5лекция.ppt
- Количество слайдов: 66
 Disperse systems. Forming a solution. Solutions of electrolytes and nonelectrolytes. Hydrolysis salts.
Disperse systems. Forming a solution. Solutions of electrolytes and nonelectrolytes. Hydrolysis salts.
 Basic Concepts Disperse systems - a mixture of different substances. They consist of a dispersed phase and the dispersion medium and are classified according to particle sizes of dispersible components.
Basic Concepts Disperse systems - a mixture of different substances. They consist of a dispersed phase and the dispersion medium and are classified according to particle sizes of dispersible components.
 Depending on the particle size of the dispersed systems are divided into groups: slurry (suspension, emulsion) - in which the particles have a size of 1000 nm (10 -6 m) or more; colloidal systems - the particle size of 1 -500 nm (10 -9 ÷ 5 · 10 -7 m) exist, if the particles have a charge. They are characterized by light scattering (Tyndall effect). Dispersions are also classified by aggregate states of the dispersed phase and the dispersion medium.
Depending on the particle size of the dispersed systems are divided into groups: slurry (suspension, emulsion) - in which the particles have a size of 1000 nm (10 -6 m) or more; colloidal systems - the particle size of 1 -500 nm (10 -9 ÷ 5 · 10 -7 m) exist, if the particles have a charge. They are characterized by light scattering (Tyndall effect). Dispersions are also classified by aggregate states of the dispersed phase and the dispersion medium.
 The dispersion medium - continuous phase, in which the amount allocated to the other (dispersed) phase in the form of fine solid particles, liquid droplets or gas bubbles. The dispersed phase - a set of homogeneous small solid particles, liquid droplets or gas bubbles, evenly distributed in the environment (dispersion) environment.
The dispersion medium - continuous phase, in which the amount allocated to the other (dispersed) phase in the form of fine solid particles, liquid droplets or gas bubbles. The dispersed phase - a set of homogeneous small solid particles, liquid droplets or gas bubbles, evenly distributed in the environment (dispersion) environment.
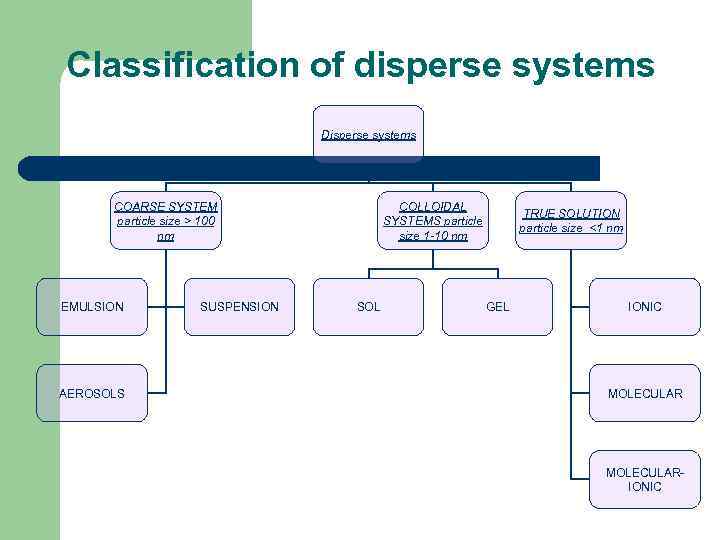 Classification of disperse systems Disperse systems COARSE SYSTEM particle size > 100 nm EMULSION AEROSOLS SUSPENSION COLLOIDAL SYSTEMS particle size 1 -10 nm SOL GEL TRUE SOLUTION particle size <1 nm IONIC MOLECULAR- IONIC
Classification of disperse systems Disperse systems COARSE SYSTEM particle size > 100 nm EMULSION AEROSOLS SUSPENSION COLLOIDAL SYSTEMS particle size 1 -10 nm SOL GEL TRUE SOLUTION particle size <1 nm IONIC MOLECULAR- IONIC
 Coarse system (suspension) ь Emulsions - it dispersions in which the dispersed phase and dispersion medium and fluids are mutually immiscible. For example, water, oil, milk, wherein small beads of fat floating in the liquid. ь Suspensions - a dispersion system in which the dispersed phase is solid and the dispersing medium - liquid - solid and substantially insoluble in the liquid. For example, agitating the clay in water. Over time, the particles will fall to the bottom of the vessel. Obviously, the smaller the particles, the more will the suspension. ь Aerosols - suspension of fine particles in the gas liquids or solids.
Coarse system (suspension) ь Emulsions - it dispersions in which the dispersed phase and dispersion medium and fluids are mutually immiscible. For example, water, oil, milk, wherein small beads of fat floating in the liquid. ь Suspensions - a dispersion system in which the dispersed phase is solid and the dispersing medium - liquid - solid and substantially insoluble in the liquid. For example, agitating the clay in water. Over time, the particles will fall to the bottom of the vessel. Obviously, the smaller the particles, the more will the suspension. ь Aerosols - suspension of fine particles in the gas liquids or solids.
 Colloidal solutions ь ь Sols (colloids) - liquid colloidal systems the particles of dispersed phase (micelles), freely and independently moving in Brownian motion. Sols with aqueous dispersion medium is called hydrosol, organic - organosol. Gels. Under certain conditions coagulation (coalescence phenomenon of the colloidal particles and deposition of a precipitate) sols leads to the formation of the gelatinous mass, called a gel. In this case, the entire mass of the colloidal particles, linking solvent goes into a kind of semi -liquid, semi-solid state. - Gelatin, jelly, marmalade.
Colloidal solutions ь ь Sols (colloids) - liquid colloidal systems the particles of dispersed phase (micelles), freely and independently moving in Brownian motion. Sols with aqueous dispersion medium is called hydrosol, organic - organosol. Gels. Under certain conditions coagulation (coalescence phenomenon of the colloidal particles and deposition of a precipitate) sols leads to the formation of the gelatinous mass, called a gel. In this case, the entire mass of the colloidal particles, linking solvent goes into a kind of semi -liquid, semi-solid state. - Gelatin, jelly, marmalade.
 Tyndall effect Tyndall effect - an optical effect, the scattering of light by passing light beam through an optically inhomogeneous medium. Usually, there is a luminous cone (cone Tyndall) visible on a dark background. Typical for solutions of colloidal systems (e. g. , sols, metals, dilute latexes tobacco smoke), in which the particles and their environment are different in refractive index. Tyndall effect based on a number of optical methods for determining the size, shape and concentration of colloidal particles and macromolecules. Tyndall Effect is named after its discoverer, John Tyndall.
Tyndall effect Tyndall effect - an optical effect, the scattering of light by passing light beam through an optically inhomogeneous medium. Usually, there is a luminous cone (cone Tyndall) visible on a dark background. Typical for solutions of colloidal systems (e. g. , sols, metals, dilute latexes tobacco smoke), in which the particles and their environment are different in refractive index. Tyndall effect based on a number of optical methods for determining the size, shape and concentration of colloidal particles and macromolecules. Tyndall Effect is named after its discoverer, John Tyndall.
