e0f6a6719a49f1db1fbf50d1ed025146.ppt
- Количество слайдов: 63
 DISINFECTION & STERILIZATION
DISINFECTION & STERILIZATION
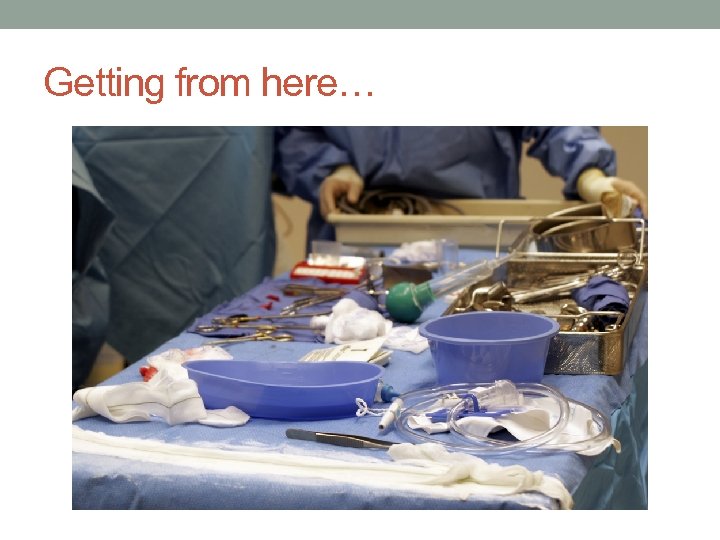 Getting from here…
Getting from here…
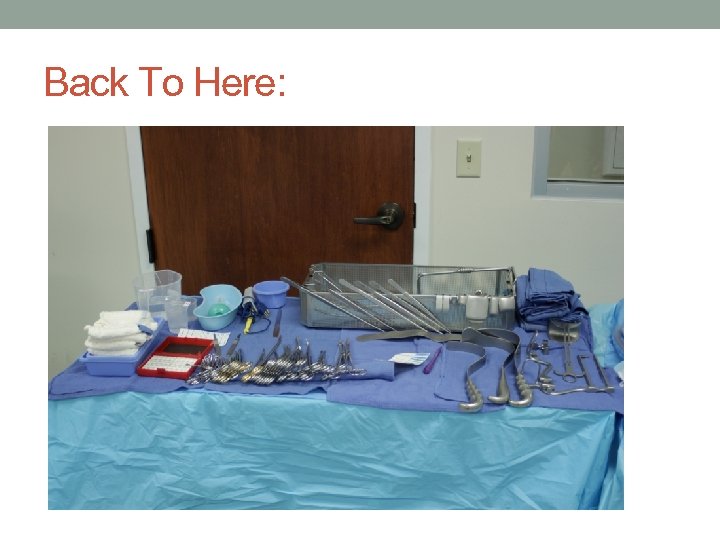 Back To Here:
Back To Here:
 Outline • Cleaning & Decontamination • Disinfection • Prep & Pack • Sterilization • Terminal • Point of Use • Quality Assurance & Monitoring • Sterilization Process Failure
Outline • Cleaning & Decontamination • Disinfection • Prep & Pack • Sterilization • Terminal • Point of Use • Quality Assurance & Monitoring • Sterilization Process Failure
 Good old Dr. Earle Spaulding • The selection of a disinfection or sterilization method depends on the intended use of the item. • Non-critical requires low level disinfection • Semi-critical requires high level disinfection • Critical requires sterilization
Good old Dr. Earle Spaulding • The selection of a disinfection or sterilization method depends on the intended use of the item. • Non-critical requires low level disinfection • Semi-critical requires high level disinfection • Critical requires sterilization
 Definitions • Cleaning—Removal of contamination from an item to the extent necessary for further processing or for intended use. • Decontamination—The use of physical or chemical means to remove, inactivate, or destroy blood-borne pathogens on a surface or item to the point where they are no longer capable of transmitting infection and the item is rendered safe to handle with the ungloved hand.
Definitions • Cleaning—Removal of contamination from an item to the extent necessary for further processing or for intended use. • Decontamination—The use of physical or chemical means to remove, inactivate, or destroy blood-borne pathogens on a surface or item to the point where they are no longer capable of transmitting infection and the item is rendered safe to handle with the ungloved hand.
 Definitions • Disinfection—The process that kills pathogenic and other microorganisms by physical or chemical means. • Low • Intermediate • High Level • Sterilization—Free from viable microorganisms. • This is actually just a probability. • Sterility Assurance Level (SAL)= 10 -6 th power, or 1: 1, 000 chance that a single viable microorganism is present on a “sterilized” item.
