition coefficient often used in formulation development Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Partition Coefficient • Octanol: water partition coefficient often used in formulation development Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 p. Ka/Dissociation Constants • Extent of dissociation or ionization • Dependent on p. H of medium • Can affect absorption, distribution, and elimination Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
p. Ka/Dissociation Constants • Extent of dissociation or ionization • Dependent on p. H of medium • Can affect absorption, distribution, and elimination Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Drug and Drug Product Stability • Physical stability • Chemical stability • Shelf life of 2 -3 years is generally desired Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Drug and Drug Product Stability • Physical stability • Chemical stability • Shelf life of 2 -3 years is generally desired Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Drug Stability: Mechanisms of Degradation • Hydrolysis, solvolysis • Oxidation • Other processes Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Drug Stability: Mechanisms of Degradation • Hydrolysis, solvolysis • Oxidation • Other processes Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Drug and Drug Product Stability: Kinetics and Shelf Life • Chemical stability • Physical stability • Microbiological stability • Therapeutic stability • Toxicologic stability Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Drug and Drug Product Stability: Kinetics and Shelf Life • Chemical stability • Physical stability • Microbiological stability • Therapeutic stability • Toxicologic stability Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Rate Reactions • Change of drug concentration with respect to time Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Rate Reactions • Change of drug concentration with respect to time Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Q 10 Method of Shelf Life Estimation • Shelf life estimation • Q 10 = e{(Ea/R)[(1/T + 10) – (1/T)]} Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Q 10 Method of Shelf Life Estimation • Shelf life estimation • Q 10 = e{(Ea/R)[(1/T + 10) – (1/T)]} Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Enhancing Stability of Drug Products • Excipients may be added to protect the drug – Antioxidants – Preservatives – Chelating agents – Buffering agents Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Enhancing Stability of Drug Products • Excipients may be added to protect the drug – Antioxidants – Preservatives – Chelating agents – Buffering agents Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Stability Testing • Done at each stage of product development • Product containers and closures must be considered • Temperature and humidity studies • Light studies • Changes in physical appearance, color, odor, taste, texture • Chemical changes of drug degradation • Pharmacist is last professional to check for quality and stability prior to dispensing Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Stability Testing • Done at each stage of product development • Product containers and closures must be considered • Temperature and humidity studies • Light studies • Changes in physical appearance, color, odor, taste, texture • Chemical changes of drug degradation • Pharmacist is last professional to check for quality and stability prior to dispensing Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Kinetics and Drug Stability Kinetics is important in all phases of the drug development process as well as in quality control, stability, bioavailability, and therapeutic drug monitoring. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Kinetics and Drug Stability Kinetics is important in all phases of the drug development process as well as in quality control, stability, bioavailability, and therapeutic drug monitoring. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Kinetics • The study of the rate of chemical change and the way this rate is influenced by conditions of concentration of reactants, products, and other chemical species that may be present and by factors such as solvent, pressure, and temperature Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Kinetics • The study of the rate of chemical change and the way this rate is influenced by conditions of concentration of reactants, products, and other chemical species that may be present and by factors such as solvent, pressure, and temperature Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Importance of Kinetics 1. Selection of proper storage temperature – Temperature – Light – Advising patient on storage conditions 2. Selection of proper container for dispensing – Glass vs. plastic – Clear vs. amber vs. opaque – Cap liner selection Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Importance of Kinetics 1. Selection of proper storage temperature – Temperature – Light – Advising patient on storage conditions 2. Selection of proper container for dispensing – Glass vs. plastic – Clear vs. amber vs. opaque – Cap liner selection Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Importance of Kinetics (cont’d) 3. Anticipation of interactions when mixing drugs and dosage forms (incompatibilities) – Active drugs – Excipients 4. Dissolution determinations Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Importance of Kinetics (cont’d) 3. Anticipation of interactions when mixing drugs and dosage forms (incompatibilities) – Active drugs – Excipients 4. Dissolution determinations Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Importance of Kinetics (cont’d) 5. ADME Processes in pharmacokinetics – A = Absorption – D = Distribution – M = Metabolism/Biotransformation – E = Excretion 6. Drug action at the molecular level Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Importance of Kinetics (cont’d) 5. ADME Processes in pharmacokinetics – A = Absorption – D = Distribution – M = Metabolism/Biotransformation – E = Excretion 6. Drug action at the molecular level Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Responsibility of the Pharmacist • Dispense oldest stock first and observe expiration dates. • Store products under conditions stated in USP monographs and/or labeling. • Observe products for evidence of instability. • Properly treat/label products that are repackaged, diluted, or mixed with other products. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Responsibility of the Pharmacist • Dispense oldest stock first and observe expiration dates. • Store products under conditions stated in USP monographs and/or labeling. • Observe products for evidence of instability. • Properly treat/label products that are repackaged, diluted, or mixed with other products. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Responsibility of the Pharmacist (cont’d) • Dispensing in proper container with proper closure • Informing/educating patients concerning proper storage and use of products, including the disposition of outdated or excessively aged prescriptions Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Responsibility of the Pharmacist (cont’d) • Dispensing in proper container with proper closure • Informing/educating patients concerning proper storage and use of products, including the disposition of outdated or excessively aged prescriptions Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Why Do We Need Shelf Life Estimates? • Expiration date given at room temperature: – What is the expiration extension if refrigerated? • Expiration date for refrig temperature given: – How long if left at room temperature? • Expiration date for room temperature given and it is desired to heat (autoclave): – What is the % decomposition? Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Why Do We Need Shelf Life Estimates? • Expiration date given at room temperature: – What is the expiration extension if refrigerated? • Expiration date for refrig temperature given: – How long if left at room temperature? • Expiration date for room temperature given and it is desired to heat (autoclave): – What is the % decomposition? Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Why Do We Need Shelf Life Estimates? (cont’d) • Expiration date for refrigerated temperature given; product stored at room temperature and then returned to refrigerator: – What is the new expiration date? Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Why Do We Need Shelf Life Estimates? (cont’d) • Expiration date for refrigerated temperature given; product stored at room temperature and then returned to refrigerator: – What is the new expiration date? Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Stability: USP • The extent to which a product retains, within specified limits, and throughout its period of storage and use (i. e. , its shelf life), the same properties and characteristics that it possessed at the time of manufacture Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Stability: USP • The extent to which a product retains, within specified limits, and throughout its period of storage and use (i. e. , its shelf life), the same properties and characteristics that it possessed at the time of manufacture Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Definitions • Accelerated Testing – Studies designed to increase the rate of chemical or physical degradation by using exaggerated storage conditions • Bulk Drug Substance – Active drug before formulation • Drug Product – Finished dosage form Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Definitions • Accelerated Testing – Studies designed to increase the rate of chemical or physical degradation by using exaggerated storage conditions • Bulk Drug Substance – Active drug before formulation • Drug Product – Finished dosage form Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Definitions (cont’d) • Expiration Date – The date placed on the immediate container label of a drug product that designates the date through which the product is expected to remain within specifications • Expiration Dating Period – The interval that a drug product is expected to remain within the approved specifications after manufacture Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Definitions (cont’d) • Expiration Date – The date placed on the immediate container label of a drug product that designates the date through which the product is expected to remain within specifications • Expiration Dating Period – The interval that a drug product is expected to remain within the approved specifications after manufacture Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Definitions (cont’d) • Primary Stability Data – Data on the drug product stored in the proposed container-closure for marketing under storage conditions that support the proposed expiration date Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Definitions (cont’d) • Primary Stability Data – Data on the drug product stored in the proposed container-closure for marketing under storage conditions that support the proposed expiration date Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Definitions (cont’d) • Stability-Indicating Methodology – Quantitative analytical methods based on the characteristic structural, chemical, or biological properties of each active ingredient of a drug product, and that will distinguish each active ingredient from its degradation products so that the active ingredient content can be accurately measured Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Definitions (cont’d) • Stability-Indicating Methodology – Quantitative analytical methods based on the characteristic structural, chemical, or biological properties of each active ingredient of a drug product, and that will distinguish each active ingredient from its degradation products so that the active ingredient content can be accurately measured Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Definitions (cont’d) • Stability – The capacity of a drug product to remain within specifications established to ensure its identity, strength, quality, and purity • Strength – A quantitative measure of active ingredient, as well as other ingredients requiring quantitation • Supportive Stability Data – Data other than primary stability data Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Definitions (cont’d) • Stability – The capacity of a drug product to remain within specifications established to ensure its identity, strength, quality, and purity • Strength – A quantitative measure of active ingredient, as well as other ingredients requiring quantitation • Supportive Stability Data – Data other than primary stability data Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Physical Paths of Instability • 1. Polymorphs – Cocoa butter, Cortisone