55e0210bf7635464959793b05d11cf31.ppt
- Количество слайдов: 85
 Diagnosis and Management of Craniofacial Pain Richard K. Osenbach, M. D. Director, Neurosurgical Services Cape Fear Valley Medical Center
Diagnosis and Management of Craniofacial Pain Richard K. Osenbach, M. D. Director, Neurosurgical Services Cape Fear Valley Medical Center
 Key Points All facial pain IS NOT trigeminal neuralgia There are no tests for trigeminal neuralgia or for that matter most causes of facial pain The wrong diagnosis can lead to the wrong treatment Despite all the advancements in medicine, it is not possible to cure all pain problems
Key Points All facial pain IS NOT trigeminal neuralgia There are no tests for trigeminal neuralgia or for that matter most causes of facial pain The wrong diagnosis can lead to the wrong treatment Despite all the advancements in medicine, it is not possible to cure all pain problems
 Approach to the patient with craniofacial pain Specific pain syndromes Pharmacological Management Surgical Treatments
Approach to the patient with craniofacial pain Specific pain syndromes Pharmacological Management Surgical Treatments
 Approach to the Patient with Craniofacial Pain Single most important aspect is to ESTABLISH THE CORRECT DIAGNOSIS Careful detailed pain history Location Duration Temporal characteristics Quality Severity Circumstances of onset Influencing factors Neurological symptoms Response to medications The more paroxysmal the pain, the more likely that surgery may be beneficial
Approach to the Patient with Craniofacial Pain Single most important aspect is to ESTABLISH THE CORRECT DIAGNOSIS Careful detailed pain history Location Duration Temporal characteristics Quality Severity Circumstances of onset Influencing factors Neurological symptoms Response to medications The more paroxysmal the pain, the more likely that surgery may be beneficial
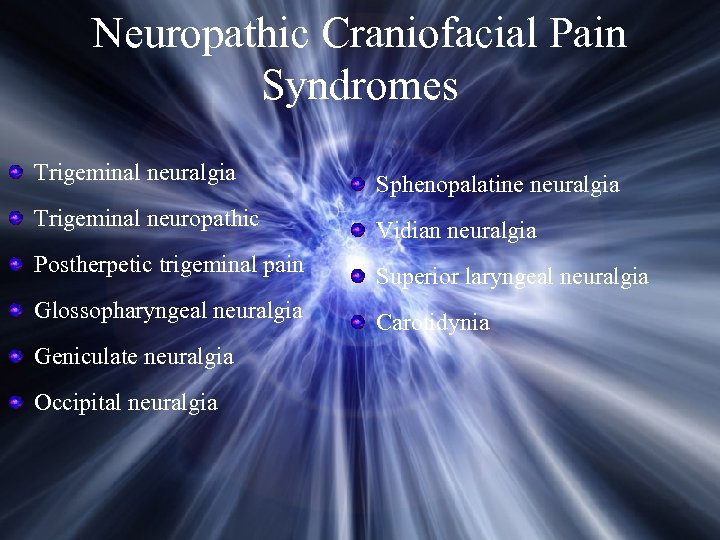 Neuropathic Craniofacial Pain Syndromes Trigeminal neuralgia Sphenopalatine neuralgia Trigeminal neuropathic Vidian neuralgia Postherpetic trigeminal pain Superior laryngeal neuralgia Glossopharyngeal neuralgia Carotidynia Geniculate neuralgia Occipital neuralgia
Neuropathic Craniofacial Pain Syndromes Trigeminal neuralgia Sphenopalatine neuralgia Trigeminal neuropathic Vidian neuralgia Postherpetic trigeminal pain Superior laryngeal neuralgia Glossopharyngeal neuralgia Carotidynia Geniculate neuralgia Occipital neuralgia
 Headache Syndromes Classic migraine Common migraine Migraine variants Chronic daily headache Cluster headache Muscle tension headache Post-traumatic headache Chronic paroxysmal hemicrania Headache caused by other disorders Eg. Brain tumor, hydrocephalus, etc.
Headache Syndromes Classic migraine Common migraine Migraine variants Chronic daily headache Cluster headache Muscle tension headache Post-traumatic headache Chronic paroxysmal hemicrania Headache caused by other disorders Eg. Brain tumor, hydrocephalus, etc.
 Ocular and Periocular Disorders Tolosa-Hunt Syndrome Raeder’s paratrigeminal syndrome Orbital apex syndrome Cavernous sinus syndrome Parasellar syndrome Corneal pathology Angle closure glaucoma Optic neuritis Orbital cellulits
Ocular and Periocular Disorders Tolosa-Hunt Syndrome Raeder’s paratrigeminal syndrome Orbital apex syndrome Cavernous sinus syndrome Parasellar syndrome Corneal pathology Angle closure glaucoma Optic neuritis Orbital cellulits
 Otologic Problems Otitis externa and interna Ramsey-Hunt Syndrome Bullous myringitis Tumors Mastoiditis
Otologic Problems Otitis externa and interna Ramsey-Hunt Syndrome Bullous myringitis Tumors Mastoiditis
 Dental and Periodontal Pathology Periodontal abscess Bruxism Burning mouth syndrome Temporomandibular joint disorders
Dental and Periodontal Pathology Periodontal abscess Bruxism Burning mouth syndrome Temporomandibular joint disorders
 What’s The Point? SUCCESSFUL TREATMENT DEPENDS ON MAKING THE CORRECT DIAGNOSIS
What’s The Point? SUCCESSFUL TREATMENT DEPENDS ON MAKING THE CORRECT DIAGNOSIS
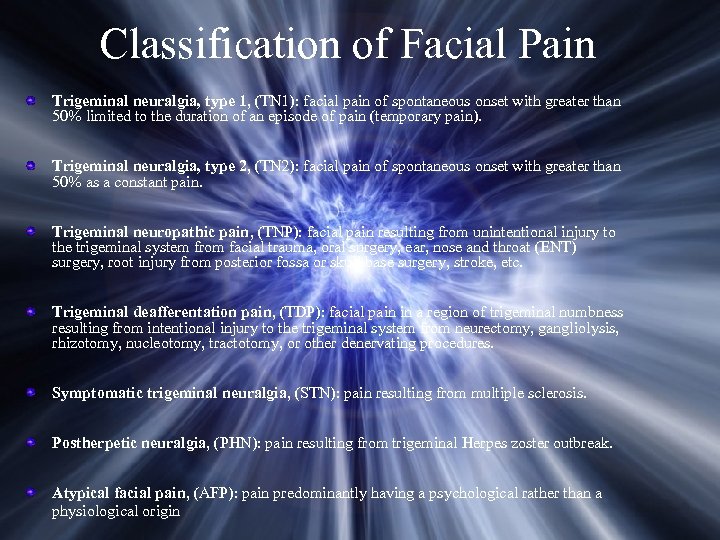 Classification of Facial Pain Trigeminal neuralgia, type 1, (TN 1): facial pain of spontaneous onset with greater than 50% limited to the duration of an episode of pain (temporary pain). Trigeminal neuralgia, type 2, (TN 2): facial pain of spontaneous onset with greater than 50% as a constant pain. Trigeminal neuropathic pain, (TNP): facial pain resulting from unintentional injury to the trigeminal system from facial trauma, oral surgery, ear, nose and throat (ENT) surgery, root injury from posterior fossa or skull base surgery, stroke, etc. Trigeminal deafferentation pain, (TDP): facial pain in a region of trigeminal numbness resulting from intentional injury to the trigeminal system from neurectomy, gangliolysis, rhizotomy, nucleotomy, tractotomy, or other denervating procedures. Symptomatic trigeminal neuralgia, (STN): pain resulting from multiple sclerosis. Postherpetic neuralgia, (PHN): pain resulting from trigeminal Herpes zoster outbreak. Atypical facial pain, (AFP): pain predominantly having a psychological rather than a physiological origin
Classification of Facial Pain Trigeminal neuralgia, type 1, (TN 1): facial pain of spontaneous onset with greater than 50% limited to the duration of an episode of pain (temporary pain). Trigeminal neuralgia, type 2, (TN 2): facial pain of spontaneous onset with greater than 50% as a constant pain. Trigeminal neuropathic pain, (TNP): facial pain resulting from unintentional injury to the trigeminal system from facial trauma, oral surgery, ear, nose and throat (ENT) surgery, root injury from posterior fossa or skull base surgery, stroke, etc. Trigeminal deafferentation pain, (TDP): facial pain in a region of trigeminal numbness resulting from intentional injury to the trigeminal system from neurectomy, gangliolysis, rhizotomy, nucleotomy, tractotomy, or other denervating procedures. Symptomatic trigeminal neuralgia, (STN): pain resulting from multiple sclerosis. Postherpetic neuralgia, (PHN): pain resulting from trigeminal Herpes zoster outbreak. Atypical facial pain, (AFP): pain predominantly having a psychological rather than a physiological origin
 Pharmacological Therapy Anti-epileptics drugs (AEDs) Antidepressant medications Opiates Neuroleptics Antispasmodics Miscellaneous drugs Botox
Pharmacological Therapy Anti-epileptics drugs (AEDs) Antidepressant medications Opiates Neuroleptics Antispasmodics Miscellaneous drugs Botox
 General Principles of Pharmacological Management Rule out surgical lesions (tumor, etc. ) Neuropathic vs. nociceptive? Develop a strategy Lay out a plan Conservative initial dosing to avoid side effects Monotherapy is preferable if possible Escalate dose to effect or toxicity If second drug needed, choose agent in different class Na+ channel blcoker, GABA agonist, etc.
General Principles of Pharmacological Management Rule out surgical lesions (tumor, etc. ) Neuropathic vs. nociceptive? Develop a strategy Lay out a plan Conservative initial dosing to avoid side effects Monotherapy is preferable if possible Escalate dose to effect or toxicity If second drug needed, choose agent in different class Na+ channel blcoker, GABA agonist, etc.
