fa2cc9c6364f83651c228aacb3509c12.ppt
- Количество слайдов: 40
 Diagnostic in Hadron Therapy 1 st International Conference on Frontiers in Diagnostic Technologies Frascati (Roma), Italy November, 27, 2009 Wolfgang Enghardt Onco. Ray – Center for Radiation Research in Oncology Technische Universität Dresden, Germany and Forschungszentrum Dresden-Rossendorf, Dresden, Germany Institute of Radiation Physics
Diagnostic in Hadron Therapy 1 st International Conference on Frontiers in Diagnostic Technologies Frascati (Roma), Italy November, 27, 2009 Wolfgang Enghardt Onco. Ray – Center for Radiation Research in Oncology Technische Universität Dresden, Germany and Forschungszentrum Dresden-Rossendorf, Dresden, Germany Institute of Radiation Physics
 Outline 1. Diagnostic imaging for radiotherapy 2. Image guided radiotherapy 3. Image based in-vivo dosimetry 4. Particle therapy positron emission tomography 5. In-beam single photon tomography
Outline 1. Diagnostic imaging for radiotherapy 2. Image guided radiotherapy 3. Image based in-vivo dosimetry 4. Particle therapy positron emission tomography 5. In-beam single photon tomography
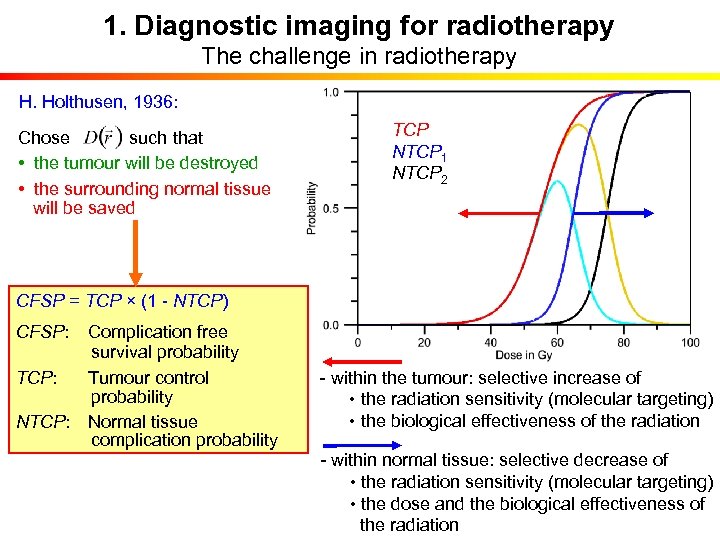 1. Diagnostic imaging for radiotherapy The challenge in radiotherapy H. Holthusen, 1936: Chose such that • the tumour will be destroyed • the surrounding normal tissue will be saved TCP NTCP 1 NTCP 2 CFSP = TCP × (1 - NTCP) CFSP: Complication free survival probability TCP: Tumour control probability NTCP: Normal tissue complication probability - within the tumour: selective increase of • the radiation sensitivity (molecular targeting) • the biological effectiveness of the radiation - within normal tissue: selective decrease of • the radiation sensitivity (molecular targeting) • the dose and the biological effectiveness of the radiation
1. Diagnostic imaging for radiotherapy The challenge in radiotherapy H. Holthusen, 1936: Chose such that • the tumour will be destroyed • the surrounding normal tissue will be saved TCP NTCP 1 NTCP 2 CFSP = TCP × (1 - NTCP) CFSP: Complication free survival probability TCP: Tumour control probability NTCP: Normal tissue complication probability - within the tumour: selective increase of • the radiation sensitivity (molecular targeting) • the biological effectiveness of the radiation - within normal tissue: selective decrease of • the radiation sensitivity (molecular targeting) • the dose and the biological effectiveness of the radiation
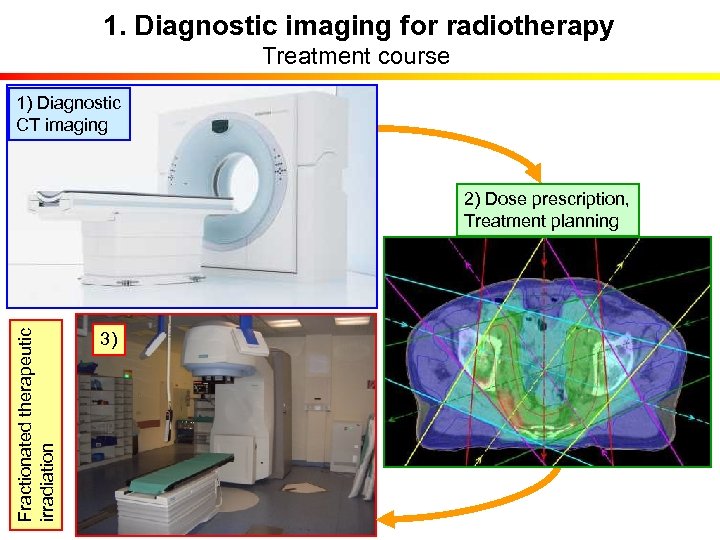 1. Diagnostic imaging for radiotherapy Treatment course 1) Diagnostic CT imaging Fractionated therapeutic irradiation 2) Dose prescription, Treatment planning 3)
1. Diagnostic imaging for radiotherapy Treatment course 1) Diagnostic CT imaging Fractionated therapeutic irradiation 2) Dose prescription, Treatment planning 3)
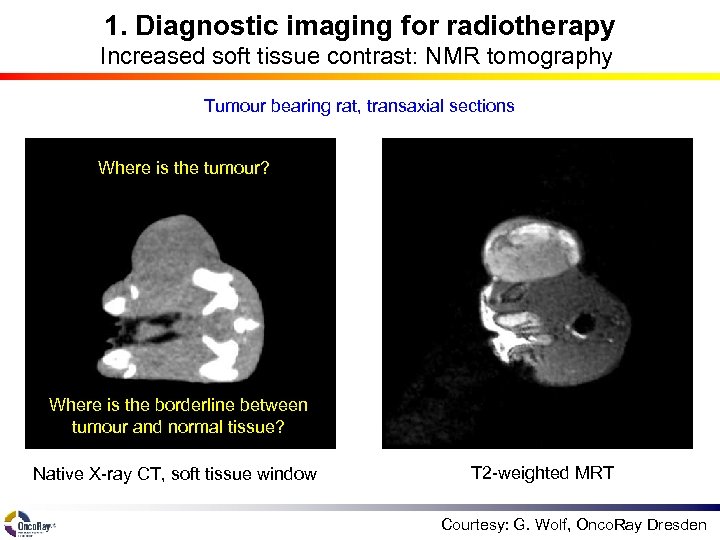 1. Diagnostic imaging for radiotherapy Increased soft tissue contrast: NMR tomography Tumour bearing rat, transaxial sections Where is the tumour? Where is the borderline between tumour and normal tissue? Native X-ray CT, soft tissue window T 2 -weighted MRT Courtesy: G. Wolf, Onco. Ray Dresden
1. Diagnostic imaging for radiotherapy Increased soft tissue contrast: NMR tomography Tumour bearing rat, transaxial sections Where is the tumour? Where is the borderline between tumour and normal tissue? Native X-ray CT, soft tissue window T 2 -weighted MRT Courtesy: G. Wolf, Onco. Ray Dresden
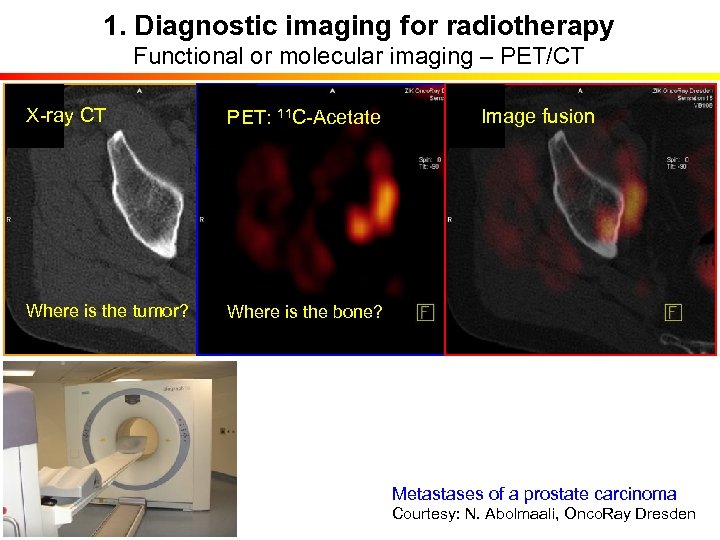 1. Diagnostic imaging for radiotherapy Functional or molecular imaging – PET/CT X-ray CT PET: 11 C-Acetate Where is the tumor? Where is the bone? Image fusion Metastases of a prostate carcinoma Courtesy: N. Abolmaali, Onco. Ray Dresden
1. Diagnostic imaging for radiotherapy Functional or molecular imaging – PET/CT X-ray CT PET: 11 C-Acetate Where is the tumor? Where is the bone? Image fusion Metastases of a prostate carcinoma Courtesy: N. Abolmaali, Onco. Ray Dresden