Acetate • 2. Crystallization – Solutions, suspensions • 3. Vaporization – Flavoring agents, cosolvents, nitroglycerin • 4. Particle sedimentation – Suspensions Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Physical Paths of Instability • 1. Polymorphs – Cocoa butter, Cortisone Acetate • 2. Crystallization – Solutions, suspensions • 3. Vaporization – Flavoring agents, cosolvents, nitroglycerin • 4. Particle sedimentation – Suspensions Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Observing Products for Evidence of Instability • Solid Dosage Forms – Hard/soft gelatin capsules – Uncoated tablets – Coated tablets – Dry powders and granules – Powders/granules for solution/suspension – Effervescent tablets/granules/powders Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Observing Products for Evidence of Instability • Solid Dosage Forms – Hard/soft gelatin capsules – Uncoated tablets – Coated tablets – Dry powders and granules – Powders/granules for solution/suspension – Effervescent tablets/granules/powders Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Observing Products for Evidence of Instability (cont’d) • Liquid Dosage Forms – Solutions/elixirs/syrups – Emulsions – Suspensions – Tinctures/fluid extracts – Sterile liquids • Semisolids – Creams – Ointments – Suppositories Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Observing Products for Evidence of Instability (cont’d) • Liquid Dosage Forms – Solutions/elixirs/syrups – Emulsions – Suspensions – Tinctures/fluid extracts – Sterile liquids • Semisolids – Creams – Ointments – Suppositories Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Reaction Kinetics • Want two things from kinetic data: – Reaction order – Reaction rate • In considering the chemical stability of a pharmaceutical, we need to know the REACTION ORDER, which is obtained experimentally by measuring the REACTION RATE as a function of concentration of the degrading drug. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Reaction Kinetics • Want two things from kinetic data: – Reaction order – Reaction rate • In considering the chemical stability of a pharmaceutical, we need to know the REACTION ORDER, which is obtained experimentally by measuring the REACTION RATE as a function of concentration of the degrading drug. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Reaction Kinetics • The overall ORDER of a reaction is the SUM of the EXPONENTS of the CONCENTRATION terms of the RATE EXPRESSION. • The ORDER with respect to EACH REACTANT is the EXPONENT of the INDIVIDUAL CONCENTRATION terms in the RATE EXPRESSION. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Reaction Kinetics • The overall ORDER of a reaction is the SUM of the EXPONENTS of the CONCENTRATION terms of the RATE EXPRESSION. • The ORDER with respect to EACH REACTANT is the EXPONENT of the INDIVIDUAL CONCENTRATION terms in the RATE EXPRESSION. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Order of a Reaction • An experimental quantity; merely provides information about the way in which the rate depends on concentration Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Order of a Reaction • An experimental quantity; merely provides information about the way in which the rate depends on concentration Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Factors Affecting Reaction Rates • Temperature • Dielectric Constant • Ionic Strength • Solvent Effect • Catalysis • Light Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Factors Affecting Reaction Rates • Temperature • Dielectric Constant • Ionic Strength • Solvent Effect • Catalysis • Light Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
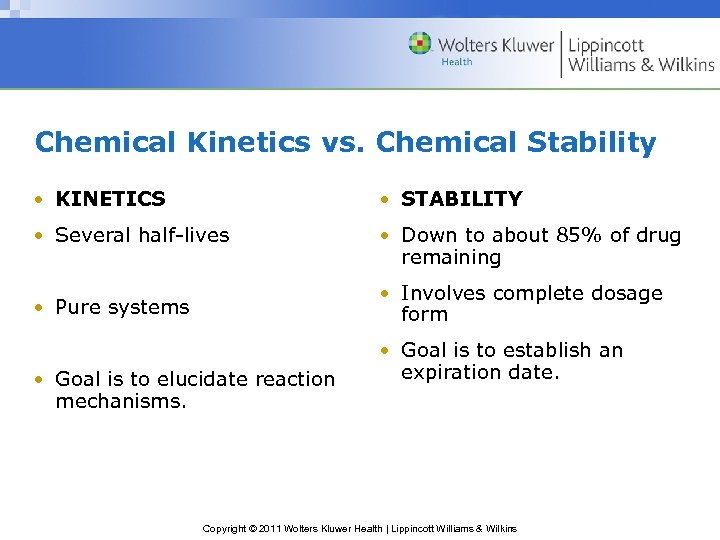 Chemical Kinetics vs. Chemical Stability • KINETICS • STABILITY • Several half-lives • Down to about 85% of drug remaining • Involves complete dosage form • Pure systems • Goal is to elucidate reaction mechanisms. • Goal is to establish an expiration date. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Chemical Kinetics vs. Chemical Stability • KINETICS • STABILITY • Several half-lives • Down to about 85% of drug remaining • Involves complete dosage form • Pure systems • Goal is to elucidate reaction mechanisms. • Goal is to establish an expiration date. Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 First-Order Rate Reaction –dt d. C = k. C Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