 Antiepileptic Agents Tegretol (carbamazepine) Topamax (topirimate) Trileptal (oxcarbazepine) Lamictal (lamotrigene) Neurontin (gabpentin) Keppra (levateracitam) Lyrica (pregabalin) Gabatril Dilantin (phenytoin) Benzodiazepines Depakote (valproic acid)
Antiepileptic Agents Tegretol (carbamazepine) Topamax (topirimate) Trileptal (oxcarbazepine) Lamictal (lamotrigene) Neurontin (gabpentin) Keppra (levateracitam) Lyrica (pregabalin) Gabatril Dilantin (phenytoin) Benzodiazepines Depakote (valproic acid)
 Antiepileptic Drugs (AEDS) Similarities in pathophysiology of neuropathic pain and epilepsy All AEDS ultimately act on ion channels Efficacy of AEDS most clearly established for neuropathic conditions characterized by episodic lancinating pain Most clinical studies have focused on DPN and PHN Use of AEDS in patients with FBSS is nearly entirely empiric
Antiepileptic Drugs (AEDS) Similarities in pathophysiology of neuropathic pain and epilepsy All AEDS ultimately act on ion channels Efficacy of AEDS most clearly established for neuropathic conditions characterized by episodic lancinating pain Most clinical studies have focused on DPN and PHN Use of AEDS in patients with FBSS is nearly entirely empiric
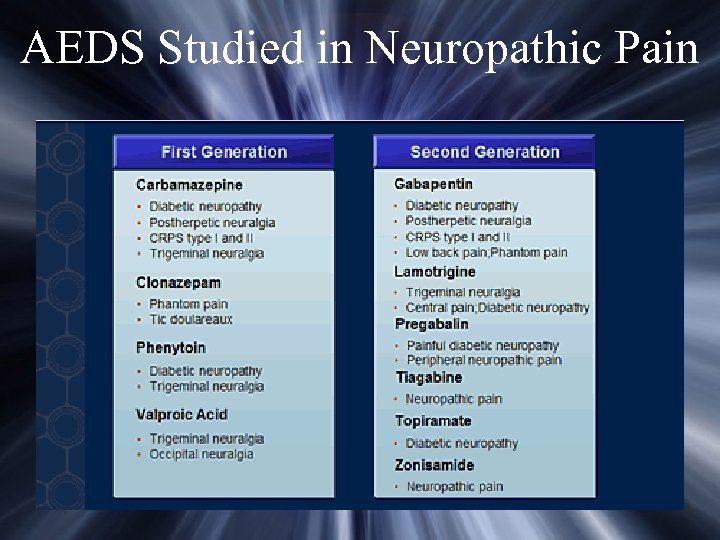 AEDS Studied in Neuropathic Pain
AEDS Studied in Neuropathic Pain
 Mechanisms of Selected AEDS Carbamazepine (Tegretol) Modulates voltage-gated Na+ channels Reduces spontaneous activity in experimental neuromas Inhibits NE uptake; promotes endogenous descending inhibitory mechanisms Oxcarbazepine (Trileptal) Modulates Na+ and Ca+2 channels, incease K+ conductance Lacks toxicity of epoxide metabolites Lamotrigine Blocks voltage-gated Na+ channels Inhibits glutamate release from pre-synaptic neurons Gabapentin (Neurontin) Structural analog of GABA Binds to voltage-dependent calcium channels Inhibits EAA release; Interacts with NMDA receptor at glycine site Pregabalin (Lyrica) Binds to voltage-gated calcium channels
Mechanisms of Selected AEDS Carbamazepine (Tegretol) Modulates voltage-gated Na+ channels Reduces spontaneous activity in experimental neuromas Inhibits NE uptake; promotes endogenous descending inhibitory mechanisms Oxcarbazepine (Trileptal) Modulates Na+ and Ca+2 channels, incease K+ conductance Lacks toxicity of epoxide metabolites Lamotrigine Blocks voltage-gated Na+ channels Inhibits glutamate release from pre-synaptic neurons Gabapentin (Neurontin) Structural analog of GABA Binds to voltage-dependent calcium channels Inhibits EAA release; Interacts with NMDA receptor at glycine site Pregabalin (Lyrica) Binds to voltage-gated calcium channels
 Adverse Effects of AEDs Allergic reaction Up to 7% with CBZ Some cross-reactivity between CBZ and PHT Cognitive changes Sedation Nystagmus, ataxia, diplopia, dizziness Nausea, vomiting, headache
Adverse Effects of AEDs Allergic reaction Up to 7% with CBZ Some cross-reactivity between CBZ and PHT Cognitive changes Sedation Nystagmus, ataxia, diplopia, dizziness Nausea, vomiting, headache
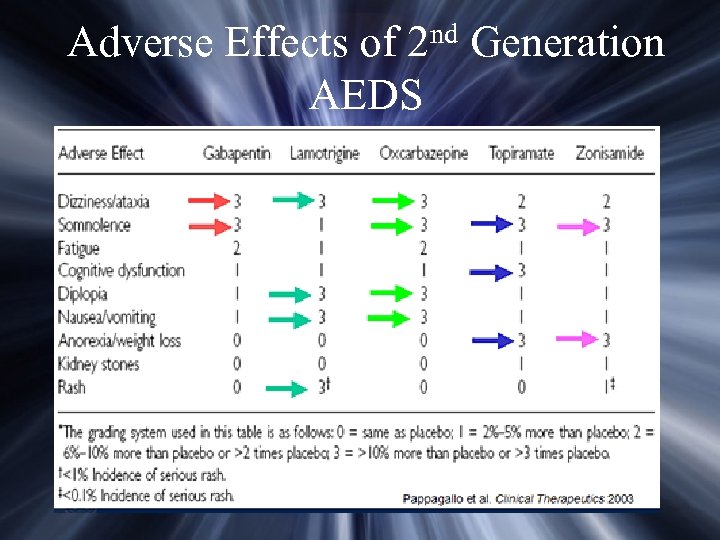 nd 2 Adverse Effects of AEDS Generation
nd 2 Adverse Effects of AEDS Generation
 Antidepressant Analgesics “The results suggest to us that antidepressants may have an analgesic action which is independent of their mood-altering effects” Merskey & Hester 1972
Antidepressant Analgesics “The results suggest to us that antidepressants may have an analgesic action which is independent of their mood-altering effects” Merskey & Hester 1972
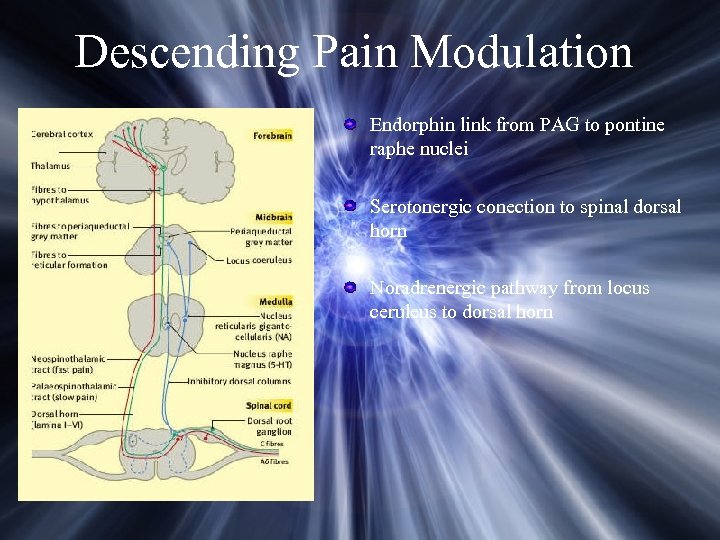 Descending Pain Modulation Endorphin link from PAG to pontine raphe nuclei Serotonergic conection to spinal dorsal horn Noradrenergic pathway from locus ceruleus to dorsal horn
Descending Pain Modulation Endorphin link from PAG to pontine raphe nuclei Serotonergic conection to spinal dorsal horn Noradrenergic pathway from locus ceruleus to dorsal horn
 Antidepressant Analgesics Current Evidence Relieves all components of neuropathic pain RCT - clear separation of analgesic and antidepressant effects Although other agents (eg anti-epileptics)) may be regarded as 1 st line therapy over antidepressants, there is no good evidence for this practice More selective agents are either less effective or not useful (serotonergic, noradrenergic) Because of incomplete efficacy, combination therapy may be needed Comparative data regarding other drugs using NNT figures now exists
Antidepressant Analgesics Current Evidence Relieves all components of neuropathic pain RCT - clear separation of analgesic and antidepressant effects Although other agents (eg anti-epileptics)) may be regarded as 1 st line therapy over antidepressants, there is no good evidence for this practice More selective agents are either less effective or not useful (serotonergic, noradrenergic) Because of incomplete efficacy, combination therapy may be needed Comparative data regarding other drugs using NNT figures now exists
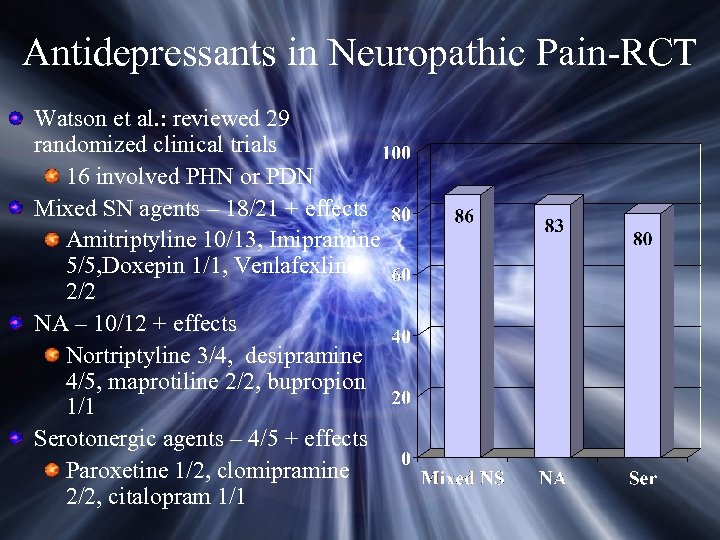 Antidepressants in Neuropathic Pain-RCT Watson et al. : reviewed 29 randomized clinical trials 16 involved PHN or PDN Mixed SN agents – 18/21 + effects Amitriptyline 10/13, Imipramine 5/5, Doxepin 1/1, Venlafexline 2/2 NA – 10/12 + effects Nortriptyline 3/4, desipramine 4/5, maprotiline 2/2, bupropion 1/1 Serotonergic agents – 4/5 + effects Paroxetine 1/2, clomipramine 2/2, citalopram 1/1
Antidepressants in Neuropathic Pain-RCT Watson et al. : reviewed 29 randomized clinical trials 16 involved PHN or PDN Mixed SN agents – 18/21 + effects Amitriptyline 10/13, Imipramine 5/5, Doxepin 1/1, Venlafexline 2/2 NA – 10/12 + effects Nortriptyline 3/4, desipramine 4/5, maprotiline 2/2, bupropion 1/1 Serotonergic agents – 4/5 + effects Paroxetine 1/2, clomipramine 2/2, citalopram 1/1
 Adverse Effect of Antidepressants Anti-cholinergic autonomic effects (TCAs) Allergic and hypresensitivity reactions Cardiovascular effects Orthostatic hypotension (avoid imipramine in elderly) Quinidine-like cardiac effects CNS effects Sedation, tremor, seizures, atropine-like delerium, exacerbation of schizophrenia/mania Acute overdose may be fatal (>2000 mg) Withdrawal reactions
Adverse Effect of Antidepressants Anti-cholinergic autonomic effects (TCAs) Allergic and hypresensitivity reactions Cardiovascular effects Orthostatic hypotension (avoid imipramine in elderly) Quinidine-like cardiac effects CNS effects Sedation, tremor, seizures, atropine-like delerium, exacerbation of schizophrenia/mania Acute overdose may be fatal (>2000 mg) Withdrawal reactions
 Guidelines for Use of Antidepressants in Pain Management Eliminate all other ineffective analgesics Start low and titrate slowly to effect or toxicity Nortriptyline or amitriptyline for initial treatment Move to agents with more noradrenergic effects Consider trazadone in patients with poor sleep pattern Try more selective agents if mixed agents ineffective Do NOT prescribe monoamine oxidase inhibitors Tolerance to anti-muscarinic side effects usually takes weeks to develop Withdraw therapy gradually to avoid withdrawal syndrome
Guidelines for Use of Antidepressants in Pain Management Eliminate all other ineffective analgesics Start low and titrate slowly to effect or toxicity Nortriptyline or amitriptyline for initial treatment Move to agents with more noradrenergic effects Consider trazadone in patients with poor sleep pattern Try more selective agents if mixed agents ineffective Do NOT prescribe monoamine oxidase inhibitors Tolerance to anti-muscarinic side effects usually takes weeks to develop Withdraw therapy gradually to avoid withdrawal syndrome
