c36e86e6a06690ae45e1f22fc6181089.ppt
- Количество слайдов: 21

DEVELOPING FLUORESCENCE LIFETIME IMAGING ENDOSCOPES FOR BIOMEDICAL APPLICATIONS Hugh Sparks 1, Ian Munro 1, Douglas Kelly 1, Sean Warren 1, Gordon Kennedy 1, Eishu Hirata 2, Esra Nigar 3, Eric Sahai 2, Taran Tatla 3, Christopher Dunsby 1 and Paul French 1 1 Photonics 2 Cancer 3 North Group, Department of Physics , Imperial College London Research UK, London Research Institute, United Kingdom West London Hospitals NHS Trust, United Kingdom

Overview Section. 1 Wide-field FLIM flexible endoscopy – Wide-field FLIM of autofluorescence for clinical applications • • Basics of FLIM Origins of tissue autofluorescence FLIM endoscopy of autofluorescence and a brief look at past research Time-domain FLIM using time-gated detection – Wide-field FLIM endoscopy of tissue autofluorescence • Macroscopic imaging of ex vivo head & neck tissue samples • A prototype FLIM endoscope targeting autofluorescence • Summary of work and future outlook Section. 2 Confocal laser scanning endoscope (CLSE) adapted for FLIM – Introduction to confocal FLIM for biomedical applications • Basics of Time Correlated Single Photon Counting (TCSPC) • Basics of Forster Resonance Energy Transfer (FRET) – Developing CLSE FLIM for mentoring protein interactions by FLIM FRET • A commercial CLSE system adapted for TCSPC FLIM • Demonstration of CLSE FLIM FRET in vitro
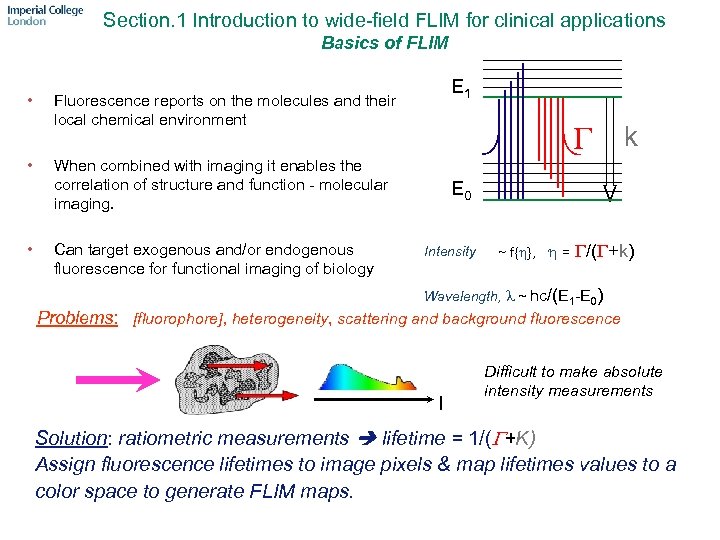
Section. 1 Introduction to wide-field FLIM for clinical applications Basics of FLIM • • • E 1 Fluorescence reports on the molecules and their local chemical environment G k When combined with imaging it enables the correlation of structure and function - molecular imaging. Can target exogenous and/or endogenous fluorescence for functional imaging of biology E 0 Intensity ~ f{h}, h = G/(G+k) Wavelength, l ~ hc/(E 1 -E 0) Problems: [fluorophore], heterogeneity, scattering and background fluorescence l Difficult to make absolute intensity measurements Solution: ratiometric measurements lifetime = 1/(G+K) Assign fluorescence lifetimes to image pixels & map lifetimes values to a color space to generate FLIM maps.