Definitions • Disinfection—The process that kills pathogenic and other microorganisms by physical or chemical means. • Low • Intermediate • High Level • Sterilization—Free from viable microorganisms. • This is actually just a probability. • Sterility Assurance Level (SAL)= 10 -6 th power, or 1: 1, 000 chance that a single viable microorganism is present on a “sterilized” item.
 AORN Standards & Recommended Practices Recommendation #1 “Items to be processed should be categorized as critical, semicritical, and noncritical. ” • Non. Critical: Contact with intact skin but not mucous membranes • Clean with low-level disinfectant : kill bacteria, fungi and some viruses, won’t kill spores • Ex Alcohol, quaternary ammonium (virex)
AORN Standards & Recommended Practices Recommendation #1 “Items to be processed should be categorized as critical, semicritical, and noncritical. ” • Non. Critical: Contact with intact skin but not mucous membranes • Clean with low-level disinfectant : kill bacteria, fungi and some viruses, won’t kill spores • Ex Alcohol, quaternary ammonium (virex)
 • Intermediate level disinfection is also mainly for environmental surfaces • Used on noncritical items that have been exposed to pathogens that aren’t killed by low level disinfection. • Examples: Halogens (chlorine, iodine), Phenolics (carbolic acid) • Relatively fast acting • Relatively broad spectrum
• Intermediate level disinfection is also mainly for environmental surfaces • Used on noncritical items that have been exposed to pathogens that aren’t killed by low level disinfection. • Examples: Halogens (chlorine, iodine), Phenolics (carbolic acid) • Relatively fast acting • Relatively broad spectrum
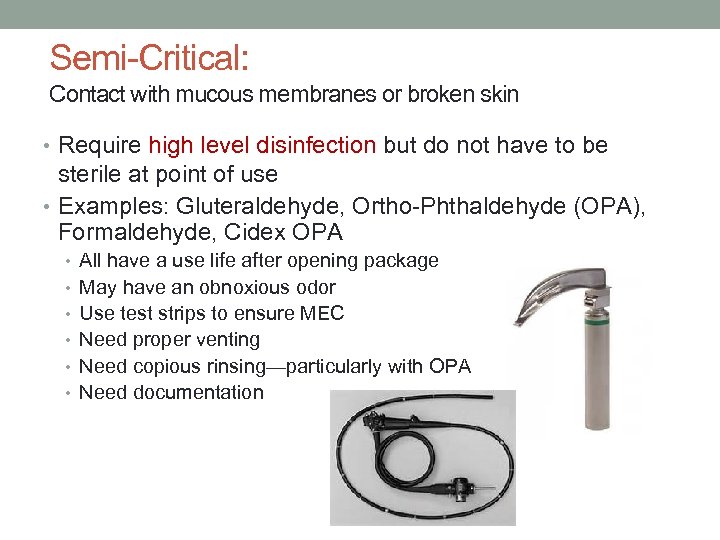 Semi-Critical: Contact with mucous membranes or broken skin • Require high level disinfection but do not have to be sterile at point of use • Examples: Gluteraldehyde, Ortho-Phthaldehyde (OPA), Formaldehyde, Cidex OPA • All have a use life after opening package • May have an obnoxious odor • Use test strips to ensure MEC • Need proper venting • Need copious rinsing—particularly with OPA • Need documentation
Semi-Critical: Contact with mucous membranes or broken skin • Require high level disinfection but do not have to be sterile at point of use • Examples: Gluteraldehyde, Ortho-Phthaldehyde (OPA), Formaldehyde, Cidex OPA • All have a use life after opening package • May have an obnoxious odor • Use test strips to ensure MEC • Need proper venting • Need copious rinsing—particularly with OPA • Need documentation
