62b7fd8ec0aa598975be3e1193ccb322.ppt
- Количество слайдов: 31
 DNA methylation
DNA methylation
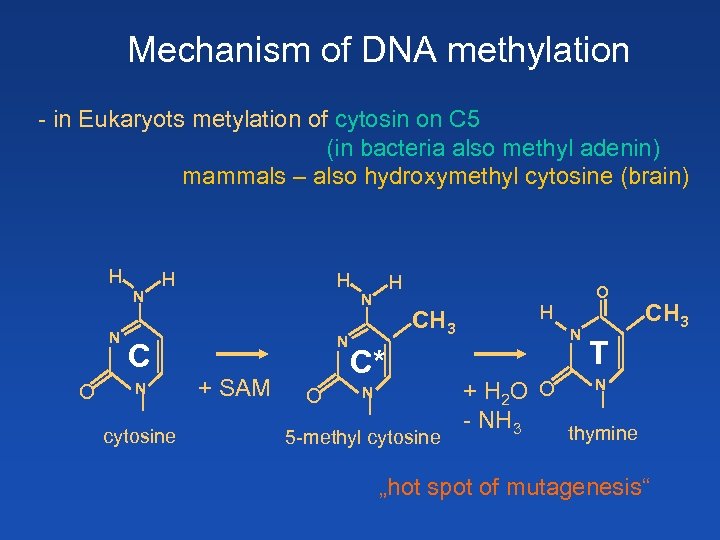 Mechanism of DNA methylation - in Eukaryots metylation of cytosin on C 5 (in bacteria also methyl adenin) mammals – also hydroxymethyl cytosine (brain) H N N O H H N N C N cytosine H + SAM O CH 3 C* N 5 -methyl cytosine O H N + H 2 O O - NH 3 CH 3 T N thymine „hot spot of mutagenesis“
Mechanism of DNA methylation - in Eukaryots metylation of cytosin on C 5 (in bacteria also methyl adenin) mammals – also hydroxymethyl cytosine (brain) H N N O H H N N C N cytosine H + SAM O CH 3 C* N 5 -methyl cytosine O H N + H 2 O O - NH 3 CH 3 T N thymine „hot spot of mutagenesis“
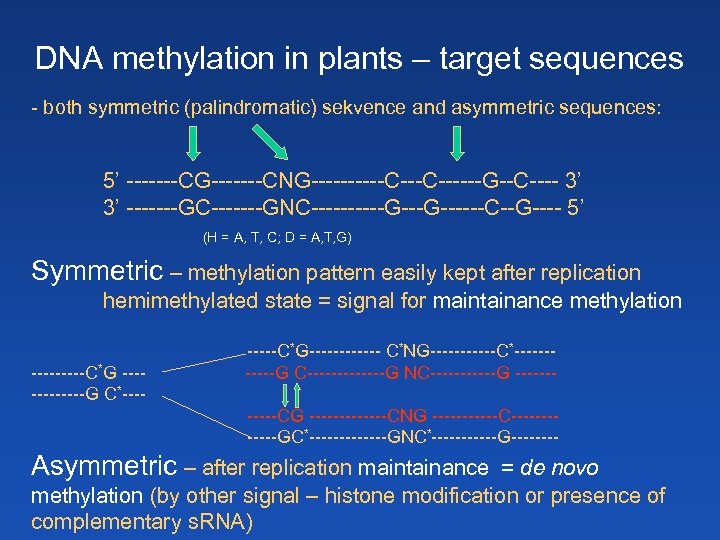 DNA methylation in plants – target sequences - both symmetric (palindromatic) sekvence and asymmetric sequences: 5’ -------CG-------CNG-----C------G--C---- 3’ 3’ -------GC-------GNC-----G------C--G---- 5’ (H = A, T, C; D = A, T, G) Symmetric – methylation pattern easily kept after replication hemimethylated state = signal for maintainance methylation -----C*G ------G C*-----C*G------ C*NG------C*------G C-------G NC------G ------CG -------CNG ------C------GC*-------GNC*------G---- Asymmetric – after replication maintainance = de novo methylation (by other signal – histone modification or presence of complementary s. RNA)
DNA methylation in plants – target sequences - both symmetric (palindromatic) sekvence and asymmetric sequences: 5’ -------CG-------CNG-----C------G--C---- 3’ 3’ -------GC-------GNC-----G------C--G---- 5’ (H = A, T, C; D = A, T, G) Symmetric – methylation pattern easily kept after replication hemimethylated state = signal for maintainance methylation -----C*G ------G C*-----C*G------ C*NG------C*------G C-------G NC------G ------CG -------CNG ------C------GC*-------GNC*------G---- Asymmetric – after replication maintainance = de novo methylation (by other signal – histone modification or presence of complementary s. RNA)
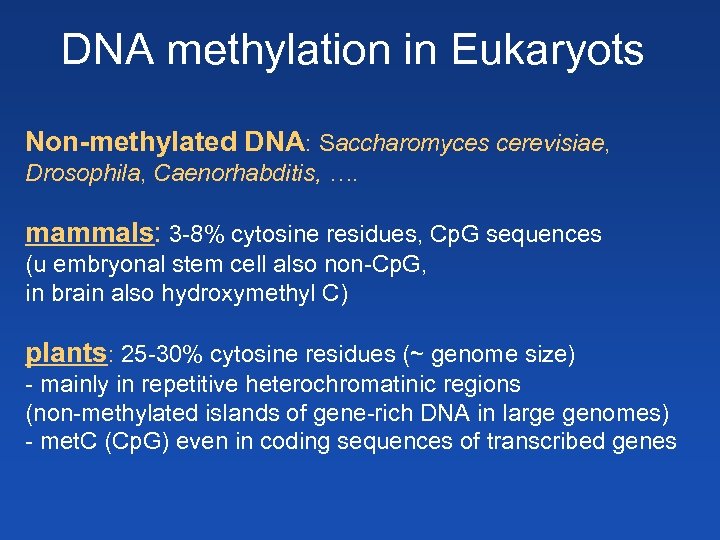 DNA methylation in Eukaryots Non-methylated DNA: Saccharomyces cerevisiae, Drosophila, Caenorhabditis, …. mammals: 3 -8% cytosine residues, Cp. G sequences (u embryonal stem cell also non-Cp. G, in brain also hydroxymethyl C) plants: 25 -30% cytosine residues (~ genome size) - mainly in repetitive heterochromatinic regions (non-methylated islands of gene-rich DNA in large genomes) - met. C (Cp. G) even in coding sequences of transcribed genes
DNA methylation in Eukaryots Non-methylated DNA: Saccharomyces cerevisiae, Drosophila, Caenorhabditis, …. mammals: 3 -8% cytosine residues, Cp. G sequences (u embryonal stem cell also non-Cp. G, in brain also hydroxymethyl C) plants: 25 -30% cytosine residues (~ genome size) - mainly in repetitive heterochromatinic regions (non-methylated islands of gene-rich DNA in large genomes) - met. C (Cp. G) even in coding sequences of transcribed genes
