7cdaa35b568bfb2a611625a650708e6d.ppt
- Количество слайдов: 177

Diabetes mellitus By Dr. Mohamed Abd Almoneim Attia
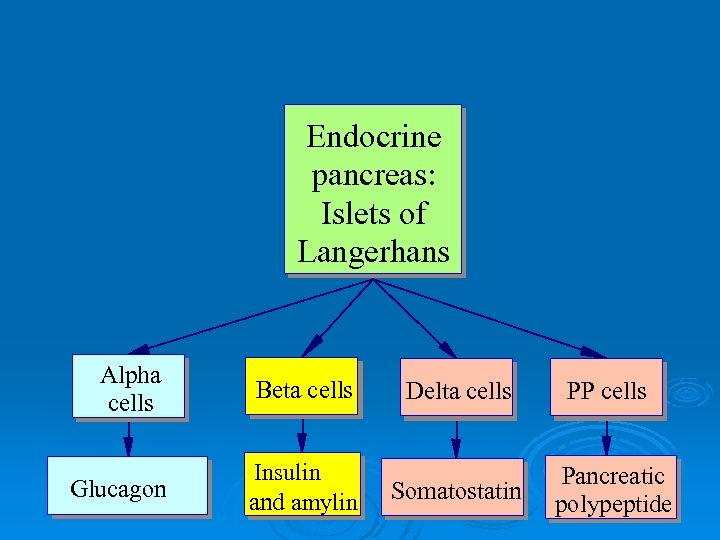
Endocrine pancreas: Islets of Langerhans Alpha cells Beta cells Glucagon Insulin and amylin Delta cells PP cells Somatostatin Pancreatic polypeptide

Functions of Pancreatic Hormones Glucagon: causes cells to release stored glucose into the blood Insulin is the only hormone known to have a direct effect in lowering blood glucose levels. Insulin: allows cells to take up glucose from the blood Amylin: slows glucose absorption in small intestine; suppresses glucagon secretion Somatostatin: decreases GI activity; suppresses glucagon and insulin secretion
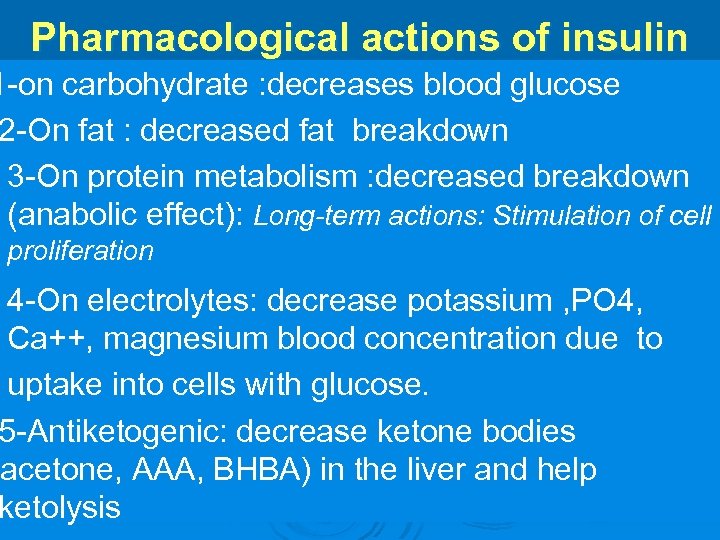
Pharmacological actions of insulin 1 -on carbohydrate : decreases blood glucose 2 -On fat : decreased fat breakdown 3 -On protein metabolism : decreased breakdown (anabolic effect): Long-term actions: Stimulation of cell proliferation 4 -On electrolytes: decrease potassium , PO 4, Ca++, magnesium blood concentration due to uptake into cells with glucose. 5 -Antiketogenic: decrease ketone bodies (acetone, AAA, BHBA) in the liver and help ketolysis

Amylin, Somatostatin, and Gut. Derived Hormones A polypeptide that is co-secreted with insulin from the beta cells. Plasma levels of amylin increase in response to nutritional stimuli to produce inhibition of gastric emptying and glucagon secretion.

Incretins Several gut derived hormones have been identified as having what is termed an incretin effect, meaning that they increase insulin release after an oral nutrient load. =Augmentation of insulin after oral glucose. The two hormones that account for about 90% of the incretin effect are glucagon like peptide-1, which is released from L cells in the distal small bowel, and glucose-dependent insulinotropic polypeptide. GLP -1 + GIP = incretin effect
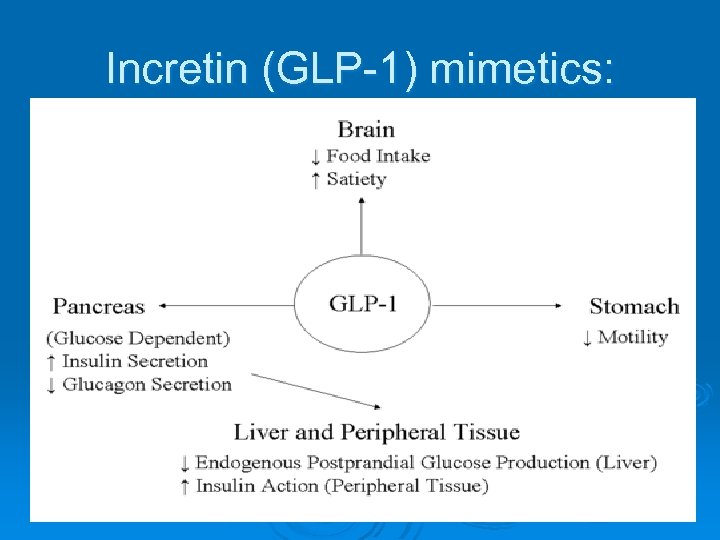
Incretin (GLP-1) mimetics:

Counter regulatory Hormones They counteract the storage functions of insulin in regulating blood glucose levels during periods of fasting, exercise, and other situations that either limit glucose intake or deplete glucose stores. 1 -The catecholamines, 2 -Growth hormone, and 3 - Glucocorticoids.

Epinephrine helps to maintain blood glucose levels during periods of stress. Glycogenolysis in the liver Inhibits insulin release Increasing the breakdown of muscle glycogen stores. A direct lipolytic effect on adipose cells,

Growth Hormone Increases protein synthesis Mobilizes fatty acids from adipose tissue, and antagonizes the effects of insulin. Chronic hypersecretion of growth hormone, as occurs in acromegaly, can lead to glucose intolerance and the development of diabetes mellitus.

Glucocorticoid Hormones Gluconeogenesis by the liver Hypoglycemia is a potent stimulus for cortisol secretion. In predisposed persons, the prolonged elevation of glucocorticoid hormones can lead to hyperglycemia and the development of diabetes mellitus.
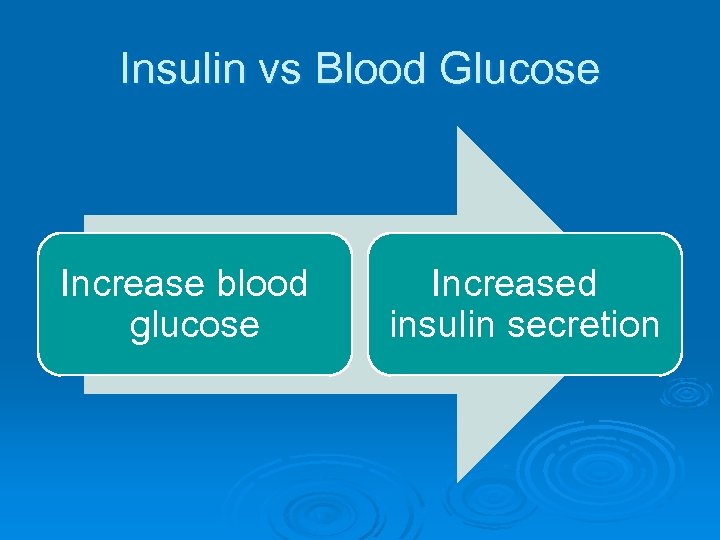
Insulin vs Blood Glucose Increase blood glucose Increased insulin secretion
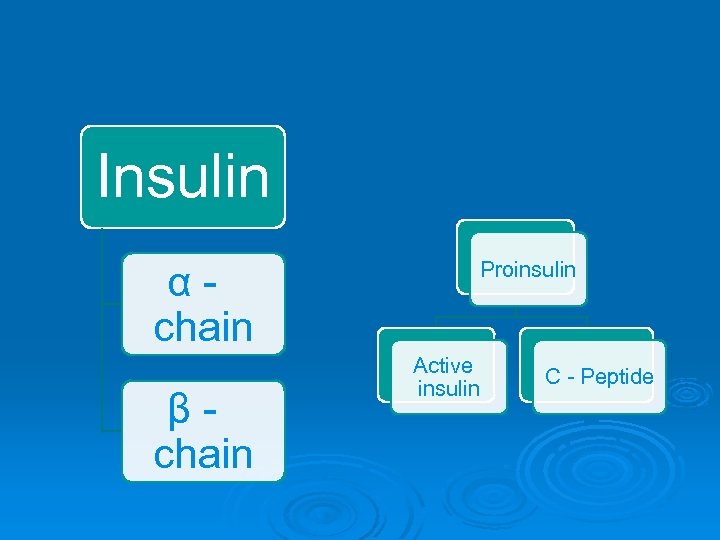
Insulin α - chain β - chain Proinsulin Active insulin C - Peptide
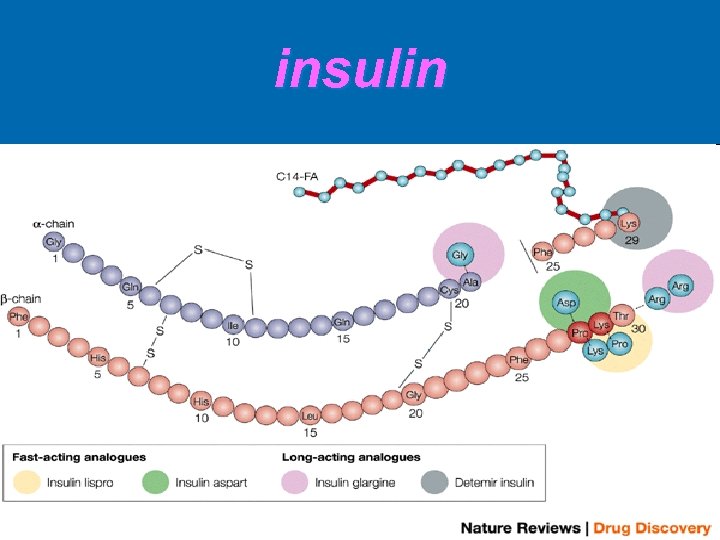
insulin

Ø Ø insulin polypeptide hormone secreted from beta-cells of islets of Langerhans. It consists of 51 amino acids connected with each other with Cpeptides in 2 chains ( A ; 21 aa & B; 30 aa) , linked by 2 disulphide bridges. Insulin is secreted as pre-pro-insulin & then is converted to proinsulin. In stimulation it is hydrolyzed into insulin & C peptide. The C-peptide chains can be measured clinically, and this measurement can be used to study beta cell function (i. e. , persons with type 1 diabetes with very little or no remaining beta cell function will have very low or nonexistent levels of Cpeptide in their blood, and thus will likely need insulin replacement for treatment).

*basal secretion of insulin occurs in a pulsatile fashion Basal release (in pulses every 15 -30 minutes) Glucose stimulated release: After exposure to glucose, which is a nutrient secretagogue, 1 - Early, rapid phase (stored insulin is secreted) a First-phase release of stored preformed insulin occurs, followed by 2 - Later, slower phase (newly synthesized insulin is secreted). a second-phase release of newly synthesized insulin. Serum insulin levels begin to rise within minutes after a meal, reach a peak in approximately 3 to 5 minutes, and then return to baseline levels within 2 to 3 hours. Insulin has a half-life of approximately 15 minutes once it is released into the general circulation.
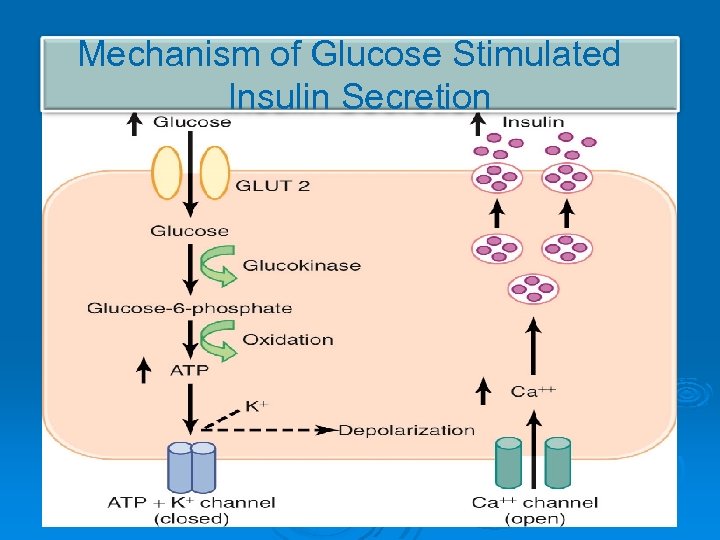
Mechanism of Glucose Stimulated Insulin Secretion
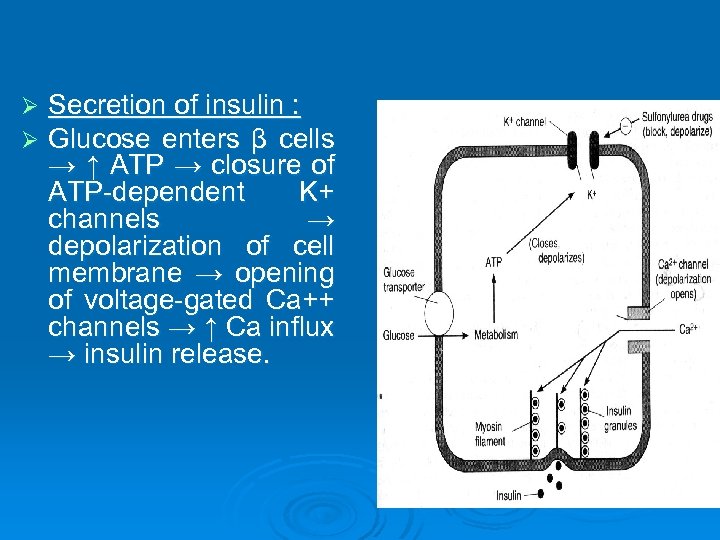
Ø Ø Secretion of insulin : Glucose enters β cells → ↑ ATP → closure of ATP-dependent K+ channels → depolarization of cell membrane → opening of voltage-gated Ca++ channels → ↑ Ca influx → insulin release.