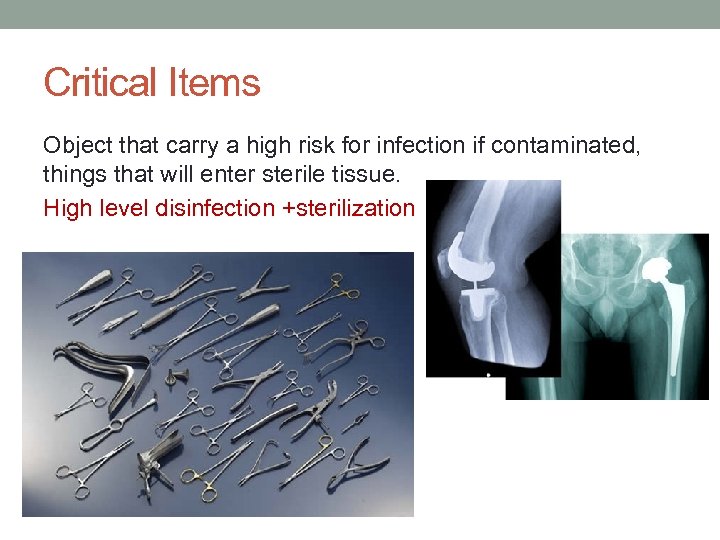 Critical Items Object that carry a high risk for infection if contaminated, things that will enter sterile tissue. High level disinfection +sterilization
Critical Items Object that carry a high risk for infection if contaminated, things that will enter sterile tissue. High level disinfection +sterilization
 It all starts with cleaning • Items can’t be disinfected or sterilized unless they are properly cleaned. • AORN: “Cleaning and decontamination are the initial and most critical steps in breaking the chain of disease transmission. ” 2011 pg 401
It all starts with cleaning • Items can’t be disinfected or sterilized unless they are properly cleaned. • AORN: “Cleaning and decontamination are the initial and most critical steps in breaking the chain of disease transmission. ” 2011 pg 401
 Cleaning & Decontamination • Cleaning begins at the point of use. • AORN RP: Care of Instruments Recommendation #4— Instruments should be kept free of gross soils during surgical procedures • Use enzymatic spray at the end of the procedure
Cleaning & Decontamination • Cleaning begins at the point of use. • AORN RP: Care of Instruments Recommendation #4— Instruments should be kept free of gross soils during surgical procedures • Use enzymatic spray at the end of the procedure
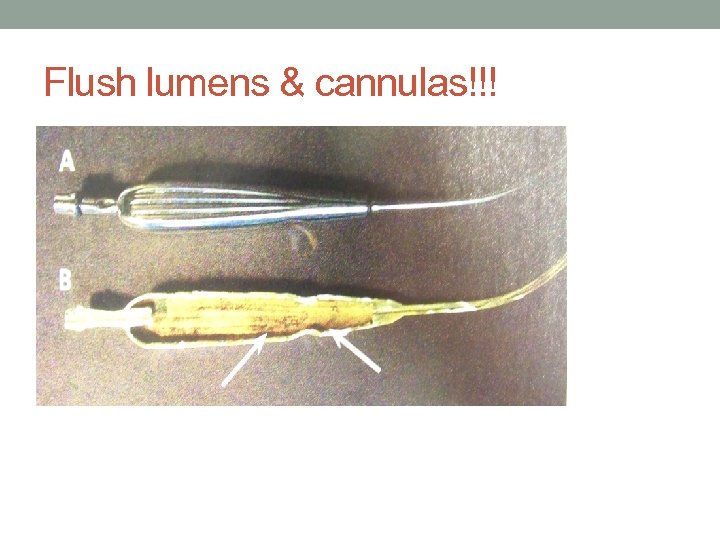 Flush lumens & cannulas!!!
Flush lumens & cannulas!!!
 Cleaning & Decontamination • Soiled instruments need to be contained and confined for transportation to the soiled utility room or to SPD.
Cleaning & Decontamination • Soiled instruments need to be contained and confined for transportation to the soiled utility room or to SPD.
 Cleaning & Decontamination—after the procedure • The first step is to remove all visible and invisible soils • Must be done according to manufacturer’s guidelines • Neutral PH enzymatic detergent, mechanical friction • Next step is generally the mechanical washer
Cleaning & Decontamination—after the procedure • The first step is to remove all visible and invisible soils • Must be done according to manufacturer’s guidelines • Neutral PH enzymatic detergent, mechanical friction • Next step is generally the mechanical washer

 Cleaning & Decontamination • AORN RP: Care of Instruments, Recommendation #5— Cleaning and decontamination should occur as soon as possible after the instruments are used. • AAMI: Instruments are decontaminated within 15 -60 minutes after use.
Cleaning & Decontamination • AORN RP: Care of Instruments, Recommendation #5— Cleaning and decontamination should occur as soon as possible after the instruments are used. • AAMI: Instruments are decontaminated within 15 -60 minutes after use.
