3dd36f799c79eab3d3b9fc2edb58c0cb.ppt
- Количество слайдов: 21
 Does quality matter? Peter Hall Chief Executive Officer Concept Foundation Bangkok, Thailand Geneva, Switzerland Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Does quality matter? Peter Hall Chief Executive Officer Concept Foundation Bangkok, Thailand Geneva, Switzerland Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 Does quality matter to the people that use the products? Poor quality product can: • Contain extraneous impurities or increased levels of known degradation products from the API, improper manufacture or inappropriate storage - can lead to increase in known side-effects; other adverse events; and in extreme circumstances, serious adverse events, such as anaphylactic shock or death. • Have poor content uniformity - leading to overdosing or failure of treatment. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Does quality matter to the people that use the products? Poor quality product can: • Contain extraneous impurities or increased levels of known degradation products from the API, improper manufacture or inappropriate storage - can lead to increase in known side-effects; other adverse events; and in extreme circumstances, serious adverse events, such as anaphylactic shock or death. • Have poor content uniformity - leading to overdosing or failure of treatment. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 Does quality matter to the people that use the products? This means poor quality hormonal contraceptives can: • Give rise to adverse events BUT are adverse events recorded? Adverse events are recorded in clinical trials but recording is virtually impossible in routine use in many countries. Even if we could – we counsel women to put up with side-effects! • Lead to failure BUT we accept there is lower efficacy in routine use rather than “ideal” use – we blame it on the woman! The impact of poor quality hormonal contraceptives is hard to measure BUT we know that by ensuring availability of quality products the risk is minimized. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Does quality matter to the people that use the products? This means poor quality hormonal contraceptives can: • Give rise to adverse events BUT are adverse events recorded? Adverse events are recorded in clinical trials but recording is virtually impossible in routine use in many countries. Even if we could – we counsel women to put up with side-effects! • Lead to failure BUT we accept there is lower efficacy in routine use rather than “ideal” use – we blame it on the woman! The impact of poor quality hormonal contraceptives is hard to measure BUT we know that by ensuring availability of quality products the risk is minimized. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 Does quality matter to the people that buy the products? Poor quality product leads to: • • • Additional testing and rejection of batches Loss of time Waste of product and money Legal fees Stock outs Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Does quality matter to the people that buy the products? Poor quality product leads to: • • • Additional testing and rejection of batches Loss of time Waste of product and money Legal fees Stock outs Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 Does quality matter to the people that buy the products? • The extent of the problem of contraceptive need and the way we address it means that the buying community is concerned with getting the greatest amount of product for the lowest price. • Many manufacturers are willing to compete at low cost and bear any resulting risks/costs. • BUT this is false economics – designing and assuring quality products may mean higher production costs but lower overall costs! Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Does quality matter to the people that buy the products? • The extent of the problem of contraceptive need and the way we address it means that the buying community is concerned with getting the greatest amount of product for the lowest price. • Many manufacturers are willing to compete at low cost and bear any resulting risks/costs. • BUT this is false economics – designing and assuring quality products may mean higher production costs but lower overall costs! Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 Why quality matters – the people that buy the products • Purchasing a product that has been approved by a stringent drug regulatory agency or prequalified by the WHO programme means that all aspects of quality and safety and efficacy of the product have been assessed. • This can never be a 100% guarantee but it is an enormous step forward to eliminate poor quality products. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Why quality matters – the people that buy the products • Purchasing a product that has been approved by a stringent drug regulatory agency or prequalified by the WHO programme means that all aspects of quality and safety and efficacy of the product have been assessed. • This can never be a 100% guarantee but it is an enormous step forward to eliminate poor quality products. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 Nearly twenty years ago “Numerous examples of problems relating to the quality of hormonal contraceptive formulations from various developing country manufacturer have been reported following observation and analysis of products obtained from pharmacies or other service delivery points (P. E. Hall, S. Matlin and M. Morrow, personal communication). Examples have included: Oral contraceptives • tablets with highly variable active ingredient content. In one example, these ranged from 14% (4. 2μg) to 141% (42μg) of labelled content. In another, tablets contained up to 1, 000μg instead of 50μg of ethinyl estradiol; • tablets that crumbled in the packaging; • discoloured tablets; • tablets with active ingredients misplaced in the placebo tablet row; • faulty packaging. Injectable contraceptives • active ingredients that were clumped, preventing them from being resuspended in the vehicle. ” Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Nearly twenty years ago “Numerous examples of problems relating to the quality of hormonal contraceptive formulations from various developing country manufacturer have been reported following observation and analysis of products obtained from pharmacies or other service delivery points (P. E. Hall, S. Matlin and M. Morrow, personal communication). Examples have included: Oral contraceptives • tablets with highly variable active ingredient content. In one example, these ranged from 14% (4. 2μg) to 141% (42μg) of labelled content. In another, tablets contained up to 1, 000μg instead of 50μg of ethinyl estradiol; • tablets that crumbled in the packaging; • discoloured tablets; • tablets with active ingredients misplaced in the placebo tablet row; • faulty packaging. Injectable contraceptives • active ingredients that were clumped, preventing them from being resuspended in the vehicle. ” Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 Four years ago – company review • A review of 47 companies showed that most companies manufacture products according to outdated requirements that do not conform to CGMP, or even outside GMP. Many have inadequate understanding and ability to implement and invest in quality assurance. Some should be closed! • Most are not in a position to supply products into international markets, not having considered regulatory approval for their products outside their home markets. (Hall et al, Contraception, 2007: 75, 311 -317) Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Four years ago – company review • A review of 47 companies showed that most companies manufacture products according to outdated requirements that do not conform to CGMP, or even outside GMP. Many have inadequate understanding and ability to implement and invest in quality assurance. Some should be closed! • Most are not in a position to supply products into international markets, not having considered regulatory approval for their products outside their home markets. (Hall et al, Contraception, 2007: 75, 311 -317) Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 Four years ago – prequalification of essential RH medicines • In January 2006, the MDAWG held a meeting of a Subgroup on generic manufacturers • Its findings and recommendations were resulted in commitment to prequalification at the RHSC meeting in Bonn in October 2006. • The first Expression of Interest for products for reproductive health was issued by WHO’s Prequalification Programme in October 2006. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Four years ago – prequalification of essential RH medicines • In January 2006, the MDAWG held a meeting of a Subgroup on generic manufacturers • Its findings and recommendations were resulted in commitment to prequalification at the RHSC meeting in Bonn in October 2006. • The first Expression of Interest for products for reproductive health was issued by WHO’s Prequalification Programme in October 2006. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 So what is the situation today? There has been significant work undertaken to improve quality. In particular, the PQ programme has received some 37 product applications. Three contraceptive products have been prequalified and a further 11 are under review (+ 1 oxytocin). There has been an increasing awareness on the part of both procurers and suppliers that quality matters. Moreover, as we will hear from the Global Fund, WHO prequalification of products yields results. So what are the challenges that remain and why are we not further forward? Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
So what is the situation today? There has been significant work undertaken to improve quality. In particular, the PQ programme has received some 37 product applications. Three contraceptive products have been prequalified and a further 11 are under review (+ 1 oxytocin). There has been an increasing awareness on the part of both procurers and suppliers that quality matters. Moreover, as we will hear from the Global Fund, WHO prequalification of products yields results. So what are the challenges that remain and why are we not further forward? Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 So what is the situation today? Many generic manufacturers have not: • appreciated that national GMP approval, is not always CGMP • understood the process of prequalification • been able to compile appropriate dossiers or have inadequate data to do so • have not been willing or able to undertake bioequivalence studies • had the incentive to invest in upgrading their facilities or processes or have still been getting orders for existing product. We are on the cusp of change – it has taken time but the ability to purchase quality assured products will happen! Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
So what is the situation today? Many generic manufacturers have not: • appreciated that national GMP approval, is not always CGMP • understood the process of prequalification • been able to compile appropriate dossiers or have inadequate data to do so • have not been willing or able to undertake bioequivalence studies • had the incentive to invest in upgrading their facilities or processes or have still been getting orders for existing product. We are on the cusp of change – it has taken time but the ability to purchase quality assured products will happen! Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 What are the issues? Hormonal contraceptives • contain highly potent drugs • require separate manufacturing facility with own HVAC, water • require worker protection • oral contraceptives contain tiny amounts of API • injectables must be sterile • content uniformity can be a problem for both tablets and injectables Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
What are the issues? Hormonal contraceptives • contain highly potent drugs • require separate manufacturing facility with own HVAC, water • require worker protection • oral contraceptives contain tiny amounts of API • injectables must be sterile • content uniformity can be a problem for both tablets and injectables Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
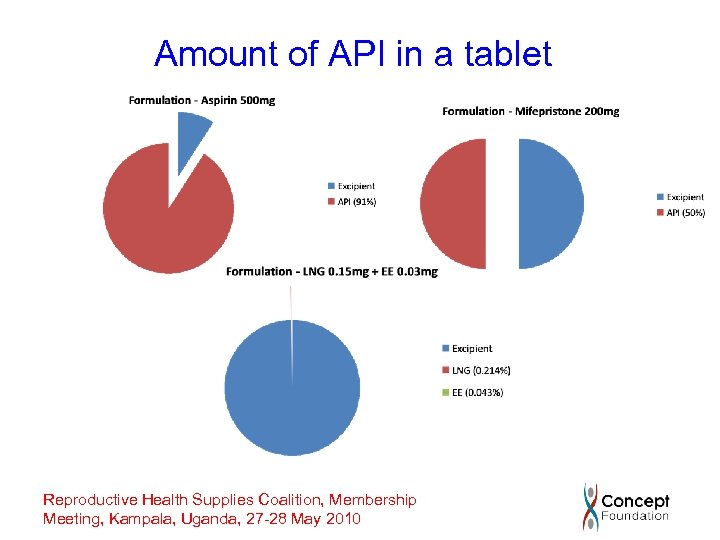 Amount of API in a tablet Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Amount of API in a tablet Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
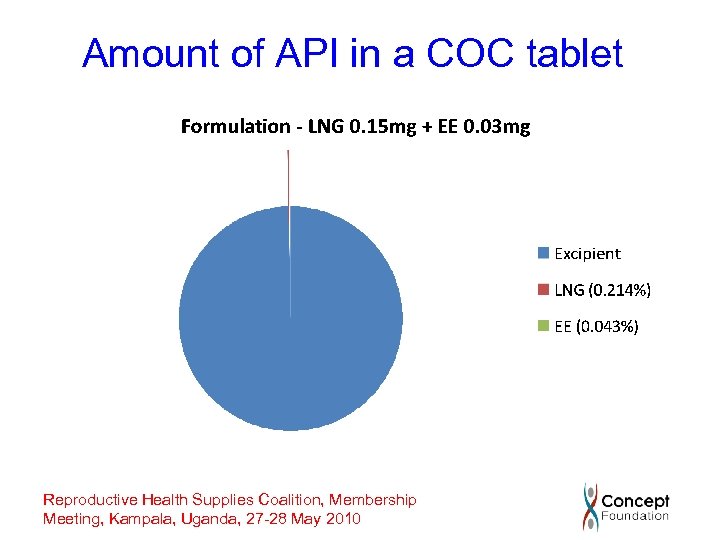 Amount of API in a COC tablet Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Amount of API in a COC tablet Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
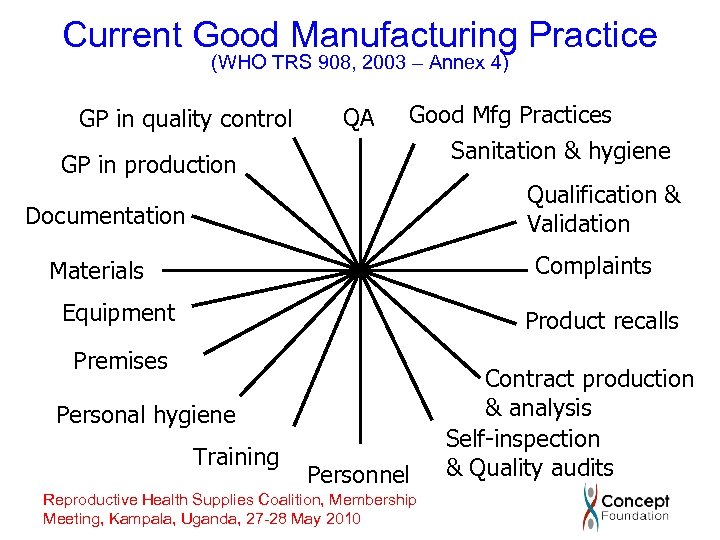 Current Good Manufacturing Practice (WHO TRS 908, 2003 – Annex 4) GP in quality control GP in production QA Good Mfg Practices Sanitation & hygiene Qualification & Validation Documentation Complaints Materials Equipment Product recalls Premises Personal hygiene Training Personnel Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010 Contract production & analysis Self-inspection & Quality audits