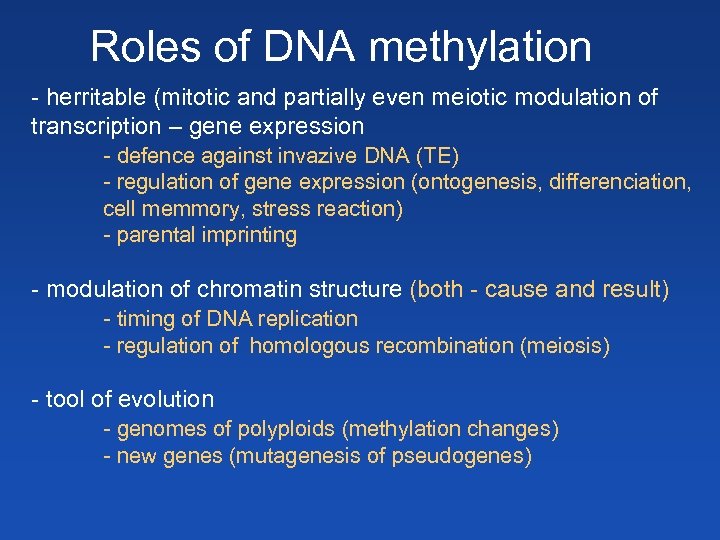 Roles of DNA methylation - herritable (mitotic and partially even meiotic modulation of transcription – gene expression - defence against invazive DNA (TE) - regulation of gene expression (ontogenesis, differenciation, cell memmory, stress reaction) - parental imprinting - modulation of chromatin structure (both - cause and result) - timing of DNA replication - regulation of homologous recombination (meiosis) - tool of evolution - genomes of polyploids (methylation changes) - new genes (mutagenesis of pseudogenes)
Roles of DNA methylation - herritable (mitotic and partially even meiotic modulation of transcription – gene expression - defence against invazive DNA (TE) - regulation of gene expression (ontogenesis, differenciation, cell memmory, stress reaction) - parental imprinting - modulation of chromatin structure (both - cause and result) - timing of DNA replication - regulation of homologous recombination (meiosis) - tool of evolution - genomes of polyploids (methylation changes) - new genes (mutagenesis of pseudogenes)
 Targets of DNA methylation in chromatine structure Stronger methylation of exons (CG) - increased frequency of nucleosomes on exons (role in defining the exon-intron borders) - increased occupancy with Pol II – braking by nucleosome met. C preferentionally at outer site of nucleosomes - higher frequency of Cp. G on outer site (steric reasons) - better available for methyltransferases
Targets of DNA methylation in chromatine structure Stronger methylation of exons (CG) - increased frequency of nucleosomes on exons (role in defining the exon-intron borders) - increased occupancy with Pol II – braking by nucleosome met. C preferentionally at outer site of nucleosomes - higher frequency of Cp. G on outer site (steric reasons) - better available for methyltransferases
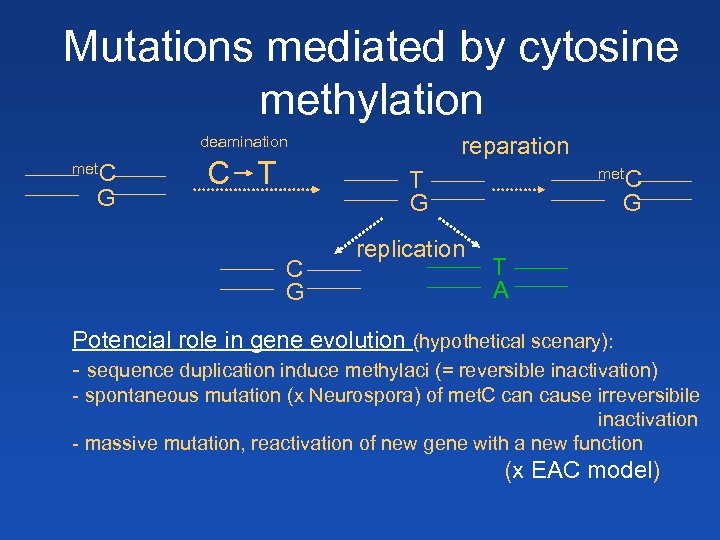 Mutations mediated by cytosine methylation deamination met. C G C T reparation met. C T G C G replication G T A Potencial role in gene evolution (hypothetical scenary): - sequence duplication induce methylaci (= reversible inactivation) - spontaneous mutation (x Neurospora) of met. C can cause irreversibile inactivation - massive mutation, reactivation of new gene with a new function (x EAC model)
Mutations mediated by cytosine methylation deamination met. C G C T reparation met. C T G C G replication G T A Potencial role in gene evolution (hypothetical scenary): - sequence duplication induce methylaci (= reversible inactivation) - spontaneous mutation (x Neurospora) of met. C can cause irreversibile inactivation - massive mutation, reactivation of new gene with a new function (x EAC model)