 How fast do microorganisms reproduce? ? • 12: 00 • 12: 20 • 12: 40 • 13: 00 • 14: 00 • 15: 00 • 16: 00 • 17: 00 • 18: 00 • 19: 00 1 2 4 8 64 512 4, 096 32, 768 262, 144 2, 097, 152
How fast do microorganisms reproduce? ? • 12: 00 • 12: 20 • 12: 40 • 13: 00 • 14: 00 • 15: 00 • 16: 00 • 17: 00 • 18: 00 • 19: 00 1 2 4 8 64 512 4, 096 32, 768 262, 144 2, 097, 152
 Final point on cleaning and decontamination • Anything that can be disassembled must be disassembled for cleaning, decontamination, and sterilization • Copious rinse with RO, DI or distilled water
Final point on cleaning and decontamination • Anything that can be disassembled must be disassembled for cleaning, decontamination, and sterilization • Copious rinse with RO, DI or distilled water
 What’s next? • Disinfection • Use a germicidal solution • Follow manufacturer’s guidelines regarding dilution • Must come in contact with all surfaces • Must remain in wet contact for the prescribed amount of time—read the label
What’s next? • Disinfection • Use a germicidal solution • Follow manufacturer’s guidelines regarding dilution • Must come in contact with all surfaces • Must remain in wet contact for the prescribed amount of time—read the label
 Disinfection • Thermal!!! Using Mechanical Washer-Sterilizer • Washes with cold water then fills chamber with steam • Heat denatures proteins at lower temperatures if steam is present • Pressure is used to achieve temp of 270 deg. • Goal is steam saturation of 100%
Disinfection • Thermal!!! Using Mechanical Washer-Sterilizer • Washes with cold water then fills chamber with steam • Heat denatures proteins at lower temperatures if steam is present • Pressure is used to achieve temp of 270 deg. • Goal is steam saturation of 100%

 Inspect & Assemble • Regardless of who is processing the instruments, they need to be inspected to make sure they’re clean and in good working order before they go into a sterilizer
Inspect & Assemble • Regardless of who is processing the instruments, they need to be inspected to make sure they’re clean and in good working order before they go into a sterilizer
 Packaging • Instruments that are terminally sterilized are placed into some kind of package before they go into a sterilizer • Rigid container • Flat wrapped • Peel pouch
Packaging • Instruments that are terminally sterilized are placed into some kind of package before they go into a sterilizer • Rigid container • Flat wrapped • Peel pouch
 Sterilization • Terminal Sterilization—sterilized in a package, has a shelf life • Point of Use Sterilization—no shelf life, needs to be delivered to the sterile field at the completion of the sterilization cycle.
Sterilization • Terminal Sterilization—sterilized in a package, has a shelf life • Point of Use Sterilization—no shelf life, needs to be delivered to the sterile field at the completion of the sterilization cycle.
 Steam Sterilization • Most common method of sterilization • Has been used for many years • Relatively safe • Relatively inexpensive • Can be used for the majority of surgical instruments
Steam Sterilization • Most common method of sterilization • Has been used for many years • Relatively safe • Relatively inexpensive • Can be used for the majority of surgical instruments

 Steam Sterilization • Phases • Condition • Sterilize • Exhaust • Dry—needs to cool to room temperature before handling (sterility assurance and condensation) • Need to make sure all parameters for sterilization have been met prior to releasing the instruments for patient use.
Steam Sterilization • Phases • Condition • Sterilize • Exhaust • Dry—needs to cool to room temperature before handling (sterility assurance and condensation) • Need to make sure all parameters for sterilization have been met prior to releasing the instruments for patient use.