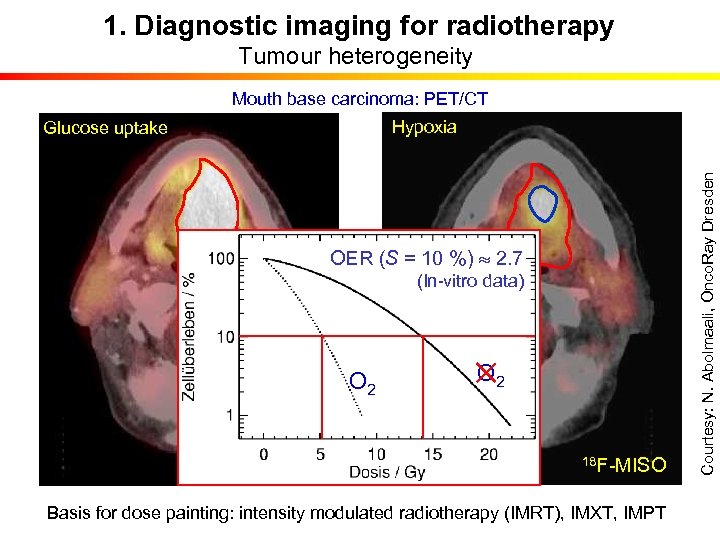 1. Diagnostic imaging for radiotherapy Tumour heterogeneity Mouth base carcinoma: PET/CT OER (S = 10 %) 2. 7 (In-vitro data) O 2 18 F-FDG O 2 18 F-MISO Basis for dose painting: intensity modulated radiotherapy (IMRT), IMXT, IMPT Courtesy: N. Abolmaali, Onco. Ray Dresden Hypoxia Glucose uptake
1. Diagnostic imaging for radiotherapy Tumour heterogeneity Mouth base carcinoma: PET/CT OER (S = 10 %) 2. 7 (In-vitro data) O 2 18 F-FDG O 2 18 F-MISO Basis for dose painting: intensity modulated radiotherapy (IMRT), IMXT, IMPT Courtesy: N. Abolmaali, Onco. Ray Dresden Hypoxia Glucose uptake
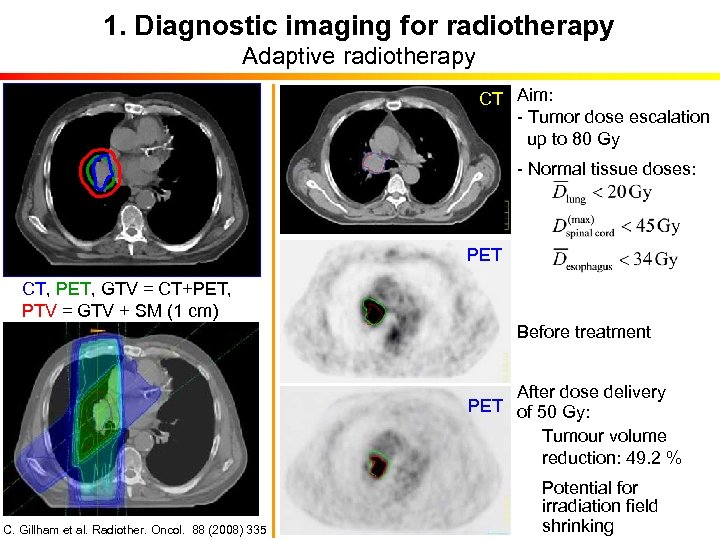 1. Diagnostic imaging for radiotherapy Adaptive radiotherapy CT Aim: - Tumor dose escalation up to 80 Gy - Normal tissue doses: PET CT, PET, GTV = CT+PET, PTV = GTV + SM (1 cm) Before treatment After dose delivery PET of 50 Gy: Tumour volume reduction: 49. 2 % C. Gillham et al. Radiother. Oncol. 88 (2008) 335 Potential for irradiation field shrinking
1. Diagnostic imaging for radiotherapy Adaptive radiotherapy CT Aim: - Tumor dose escalation up to 80 Gy - Normal tissue doses: PET CT, PET, GTV = CT+PET, PTV = GTV + SM (1 cm) Before treatment After dose delivery PET of 50 Gy: Tumour volume reduction: 49. 2 % C. Gillham et al. Radiother. Oncol. 88 (2008) 335 Potential for irradiation field shrinking
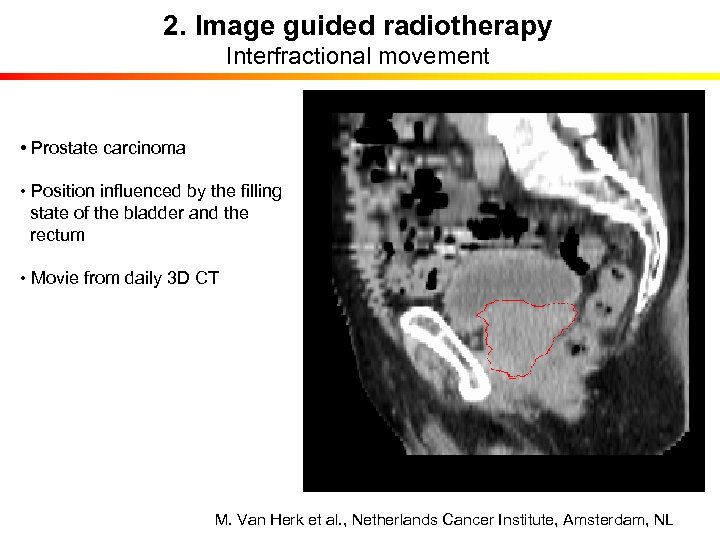 2. Image guided radiotherapy Interfractional movement • Prostate carcinoma • Position influenced by the filling state of the bladder and the rectum • Movie from daily 3 D CT M. Van Herk et al. , Netherlands Cancer Institute, Amsterdam, NL
2. Image guided radiotherapy Interfractional movement • Prostate carcinoma • Position influenced by the filling state of the bladder and the rectum • Movie from daily 3 D CT M. Van Herk et al. , Netherlands Cancer Institute, Amsterdam, NL
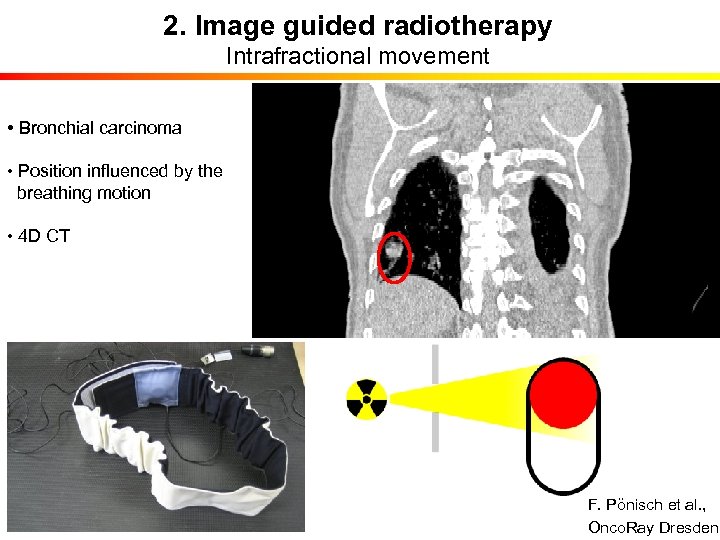 2. Image guided radiotherapy Intrafractional movement • Bronchial carcinoma • Position influenced by the breathing motion • 4 D CT F. Pönisch et al. , Onco. Ray Dresden
2. Image guided radiotherapy Intrafractional movement • Bronchial carcinoma • Position influenced by the breathing motion • 4 D CT F. Pönisch et al. , Onco. Ray Dresden
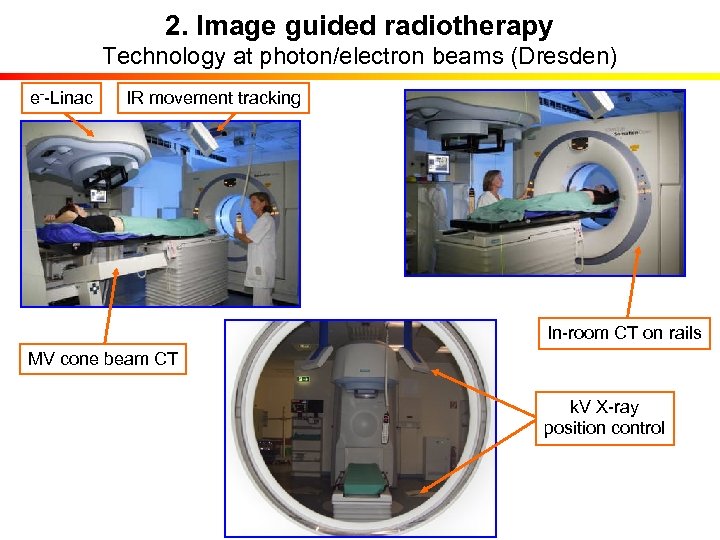 2. Image guided radiotherapy Technology at photon/electron beams (Dresden) e--Linac IR movement tracking In-room CT on rails MV cone beam CT k. V X-ray position control
2. Image guided radiotherapy Technology at photon/electron beams (Dresden) e--Linac IR movement tracking In-room CT on rails MV cone beam CT k. V X-ray position control
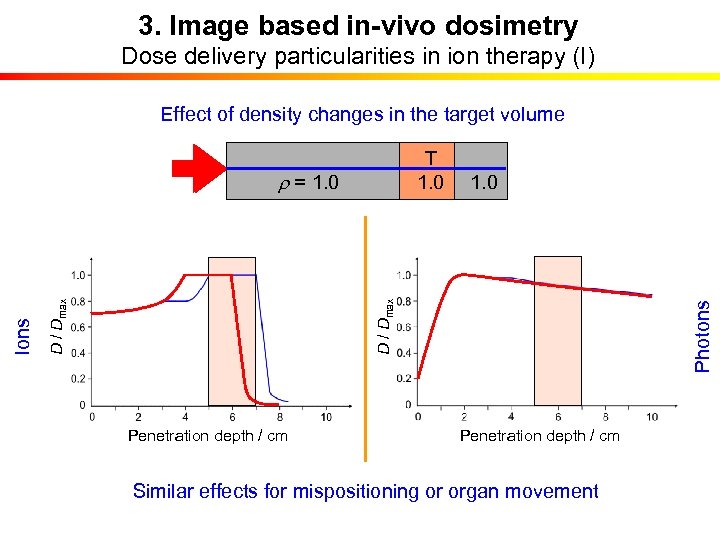 3. Image based in-vivo dosimetry Dose delivery particularities in ion therapy (I) Effect of density changes in the target volume T T 1. 0 Penetration depth / cm Photons 1. 0 D / Dmax Ions r = 1. 0 0 r = 1. 0 Penetration depth / cm Similar effects for mispositioning or organ movement
3. Image based in-vivo dosimetry Dose delivery particularities in ion therapy (I) Effect of density changes in the target volume T T 1. 0 Penetration depth / cm Photons 1. 0 D / Dmax Ions r = 1. 0 0 r = 1. 0 Penetration depth / cm Similar effects for mispositioning or organ movement
 3. Image based in-vivo dosimetry Dose delivery particularities in ion therapy (II) The dose distribution deposited by ions is extremely sensitive to the ion range in vivo The accuracy of the ion range is influenced by (1) Systematic errors in the physical beam model used for treatment (2) planning: R = R(HU) (2) Random errors like - mispositioning - patient- or organ movement - density changes within the irradiated volume - treatment mistakes and accidents Desirable: A procedure for the verification - of the irradiation field position - of the particle range - simultaneous with therapeutic irradiation and, in particular a procedure for the quantification of the dose distribution - in-situ - in-vivo - in real time.