pharmacokinetics Ø Insulin is not taken orally due to its rapid destruction by proteolytic enzymes of GIT, thus, it is used by injection. Ø It is metabolized by insulinase enzyme in liver. Ø Half life of insulin in plasma ( if taken IV) is about 5 minutes. Ø 60% of endogenous insulin removed by liver & 40% by the kidney. Ø In exogenous insulin the ratio is reversed.

pharmacokinetics ABSORPTION SC, IM, IV : good. ✐ Nasal: good (investigational). ✐ Half-life: 5 -10 minutes Estimation of blood level of insulin is by RIA; its level is about 515/u units/ml ( fasting) , 60 -90/u units/ml (post-prandial) Ø NB: to assess function of beta cells : 1 - estimation of insulin by RIA. 2 - estimation of C-peptide. Ø
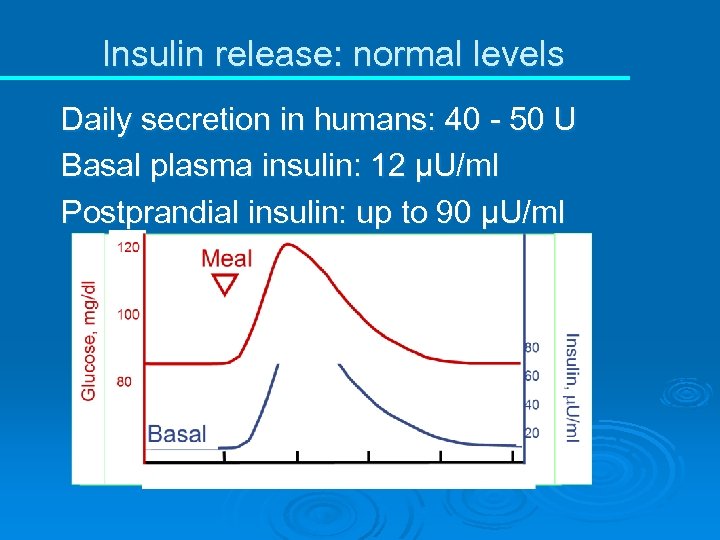
Insulin release: normal levels Daily secretion in humans: 40 - 50 U Basal plasma insulin: 12 µU/ml Postprandial insulin: up to 90 µU/ml
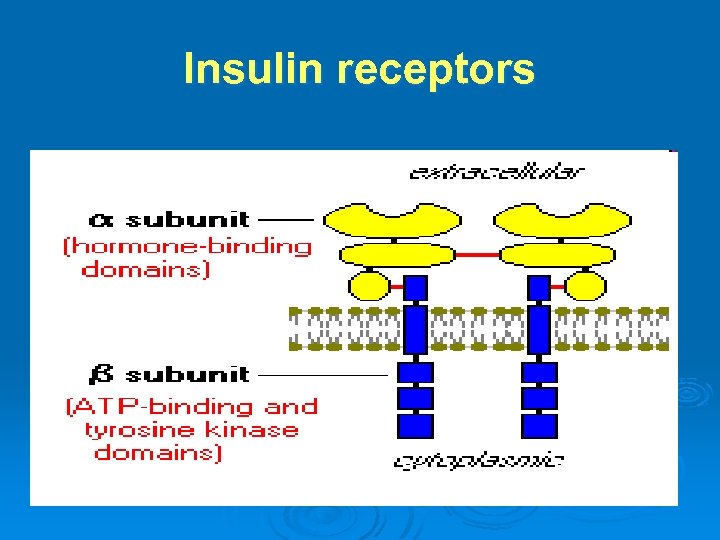
Insulin receptors
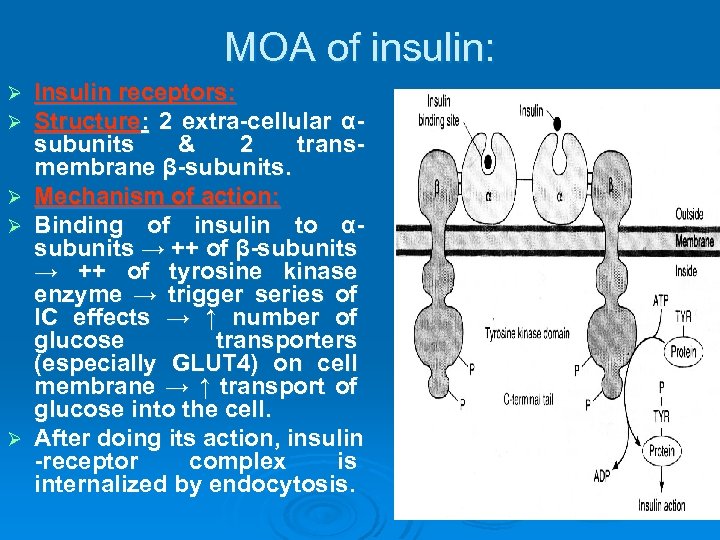
MOA of insulin: Ø Ø Ø Insulin receptors: Structure: 2 extra-cellular αsubunits & 2 transmembrane β-subunits. Mechanism of action: Binding of insulin to αsubunits → ++ of β-subunits → ++ of tyrosine kinase enzyme → trigger series of IC effects → ↑ number of glucose transporters (especially GLUT 4) on cell membrane → ↑ transport of glucose into the cell. After doing its action, insulin -receptor complex is internalized by endocytosis.
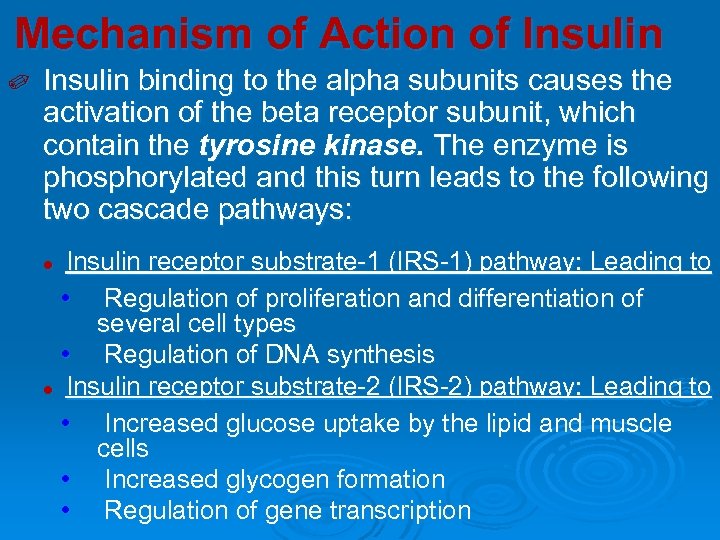
Mechanism of Action of Insulin ✐ Insulin binding to the alpha subunits causes the activation of the beta receptor subunit, which contain the tyrosine kinase. The enzyme is phosphorylated and this turn leads to the following two cascade pathways: Insulin receptor substrate-1 (IRS-1) pathway: Leading to • Regulation of proliferation and differentiation of several cell types • Regulation of DNA synthesis l Insulin receptor substrate-2 (IRS-2) pathway: Leading to • Increased glucose uptake by the lipid and muscle cells • Increased glycogen formation • Regulation of gene transcription l

Cell membranes are impermeable to glucose, they require a special carrier, called a glucose transporter, to move glucose from the blood into the cell. GLUT-2 is the major transporter of glucose into beta cells and liver cells. It has a low affinity for glucose and acts as a transporter only when plasma glucose levels are relatively high, such as after a meal. GLUT-1 is present in all tissues. It does not require the actions of insulin and is important in transport of glucose into cells of the nervous system. GLUT 1, GLUT 2, GLUT 3, GLUT 4. GLUT-4 is the insulin-dependent glucose transporter for skeletal muscle and adipose tissue

Factors that ↑ affinity of insulin receptors (up-regulation): Ø Insulin deficiency (e. g. starvation) → ↑ number of receptor. Ø Drugs: l l Thiazolidinediones: e. g. , . . . . pioglitazone, rosiglitazone. Sulphonylureas: e. g……………… gliclazide. Biguanides e. g ……………. Trace elements: e. g……. selenium and chromium.

Factors that ↓ affinity of insulin receptors (down-regulation): Ø Insulin excess: → ↓ number of receptors. Ø Obesity Ø Drugs: e. g. Corticosteroids…….

Sources of insulin: Ø Traditional (animal) insulin: prepared from: animals (beef and pork). Ø Pork insulin differs from human insulin in 1 aa (alanine in pork and threonine in human) while beef insulin differs from human in 3 aa. Ø Human insulin:

Ø Human insulin: prepared by 2 methods: l l From pork insulin by chemical replacement of alanine by threonine. Recombinant DNA technology (genetic engineering). Advantages of human insulin: Ø Highly purified Ø least antigenic. Ø Rare resistance. Ø Used in insulin resistance and gestational DM. Ø

Insulin Therapy Administration Insulin is administered either IV, IM or SC. ✐ Administration of insulin differs from physiological secretion of insulin because: ✐ *The kinetics does not reproduce the normal rapid rise and decline of insulin secretion in response of ingestion of nutrients. *The insulin diffuses into the peripheral circulation instead of being released into the portal circulation. Therefore the direct effects of insulin on hepatic metabolic processes are eliminated.

Insulin Therapy The duration of action of insulin can be varied by: Modification of the insulin molecule (by recombinant technology) 2. Conjugation of insulin with protamine in a low soluble complex. After injection proteolytic enzymes degrade protamine so allowing absorption of insulin. 3. Combination of insulin with zinc, to form zinc salts. After injection the salt precipitates and insulin is slowly released. 1. All available insulin preparations are either human insulin (produced by recombinant DNA techniques) or human analog insulin (some amino acids in the molecule are substituted or changed in position)

Different PREPARATIONS of insulin: 1 - Ultrashort -ACTING INSULIN: Ø Insulin lispro: Ø It is a semisynthetic analog of human insulin. Ø It is identical to human insulin except for the transposition of proline and lysine at positions B 28 and B 29 in the B chain. Ø When it is injected s. c, it dissociates quickly into monomers and is rapidly absorbed. Ø Onset of action 5 -15 min. Ø Peak activity in 1 hr. Ø Duration of action 3 -5 hrs. Ø (i. e. , rapid onset and short duration).

Insulin Aspart substitution of the B 28 proline with a negatively charged aspartic acid. When they are injected subcutaneously, the drugs are rapidly absorbed with onset of action within 5– 15 minutes, and reaching peak activity as early as 1 hour- short duration of action.