 Steam Sterilization • Cycle selection needs to be in accordance with manufacturer’s guidelines • Terminal sterilization cycles all take around an hour. • Common Cycles • Prevacuum 270’ 4 minutes • Prevacuum 270’ 8 minutes • Gravity 250’ 30 minutes
Steam Sterilization • Cycle selection needs to be in accordance with manufacturer’s guidelines • Terminal sterilization cycles all take around an hour. • Common Cycles • Prevacuum 270’ 4 minutes • Prevacuum 270’ 8 minutes • Gravity 250’ 30 minutes
 Hydrogen Peroxide Gas Plasma • Used for heat and pressure sensitive instruments • Relatively safe • More expensive than steam sterilization • Fast cycle times— 48 -75 minutes • Good for batteries, shavers, fiber optic scopes • Not for use with paper or cloth
Hydrogen Peroxide Gas Plasma • Used for heat and pressure sensitive instruments • Relatively safe • More expensive than steam sterilization • Fast cycle times— 48 -75 minutes • Good for batteries, shavers, fiber optic scopes • Not for use with paper or cloth
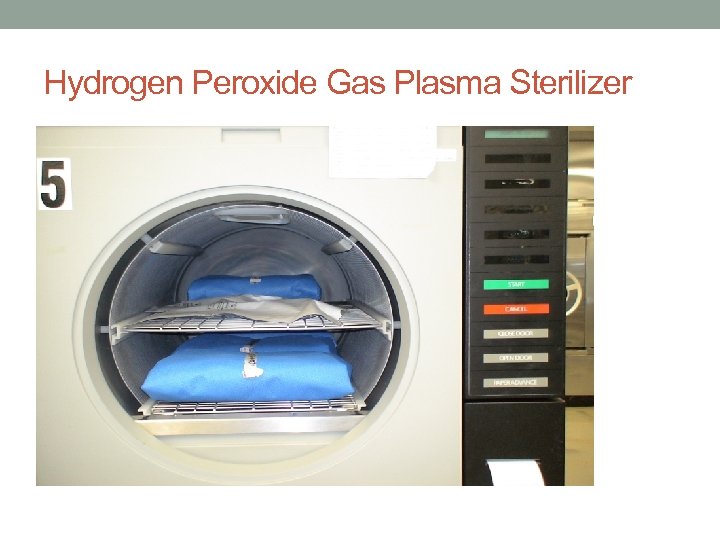 Hydrogen Peroxide Gas Plasma Sterilizer
Hydrogen Peroxide Gas Plasma Sterilizer
 Hydrogen Peroxide Gas Plasma • Cycles: • Non-lumen: 20 -40 minute cycle for batteries & other non-lumened items. • Lumen cycle: 55 -75 minutes for items containing lumens, mixed loads, complex items • Flexible scope cycle: 75 minutes, allows for terminal sterilization of selected flexible endoscopes
Hydrogen Peroxide Gas Plasma • Cycles: • Non-lumen: 20 -40 minute cycle for batteries & other non-lumened items. • Lumen cycle: 55 -75 minutes for items containing lumens, mixed loads, complex items • Flexible scope cycle: 75 minutes, allows for terminal sterilization of selected flexible endoscopes
 Point of Use Sterilization • Immediate use steam (“Flash”) sterilization • Paracetic Acid Sterilizers • Hydrogen Peroxide Sterilizers • Automated Endoscope Reprocessors
Point of Use Sterilization • Immediate use steam (“Flash”) sterilization • Paracetic Acid Sterilizers • Hydrogen Peroxide Sterilizers • Automated Endoscope Reprocessors
 Flash Sterilizer
Flash Sterilizer
 Immediate Use Steam Sterilization • Will sterilize an item in 10 minutes. • Item will come out of the sterilizer hot and wet; • need to be transported to the sterile field at the end of the cycle in a manner that doesn’t compromise sterility • Items need to cool before coming in contact with the patient—force cooling can cause instruments to crack & break
Immediate Use Steam Sterilization • Will sterilize an item in 10 minutes. • Item will come out of the sterilizer hot and wet; • need to be transported to the sterile field at the end of the cycle in a manner that doesn’t compromise sterility • Items need to cool before coming in contact with the patient—force cooling can cause instruments to crack & break
 Immediate Use Steam Sterilization • Need to review load printout to make sure all parameters for sterilization were met • Processing implants in an immediate use sterilizer should be avoided at all costs. • If using a “Flashguard” container, read instructions carefully!
Immediate Use Steam Sterilization • Need to review load printout to make sure all parameters for sterilization were met • Processing implants in an immediate use sterilizer should be avoided at all costs. • If using a “Flashguard” container, read instructions carefully!
 Paracetic Acid Sterilizer • Steris System 1 E • Relatively safe for staff • Relatively fast— 38 -43 minutes • Sensitive to water temperature & pressure fluctuations • Use for choledochoscope, bronchoscope, etc.
Paracetic Acid Sterilizer • Steris System 1 E • Relatively safe for staff • Relatively fast— 38 -43 minutes • Sensitive to water temperature & pressure fluctuations • Use for choledochoscope, bronchoscope, etc.