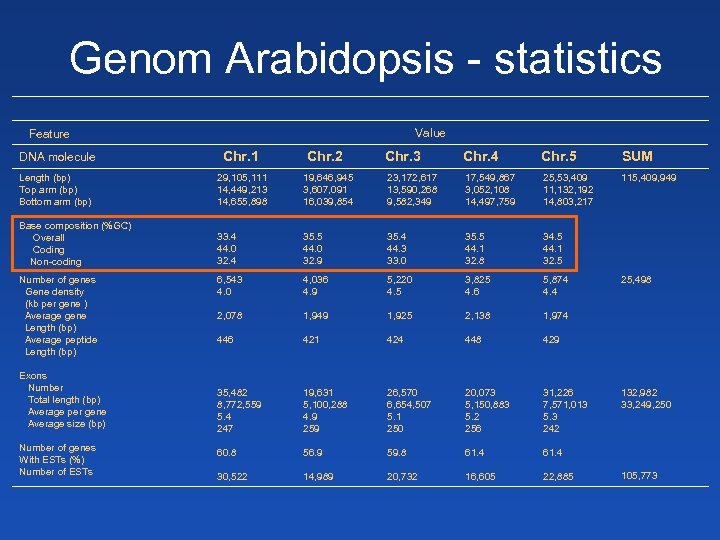 Genom Arabidopsis - statistics Value Feature DNA molecule Chr. 1 Length (bp) Top arm (bp) Bottom arm (bp) 29, 105, 111 14, 449, 213 14, 655, 898 Base composition (%GC) Overall Coding Non-coding Number of genes Gene density (kb per gene ) Average gene Length (bp) Average peptide Length (bp) Exons Number Total length (bp) Average per gene Average size (bp) Number of genes With ESTs (%) Number of ESTs Chr. 2 Chr. 3 Chr. 4 Chr. 5 SUM 19, 646, 945 3, 607, 091 16, 039, 854 23, 172, 617 13, 590, 268 9, 582, 349 17, 549, 867 3, 052, 108 14, 497, 759 25, 53, 409 11, 132, 192 14, 803, 217 115, 409, 949 33. 4 44. 0 32. 4 35. 5 44. 0 32. 9 35. 4 44. 3 33. 0 35. 5 44. 1 32. 8 34. 5 44. 1 32. 5 6, 543 4. 0 4, 036 4. 9 5, 220 4. 5 3, 825 4. 6 5, 874 4. 4 2, 078 1, 949 1, 925 2, 138 1, 974 446 421 424 448 429 35, 482 8, 772, 559 5. 4 247 19, 631 5, 100, 288 4. 9 259 26, 570 6, 654, 507 5. 1 250 20, 073 5, 150, 883 5. 2 256 31, 226 7, 571, 013 5. 3 242 60. 8 56. 9 59. 8 61. 4 30, 522 14, 989 20, 732 16, 605 22, 885 25, 498 132, 982 33, 249, 250 105, 773
Genom Arabidopsis - statistics Value Feature DNA molecule Chr. 1 Length (bp) Top arm (bp) Bottom arm (bp) 29, 105, 111 14, 449, 213 14, 655, 898 Base composition (%GC) Overall Coding Non-coding Number of genes Gene density (kb per gene ) Average gene Length (bp) Average peptide Length (bp) Exons Number Total length (bp) Average per gene Average size (bp) Number of genes With ESTs (%) Number of ESTs Chr. 2 Chr. 3 Chr. 4 Chr. 5 SUM 19, 646, 945 3, 607, 091 16, 039, 854 23, 172, 617 13, 590, 268 9, 582, 349 17, 549, 867 3, 052, 108 14, 497, 759 25, 53, 409 11, 132, 192 14, 803, 217 115, 409, 949 33. 4 44. 0 32. 4 35. 5 44. 0 32. 9 35. 4 44. 3 33. 0 35. 5 44. 1 32. 8 34. 5 44. 1 32. 5 6, 543 4. 0 4, 036 4. 9 5, 220 4. 5 3, 825 4. 6 5, 874 4. 4 2, 078 1, 949 1, 925 2, 138 1, 974 446 421 424 448 429 35, 482 8, 772, 559 5. 4 247 19, 631 5, 100, 288 4. 9 259 26, 570 6, 654, 507 5. 1 250 20, 073 5, 150, 883 5. 2 256 31, 226 7, 571, 013 5. 3 242 60. 8 56. 9 59. 8 61. 4 30, 522 14, 989 20, 732 16, 605 22, 885 25, 498 132, 982 33, 249, 250 105, 773
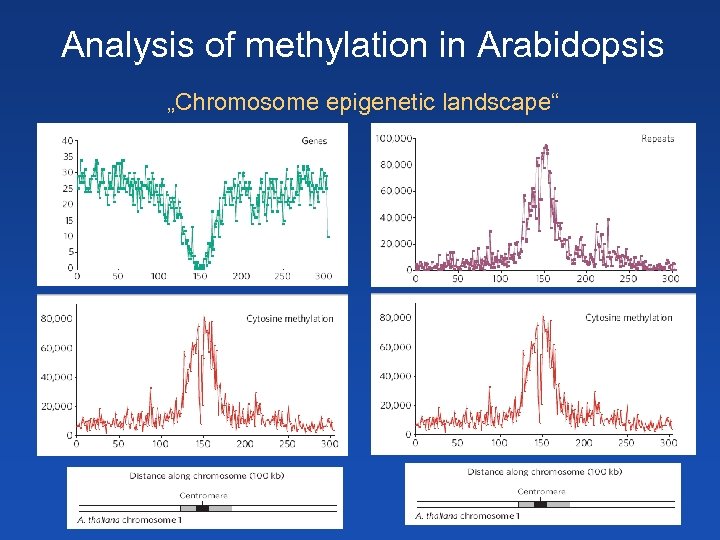 Analysis of methylation in Arabidopsis „Chromosome epigenetic landscape“
Analysis of methylation in Arabidopsis „Chromosome epigenetic landscape“
 Analysis of DNA methylation in Arabidopsis Strong methylation in heterochromatin near centromeres Metylation in promotors of developmentally regulated genes (less than 5 % genes) – inactive state Met. C (in Cp. G!) even in coding sequences of expressed genes! - more than 30 % genes (actively transcribed)
Analysis of DNA methylation in Arabidopsis Strong methylation in heterochromatin near centromeres Metylation in promotors of developmentally regulated genes (less than 5 % genes) – inactive state Met. C (in Cp. G!) even in coding sequences of expressed genes! - more than 30 % genes (actively transcribed)
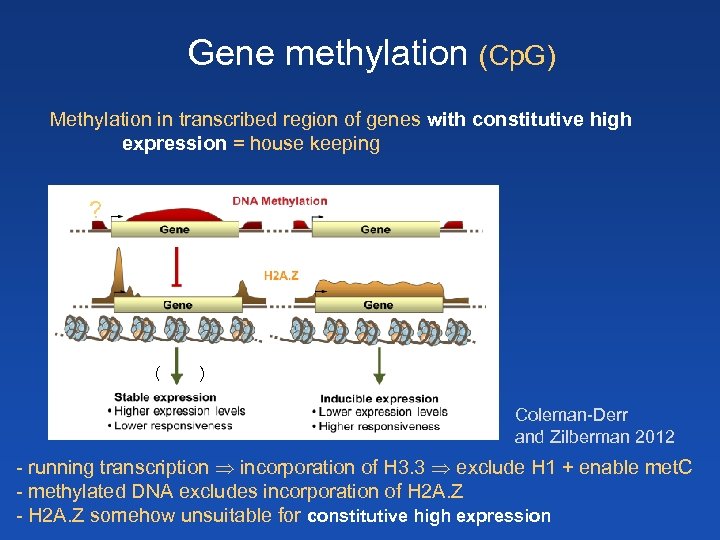 Gene methylation (Cp. G) Methylation in transcribed region of genes with constitutive high expression = house keeping ? ( ) Coleman-Derr and Zilberman 2012 - running transcription incorporation of H 3. 3 exclude H 1 + enable met. C - methylated DNA excludes incorporation of H 2 A. Z - H 2 A. Z somehow unsuitable for constitutive high expression
