7f51595da15e7aaa768dcb1bbf7373c7.ppt
- Количество слайдов: 16
Difference between HPV positive and negative non-oropharyngeal cancer; texture analysis features on CT Akifumi Fujita, MD 1, 4, Karen Buch, MD 1, Baojun Li, Ph. D 1, Yusuke Kawashima, DDS 1, 5, Muhammad M. Qureshi, MBBS 1, 3 Osamu Sakai, MD, Ph. D 1, 2, 3 Departments of 1 Radiology, 2 Otolaryngology – Head and Neck Surgery, and 3 Radiation Oncology Boston Medical Center, Boston University School of Medicine 4 Departments of Radiology, Jichi Medical University School of Medicine 5 Departments of Radiology, Nihon University School of Dentistry at Matsudo
Background • Although the prevalence of humanpapilloma virus (HPV) infection is not as high as oropharyngeal cancer (OPC), non-oropharyngeal cancer (non-OPC) has reported association with HPV infection in 4. 1 -23. 7% (6 -9). – non-OPC; oral cavity (tongue, floor of mouth, and buccal mucosa), larynx, and hypopharynx • Several articles shows that HPV positive (HPV+) non. OPC also tends to show better clinical outcomes than HPV negative (HPV-) non-OPC (2, 9). • However, the influence on treatment options and impact on prognosis of HPV is not established among non-OPC patients (7, 8, 10).
Background • Texture analysis is a quantitative means for extracting image features for comparative analysis – Post-processing step – Evaluated in prior studies: liver, cartilage, brain, myocardium • Texture features – Histogram features: spatially invariant – Gray level co-occurrence matrix (GLCM) features: highly spatially dependent, # times a given gray tone i is adjacent to gray tone j – Gray level run-length matrix (GLRL) features: spatially dependent, # pixel line segments in a given run-length and tone – Gray level gradient matrix (GLGM) features: mathematical summary of gradient values of pixels in the ROI

Purpose • We hypothesized that there may also be underlying morphologic difference in the texture of non-OPC between HPV+ and HPV- patients. • The purpose of this study is to investigate and identify if there is any specific texture parameter that may distinguish between HPV+ and HPV- patients in non-OPC.