3. Image based in-vivo dosimetry Dose delivery particularities in ion therapy (II) The dose distribution deposited by ions is extremely sensitive to the ion range in vivo The accuracy of the ion range is influenced by (1) Systematic errors in the physical beam model used for treatment (2) planning: R = R(HU) (2) Random errors like - mispositioning - patient- or organ movement - density changes within the irradiated volume - treatment mistakes and accidents Desirable: A procedure for the verification - of the irradiation field position - of the particle range - simultaneous with therapeutic irradiation and, in particular a procedure for the quantification of the dose distribution - in-situ - in-vivo - in real time.
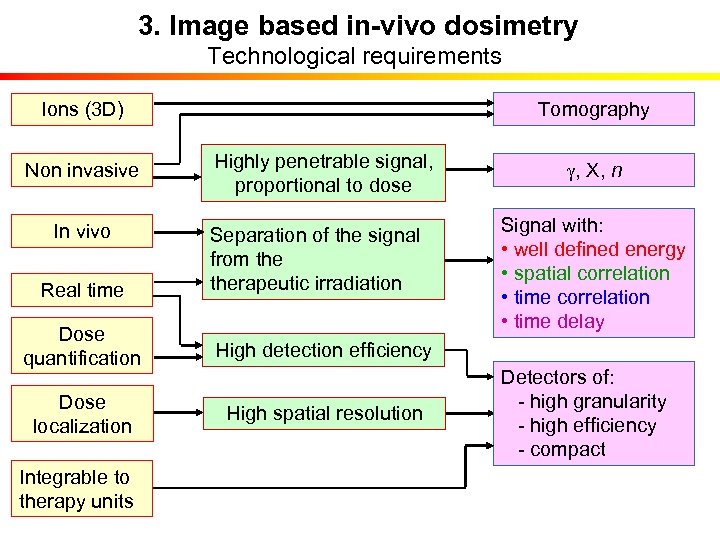 3. Image based in-vivo dosimetry Technological requirements Tomography Ions (3 D) Non invasive Highly penetrable signal, proportional to dose In vivo Real time Separation of the signal from therapeutic irradiation Dose quantification High detection efficiency Dose localization High spatial resolution Integrable to therapy units g, X, n Signal with: • well defined energy • spatial correlation • time delay Detectors of: - high granularity - high efficiency - compact
3. Image based in-vivo dosimetry Technological requirements Tomography Ions (3 D) Non invasive Highly penetrable signal, proportional to dose In vivo Real time Separation of the signal from therapeutic irradiation Dose quantification High detection efficiency Dose localization High spatial resolution Integrable to therapy units g, X, n Signal with: • well defined energy • spatial correlation • time delay Detectors of: - high granularity - high efficiency - compact
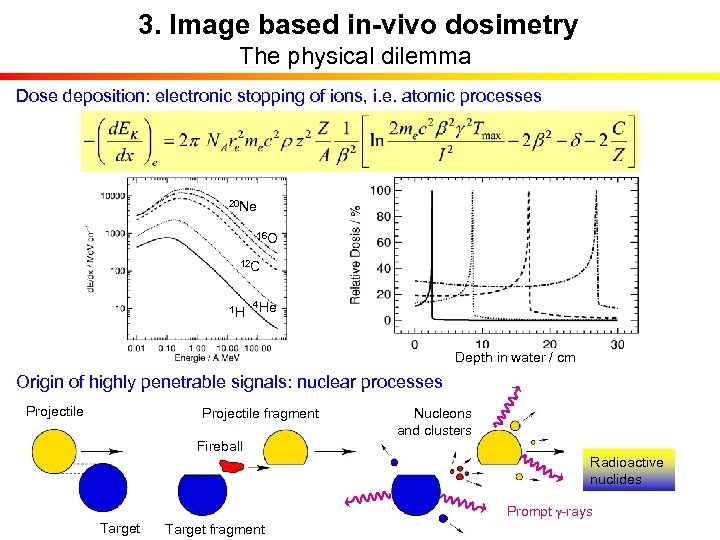 3. Image based in-vivo dosimetry The physical dilemma Dose deposition: electronic stopping of ions, i. e. atomic processes 20 Ne 16 O 12 C 1 H 4 He Depth in water / cm Origin of highly penetrable signals: nuclear processes Projectile fragment Nucleons and clusters Fireball Radioactive nuclides Prompt g-rays Target fragment
3. Image based in-vivo dosimetry The physical dilemma Dose deposition: electronic stopping of ions, i. e. atomic processes 20 Ne 16 O 12 C 1 H 4 He Depth in water / cm Origin of highly penetrable signals: nuclear processes Projectile fragment Nucleons and clusters Fireball Radioactive nuclides Prompt g-rays Target fragment
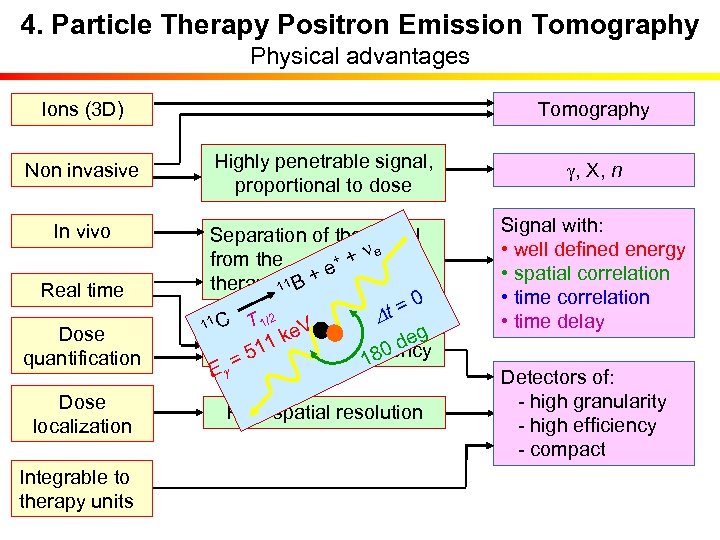 4. Particle Therapy Positron Emission Tomography Physical advantages Tomography Ions (3 D) Non invasive In vivo Real time Dose quantification Dose localization Integrable to therapy units Highly penetrable signal, proportional to dose Separation of the signal ne + + from the + e therapeutic irradiation 11 B 0 t = T 1/2 D 11 C e. V g k de 11 High detection efficiency 180 = 5 Eg High spatial resolution g, X, n Signal with: • well defined energy • spatial correlation • time delay Detectors of: - high granularity - high efficiency - compact
4. Particle Therapy Positron Emission Tomography Physical advantages Tomography Ions (3 D) Non invasive In vivo Real time Dose quantification Dose localization Integrable to therapy units Highly penetrable signal, proportional to dose Separation of the signal ne + + from the + e therapeutic irradiation 11 B 0 t = T 1/2 D 11 C e. V g k de 11 High detection efficiency 180 = 5 Eg High spatial resolution g, X, n Signal with: • well defined energy • spatial correlation • time delay Detectors of: - high granularity - high efficiency - compact