Gene methylation (Cp. G) Methylation in transcribed region of genes with constitutive high expression = house keeping ? ( ) Coleman-Derr and Zilberman 2012 - running transcription incorporation of H 3. 3 exclude H 1 + enable met. C - methylated DNA excludes incorporation of H 2 A. Z - H 2 A. Z somehow unsuitable for constitutive high expression
 Plant cytosine 5 -methyltransferases MET 1 (metyltransferase) CMT 3 a CMT 2 (chromomethyltransferases) DRM 2 (a DRM 1) (domain rearranged methyltransferase)
Plant cytosine 5 -methyltransferases MET 1 (metyltransferase) CMT 3 a CMT 2 (chromomethyltransferases) DRM 2 (a DRM 1) (domain rearranged methyltransferase)
 MET 1 (metyltransferase 1) - related to mammalian Dnmt 1 - maintenance methylation of symmetric CG - asociated with replication - involvement of nucleosom remodeling complex (DDM 1) (in heterochromatin) Signal – hemimethylated CG (Cp. G) -----C*G ------G C*----C*G------- C*HG----- C* ----- G C------ G DC------G ------CG -------CHG ------C------GC* -------GDC*------G----
MET 1 (metyltransferase 1) - related to mammalian Dnmt 1 - maintenance methylation of symmetric CG - asociated with replication - involvement of nucleosom remodeling complex (DDM 1) (in heterochromatin) Signal – hemimethylated CG (Cp. G) -----C*G ------G C*----C*G------- C*HG----- C* ----- G C------ G DC------G ------CG -------CHG ------C------GC* -------GDC*------G----
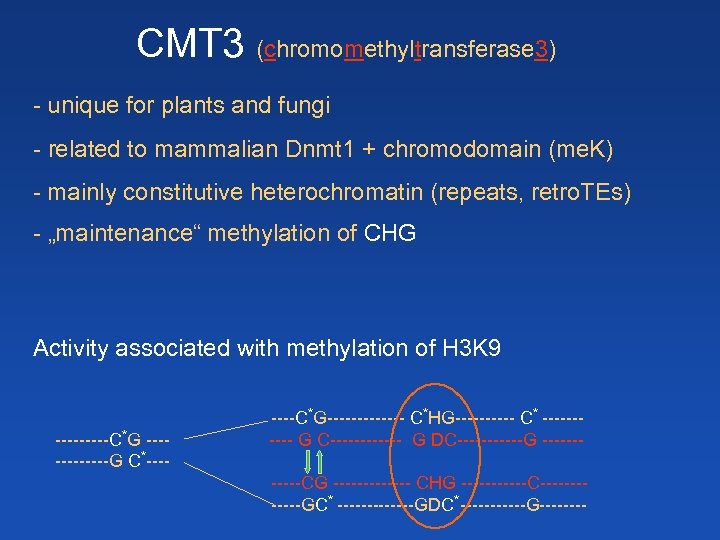 CMT 3 (chromomethyltransferase 3) - unique for plants and fungi - related to mammalian Dnmt 1 + chromodomain (me. K) - mainly constitutive heterochromatin (repeats, retro. TEs) - „maintenance“ methylation of CHG Activity associated with methylation of H 3 K 9 -----C*G ------G C*----C*G------- C*HG----- C* ----- G C------ G DC------G ------CG ------- CHG ------C------GC* -------GDC*------G----
CMT 3 (chromomethyltransferase 3) - unique for plants and fungi - related to mammalian Dnmt 1 + chromodomain (me. K) - mainly constitutive heterochromatin (repeats, retro. TEs) - „maintenance“ methylation of CHG Activity associated with methylation of H 3 K 9 -----C*G ------G C*----C*G------- C*HG----- C* ----- G C------ G DC------G ------CG ------- CHG ------C------GC* -------GDC*------G----
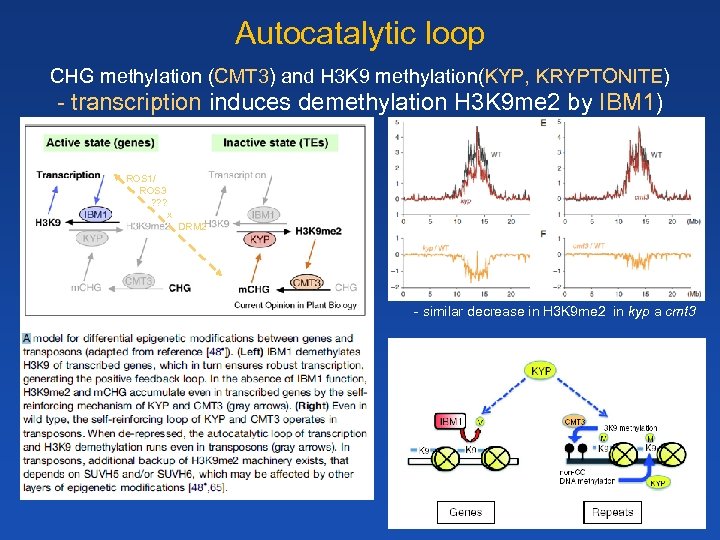 Autocatalytic loop CHG methylation (CMT 3) and H 3 K 9 methylation(KYP, KRYPTONITE) - transcription induces demethylation H 3 K 9 me 2 by IBM 1) ROS 1/ ROS 3 ? ? ? x DRM 2 - similar decrease in H 3 K 9 me 2 in kyp a cmt 3
Autocatalytic loop CHG methylation (CMT 3) and H 3 K 9 methylation(KYP, KRYPTONITE) - transcription induces demethylation H 3 K 9 me 2 by IBM 1) ROS 1/ ROS 3 ? ? ? x DRM 2 - similar decrease in H 3 K 9 me 2 in kyp a cmt 3