2 - SHORT-ACTING INSULIN 1. Regular, neutral, soluble. It is a short-acting soluble crystalline zinc insulin made by recombinant DNA techniques to produce a molecule identical to human insulin. could be given intravenously in Emergency conditions (e. g. diabetic ketoacidosis) or When insulin requirement is changing rapidly, e. g. after surgery or during acute infections. Also aspart and lispro Ø 2. Semi-lente. It is short-acting crystalline insulin made by recombinant DNA techniques. Ø
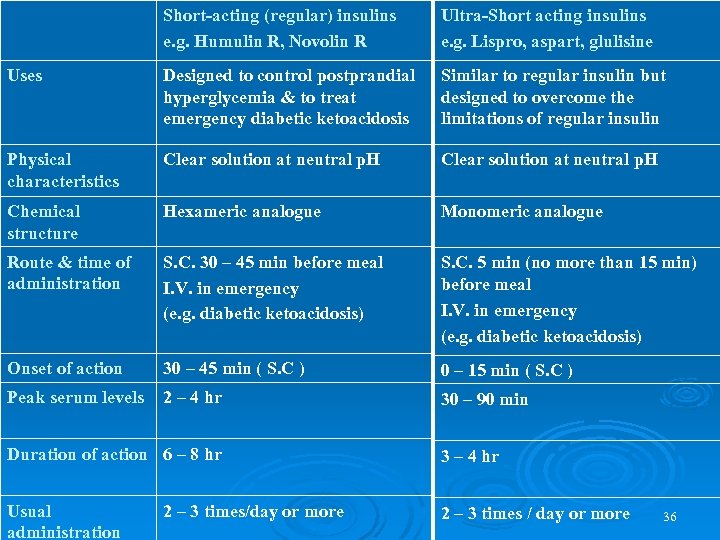
Short-acting (regular) insulins e. g. Humulin R, Novolin R Ultra-Short acting insulins e. g. Lispro, aspart, glulisine Uses Designed to control postprandial hyperglycemia & to treat emergency diabetic ketoacidosis Similar to regular insulin but designed to overcome the limitations of regular insulin Physical characteristics Clear solution at neutral p. H Chemical structure Hexameric analogue Monomeric analogue Route & time of administration S. C. 30 – 45 min before meal I. V. in emergency (e. g. diabetic ketoacidosis) S. C. 5 min (no more than 15 min) before meal I. V. in emergency (e. g. diabetic ketoacidosis) Onset of action 30 – 45 min ( S. C ) 0 – 15 min ( S. C ) Peak serum levels 2 – 4 hr 30 – 90 min Duration of action 6 – 8 hr 3 – 4 hr Usual administration 2 – 3 times / day or more 2 – 3 times/day or more 36
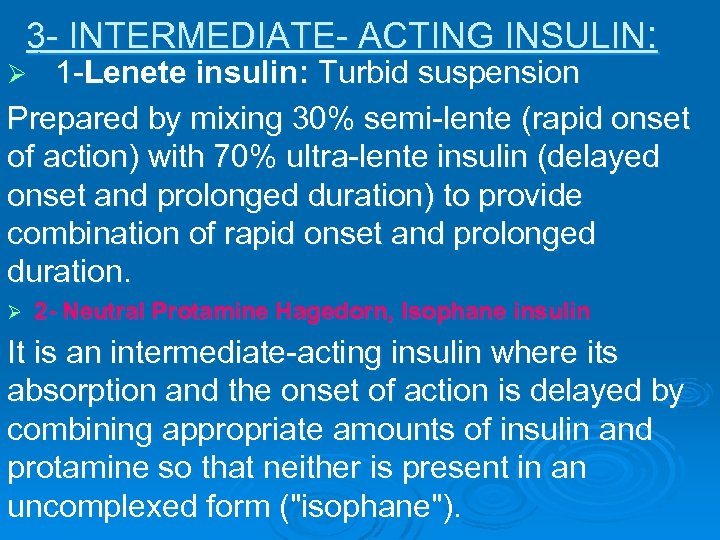
3 - INTERMEDIATE- ACTING INSULIN: 1 -Lenete insulin: Turbid suspension Prepared by mixing 30% semi-lente (rapid onset of action) with 70% ultra-lente insulin (delayed onset and prolonged duration) to provide combination of rapid onset and prolonged duration. Ø Ø 2 - Neutral Protamine Hagedorn, Isophane insulin It is an intermediate-acting insulin where its absorption and the onset of action is delayed by combining appropriate amounts of insulin and protamine so that neither is present in an uncomplexed form ("isophane").

4 -LONG-ACTING INSULIN: Human insulin with modified aa or added to more zinc to make them long acting “peakless” Given once daily. Examples: Ø 1 - Ultra-lente insulin: insulin zinc suspension : Turbid suspension Combination of regular insulin e excess zinc. Has a delayed onset and prolonged duration of action.

2 -Insulin glargine: Ø Ø Ø It is ultralong-acting insulin analog. Prepared by substitution of glycine for asparagine at the A 21 position and adding 2 arginine molecules to the B chain terminal. Insulin molecules dissolve slowly from the injection site and provide a low, continuous level of circulating insulin. Insulin glargine has: Slow onset: 1 -1. 5 hrs. Peak after 4 -5 hrs. Duration of action: 11 -24 hrs. Ø Advantages over intermediate-acting insulins: Constant circulating insulin over 24 hr with no pronounced peak. More safe than NPH& Lente insulins due to reduced risk of hypoglycemia (esp. nocturnal hypoglycemia). Clear solution that does not require resuspension before administration 3 -Insulin Detemir: ……………
Glargine 41
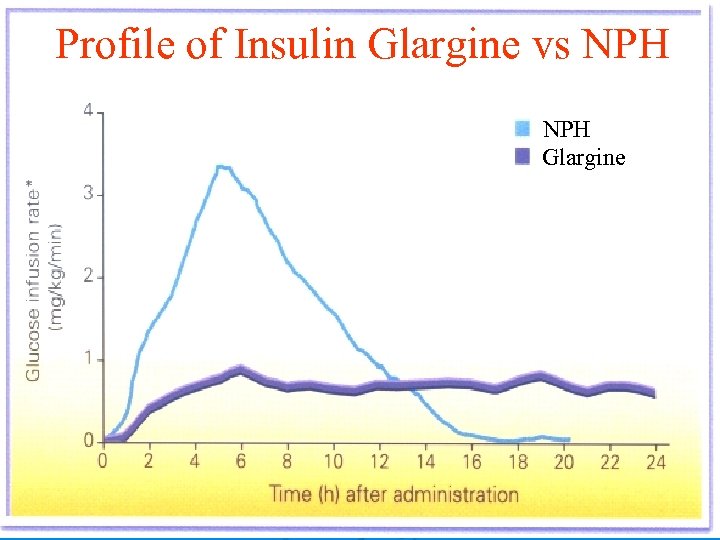
Profile of Insulin Glargine vs NPH Glargine 42
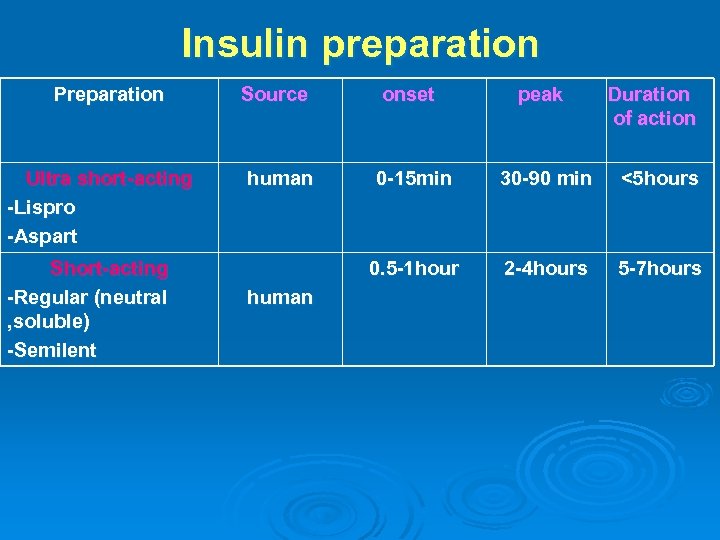
Insulin preparation Preparation Ultra short-acting -Lispro -Aspart Short-acting -Regular (neutral , soluble) -Semilent Source onset peak Duration of action human 0 -15 min 30 -90 min <5 hours 0. 5 -1 hour 2 -4 hours 5 -7 hours human
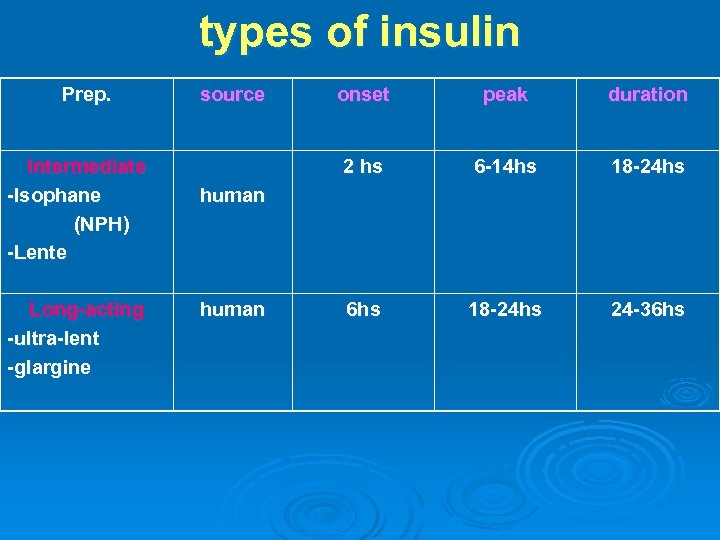
types of insulin Prep. Intermediate -Isophane (NPH) -Lente Long-acting -ultra-lent -glargine source onset peak duration 2 hs 6 -14 hs 18 -24 hs 6 hs 18 -24 hs 24 -36 hs human
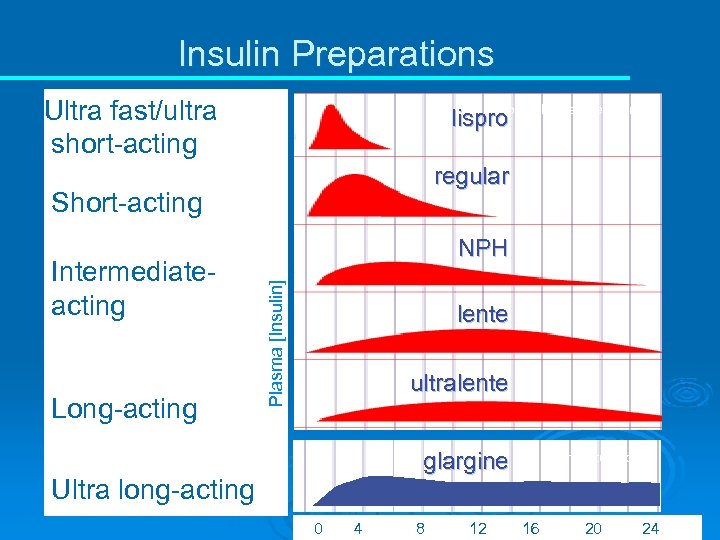
Insulin Preparations Ultra fast/ultra short-acting from Lilly. Diabetes. com lispro regular Short-acting Long-acting Ultra long-acting Plasma [Insulin] Intermediateacting NPH lente ultralente glargine from lantus. com 0 4 8 12 16 20 24

MIXTURES OF INSULIN: Pre-mixed formulations (Biphasic) of 30% regular (short) and 70% NPH (intermediate): provide rapid onset and prolonged duration. Ø Insulin lispro and aspart can be mixed (i. e, just before injection) with either NPH or ultralente insulin without affecting their rapid absorption. e. g: Ø Humalin 70/30 (NPH/Reg. )= (mixtard). Ø Humalog mix 75/25 (NPH/Lispro). Rapid onset , Long duration, Given twice daily, Ø

Definition of DM: Ø is a clinical syndrome characterized by disturbance in carbohydrates, fat, and proteins metabolism due to insulin deficiency or insulin resistance. diabainein, Gr- to pass through mel, L- honey

DIABETES MELLITUS Affects approx. 5 – 8 % Mostly asymptomatic Tendency increase with obesity One of the leading cause of death by disease One of the leading cause of blindness One of the leading cause of renal failure

EPIDEMIOLOGY of DM In 2013, according to International Diabetes Federation, an estimated 381 million people had diabetes. Its prevalence is increasing rapidly, and by 2030, this number is estimated to almost double. Diabetes mellitus occurs throughout the world, but is more common (especially type 2 (NIDDM) in the more developed countries.

THE CLASSIFICATI ON OF DM

Types Of Diabetes Mellitus Type I alled insulin-dependent diabetes an autoimmune condition. Occur in childhood Rapid onset, Complete insulin deficiency Absolute insulin requirement. Autoimmunity is the major factor in the pathophysiology of type 1 DM. Approximately 85% of type 1 DM patients have circulating islet cell antibodies. The most commonly found islet cell antibodies are those directed against glutamic acid decarboxylase (GAD) which is an enzyme found within pancreatic beta cells Moreover, in a genetically susceptible individual, a viral infection may stimulate the production of antibodies against a viral protein that trigger an autoimmune response against antigenically similar beta cell molecules. There is lymphocytic infiltration and destruction of pancreatic beta cells. Once about 80 -90% of the beta cells are destroyed, hyperglycemia develops and diabetes manifests.