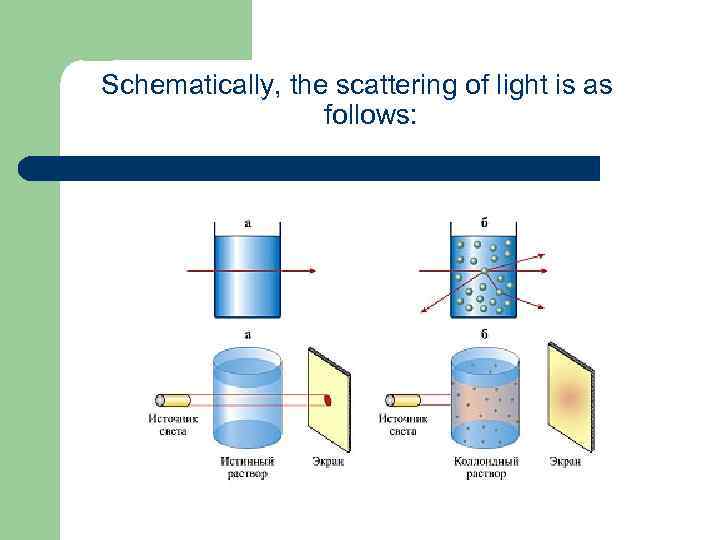 Schematically, the scattering of light is as follows:
Schematically, the scattering of light is as follows:
 Classification the state of aggregation of the dispersion medium and dispersed phase: - Solid substance - Gas - Liquid
Classification the state of aggregation of the dispersion medium and dispersed phase: - Solid substance - Gas - Liquid
 The dispersion medium: solid l l The dispersed phase - gas: Soil, textile fabrics, bricks and ceramics, porous chocolate powders. n n The dispersed phase - liquid: Wet soil, medical and cosmetic products. Dispersed phase - solid: Rocks, colored glass, some alloys.
The dispersion medium: solid l l The dispersed phase - gas: Soil, textile fabrics, bricks and ceramics, porous chocolate powders. n n The dispersed phase - liquid: Wet soil, medical and cosmetic products. Dispersed phase - solid: Rocks, colored glass, some alloys.
 Dispersion medium: gas l l The dispersed phase - gas: Always homogeneous mixture (air, gas) n n The dispersed phase - liquid: Fog, associated gas from oil droplets, aerosols. Dispersed phase - solid: Dust in the air, smoke, smog, dust storms.
Dispersion medium: gas l l The dispersed phase - gas: Always homogeneous mixture (air, gas) n n The dispersed phase - liquid: Fog, associated gas from oil droplets, aerosols. Dispersed phase - solid: Dust in the air, smoke, smog, dust storms.
 The dispersion medium: liquid l l The dispersed phase - gas: Fizzy drinks foam. n n The dispersed phase - liquid: Emulsions: oil, cream, milk; body fluids, the liquid contents of the cells. Dispersed phase - solid: Sols, gels, pastes. Construction solutions.
The dispersion medium: liquid l l The dispersed phase - gas: Fizzy drinks foam. n n The dispersed phase - liquid: Emulsions: oil, cream, milk; body fluids, the liquid contents of the cells. Dispersed phase - solid: Sols, gels, pastes. Construction solutions.
 True solutions - contain atoms and molecules whose size is typically less than 5 x 10 -9 m - is thermodynamically stable singlephase multicomponent systems
True solutions - contain atoms and molecules whose size is typically less than 5 x 10 -9 m - is thermodynamically stable singlephase multicomponent systems
 True solutions ь ь ь Molecular - this aqueous solutions of nonelectrolytes - organic substances (such as alcohol, glucose, sucrose, etc. ); Ionic - are solutions of strong electrolytes (alkali salts, acid - Na. OH, K 2 SO 4. HNO 3, HCl. O 4); Molecular - ionic - a solution of weak electrolytes (nitrous, hydrogen sulphide, and others. ).
True solutions ь ь ь Molecular - this aqueous solutions of nonelectrolytes - organic substances (such as alcohol, glucose, sucrose, etc. ); Ionic - are solutions of strong electrolytes (alkali salts, acid - Na. OH, K 2 SO 4. HNO 3, HCl. O 4); Molecular - ionic - a solution of weak electrolytes (nitrous, hydrogen sulphide, and others. ).
 l Solution - homogeneous system variable composition consisting of one or more components. l Each solution consists of a solvent and solute.
l Solution - homogeneous system variable composition consisting of one or more components. l Each solution consists of a solvent and solute.
 l. The solvent - is one component of aggregate state, which does not change during the formation of the solution. l. Solubility - is the ability of the substance to dissolve in a given solvent.
l. The solvent - is one component of aggregate state, which does not change during the formation of the solution. l. Solubility - is the ability of the substance to dissolve in a given solvent.
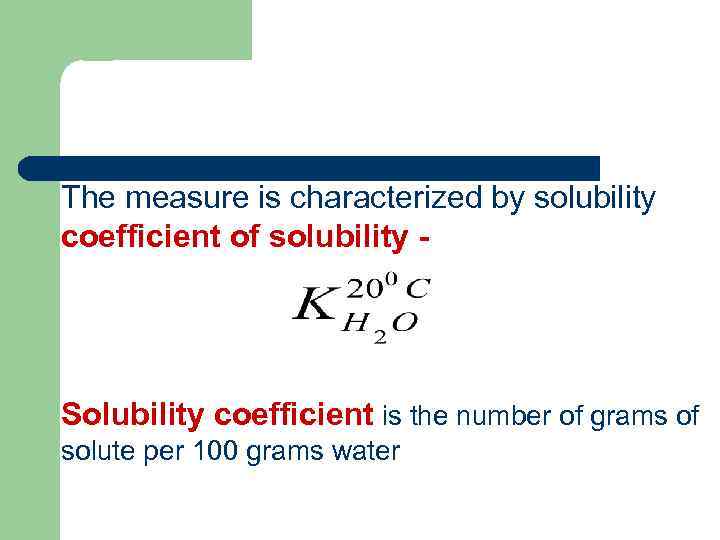 The measure is characterized by solubility coefficient of solubility - Solubility coefficient is the number of grams of solute per 100 grams water
The measure is characterized by solubility coefficient of solubility - Solubility coefficient is the number of grams of solute per 100 grams water
 Basic Concepts The solubility product (PR) - is the part of the substance which is soluble and dissociates into ions in solution.
Basic Concepts The solubility product (PR) - is the part of the substance which is soluble and dissociates into ions in solution.
 Basic provisions Dissolution - a physical-chemical process. l The physical side - dissolving the substance loses its structure collapses. l Chemical side - solute interacts with the solvent - solvation - the solvates are formed when dissolved in water is then called hydration process - formed hydrates. Saturated solution - a solution which is in equilibrium with a solute. l
Basic provisions Dissolution - a physical-chemical process. l The physical side - dissolving the substance loses its structure collapses. l Chemical side - solute interacts with the solvent - solvation - the solvates are formed when dissolved in water is then called hydration process - formed hydrates. Saturated solution - a solution which is in equilibrium with a solute. l
 Solution concentration - this amount of solute contained in a unit of weight and volume of the solution or solvent.
Solution concentration - this amount of solute contained in a unit of weight and volume of the solution or solvent.
