0ae19f07f9cf4bd95b57caf6740fd488.ppt
- Количество слайдов: 18

Development of an Institutional Knowledge-base at FDA’s Center for Food Safety and Applied Nutrition Kirk B. Arvidson 1, Annette Mc. Carthy 1, Chihae Yang 2, Dimitar Hristozov 1 1)U. S. Food and Drug Administration (FDA) Center for Food Safety and Applied Nutrition (CFSAN) Office of Food Additive Safety (OFAS) 2) The Ohio State University, Columbus, Ohio

Outline • • • Introduction CERES workflow Database scheme Query Results QSAR models Q&A

Office Of Food Additive Safety (OFAS) • OFAS is a program office within CFSAN – Ensure the safety of food additives and packaging in U. S. • Evaluate safety information in industry submissions for various categories of food ingredients – – Direct food additives (e. g. , high intensity sweeteners) Biotech foods (e. g. , herbicide-ready soybeans) Generally Recognized as Safe (GRAS – phosphoric acid) Food contact substances (e. g. , plastic bottles, sanitizers)

Pre- and Post-market Evaluations • Shortfall of current processes – – – Rely on institutional knowledge Data is sequestered in multiple small databases Accessing/locating data can be difficult and time consuming Difficult to keep data up to date-time and resource intensive No systematic method to bring together disparate tox. data on a compound or its structural and biological analogues • Current needs – – – A centralized knowledgebase accessible from desktop computer Real time access to data-both internal and external sources Relate new and existing data in new ways Leveraging knowledge more efficiently Make sound decisions faster

Chemical Evaluation and Risk Estimation System (CERES) • Food additives knowledge-base – Captures institutional knowledge – Chemical centric – Structured data/controlled vocabulary • Desktop access to: – – Internal and external chemical and toxicity data Structure analog searching and data retrieval QSAR Models Threshold of Toxicological Concern evaluations
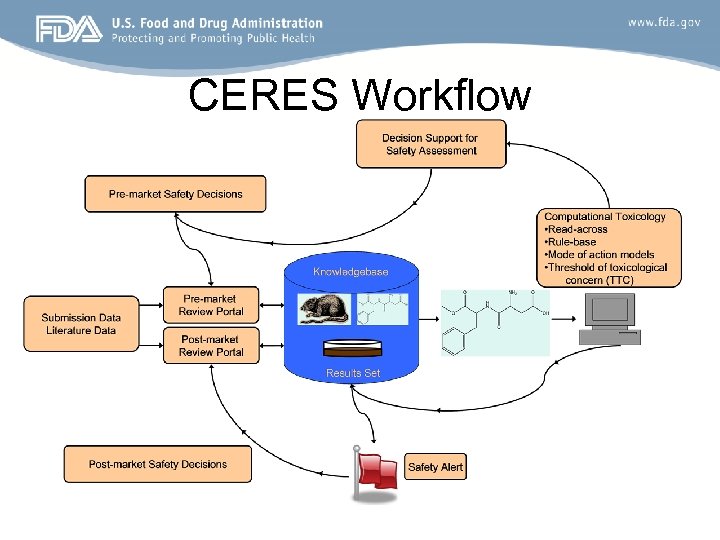
CERES Workflow
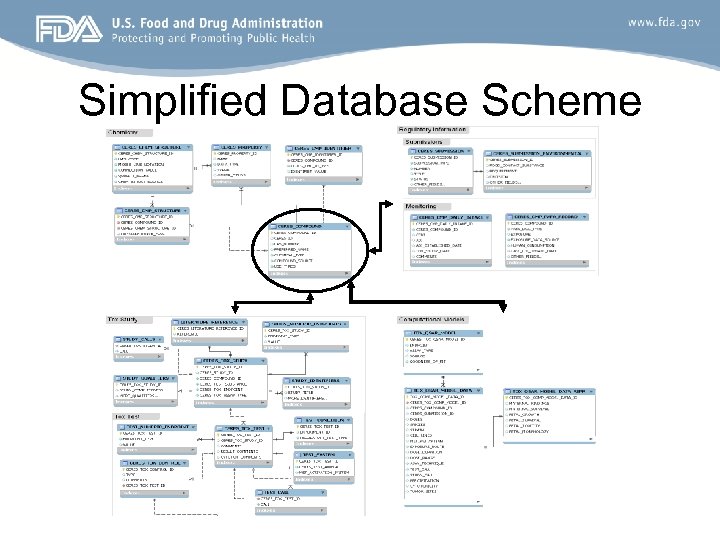
Simplified Database Scheme
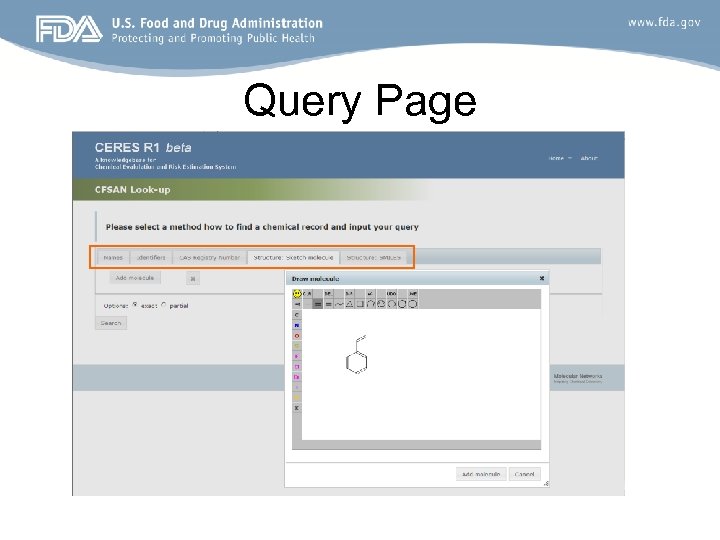
Query Page
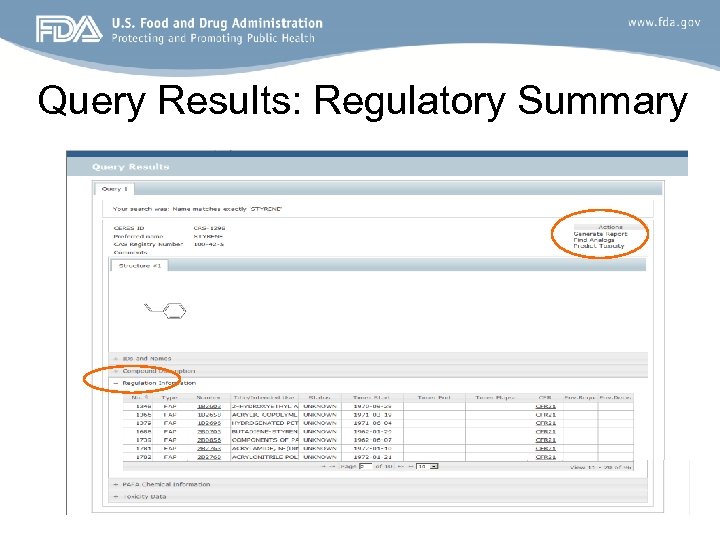
Query Results: Regulatory Summary
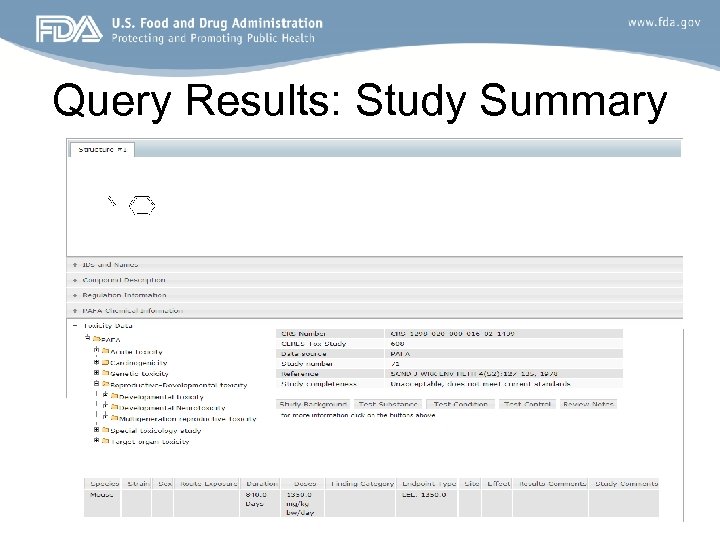
Query Results: Study Summary
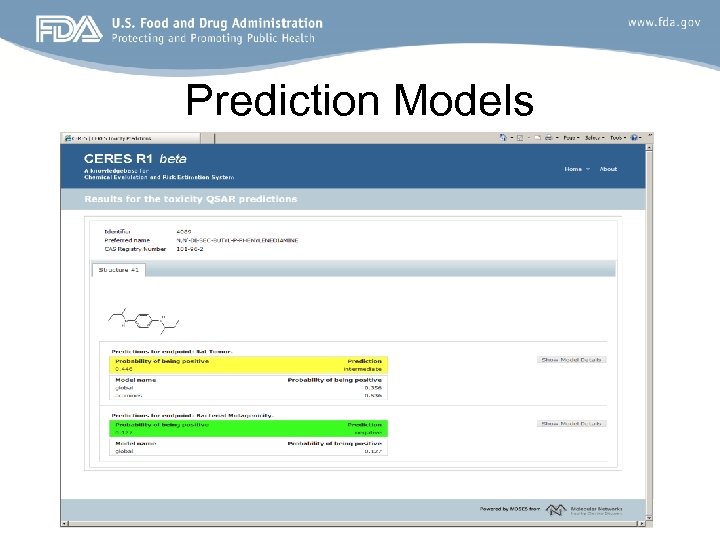
Prediction Models
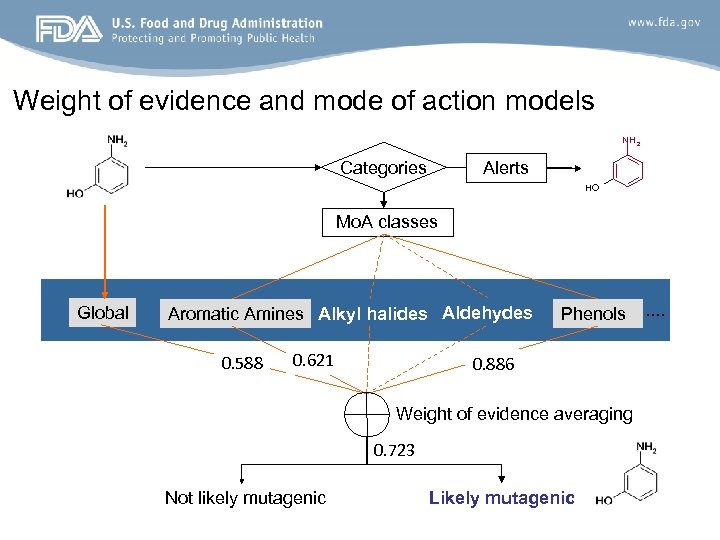
Weight of evidence and mode of action models NH 2 Categories Alerts HO Mo. A classes Global Aromatic Amines Alkyl halides Aldehydes 0. 588 0. 621 Phenols 0. 886 Weight of evidence averaging 0. 723 Not likely mutagenic Likely mutagenic . .
