529dcec49dbbce361d3ddcb18794a5d9.ppt
- Количество слайдов: 11

Document Control Dr. W. Huisman, Cairo, november 19 th, 2012
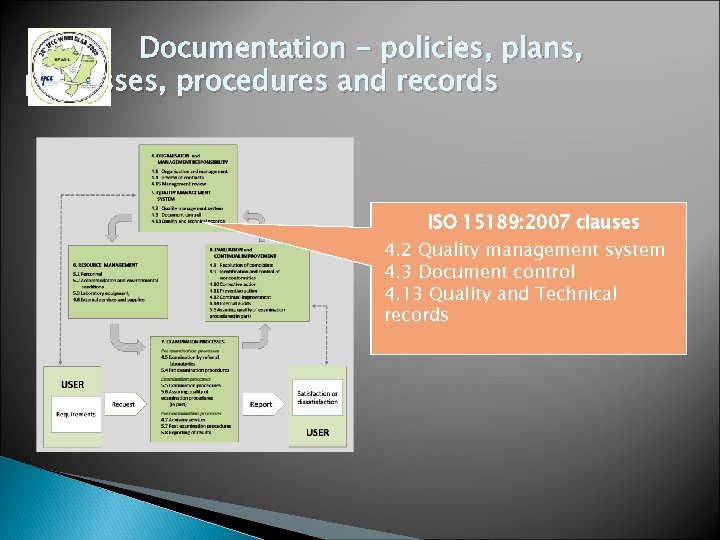
Documentation - policies, plans, processes, procedures and records ISO 15189: 2007 clauses 4. 2 Quality management system 4. 3 Document control 4. 13 Quality and Technical records

4. 3 Document control In third edition this chapter is much clearer, at least in my opinion 4. 3 Documentation requirements 4. 3. 1 General 4. 3. 2 Quality manual 4. 3. 3 Document control 4. 1. 3 Control of records

Document hierarchy On top Policies: What we do and why In the middle: Processes: How it happens- who does what and when At the base: Procedures (Instructions) and Forms: How to do it and record the results Documents tell us what to do and how to do it-policies, processes, procedures Records tell us what happened, when we did it- objective evidence

Document hierarchy Examples Policies: documents and records the policy Processes: Examples: Creation, review and approval process; document change process; records storage process; records retention schedule Forms: document templates; record change procedure Forms ate blank request, worksheet, logs, and computer screens Records are the filled-in forms, logs and instrument printouts

Quality manual contents Quality policy Quality objectives Quality processes and procedures for: personell, accomodation and environmental condition, laboratory equipment, reagents and materials, information system (paper and electronic), external supplies and services, documentation and records, pre-examination and post-examination processes, quality control and interlaboratory comparisons, non-conformities and complaints, internal indicators and internal audits, communications, ethics
Smart quality objectives Specific Measurable Achievable Relevant Timebound Examples: We will reduce our turnaround time for chemistry results by 30% within 6 months We will do at least 3 root cause analyses this year for our top 3 nonconformities

Role of the quality manual To inform staff about the laboratory quality management system To train laboratory management staff in administrative functions To describe the laboratory’s quality management system to assessors/inspectors/auditors To describe the laboratory quality management system to potential custoners
Role of Technical Procedures To provide approved instructions for preexamination, and post-examination activities To serve as the basis for new employee training and competence assessment To ensure consistency in the performance of laboratory technical activities “Quality is lack of variations

4. 3 Document control Your laboratory needs to have: Processes/procedures for managing the 8 elements of document control Review and approval before use Document master log Availability of only current authorised versions ( take care about consistency between paper and electronic versions) Periodic review, revision and approval Removal of invalid documents Identification of superseded documents Amendment of documents by hand ( if allowed) Amendment to documents in computer system

4. 13 Quality and Technical records Your laboratory needs to have Procedures for ident. Iification, collecting, indexing, access, storage, maintenance, safe disposal Records that are legible Handwriting, printer ink on paper, newer version of software reads older elctronic copies Records retention schedule Paper and/or storage environment to prevent Unauthorised access, damage, detoriation, loss
529dcec49dbbce361d3ddcb18794a5d9.ppt