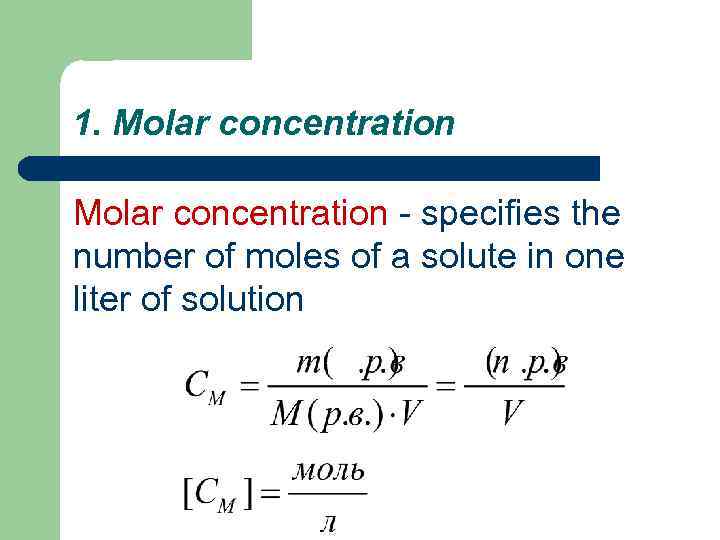 1. Molar concentration - specifies the number of moles of a solute in one liter of solution
1. Molar concentration - specifies the number of moles of a solute in one liter of solution
 m (m. s. ) - the mass of the solute, in grams; M (m. s. ) - the molar mass of the solute in g / mol; V - the volume of solution, l.
m (m. s. ) - the mass of the solute, in grams; M (m. s. ) - the molar mass of the solute in g / mol; V - the volume of solution, l.
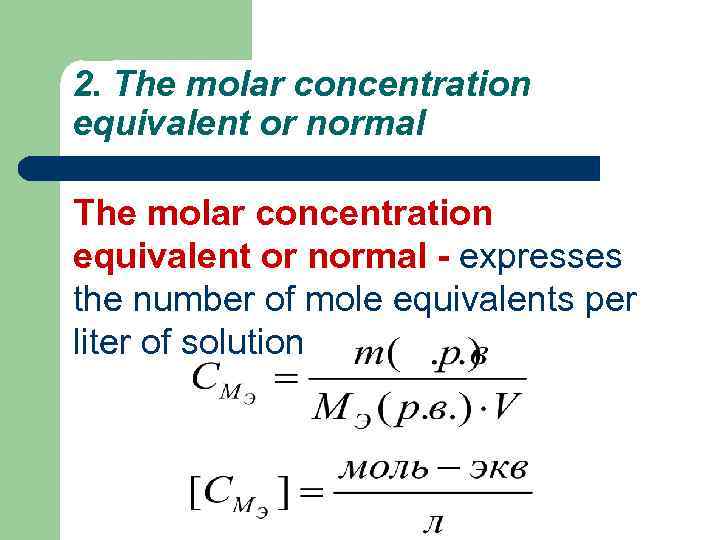 2. The molar concentration equivalent or normal - expresses the number of mole equivalents per liter of solution
2. The molar concentration equivalent or normal - expresses the number of mole equivalents per liter of solution
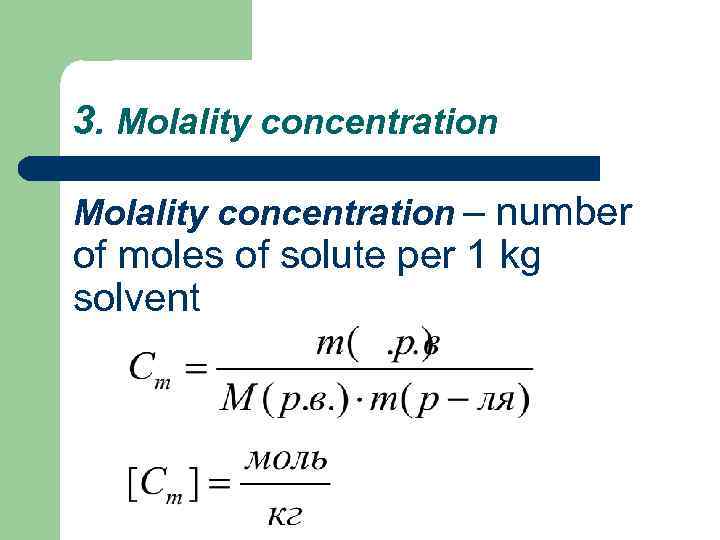 3. Molality concentration – number of moles of solute per 1 kg solvent
3. Molality concentration – number of moles of solute per 1 kg solvent
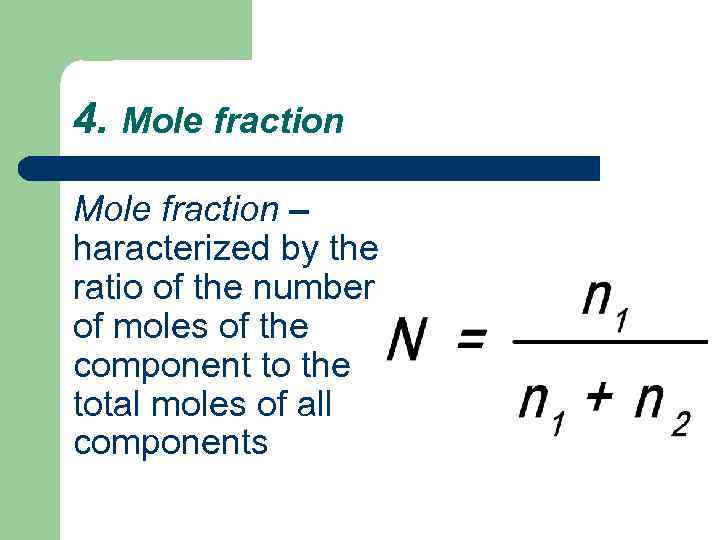 4. Mole fraction – haracterized by the ratio of the number of moles of the component to the total moles of all components
4. Mole fraction – haracterized by the ratio of the number of moles of the component to the total moles of all components
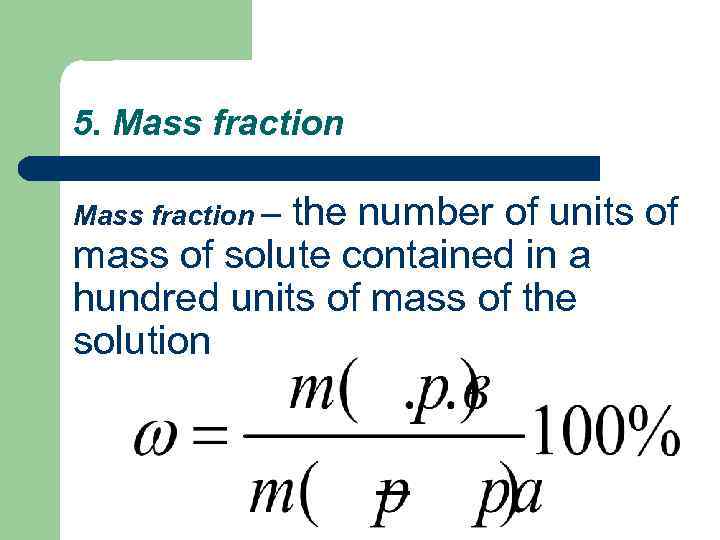 5. Mass fraction – the number of units of mass of solute contained in a hundred units of mass of the solution
5. Mass fraction – the number of units of mass of solute contained in a hundred units of mass of the solution
 6. Titer of solution – the mass of the dissolved substance in 1 ml of solution
6. Titer of solution – the mass of the dissolved substance in 1 ml of solution
 Ideal solution. Raoult's law
Ideal solution. Raoult's law
 l Ideal solutions - these solutions, the formation of which there is no change in the volume and thermal effect. (∆H = 0, ∆ V = 0), only due to the increase of entropy. l The ideal solution - a solution in which neglect intermolecular interaction.
l Ideal solutions - these solutions, the formation of which there is no change in the volume and thermal effect. (∆H = 0, ∆ V = 0), only due to the increase of entropy. l The ideal solution - a solution in which neglect intermolecular interaction.
