de3e165b5b302de23ccffcbf54f508e8.ppt
- Количество слайдов: 33
 Diffuse scattering and disorder in relaxor ferroelectrics. T. R. Welberry, D. J. Goossens Pb. Zn 1/3 Nb 2/3 O 3, (PZN)
Diffuse scattering and disorder in relaxor ferroelectrics. T. R. Welberry, D. J. Goossens Pb. Zn 1/3 Nb 2/3 O 3, (PZN)
 Relaxor ferroelectrics Pb. Mg 1/3 Nb 2/3 O 3 (PMN) Pb. Zn 1/3 Nb 2/3 O 3 (PZN) • • • computer disks high dielectric constant dispersion over broad range of frequencies and wide temperature range • • evidence of polar nanostructure plays essential role in piezo-electric properties • no consensus on exact nature of polar nanostructure
Relaxor ferroelectrics Pb. Mg 1/3 Nb 2/3 O 3 (PMN) Pb. Zn 1/3 Nb 2/3 O 3 (PZN) • • • computer disks high dielectric constant dispersion over broad range of frequencies and wide temperature range • • evidence of polar nanostructure plays essential role in piezo-electric properties • no consensus on exact nature of polar nanostructure
![Perovskite structure [001] [110] Pb O Zn/Nb important to see oxygens use neutron scattering Perovskite structure [001] [110] Pb O Zn/Nb important to see oxygens use neutron scattering](https://present5.com/presentation/de3e165b5b302de23ccffcbf54f508e8/image-3.jpg) Perovskite structure [001] [110] Pb O Zn/Nb important to see oxygens use neutron scattering
Perovskite structure [001] [110] Pb O Zn/Nb important to see oxygens use neutron scattering
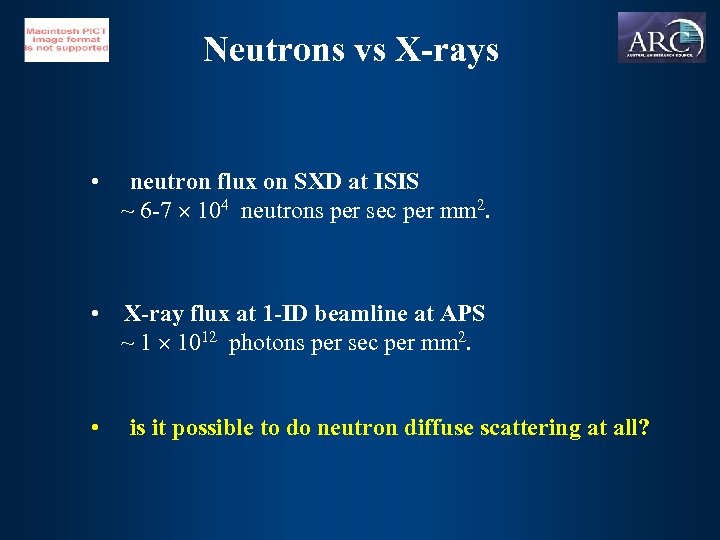 Neutrons vs X-rays • neutron flux on SXD at ISIS ~ 6 -7 104 neutrons per sec per mm 2. • X-ray flux at 1 -ID beamline at APS ~ 1 1012 photons per sec per mm 2. • is it possible to do neutron diffuse scattering at all?
Neutrons vs X-rays • neutron flux on SXD at ISIS ~ 6 -7 104 neutrons per sec per mm 2. • X-ray flux at 1 -ID beamline at APS ~ 1 1012 photons per sec per mm 2. • is it possible to do neutron diffuse scattering at all?