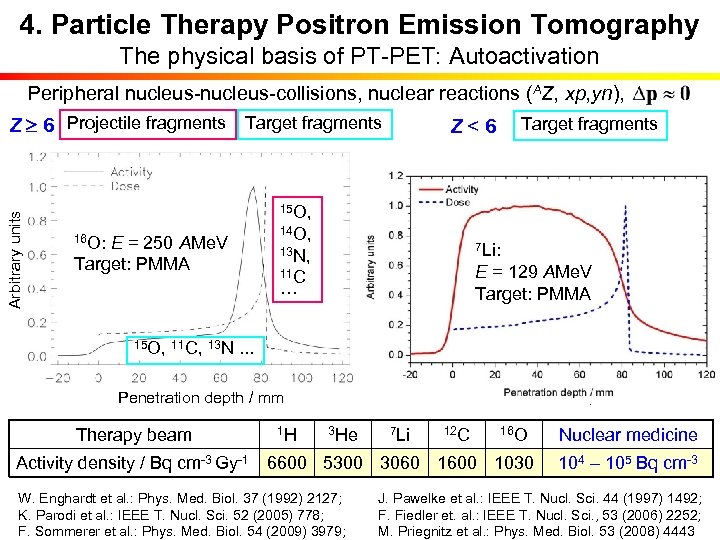 4. Particle Therapy Positron Emission Tomography The physical basis of PT-PET: Autoactivation Peripheral nucleus-collisions, nuclear reactions (AZ, xp, yn), 15 O, 12 C: E = 212 AMe. V 16 O: E = 250 AMe. V Target: PMMA 14 O, 11 C, 13 N, 10 C 11 C … 3 He: E = 130 AMe. V 7 Li: 1 H: E = 110 Me. V Target: PMMA ETarget: PMMA = 129 AMe. V Target: PMMA 15 O, 11 C, 13 N. . . Penetration depth / mm Therapy beam Target fragments Z<6 Arbitrary units Z 6 Projectile fragments Target fragments 1 H 3 He 7 Li 12 C 16 O Activity density / Bq cm-3 Gy-1 6600 5300 3060 1600 1030 Nuclear medicine 104 – 105 Bq cm-3 W. Enghardt et al. : Phys. Med. Biol. 37 (1992) 2127; J. Pawelke et al. : IEEE T. Nucl. Sci. 44 (1997) 1492; K. Parodi et al. : IEEE T. Nucl. Sci. 52 (2005) 778; F. Fiedler et. al. : IEEE T. Nucl. Sci. , 53 (2006) 2252; F. Sommerer et al. : Phys. Med. Biol. 54 (2009) 3979; M. Priegnitz et al. : Phys. Med. Biol. 53 (2008) 4443
4. Particle Therapy Positron Emission Tomography The physical basis of PT-PET: Autoactivation Peripheral nucleus-collisions, nuclear reactions (AZ, xp, yn), 15 O, 12 C: E = 212 AMe. V 16 O: E = 250 AMe. V Target: PMMA 14 O, 11 C, 13 N, 10 C 11 C … 3 He: E = 130 AMe. V 7 Li: 1 H: E = 110 Me. V Target: PMMA ETarget: PMMA = 129 AMe. V Target: PMMA 15 O, 11 C, 13 N. . . Penetration depth / mm Therapy beam Target fragments Z<6 Arbitrary units Z 6 Projectile fragments Target fragments 1 H 3 He 7 Li 12 C 16 O Activity density / Bq cm-3 Gy-1 6600 5300 3060 1600 1030 Nuclear medicine 104 – 105 Bq cm-3 W. Enghardt et al. : Phys. Med. Biol. 37 (1992) 2127; J. Pawelke et al. : IEEE T. Nucl. Sci. 44 (1997) 1492; K. Parodi et al. : IEEE T. Nucl. Sci. 52 (2005) 778; F. Fiedler et. al. : IEEE T. Nucl. Sci. , 53 (2006) 2252; F. Sommerer et al. : Phys. Med. Biol. 54 (2009) 3979; M. Priegnitz et al. : Phys. Med. Biol. 53 (2008) 4443
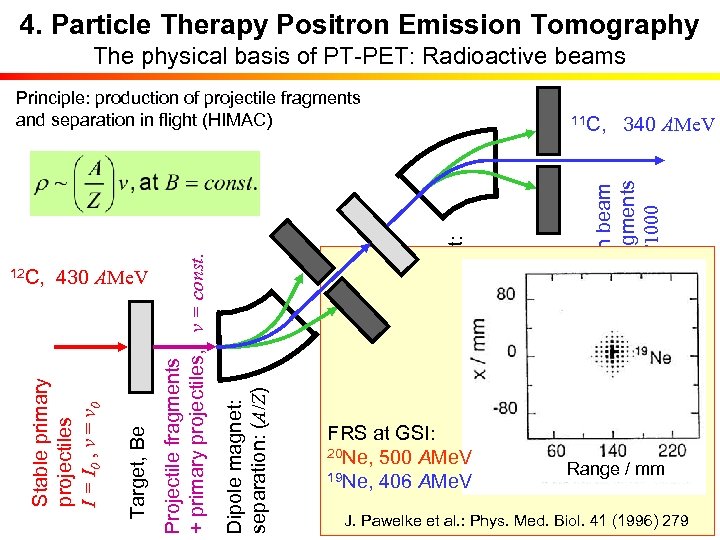 4. Particle Therapy Positron Emission Tomography The physical basis of PT-PET: Radioactive beams FRS at GSI: 20 Ne, 500 AMe. V 19 Ne, 406 AMe. V Clean beam of fragments I ≈ I 0/1000 Dipole magnet: separation: v 11 C, 340 AMe. V Degrader: DE Z 2 Aperture Dipole magnet: separation: (A/Z) Target, Be Stable primary projectiles I = I 0 , v = v 0 12 C, 430 AMe. V Projectile fragments + primary projectiles, v = const. Principle: production of projectile fragments and separation in flight (HIMAC) Range / mm E. Urakabe et al. : Jpn. J. Appl. Phys. 40 (2001) 2540 J. Pawelke et al. : Phys. Med. Biol. 41 (1996) 279 M. Kanazawa et al. : Nucl. Phys. A 701 (2002) 244 c
4. Particle Therapy Positron Emission Tomography The physical basis of PT-PET: Radioactive beams FRS at GSI: 20 Ne, 500 AMe. V 19 Ne, 406 AMe. V Clean beam of fragments I ≈ I 0/1000 Dipole magnet: separation: v 11 C, 340 AMe. V Degrader: DE Z 2 Aperture Dipole magnet: separation: (A/Z) Target, Be Stable primary projectiles I = I 0 , v = v 0 12 C, 430 AMe. V Projectile fragments + primary projectiles, v = const. Principle: production of projectile fragments and separation in flight (HIMAC) Range / mm E. Urakabe et al. : Jpn. J. Appl. Phys. 40 (2001) 2540 J. Pawelke et al. : Phys. Med. Biol. 41 (1996) 279 M. Kanazawa et al. : Nucl. Phys. A 701 (2002) 244 c
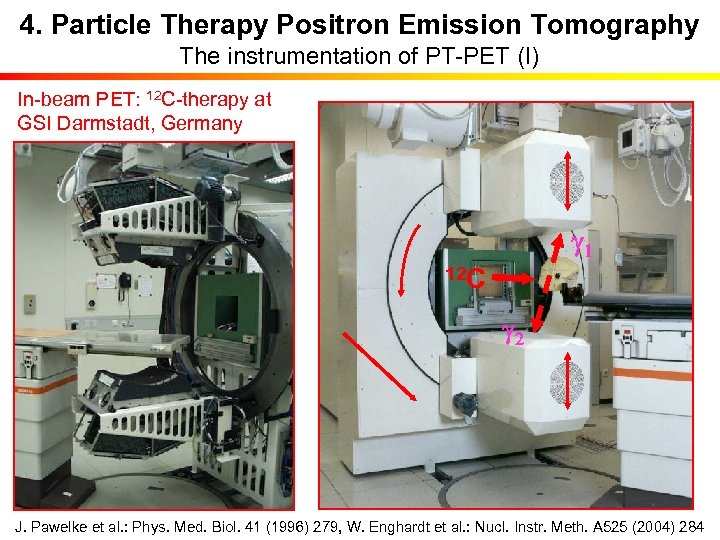 4. Particle Therapy Positron Emission Tomography The instrumentation of PT-PET (I) In-beam PET: 12 C-therapy at GSI Darmstadt, Germany g 1 12 C g 2 J. Pawelke et al. : Phys. Med. Biol. 41 (1996) 279, W. Enghardt et al. : Nucl. Instr. Meth. A 525 (2004) 284