 Steris System 1 E
Steris System 1 E
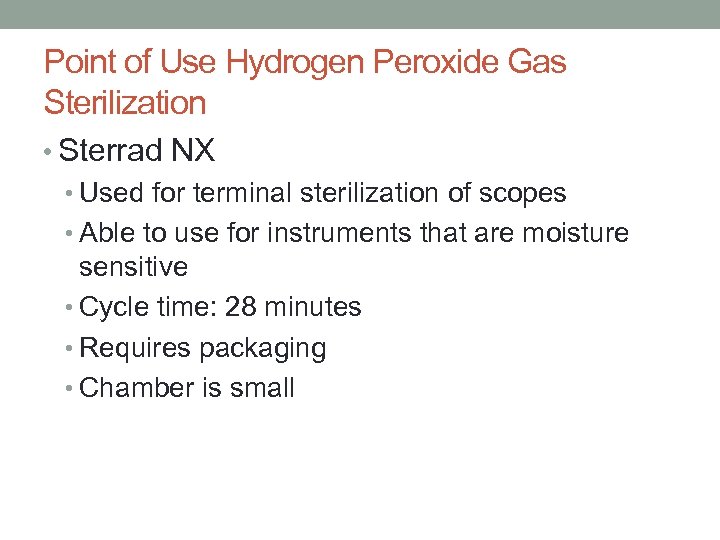 Point of Use Hydrogen Peroxide Gas Sterilization • Sterrad NX • Used for terminal sterilization of scopes • Able to use for instruments that are moisture sensitive • Cycle time: 28 minutes • Requires packaging • Chamber is small
Point of Use Hydrogen Peroxide Gas Sterilization • Sterrad NX • Used for terminal sterilization of scopes • Able to use for instruments that are moisture sensitive • Cycle time: 28 minutes • Requires packaging • Chamber is small
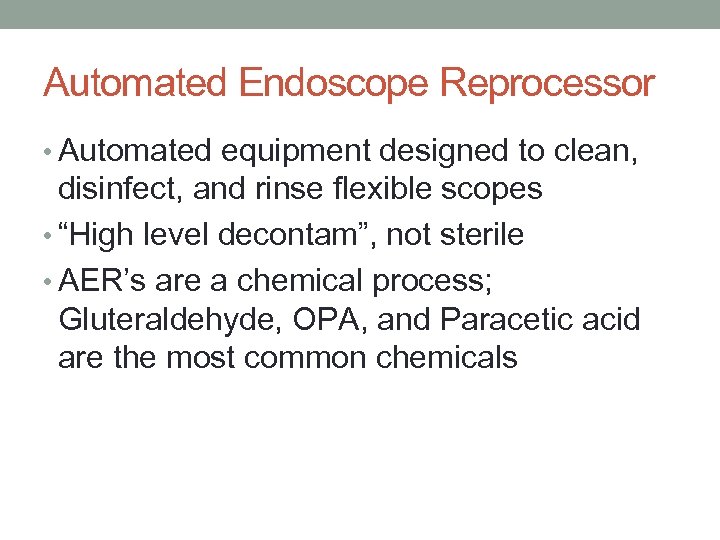 Automated Endoscope Reprocessor • Automated equipment designed to clean, disinfect, and rinse flexible scopes • “High level decontam”, not sterile • AER’s are a chemical process; Gluteraldehyde, OPA, and Paracetic acid are the most common chemicals
Automated Endoscope Reprocessor • Automated equipment designed to clean, disinfect, and rinse flexible scopes • “High level decontam”, not sterile • AER’s are a chemical process; Gluteraldehyde, OPA, and Paracetic acid are the most common chemicals
 Automated Endoscope Reprocessor
Automated Endoscope Reprocessor
 Sterilization Quality Control • How do I know my item is safe for the patient? ? ?
Sterilization Quality Control • How do I know my item is safe for the patient? ? ?