First-Order Rate Reaction –dt d. C = k. C Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Half-Life • Is meaningless to attempt to describe the time required for ALL material to decompose (i. e. , infinity) • Therefore, reaction rate can be described by K or halflife, t 1/2 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Half-Life • Is meaningless to attempt to describe the time required for ALL material to decompose (i. e. , infinity) • Therefore, reaction rate can be described by K or halflife, t 1/2 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
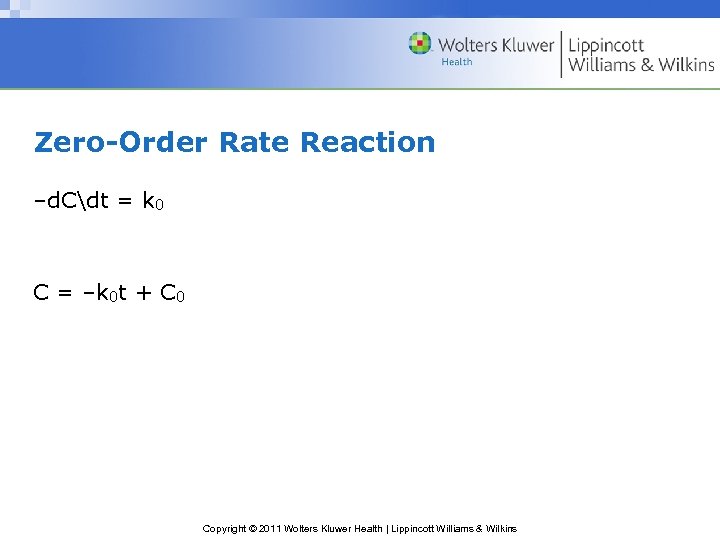 Zero-Order Rate Reaction –d. Cdt = k 0 C = –k 0 t + C 0 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Zero-Order Rate Reaction –d. Cdt = k 0 C = –k 0 t + C 0 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Arrhenius Equation log = k 2k 1 = Ea (t 2 – T 1)2. 3 RT 1 T 2 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Arrhenius Equation log = k 2k 1 = Ea (t 2 – T 1)2. 3 RT 1 T 2 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Arrhenius Equation (cont’d) • Energy of activation calculations Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Arrhenius Equation (cont’d) • Energy of activation calculations Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
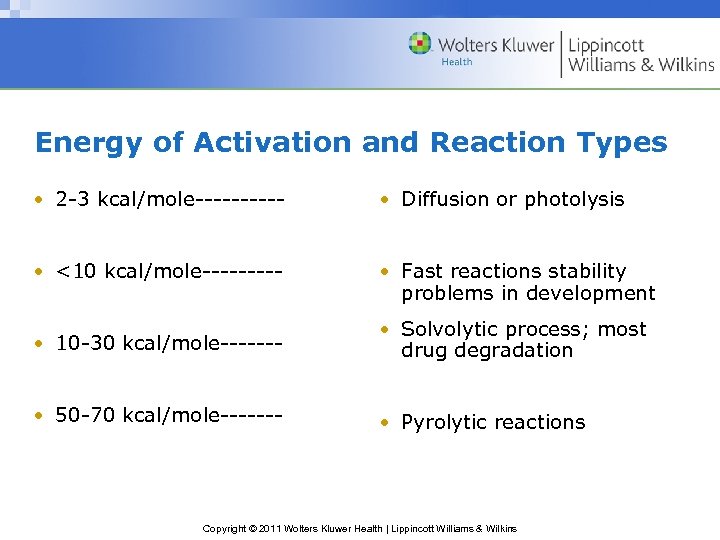 Energy of Activation and Reaction Types • 2 -3 kcal/mole----- • Diffusion or photolysis • <10 kcal/mole----- • Fast reactions stability problems in development • 10 -30 kcal/mole------- • Solvolytic process; most drug degradation • 50 -70 kcal/mole------- • Pyrolytic reactions Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Energy of Activation and Reaction Types • 2 -3 kcal/mole----- • Diffusion or photolysis • <10 kcal/mole----- • Fast reactions stability problems in development • 10 -30 kcal/mole------- • Solvolytic process; most drug degradation • 50 -70 kcal/mole------- • Pyrolytic reactions Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
![Shelf Life Estimates • Q 10 = [K(T+10)]/KT • =e[-(Ea/R) ({1/T+10} - {1/T}] • Shelf Life Estimates • Q 10 = [K(T+10)]/KT • =e[-(Ea/R) ({1/T+10} - {1/T}] •](https://present5.com/presentation/eaddc057d30ea8a5fcfa35da3a455226/image-70.jpg) Shelf Life Estimates • Q 10 = [K(T+10)]/KT • =e[-(Ea/R) ({1/T+10} - {1/T}] • Q 10 =2 Lower limit • Q 10 = 3 Average, best estimate • Q 10 = 4 Upper limit Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Shelf Life Estimates • Q 10 = [K(T+10)]/KT • =e[-(Ea/R) ({1/T+10} - {1/T}] • Q 10 =2 Lower limit • Q 10 = 3 Average, best estimate • Q 10 = 4 Upper limit Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
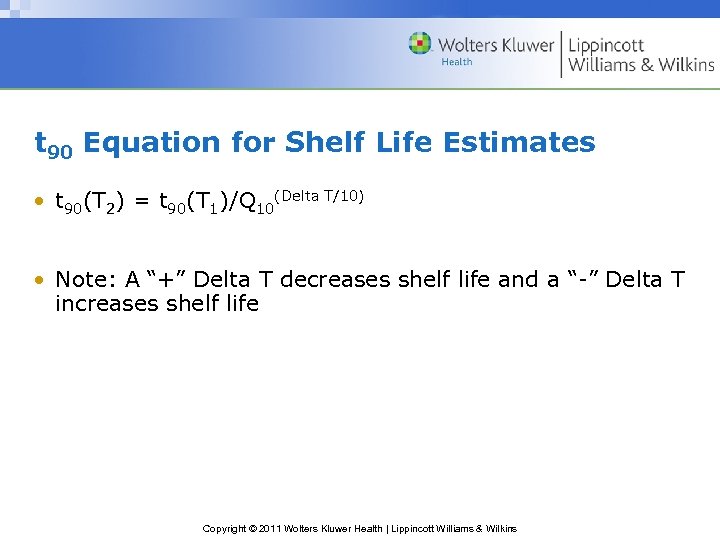 t 90 Equation for Shelf Life Estimates • t 90(T 2) = t 90(T 1)/Q 10(Delta T/10) • Note: A “+” Delta T decreases shelf life and a “-” Delta T increases shelf life Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
t 90 Equation for Shelf Life Estimates • t 90(T 2) = t 90(T 1)/Q 10(Delta T/10) • Note: A “+” Delta T decreases shelf life and a “-” Delta T increases shelf life Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Pharmaceutical Ingredients and Excipients Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Pharmaceutical Ingredients and Excipients Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Definitions and Types • Active pharmaceutical ingredients • Pharmaceutical ingredients added to prepare a dosage form Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Definitions and Types • Active pharmaceutical ingredients • Pharmaceutical ingredients added to prepare a dosage form Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