 Opioids for Chronic Non-Malignant Pain Well-established and accepted for acute/cancer pain Extrapolation of outcomes to non-malignant pain flawed Information is more anecdotal, contradictory, philosophical, and/or emotional than scientific Limited number of well-designed RCT with inconclusive results Reduction in pain scores of around 20% without major benefits on function or psychological outcomes
Opioids for Chronic Non-Malignant Pain Well-established and accepted for acute/cancer pain Extrapolation of outcomes to non-malignant pain flawed Information is more anecdotal, contradictory, philosophical, and/or emotional than scientific Limited number of well-designed RCT with inconclusive results Reduction in pain scores of around 20% without major benefits on function or psychological outcomes
 Principles of Opioid Therapy in Chronic Non-Malignant Pain Opioids provide analgesic benefit for a selected subpopulation of patients Less evidence exists regarding improvement in function Benefits outweigh risks in well-selected patients Most benefit in patients with pain from established nociceptive/neuropathic conditions Identification of other appropriate patients is problematic, and valid diagnostic criteria do not exist
Principles of Opioid Therapy in Chronic Non-Malignant Pain Opioids provide analgesic benefit for a selected subpopulation of patients Less evidence exists regarding improvement in function Benefits outweigh risks in well-selected patients Most benefit in patients with pain from established nociceptive/neuropathic conditions Identification of other appropriate patients is problematic, and valid diagnostic criteria do not exist
 Implementation of Opioid Therapy Prerequisites Failure of pain management alternatives; but not a last resort Opioids should only be use as part of a multimodality approach Identification of realistic goals of treatment Physical and psychosocial assessment by multidisciplinary team Consider history of substance abuse as a relative contraindication Decision to prescribe by multidisciplinary team or at least two practitioners Informed written consent Best practice – prescribe a trial of opioids and withdraw use if the provision of analgesia does not result in functional improvement
Implementation of Opioid Therapy Prerequisites Failure of pain management alternatives; but not a last resort Opioids should only be use as part of a multimodality approach Identification of realistic goals of treatment Physical and psychosocial assessment by multidisciplinary team Consider history of substance abuse as a relative contraindication Decision to prescribe by multidisciplinary team or at least two practitioners Informed written consent Best practice – prescribe a trial of opioids and withdraw use if the provision of analgesia does not result in functional improvement
 Implementation of Opioid Therapy Therapeutic Trial Period Appropriate oral or transdermal drug selection Defined trial period with regular assessment and review Opioid dose adjustment or rotation as needed Decision for long-term treatment predicated upon demonstration of pain relief and/or functional improvement
Implementation of Opioid Therapy Therapeutic Trial Period Appropriate oral or transdermal drug selection Defined trial period with regular assessment and review Opioid dose adjustment or rotation as needed Decision for long-term treatment predicated upon demonstration of pain relief and/or functional improvement
 Implementation of Opioid Therapy Long-Term Therapy Opioid contract Single defined prescriber Regular assessment and review Routine urine and serum drug screen Ongoing effort to improve physical, psychological, and social function as a result of pain relief Continued multidisciplinary approach to pain Defined responses to psychosocial or behavioral problems (addiction, diversion, etc)
Implementation of Opioid Therapy Long-Term Therapy Opioid contract Single defined prescriber Regular assessment and review Routine urine and serum drug screen Ongoing effort to improve physical, psychological, and social function as a result of pain relief Continued multidisciplinary approach to pain Defined responses to psychosocial or behavioral problems (addiction, diversion, etc)
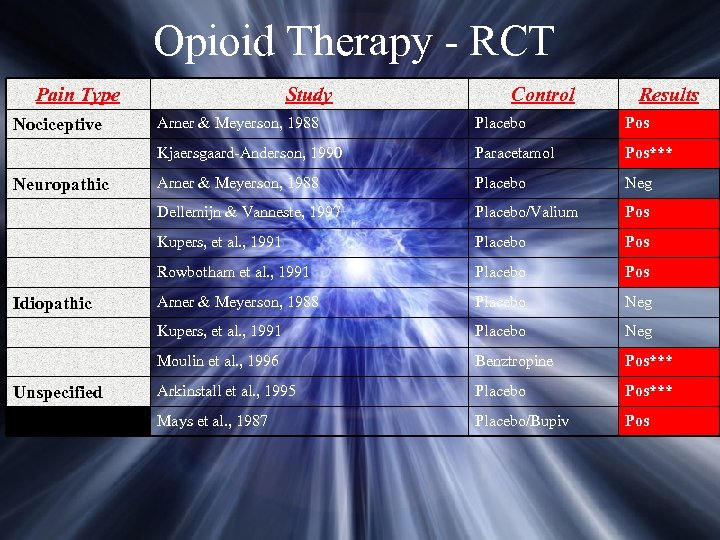 Opioid Therapy - RCT Pain Type Study Control Results Paracetamol Pos*** Arner & Meyerson, 1988 Placebo Neg Placebo/Valium Pos Placebo Pos Rowbotham et al. , 1991 Placebo Pos Arner & Meyerson, 1988 Placebo Neg Kupers, et al. , 1991 Placebo Neg Moulin et al. , 1996 Unspecified Pos Kupers, et al. , 1991 Idiopathic Placebo Dellemijn & Vanneste, 1997 Neuropathic Arner & Meyerson, 1988 Kjaersgaard-Anderson, 1990 Nociceptive Benztropine Pos*** Arkinstall et al. , 1995 Placebo Pos*** Mays et al. , 1987 Placebo/Bupiv Pos
Opioid Therapy - RCT Pain Type Study Control Results Paracetamol Pos*** Arner & Meyerson, 1988 Placebo Neg Placebo/Valium Pos Placebo Pos Rowbotham et al. , 1991 Placebo Pos Arner & Meyerson, 1988 Placebo Neg Kupers, et al. , 1991 Placebo Neg Moulin et al. , 1996 Unspecified Pos Kupers, et al. , 1991 Idiopathic Placebo Dellemijn & Vanneste, 1997 Neuropathic Arner & Meyerson, 1988 Kjaersgaard-Anderson, 1990 Nociceptive Benztropine Pos*** Arkinstall et al. , 1995 Placebo Pos*** Mays et al. , 1987 Placebo/Bupiv Pos
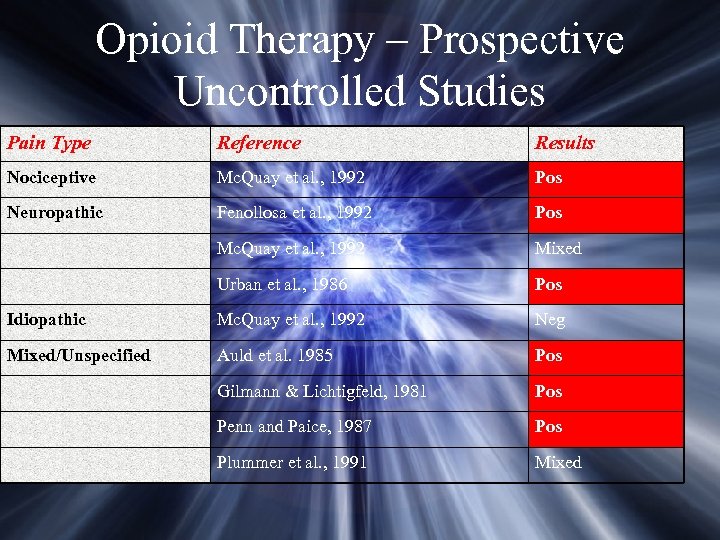 Opioid Therapy – Prospective Uncontrolled Studies Pain Type Reference Results Nociceptive Mc. Quay et al. , 1992 Pos Neuropathic Fenollosa et al. , 1992 Pos Mc. Quay et al. , 1992 Mixed Urban et al. , 1986 Pos Idiopathic Mc. Quay et al. , 1992 Neg Mixed/Unspecified Auld et al. 1985 Pos Gilmann & Lichtigfeld, 1981 Pos Penn and Paice, 1987 Pos Plummer et al. , 1991 Mixed
Opioid Therapy – Prospective Uncontrolled Studies Pain Type Reference Results Nociceptive Mc. Quay et al. , 1992 Pos Neuropathic Fenollosa et al. , 1992 Pos Mc. Quay et al. , 1992 Mixed Urban et al. , 1986 Pos Idiopathic Mc. Quay et al. , 1992 Neg Mixed/Unspecified Auld et al. 1985 Pos Gilmann & Lichtigfeld, 1981 Pos Penn and Paice, 1987 Pos Plummer et al. , 1991 Mixed
 Adverse Effects of Opioids Common Nausea/vomiting Constipation Urinary retention Sedation Cognitive impairment Pruritis Occasional Hallucinations Myoclonus Mood changes Anxiety Rigidity Dry mouth Gastric stasis Bronchoconstriction Rare Respiratory dep. Seizures Delerium Hyperalgesia Allodynia Tolerance, Physical Dependence, Addiction
Adverse Effects of Opioids Common Nausea/vomiting Constipation Urinary retention Sedation Cognitive impairment Pruritis Occasional Hallucinations Myoclonus Mood changes Anxiety Rigidity Dry mouth Gastric stasis Bronchoconstriction Rare Respiratory dep. Seizures Delerium Hyperalgesia Allodynia Tolerance, Physical Dependence, Addiction
 Miscellaneous Agents Antiarrhythmics - Mexilitene Na+ channel blockade Reduce neuronal hyperexcitability Possible predictive effect of IV lidocaine challenge May worsen AV conduction block Monitor EKG, LFT, renal fxn Significant incidence of treatment-limiting side effects Baclofen GABAB receptor antagonist Efficacious in TN Start 10 mg QD and titrate until effect or sedation Cannot abruptly withdraw drug!