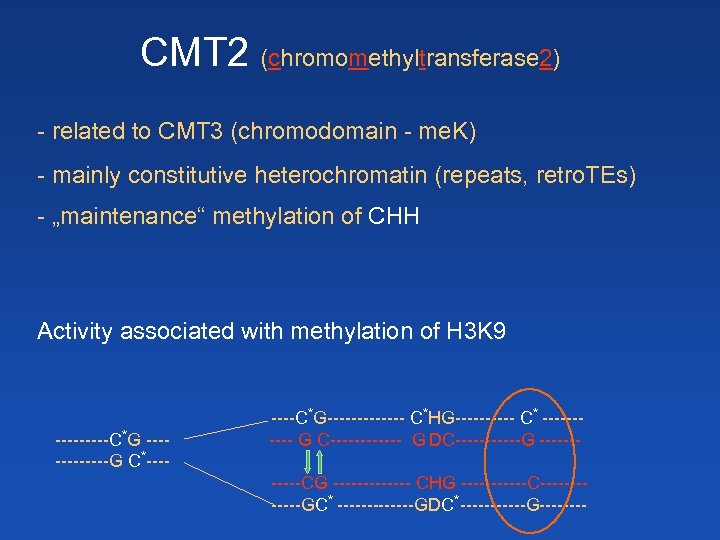 CMT 2 (chromomethyltransferase 2) - related to CMT 3 (chromodomain - me. K) - mainly constitutive heterochromatin (repeats, retro. TEs) - „maintenance“ methylation of CHH Activity associated with methylation of H 3 K 9 -----C*G ------G C*----C*G------- C*HG----- C* ----- G C------ G DC------G ------CG ------- CHG ------C------GC* -------GDC*------G----
CMT 2 (chromomethyltransferase 2) - related to CMT 3 (chromodomain - me. K) - mainly constitutive heterochromatin (repeats, retro. TEs) - „maintenance“ methylation of CHH Activity associated with methylation of H 3 K 9 -----C*G ------G C*----C*G------- C*HG----- C* ----- G C------ G DC------G ------CG ------- CHG ------C------GC* -------GDC*------G----
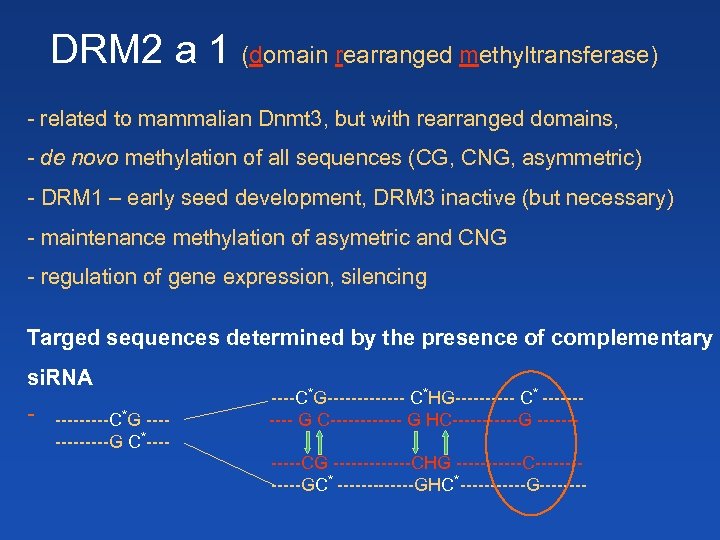 DRM 2 a 1 (domain rearranged methyltransferase) - related to mammalian Dnmt 3, but with rearranged domains, - de novo methylation of all sequences (CG, CNG, asymmetric) - DRM 1 – early seed development, DRM 3 inactive (but necessary) - maintenance methylation of asymetric and CNG - regulation of gene expression, silencing Targed sequences determined by the presence of complementary si. RNA - -----C*G ------G C*----C*G------- C*HG----- C* ----- G C------ G HC------G ------CG -------CHG ------C------GC* -------GHC*------G----
DRM 2 a 1 (domain rearranged methyltransferase) - related to mammalian Dnmt 3, but with rearranged domains, - de novo methylation of all sequences (CG, CNG, asymmetric) - DRM 1 – early seed development, DRM 3 inactive (but necessary) - maintenance methylation of asymetric and CNG - regulation of gene expression, silencing Targed sequences determined by the presence of complementary si. RNA - -----C*G ------G C*----C*G------- C*HG----- C* ----- G C------ G HC------G ------CG -------CHG ------C------GC* -------GHC*------G----
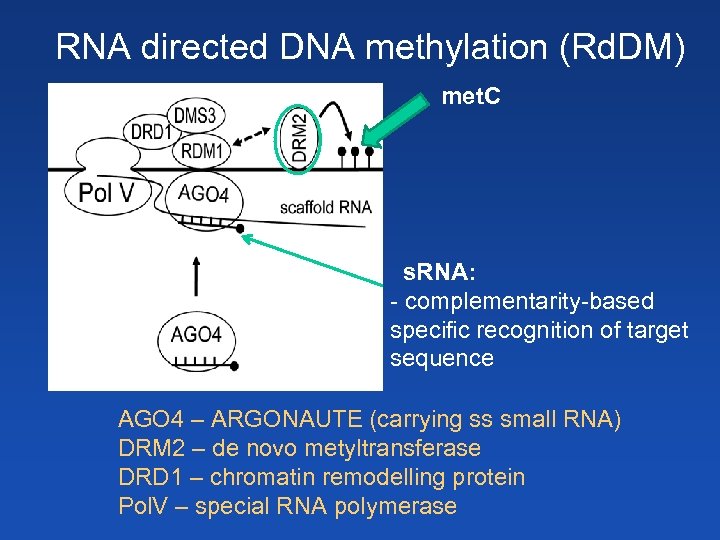 RNA directed DNA methylation (Rd. DM) met. C s. RNA: - complementarity-based specific recognition of target sequence AGO 4 – ARGONAUTE (carrying ss small RNA) DRM 2 – de novo metyltransferase DRD 1 – chromatin remodelling protein Pol. V – special RNA polymerase
RNA directed DNA methylation (Rd. DM) met. C s. RNA: - complementarity-based specific recognition of target sequence AGO 4 – ARGONAUTE (carrying ss small RNA) DRM 2 – de novo metyltransferase DRD 1 – chromatin remodelling protein Pol. V – special RNA polymerase
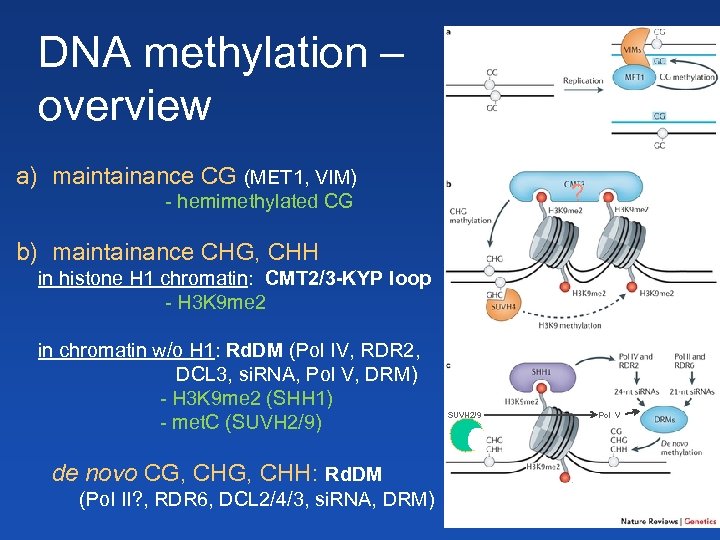 DNA methylation – overview a) maintainance CG (MET 1, VIM) ? - hemimethylated CG b) maintainance CHG, CHH in histone H 1 chromatin: CMT 2/3 -KYP loop - H 3 K 9 me 2 in chromatin w/o H 1: Rd. DM (Pol IV, RDR 2, DCL 3, si. RNA, Pol V, DRM) - H 3 K 9 me 2 (SHH 1) - met. C (SUVH 2/9) de novo CG, CHH: Rd. DM (Pol II? , RDR 6, DCL 2/4/3, si. RNA, DRM) SUVH 2/9 Pol V
DNA methylation – overview a) maintainance CG (MET 1, VIM) ? - hemimethylated CG b) maintainance CHG, CHH in histone H 1 chromatin: CMT 2/3 -KYP loop - H 3 K 9 me 2 in chromatin w/o H 1: Rd. DM (Pol IV, RDR 2, DCL 3, si. RNA, Pol V, DRM) - H 3 K 9 me 2 (SHH 1) - met. C (SUVH 2/9) de novo CG, CHH: Rd. DM (Pol II? , RDR 6, DCL 2/4/3, si. RNA, DRM) SUVH 2/9 Pol V