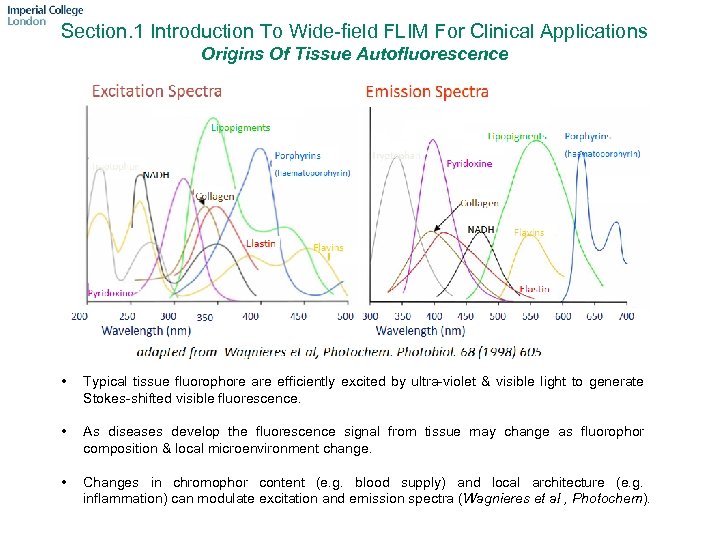
Section. 1 Introduction To Wide-field FLIM For Clinical Applications Origins Of Tissue Autofluorescence • Typical tissue fluorophore are efficiently excited by ultra-violet & visible light to generate Stokes-shifted visible fluorescence. • As diseases develop the fluorescence signal from tissue may change as fluorophor composition & local microenvironment change. • Changes in chromophor content (e. g. blood supply) and local architecture (e. g. inflammation) can modulate excitation and emission spectra (Wagnieres et al , Photochem).
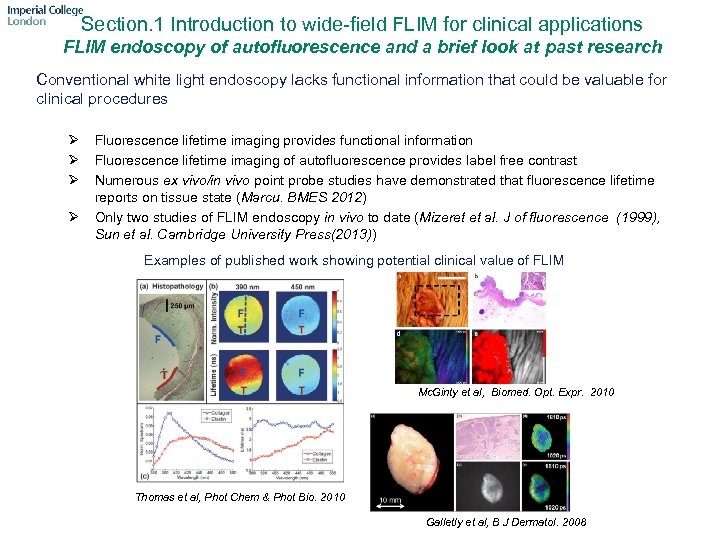
Section. 1 Introduction to wide-field FLIM for clinical applications FLIM endoscopy of autofluorescence and a brief look at past research Conventional white light endoscopy lacks functional information that could be valuable for clinical procedures Ø Ø Fluorescence lifetime imaging provides functional information Fluorescence lifetime imaging of autofluorescence provides label free contrast Numerous ex vivo/in vivo point probe studies have demonstrated that fluorescence lifetime reports on tissue state (Marcu. BMES 2012) Only two studies of FLIM endoscopy in vivo to date (Mizeret et al. J of fluorescence (1999), Sun et al. Cambridge University Press(2013)) Examples of published work showing potential clinical value of FLIM Mc. Ginty et al, Biomed. Opt. Expr. 2010 Thomas et al, Phot Chem & Phot Bio. 2010 Galletly et al, B J Dermatol. 2008
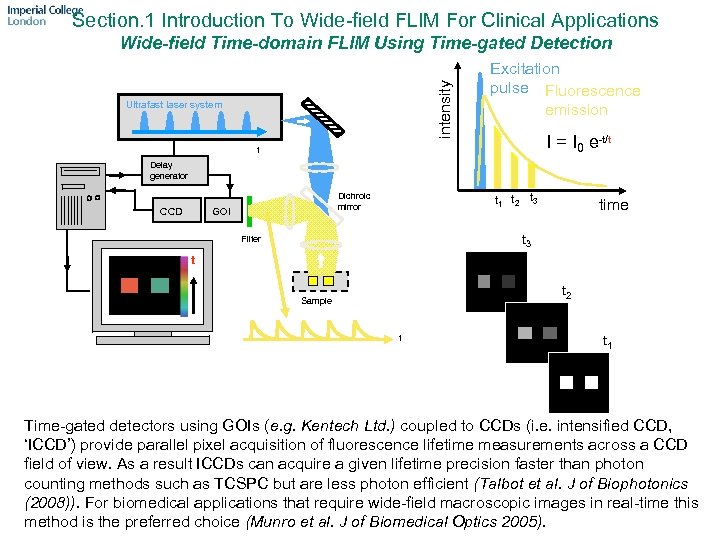
Section. 1 Introduction To Wide-field FLIM For Clinical Applications intensity Wide-field Time-domain FLIM Using Time-gated Detection Ultrafast laser system Excitation pulse Fluorescence emission I = I 0 e-t/t t Delay generator CCD t 1 t 2 t 3 Dichroic mirror GOI time t 3 Filter t t 2 Sample t t 1 Time-gated detectors using GOIs (e. g. Kentech Ltd. ) coupled to CCDs (i. e. intensified CCD, ‘ICCD’) provide parallel pixel acquisition of fluorescence lifetime measurements across a CCD field of view. As a result ICCDs can acquire a given lifetime precision faster than photon counting methods such as TCSPC but are less photon efficient (Talbot et al. J of Biophotonics (2008)). For biomedical applications that require wide-field macroscopic images in real-time this method is the preferred choice (Munro et al. J of Biomedical Optics 2005).