 The ideal solutions solute particles are at a great distance from each other and their mutual influence can be eliminated, and the solvent practically does not change its properties.
The ideal solutions solute particles are at a great distance from each other and their mutual influence can be eliminated, and the solvent practically does not change its properties.
 l Dilute solutions are close to ideal. l From real solutions dilute solutions of non-electrolytes can be on the properties closer to the ideal.
l Dilute solutions are close to ideal. l From real solutions dilute solutions of non-electrolytes can be on the properties closer to the ideal.
 The solutions are not conducting electrical current are called non-electrolytes. Weak electrolytes in solution do not dissociate into ions.
The solutions are not conducting electrical current are called non-electrolytes. Weak electrolytes in solution do not dissociate into ions.
 Some physical properties of solutions of non-electrolytes depend only on the particle concentration of the solute and the solvent nature and do not depend on the nature of the solute. These properties are called colligative properties.
Some physical properties of solutions of non-electrolytes depend only on the particle concentration of the solute and the solvent nature and do not depend on the nature of the solute. These properties are called colligative properties.
 - Colligative properties: Lowering the vapor pressure of the solvent Boiling point elevation, freezing point lowering Osmotic pressure
- Colligative properties: Lowering the vapor pressure of the solvent Boiling point elevation, freezing point lowering Osmotic pressure
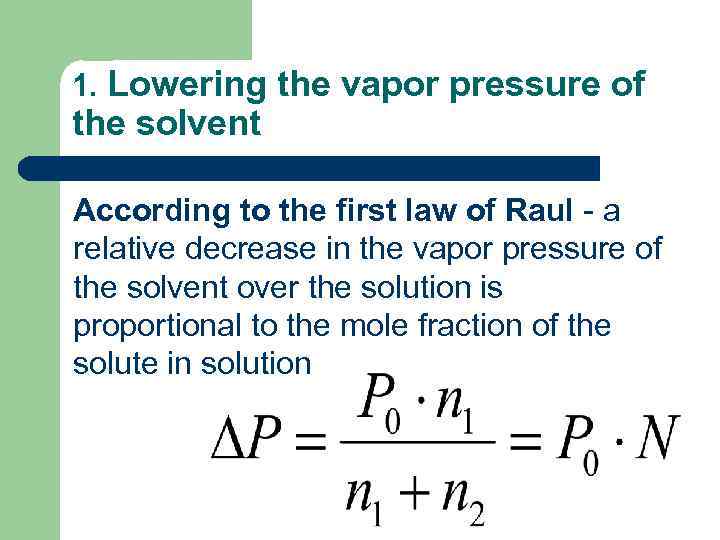 1. Lowering the vapor pressure of the solvent According to the first law of Raul - a relative decrease in the vapor pressure of the solvent over the solution is proportional to the mole fraction of the solute in solution
1. Lowering the vapor pressure of the solvent According to the first law of Raul - a relative decrease in the vapor pressure of the solvent over the solution is proportional to the mole fraction of the solute in solution
 Р 0 – saturated vapor pressure of the pure solvent; N – the mole fraction of the solute in solution.
Р 0 – saturated vapor pressure of the pure solvent; N – the mole fraction of the solute in solution.
 2 а. Increasing the reflux temperature The second law of Raul: а) Raising the boiling point of the solution is proportional to ∆Tboil. molal concentration of the solution
2 а. Increasing the reflux temperature The second law of Raul: а) Raising the boiling point of the solution is proportional to ∆Tboil. molal concentration of the solution
 Еboil. – is the molar constant increase the boiling point of the solvent or ebulioskopic constant that depends on the nature of the solvent. l Еboil. refer to the manuals. l
Еboil. – is the molar constant increase the boiling point of the solvent or ebulioskopic constant that depends on the nature of the solvent. l Еboil. refer to the manuals. l
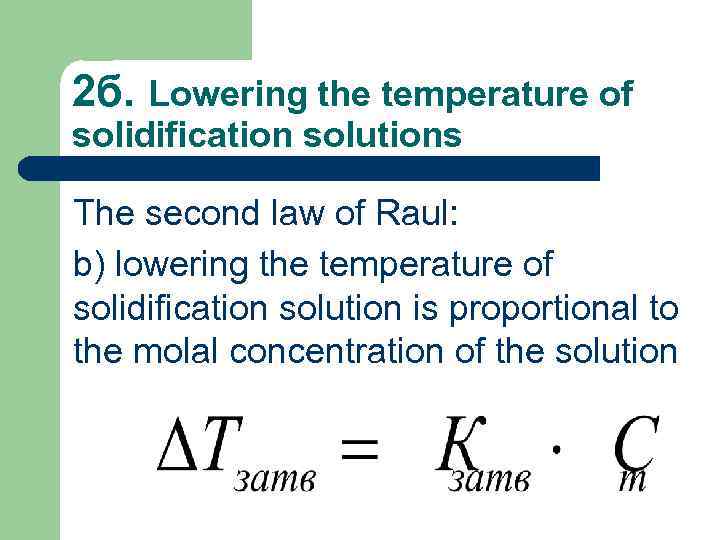 2 б. Lowering the temperature of solidification solutions The second law of Raul: b) lowering the temperature of solidification solution is proportional to the molal concentration of the solution
2 б. Lowering the temperature of solidification solutions The second law of Raul: b) lowering the temperature of solidification solution is proportional to the molal concentration of the solution
 Кsolid. – is the molal constant of lowering the freezing point or freezing point depression constant (solvent).
Кsolid. – is the molal constant of lowering the freezing point or freezing point depression constant (solvent).
 3. Osmotic pressure l A solution is a homogeneous system. l Particles of the solute and solvent are disordered heat motion and uniformly distributed throughout the volume of the solution.
3. Osmotic pressure l A solution is a homogeneous system. l Particles of the solute and solvent are disordered heat motion and uniformly distributed throughout the volume of the solution.
 l The molecules of the solvent and solute will diffuse predominantly in the direction where their concentration is lower. l This will lead to a two-way diffusion, and equalize the concentration С 1=С 2.
l The molecules of the solvent and solute will diffuse predominantly in the direction where their concentration is lower. l This will lead to a two-way diffusion, and equalize the concentration С 1=С 2.
 However, diffusion is one-sided, if the solution is separated by a semipermeable wall permeable only to solvent molecules. l Under this condition, that C 2 > C 1 molecules of solvent at a higher rate will diffuse towards S 1→S 2 and the volume of solution with a concentration C 2 to increase slightly. This one-sided diffusion called osmosis. l
However, diffusion is one-sided, if the solution is separated by a semipermeable wall permeable only to solvent molecules. l Under this condition, that C 2 > C 1 molecules of solvent at a higher rate will diffuse towards S 1→S 2 and the volume of solution with a concentration C 2 to increase slightly. This one-sided diffusion called osmosis. l
 l. To quantify the characteristics of osmotic properties introduces the concept of osmotic pressure. l. Osmotic pressure - it is the pressure that must be applied to osmosis stopped.
l. To quantify the characteristics of osmotic properties introduces the concept of osmotic pressure. l. Osmotic pressure - it is the pressure that must be applied to osmosis stopped.