4. Particle Therapy Positron Emission Tomography The instrumentation of PT-PET (I) In-beam PET: 12 C-therapy at GSI Darmstadt, Germany g 1 12 C g 2 J. Pawelke et al. : Phys. Med. Biol. 41 (1996) 279, W. Enghardt et al. : Nucl. Instr. Meth. A 525 (2004) 284
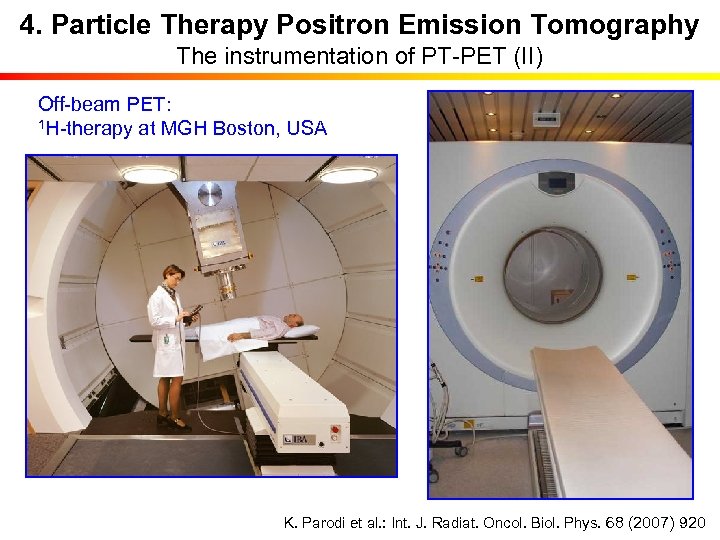 4. Particle Therapy Positron Emission Tomography The instrumentation of PT-PET (II) Off-beam PET: 1 H-therapy at MGH Boston, USA K. Parodi et al. : Int. J. Radiat. Oncol. Biol. Phys. 68 (2007) 920
4. Particle Therapy Positron Emission Tomography The instrumentation of PT-PET (II) Off-beam PET: 1 H-therapy at MGH Boston, USA K. Parodi et al. : Int. J. Radiat. Oncol. Biol. Phys. 68 (2007) 920
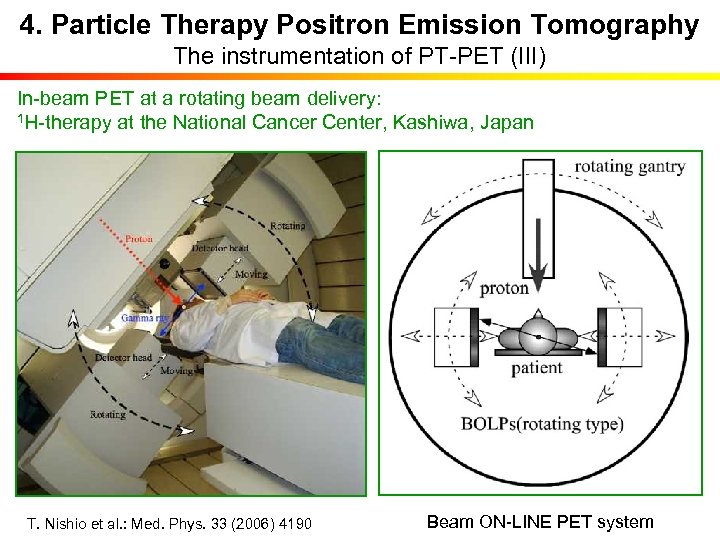 4. Particle Therapy Positron Emission Tomography The instrumentation of PT-PET (III) In-beam PET at a rotating beam delivery: 1 H-therapy at the National Cancer Center, Kashiwa, Japan T. Nishio et al. : Med. Phys. 33 (2006) 4190 Beam ON-LINE PET system
4. Particle Therapy Positron Emission Tomography The instrumentation of PT-PET (III) In-beam PET at a rotating beam delivery: 1 H-therapy at the National Cancer Center, Kashiwa, Japan T. Nishio et al. : Med. Phys. 33 (2006) 4190 Beam ON-LINE PET system
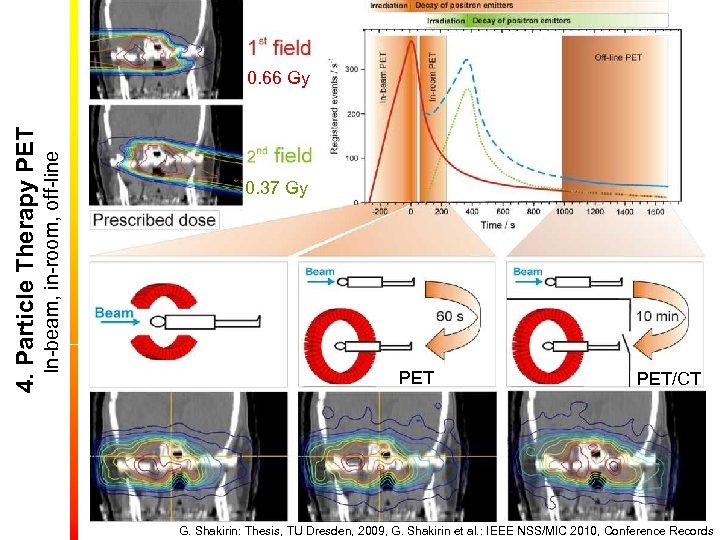 In-beam, in-room, off-line 4. Particle Therapy PET 0. 66 Gy 0. 37 Gy PET/CT G. Shakirin: Thesis, TU Dresden, 2009, G. Shakirin et al. : IEEE NSS/MIC 2010, Conference Records
In-beam, in-room, off-line 4. Particle Therapy PET 0. 66 Gy 0. 37 Gy PET/CT G. Shakirin: Thesis, TU Dresden, 2009, G. Shakirin et al. : IEEE NSS/MIC 2010, Conference Records
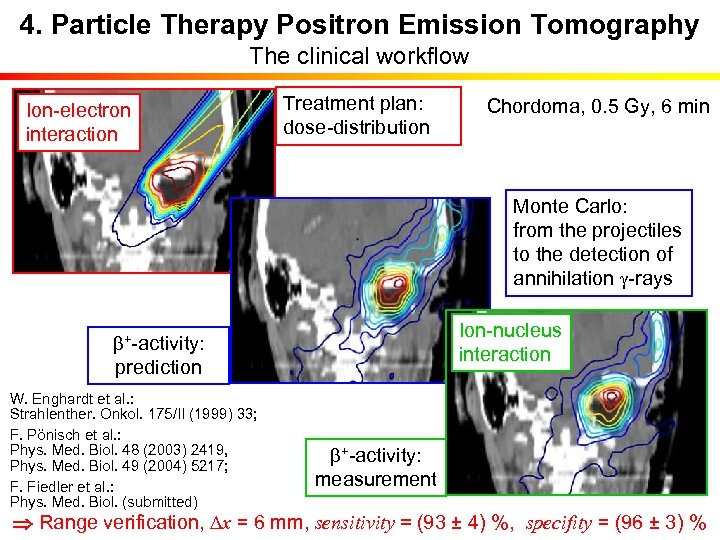 4. Particle Therapy Positron Emission Tomography The clinical workflow Ion-electron interaction b+-activity: Treatment plan: dose-distribution prediction W. Enghardt et al. : Strahlenther. Onkol. 175/II (1999) 33; F. Pönisch et al. : Phys. Med. Biol. 48 (2003) 2419, Phys. Med. Biol. 49 (2004) 5217; F. Fiedler et al. : Phys. Med. Biol. (submitted) Chordoma, 0. 5 Gy, 6 min Monte Carlo: from the projectiles to the detection of annihilation g-rays Ion-nucleus interaction b+-activity: measurement Range verification, Dx = 6 mm, sensitivity = (93 ± 4) %, specifity = (96 ± 3) %
4. Particle Therapy Positron Emission Tomography The clinical workflow Ion-electron interaction b+-activity: Treatment plan: dose-distribution prediction W. Enghardt et al. : Strahlenther. Onkol. 175/II (1999) 33; F. Pönisch et al. : Phys. Med. Biol. 48 (2003) 2419, Phys. Med. Biol. 49 (2004) 5217; F. Fiedler et al. : Phys. Med. Biol. (submitted) Chordoma, 0. 5 Gy, 6 min Monte Carlo: from the projectiles to the detection of annihilation g-rays Ion-nucleus interaction b+-activity: measurement Range verification, Dx = 6 mm, sensitivity = (93 ± 4) %, specifity = (96 ± 3) %
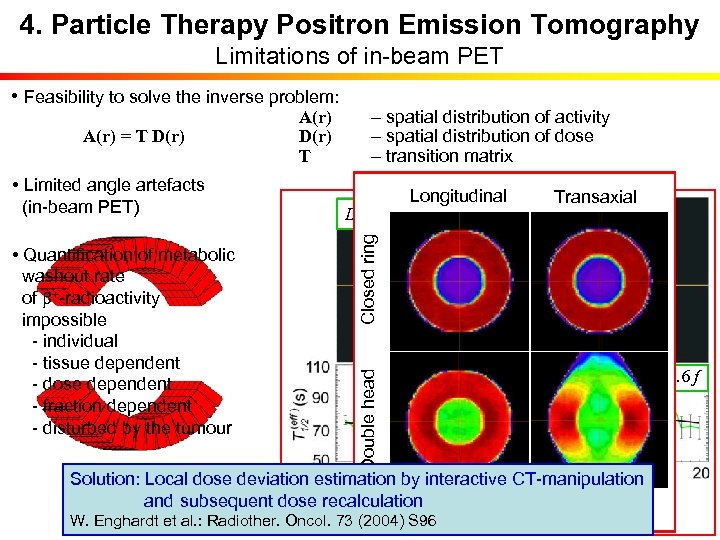 4. Particle Therapy Positron Emission Tomography Limitations of in-beam PET • Feasibility to solve the inverse problem: K. Parodi, T. Bortfeld: • Limited angle artefacts (in-beam PET) • Quantification of metabolic washout rate of b+-radioactivity impossible - individual - tissue dependent - dose dependent - fraction dependent - disturbed by the tumour – spatial distribution of activity – spatial distribution of dose – transition matrix Longitudinal Transaxial Phys. Med. Biol. 51 (2006) 1991 D < 0. 9 Dmax D > 0. 9 Dmax Dose Closed ring A(r) = T D(r) A(r) D(r) T T 1/2/s = 74. 3 – 0. 2 f Double head b+-activity T 1/2/s = 86. 3 – 0. 6 f Solution: Local dose deviation estimation by interactive CT-manipulation P. Crespo et al. : and subsequent dose recalculation K. Parodi et al. : IJROBP 68 (2007) 920 Phys. Med. Biol. 51 (2006) 2143 F. Fiedler et al. : Acta Oncol. 47 (2008) 1077 W. Enghardt et al. : Radiother. Oncol. 73 (2004) S 96