RISK FACTORS TYPE I Family history Environmental factors The presence of damaging immune system cells (autoantibodies). Dietary factors
Type 2 diabetes mellitus Ranges from predominantly insulin resistance with relative insulin deficiency to a predominantly secretory defect with insulin resistance Insulin required in 20 -30% of patients Heterogeneous polygenic disease Progression gradual

Type II It develops when the body cells are unable to efficiently use the insulin (insulin resistance). The pancreas initially tries to make more insulin (hyperinsulinemia) in order to overcome insulin resistance, but eventually there is beta cell exhaustion and failure and then the beta cells don’t make enough insulin leading to relative or absolute insulin deficiency (especially if Type 2 DM has been present for a long time) Called non-insulin dependent Most common Used to be called adult-onset diabetes, but with the epidemic of obese and overweight kids, more teenagers are now developing type 2 diabetes often a milder form of diabetes than type 1

Risk factors for TYPE II Weight. Inactivity. Family history. Age Gestational diabetes. Polycystic ovary syndrome.
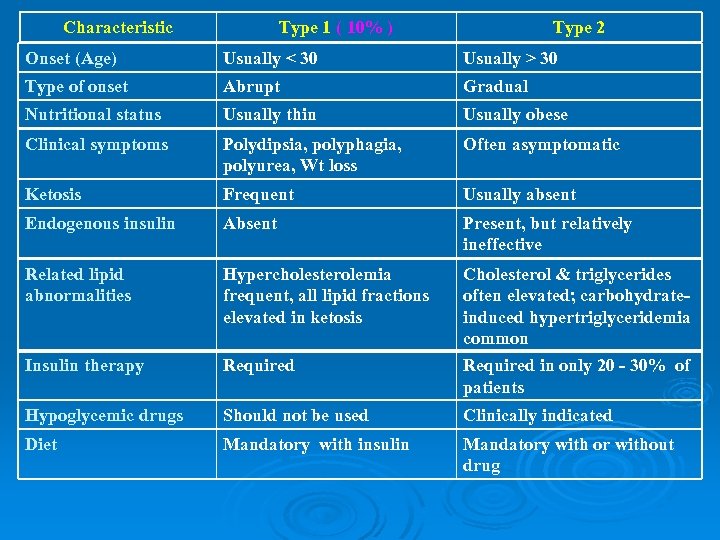
Characteristic Type 1 ( 10% ) Type 2 Onset (Age) Usually < 30 Usually > 30 Type of onset Abrupt Gradual Nutritional status Usually thin Usually obese Clinical symptoms Polydipsia, polyphagia, polyurea, Wt loss Often asymptomatic Ketosis Frequent Usually absent Endogenous insulin Absent Present, but relatively ineffective Related lipid abnormalities Hypercholesterolemia frequent, all lipid fractions elevated in ketosis Insulin therapy Required Cholesterol & triglycerides often elevated; carbohydrateinduced hypertriglyceridemia common Required in only 20 - 30% of patients Hypoglycemic drugs Should not be used Clinically indicated Diet Mandatory with insulin Mandatory with or without drug
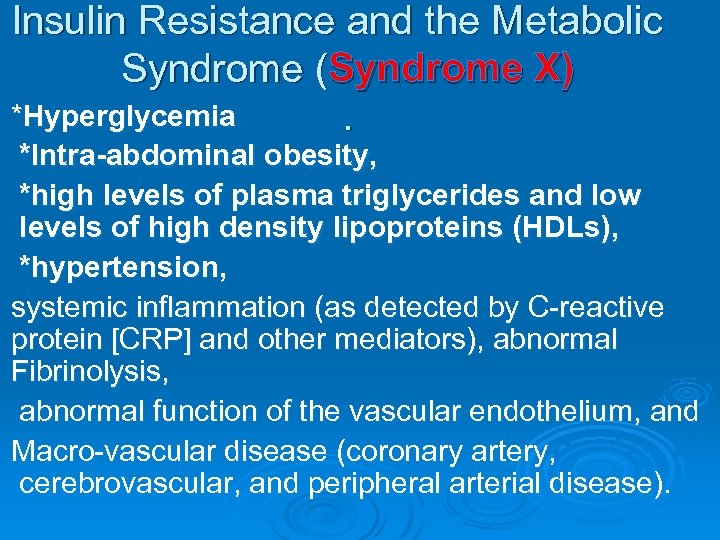
Insulin Resistance and the Metabolic Syndrome (Syndrome X) *Hyperglycemia . *Intra-abdominal obesity, *high levels of plasma triglycerides and low levels of high density lipoproteins (HDLs), *hypertension, systemic inflammation (as detected by C-reactive protein [CRP] and other mediators), abnormal Fibrinolysis, abnormal function of the vascular endothelium, and Macro-vascular disease (coronary artery, cerebrovascular, and peripheral arterial disease).

Gestational Diabetes hat's triggered by pregnancy Often diagnosed in middle or late pregnancy Having gestational diabetes does, however, put mothers at risk for developing type 2 diabetes later in life Can occur anywhere from a few weeks after delivery to months or years later Risks to the unborn baby are even greater than risks to the mother Any degree of glucose intolerance with onset during pregnancy Return to normal glucose regulation after delivery is common Increased perinatal morbidity and mortality if untreated

Less common types of DM: Latent autoimmune diabetes of adults (LADA): type 1 DM develops in adults. Ø Maturity onset diabetes of the young (MODY): type 2 DM develops in children or young adults who develop hyperglycemia without diabetic ketosis. N. B. LADA or MODY are misdiagnosed Ø Secondary DM: to drugs, infections, endocrine diseases…. Ø

Manifestations of diabetes mellitus: Acute manifestations Asymptomatic Polyurea. Polydepsia. Weigh Loss despite of food intake (polyphagia). Formation of ketone bodies. Fatigue and loss of concentration. Recurrent infection e. g. vaginitis or pruritis valvae in female (glucose is an excellent medium for macro-organism). Increased lipolysis leads to formation of ketone bodies in the body due to lack of insulin (more common in type I). Blurred vision due to osmotically induced changes in the eye lens.

Laboratory investigations: 1. Urine analysis for glucosuria & ketonuria. 2. Blood glucose: fasting and 2 -hr after oral 75 g glucose. Fasting BG > 126 mg/dl OR 2 hr postprandial BG > 200 mg/dl. 3. Glycated hemoglobin (Hb. A 1 C): Indicates average blood sugar level for the past 2 -3 months. > 8% (elevated levels mean uncontrolled DM for long period). 4. Test for lipid profile especially triglycerides and cholesterol. Ø
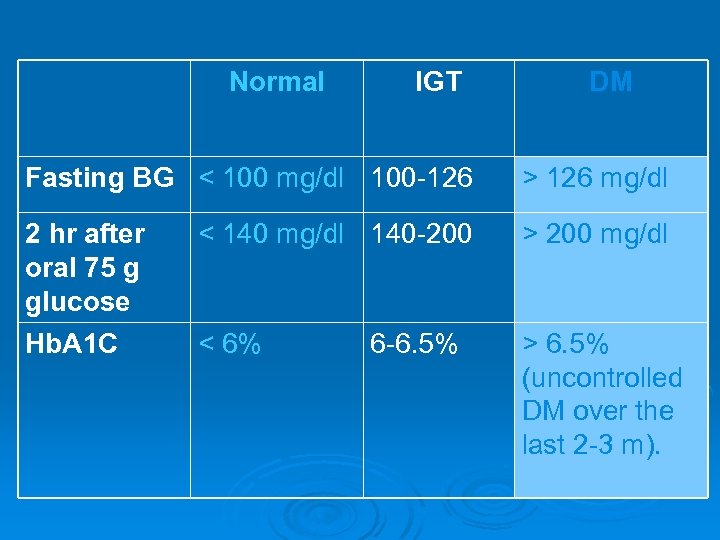
Normal IGT DM Fasting BG < 100 mg/dl 100 -126 > 126 mg/dl 2 hr after oral 75 g glucose < 140 mg/dl 140 -200 > 200 mg/dl Hb. A 1 C < 6% > 6. 5% (uncontrolled DM over the last 2 -3 m). 6 -6. 5%

Goals of therapy: 1 -Try to maintain patient as close to euglycemia as possible. 2 -Try to keep the patient free of symptoms associated with hyperglycemia. 3 -Try to eliminate or minimize all cardiovascular risk factors and other complications of diabetes. 4 -Normal growth and development should be maintained in Children (Type I) i. e no restrict diet control.

Lines of treatment: Patient education about the disease and its complications. 2. Exercise: to enhance peripheral utilization of glucose and ↓ insulin requirements. 3. Diet control. 4. Diet + insulin (type 1). 5. Diet + oral antidiabetic drugs ± insulin (type 2). 1.

Intensive patient education about: 1 -Manifestations of disease and its complications (including hyper and hypoglycemic manifestations). 2 -How to test urine for glucose. 3 -Disease treatment (non-drug therapy, type of drug therapy, where and how to inject insulin preparations). 4 -Personal hygiene with regard of skin, feet and teeth

5 -Simple pathophysiology 6 -Treatment modalities 7 -Recognition, treatment and prevention of acute complications 8 -When to call the doctor 9 -Foot care, eye care, general hygiene, risk factor management

Exercise: because it promotes peripheral utilization of glucose, improves utilization of fat and reduces insulin requirements. It also prevents CVS complications. Diet control alone (Mild type of NIDDM) Diet control + insulin (IDDM) Diet control + oral antidiabetic drugs (NIDDM)

Diet regime Ø Children and underweight patients need 40 calories /kg/day. Ø Middle age (non-obese) need 30 calories /kg/day. Ø Overweight (obese) adult needs 20 calories /kg/clay.

Distribution of diet is as follows: 20% of calories from proteins Ø (1. 5 gm/kg/day) Ø (Igm=4 calories). Ø 40% of calories from carbohydrates Ø (2. 5 gm/kg/day) Ø (Igm=4 calories). Ø 40% of calories from fat Ø (1 gm/kg/day) Ø (Igm = 9 calories). Ø

Indications of insulin: 1. 2. 3. 4. 5. 6. 7. 8. 9. IDDM. NIDDM in some conditions: In combination with diet and oral hypoglycemics after their failure Stress conditions e. g. infections, surgery, and pregnancy. Diabetic ketoacidosis: regular insulin is the only type used i. v. Other uses: Hyperkalemia (insulin + glucose): insulin ↑K+ shift from blood to cells. Anorexia nervosa: to stimulate appetite. Test for vagotomy: if there is hunger pain → vagotomy is not complete.
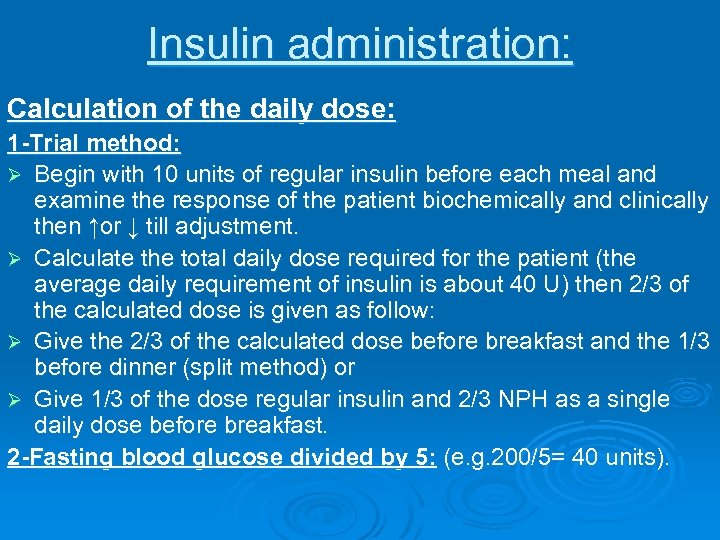
Insulin administration: Calculation of the daily dose: 1 -Trial method: Ø Begin with 10 units of regular insulin before each meal and examine the response of the patient biochemically and clinically then ↑or ↓ till adjustment. Ø Calculate the total daily dose required for the patient (the average daily requirement of insulin is about 40 U) then 2/3 of the calculated dose is given as follow: Ø Give the 2/3 of the calculated dose before breakfast and the 1/3 before dinner (split method) or Ø Give 1/3 of the dose regular insulin and 2/3 NPH as a single daily dose before breakfast. 2 -Fasting blood glucose divided by 5: (e. g. 200/5= 40 units).

Ø 3 -Multiple daily injections= Basal-Bolus regimen : give one injection of long acting insulin (ultralente) at bed time; plus three daily injection of short acting insulin before each meal. The ultralente insulin provides basal level of insulin that controls blood glucose during night and between meals, and the other three injections control postprandial hyperglycemia. Commonly used for patients with Type -1 DM and in pregnant women with diabetes. 4 - B)Twice-daily biphasic insulin regimen Use biphasic insulin. - Give the 2/3 of the TDD in the morning and the 1/3 at evening. It is commonly used for patients with Type-2 DM.