Materials & Methods – 110 patients with non-OPC SCC and known HPV status who had contrast enhanced CT (CECT) for initial staging between December 2009 -August 2014 – CECT examinations were performed either independently or combined with FDG-PET examination, acquired by 64 - or 16 -detector row CT scanners • Lightspeed VCT • Discovery STE-16 PET/CT (GE Healthcare, Milwaukee, Wisconsin) – Exclusion criteria: • Severely motion limited studies • Significant artifact generated from dental hardware • patients with very small primary tumors (less than 5 mm)
Materials & Methods • Electronic Medical Record Review: • Age and gender • HPV status • Tumor site • Primary Tumor Segmentation and Texture Analysis – Segmentation was performed on a dedicated AW workstation (GE Healthcare, Milwaukee, WI) with a semiautomated graphical-user-interface (GUI) – In-house developed, MATLAB-based texture analysis program was employed to extract 42 texture features from each segmented volume • Statistical Analysis – t-test to compare differences in texture parameters between HPV+ and HPV- non-OPC SCC
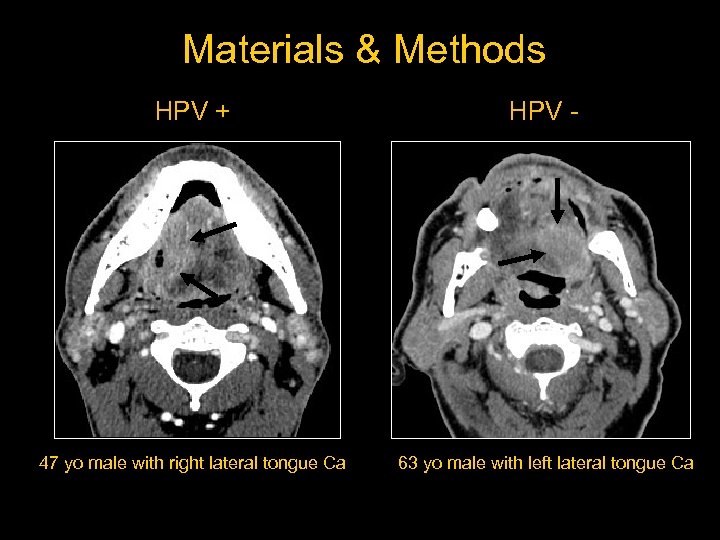
Materials & Methods HPV + HPV - 47 yo male with right lateral tongue Ca 63 yo male with left lateral tongue Ca
Results • Patient characteristics – 64 patients were excluded fro the analysis, and ultimately 46 CECT exams and corresponding medical records were reviewed – 31 males, 15 females – Age range: 39 to 86 years (59. 6± 7. 9) • HPV Status – 10 patients with HPV+ non-OPC • 5 oral cavity, 5 larynx – 36 patients with HPV- non-OPC • 19 oral cavity, 12 larynx, 5 hypopharynx
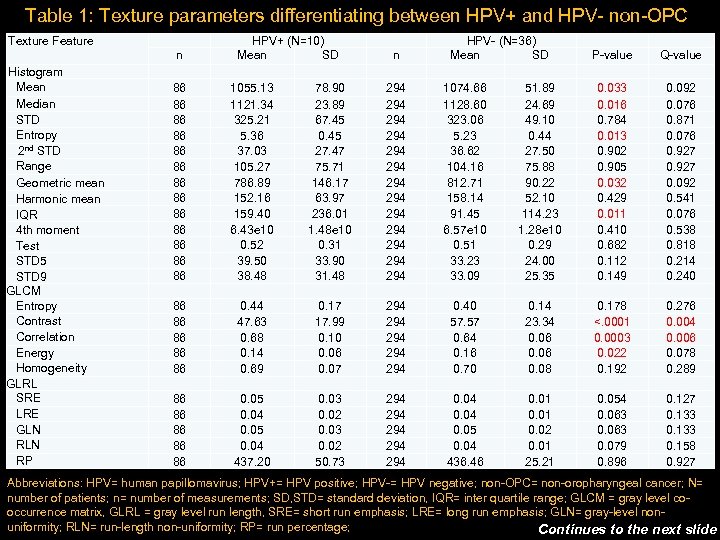
Table 1: Texture parameters differentiating between HPV+ and HPV- non-OPC Texture Feature Histogram Mean Median STD Entropy 2 nd STD Range Geometric mean Harmonic mean IQR 4 th moment Test STD 5 STD 9 GLCM Entropy Contrast Correlation Energy Homogeneity GLRL SRE LRE GLN RP n 86 86 86 86 86 86 86 HPV+ (N=10) Mean SD 1055. 13 78. 90 1121. 34 23. 89 325. 21 67. 45 5. 36 0. 45 37. 03 27. 47 105. 27 75. 71 786. 89 146. 17 152. 16 63. 97 159. 40 236. 01 6. 43 e 10 1. 48 e 10 0. 52 0. 31 39. 50 33. 90 38. 48 31. 48 0. 44 0. 17 47. 63 17. 99 0. 68 0. 10 0. 14 0. 06 0. 69 0. 07 0. 05 0. 03 0. 04 0. 