Current Good Manufacturing Practice (WHO TRS 908, 2003 – Annex 4) GP in quality control GP in production QA Good Mfg Practices Sanitation & hygiene Qualification & Validation Documentation Complaints Materials Equipment Product recalls Premises Personal hygiene Training Personnel Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010 Contract production & analysis Self-inspection & Quality audits
 Challenges to procuring quality products • Since there are no generic products yet PQ’d, procurers are still having to undertake their own risk management. • This is not a uniform process nor do different procurers have a common definition of risk. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Challenges to procuring quality products • Since there are no generic products yet PQ’d, procurers are still having to undertake their own risk management. • This is not a uniform process nor do different procurers have a common definition of risk. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 Challenges to procuring quality products • Were there ordering time constraints to be met? • Were there agreed criteria for the audit? • What is the background of the auditor? Have they had any production experience? • Was the production facility functioning and, if so, was the product being manufactured? • Can a single auditor on a short trip address all “ 17 GMP issues” in depth? • How was the report written – were there conditions to be met before purchase could be considered? Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Challenges to procuring quality products • Were there ordering time constraints to be met? • Were there agreed criteria for the audit? • What is the background of the auditor? Have they had any production experience? • Was the production facility functioning and, if so, was the product being manufactured? • Can a single auditor on a short trip address all “ 17 GMP issues” in depth? • How was the report written – were there conditions to be met before purchase could be considered? Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
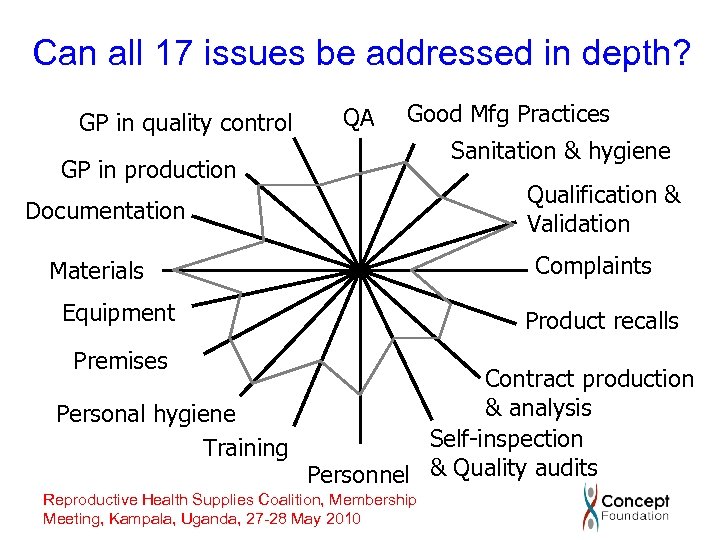 Can all 17 issues be addressed in depth? GP in quality control QA Good Mfg Practices GP in production Documentation Materials Equipment Premises Sanitation & hygiene Qualification & Validation Complaints Product recalls Contract production & analysis Personal hygiene Self-inspection Training Personnel & Quality audits Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Can all 17 issues be addressed in depth? GP in quality control QA Good Mfg Practices GP in production Documentation Materials Equipment Premises Sanitation & hygiene Qualification & Validation Complaints Product recalls Contract production & analysis Personal hygiene Self-inspection Training Personnel & Quality audits Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 Addressing these challenges • Prequalification helps minimize risk and allows procurers greater confidence in their risk management strategies. • Meantime, it would help procurers both in terms of risk management and avoiding duplication of effort to work towards a harmonized procurement and quality assurance strategy, in particular, the development of a common set of approaches to assessing manufacturers and their products. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Addressing these challenges • Prequalification helps minimize risk and allows procurers greater confidence in their risk management strategies. • Meantime, it would help procurers both in terms of risk management and avoiding duplication of effort to work towards a harmonized procurement and quality assurance strategy, in particular, the development of a common set of approaches to assessing manufacturers and their products. Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 To obtain products of assured quality Quality cannot be assessed, tested or inspected in Quality has to be built in! Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
To obtain products of assured quality Quality cannot be assessed, tested or inspected in Quality has to be built in! Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
 Does quality matter? Yes! Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010
Does quality matter? Yes! Reproductive Health Supplies Coalition, Membership Meeting, Kampala, Uganda, 27 -28 May 2010