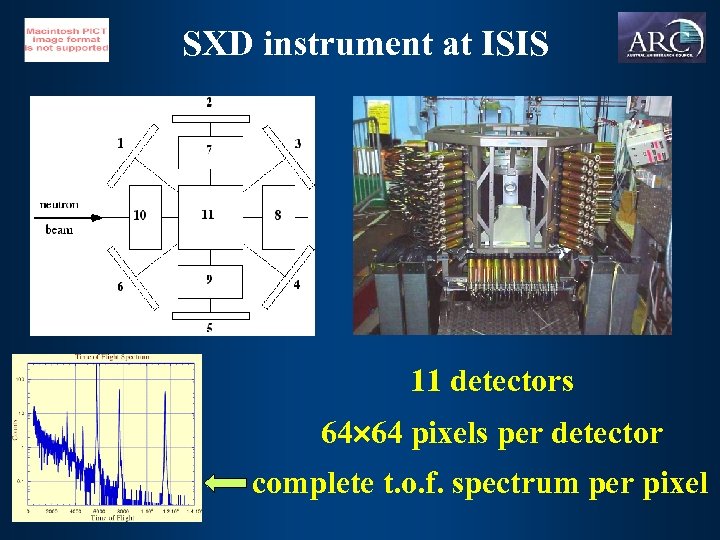 SXD instrument at ISIS 11 detectors 64 64 pixels per detector complete t. o. f. spectrum per pixel
SXD instrument at ISIS 11 detectors 64 64 pixels per detector complete t. o. f. spectrum per pixel
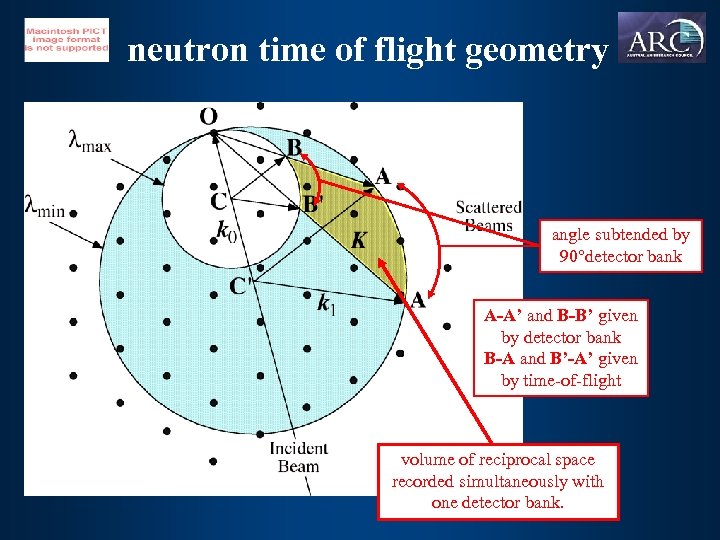 neutron time of flight geometry angle subtended by 90 detector bank A-A’ and B-B’ given by detector bank B-A and B’-A’ given by time-of-flight volume of reciprocal space recorded simultaneously with one detector bank.
neutron time of flight geometry angle subtended by 90 detector bank A-A’ and B-B’ given by detector bank B-A and B’-A’ given by time-of-flight volume of reciprocal space recorded simultaneously with one detector bank.
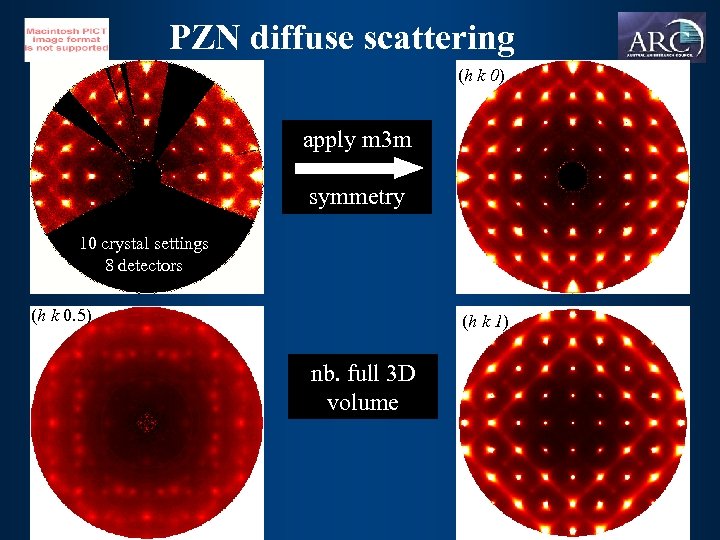 PZN diffuse scattering (h k 0) apply m 3 m symmetry 10 crystal settings 8 detectors (h k 0. 5) (h k 1) nb. full 3 D volume
PZN diffuse scattering (h k 0) apply m 3 m symmetry 10 crystal settings 8 detectors (h k 0. 5) (h k 1) nb. full 3 D volume
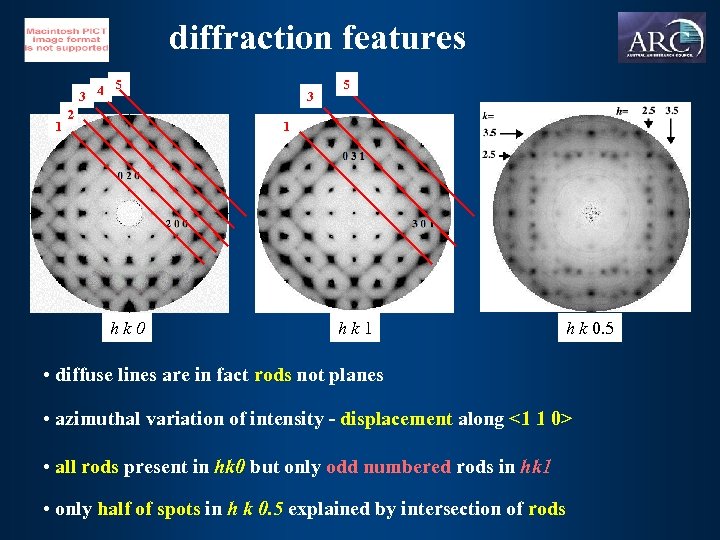 diffraction features 5 3 4 1 2 3 5 1 hk 0 hk 1 h k 0. 5 • diffuse lines are in fact rods not planes • azimuthal variation of intensity - displacement along <1 1 0> • all rods present in hk 0 but only odd numbered rods in hk 1 • only half of spots in h k 0. 5 explained by intersection of rods
diffraction features 5 3 4 1 2 3 5 1 hk 0 hk 1 h k 0. 5 • diffuse lines are in fact rods not planes • azimuthal variation of intensity - displacement along <1 1 0> • all rods present in hk 0 but only odd numbered rods in hk 1 • only half of spots in h k 0. 5 explained by intersection of rods