Insulin administration: All insulin are given by s. c. injection. Ø Regular insulin is the only type that can be given i. v. in diabetic emergencies. Ø The standard insulin concentration is 100 units/m. L (U 100). Ø It should be injected with a standard U-100 syringe. Ø Under Clinical Trials Oral tablets Inhaled aerosol Intranasal, Transdermal
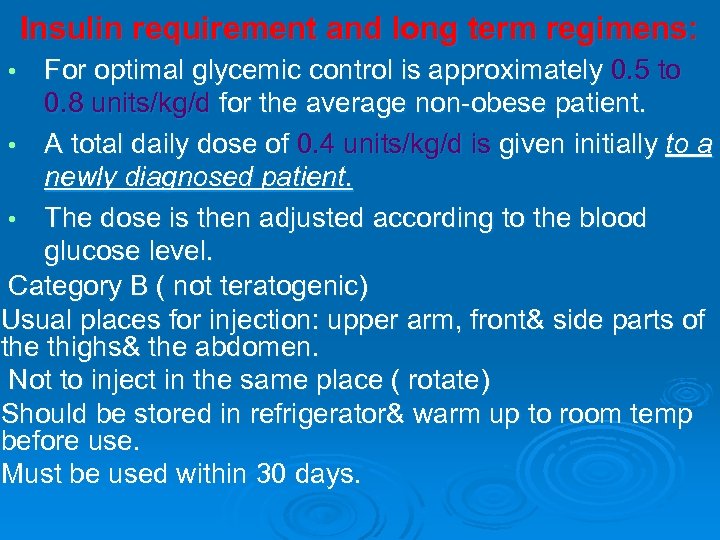
Insulin requirement and long term regimens: For optimal glycemic control is approximately 0. 5 to 0. 8 units/kg/d for the average non-obese patient. • A total daily dose of 0. 4 units/kg/d is given initially to a newly diagnosed patient. • The dose is then adjusted according to the blood glucose level. Category B ( not teratogenic) Usual places for injection: upper arm, front& side parts of the thighs& the abdomen. Not to inject in the same place ( rotate) Should be stored in refrigerator& warm up to room temp before use. Must be used within 30 days. •

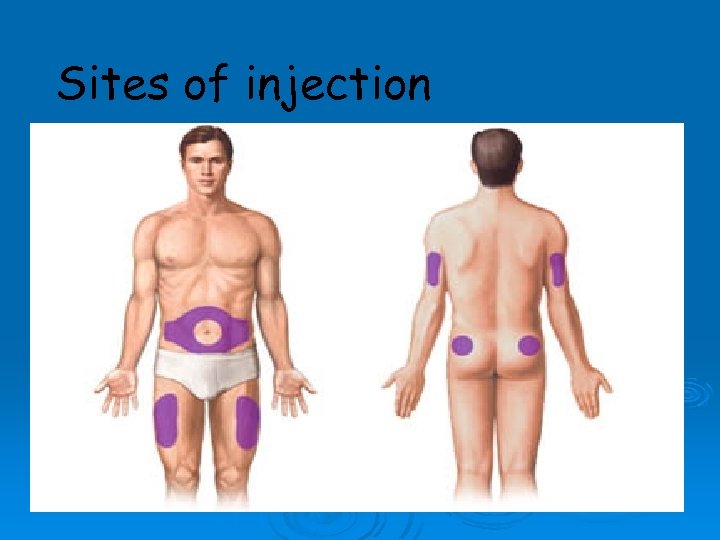
Sites of injection Arms n Legs n Buttocks n Abdomen n
78
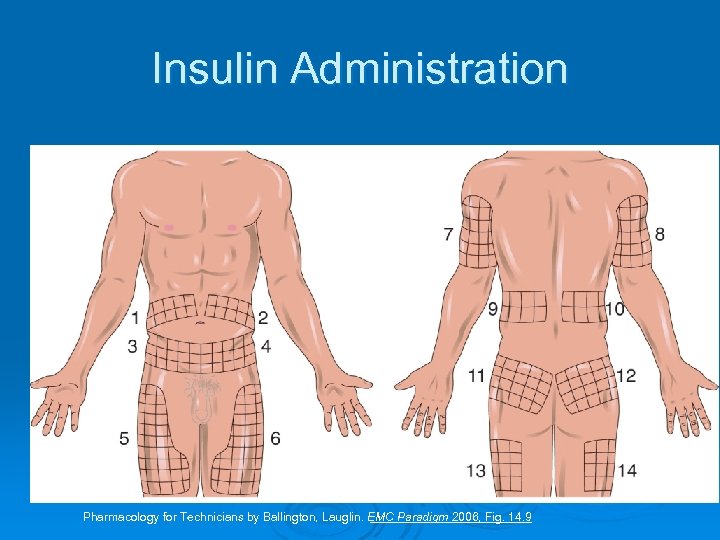
Insulin Administration Pharmacology for Technicians by Ballington, Lauglin. EMC Paradigm 2006, Fig. 14. 9

Methods of administration: Ø Ø Ø Subcutaneous injection (commonest method). Portable pen injector. Continuous insulin infusion (insulin pump = artificial pancreas): See below…………………. . Intranasal administration: is still under investigation.

Continuous insulin infusion (artificial pancreas) Closed loop method: It can measure blood glucose level by automatic glucose sensor and according to it, it releases insulin. Used mainly in acute cases e. g. ketoacidosis, before surgery. Ø Open loop method: This type can’t measure blood glucose level (has no sensor). It releases insulin according to a calculated rate. Used mainly in chronic administration. Ø Complications of artificial pancreas: 1. Hypoglycemia (excess insulin delivery) 2. Ketosis (pump failure) 3. Infection (locally).
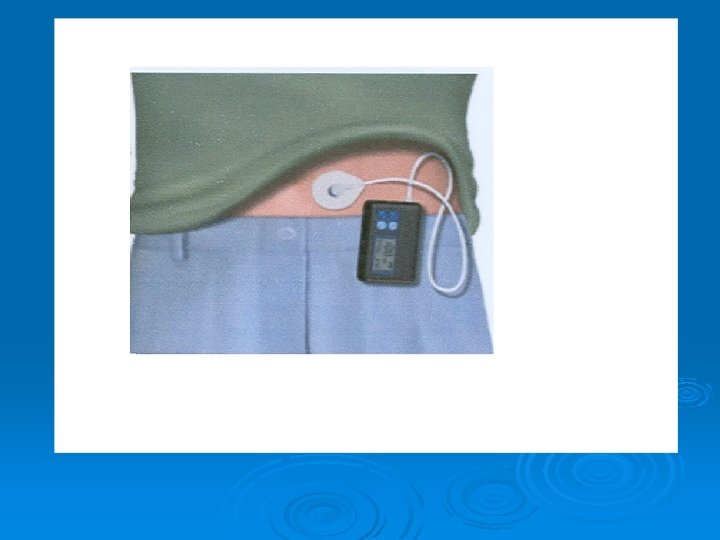

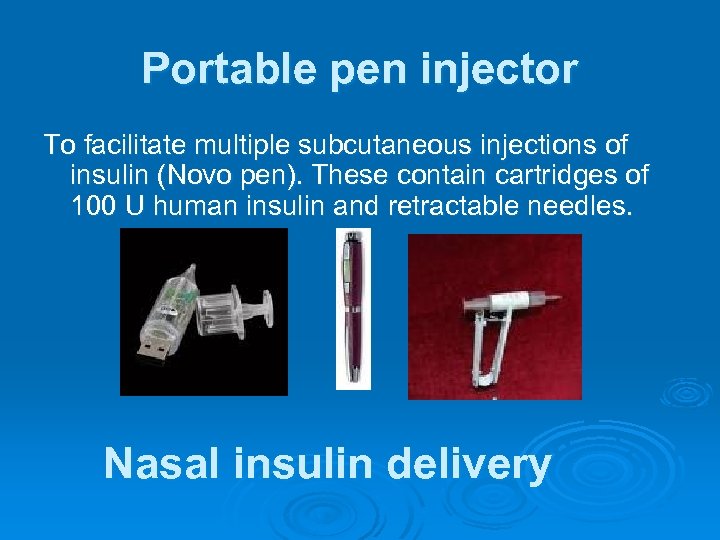
Portable pen injector To facilitate multiple subcutaneous injections of insulin (Novo pen). These contain cartridges of 100 U human insulin and retractable needles. Nasal insulin delivery

Follow up of insulin therapy: 1. By estimation of glucose in urine using urine dipsticks 2. From capillary blood glucose level using portable glucometers. Ø N. B Ø By estimation of glucose in urine: If Ø + Add no insulin to your total daily dose. Ø ++ Add 10 units insulin to your total daily dose. Ø +++ Add 20 units insulin to your total daily dose. Ø ++++ Add 30 units insulin to your total daily dose. Ø
Side effects of insulin: Local side effects: Allergy: at the site of injection (due to formation of Ig. GE). Treatment: Ø l l Change the type of insulin to more purified preparation. Local corticosteroids or antihistamines. Lipodystrophy: atrophy or hypertrophy of s. c tissue after repeated injections. Treatment: Ø Change the site of injection regularly. Ø In lipoatrophy, give cone purified regular insulin in the center and periphery of the atrophic area to stimulate lipogenesis. Ø Ø Local infection.

Systemic side effects: Ø Hypoglycemia: the most common and dangerous side effect. Ø Hypokalemia: insulin causes shift of K+ from extracellular to intracellular fluid. Ø Hypersensitivity reactions: urticaria, angioedema or anaphylactic shock. Ø Weight gain

Insulin resistance (IR): Ø Ø Ø Definition: failure of the body cells to respond to endogenous or exogenous insulin. Diagnosis: By homeostasis model assessment (HOMA Formula): IR index = Fasting insulin (u. U/ml) x Fasting glucose (mg/dl)/ 405 HOMA< 20= insulin sensitivity. HOMA > 20 = insulin resistance.

Causes and mechanism: Immunological (pre-receptors defect): due to formation of Ig. A or Ig. G antibodies against insulin. Ø Non-Immunological(receptors defect): occurs due to down-regulation of insulin receptors) such as: Ø The metabolic syndrome: it means association of central obesity, hypercholesterolemia, hyperinsulinemia, and insulin resistance. Ø Obesity. Ø Pregnancy. Ø Severe infection or stress. Ø Corticosteroids. Ø

3 - Post-receptor defect (intracellular): abnormal signal transduction due to genetic defect. 4 - Local insulin resistance: due to degradation of insulin in s. c. tissue by proteolytic enzymes. It is diagnosed by changing the route of administration to i. v. injection. If there is good response, then the resistance is local. Ø Hepatitis C makes people three to four times more likely to develop type 2 DM and insulin resistance.
Treatment: Ø Ø Ø Ø Ø Life-style modification: e. g. Diet: should have low glycemic index (i. e. ↑ vegetables and ↓ CHO). Weight reduction. Encourage physical exercise. Correction of the precipitating factors: e. g. infection. Metformin: it ↓hepatic output of glucose and ↓ intestinal glucose absorption so it ↓ plasma insulin levels. Insulin sensitizers e. g. pioglitazone or sulphonylureas to ↑ insulin receptors sensitivity. Small dose of fluorinated corticosteroids e. g dexamethazone : because it has anti-immunological effect e little or no hyperglycemic effect. In case of local insulin resistance: l l Change the site of injection regularly. Give aprotinin (trasylol) with insulin at the injection site to inhibit the proteolytic enzymes.

Pseudo-insulin resistance (Somogyi phenomenon): Definition: It is rebound hyperglycemia following hypoglycemia occurs in patients over-treated with insulin (especially children) due to release of counter-regulatory hormones. Ø Treatment: adjust insulin dose and diet control. Ø

The Somogyi effect and Dawn Phenomenon The Somogyi Effect: Hypoglycemia associated with alternate episodes of hyperglycemia. The Dawn phenomenon is characterized by increased levels of fasting blood glucose or insulin requirements, or both, between 5 and 9 AM without antecedent hypoglycemia.

✐ Events Requiring an Increase in Dosage of Insulin in Diabetic Patients Infections ✐ High fever ✐ Trauma, surgical operations ✐ Myocardial infarction ✐ Pregnancy ✐ Hyperthyroidism ✐ Diabetic ketoacidosis

Oral anti-diabetic drugs Pts with type 11 diabetes have two physiological defects: Abnormal insulin secretion Resistance to insulin action in target tissues associated with decreased number of insulin receptors Hypoglycemic drugs (insulin secretagogues): Def. : are drugs that stimulate insulin secretion and may lead to hypoglycemia. Ø Members: Ø Sulphonylureas. Ø Repaglinide. Ø Nateglinide. Anti-hyperglycemic drugs: Def. : are drugs that prevent hyperglycemia but don't induce hypoglycemia. Ø Members: Ø Biguanide: e. g. metformin. Ø Thiazolidinediones: e. g. troglitazone and pioglitazone Ø Starch blockers: acarbose.

1. Sulfonylureas Ø 1. 2. 3. Classification: First-generation compounds: chlorpropamide, tolbutamide, acetohexamide. Second-generation compounds: glibenclamide, glyclazide and glipizide; up to 200 times more potent than first-generation. Third-generation compounds: glimepiride; may interact with different cellular proteins.