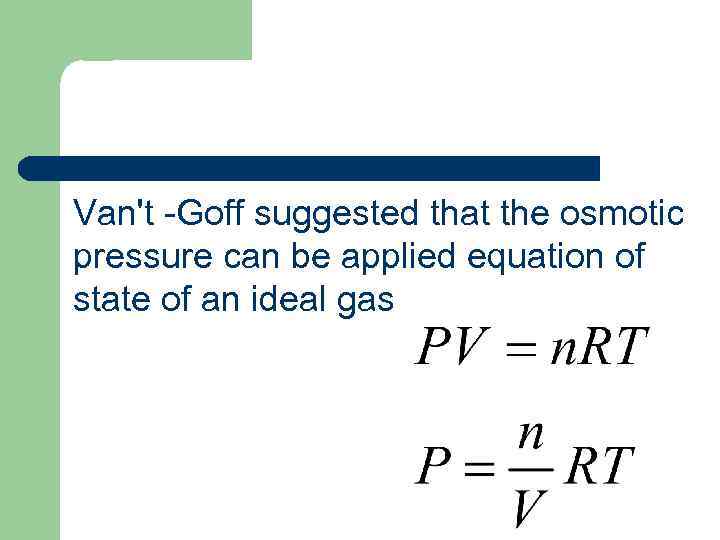 Van't -Goff suggested that the osmotic pressure can be applied equation of state of an ideal gas
Van't -Goff suggested that the osmotic pressure can be applied equation of state of an ideal gas
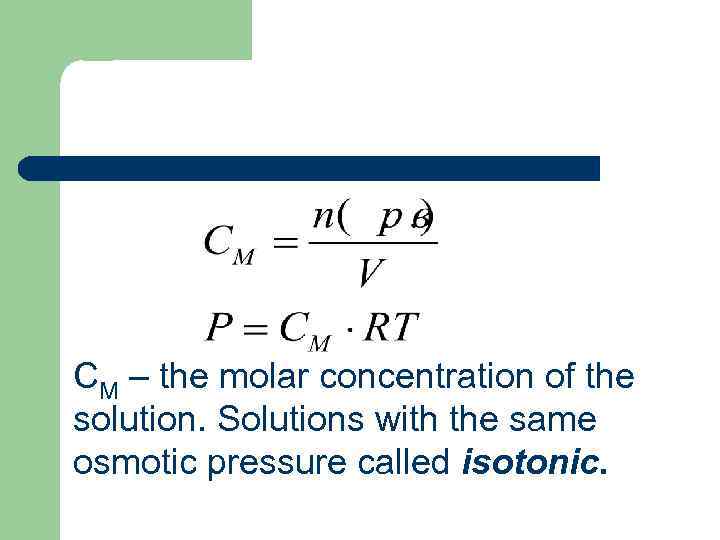 СМ – the molar concentration of the solution. Solutions with the same osmotic pressure called isotonic.
СМ – the molar concentration of the solution. Solutions with the same osmotic pressure called isotonic.
 l The process of disintegration of matter into ions when dissolved is called electrolytic dissociation. l A quantitative description of this process is the degree of electrolytic dissociation( )
l The process of disintegration of matter into ions when dissolved is called electrolytic dissociation. l A quantitative description of this process is the degree of electrolytic dissociation( )
 The degree of electrolytic dissociation( ) - the number of broken molecules into ions to the total amount of dissolved molecules.
The degree of electrolytic dissociation( ) - the number of broken molecules into ions to the total amount of dissolved molecules.
 The magnitude of distinguishes: а) strong electrolytes > 0, 3 б) electrolytes of moderate strength 0, 03 < < 0, 3 в) weak electrolytes < 0, 03
The magnitude of distinguishes: а) strong electrolytes > 0, 3 б) electrolytes of moderate strength 0, 03 < < 0, 3 в) weak electrolytes < 0, 03
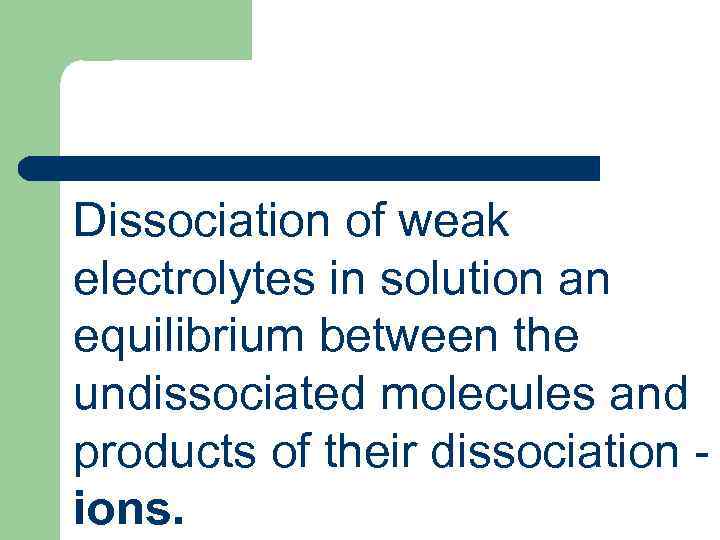 Dissociation of weak electrolytes in solution an equilibrium between the undissociated molecules and products of their dissociation - ions.
Dissociation of weak electrolytes in solution an equilibrium between the undissociated molecules and products of their dissociation - ions.
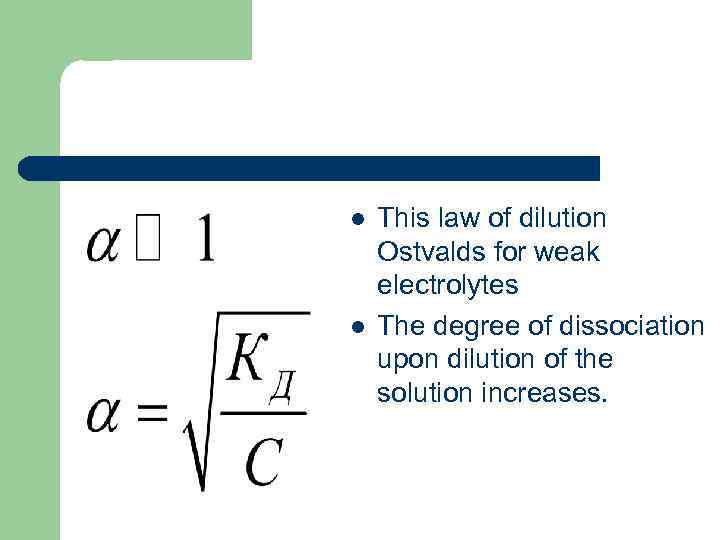 l l This law of dilution Ostvalds for weak electrolytes The degree of dissociation upon dilution of the solution increases.
l l This law of dilution Ostvalds for weak electrolytes The degree of dissociation upon dilution of the solution increases.
 Strong electrolytes in solution dissociate into ions.
Strong electrolytes in solution dissociate into ions.