 Fourier transform theory a rod of scattering in reciprocal space corresponds to a plane in real-space (normal to the rod) in this case: rods are parallel to the six <110> directions hence planes are normal to <110> azimuthal variation of intensity means: atomic displacements are within these planes and parallel to another <110> direction
Fourier transform theory a rod of scattering in reciprocal space corresponds to a plane in real-space (normal to the rod) in this case: rods are parallel to the six <110> directions hence planes are normal to <110> azimuthal variation of intensity means: atomic displacements are within these planes and parallel to another <110> direction
![Planar defects in PZN Planar defect normal to [1 -1 0] cation displacements in Planar defects in PZN Planar defect normal to [1 -1 0] cation displacements in](https://present5.com/presentation/de3e165b5b302de23ccffcbf54f508e8/image-10.jpg) Planar defects in PZN Planar defect normal to [1 -1 0] cation displacements in planar defect are parallel to [1 1 0]
Planar defects in PZN Planar defect normal to [1 -1 0] cation displacements in planar defect are parallel to [1 1 0]
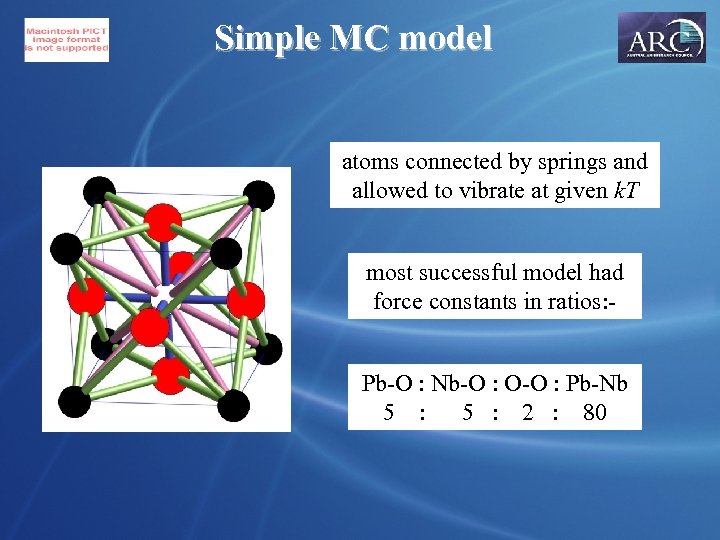 Simple MC model atoms connected by springs and allowed to vibrate at given k. T most successful model had force constants in ratios: Pb-O : Nb-O : O-O : Pb-Nb 5 : 2 : 80