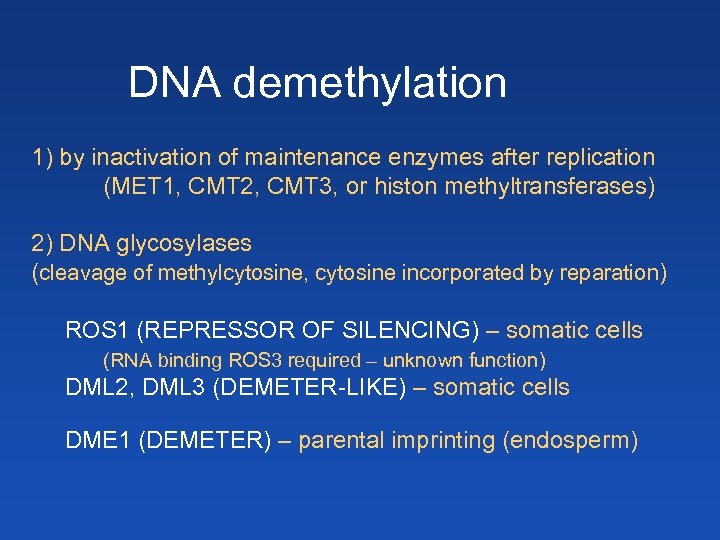 DNA demethylation 1) by inactivation of maintenance enzymes after replication (MET 1, CMT 2, CMT 3, or histon methyltransferases) 2) DNA glycosylases (cleavage of methylcytosine, cytosine incorporated by reparation) ROS 1 (REPRESSOR OF SILENCING) – somatic cells (RNA binding ROS 3 required – unknown function) DML 2, DML 3 (DEMETER-LIKE) – somatic cells DME 1 (DEMETER) – parental imprinting (endosperm)
DNA demethylation 1) by inactivation of maintenance enzymes after replication (MET 1, CMT 2, CMT 3, or histon methyltransferases) 2) DNA glycosylases (cleavage of methylcytosine, cytosine incorporated by reparation) ROS 1 (REPRESSOR OF SILENCING) – somatic cells (RNA binding ROS 3 required – unknown function) DML 2, DML 3 (DEMETER-LIKE) – somatic cells DME 1 (DEMETER) – parental imprinting (endosperm)
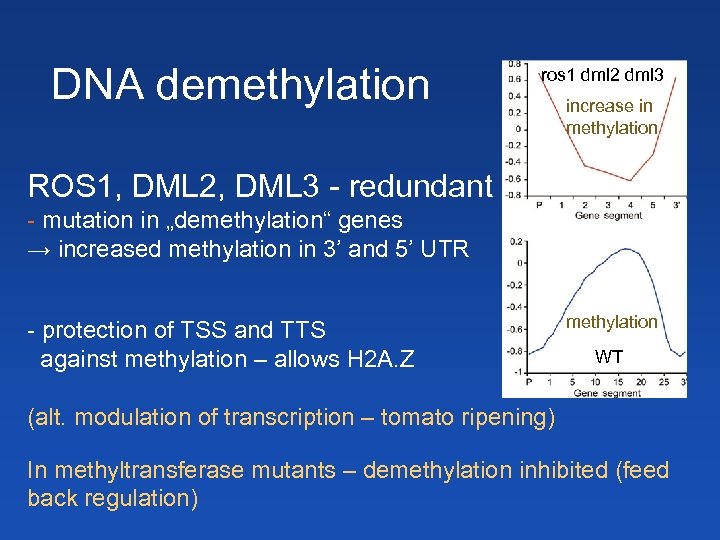 DNA demethylation ros 1 dml 2 dml 3 increase in methylation ROS 1, DML 2, DML 3 - redundant - mutation in „demethylation“ genes → increased methylation in 3’ and 5’ UTR - protection of TSS and TTS against methylation – allows H 2 A. Z methylation WT (alt. modulation of transcription – tomato ripening) In methyltransferase mutants – demethylation inhibited (feed back regulation)
DNA demethylation ros 1 dml 2 dml 3 increase in methylation ROS 1, DML 2, DML 3 - redundant - mutation in „demethylation“ genes → increased methylation in 3’ and 5’ UTR - protection of TSS and TTS against methylation – allows H 2 A. Z methylation WT (alt. modulation of transcription – tomato ripening) In methyltransferase mutants – demethylation inhibited (feed back regulation)
 Modulation of DNA methylation - inactivation of methyltransferase genes MET 1, DDM 1, CMT 3 - treatment with: 5 -azacytidine – inhibits MET 1 dihydroxypropyladenine (DHPA) – modulates [SAM] Fenotypic changes connected with demethylation: - variable: from negligible to strong (caused by activated TE) - developmental changes: - sex reversion - cycloidea phenotype (hypermethylation)
Modulation of DNA methylation - inactivation of methyltransferase genes MET 1, DDM 1, CMT 3 - treatment with: 5 -azacytidine – inhibits MET 1 dihydroxypropyladenine (DHPA) – modulates [SAM] Fenotypic changes connected with demethylation: - variable: from negligible to strong (caused by activated TE) - developmental changes: - sex reversion - cycloidea phenotype (hypermethylation)
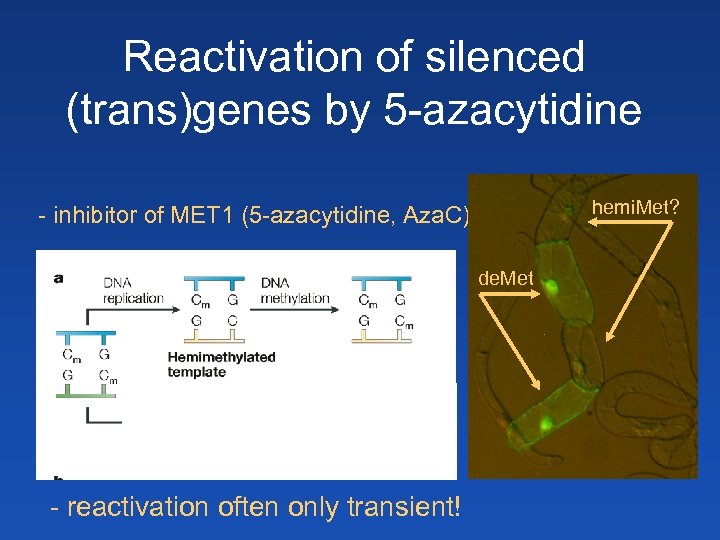 Reactivation of silenced (trans)genes by 5 -azacytidine hemi. Met? - inhibitor of MET 1 (5 -azacytidine, Aza. C) de. Met + Aza. C Demethylated - reactivation often only transient!
Reactivation of silenced (trans)genes by 5 -azacytidine hemi. Met? - inhibitor of MET 1 (5 -azacytidine, Aza. C) de. Met + Aza. C Demethylated - reactivation often only transient!