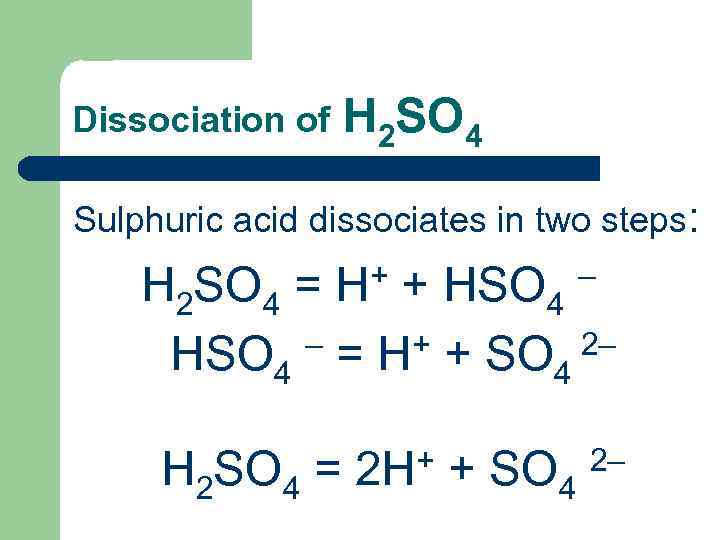 Dissociation of H 2 SO 4 Sulphuric acid dissociates in two steps: + + HSO – H 2 SO 4 = H 4 – = H+ + SO 2– HSO 4 4 + + SO 2– H 2 SO 4 = 2 H 4
Dissociation of H 2 SO 4 Sulphuric acid dissociates in two steps: + + HSO – H 2 SO 4 = H 4 – = H+ + SO 2– HSO 4 4 + + SO 2– H 2 SO 4 = 2 H 4
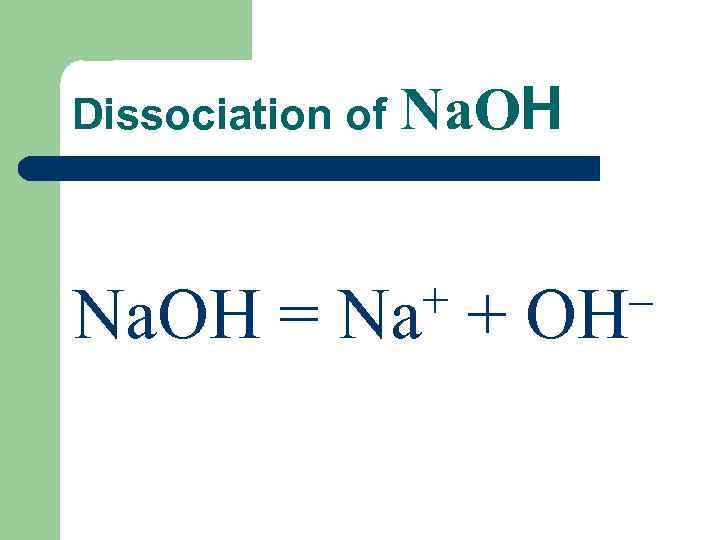 Dissociation of Na. OН + + OH– Na. ОH = Na
Dissociation of Na. OН + + OH– Na. ОH = Na
 Dissociation of the salts KCI = K+ + CI– 3+ + 3 SO 2– Al 2(SO 4)3 = 2 Al 4
Dissociation of the salts KCI = K+ + CI– 3+ + 3 SO 2– Al 2(SO 4)3 = 2 Al 4
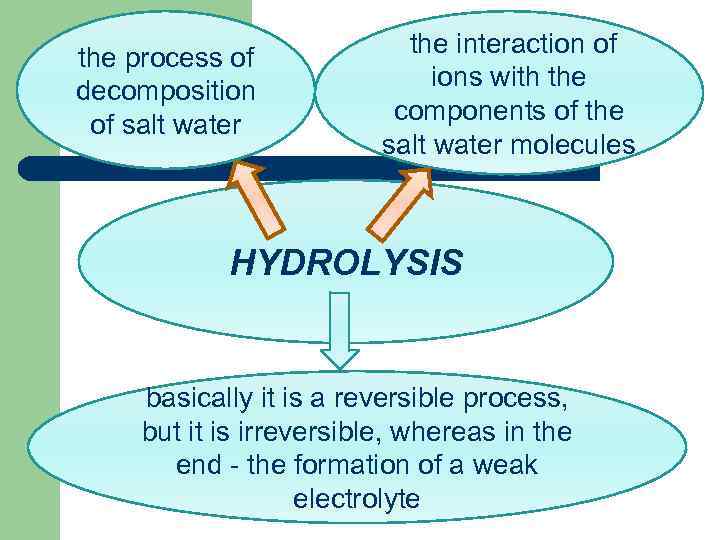 the process of decomposition of salt water the interaction of ions with the components of the salt water molecules HYDROLYSIS basically it is a reversible process, but it is irreversible, whereas in the end - the formation of a weak electrolyte
the process of decomposition of salt water the interaction of ions with the components of the salt water molecules HYDROLYSIS basically it is a reversible process, but it is irreversible, whereas in the end - the formation of a weak electrolyte
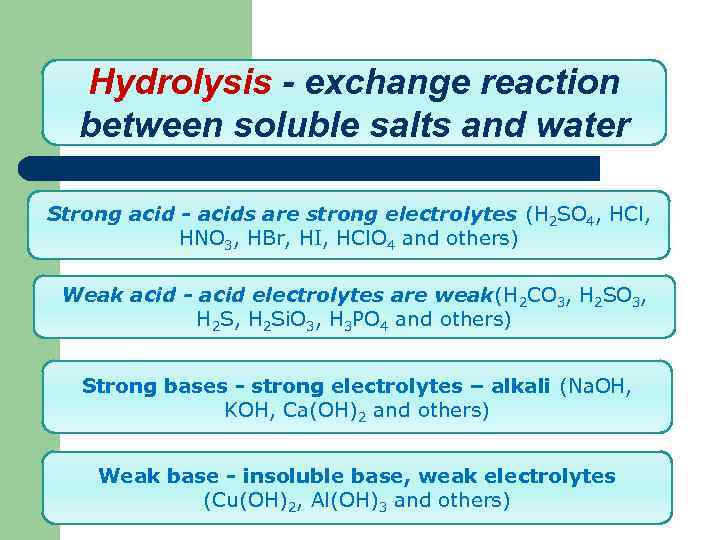 Hydrolysis - exchange reaction between soluble salts and water Strong acid - acids are strong electrolytes (H 2 SO 4, HCl, HNO 3, HBr, HI, HCl. O 4 and others) Weak acid - acid electrolytes are weak(H 2 CO 3, H 2 S, H 2 Si. O 3, H 3 PO 4 and others) Strong bases - strong electrolytes – alkali (Na. OH, KOH, Ca(OH)2 and others) Weak base - insoluble base, weak electrolytes (Cu(OH)2, Al(OH)3 and others)