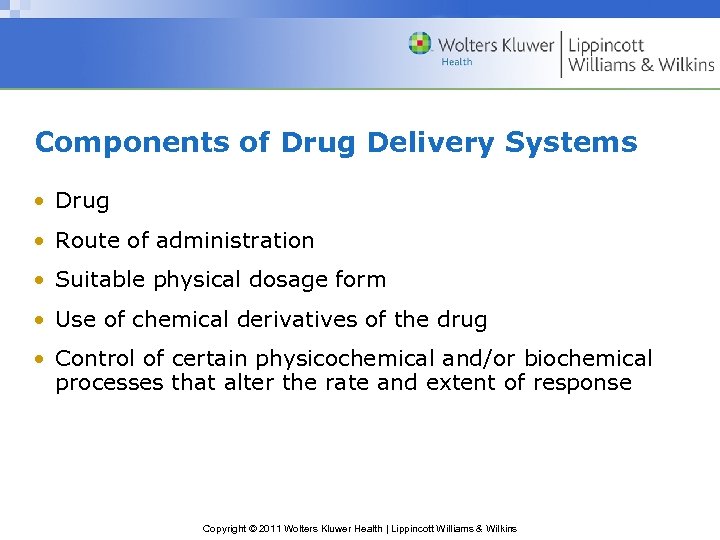 Components of Drug Delivery Systems • Drug • Route of administration • Suitable physical dosage form • Use of chemical derivatives of the drug • Control of certain physicochemical and/or biochemical processes that alter the rate and extent of response Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Components of Drug Delivery Systems • Drug • Route of administration • Suitable physical dosage form • Use of chemical derivatives of the drug • Control of certain physicochemical and/or biochemical processes that alter the rate and extent of response Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Excipients • Coloring agents • Thickening agents • Sweetening agents • Suspending agents • Flavoring agents • Binding agents • Surfactants • Solvents • Solubilizing agents • Lubricants • Antioxidants • Perfumes • Preservatives • Fats and oils Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Excipients • Coloring agents • Thickening agents • Sweetening agents • Suspending agents • Flavoring agents • Binding agents • Surfactants • Solvents • Solubilizing agents • Lubricants • Antioxidants • Perfumes • Preservatives • Fats and oils Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Handbook of Pharmaceutical Excipients • Monographs on more than 250 excipients used in dosage form preparation • Additional excipients listed in Food Chemicals Codex and National Formulary Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Handbook of Pharmaceutical Excipients • Monographs on more than 250 excipients used in dosage form preparation • Additional excipients listed in Food Chemicals Codex and National Formulary Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Harmonization of Standards • International harmonization of excipients • Pharmaceutical industry is multinational • Uniform standards needed Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Harmonization of Standards • International harmonization of excipients • Pharmaceutical industry is multinational • Uniform standards needed Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
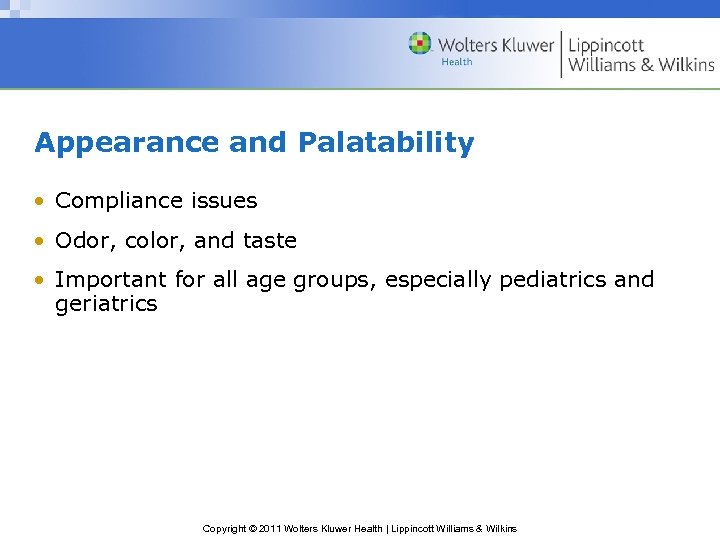 Appearance and Palatability • Compliance issues • Odor, color, and taste • Important for all age groups, especially pediatrics and geriatrics Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Appearance and Palatability • Compliance issues • Odor, color, and taste • Important for all age groups, especially pediatrics and geriatrics Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
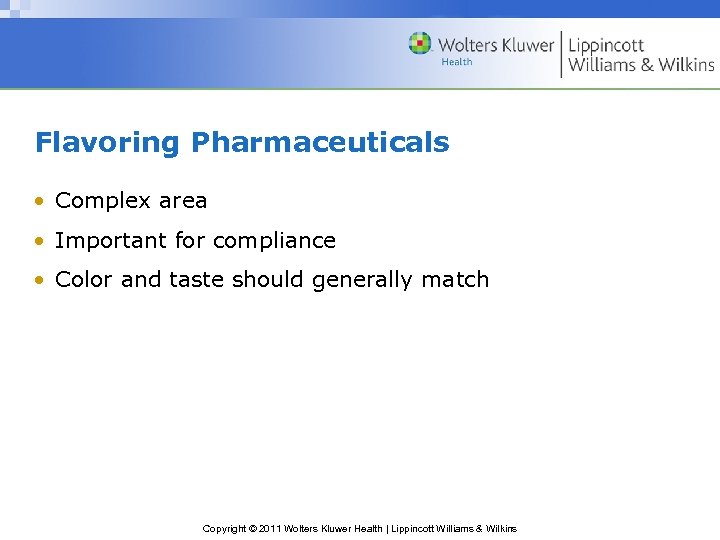 Flavoring Pharmaceuticals • Complex area • Important for compliance • Color and taste should generally match Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Flavoring Pharmaceuticals • Complex area • Important for compliance • Color and taste should generally match Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Flavor • Taste • Touch • Smell • Sight • Sound Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Flavor • Taste • Touch • Smell • Sight • Sound Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Four Primary Tastes • Sweet • Bitter • Sour • Salty Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Four Primary Tastes • Sweet • Bitter • Sour • Salty Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
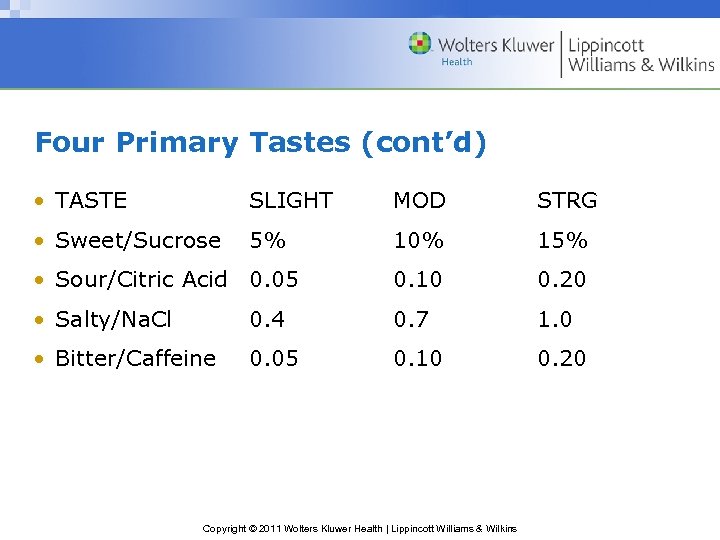 Four Primary Tastes (cont’d) • TASTE SLIGHT MOD STRG • Sweet/Sucrose 5% 10% 15% • Sour/Citric Acid 0. 05 0. 10 0. 20 • Salty/Na. Cl 0. 4 0. 7 1. 0 • Bitter/Caffeine 0. 05 0. 10 0. 20 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Four Primary Tastes (cont’d) • TASTE SLIGHT MOD STRG • Sweet/Sucrose 5% 10% 15% • Sour/Citric Acid 0. 05 0. 10 0. 20 • Salty/Na. Cl 0. 4 0. 7 1. 0 • Bitter/Caffeine 0. 05 0. 10 0. 20 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Causative Factors for Taste • Hot Mild, counterirritant • Astringent Tannins, acids • Coarseness/Grittiness Texture • Coolness Neg heat of solution • Greater sensitivity to odors than to tastes • Females have greater sensitivity to odors than males Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Causative Factors for Taste • Hot Mild, counterirritant • Astringent Tannins, acids • Coarseness/Grittiness Texture • Coolness Neg heat of solution • Greater sensitivity to odors than to tastes • Females have greater sensitivity to odors than males Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
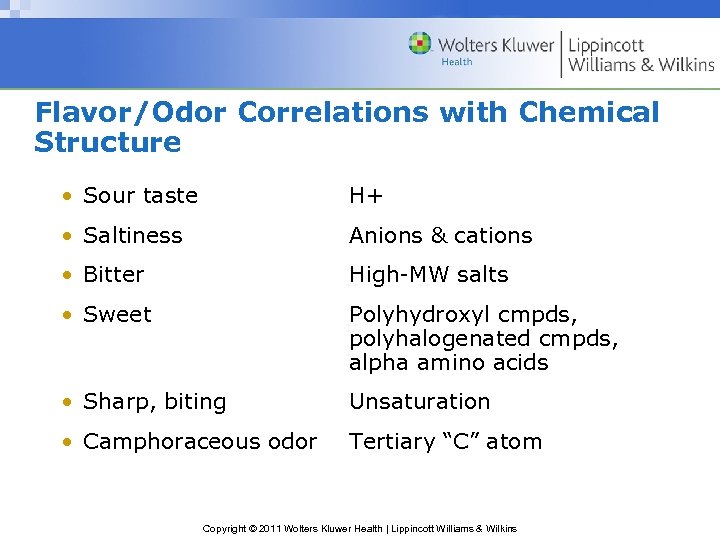 Flavor/Odor Correlations with Chemical Structure • Sour taste H+ • Saltiness Anions & cations • Bitter High-MW salts • Sweet Polyhydroxyl cmpds, polyhalogenated cmpds, alpha amino acids • Sharp, biting Unsaturation • Camphoraceous odor Tertiary “C” atom Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Flavor/Odor Correlations with Chemical Structure • Sour taste H+ • Saltiness Anions & cations • Bitter High-MW salts • Sweet Polyhydroxyl cmpds, polyhalogenated cmpds, alpha amino acids • Sharp, biting Unsaturation • Camphoraceous odor Tertiary “C” atom Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