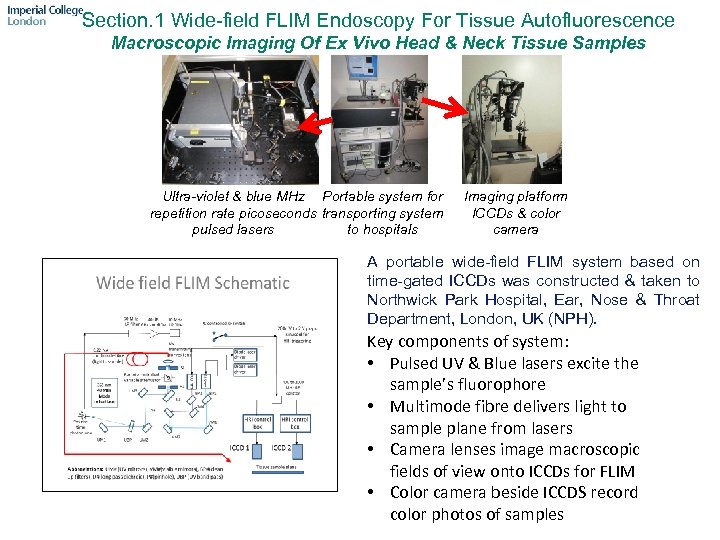
Section. 1 Wide-field FLIM Endoscopy For Tissue Autofluorescence Macroscopic Imaging Of Ex Vivo Head & Neck Tissue Samples Ultra-violet & blue MHz Portable system for repetition rate picoseconds transporting system pulsed lasers to hospitals Imaging platform ICCDs & color camera A portable wide-field FLIM system based on time-gated ICCDs was constructed & taken to Northwick Park Hospital, Ear, Nose & Throat Department, London, UK (NPH). Key components of system: • Pulsed UV & Blue lasers excite the sample’s fluorophore • Multimode fibre delivers light to sample plane from lasers • Camera lenses image macroscopic fields of view onto ICCDs for FLIM • Color camera beside ICCDS record color photos of samples
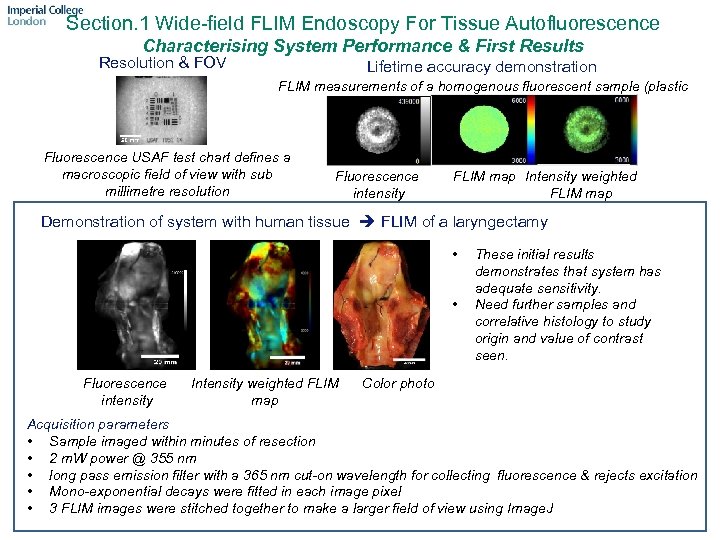
Section. 1 Wide-field FLIM Endoscopy For Tissue Autofluorescence Characterising System Performance & First Results Resolution & FOV Lifetime accuracy demonstration FLIM measurements of a homogenous fluorescent sample (plastic sheet) Fluorescence USAF test chart defines a macroscopic field of view with sub millimetre resolution Fluorescence intensity FLIM map Intensity weighted FLIM map Demonstration of system with human tissue FLIM of a laryngectamy • • Fluorescence intensity Intensity weighted FLIM map These initial results demonstrates that system has adequate sensitivity. Need further samples and correlative histology to study origin and value of contrast seen. Color photo Acquisition parameters • Sample imaged within minutes of resection • 2 m. W power @ 355 nm • long pass emission filter with a 365 nm cut-on wavelength for collecting fluorescence & rejects excitation • Mono-exponential decays were fitted in each image pixel • 3 FLIM images were stitched together to make a larger field of view using Image. J