Simple MC model atoms connected by springs and allowed to vibrate at given k. T most successful model had force constants in ratios: Pb-O : Nb-O : O-O : Pb-Nb 5 : 2 : 80
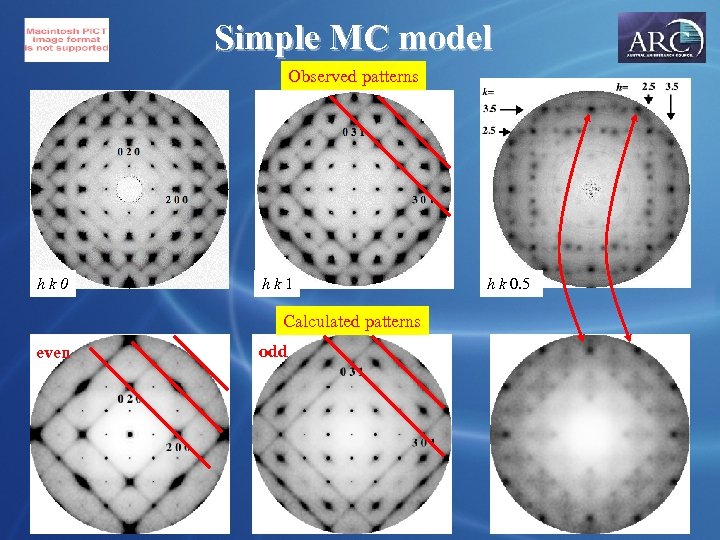 Simple MC model Observed patterns hk 0 hk 1 Calculated patterns even odd h k 0. 5
Simple MC model Observed patterns hk 0 hk 1 Calculated patterns even odd h k 0. 5
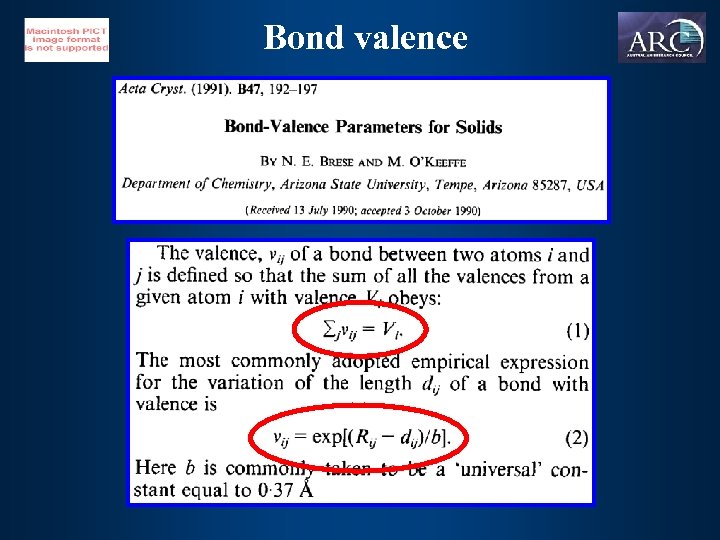 Bond valence
Bond valence
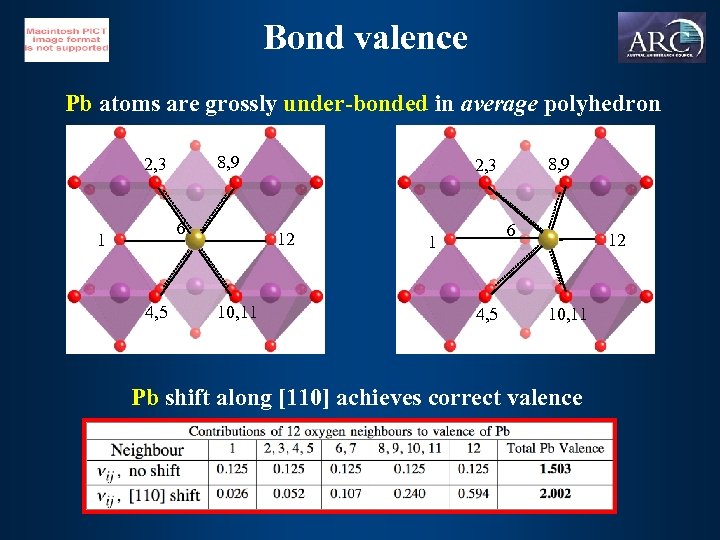 Bond valence Pb atoms are grossly under-bonded in average polyhedron 8, 9 2, 3 6 1 4, 5 12 10, 11 Pb shift along [110] achieves correct valence
Bond valence Pb atoms are grossly under-bonded in average polyhedron 8, 9 2, 3 6 1 4, 5 12 10, 11 Pb shift along [110] achieves correct valence
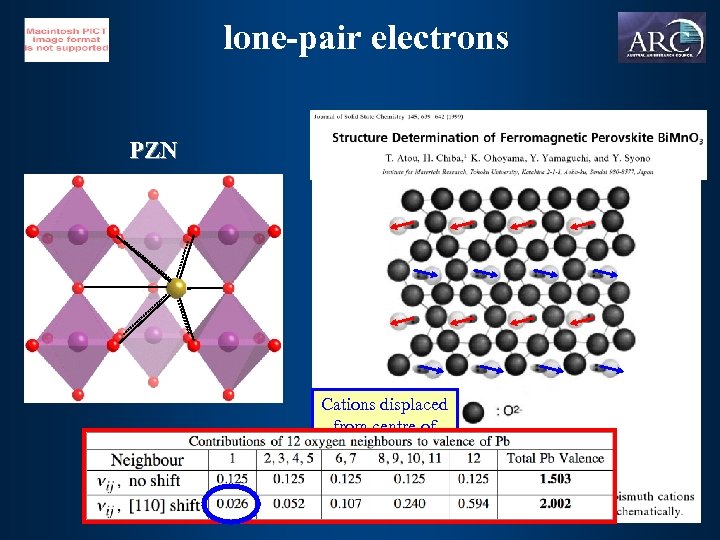 lone-pair electrons PZN Cations displaced from centre of coordination polyhedra
lone-pair electrons PZN Cations displaced from centre of coordination polyhedra
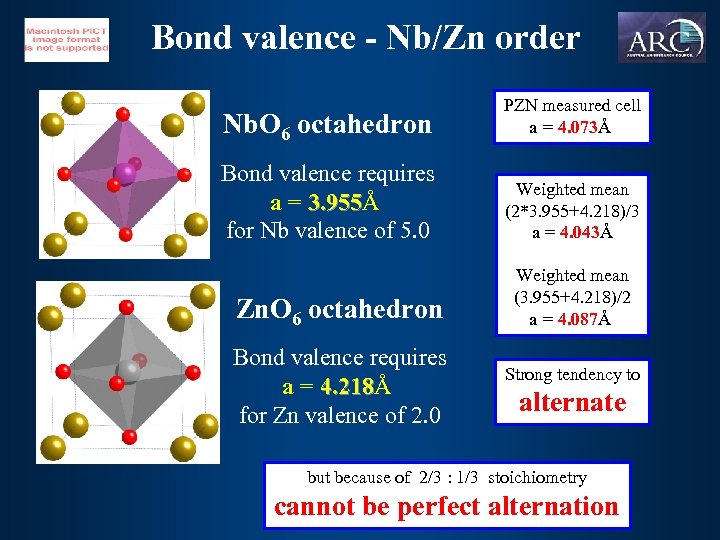 Bond valence - Nb/Zn order Nb. O 6 octahedron PZN measured cell a = 4. 073Å 4. 073 Bond valence requires a = 3. 955Å 3. 955 for Nb valence of 5. 0 Weighted mean (2*3. 955+4. 218)/3 a = 4. 043Å 4. 043 Zn. O 6 octahedron Bond valence requires a = 4. 218Å 4. 218 for Zn valence of 2. 0 Weighted mean (3. 955+4. 218)/2 a = 4. 087Å 4. 087 Strong tendency to alternate but because of 2/3 : 1/3 stoichiometry cannot be perfect alternation