4. Particle Therapy Positron Emission Tomography Limitations of in-beam PET • Feasibility to solve the inverse problem: K. Parodi, T. Bortfeld: • Limited angle artefacts (in-beam PET) • Quantification of metabolic washout rate of b+-radioactivity impossible - individual - tissue dependent - dose dependent - fraction dependent - disturbed by the tumour – spatial distribution of activity – spatial distribution of dose – transition matrix Longitudinal Transaxial Phys. Med. Biol. 51 (2006) 1991 D < 0. 9 Dmax D > 0. 9 Dmax Dose Closed ring A(r) = T D(r) A(r) D(r) T T 1/2/s = 74. 3 – 0. 2 f Double head b+-activity T 1/2/s = 86. 3 – 0. 6 f Solution: Local dose deviation estimation by interactive CT-manipulation P. Crespo et al. : and subsequent dose recalculation K. Parodi et al. : IJROBP 68 (2007) 920 Phys. Med. Biol. 51 (2006) 2143 F. Fiedler et al. : Acta Oncol. 47 (2008) 1077 W. Enghardt et al. : Radiother. Oncol. 73 (2004) S 96
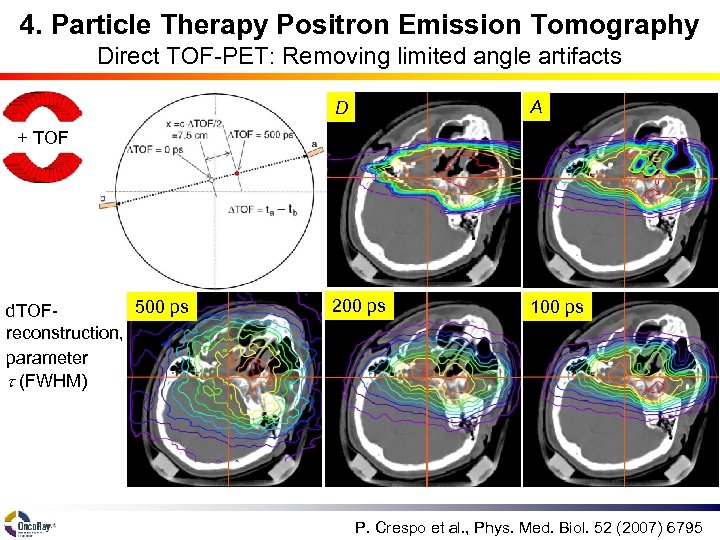 4. Particle Therapy Positron Emission Tomography Direct TOF-PET: Removing limited angle artifacts D A 200 ps 100 ps + TOF 500 ps d. TOFreconstruction, parameter t (FWHM) P. Crespo et al. , Phys. Med. Biol. 52 (2007) 6795
4. Particle Therapy Positron Emission Tomography Direct TOF-PET: Removing limited angle artifacts D A 200 ps 100 ps + TOF 500 ps d. TOFreconstruction, parameter t (FWHM) P. Crespo et al. , Phys. Med. Biol. 52 (2007) 6795
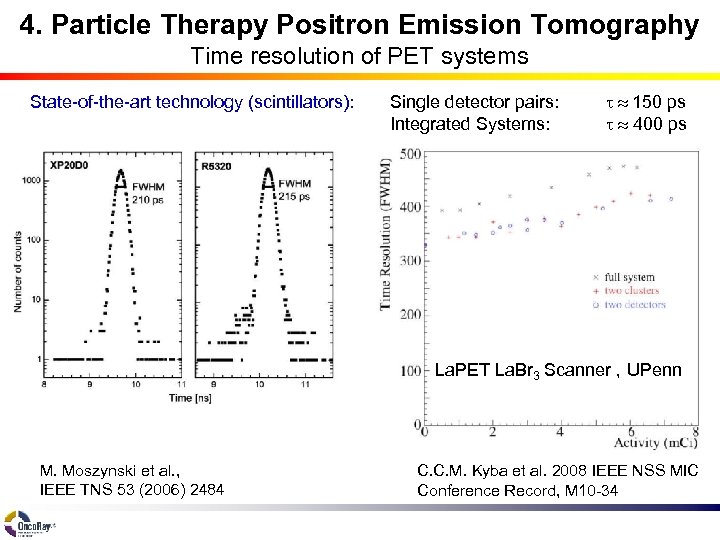 4. Particle Therapy Positron Emission Tomography Time resolution of PET systems State-of-the-art technology (scintillators): Single detector pairs: Integrated Systems: t 150 ps t 400 ps La. PET La. Br 3 Scanner , UPenn M. Moszynski et al. , IEEE TNS 53 (2006) 2484 C. C. M. Kyba et al. 2008 IEEE NSS MIC Conference Record, M 10 -34
4. Particle Therapy Positron Emission Tomography Time resolution of PET systems State-of-the-art technology (scintillators): Single detector pairs: Integrated Systems: t 150 ps t 400 ps La. PET La. Br 3 Scanner , UPenn M. Moszynski et al. , IEEE TNS 53 (2006) 2484 C. C. M. Kyba et al. 2008 IEEE NSS MIC Conference Record, M 10 -34
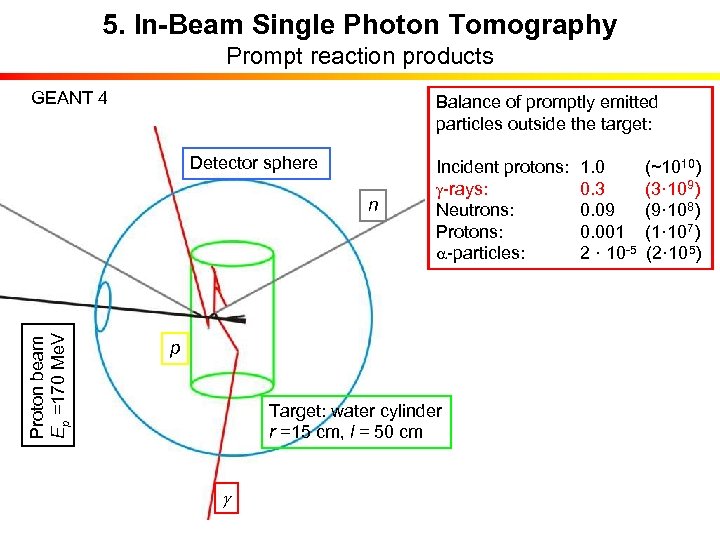 5. In-Beam Single Photon Tomography Prompt reaction products GEANT 4 Balance of promptly emitted particles outside the target: Detector sphere Proton beam Ep =170 Me. V n Incident protons: g-rays: Neutrons: Protons: a-particles: p Target: water cylinder r =15 cm, l = 50 cm g 1. 0 (~1010) 0. 3 (3· 109) 0. 09 (9· 108) 0. 001 (1· 107) 2 · 10 -5 (2· 105)
5. In-Beam Single Photon Tomography Prompt reaction products GEANT 4 Balance of promptly emitted particles outside the target: Detector sphere Proton beam Ep =170 Me. V n Incident protons: g-rays: Neutrons: Protons: a-particles: p Target: water cylinder r =15 cm, l = 50 cm g 1. 0 (~1010) 0. 3 (3· 109) 0. 09 (9· 108) 0. 001 (1· 107) 2 · 10 -5 (2· 105)
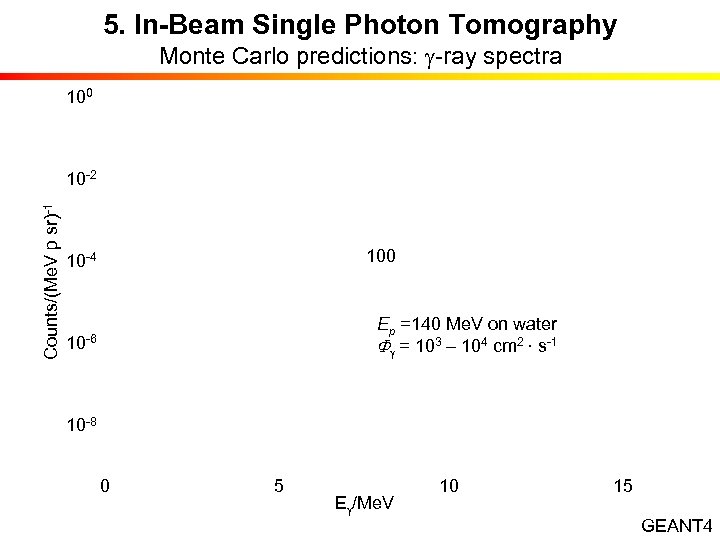 5. In-Beam Single Photon Tomography Monte Carlo predictions: g-ray spectra 100 Counts/(Me. V p sr)-1 10 -2 10 -4 10 -6 100 Ep =140 Me. V on water Fg = 103 – 104 cm 2 · s-1 10 -8 0 5 10 15 Eg/Me. V GEANT 4
5. In-Beam Single Photon Tomography Monte Carlo predictions: g-ray spectra 100 Counts/(Me. V p sr)-1 10 -2 10 -4 10 -6 100 Ep =140 Me. V on water Fg = 103 – 104 cm 2 · s-1 10 -8 0 5 10 15 Eg/Me. V GEANT 4