 Sterilization Quality Control • Physical monitoring—instruments within the sterilizer that tell us the temperature, pressure, and duration of a sterilization cycle. • Process indicators—let us know whether or not the item has been processed
Sterilization Quality Control • Physical monitoring—instruments within the sterilizer that tell us the temperature, pressure, and duration of a sterilization cycle. • Process indicators—let us know whether or not the item has been processed
 External Chemical Indicator Tape Before Processing After Processing
External Chemical Indicator Tape Before Processing After Processing
 Sterilization Quality Control • Tamper evident device—container locks, tape, arrows • Internal Pack Control—chemical indicators placed inside the tray, let us know that several parameters for sterilization have been met
Sterilization Quality Control • Tamper evident device—container locks, tape, arrows • Internal Pack Control—chemical indicators placed inside the tray, let us know that several parameters for sterilization have been met
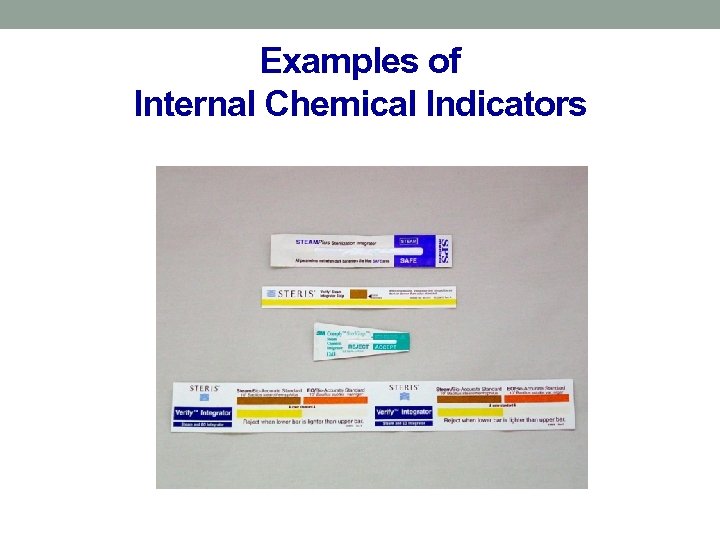 Examples of Internal Chemical Indicators
Examples of Internal Chemical Indicators
 Sterilization Quality Control • Biological Monitoring—spores inside a test package that measure the lethality of a sterilization cycle • Always run a BI with implants
Sterilization Quality Control • Biological Monitoring—spores inside a test package that measure the lethality of a sterilization cycle • Always run a BI with implants
 Biological Indicators • Contain Geobacillus stearothermophillus- Highly heat resistant, spore-forming bacteria does not produce toxins and is non-pathogenic.
Biological Indicators • Contain Geobacillus stearothermophillus- Highly heat resistant, spore-forming bacteria does not produce toxins and is non-pathogenic.
 Sterilization Quality Control • Bowie Dick Test—used to assure the air removal system is working properly in a prevacuum sterilizer • Daily
Sterilization Quality Control • Bowie Dick Test—used to assure the air removal system is working properly in a prevacuum sterilizer • Daily
 Sterilization Quality Control • Lot control number—includes sterilizer identification, cycle number, date of sterilization • This is how we achieve lot traceability • It’s up to the clinical staff to record lot control number in the patient’s record.
Sterilization Quality Control • Lot control number—includes sterilizer identification, cycle number, date of sterilization • This is how we achieve lot traceability • It’s up to the clinical staff to record lot control number in the patient’s record.
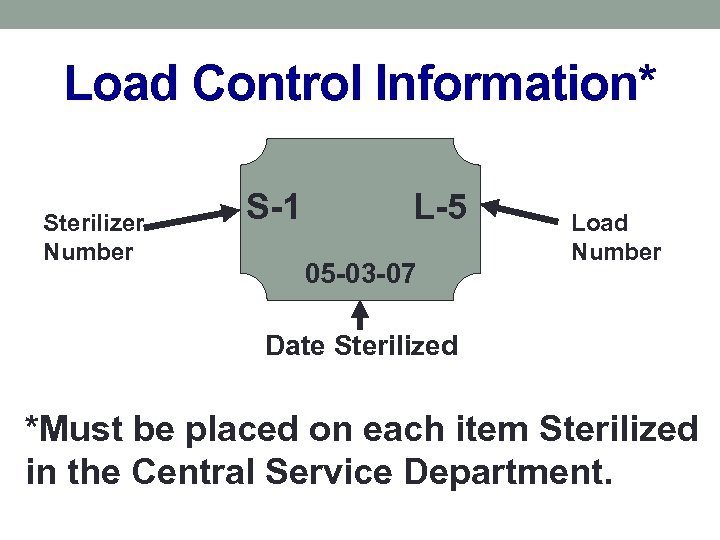 Load Control Information* Sterilizer Number S-1 L-5 05 -03 -07 Load Number Date Sterilized *Must be placed on each item Sterilized in the Central Service Department.
Load Control Information* Sterilizer Number S-1 L-5 05 -03 -07 Load Number Date Sterilized *Must be placed on each item Sterilized in the Central Service Department.
 Sterilization Quality Control • In order for a sterilizer load to be released for patient use: • Sterilizer tests complete • Parameters for sterilization must be met • Process indicator (tape) must pass • Tamper evident devices present • Lot control present & accurate • Packages cooled to room temperature if terminally sterilized • If implants, BI must pass
Sterilization Quality Control • In order for a sterilizer load to be released for patient use: • Sterilizer tests complete • Parameters for sterilization must be met • Process indicator (tape) must pass • Tamper evident devices present • Lot control present & accurate • Packages cooled to room temperature if terminally sterilized • If implants, BI must pass
 Sterilization Quality Control • What can go wrong? ? ?
Sterilization Quality Control • What can go wrong? ? ?