Bond valence - Nb/Zn order Nb. O 6 octahedron PZN measured cell a = 4. 073Å 4. 073 Bond valence requires a = 3. 955Å 3. 955 for Nb valence of 5. 0 Weighted mean (2*3. 955+4. 218)/3 a = 4. 043Å 4. 043 Zn. O 6 octahedron Bond valence requires a = 4. 218Å 4. 218 for Zn valence of 2. 0 Weighted mean (3. 955+4. 218)/2 a = 4. 087Å 4. 087 Strong tendency to alternate but because of 2/3 : 1/3 stoichiometry cannot be perfect alternation
 SRO of Nb/Zn Two models tested: 1. random occupancy of Nb and Zn ? 2. tendency to alternate? maximal Nb/Zn ordering Peaks due to cation displacements random Nb/Zn 0 (h k 0. 5) layer Extra peaks due to Nb/Zn ordering B-site occupancy is 2/3 Nb and 1/3 Zn complete alternation not possible - max corr. = -0. 5 • Nb certainly follows Zn but • after Nb sometimes Zn sometimes Nb
SRO of Nb/Zn Two models tested: 1. random occupancy of Nb and Zn ? 2. tendency to alternate? maximal Nb/Zn ordering Peaks due to cation displacements random Nb/Zn 0 (h k 0. 5) layer Extra peaks due to Nb/Zn ordering B-site occupancy is 2/3 Nb and 1/3 Zn complete alternation not possible - max corr. = -0. 5 • Nb certainly follows Zn but • after Nb sometimes Zn sometimes Nb
![Planar defects cation displacements in planar defect are parallel to [1 1 0] random Planar defects cation displacements in planar defect are parallel to [1 1 0] random](https://present5.com/presentation/de3e165b5b302de23ccffcbf54f508e8/image-18.jpg) Planar defects cation displacements in planar defect are parallel to [1 1 0] random variables to represent cation displacements
Planar defects cation displacements in planar defect are parallel to [1 1 0] random variables to represent cation displacements