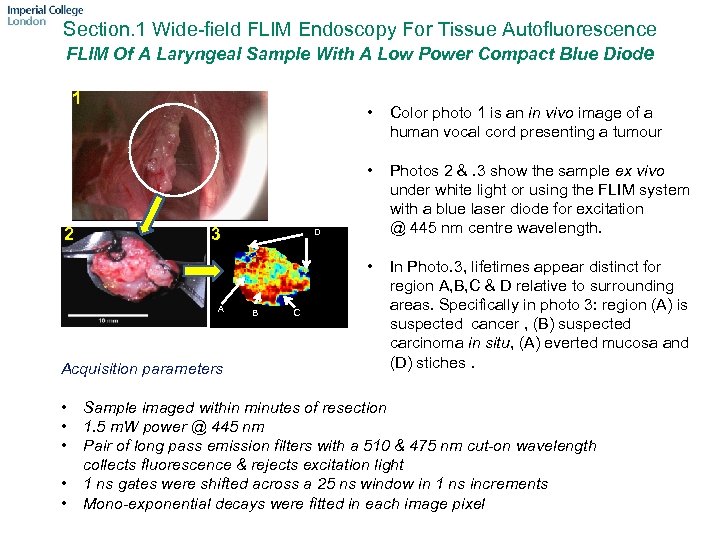
Section. 1 Wide-field FLIM Endoscopy For Tissue Autofluorescence FLIM Of A Laryngeal Sample With A Low Power Compact Blue Diode 1 A Acquisition parameters • • • Photos 2 &. 3 show the sample ex vivo under white light or using the FLIM system with a blue laser diode for excitation @ 445 nm centre wavelength. • 3 Color photo 1 is an in vivo image of a human vocal cord presenting a tumour • 2 3 • In Photo. 3, lifetimes appear distinct for region A, B, C & D relative to surrounding areas. Specifically in photo 3: region (A) is suspected cancer , (B) suspected carcinoma in situ, (A) everted mucosa and (D) stiches. D B C Sample imaged within minutes of resection 1. 5 m. W power @ 445 nm Pair of long pass emission filters with a 510 & 475 nm cut-on wavelength collects fluorescence & rejects excitation light 1 ns gates were shifted across a 25 ns window in 1 ns increments Mono-exponential decays were fitted in each image pixel
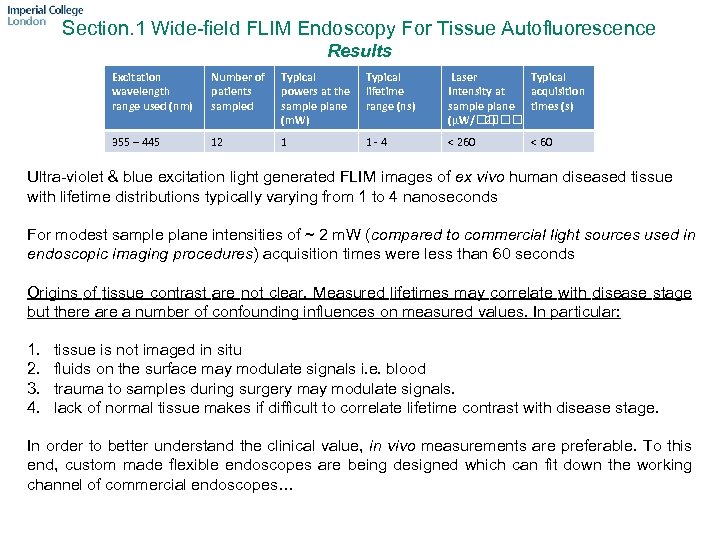
Section. 1 Wide-field FLIM Endoscopy For Tissue Autofluorescence Results Excitation wavelength range used (nm) Number of patients sampled Typical powers at the sample plane (m. W) Typical lifetime range (ns) Laser Typical Intensity at acquisition sample plane times (s) (μW/ 2) 355 – 445 12 1 1 - 4 < 260 < 60 Ultra-violet & blue excitation light generated FLIM images of ex vivo human diseased tissue with lifetime distributions typically varying from 1 to 4 nanoseconds For modest sample plane intensities of ~ 2 m. W (compared to commercial light sources used in endoscopic imaging procedures) acquisition times were less than 60 seconds Origins of tissue contrast are not clear. Measured lifetimes may correlate with disease stage but there a number of confounding influences on measured values. In particular: 1. 