Miscellaneous Agents Antiarrhythmics - Mexilitene Na+ channel blockade Reduce neuronal hyperexcitability Possible predictive effect of IV lidocaine challenge May worsen AV conduction block Monitor EKG, LFT, renal fxn Significant incidence of treatment-limiting side effects Baclofen GABAB receptor antagonist Efficacious in TN Start 10 mg QD and titrate until effect or sedation Cannot abruptly withdraw drug!
 Trigeminal Branch Stimulation
Trigeminal Branch Stimulation
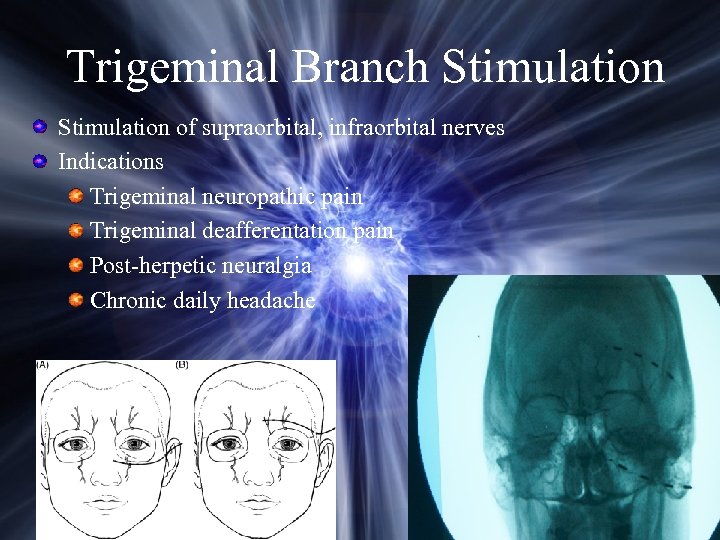 Trigeminal Branch Stimulation of supraorbital, infraorbital nerves Indications Trigeminal neuropathic pain Trigeminal deafferentation pain Post-herpetic neuralgia Chronic daily headache
Trigeminal Branch Stimulation of supraorbital, infraorbital nerves Indications Trigeminal neuropathic pain Trigeminal deafferentation pain Post-herpetic neuralgia Chronic daily headache
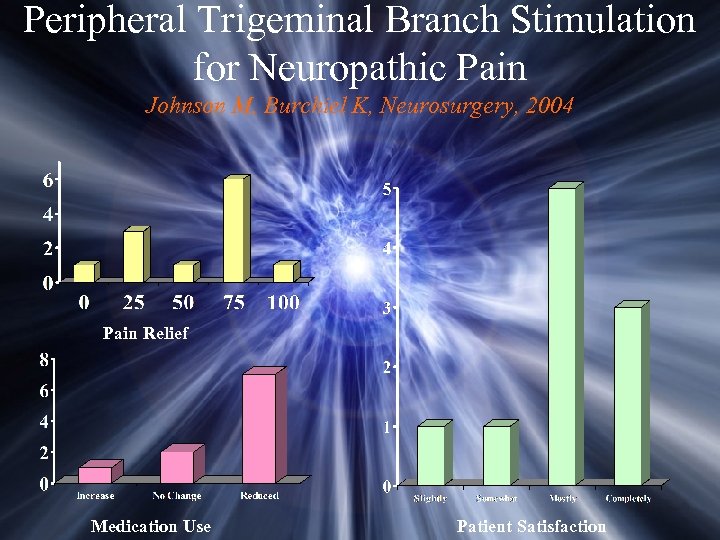 Peripheral Trigeminal Branch Stimulation for Neuropathic Pain Johnson M, Burchiel K, Neurosurgery, 2004 Pain Relief Medication Use Patient Satisfaction
Peripheral Trigeminal Branch Stimulation for Neuropathic Pain Johnson M, Burchiel K, Neurosurgery, 2004 Pain Relief Medication Use Patient Satisfaction
 Peripheral Trigeminal Branch Stimulation for Neuropathic Pain Effective for trigeminal neuropathic pain Less effective for PHN Simple, low morbidity Pain relief seems relatively durable Major problem is erosion of connector
Peripheral Trigeminal Branch Stimulation for Neuropathic Pain Effective for trigeminal neuropathic pain Less effective for PHN Simple, low morbidity Pain relief seems relatively durable Major problem is erosion of connector
 Motor Cortex Stimulation Motor cortex stimulation is NOT FDA approved and represents an off-label use of the implanted device
Motor Cortex Stimulation Motor cortex stimulation is NOT FDA approved and represents an off-label use of the implanted device
 History of MCS Developed by Tsubokawa and colleagues during 1980 s Treatment of central deafferentation pain Poststroke pain Thalamic pain Bulbar pain Alternative to other methods of neuromodulation for SCS DBS Discovered that stimulation of motor rather than sensory cortex produced better pain relief
History of MCS Developed by Tsubokawa and colleagues during 1980 s Treatment of central deafferentation pain Poststroke pain Thalamic pain Bulbar pain Alternative to other methods of neuromodulation for SCS DBS Discovered that stimulation of motor rather than sensory cortex produced better pain relief
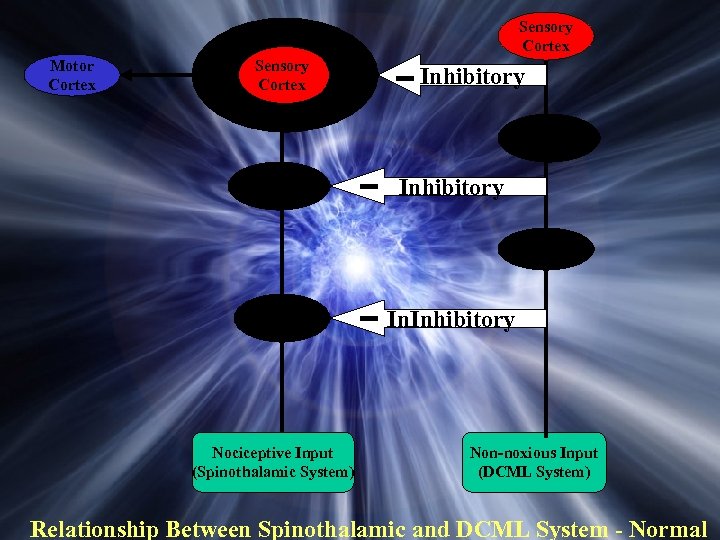 Sensory Cortex Motor Cortex Sensory Cortex Inhibitory Thalamus Inhibitory DCN Dorsal Horn Nociceptive Input (Spinothalamic System) In. Inhibitory Non-noxious Input (DCML System) Relationship Between Spinothalamic and DCML System - Normal
Sensory Cortex Motor Cortex Sensory Cortex Inhibitory Thalamus Inhibitory DCN Dorsal Horn Nociceptive Input (Spinothalamic System) In. Inhibitory Non-noxious Input (DCML System) Relationship Between Spinothalamic and DCML System - Normal