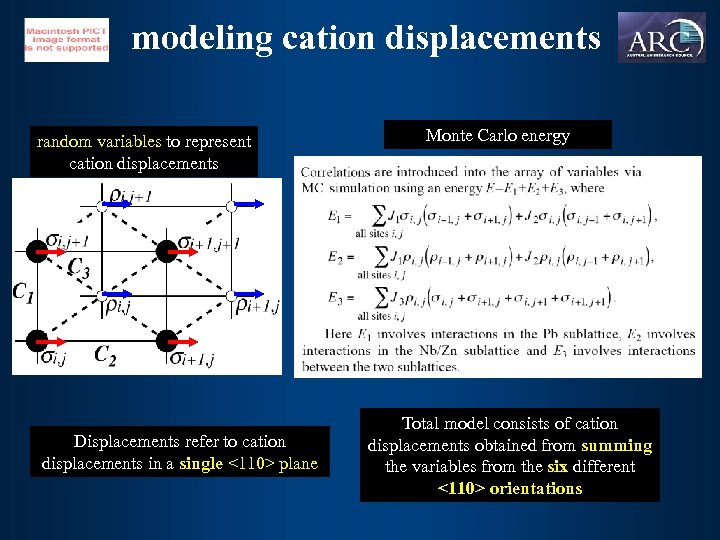 modeling cation displacements random variables to represent cation displacements Displacements refer to cation displacements in a single <110> plane Monte Carlo energy Total model consists of cation displacements obtained from summing the variables from the six different <110> orientations
modeling cation displacements random variables to represent cation displacements Displacements refer to cation displacements in a single <110> plane Monte Carlo energy Total model consists of cation displacements obtained from summing the variables from the six different <110> orientations
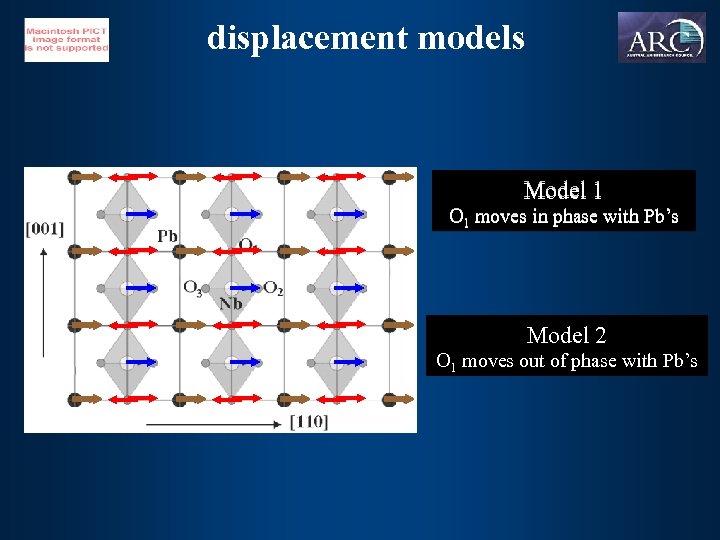 displacement models Model 1 O 1 moves in phase with Pb’s Model 2 O 1 moves out of phase with Pb’s
displacement models Model 1 O 1 moves in phase with Pb’s Model 2 O 1 moves out of phase with Pb’s
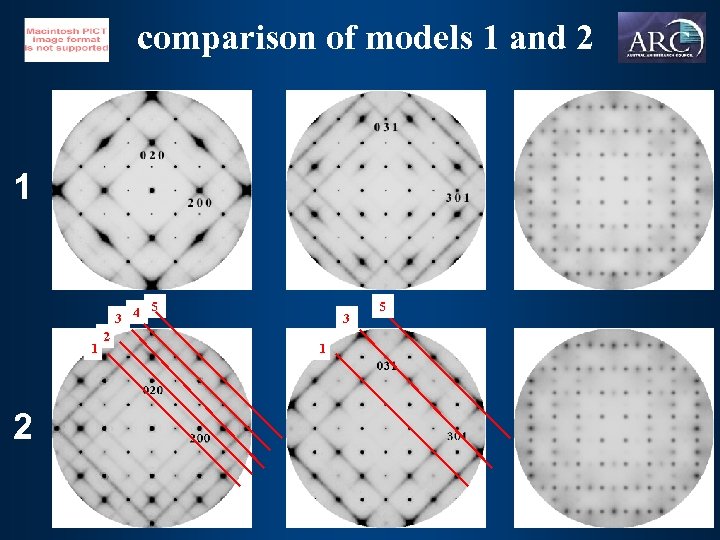 comparison of models 1 and 2 1 5 3 4 1 2 2 3 1 5
comparison of models 1 and 2 1 5 3 4 1 2 2 3 1 5
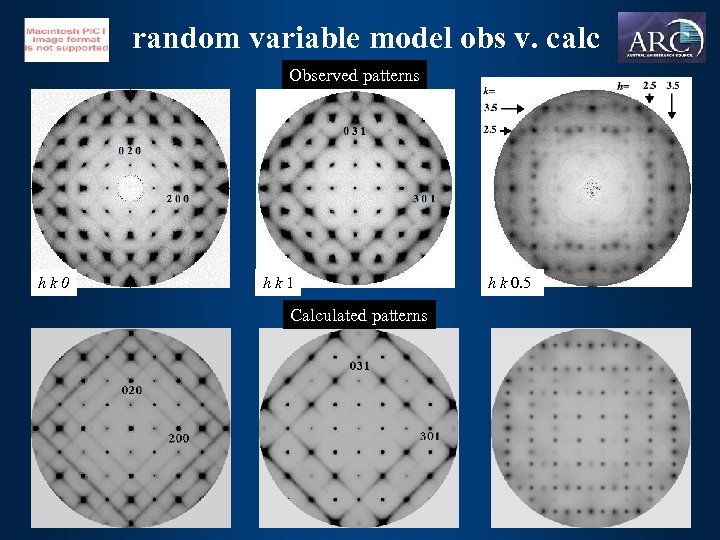 random variable model obs v. calc Observed patterns hk 0 hk 1 Calculated patterns h k 0. 5
random variable model obs v. calc Observed patterns hk 0 hk 1 Calculated patterns h k 0. 5
 Summary of Gaussian Variable models 1. planar nanodomains normal to <110> 2. atomic displacements parallel to <110> 3. atomic displacements within domains correlated 4. Pb & Nb/Zn displacements in phase 5. O 1 displacements out of phase with Pb can we construct an atomistic model satisfying these criteria?