02 437. 20 50. 73 n 294 294 294 294 294 294 HPV- (N=36) Mean SD 1074. 66 51. 89 1128. 60 24. 69 323. 06 49. 10 5. 23 0. 44 36. 62 27. 50 104. 16 75. 88 812. 71 90. 22 158. 14 52. 10 91. 45 114. 23 6. 57 e 10 1. 28 e 10 0. 51 0. 29 33. 23 24. 00 33. 09 25. 35 0. 40 0. 14 57. 57 23. 34 0. 64 0. 06 0. 16 0. 06 0. 70 0. 08 0. 04 0. 01 0. 05 0. 02 0. 04 0. 01 436. 46 25. 21 P-value 0. 033 0. 016 0. 784 0. 013 0. 902 0. 905 0. 032 0. 429 0. 011 0. 410 0. 682 0. 112 0. 149 0. 178 <. 0001 0. 0003 0. 022 0. 192 0. 054 0. 063 0. 079 0. 896 Q-value 0. 092 0. 076 0. 871 0. 076 0. 927 0. 092 0. 541 0. 076 0. 538 0. 818 0. 214 0. 240 0. 276 0. 004 0. 006 0. 078 0. 289 0. 127 0. 133 0. 158 0. 927 Abbreviations: HPV= human papillomavirus; HPV+= HPV positive; HPV-= HPV negative; non-OPC= non-oropharyngeal cancer; N= number of patients; n= number of measurements; SD, STD= standard deviation, IQR= inter quartile range; GLCM = gray level cooccurrence matrix, GLRL = gray level run length, SRE= short run emphasis; LRE= long run emphasis; GLN= gray-level nonuniformity; RLN= run-length non-uniformity; RP= run percentage; Continues to the next slide
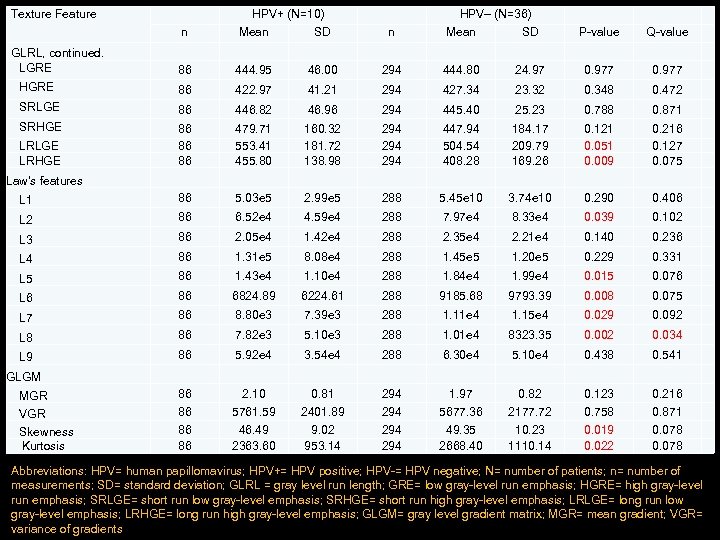
Texture Feature GLRL, continued. LGRE n HPV+ (N=10) Mean SD 86 444. 95 46. 00 HGRE 86 422. 97 SRLGE 86 SRHGE Law's features 86 86 86 L 1 n HPV– (N=36) Mean SD P-value Q-value 0. 977 294 444. 80 24. 97 0. 977 41. 21 294 427. 34 23. 32 0. 348 0. 472 446. 82 46. 96 294 445. 40 25. 23 0. 788 0. 871 479. 71 553. 41 455. 80 160. 32 181. 72 138. 98 447. 94 504. 54 408. 28 184. 17 209. 79 169. 26 0. 121 0. 051 0. 009 2. 99 e 5 3. 74 e 10 0. 290 288 5. 45 e 10 0. 216 0. 127 0. 075 86 5. 03 e 5 294 294 0. 406 L 2 86 6. 52 e 4 4. 59 e 4 288 7. 97 e 4 8. 33 e 4 0. 039 0. 102 L 3 86 2. 05 e 4 1. 42 e 4 288 2. 35 e 4 2. 21 e 4 0. 140 0. 236 L 4 86 1. 31 e 5 8. 08 e 4 288 1. 45 e 5 1. 20 e 5 0. 229 0. 331 L 5 86 1. 43 e 4 1. 10 e 4 288 1. 84 e 4 1. 99 e 4 0. 015 0. 076 L 6 86 6824. 89 6224. 61 288 9185. 68 9793. 39 0. 008 0. 075 L 7 86 8. 80 e 3 7. 39 e 3 288 1. 11 e 4 1. 15 e 4 0. 029 0. 092 L 8 86 7. 82 e 3 5. 10 e 3 288 1. 01 e 4 8323. 35 0. 002 0. 034 L 9 86 5. 92 e 4 3. 54 e 4 6. 30 e 4 5. 10 e 4 0. 438 86 0. 