Pharmacokinetics: Ø Absorbed from GIT. Given 30 minutes before breakfast. Ø Food can reduce the absorption. Ø Plasma protein binding is high 90 – 99 %. Metabolized by liver and their metabolites are excreted in urine with about 20 % excreted unchanged.
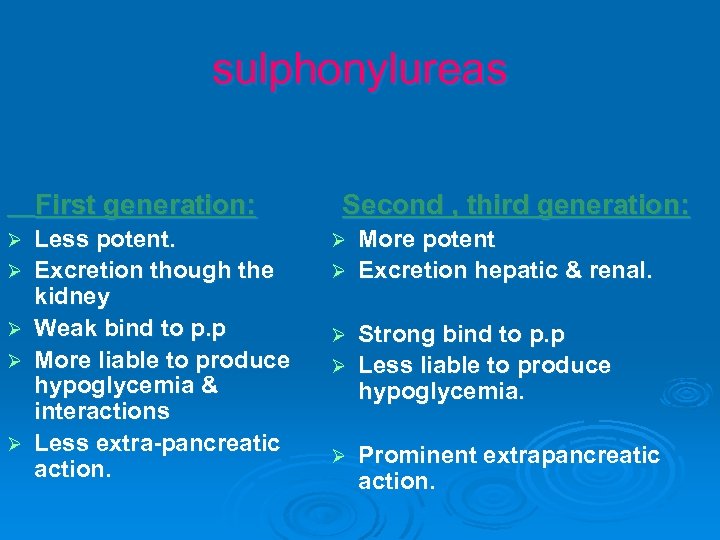
sulphonylureas First generation: Ø Ø Ø Less potent. Excretion though the kidney Weak bind to p. p More liable to produce hypoglycemia & interactions Less extra-pancreatic action. Second , third generation: More potent Ø Excretion hepatic & renal. Ø Strong bind to p. p Ø Less liable to produce hypoglycemia. Ø Ø Prominent extrapancreatic action.
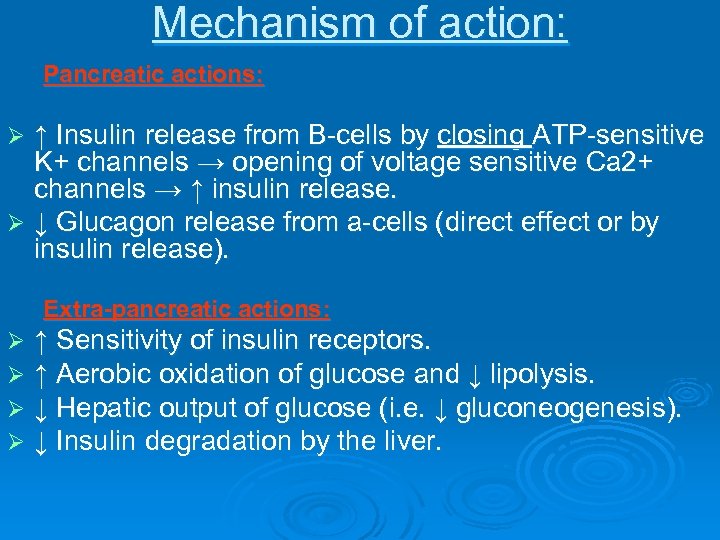
Mechanism of action: Pancreatic actions: ↑ Insulin release from B-cells by closing ATP-sensitive K+ channels → opening of voltage sensitive Ca 2+ channels → ↑ insulin release. Ø ↓ Glucagon release from a-cells (direct effect or by insulin release). Ø Extra-pancreatic actions: Ø Ø ↑ Sensitivity of insulin receptors. ↑ Aerobic oxidation of glucose and ↓ lipolysis. ↓ Hepatic output of glucose (i. e. ↓ gluconeogenesis). ↓ Insulin degradation by the liver.

Therapeutic uses: Ø NIDDM after failure of diet regime. Ø With insulin in special cases. Ø Chloropropamide is used in ttt of nephrogenic diabetes insipidus (it ↑ sensitivity of renal tubules to the effect of ADH).

Side effects: Ø Ø Ø Hypoglycemia: especially with long acting drugs or in elderly patients with hepatic or renal dysfunction. Cholestatic jaundice. Allergy: due to presence of sulfur (sulphonylureas). There is no cross reactions between different drugs. Teratogenicity. Tachyphylaxis and secondary failure to sulfonylureas: Def. : failure to maintain good response to sulfonylurea therapy over long term. ↑ by: Progressive ↓ in B-cell mass ↓ physical activity. ↓ in lean body mass. ↑ increase ectopic fat deposition in type 2 DM.

Contraindications: Ø Presence of complications: e. g. ketoacidosis and infections. Ø Pregnancy. Ø Hepatic or renal dysfunctions.

Repaglinide and nateglinide Ø Def. : are non-sulphonylureas that ↑ insulin release. Ø Characters: l l l Very fast onset. Short duration (so must be given just before meals) Taken before each meal, 3 times / day Hypoglycemia can occur if the meal is delayed or skipped. T 1/2 of both = 2 hrs. Duration of action = 4 -6 hrs.

Ø Mechanism of action: Ø ++ very rapid and transient release of insulin from B-cells through closure of ATP-sensitive K+ channel. (the mechanism is very close to that of sulfonylureas) Ø It also partially restores initial insulin release significant advantage of the drug because type 2 DM is associated e loss of this initial insulin response. (Restoration of more normal insulin secretion)

Uses: Useful in controlling post-prandial hyperglycemia or in patients whose diet varies from day to day. l Don't contain sulfur so can be used in patients allergic to sulphonylureas. l Used as monotherapy or in combination with biguanides.

Adverse effects Hypoglycemic reaction ✐ Upper respiratory tract infections ✐ Contraindications and precautions Type I diabetes (alone) ✐ Severe hepatic disease. ✐ Pregnancy ✐
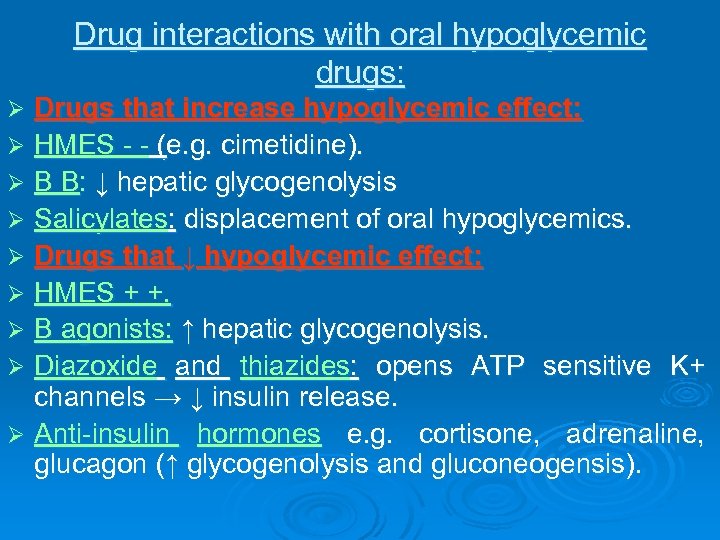
Drug interactions with oral hypoglycemic drugs: Drugs that increase hypoglycemic effect: Ø HMES - - (e. g. cimetidine). Ø B B: ↓ hepatic glycogenolysis Ø Salicylates: displacement of oral hypoglycemics. Ø Drugs that ↓ hypoglycemic effect: Ø HMES + +. Ø B agonists: ↑ hepatic glycogenolysis. Ø Diazoxide and thiazides: opens ATP sensitive K+ channels → ↓ insulin release. Ø Anti-insulin hormones e. g. cortisone, adrenaline, glucagon (↑ glycogenolysis and gluconeogensis). Ø
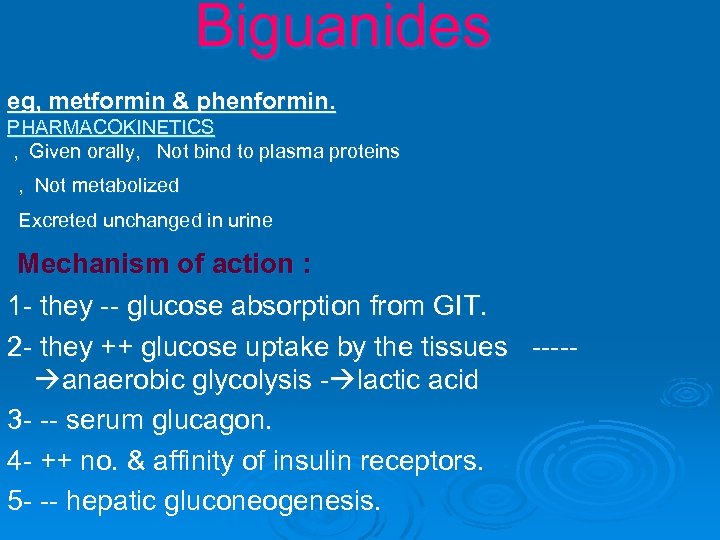
Biguanides eg, metformin & phenformin. PHARMACOKINETICS , Given orally, Not bind to plasma proteins , Not metabolized Excreted unchanged in urine Mechanism of action : 1 - they -- glucose absorption from GIT. 2 - they ++ glucose uptake by the tissues ---- anaerobic glycolysis - lactic acid 3 - -- serum glucagon. 4 - ++ no. & affinity of insulin receptors. 5 - -- hepatic gluconeogenesis.

Uses 1 - Type 2 DM either alone (in mild cases) or in combination with other drugs (SU). obese pt with t 2 dm 2 - to enhance weight loss in overweight patient 3 - with insulin in case of insulin resistance. Ø Polycystic ovary syndrome; it lowers serum androgens and restores normal menstrual cycles and ovulation. Advantages of Metformin over SUs Does not cause hypoglycemia ( why ? ) ( Ideal for obese pts) Does not result in wt gain ( why ? )
Side effects: Ø Anorexia, vomiting, diarrhoea. Ø Metallic taste. Ø ↓ Absorption of vitamin B 12 (→ megaloblastic anaemia). Ø Lactic acidosis: common e phenformin and less e metformin; especially in renal or hepatic diseases. Lactic acidosis (rare but fatal in 50% of cases) (by inhibiting gluconeogenesis the drug impairs the hepatic uptake of lactic acid)

thiazolidinediones (Tzds) eg. pioglitazone & roseglitazone and troglitazone. Pharmacokinetics: Ø They are absorbed within 2 hours of ingestion Ø They are taken once a day Ø It is bound to plasma proteins by more than 99 % Ø metabolized in the liver by micosomal enzymes
Mechanism of action: Ø Ø Thiazolidinediones act to decrease insulin resistance. The overall effect is an enhancement of tissue sensitivity to insulin (that is a reduction in insulin resistance). Therefore the need of exogenous insulin is reduced. Their primary action is the nuclear regulation of genes involved in glucose and lipid metabolism and adipocyte differentiation. Tzds are ligands of peroxisome proliferator-activated receptor-gamma (PPAR-γ). They have a slow onset and offset of activity over weeks or even months. These drugs are ‘insulin sensitizers’. They bind to a nuclear receptor (peroxisome proliferator activated receptor, PPAR), located mainly in adipose tissue, skeletal muscle and liver, which regulates the transcription of several insulin responsive genes.

Pharmacological actions They act by increasing the member of GLUT 4 glucose transportars in cell membrane of muscles and adipose tissue. -- increases the uptake and utilization of glucose in these tissues. Ø it also suppresses hepatic glucose output. Ø They increase HDL and decrease triglycerides Ø Tzds also have significant effects on vascular endothelium, the immune system, the ovaries, and tumor cells (Reduction of hyperglycemia, hyperinsulinemia and hypertriglyceridemia that are characteristic of insulinresistant states). Ø

The drugs are antihyperglycemic, not hypoglycemic. They do not cause hypoglycemia (similar to metformin and acarbose ) They do not cause hypoglycemia when given alone, but can prevent postprandial hyperglycemia. Clinical effect is not observed for 6 to 12 weeks. Therapeutic uses Ø In combination with insulin, biguanides or sulfonylureas, in type II diabetes which exhibits insulin resistance.

Side effects: fluid retention, peripheral edema. 2. weight gain. 3. Anovulatory women may resume ovulation and increased risk of pregnancy. 4. Hepatotoxicity Contraindication: pregnancy, liver disease, heart failure. Type 1 DM 1.

. αAlpha-Glucosidase inhibitors (starch blockers): Eg. Acarbose They act by competitive inhibition of intestinal αglucosidase enzyme → ↓ digestion & absorption of carbohydrates. Ø Given as monotherapy or in combination with sulphonylureas in type II. Ø Sl. E: Flatulance , abdominal pain & diarrhea may occur. Ø GIT side effects are common: flatulence, diarrhea, abdominal pain. Ø They should be avoided in patients with inflammatory bowel disease or renal impairment. Ø

s rug r e d w ic e t N be ia id t n a

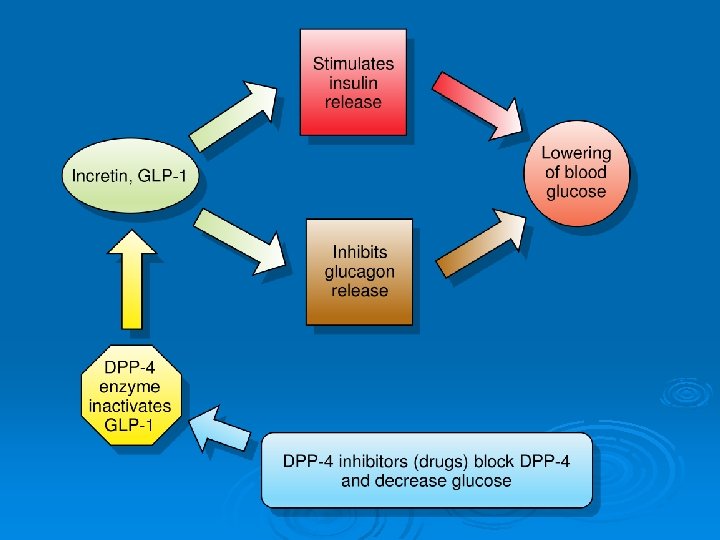
1. Incretin (GLP-1) mimetics: Ø Endogenous human incretins, as glucagon-like peptide-1 (GLP-1), are released from the gut and ↑ insulin secretion in response to oral glucose. Ø Exenatide is a synthetic GLP-1 analog; liraglutide is longer acting analog and more resistant to metabolism by DPP IV. Mechanism of action: 1. They ↑ insulin secretion and ↓ glucagon secretion. 2. They slow gastric emptying and ↓ appetite. 3. Improve insulin sensitivity. 4. ↑ Beta cells number.