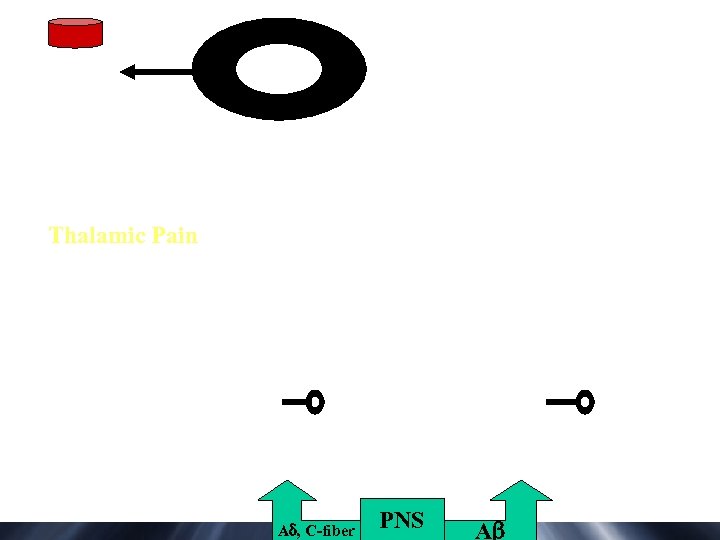 Thalamic Pain A , C-fiber PNS A
Thalamic Pain A , C-fiber PNS A
 Motor Cortex Stimulation Clinical Indications Post-stroke pain Post-herpetic neuralgia Trigeminal neuropathic pain Trigeminal deafferentation pain
Motor Cortex Stimulation Clinical Indications Post-stroke pain Post-herpetic neuralgia Trigeminal neuropathic pain Trigeminal deafferentation pain
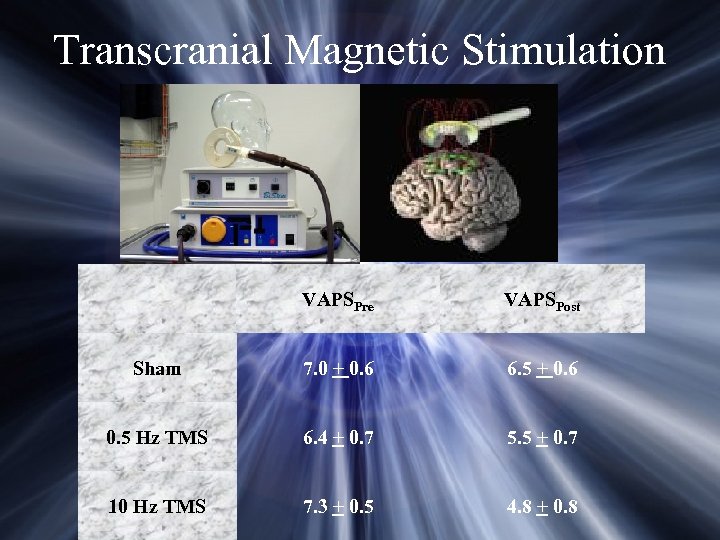 Transcranial Magnetic Stimulation VAPSPre VAPSPost Sham 7. 0 + 0. 6 6. 5 + 0. 6 0. 5 Hz TMS 6. 4 + 0. 7 5. 5 + 0. 7 10 Hz TMS 7. 3 + 0. 5 4. 8 + 0. 8
Transcranial Magnetic Stimulation VAPSPre VAPSPost Sham 7. 0 + 0. 6 6. 5 + 0. 6 0. 5 Hz TMS 6. 4 + 0. 7 5. 5 + 0. 7 10 Hz TMS 7. 3 + 0. 5 4. 8 + 0. 8
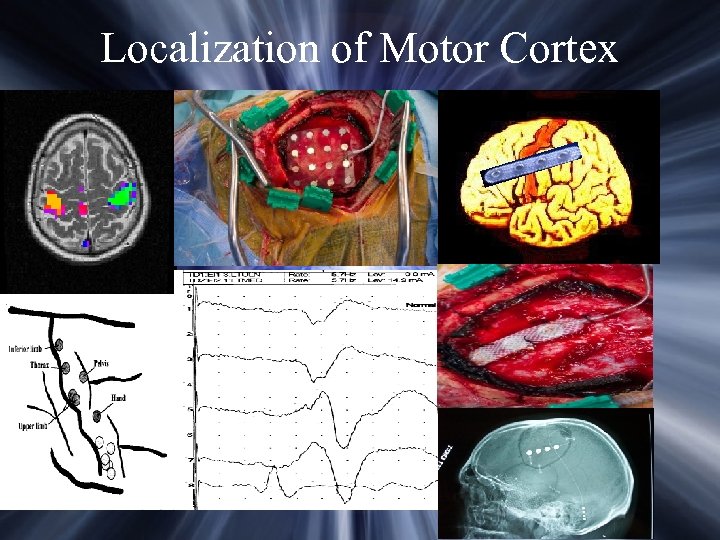 Localization of Motor Cortex
Localization of Motor Cortex
 Complications Stimulation-induced seizures Pain at stimulation site Epidural hematoma CSF leak Electrode fracture or migration Infection
Complications Stimulation-induced seizures Pain at stimulation site Epidural hematoma CSF leak Electrode fracture or migration Infection
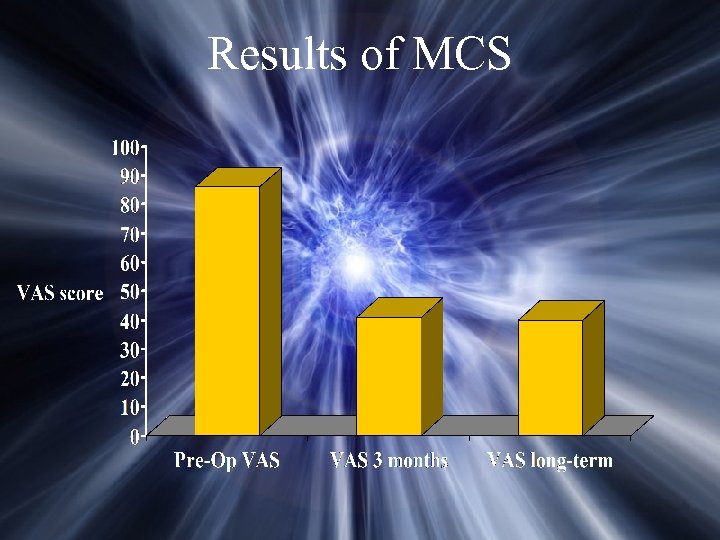
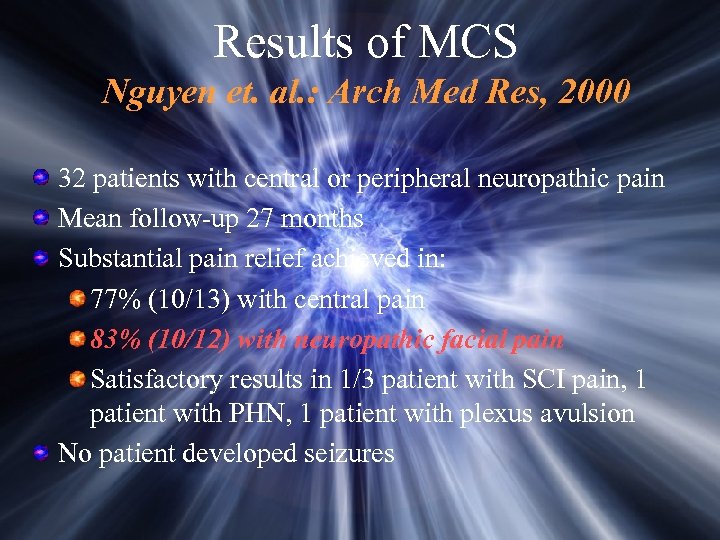 Results of MCS Nguyen et. al. : Arch Med Res, 2000 32 patients with central or peripheral neuropathic pain Mean follow-up 27 months Substantial pain relief achieved in: 77% (10/13) with central pain 83% (10/12) with neuropathic facial pain Satisfactory results in 1/3 patient with SCI pain, 1 patient with PHN, 1 patient with plexus avulsion No patient developed seizures
Results of MCS Nguyen et. al. : Arch Med Res, 2000 32 patients with central or peripheral neuropathic pain Mean follow-up 27 months Substantial pain relief achieved in: 77% (10/13) with central pain 83% (10/12) with neuropathic facial pain Satisfactory results in 1/3 patient with SCI pain, 1 patient with PHN, 1 patient with plexus avulsion No patient developed seizures
 Results of MCS
Results of MCS
 Unanswered Questions What are the best indications for MCS? What is the value of preoperative pharmacological testing? Is there a predictive value to TMS? What is the optimum electrode location? Is there any value to using multiple electrodes? Are there optimum stimulation parameters? How often should stimulation be applied and for how long? Can long-term reduction in pain be explained by adaptation of the brain to chronic stimulation?
Unanswered Questions What are the best indications for MCS? What is the value of preoperative pharmacological testing? Is there a predictive value to TMS? What is the optimum electrode location? Is there any value to using multiple electrodes? Are there optimum stimulation parameters? How often should stimulation be applied and for how long? Can long-term reduction in pain be explained by adaptation of the brain to chronic stimulation?