 Analysis of DNA methylation Direct: - spectrophotometry, HPLC - %met. C - anti met. C antibodies (in situ on chromosome …) - localization of met. C-rich regions - methylation sensitive endonucleases - detection of met. C in selected sequence - bisulfite conversion/sequencing - precise detection of met. C in certain sequence or - whole genome (NGS techniques) - direct sequencing of non-amplified genomic DNA Indirect – relationships between methylation and gene expression – e. g. monitoring gene reactivation (=likely demethyl)
Analysis of DNA methylation Direct: - spectrophotometry, HPLC - %met. C - anti met. C antibodies (in situ on chromosome …) - localization of met. C-rich regions - methylation sensitive endonucleases - detection of met. C in selected sequence - bisulfite conversion/sequencing - precise detection of met. C in certain sequence or - whole genome (NGS techniques) - direct sequencing of non-amplified genomic DNA Indirect – relationships between methylation and gene expression – e. g. monitoring gene reactivation (=likely demethyl)
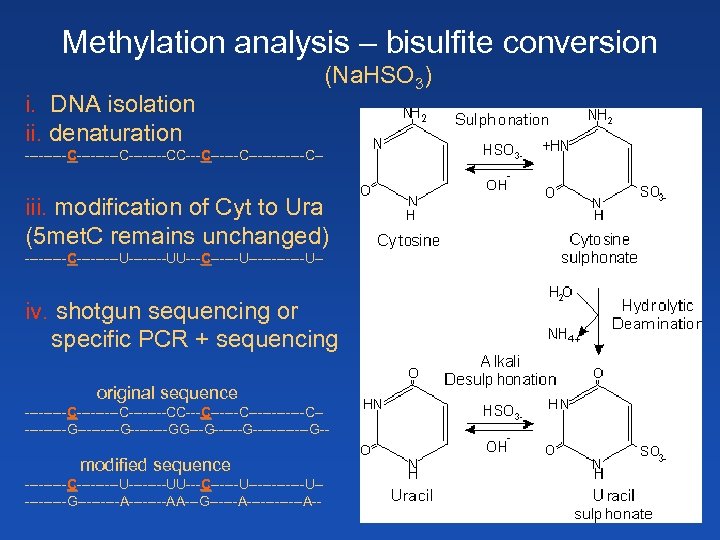 Methylation analysis – bisulfite conversion i. DNA isolation ii. denaturation (Na. HSO 3) ---------C----CC------C------C-- iii. modification of Cyt to Ura (5 met. C remains unchanged) -----C-----U----UU---C------U------U-- iv. shotgun sequencing or specific PCR + sequencing original sequence ---------C----CC------C----------G--------GG------G------G-- modified sequence -----C-----U----UU---C------U----------G-----A----AA---G------A------A--
Methylation analysis – bisulfite conversion i. DNA isolation ii. denaturation (Na. HSO 3) ---------C----CC------C------C-- iii. modification of Cyt to Ura (5 met. C remains unchanged) -----C-----U----UU---C------U------U-- iv. shotgun sequencing or specific PCR + sequencing original sequence ---------C----CC------C----------G--------GG------G------G-- modified sequence -----C-----U----UU---C------U----------G-----A----AA---G------A------A--
 Interpretation of DNA methylation - steric consequences of cytosine methylation • changes at the histone level - posttranslational modifications of histones - SRA domains: SUVH family – H 3 methylation - Metyl Cp. G-binding d. : deacetylation (HDAC) - presence of histone forms - no H 2 A. Z (mechanism? ) • changes in binding of other interacting proteins - regulators of transcription - proteins involved in structural modifications of chromatin
Interpretation of DNA methylation - steric consequences of cytosine methylation • changes at the histone level - posttranslational modifications of histones - SRA domains: SUVH family – H 3 methylation - Metyl Cp. G-binding d. : deacetylation (HDAC) - presence of histone forms - no H 2 A. Z (mechanism? ) • changes in binding of other interacting proteins - regulators of transcription - proteins involved in structural modifications of chromatin