 Parameters for sterilization not met • Parameters: Time, Temperature, Pressure, Concentration • Reprocess the load in another sterilizer • Take the sterilizer out of service until the cause can be identified and corrected • Usually something has gone wrong with the sterilizer & sometimes the plant
Parameters for sterilization not met • Parameters: Time, Temperature, Pressure, Concentration • Reprocess the load in another sterilizer • Take the sterilizer out of service until the cause can be identified and corrected • Usually something has gone wrong with the sterilizer & sometimes the plant
 Sterilization Process Failure • Positive Biological Indicator • Need to recall back to the last negative biological • Take the device out of service until the cause of the problem can be identified and corrected • Most of the time it’s user error • The rest of the time it’s caused by a problem with the sterilizer or the plant
Sterilization Process Failure • Positive Biological Indicator • Need to recall back to the last negative biological • Take the device out of service until the cause of the problem can be identified and corrected • Most of the time it’s user error • The rest of the time it’s caused by a problem with the sterilizer or the plant
 Sterilization Process Failure • Wet load/wet pack • May be inside the chamber • User error—improper loading of the chamber • Sterilizer—valve or trap problem • Plant—steam too wet • Reprocess the load in another sterilizer; take the sterilizer out of service until the cause can be identified and corrected
Sterilization Process Failure • Wet load/wet pack • May be inside the chamber • User error—improper loading of the chamber • Sterilizer—valve or trap problem • Plant—steam too wet • Reprocess the load in another sterilizer; take the sterilizer out of service until the cause can be identified and corrected
 Sterilization Process Failure • Wet load/wet pack • May be inside the packages—can’t tell until they are opened. • Usually a condensation issue • Items moved before they have cooled to room temperature • Improper configuration of tray • Improper configuration of sterilizer load • Excessively heavy tray • Mixed metals and plastic • Trays with moisture are considered contaminated—notify SPD and replace the tray. SPD needs lot control # in order to conduct a recall
Sterilization Process Failure • Wet load/wet pack • May be inside the packages—can’t tell until they are opened. • Usually a condensation issue • Items moved before they have cooled to room temperature • Improper configuration of tray • Improper configuration of sterilizer load • Excessively heavy tray • Mixed metals and plastic • Trays with moisture are considered contaminated—notify SPD and replace the tray. SPD needs lot control # in order to conduct a recall
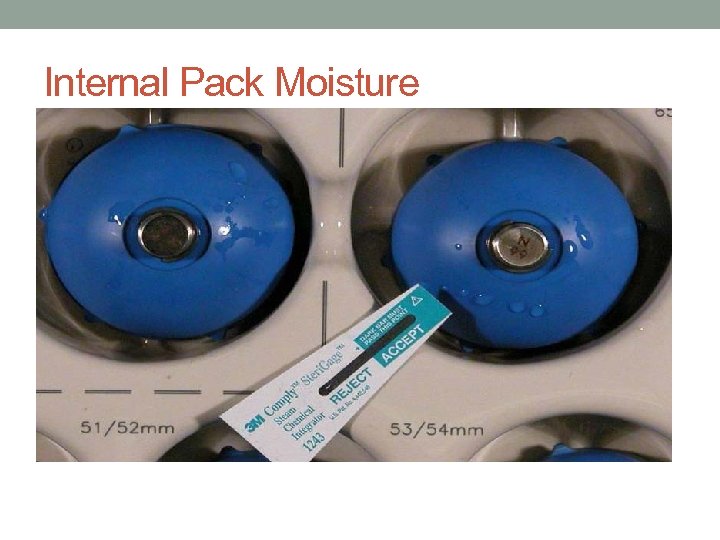 Internal Pack Moisture
Internal Pack Moisture
 Other things that can go wrong • Hole in wrapper • Items get handled many times and every time they are touched, there’s a chance the wrapper may get damaged • Lift—don’t drag • Watch out for trays that have feet or are very heavy • Rigid Container Defect • All parts have to be intact
Other things that can go wrong • Hole in wrapper • Items get handled many times and every time they are touched, there’s a chance the wrapper may get damaged • Lift—don’t drag • Watch out for trays that have feet or are very heavy • Rigid Container Defect • All parts have to be intact
 Other things that can go wrong • Peel pack has wrinkles in the seal • Tamper evident device comes off • Indicators missing • Item falls on the floor
Other things that can go wrong • Peel pack has wrinkles in the seal • Tamper evident device comes off • Indicators missing • Item falls on the floor

 • Questions? ?
• Questions? ?