 Deep Brain Stimulation Deep brain stimulation is NOT FDA approved for pain and represents an off-label use of the implanted device
Deep Brain Stimulation Deep brain stimulation is NOT FDA approved for pain and represents an off-label use of the implanted device
 Stimulation-Produced Analgesia Reynolds, 1969: science Electrical stimulation of rat midbrain results in profound analgesia without concurrent administration of analgesic drugs Relationship between SPA and endogenous opioid system Richardson, 1973 1 st published report of PAG-PVG stimulation in humans
Stimulation-Produced Analgesia Reynolds, 1969: science Electrical stimulation of rat midbrain results in profound analgesia without concurrent administration of analgesic drugs Relationship between SPA and endogenous opioid system Richardson, 1973 1 st published report of PAG-PVG stimulation in humans
 DBS Pain Targets PVG AND PAG Activation of endogenous opiate systems Descending modulatory pathways Best for nociceptive pain LEMNISCAL SYSTEM Vc (VPL, VPm) nucleus, medial lemniscus, IC Paresthesia-producing stimulation Best for neuropathic pain
DBS Pain Targets PVG AND PAG Activation of endogenous opiate systems Descending modulatory pathways Best for nociceptive pain LEMNISCAL SYSTEM Vc (VPL, VPm) nucleus, medial lemniscus, IC Paresthesia-producing stimulation Best for neuropathic pain
 Results of DBS Overall results variable 30% to 85% excellent/good pain relief Richardson (Neurosurgery, 1977) 85% effective short-term; 65% at 1 year Gybels & Kupers (Neurophys Clin, 1990) initial 61%; 4 years 30% Plotkin (Appl Neurophys, 1982) 60 -65% good results
Results of DBS Overall results variable 30% to 85% excellent/good pain relief Richardson (Neurosurgery, 1977) 85% effective short-term; 65% at 1 year Gybels & Kupers (Neurophys Clin, 1990) initial 61%; 4 years 30% Plotkin (Appl Neurophys, 1982) 60 -65% good results
 Results of Deep Brain Stimulation Gybels and Kupers Literature review through 1998 1, 863 patients (38 reports) Latest results analyzed Success defined as: Pain relief scores of 50% or more Verbal ratings of “good” or “excellent” Lack of relief during trial considered failure
Results of Deep Brain Stimulation Gybels and Kupers Literature review through 1998 1, 863 patients (38 reports) Latest results analyzed Success defined as: Pain relief scores of 50% or more Verbal ratings of “good” or “excellent” Lack of relief during trial considered failure
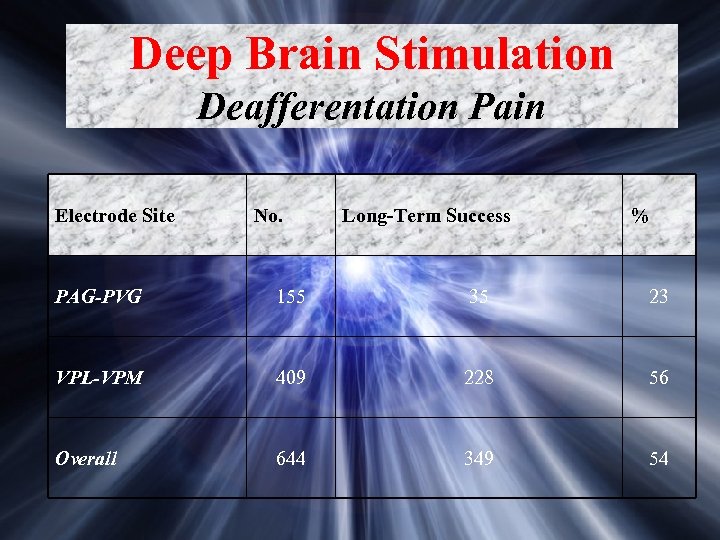 Deep Brain Stimulation Deafferentation Pain Electrode Site No. Long-Term Success % PAG-PVG 155 35 23 VPL-VPM 409 228 56 Overall 644 349 54
Deep Brain Stimulation Deafferentation Pain Electrode Site No. Long-Term Success % PAG-PVG 155 35 23 VPL-VPM 409 228 56 Overall 644 349 54
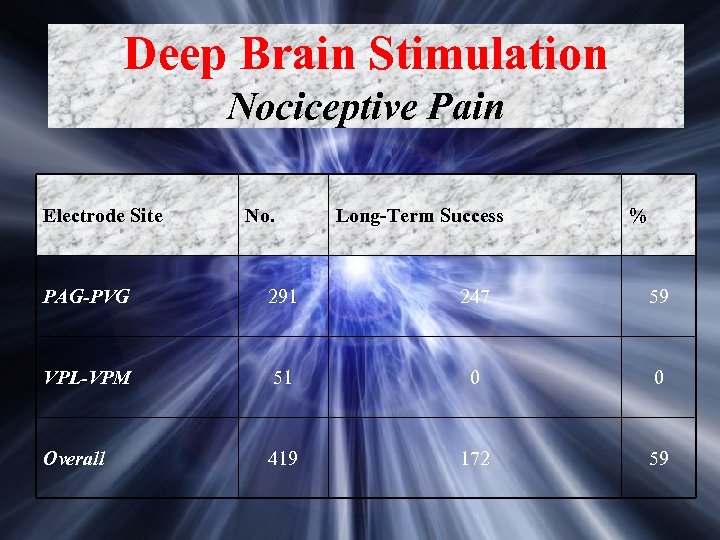 Deep Brain Stimulation Nociceptive Pain Electrode Site No. Long-Term Success % PAG-PVG 291 247 59 VPL-VPM 51 0 0 Overall 419 172 59
Deep Brain Stimulation Nociceptive Pain Electrode Site No. Long-Term Success % PAG-PVG 291 247 59 VPL-VPM 51 0 0 Overall 419 172 59
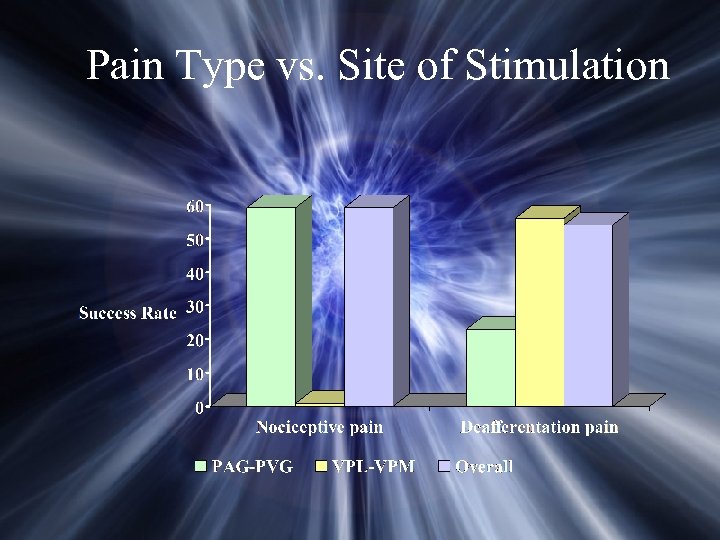 Pain Type vs. Site of Stimulation
Pain Type vs. Site of Stimulation
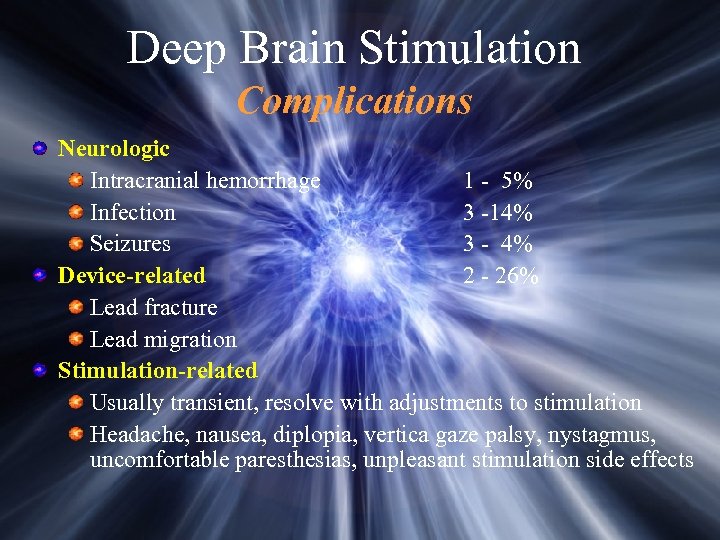 Deep Brain Stimulation Complications Neurologic Intracranial hemorrhage 1 - 5% Infection 3 -14% Seizures 3 - 4% Device-related 2 - 26% Lead fracture Lead migration Stimulation-related Usually transient, resolve with adjustments to stimulation Headache, nausea, diplopia, vertica gaze palsy, nystagmus, uncomfortable paresthesias, unpleasant stimulation side effects
Deep Brain Stimulation Complications Neurologic Intracranial hemorrhage 1 - 5% Infection 3 -14% Seizures 3 - 4% Device-related 2 - 26% Lead fracture Lead migration Stimulation-related Usually transient, resolve with adjustments to stimulation Headache, nausea, diplopia, vertica gaze palsy, nystagmus, uncomfortable paresthesias, unpleasant stimulation side effects