81 294 1. 97 0. 82 0. 123 0. 541 2. 10 288 86 86 86 5761. 59 46. 49 2363. 60 2401. 89 9. 02 953. 14 294 294 5677. 36 49. 35 2668. 40 2177. 72 10. 23 1110. 14 0. 758 0. 019 0. 022 LRLGE LRHGE GLGM MGR VGR Skewness Kurtosis 0. 216 0. 871 0. 078 Abbreviations: HPV= human papillomavirus; HPV+= HPV positive; HPV-= HPV negative; N= number of patients; n= number of measurements; SD= standard deviation; GLRL = gray level run length; GRE= low gray-level run emphasis; HGRE= high gray-level run emphasis; SRLGE= short run low gray-level emphasis; SRHGE= short run high gray-level emphasis; LRLGE= long run low gray-level emphasis; LRHGE= long run high gray-level emphasis; GLGM= gray level gradient matrix; MGR= mean gradient; VGR= variance of gradients
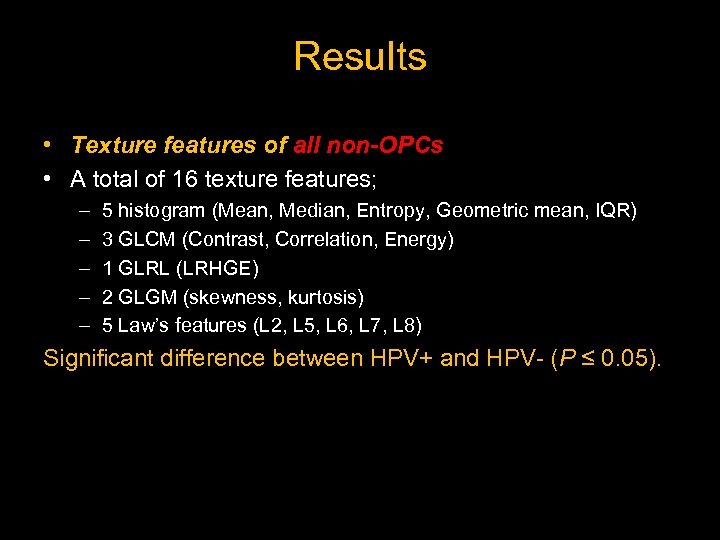
Results • Texture features of all non-OPCs • A total of 16 texture features; – – – 5 histogram (Mean, Median, Entropy, Geometric mean, IQR) 3 GLCM (Contrast, Correlation, Energy) 1 GLRL (LRHGE) 2 GLGM (skewness, kurtosis) 5 Law’s features (L 2, L 5, L 6, L 7, L 8) Significant difference between HPV+ and HPV- (P ≤ 0. 05).
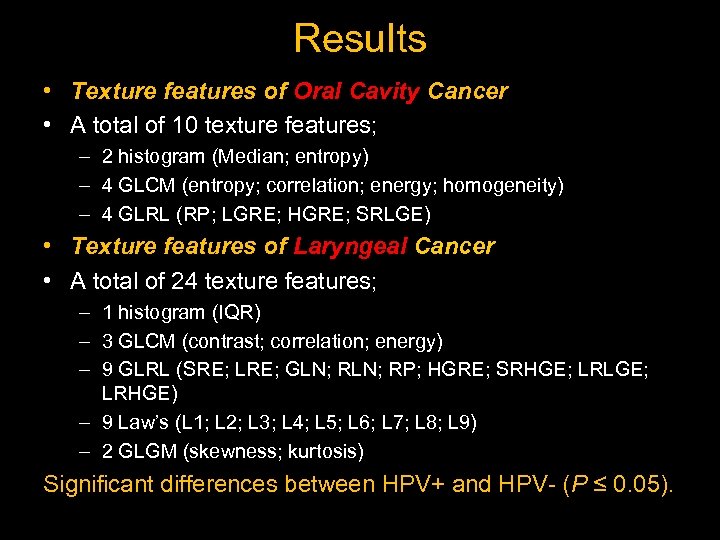
Results • Texture features of Oral Cavity Cancer • A total of 10 texture features; – 2 histogram (Median; entropy) – 4 GLCM (entropy; correlation; energy; homogeneity) – 4 GLRL (RP; LGRE; HGRE; SRLGE) • Texture features of Laryngeal Cancer • A total of 24 texture features; – 1 histogram (IQR) – 3 GLCM (contrast; correlation; energy) – 9 GLRL (SRE; LRE; GLN; RP; HGRE; SRHGE; LRLGE; LRHGE) – 9 Law’s (L 1; L 2; L 3; L 4; L 5; L 6; L 7; L 8; L 9) – 2 GLGM (skewness; kurtosis) Significant differences between HPV+ and HPV- (P ≤ 0. 05).
Discussion • A prior study showed significant differences in some texture features of OPC on CT (17), however, different classes of texture features distinguish non-OPCs. • Potentially morphologic differences exist between HPV+ and HPV- non-OPC compared to OPC. • Our findings may have a possibility to suggest HPV status does not affect prognosis of non-OPC patients, unlike those OPC patients. • May be the reason HPV+ non-OPC is still not established as a distinct subtype of non-OPC, unlike in OPC.