Hydrolysis - exchange reaction between soluble salts and water Strong acid - acids are strong electrolytes (H 2 SO 4, HCl, HNO 3, HBr, HI, HCl. O 4 and others) Weak acid - acid electrolytes are weak(H 2 CO 3, H 2 S, H 2 Si. O 3, H 3 PO 4 and others) Strong bases - strong electrolytes – alkali (Na. OH, KOH, Ca(OH)2 and others) Weak base - insoluble base, weak electrolytes (Cu(OH)2, Al(OH)3 and others)
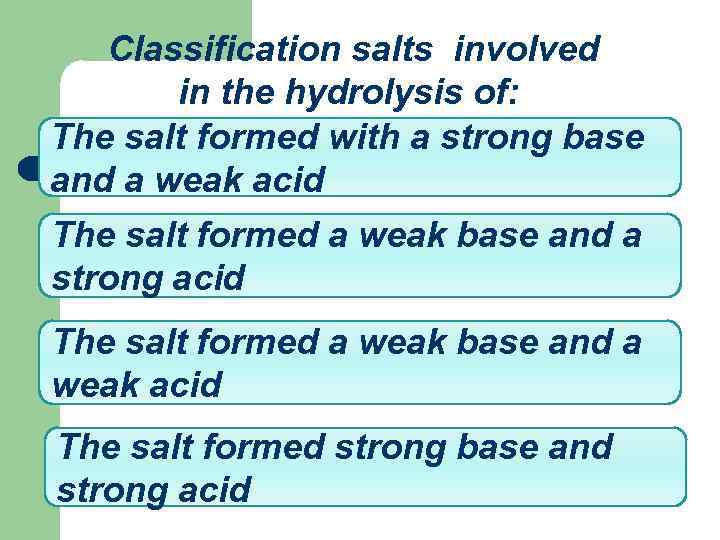 Classification salts involved in the hydrolysis of: The salt formed with a strong base and a weak acid The salt formed a weak base and a strong acid The salt formed a weak base and a weak acid The salt formed strong base and strong acid
Classification salts involved in the hydrolysis of: The salt formed with a strong base and a weak acid The salt formed a weak base and a strong acid The salt formed a weak base and a weak acid The salt formed strong base and strong acid
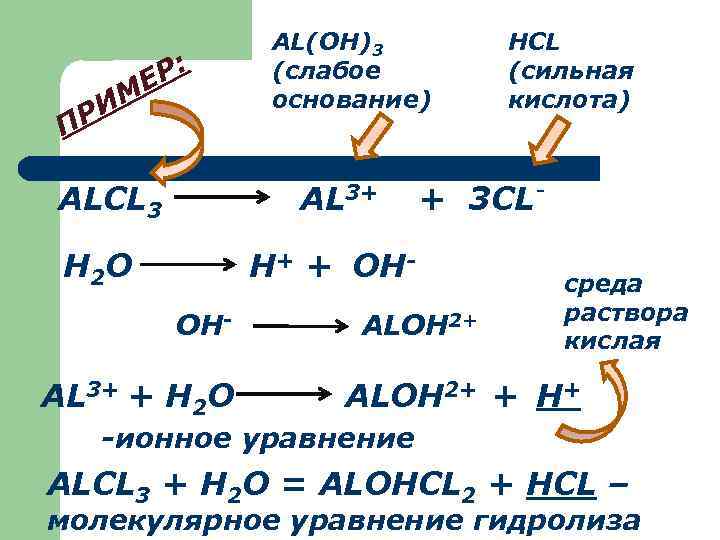 Р: Е ИМ ПР ALCL 3 AL(OH)3 (слабое основание) AL 3+ H 2 O + 3 CL- H+ + OHOH- AL 3+ + H 2 O HCL (сильная кислота) ALOH 2+ среда раствора кислая ALOH 2+ + H+ -ионное уравнение ALCL 3 + H 2 O = ALOHCL 2 + HCL – молекулярное уравнение гидролиза
Р: Е ИМ ПР ALCL 3 AL(OH)3 (слабое основание) AL 3+ H 2 O + 3 CL- H+ + OHOH- AL 3+ + H 2 O HCL (сильная кислота) ALOH 2+ среда раствора кислая ALOH 2+ + H+ -ионное уравнение ALCL 3 + H 2 O = ALOHCL 2 + HCL – молекулярное уравнение гидролиза
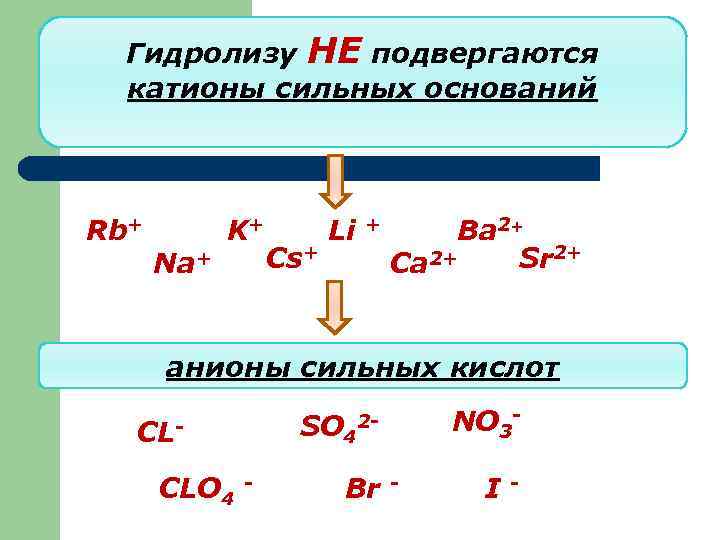 Гидролизу НЕ подвергаются катионы сильных оснований Rb+ Na+ K+ Cs+ Li + Ba 2+ 2+ Sr 2+ Ca анионы сильных кислот SO 4 CLCLO 4 - NO 3 - 2 - Br - I -
Гидролизу НЕ подвергаются катионы сильных оснований Rb+ Na+ K+ Cs+ Li + Ba 2+ 2+ Sr 2+ Ca анионы сильных кислот SO 4 CLCLO 4 - NO 3 - 2 - Br - I -
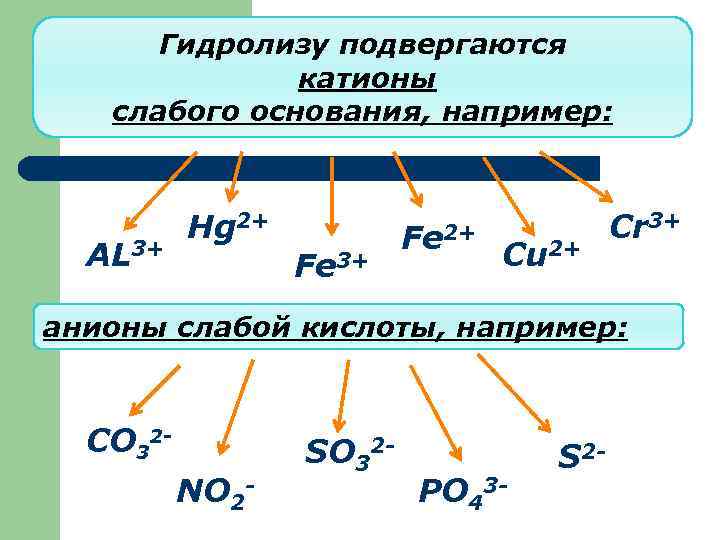 Гидролизу подвергаются катионы слабого основания, например: AL 3+ Hg 2+ Fe 3+ Fe 2+ Cu 2+ Cr 3+ анионы слабой кислоты, например: CO 32 NO 2 - SO 32 - PO 43 - S 2 -