Summary of Gaussian Variable models 1. planar nanodomains normal to <110> 2. atomic displacements parallel to <110> 3. atomic displacements within domains correlated 4. Pb & Nb/Zn displacements in phase 5. O 1 displacements out of phase with Pb can we construct an atomistic model satisfying these criteria?
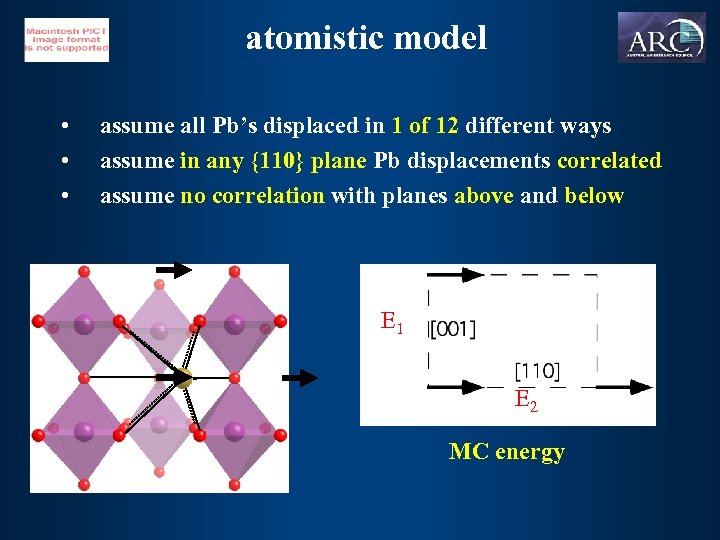 atomistic model • • • assume all Pb’s displaced in 1 of 12 different ways assume in any {110} plane Pb displacements correlated assume no correlation with planes above and below E 1 E 2 MC energy
atomistic model • • • assume all Pb’s displaced in 1 of 12 different ways assume in any {110} plane Pb displacements correlated assume no correlation with planes above and below E 1 E 2 MC energy
![development of atomistic model Single layer normal to [1 -1 0] diffraction Pb only development of atomistic model Single layer normal to [1 -1 0] diffraction Pb only](https://present5.com/presentation/de3e165b5b302de23ccffcbf54f508e8/image-25.jpg) development of atomistic model Single layer normal to [1 -1 0] diffraction Pb only [001] Note scattering around Bragg peaks as well as diffuse rods [110] Polar nanodomains 12 different orientations E 1 E 2
development of atomistic model Single layer normal to [1 -1 0] diffraction Pb only [001] Note scattering around Bragg peaks as well as diffuse rods [110] Polar nanodomains 12 different orientations E 1 E 2
![development of atomistic model two successive planes normal to [1 -1 0] Polar nanodomains development of atomistic model two successive planes normal to [1 -1 0] Polar nanodomains](https://present5.com/presentation/de3e165b5b302de23ccffcbf54f508e8/image-26.jpg) development of atomistic model two successive planes normal to [1 -1 0] Polar nanodomains 12 different orientations [001] domains do not persist in successive layers [110]
development of atomistic model two successive planes normal to [1 -1 0] Polar nanodomains 12 different orientations [001] domains do not persist in successive layers [110]
![development of atomistic model view down [0 0 1] [100] [010] Linear features do development of atomistic model view down [0 0 1] [100] [010] Linear features do](https://present5.com/presentation/de3e165b5b302de23ccffcbf54f508e8/image-27.jpg) development of atomistic model view down [0 0 1] [100] [010] Linear features do persist in successive layers