Limitations • No direct comparison between the underlying tumor histopathology and mathematical significance of the texture analysis • The sample size is relatively small, totaling 46 patients. • Few patients were eliminated due to motion artifact and streak artifact from dental hardware • Did not include areas of ulceration in the contoured volumes
Conclusions • Numerous texture features demonstrated a statistically significant difference between HPV+ and HPV- non-OPC • Non-OPC may have different morphologic features based on the HPV status of the primary tumors • Texture analysis may have the potential to explain differences between OPCs and non-OPCs based on HPV status – Different types of texture features found to be significant in OPCs compared to non-OPCs – Potentially related to differences in clinical features and prognosis

References 1. Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol 2014; 50: 380 -6 2. Thibaudeau E, Fortin B, Coutlee F, et al. HPV Prevalence and Prognostic Value in a Prospective Cohort of 255 Patients with Locally Advanced HNSCC: A Single-Centre Experience. Int J Otolaryngol 2013; 2013: 437815 3. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29: 4294 -301 4. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363: 24 -35 5. Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008; 100: 261 -9 6. Sethi S, Ali-Fehmi R, Franceschi S, et al. Characteristics and survival of head and neck cancer by HPV status: a cancer registry-based study. Int J Cancer 2012; 131: 1179 -86 7. Upile NS, Shaw RJ, Jones TM, et al. Squamous cell carcinoma of the head and neck outside the oropharynx is rarely human papillomavirus related. Laryngoscope 2014; 124: 2739 -44 8. Chung CH, Zhang Q, Kong CS, et al. p 16 Protein Expression and Human Papillomavirus Status As Prognostic Biomarkers of Nonoropharyngeal Head and Neck Squamous Cell Carcinoma. J Clin Oncol 2014; 32: 3930 -8 9. Wendt M, Romanitan M, Nasman A, et al. Presence of human papillomaviruses and p 16 expression in hypopharyngeal cancer. Head neck 2014; 36: 107 -12 10. Friedman JM, Stavas MJ, Cmelak AJ. Clinical and scientific impact of human papillomavirus on head and neck cancer. World J Clin Oncol 2014; 5: 781 -91 11. Anderson SW, Jara H, Ozonoff A, et al. Effect of disease progression on liver apparent diffusion coefficient and T 2 values in a murine model of hepatic fibrosis at 11. 7 Tesla MRI. J Magn Reson Imaging 2012; 35: 140 -6 12. de Carvalho Alegro M, Valotta Silva A, Yumi Bando S, et al. Texture analysis of high resolution MRI allows discrimination between febrile and afebrile initial precipitating injury in mesial temporal sclerosis. Magn Reson Med 2012; 68: 1647 -53 13. Fujimoto K, Tonan T, Azuma S, et al. Evaluation of the mean and entropy of apparent diffusion coefficient values in chronic hepatitis C: correlation with pathologic fibrosis stage and inflammatory activity grade. Radiology 2011; 258: 739 -48 14. Jirak D, Dezortova M, Taimr P, et al. Texture analysis of human liver. J Magn Reson Imaging 2002; 15: 68 -74 15. Mayerhoefer ME, Stelzeneder D, Bachbauer W, et al. Quantitative analysis of lumbar intervertebral disc abnormalities at 3. 0 Tesla: value of T(2) texture features and geometric parameters. NMR Biomed 2012; 25: 866 -72 16. Risse F, Pesic J, Young S, et al. A texture analysis approach to quantify ventilation changes in hyperpolarised (3)He MRI of the rat lung in an asthma model. NMR Biomed 2012; 25: 131 -41 17. Buch K, Fujita A, Li, B, et al. Using texture analysis to determine human papillomavirus status of oropharyngeal squamous cell carcinomas on CT. Am J Neuroradiol 2015 in press 18. Barry B, Buch K, Soto JA, et al. Quantifying liver fibrosis through the application of texture analysis to diffusion weighted imaging. Magn Reson Imaging 2014; 32: 84 -90 19. Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol 2014; 67: 850 -7 20. Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer 2009; 125: 362 -6 21. Panwar A, Batra R, Lydiatt WM, et al. Human papilloma virus positive oropharyngeal squamous cell carcinoma: a growing epidemic. Cancer Treat Rev 2014; 40: 215 -9 22. Chaturvedi AK. Epidemiology and clinical aspects of HPV in head and neck cancers. Head neck Pathol 2012; 6 Suppl 1: S 16 -24 23. D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356: 1944 -56 24. Westra WH. The changing face of head and neck cancer in the 21 st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head neck Pathol 2009; 3: 78 -81 25. Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human Papillomavirus and Overall Survival After Progression of Oropharyngeal Squamous Cell Carcinoma. J Clin Oncol 2014; 32: 3365 -73
7f51595da15e7aaa768dcb1bbf7373c7.ppt