Therapeutic uses: 1. Type 2 DM either alone or in combination with oral drugs. (should not be given with insulin). Ø They are given parenterally (s. c. ) 60 min before breakfast and dinner. Ø Major adverse effects: nausea, vomiting, and pancreatitis (may be serious). Ø

2. Dipeptidyl peptidase 4 inhibitors (Gliptins): Sitagliptin. , Vildagliptin Mechanism of action: Inhibits the breakdown of GLP-1 by DPP-4 therefore increasing GLP-1 levels resulting in increased glucose-dependent insulin release and decreased Ø Type 2 diabetics little incretin effect Reduced GLP-1 secretion Ø Therapeutic uses: 1. Type 2 DM either alone or in combination with metformin. Ø They are given orally; most common side effects are headache and nausea.

3. Amylin analogs: Pramlintide Ø Pramlintide is a synthetic amylin analog. Amylin is a polypeptide secreted by β-cells of the pancreas, and it acts in concert with insulin to reduce blood sugar. Ø It is injected s. c. , typically with insulin. Ø Common side effects are hypoglycemia and nausea.

Plan for treatment of Type-2 Diabetes Diet/ exercise . Oral monotherapy Oral Insulin combination +/- insulin

f o t n e ic s tm e t o n a b ti re ia ca T d li p m o c

Treatment of diabetic complications Acute complication (4 types): Ø Hypoglycemic coma. Ø Diabetic keto-acidosis (DKA). Ø Hyperosmolar non-ketotic coma (HONK). Ø Lactic acidosis (rare). Ø Chronic complications (4 types): Ø Angiopathy. Ø Neuropathy. Ø Retinopathy. Ø Nephropathy. Ø

▌Hypoglycemic coma Causes: 1. Large dose of insulin or sulfonylurea. 2. Missed meal while taking insulin or sulfonylureas. 3. Vigorous muscular exercise without dietary adjustment. Ø

Manifestations: They are first discerned at a plasma level of 60 to 80 mg/DL and include: 1. Sympathetic overactivity: tremors, cold sweating, mydriasis, tachycardia anxirety. 1. parasympathetic overactivity(hunger pain , nausea), mental confusion, convulsions, and finally coma. l They are also named neuroglycopenic symptoms (headache, mental confusion, weakness, dizziness, blurred vision, drowsiness, bizarre behavior, convulsions and coma). Ø l Severe hypoglycemia (seizure/coma) (BG < 40 mg/d. L) Therapy Conscious patients: oral glucose Ø Unconscious patients: IV glucose (25 -50%%) or glucagon IM 1 mg I. M Ø

Hyperglycemic coma (diabetic ketoacidosis; DKA): Ø Causes: Ø Ø Excess food intake. Inadequate insulin administration. Febrile illness or stress condition. Ø Manifestations: Ø Dehydration and dry shrunken tongue. Smell of acetone in breath and sugar and ketone in urine. Acidotic breathing (rapid and deep) due to acidosis. Deep coma in late cases. Ø Ø

Hyperglycemia leads to osmotic diuresis, dehydration, and a critical loss of electrolytes. Hyperosmolality of extracellular fuids from hyperglycemia leads to a shift of water and potassium from the intracellular to the extracellular compartment. Abdominal pain Fruity smell breath Hypotension and tachycardia Rate and depth of respiration increase (i. e. , Kussmaul respiration)

Treatment: Ø Fluids: 1 -2 litters of isotonic saline solution i. v. immediately, then determine the amount by urine output and clinical response of patient. Ø Regular insulin: 0. 3 U/kg i. v. then 0. 1 U/kg/h by i. v infusion. When blood glucose reaches 300 mg/dl, switch to s. c. insulin according to urine glucose. Ø Glucose 5 % is given if blood glucose falls below 300 mg/dl.

Ø Potassium: is given according to K+ level. It is better to be given as phosphate salt rather than KCl to correct associated phosphate depletion. Ø Bicarbonate (HCO 3): by i. v. i. to correct acidosis. Ø Treatment of precipitating factors e. g. antibiotics for infection.
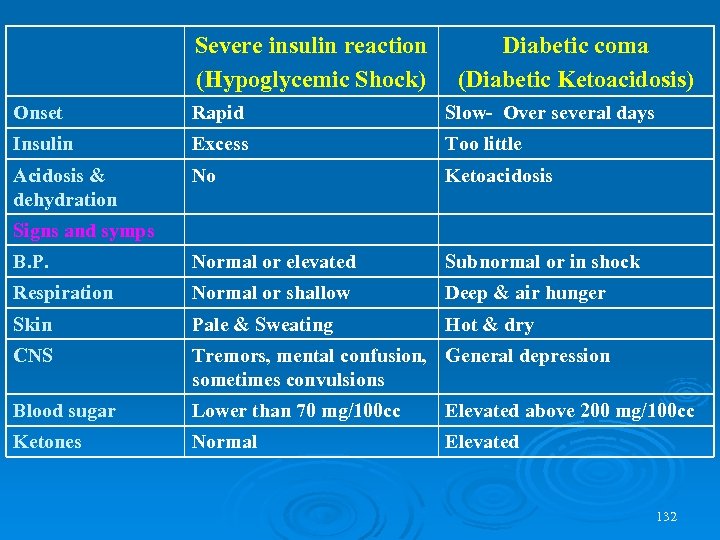
Severe insulin reaction (Hypoglycemic Shock) Diabetic coma (Diabetic Ketoacidosis) Onset Rapid Slow- Over several days Insulin Excess Too little Acidosis & dehydration No Ketoacidosis B. P. Normal or elevated Subnormal or in shock Respiration Normal or shallow Deep & air hunger Skin Pale & Sweating Hot & dry CNS Tremors, mental confusion, General depression sometimes convulsions Blood sugar Lower than 70 mg/100 cc Elevated above 200 mg/100 cc Ketones Normal Elevated Signs and symps 132

Hyperosmolar hyperglycemic state (HHS): Ø It is a life-threatening medical emergency that is more common in old age. Hyperglycemia causes to ↑ water loss (osmotic diuresis) with little sodium loss → severe dehydration and hyperosmolarity.
![Hyperosmolar Hyperglycemic State Characterized by hyperglycemia (blood glucose >600 mg/d. L[33. 3 mmol/L]), Hyperosmolarity Hyperosmolar Hyperglycemic State Characterized by hyperglycemia (blood glucose >600 mg/d. L[33. 3 mmol/L]), Hyperosmolarity](https://present5.com/presentation/7cdaa35b568bfb2a611625a650708e6d/image-134.jpg)
Hyperosmolar Hyperglycemic State Characterized by hyperglycemia (blood glucose >600 mg/d. L[33. 3 mmol/L]), Hyperosmolarity (plasma osmolarity>320 m. Osm/L) and Dehydration, the absence of ketoacidosis, and depression of the sensorium. Manifestations are weakness, dehydration, polyuria, neurologic signs and symptoms, and excessive thirst.

Treatment: similar to DKA except in: 1. 2. 3. 4. Fluids: rapid and aggressive i. v. isotonic saline: the principal treatment. Insulin is needed in smaller amount than in DKA. Insulin used without concomitant vigorous fluid replacement increases the risk of shock. Hypokalemia is usually less marked. Low dose of heparin to prevent thrombotic complications.
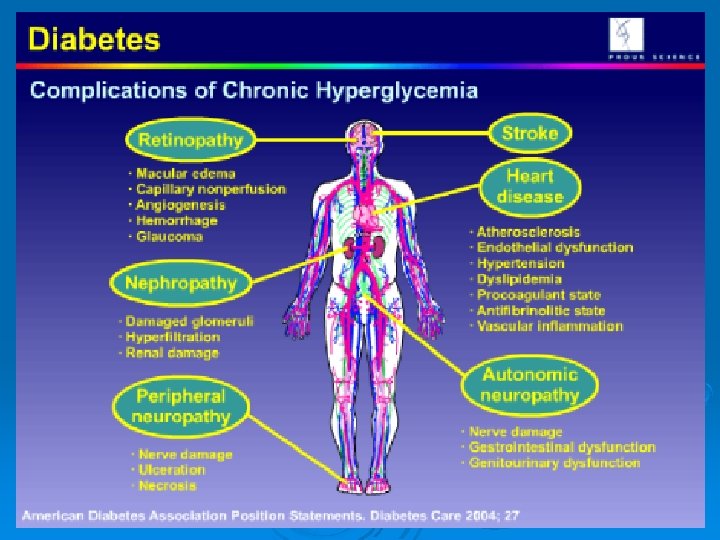
Chronic Complications of Diabetes Mellitus Increased glucose levels allow glucose to bind to proteins in: Hemoglobin Hb A 1 C has higher O 2 affinity Basement membranes of blood vessels Nephropathy Retinopathy May cause increased risk of atherosclerosis Lens cataracts
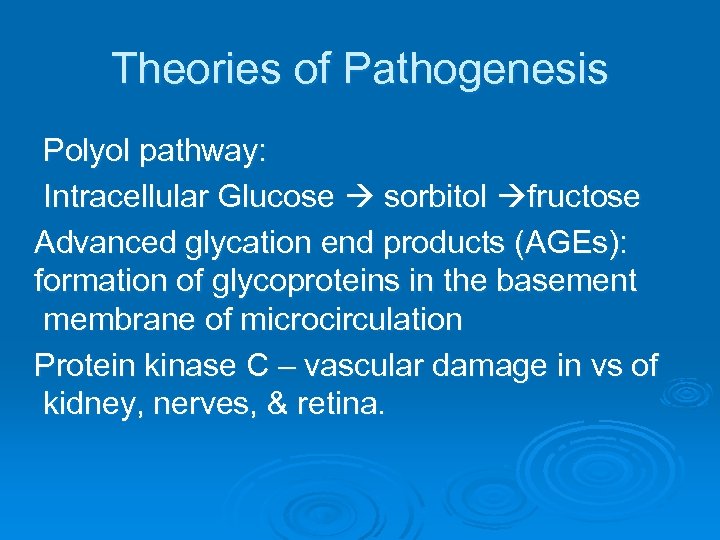
Theories of Pathogenesis Polyol pathway: Intracellular Glucose sorbitol fructose Advanced glycation end products (AGEs): formation of glycoproteins in the basement membrane of microcirculation Protein kinase C – vascular damage in vs of kidney, nerves, & retina.
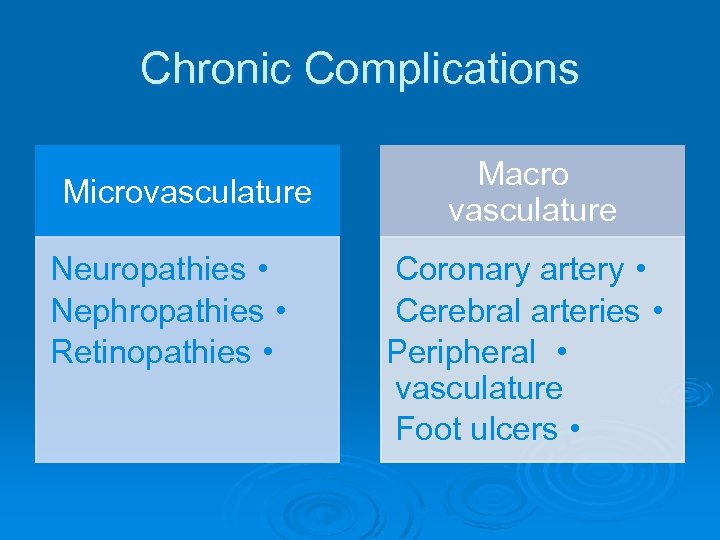
Chronic Complications Microvasculature Neuropathies • Nephropathies • Retinopathies • Macro vasculature Coronary artery • Cerebral arteries • Peripheral • vasculature Foot ulcers •

Chronic manifestation (complications): Macrovascular disease: coronary heart disease, stroke, etc. Peripheral vascular disease. Ocular Complication: cataract, glucoma, diabetic retinopathy. Nephropathy: microalbuminuria, renal failure. Neuropathy: autonomic neuropathy, peripheral neuropathy.

Chronic complications: Ø Angiopathy, neuropathy, nephropathy and retinopathy Ø Causes Ø Accumulation of sorbitol w is produced from glucose by aldose reductase enzyme → + + of cell proliferation and collagen synthesis. Ø Prevention: Ø By giving aldose reductase - - e. g. tolrestat or sorbinil to prevent formation of sorbitol.

Diabetic neuropathy Ø Diabetic neuropathy is the most common complication of diabetes mellitus (DM), affecting as many as 50% of patients with type 1 and type 2 DM. It may be cranial, sensory, sensory-motor or autonomic. DN is an irreversible damage.