Гидролизу подвергаются катионы слабого основания, например: AL 3+ Hg 2+ Fe 3+ Fe 2+ Cu 2+ Cr 3+ анионы слабой кислоты, например: CO 32 NO 2 - SO 32 - PO 43 - S 2 -
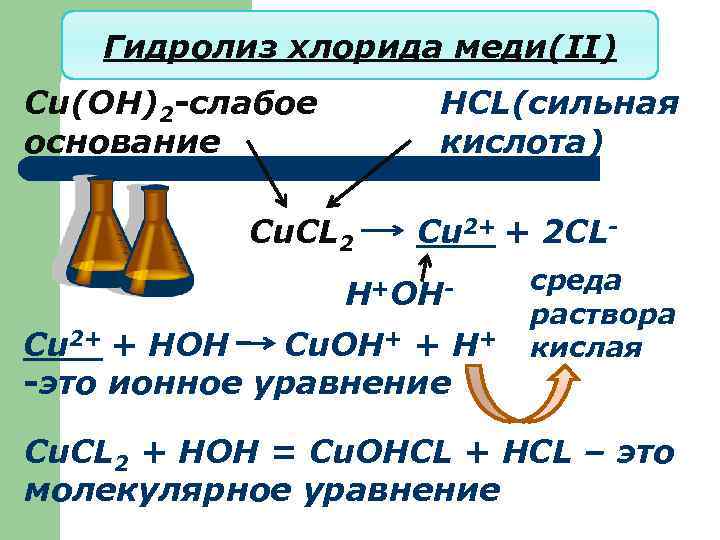 Гидролиз хлорида меди(II) Cu(OH)2 -слабое основание HCL(сильная кислота) Cu. CL 2 Cu 2+ + 2 CL- H+OHCu 2+ + HOH Cu. OH+ + H+ -это ионное уравнение среда раствора кислая Cu. CL 2 + HOH = Cu. OHCL + HCL – это молекулярное уравнение
Гидролиз хлорида меди(II) Cu(OH)2 -слабое основание HCL(сильная кислота) Cu. CL 2 Cu 2+ + 2 CL- H+OHCu 2+ + HOH Cu. OH+ + H+ -это ионное уравнение среда раствора кислая Cu. CL 2 + HOH = Cu. OHCL + HCL – это молекулярное уравнение
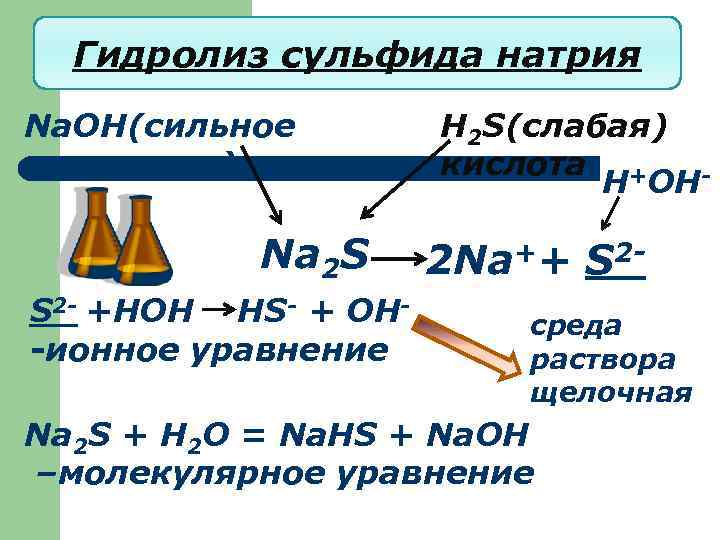 Гидролиз сульфида натрия Na. OH(сильное основание) Na 2 S S 2 - +HOH HS- + OH-ионное уравнение H 2 S(слабая) кислота + H OH- 2 Na++ S 2 среда раствора щелочная Na 2 S + H 2 O = Na. HS + Na. OH –молекулярное уравнение
Гидролиз сульфида натрия Na. OH(сильное основание) Na 2 S S 2 - +HOH HS- + OH-ионное уравнение H 2 S(слабая) кислота + H OH- 2 Na++ S 2 среда раствора щелочная Na 2 S + H 2 O = Na. HS + Na. OH –молекулярное уравнение
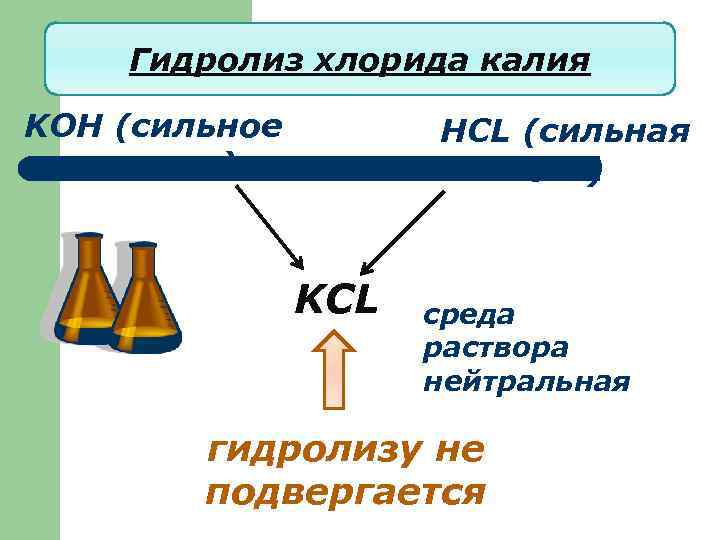 Гидролиз хлорида калия KOH (сильное основание) HCL (сильная кислота) KCL среда раствора нейтральная гидролизу не подвергается
Гидролиз хлорида калия KOH (сильное основание) HCL (сильная кислота) KCL среда раствора нейтральная гидролизу не подвергается
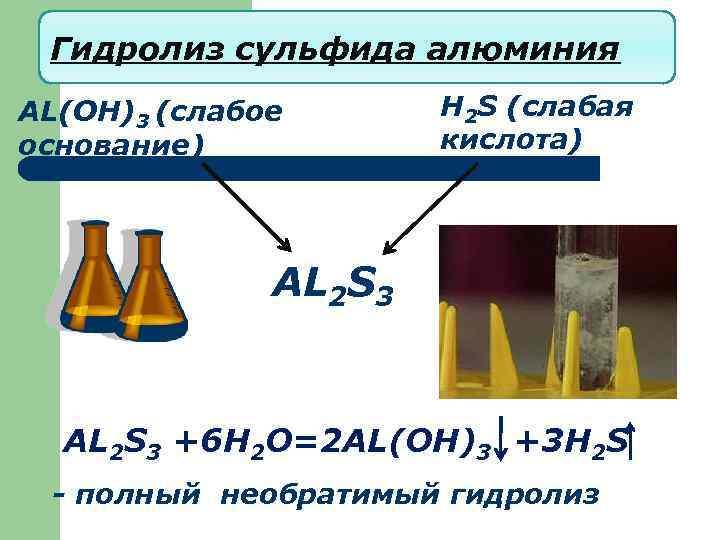 Гидролиз сульфида алюминия AL(OH)3 (слабое основание) H 2 S (слабая кислота) AL 2 S 3 +6 H 2 O=2 AL(OH)3 +3 H 2 S - полный необратимый гидролиз
Гидролиз сульфида алюминия AL(OH)3 (слабое основание) H 2 S (слабая кислота) AL 2 S 3 +6 H 2 O=2 AL(OH)3 +3 H 2 S - полный необратимый гидролиз