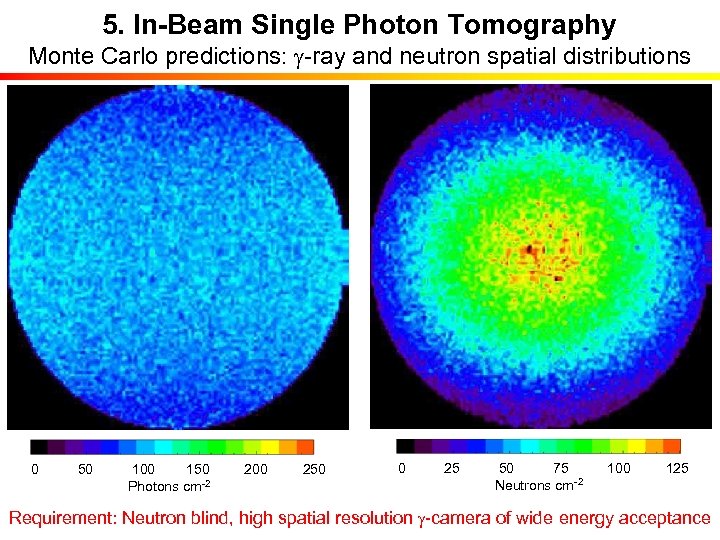 5. In-Beam Single Photon Tomography Monte Carlo predictions: g-ray and neutron spatial distributions 0 50 100 150 200 250 Photons cm-2 g cm-1 0 25 50 75 100 125 Neutrons cm-2 Requirement: Neutron blind, high spatial resolution g-camera of wide energy acceptance
5. In-Beam Single Photon Tomography Monte Carlo predictions: g-ray and neutron spatial distributions 0 50 100 150 200 250 Photons cm-2 g cm-1 0 25 50 75 100 125 Neutrons cm-2 Requirement: Neutron blind, high spatial resolution g-camera of wide energy acceptance
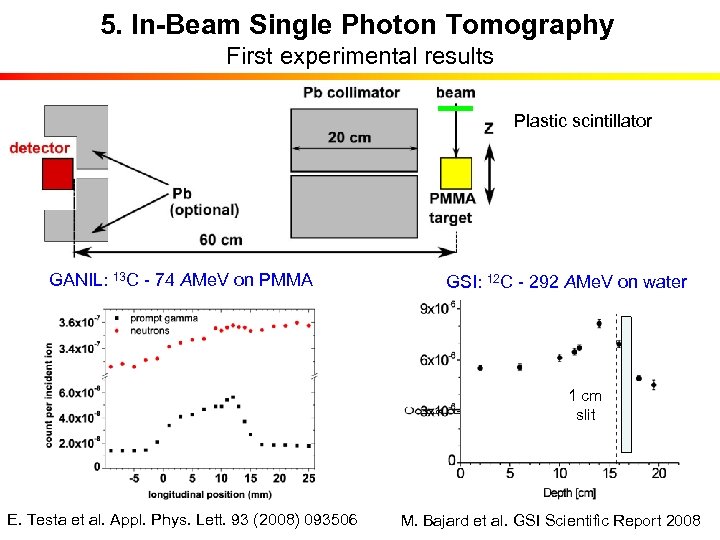 5. In-Beam Single Photon Tomography First experimental results Plastic scintillator GANIL: 13 C - 74 AMe. V on PMMA GSI: 12 C - 292 AMe. V on water 1 cm slit E. Testa et al. Appl. Phys. Lett. 93 (2008) 093506 M. Bajard et al. GSI Scientific Report 2008
5. In-Beam Single Photon Tomography First experimental results Plastic scintillator GANIL: 13 C - 74 AMe. V on PMMA GSI: 12 C - 292 AMe. V on water 1 cm slit E. Testa et al. Appl. Phys. Lett. 93 (2008) 093506 M. Bajard et al. GSI Scientific Report 2008
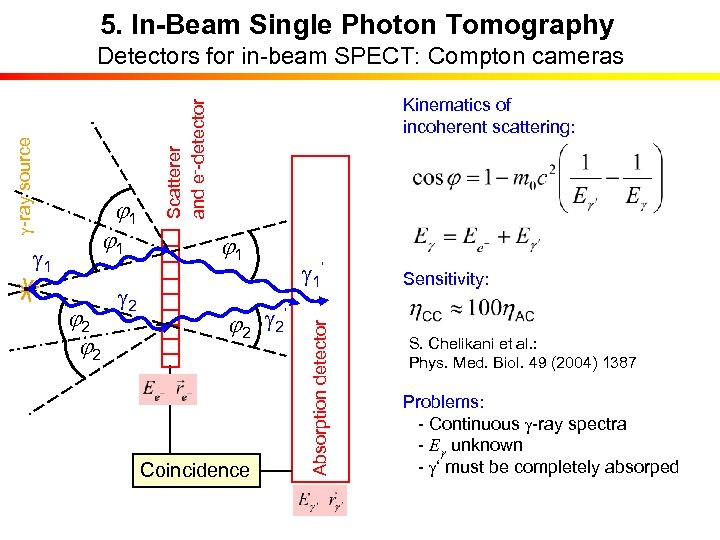 5. In-Beam Single Photon Tomography j 2 g 2 j 1 ‘ j 2 g 2 Coincidence g 1‘ Absorption detector g 1 j 1 Kinematics of incoherent scattering: Scatterer and e--detector g-ray source Detectors for in-beam SPECT: Compton cameras Sensitivity: S. Chelikani et al. : Phys. Med. Biol. 49 (2004) 1387 Problems: - Continuous g-ray spectra - Eg unknown - g‘ must be completely absorped
5. In-Beam Single Photon Tomography j 2 g 2 j 1 ‘ j 2 g 2 Coincidence g 1‘ Absorption detector g 1 j 1 Kinematics of incoherent scattering: Scatterer and e--detector g-ray source Detectors for in-beam SPECT: Compton cameras Sensitivity: S. Chelikani et al. : Phys. Med. Biol. 49 (2004) 1387 Problems: - Continuous g-ray spectra - Eg unknown - g‘ must be completely absorped
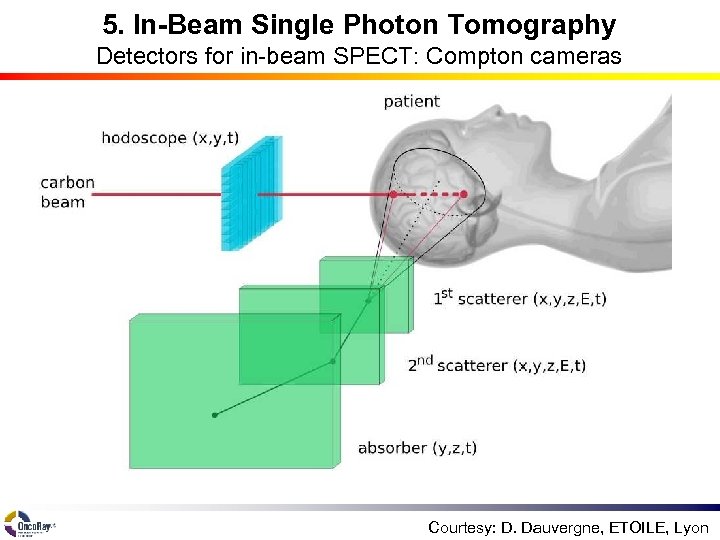 5. In-Beam Single Photon Tomography Detectors for in-beam SPECT: Compton cameras Courtesy: D. Dauvergne, ETOILE, Lyon
5. In-Beam Single Photon Tomography Detectors for in-beam SPECT: Compton cameras Courtesy: D. Dauvergne, ETOILE, Lyon
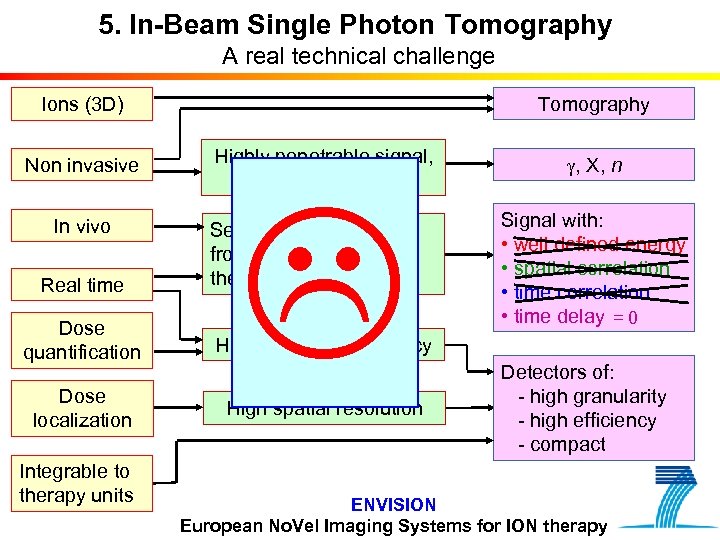 5. In-Beam Single Photon Tomography A real technical challenge Tomography Ions (3 D) Non invasive Highly penetrable signal, proportional to dose In vivo Real time Separation of the signal from therapeutic irradiation Dose quantification High detection efficiency Dose localization High spatial resolution Integrable to therapy units g, X, n Signal with: • well defined energy • spatial correlation • time delay = 0 Detectors of: - high granularity - high efficiency - compact ENVISION European No. Vel Imaging Systems for ION therapy
5. In-Beam Single Photon Tomography A real technical challenge Tomography Ions (3 D) Non invasive Highly penetrable signal, proportional to dose In vivo Real time Separation of the signal from therapeutic irradiation Dose quantification High detection efficiency Dose localization High spatial resolution Integrable to therapy units g, X, n Signal with: • well defined energy • spatial correlation • time delay = 0 Detectors of: - high granularity - high efficiency - compact ENVISION European No. Vel Imaging Systems for ION therapy
 Acknowledgment
Acknowledgment
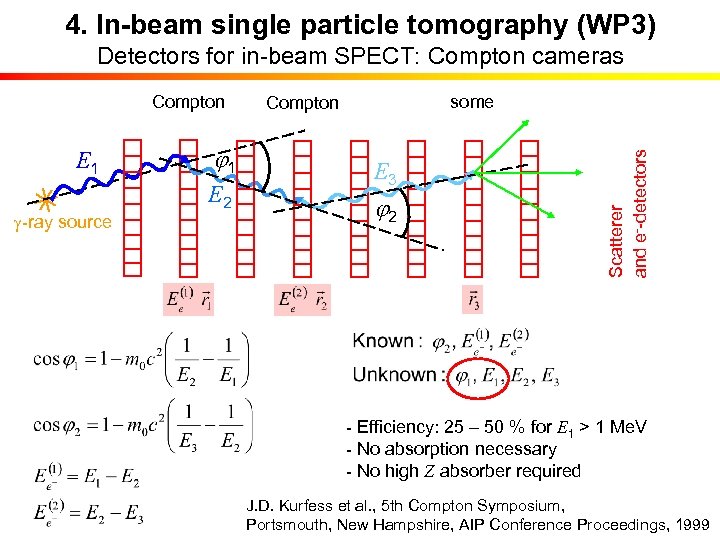 4. In-beam single particle tomography (WP 3) Detectors for in-beam SPECT: Compton cameras E 1 g-ray source j 1 E 2 some Compton E 3 j 2 Scatterer and e--detectors Compton - Efficiency: 25 – 50 % for E 1 > 1 Me. V - No absorption necessary - No high Z absorber required J. D. Kurfess et al. , 5 th Compton Symposium, Portsmouth, New Hampshire, AIP Conference Proceedings, 1999