Diabetic Neuropathy Somatic neuropathy Diminished perception of vibration, pain, and temperature Hypersensitivity to light touch; occasionally severe “burning” pain Autonomic neuropathy Defects in vasomotor and cardiac responses Impaired motility of the gastrointestinal tract Inability to empty the bladder Sexual dysfunction

Osmolarity in Diabetes Mellitus When blood glucose is high, increased blood osmolarity can cause cells to shrink Nerve cells produce intracellular osmoles to keep their osmolarity balanced with the blood When the client brings blood glucose back to normal, the nerve cells are hyperosmolar to the blood and gain water, swelling Nerve damage may be caused by swelling, demyelination, and lack of O 2 secondary to vascular disease

Treatment: Ø Tight, stable glycemic control is the most important measure. Ø For neuropathic pain: oral duloxetine or pregabalin. Ø For diabetic gastroparesis: metoclopramide Ø For erectile dysfunction: sildenafil

Retinopathies Microanurysm Neovascularization Hemorrhage Scaring Retinal detachment

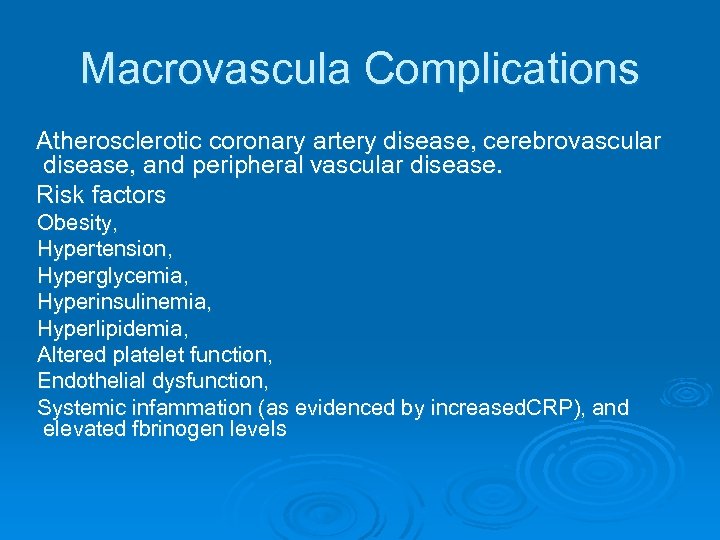
Macrovascula Complications Atherosclerotic coronary artery disease, cerebrovascular disease, and peripheral vascular disease. Risk factors Obesity, Hypertension, Hyperglycemia, Hyperinsulinemia, Hyperlipidemia, Altered platelet function, Endothelial dysfunction, Systemic infammation (as evidenced by increased. CRP), and elevated fbrinogen levels

Diabetic Foot Ulcers Effects of neuropathy and vascular insufficiency. Distal symmetric neuropathy is a major risk factor foot ulcers. People with sensory neuropathies have impaired pain sensation and often are unaware of the constant trauma to the feet caused by poorly fitting shoes, improper weight bearing, hard objects or pebbles in the shoes, or infections such as athlete’s foot.
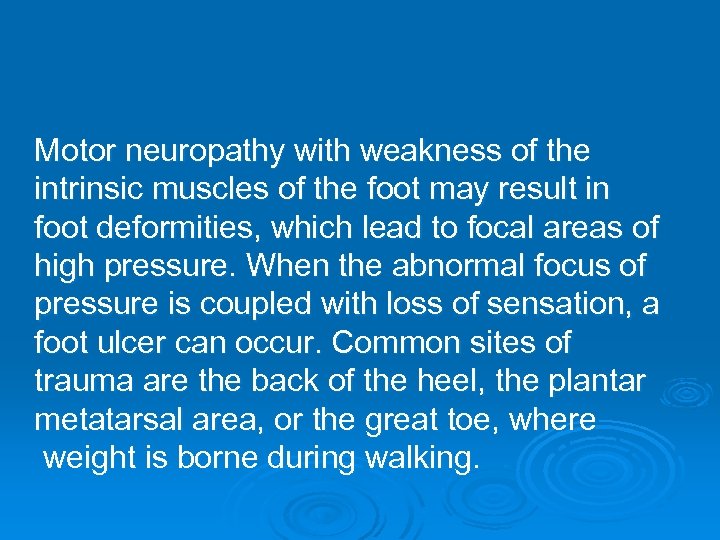
Motor neuropathy with weakness of the intrinsic muscles of the foot may result in foot deformities, which lead to focal areas of high pressure. When the abnormal focus of pressure is coupled with loss of sensation, a foot ulcer can occur. Common sites of trauma are the back of the heel, the plantar metatarsal area, or the great toe, where weight is borne during walking.

Infections Soft tissue infections of the extremities, osteomyelitis, urinary tract infections and pyelonephritis, candidal infections of the skin and mucous surfaces, dental caries and periodontal disease, and tuberculosis.

FUNGAL INFECTION BETWEEN TOES J Am Podiatr Med Assoc 98(5): 353– 356, 2008

Definition Diabetic nephropathy (DN) is a clinical syndrome in persons with diabetes characterized by : ** Albuminuria. ** Hypertension. ** Declining renal function.

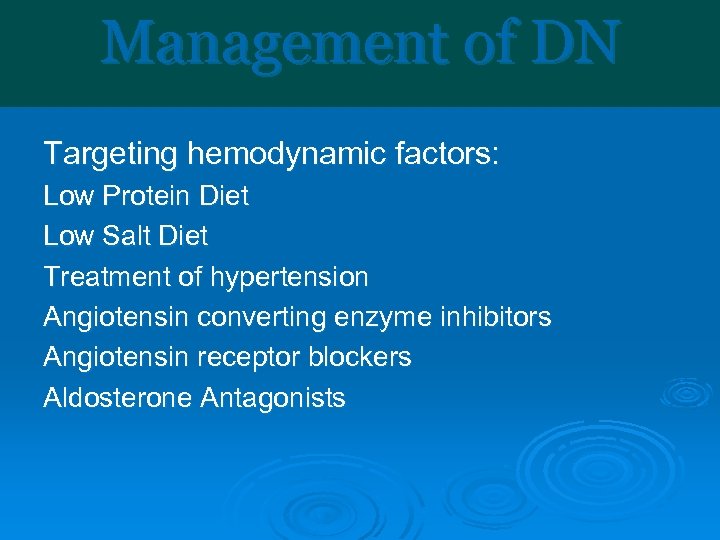
Management of DN Targeting hemodynamic factors: Low Protein Diet Low Salt Diet Treatment of hypertension Angiotensin converting enzyme inhibitors Angiotensin receptor blockers Aldosterone Antagonists

A. Glycemic control : - Prevents progression of microalbuminuria to macroalbuminuria. - Prevent deterioration of kidney function in macroalbuminuric patients. - Prevents CVS complications

- Glycemic control aims for A 1 c level <7%. - Adjust the dose of anti-hyperglycemic drugs (If albuminuria and reduced kidney function )
Exercise and smoking cessation.

Help them not reach this unpleasant stage

Case 1: 18 years old male student (wt. 40 kg and height 155 cm) complained of polyurea, polydypsia, loss of weight easy fatiguability and recurrent urinary tract infection during the last three months. Clinical and laboratory investigations reveal fasting plasma glucose 280 mg/dl, HBA 1 C 14% and glucose in urine was ++ which conclude the diagnosis of diabetes mellitus.

What are the diagnostic criteria for diabetes in this patient? What is the possible type of diabetes in this case? What are the goals of therapy? Mention the lines of treatment of this patient? What is the recommended daily caloric requirement for such patient? What is the drug of choice to start with in this case to control hyperglycemia? What is the route of administration of the drug selected? How you proceed to adjust the dose for your patient? Explain in details? What are the methods of administration of the maintenance dose of selected drug

Seven days later, he came to you suffering from severe allergy subsequent to intake of selected drug. What would be the treatment in such situation? Three months, later, the patient developed resistance to therapy although he increased the daily dose of insulin to 200 units regular insulin of pork type allover this period. What would be the most likely cause/s under such situation? Once you changed the route of administration of insulin to the I. V route his diabetic state was dramatically controlled by 40 units of regular insulin only.

How you explain such situation? How are you going to manage such situation? If the resistance to insulin therapy was of systemic type, what is the proper management of such situation? Four years after treatment you have been asked to see your patient and he was in a drowsy state, and had a carbuncle in his back, his breath smelled acetone and you discover that he has diabetic ketosis. What is the most important modification you have to do in the administration of insulin and what drugs would you like to add to treat such situation? Why?

Ø What are the most common complications of insulin therapy? Ø Name four drugs, which may increase the blood glucose level?

Case 2: 45 years old obese lady (B. W. 100 kg, height 175 cm) complained of polyurea, polydypsia and fatigue within the last few weeks. Clinical and laboratory investigations (fasting plasma glucose 200 mg/dl, Hb. A 1 C 10%, glucose in urine ++). These manifestations conclude the diagnosis of diabetes mellitus. Mention the type of diabetes in this case? If the diet regimen alone sufficient for control of hyperglycemia in this case? What is the recommended caloric intake for such patient? What is the proper duration to chick the efficiency of dietetic regimen? If your patient is in need for antidiabetic drugs for proper control of diabetic state and obesity after 4 months. Can you use metformin alone?

Ø Ø Ø Ø Mention the dose and route of administration of selected drug? What is the mechanism of action of the selected antidiabetic drug? Mention the most common side effects of the selected drugs? After one year patient came to the clinic with fasting blood glucose 300 mg% and H BA 1 C 13%. Would you like to add one of sulphonylureas to treatment regimen? Mention the dose and route of administration of selected drug in Q. 9? What is the mechanism of action of the selected drug in Q. 9? Mention the most common side effects of the selected drugs in Q. 9? After several months, the patient got fever, 39 co. On examination a gluteal abscess was found. How would you proceed to treat this condition?

Ø Ø Ø Ø Ø After the cure of the pyogenic infection, what would be your decision concerning the diabetic state? The patient was then under control for few years, but she has to have an operation for a gall stones. What are the changes in the regimen for treatment of her diabetic state? During recovery from the operation, the patient was drowsy, sweaty, had tachycardia, lack of concentration, no ketonurea, her blood glucose was 60 mg%. What is the cause of this complaint and the proper treatment of this situation? What are the common drugs that can potentiate the hypoglycemic effect of sulphonylurea drugs? What are the common drugs that can antagonize the hypoglycemic effect of sulphonylurea drugs? How to mange diabetes mellitus with pregnancy? How to mange hypertension with diabetes mellitus? How to mange IHD with diabetes mellitus? What are the stressful conditions that modify the diabetic regimen?

Case study A 12 year old boy is brought to the Emergency department of the hospital with recurrent vomiting and increasing drowsiness for the past few hours. Clinically he is found to have a fruity odour of his breath. The mother has come with the child and mentions that the child has lost weight in the last 3 months; however the mother says that he has been eating and drinking even more than usual and has been complaining of thirst all the time. On further enquiring, it is noted that the child was passing a large amount of urine frequently and was getting up frequently at night to pass urine.

Case study The lab findings for the child are as follows; Ø TLC: 11, 400/mm 3 DLC: normal Sodium: 132 m. Eq/L Chloride: 100 m. Eq/L BUN: 14 mg/d. L Creatinine: 1. 2 mg/d. L Potassium: 4. 9 m. Eq/L Glucose: 320 mg/d. L Plasma ketones 1+ Urinary glucose 4+ Urinary ketones 2+ Plasma bicarbonate level 19 Blood p. H 7. 2 Hb. A 1 c 83 mmol/mol (10%) (Normal below 48 mmol/mol (6. 5%) )

What are the important points in the patient’s history? Weight loss Excessive eating (polyphagia) Excessive drinking water (polydipsia) Passing excessive urine (polyuria) Frequently passing urine at night (nocturia)

What arte the major abnormalities in the investigation results and what do these indicate ? The patient has very high blood glucose levels with elevated ketone levels in plasma and urine. This would indicate Diabetes mellitus and also Ketosis (excess ketone production) which is commonly seen with Type 1 Diabetes mellitus. Elevated Hb. A 1 c which further supports the diagnosis of Diabetes mellitus. Slightly low sodium levels could be due to recurrent vomiting and interference of high glucose levels with lab measurement of serum sodium levels Low Bicarbonate level with low p. H indicates Metabolic acidosis The patient has high WBC count which may indicate an underlying infection or stress response to the metabolic abnormalities.

ANSWER THE FOLLOWING QUESTIONS How did the insulin deficiency lead to an increase in plasma glucose & ketone concentration? 2. What are the types of DM ? 3. What is DKA(diabetic ketoacidosis)? 4. What is the most likely diagnosis of this patient? 5. What is the treatment of type 1 DM?

case • A 38 year old lady, a bank manager, was referred to the Diabetes clinic. She was found to be obese with a Body mass index of 32 kg/m 2 • She had been diagnosed to have diabetes about 2 months ago in the primary care clinic when she presented with undue fatigue and was found to have fasting hyperglycemia with an Hb. A 1 c of 83 mmol/mol (10 %). She did not have any history of ketoacidosis at any point of time. • The patient mentioned that her father had developed DM at the age of 45 years and was currently on tablets • The patient was taking a healthy diet but was sedentary most of the time. • On further questioning the patient gave a history of having developed high blood glucose during pregnancy (Gestational Diabetes Mellitus) last year which had resolved after delivery. • Her physician had been concerned because the patient’s Hb. A 1 c ( 10 %) was high and the patient may develop complications especially renal complications.

CASE SCENARIO He had begun to increase his consumption of canned soft drinks, asking for more pocket money to spend on them, and then drinking anything available, even water! Not surprisingly, his mother noticed he was visiting the toilet much more frequently to micturate. Ian’s mother is ‘pretty certain’, with hindsight, that he has stopped putting on weight. He has lost interest in many of his usual activities. On examination, the A&E doctor finds a youth of normal stature and at the pubertal stage of development, lying in the recovery position. He is breathing both quickly (40/minute) and deeply, and his breath smells of nail varnish remover. His blood pressure is 80 / 40 mm. Hg and pulse rate 140 /minute. His bladder is full and his eyes are sunken. Tissue turgor is reduced and his mouth is dry.

Thank you
7cdaa35b568bfb2a611625a650708e6d.ppt