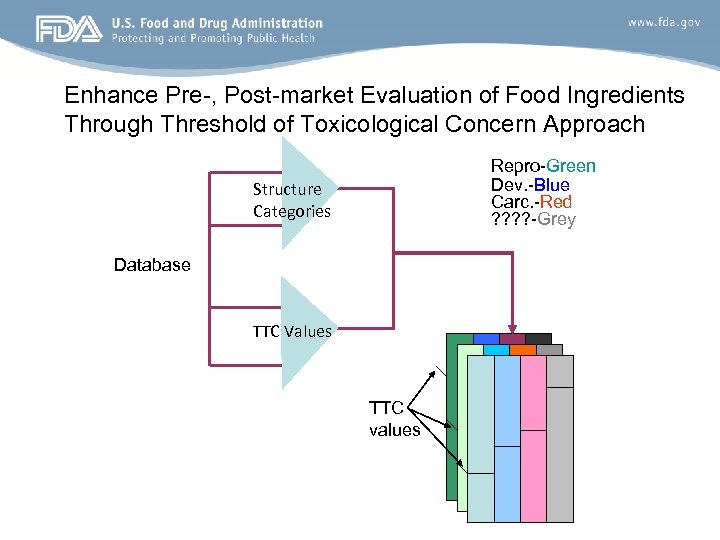
Enhance Pre-, Post-market Evaluation of Food Ingredients Through Threshold of Toxicological Concern Approach Repro-Green Dev. -Blue Carc. -Red ? ? -Grey Structure Categories Database TTC Values TTC values
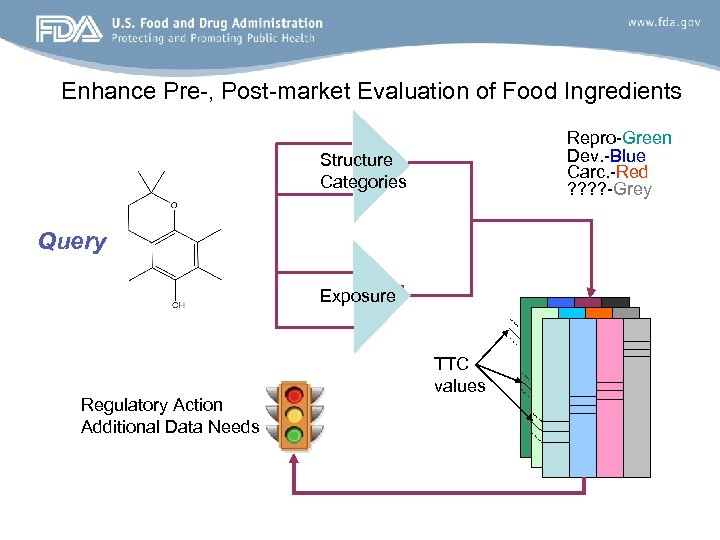
Enhance Pre-, Post-market Evaluation of Food Ingredients Repro-Green Dev. -Blue Carc. -Red ? ? -Grey Structure Categories Query Exposure Regulatory Action Additional Data Needs TTC values
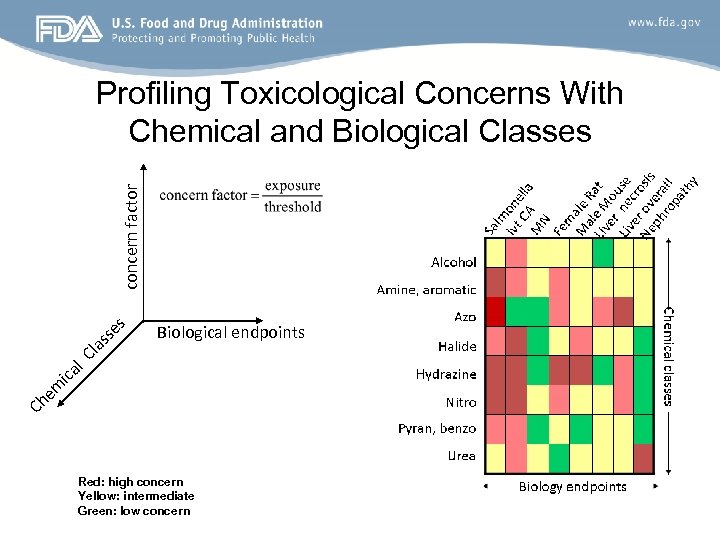
Biological endpoints Ch e m ica l. C la ss es concern factor Profiling Toxicological Concerns With Chemical and Biological Classes Red: high concern Yellow: intermediate Green: low concern
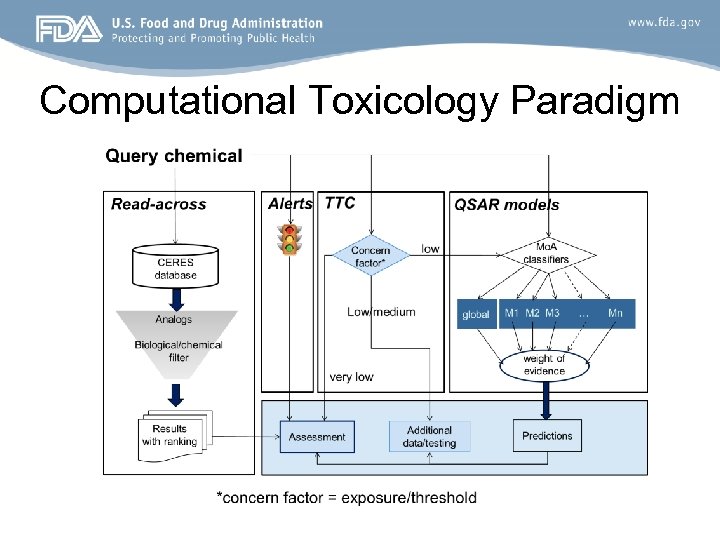
Computational Toxicology Paradigm
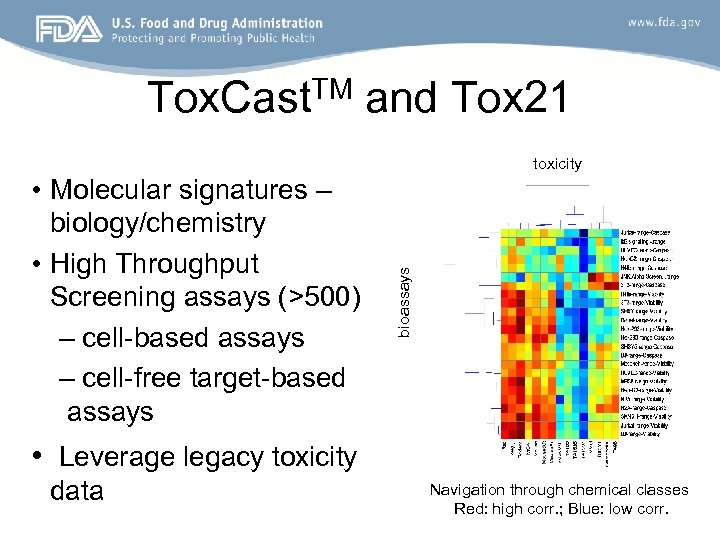
TM Tox. Cast and Tox 21 • Molecular signatures – biology/chemistry • High Throughput Screening assays (>500) – cell-based assays – cell-free target-based assays bioassays toxicity • Leverage legacy toxicity data Navigation through chemical classes Red: high corr. ; Blue: low corr.

Conclusion • CERES – Improve Pre- and Post-Market Review • Consolidates information on chemical structure, physical properties and toxicity data to allow for more robust safety analysis • Semi-automated monitoring of new safety data relative to authorized chemicals through the use of computational toxicology and TTC – Relate new and existing data in new ways • Seek biologically meaningful analogs to fill the data gaps found in many food ingredients and food contact substances • Provide molecular level mechanistic insights that eventually help us understand human effects • Metabolism knowledge will also be incorporated.
0ae19f07f9cf4bd95b57caf6740fd488.ppt