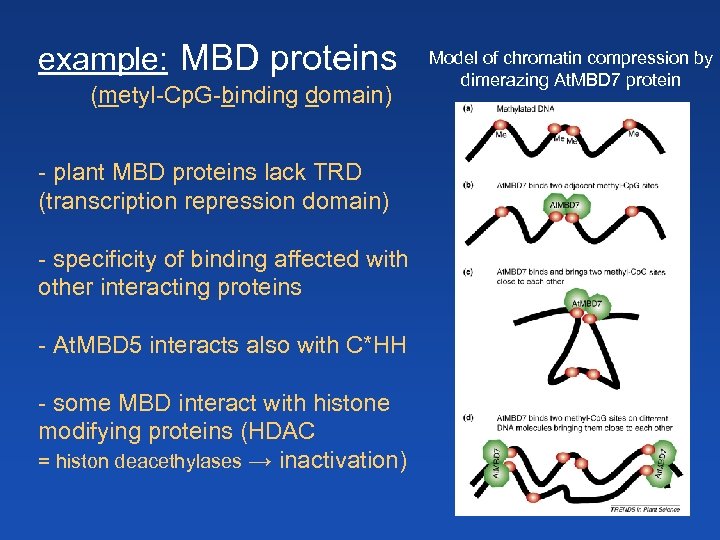 example: MBD proteins (metyl-Cp. G-binding domain) - plant MBD proteins lack TRD (transcription repression domain) - specificity of binding affected with other interacting proteins - At. MBD 5 interacts also with C*HH - some MBD interact with histone modifying proteins (HDAC = histon deacethylases → inactivation) Model of chromatin compression by dimerazing At. MBD 7 protein
example: MBD proteins (metyl-Cp. G-binding domain) - plant MBD proteins lack TRD (transcription repression domain) - specificity of binding affected with other interacting proteins - At. MBD 5 interacts also with C*HH - some MBD interact with histone modifying proteins (HDAC = histon deacethylases → inactivation) Model of chromatin compression by dimerazing At. MBD 7 protein
 DNA methylation and chromatin state - overview - correlation between DNA hypermethylation and hypoacethylation of histons and chromatin condensation (later replicated DNA) - heterochromatin - presence of histone H 2 A. Z (e. g. in promoters of active genes) excluded by DNA methylation! - presence of histone H 2 A. W supported by DNA methylation (heterochromatin)!
DNA methylation and chromatin state - overview - correlation between DNA hypermethylation and hypoacethylation of histons and chromatin condensation (later replicated DNA) - heterochromatin - presence of histone H 2 A. Z (e. g. in promoters of active genes) excluded by DNA methylation! - presence of histone H 2 A. W supported by DNA methylation (heterochromatin)!
 DNA methylation and chromatin state - overview - H 3 K 9 di methylation (KYP) signal for CHG methylation - CHG methylation signal for H 3 K 9 methylation - H 3 K 9 demethylation (IBM 1) likely induced by transcription - H 3 K 4 trimethylation (euchromatin mark), induced by transcription
DNA methylation and chromatin state - overview - H 3 K 9 di methylation (KYP) signal for CHG methylation - CHG methylation signal for H 3 K 9 methylation - H 3 K 9 demethylation (IBM 1) likely induced by transcription - H 3 K 4 trimethylation (euchromatin mark), induced by transcription

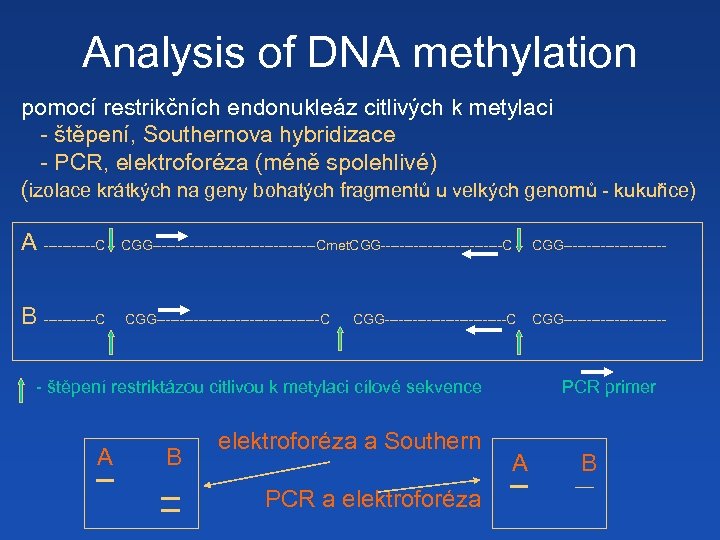 Analysis of DNA methylation pomocí restrikčních endonukleáz citlivých k metylaci - štěpení, Southernova hybridizace - PCR, elektroforéza (méně spolehlivé) (izolace krátkých na geny bohatých fragmentů u velkých genomů - kukuřice) A ------C CGG------------------Cmet. CGG-------------C CGG----------- B ------C CGG------------------C CGG--------------------------C - štěpení restriktázou citlivou k metylaci cílové sekvence A B elektroforéza a Southern PCR a elektroforéza PCR primer A B
Analysis of DNA methylation pomocí restrikčních endonukleáz citlivých k metylaci - štěpení, Southernova hybridizace - PCR, elektroforéza (méně spolehlivé) (izolace krátkých na geny bohatých fragmentů u velkých genomů - kukuřice) A ------C CGG------------------Cmet. CGG-------------C CGG----------- B ------C CGG------------------C CGG--------------------------C - štěpení restriktázou citlivou k metylaci cílové sekvence A B elektroforéza a Southern PCR a elektroforéza PCR primer A B