 Cluster Headache Unilateral headache syndrome Pain mainly located in orbitotemporal region Abrupt onset and cessation Pain last 15 – 3 hours (HIS criteria) One or multiple attacks per day Autonomic symptoms “Cluster periods” lasting weeks to months Episodic or chronic forms
Cluster Headache Unilateral headache syndrome Pain mainly located in orbitotemporal region Abrupt onset and cessation Pain last 15 – 3 hours (HIS criteria) One or multiple attacks per day Autonomic symptoms “Cluster periods” lasting weeks to months Episodic or chronic forms
 Surgical Treatment for Cluster Headache Microvascular decompression of trigeminal nerve Ablative trigeminal procedures RF rhizotomy Glycerol rhizolysis Stereotactic radiosurgery Section of nervus intermedius Destruction of sphenopalatine ganglion Deep brain stimulation
Surgical Treatment for Cluster Headache Microvascular decompression of trigeminal nerve Ablative trigeminal procedures RF rhizotomy Glycerol rhizolysis Stereotactic radiosurgery Section of nervus intermedius Destruction of sphenopalatine ganglion Deep brain stimulation
 Proposed Eligibilty Criteria for DBS in Patients with Cluster HA Diagnosis of CH according to HIS criteria Symptoms present at least 24 months CH attacks on daily basis Symptoms strictly unilateral All state-of-the-art medications have been tried singly or in combination “Normal psychological profile No medical/neurological contraindications to DBS Normal neurological exam and imaging studies Patient agrees to discontinue smoking and/or Et. OH consumption
Proposed Eligibilty Criteria for DBS in Patients with Cluster HA Diagnosis of CH according to HIS criteria Symptoms present at least 24 months CH attacks on daily basis Symptoms strictly unilateral All state-of-the-art medications have been tried singly or in combination “Normal psychological profile No medical/neurological contraindications to DBS Normal neurological exam and imaging studies Patient agrees to discontinue smoking and/or Et. OH consumption
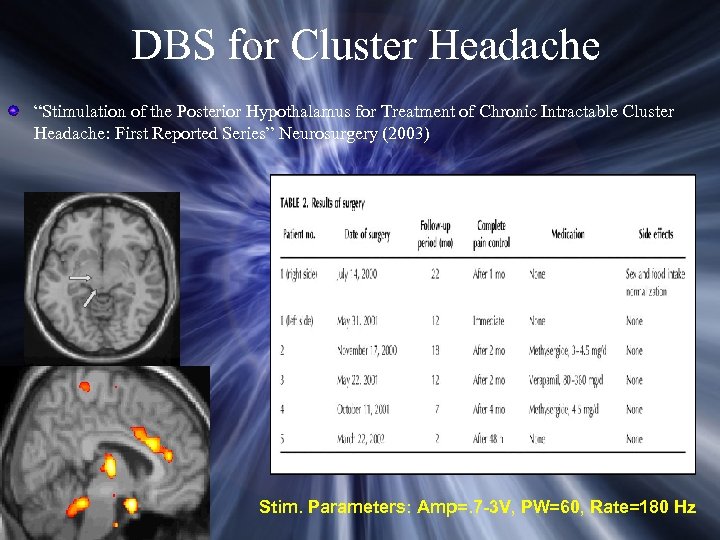 DBS for Cluster Headache “Stimulation of the Posterior Hypothalamus for Treatment of Chronic Intractable Cluster Headache: First Reported Series” Neurosurgery (2003) Stim. Parameters: Amp=. 7 -3 V, PW=60, Rate=180 Hz
DBS for Cluster Headache “Stimulation of the Posterior Hypothalamus for Treatment of Chronic Intractable Cluster Headache: First Reported Series” Neurosurgery (2003) Stim. Parameters: Amp=. 7 -3 V, PW=60, Rate=180 Hz
 Nucleus Caudalis DREZ Procedure
Nucleus Caudalis DREZ Procedure
 Indications for Caudalis DREZ Trigeminal deafferentation pain (following RF lesion) Recurrent refractory trigeminal neuralgia Trigeminal neuropathic pain (post-traumatic) Post-herpetic neuralgia Central pain following brainstem infarction Cluster headache Intractable migraine headache Atypical facial pain Cancer pain
Indications for Caudalis DREZ Trigeminal deafferentation pain (following RF lesion) Recurrent refractory trigeminal neuralgia Trigeminal neuropathic pain (post-traumatic) Post-herpetic neuralgia Central pain following brainstem infarction Cluster headache Intractable migraine headache Atypical facial pain Cancer pain
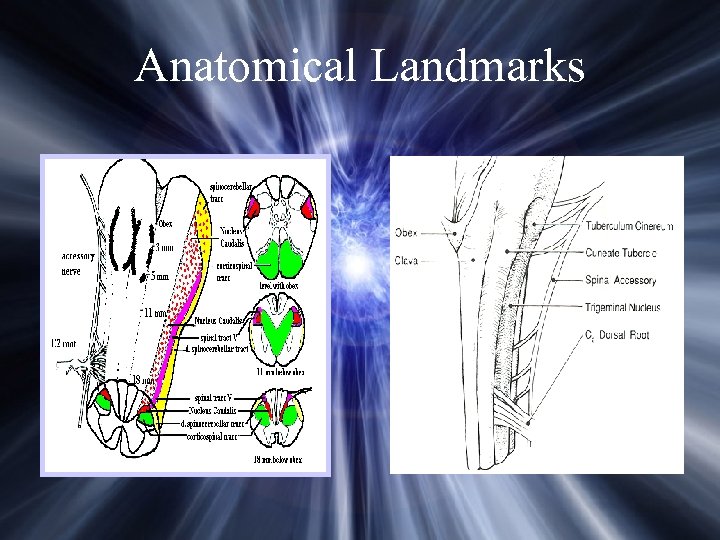 Anatomical Landmarks
Anatomical Landmarks
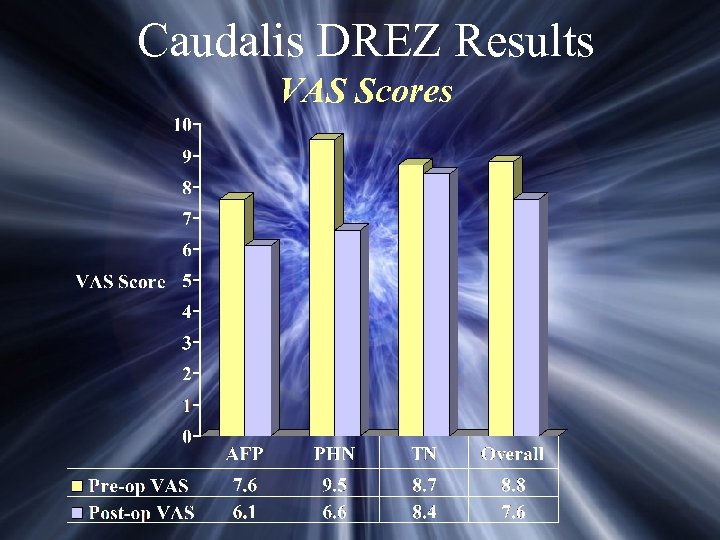 Caudalis DREZ Results VAS Scores
Caudalis DREZ Results VAS Scores
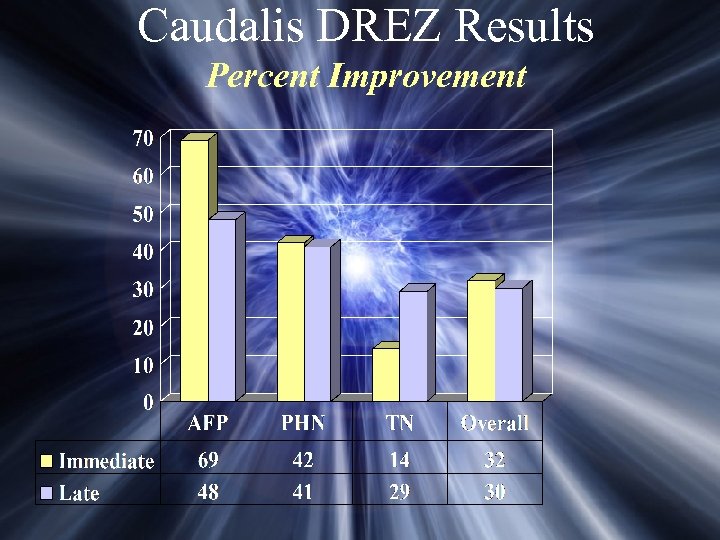 Caudalis DREZ Results Percent Improvement
Caudalis DREZ Results Percent Improvement

 Occipital Neuralgia and Occipital Headache Syndromes
Occipital Neuralgia and Occipital Headache Syndromes