4. In-beam single particle tomography (WP 3) Detectors for in-beam SPECT: Compton cameras E 1 g-ray source j 1 E 2 some Compton E 3 j 2 Scatterer and e--detectors Compton - Efficiency: 25 – 50 % for E 1 > 1 Me. V - No absorption necessary - No high Z absorber required J. D. Kurfess et al. , 5 th Compton Symposium, Portsmouth, New Hampshire, AIP Conference Proceedings, 1999
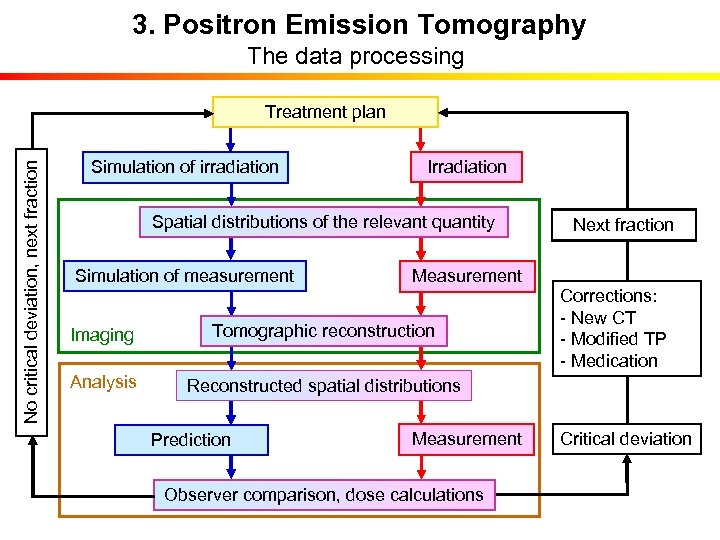 3. Positron Emission Tomography The data processing No critical deviation, next fraction Treatment plan Simulation of irradiation Irradiation Spatial distributions of the relevant quantity Simulation of measurement Measurement Imaging Tomographic reconstruction Analysis Next fraction Corrections: - New CT - Modified TP - Medication Reconstructed spatial distributions Prediction Measurement Observer comparison, dose calculations Critical deviation
3. Positron Emission Tomography The data processing No critical deviation, next fraction Treatment plan Simulation of irradiation Irradiation Spatial distributions of the relevant quantity Simulation of measurement Measurement Imaging Tomographic reconstruction Analysis Next fraction Corrections: - New CT - Modified TP - Medication Reconstructed spatial distributions Prediction Measurement Observer comparison, dose calculations Critical deviation
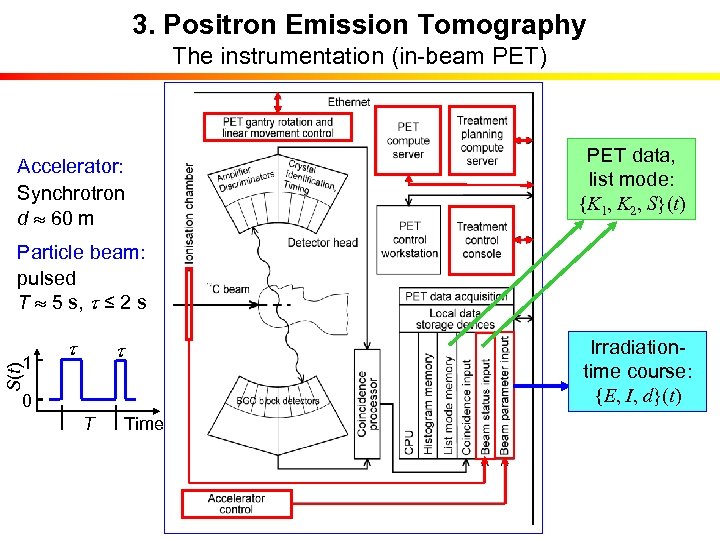 3. Positron Emission Tomography The instrumentation (in-beam PET) PET data, list mode: {K 1, K 2, S}(t) Accelerator: Synchrotron d 60 m Particle beam: pulsed T 5 s, t ≤ 2 s t S(t) 1 t 0 T Time Irradiationtime course: {E, I, d}(t)
3. Positron Emission Tomography The instrumentation (in-beam PET) PET data, list mode: {K 1, K 2, S}(t) Accelerator: Synchrotron d 60 m Particle beam: pulsed T 5 s, t ≤ 2 s t S(t) 1 t 0 T Time Irradiationtime course: {E, I, d}(t)
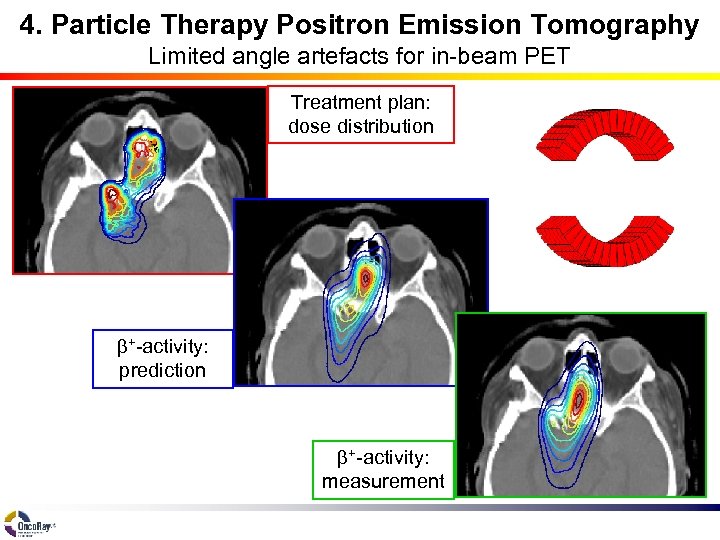 4. Particle Therapy Positron Emission Tomography Limited angle artefacts for in-beam PET Treatment plan: dose distribution b+-activity: prediction b+-activity: measurement
4. Particle Therapy Positron Emission Tomography Limited angle artefacts for in-beam PET Treatment plan: dose distribution b+-activity: prediction b+-activity: measurement
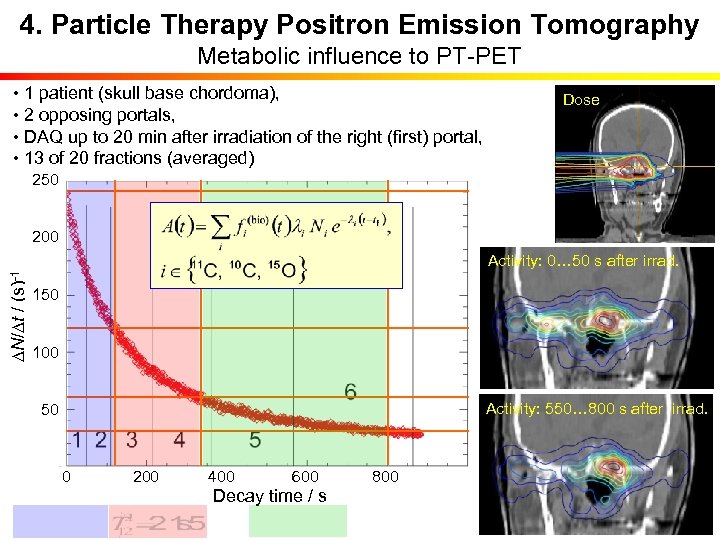 4. Particle Therapy Positron Emission Tomography Metabolic influence to PT-PET • 1 patient (skull base chordoma), • 2 opposing portals, • DAQ up to 20 min after irradiation of the right (first) portal, • 13 of 20 fractions (averaged) Dose 250 200 DN/Dt / (s)-1 Activity: 0… 50 s after irrad. 150 100 Activity: 550… 800 s after irrad. 50 0 200 400 600 800 Decay time / s
4. Particle Therapy Positron Emission Tomography Metabolic influence to PT-PET • 1 patient (skull base chordoma), • 2 opposing portals, • DAQ up to 20 min after irradiation of the right (first) portal, • 13 of 20 fractions (averaged) Dose 250 200 DN/Dt / (s)-1 Activity: 0… 50 s after irrad. 150 100 Activity: 550… 800 s after irrad. 50 0 200 400 600 800 Decay time / s
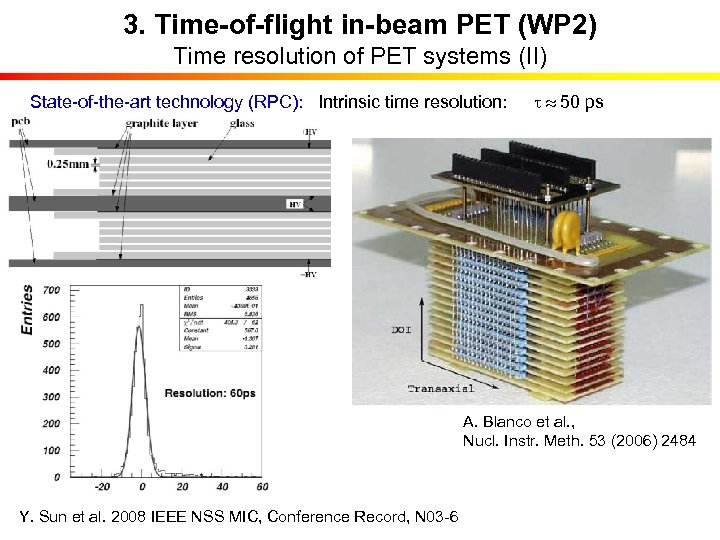 3. Time-of-flight in-beam PET (WP 2) Time resolution of PET systems (II) State-of-the-art technology (RPC): Intrinsic time resolution: t 50 ps A. Blanco et al. , Nucl. Instr. Meth. 53 (2006) 2484 Y. Sun et al. 2008 IEEE NSS MIC, Conference Record, N 03 -6
3. Time-of-flight in-beam PET (WP 2) Time resolution of PET systems (II) State-of-the-art technology (RPC): Intrinsic time resolution: t 50 ps A. Blanco et al. , Nucl. Instr. Meth. 53 (2006) 2484 Y. Sun et al. 2008 IEEE NSS MIC, Conference Record, N 03 -6