development of atomistic model view down [0 0 1] [100] [010] Linear features do persist in successive layers
![development of atomistic model [100] Linear features do neighbours attract in successive [010] persist development of atomistic model [100] Linear features do neighbours attract in successive [010] persist](https://present5.com/presentation/de3e165b5b302de23ccffcbf54f508e8/image-28.jpg) development of atomistic model [100] Linear features do neighbours attract in successive [010] persist or repel each other according to their mutual orientation layers
development of atomistic model [100] Linear features do neighbours attract in successive [010] persist or repel each other according to their mutual orientation layers
![size-effect relaxation P [110]. [101] =2 =1 [110]. [1 -1 0] = 0 smaller size-effect relaxation P [110]. [101] =2 =1 [110]. [1 -1 0] = 0 smaller](https://present5.com/presentation/de3e165b5b302de23ccffcbf54f508e8/image-29.jpg) size-effect relaxation P [110]. [101] =2 =1 [110]. [1 -1 0] = 0 smaller than average E = (d - d 0(1 - P e))2 size-effect parameter average [110]. [-1 0 -1] =-1 [110]. [-1 -1 0] =-2 bigger than average
size-effect relaxation P [110]. [101] =2 =1 [110]. [1 -1 0] = 0 smaller than average E = (d - d 0(1 - P e))2 size-effect parameter average [110]. [-1 0 -1] =-1 [110]. [-1 -1 0] =-2 bigger than average
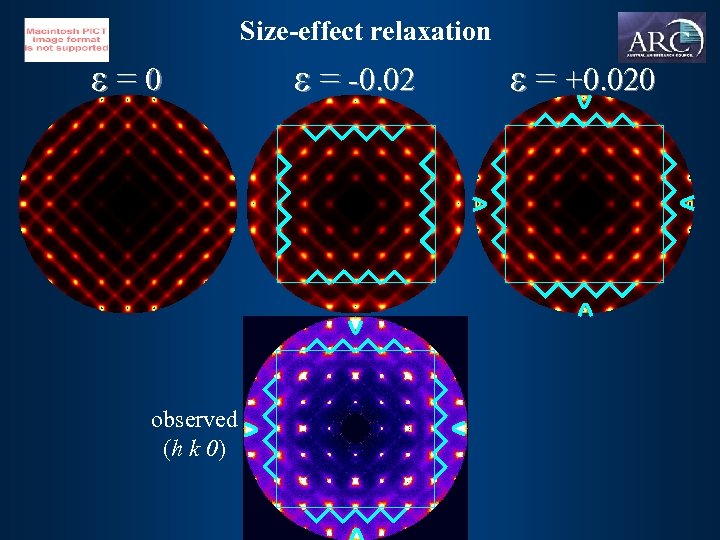 Size-effect relaxation e=0 observed (h k 0) e = -0. 02 e = +0. 020
Size-effect relaxation e=0 observed (h k 0) e = -0. 02 e = +0. 020
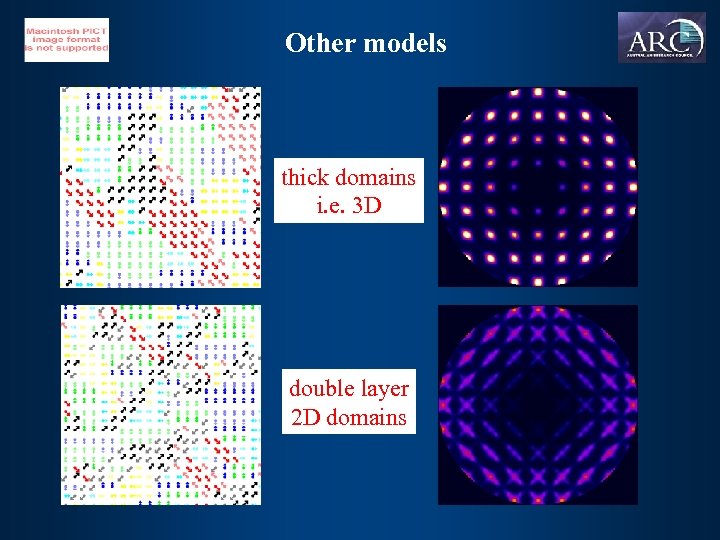 Other models thick domains i. e. 3 D double layer 2 D domains
Other models thick domains i. e. 3 D double layer 2 D domains
 Acknowledgements § § M. J. Gutmann (ISIS, UK) A. P. Heerdegen(RSC, ANU) § § H. Woo (Brookhaven N. L. ) G. Xu (Brookhaven N. L. ) § C. Stock (Toronto) § Z-G. Ye (Simon Fraser University) § AINSE { Crystal growth}
Acknowledgements § § M. J. Gutmann (ISIS, UK) A. P. Heerdegen(RSC, ANU) § § H. Woo (Brookhaven N. L. ) G. Xu (Brookhaven N. L. ) § C. Stock (Toronto) § Z-G. Ye (Simon Fraser University) § AINSE { Crystal growth}
 Go back to Disordered Materials Go to Home Page
Go back to Disordered Materials Go to Home Page