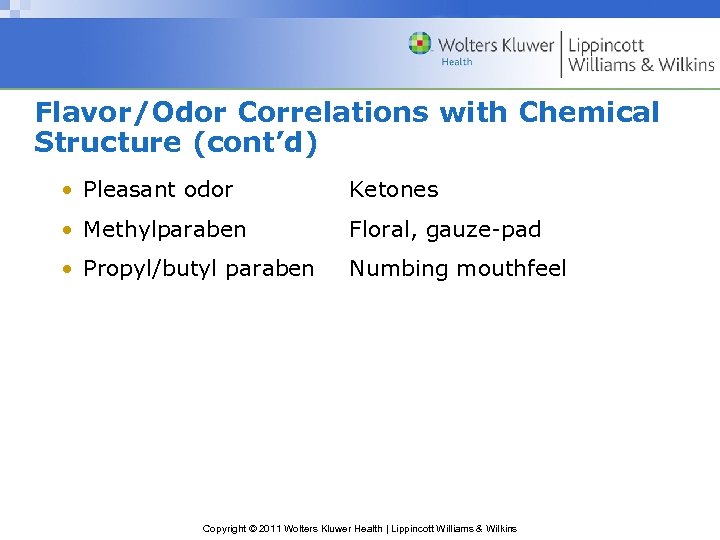 Flavor/Odor Correlations with Chemical Structure (cont’d) • Pleasant odor Ketones • Methylparaben Floral, gauze-pad • Propyl/butyl paraben Numbing mouthfeel Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Flavor/Odor Correlations with Chemical Structure (cont’d) • Pleasant odor Ketones • Methylparaben Floral, gauze-pad • Propyl/butyl paraben Numbing mouthfeel Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Flavor Selection • Immediate flavor identity • Rapid full flavor development • Acceptable mouthfeel • Short aftertaste • No undesirable sensations Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Flavor Selection • Immediate flavor identity • Rapid full flavor development • Acceptable mouthfeel • Short aftertaste • No undesirable sensations Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Flavoring Techniques • Blending – Fruit====== Sour – Salty/Sweet/Sour=== Bitter – Salty====== Decreases sourness – Salty====== Increases sweetness • Overshadowing – Methylsalicylate==== Glycyrrhiza Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Flavoring Techniques • Blending – Fruit====== Sour – Salty/Sweet/Sour=== Bitter – Salty====== Decreases sourness – Salty====== Increases sweetness • Overshadowing – Methylsalicylate==== Glycyrrhiza Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Flavoring Techniques (cont’d) • Physical – Formation of insoluble compounds – Emulsification of oils – Effervescence – High-viscosity fluids – Coating tablets Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Flavoring Techniques (cont’d) • Physical – Formation of insoluble compounds – Emulsification of oils – Effervescence – High-viscosity fluids – Coating tablets Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Flavoring Techniques (cont’d) • Chemical – Adsorption-silica gel – Complexation • Physiological – Anesthetic effect==== Menthol (mint) Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Flavoring Techniques (cont’d) • Chemical – Adsorption-silica gel – Complexation • Physiological – Anesthetic effect==== Menthol (mint) Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
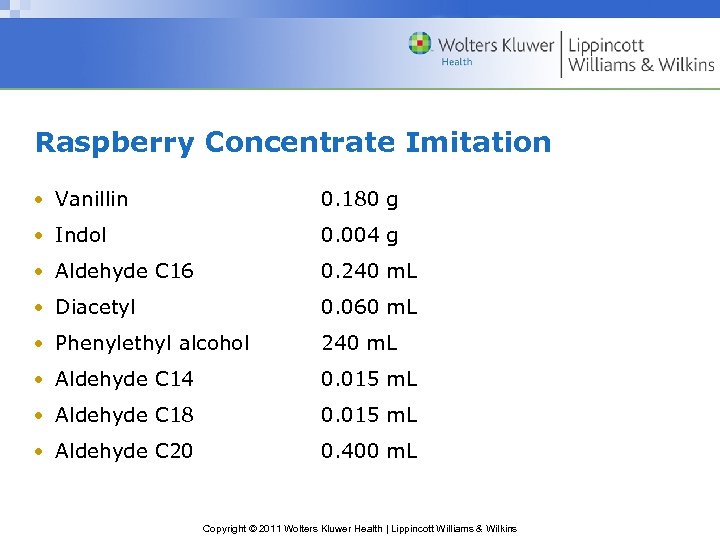 Raspberry Concentrate Imitation • Vanillin 0. 180 g • Indol 0. 004 g • Aldehyde C 16 0. 240 m. L • Diacetyl 0. 060 m. L • Phenylethyl alcohol 240 m. L • Aldehyde C 14 0. 015 m. L • Aldehyde C 18 0. 015 m. L • Aldehyde C 20 0. 400 m. L Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Raspberry Concentrate Imitation • Vanillin 0. 180 g • Indol 0. 004 g • Aldehyde C 16 0. 240 m. L • Diacetyl 0. 060 m. L • Phenylethyl alcohol 240 m. L • Aldehyde C 14 0. 015 m. L • Aldehyde C 18 0. 015 m. L • Aldehyde C 20 0. 400 m. L Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
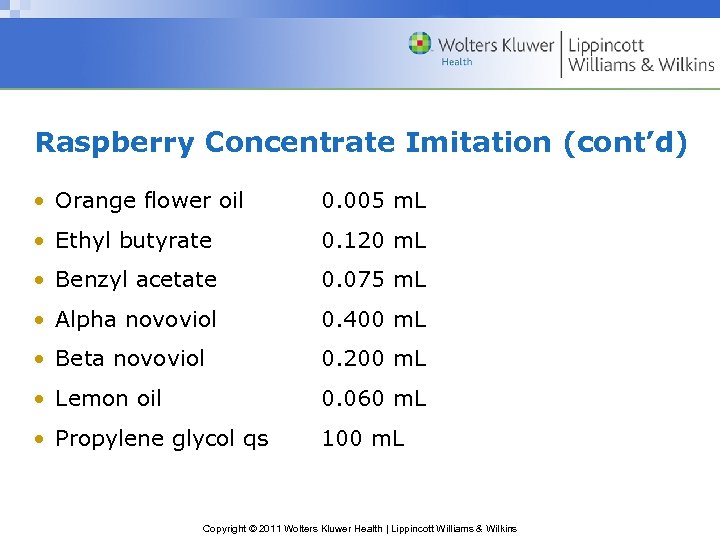 Raspberry Concentrate Imitation (cont’d) • Orange flower oil 0. 005 m. L • Ethyl butyrate 0. 120 m. L • Benzyl acetate 0. 075 m. L • Alpha novoviol 0. 400 m. L • Beta novoviol 0. 200 m. L • Lemon oil 0. 060 m. L • Propylene glycol qs 100 m. L Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Raspberry Concentrate Imitation (cont’d) • Orange flower oil 0. 005 m. L • Ethyl butyrate 0. 120 m. L • Benzyl acetate 0. 075 m. L • Alpha novoviol 0. 400 m. L • Beta novoviol 0. 200 m. L • Lemon oil 0. 060 m. L • Propylene glycol qs 100 m. L Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Flavor Selection Guide • Salty Butterscotch/Maple • Bitter Wild Cherry/Licorice Chocolate Mint • Acrid/Sour Raspberry/Fruit Berry/Acacia Syrup Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Flavor Selection Guide • Salty Butterscotch/Maple • Bitter Wild Cherry/Licorice Chocolate Mint • Acrid/Sour Raspberry/Fruit Berry/Acacia Syrup Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Flavor Selection Guide (cont’d) • Oily Peppermint/Anise Wintergreen • Sweet Fruit/Berry/Vanilla • Acid Citrus Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Flavor Selection Guide (cont’d) • Oily Peppermint/Anise Wintergreen • Sweet Fruit/Berry/Vanilla • Acid Citrus Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Sweetening Pharmaceuticals • Complex area • Natural vs. synthetic • Heat stability Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Sweetening Pharmaceuticals • Complex area • Natural vs. synthetic • Heat stability Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Sweetening Agents • Dextrose • Mannitol • Saccharin • Sorbitol • Sucrose Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Sweetening Agents • Dextrose • Mannitol • Saccharin • Sorbitol • Sucrose Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Coloring Pharmaceuticals • Lighter shades preferred Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Coloring Pharmaceuticals • Lighter shades preferred Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Coloring Agents • Dyes: FD&C, Ext D&C • Lakes: Calcium and aluminum salts • Liquids: 0. 001% to 0. 0005% • Powders: 0. 1% • Caramel • Ferric oxide: Red/yellow Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Coloring Agents • Dyes: FD&C, Ext D&C • Lakes: Calcium and aluminum salts • Liquids: 0. 001% to 0. 0005% • Powders: 0. 1% • Caramel • Ferric oxide: Red/yellow Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Coloring Agents (cont’d) • Red No. 1, Ponceau 3 R, Cherry Red – 9. 8/5 • Blue No. 1, Brilliant Blue, Blue-Green – 20/20 • Yellow No. 5, Tartrazine, Lemon Yellow – 20/18 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Coloring Agents (cont’d) • Red No. 1, Ponceau 3 R, Cherry Red – 9. 8/5 • Blue No. 1, Brilliant Blue, Blue-Green – 20/20 • Yellow No. 5, Tartrazine, Lemon Yellow – 20/18 Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Preservatives • Sterilization and Preservation • Preservative Selection • General Preservative Considerations • Mode of Action • Preservative Utilization Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Preservatives • Sterilization and Preservation • Preservative Selection • General Preservative Considerations • Mode of Action • Preservative Utilization Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Sterilization and Preservation • Some products must be sterile – Injectables – Ophthalmics • Sterilization – Autoclave – Filtration – Dry heat – Irradiation Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Sterilization and Preservation • Some products must be sterile – Injectables – Ophthalmics • Sterilization – Autoclave – Filtration – Dry heat – Irradiation Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Preservative Selection • Dosage form • Route of administration • Compatibility with excipients • Container and closure compatibility Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Preservative Selection • Dosage form • Route of administration • Compatibility with excipients • Container and closure compatibility Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 General Preservative Considerations • Range of activity • Concentration required • p. H requirements • Compatibility Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
General Preservative Considerations • Range of activity • Concentration required • p. H requirements • Compatibility Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Mode of Action • Modification of cell membrane permeability • Lysis and cytoplasmic leakage • Irreversible coagulation of cytoplasmic constituents • Inhibition of cellular metabolism • Oxidation of cellular constituents • Hydrolysis Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Mode of Action • Modification of cell membrane permeability • Lysis and cytoplasmic leakage • Irreversible coagulation of cytoplasmic constituents • Inhibition of cellular metabolism • Oxidation of cellular constituents • Hydrolysis Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
 Preservative Utilization • Benzoic acid/sodium benzoate • Alcohol • Phenylmercuric nitrate/acetate • Phenol • Cresol • Chlorobutanol • Benzalkonium chloride • Methylparaben/propylparaben • Others Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins
Preservative Utilization • Benzoic acid/sodium benzoate • Alcohol • Phenylmercuric nitrate/acetate • Phenol • Cresol • Chlorobutanol • Benzalkonium chloride • Methylparaben/propylparaben • Others Copyright © 2011 Wolters Kluwer Health | Lippincott Williams & Wilkins