 Occipital Neuralgia Pain within the distribution of the greater and/or lesser occipital nerves Neuralgic variant Sharp, shooting, electric-like pain Almost always unilateral Bursts of pain lasting for several seconds to few minutes Non-neuralgic variant Dull, aching, throbbing, pounding pain More constant pain Often bilateral Sensory dysfunction in C 2 nerve territory Responds to local blockade of occipital nerve
Occipital Neuralgia Pain within the distribution of the greater and/or lesser occipital nerves Neuralgic variant Sharp, shooting, electric-like pain Almost always unilateral Bursts of pain lasting for several seconds to few minutes Non-neuralgic variant Dull, aching, throbbing, pounding pain More constant pain Often bilateral Sensory dysfunction in C 2 nerve territory Responds to local blockade of occipital nerve
 Causes of Occipital Neuralgia Idiopathic Post-traumatic Spinal Disorders C 1 fracture C 1 -2 instability RA with cranial settling C 1 -2 arthrosis syndrome Hypertrophic facet joint Inflammatory disorders Post-Operative VP shunt Retromastoid craniectomy Mastoidectomy Chiari malformation Metabolic disorders Vascular lesions Tumors Evaluation: Plain X-rays, CT, MRI
Causes of Occipital Neuralgia Idiopathic Post-traumatic Spinal Disorders C 1 fracture C 1 -2 instability RA with cranial settling C 1 -2 arthrosis syndrome Hypertrophic facet joint Inflammatory disorders Post-Operative VP shunt Retromastoid craniectomy Mastoidectomy Chiari malformation Metabolic disorders Vascular lesions Tumors Evaluation: Plain X-rays, CT, MRI
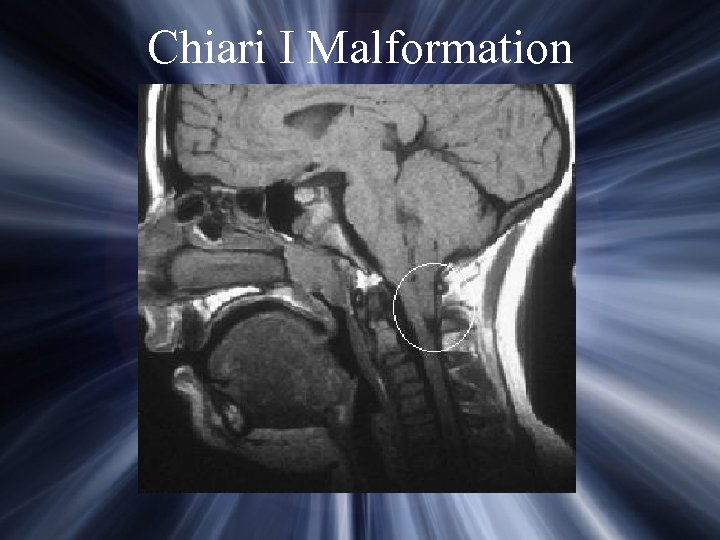 Chiari I Malformation
Chiari I Malformation
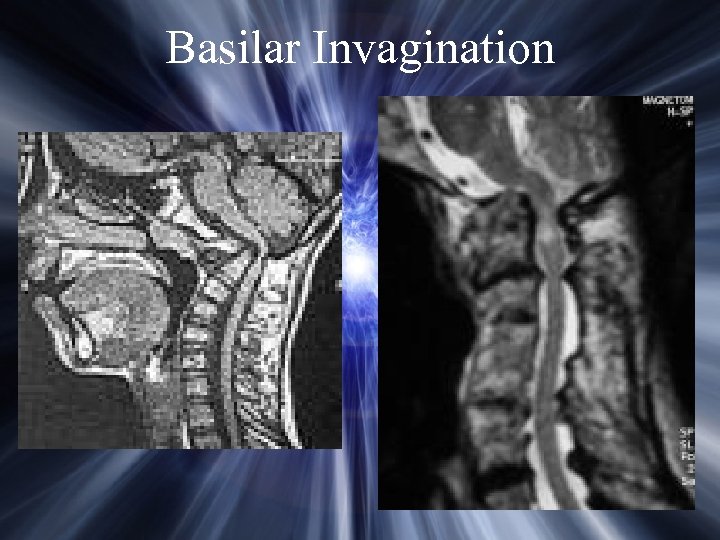 Basilar Invagination
Basilar Invagination
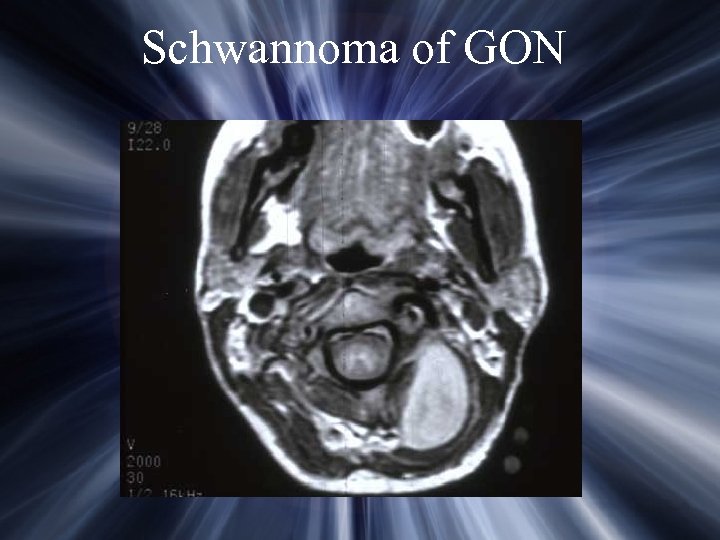 Schwannoma of GON
Schwannoma of GON
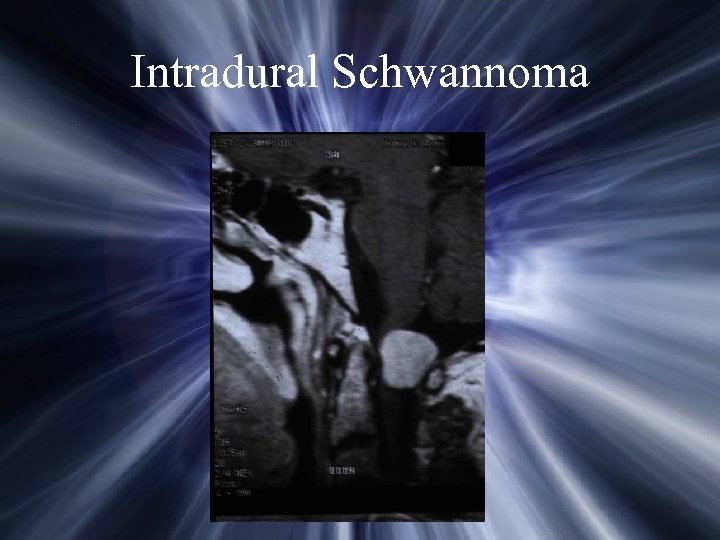 Intradural Schwannoma
Intradural Schwannoma
 Chronic Daily Headache Chronic migraine subset Headache present at least 15 days per month Near daily to continuous pain Incidence 4% to 5% Up to 50% unresponsive to medication
Chronic Daily Headache Chronic migraine subset Headache present at least 15 days per month Near daily to continuous pain Incidence 4% to 5% Up to 50% unresponsive to medication
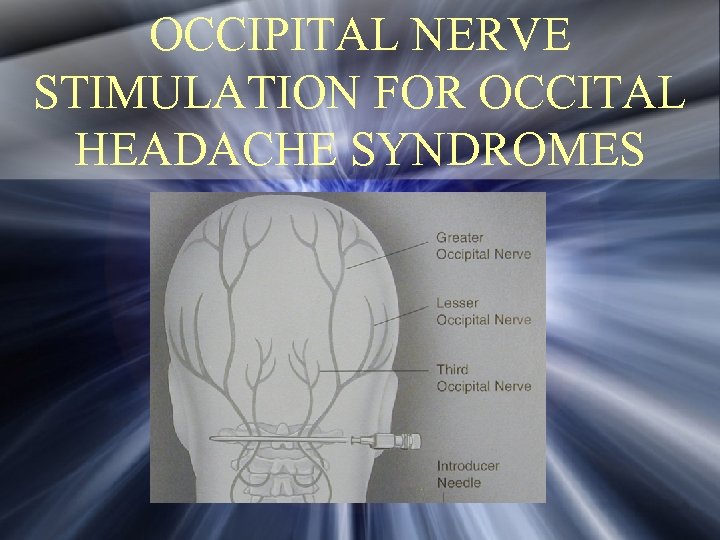 OCCIPITAL NERVE STIMULATION FOR OCCITAL HEADACHE SYNDROMES
OCCIPITAL NERVE STIMULATION FOR OCCITAL HEADACHE SYNDROMES
 Indications for ONS Appropriate clinical condition Condition refractory to non-operative therapy Acceptable psychological profile Positive response to local anesthetic block Positive response to temporary stimulation trial
Indications for ONS Appropriate clinical condition Condition refractory to non-operative therapy Acceptable psychological profile Positive response to local anesthetic block Positive response to temporary stimulation trial
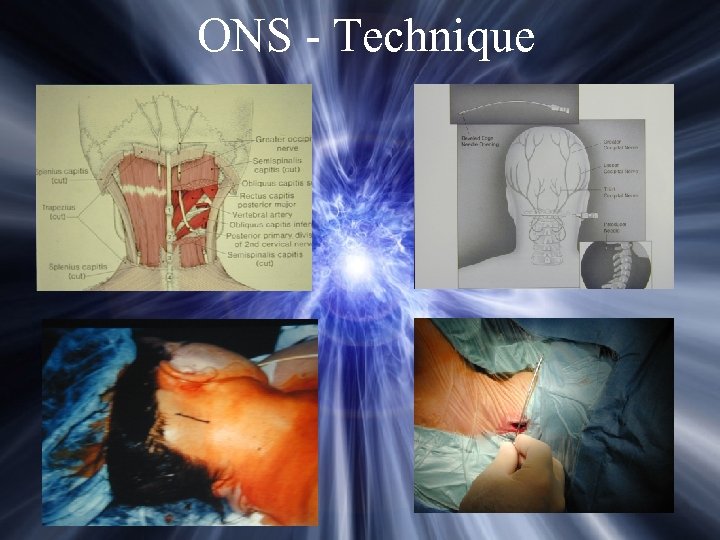 ONS - Technique
ONS - Technique
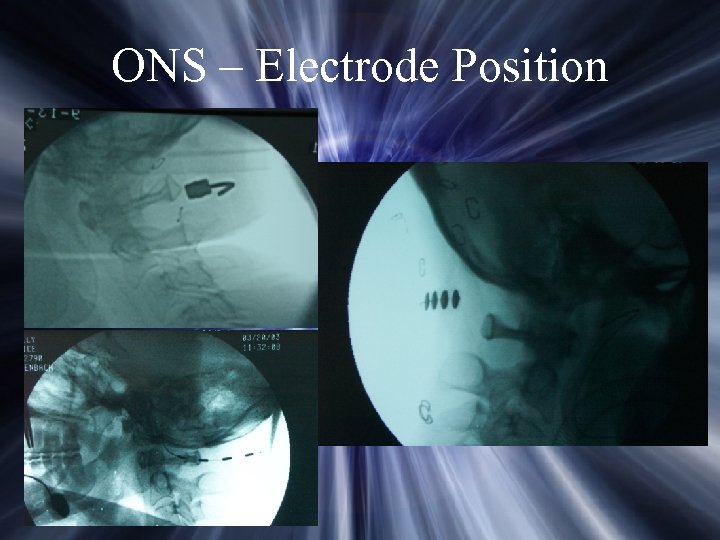 ONS – Electrode Position
ONS – Electrode Position
 Complications of ONS Infection Connector erosion Electrode migration Electrode fracture Motor stimulation Stimulation tolerance
Complications of ONS Infection Connector erosion Electrode migration Electrode fracture Motor stimulation Stimulation tolerance
 Occipital Nerve Stimulation Outcome 130 patients Average duration of symptoms – 8 years Unilateral – 88; Bilateral – 42 Mean VAS score – 9. 2 (5 -10) Weiner, R
Occipital Nerve Stimulation Outcome 130 patients Average duration of symptoms – 8 years Unilateral – 88; Bilateral – 42 Mean VAS score – 9. 2 (5 -10) Weiner, R
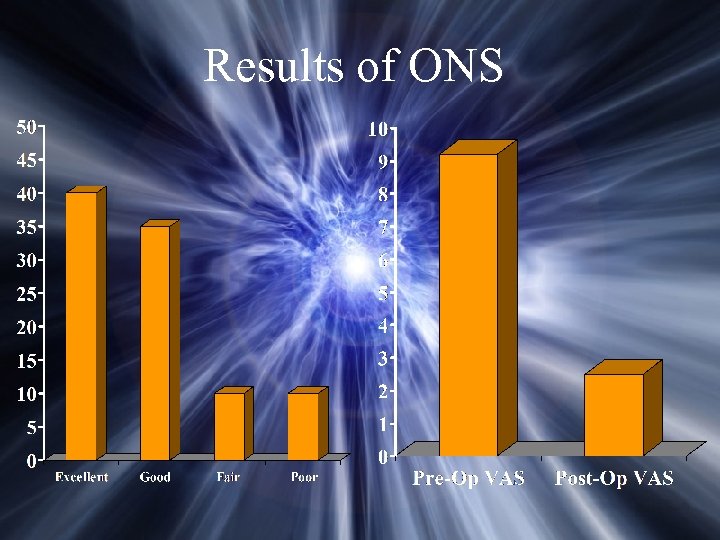 Results of ONS
Results of ONS
 Chronic Migraine
Chronic Migraine
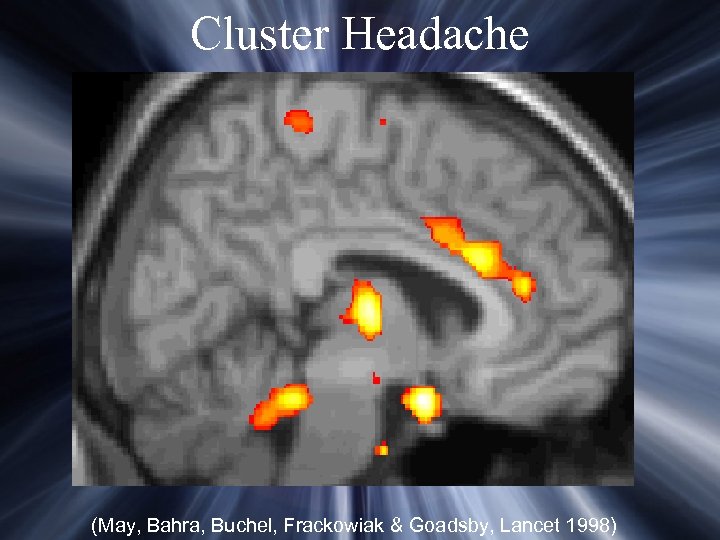 Cluster Headache (May, Bahra, Buchel, Frackowiak & Goadsby, Lancet 1998)
Cluster Headache (May, Bahra, Buchel, Frackowiak & Goadsby, Lancet 1998)