2. 3. 4. tissue is not imaged in situ fluids on the surface may modulate signals i. e. blood trauma to samples during surgery may modulate signals. lack of normal tissue makes if difficult to correlate lifetime contrast with disease stage. In order to better understand the clinical value, in vivo measurements are preferable. To this end, custom made flexible endoscopes are being designed which can fit down the working channel of commercial endoscopes…
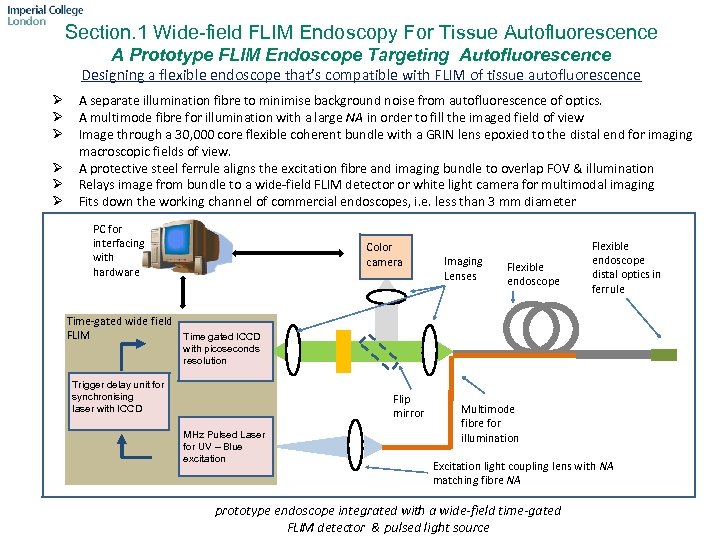
Section. 1 Wide-field FLIM Endoscopy For Tissue Autofluorescence A Prototype FLIM Endoscope Targeting Autofluorescence Designing a flexible endoscope that’s compatible with FLIM of tissue autofluorescence Ø Ø Ø A separate illumination fibre to minimise background noise from autofluorescence of optics. A multimode fibre for illumination with a large NA in order to fill the imaged field of view Image through a 30, 000 core flexible coherent bundle with a GRIN lens epoxied to the distal end for imaging macroscopic fields of view. A protective steel ferrule aligns the excitation fibre and imaging bundle to overlap FOV & illumination Relays image from bundle to a wide-field FLIM detector or white light camera for multimodal imaging Fits down the working channel of commercial endoscopes, i. e. less than 3 mm diameter PC for interfacing with hardware Color camera Imaging Lenses Flexible endoscope distal optics in ferrule Time-gated wide field FLIM Time gated ICCD with picoseconds resolution Trigger delay unit for synchronising laser with ICCD Flip mirror MHz Pulsed Laser for UV – Blue excitation Multimode fibre for illumination Excitation light coupling lens with NA matching fibre NA prototype endoscope integrated with a wide-field time-gated FLIM detector & pulsed light source
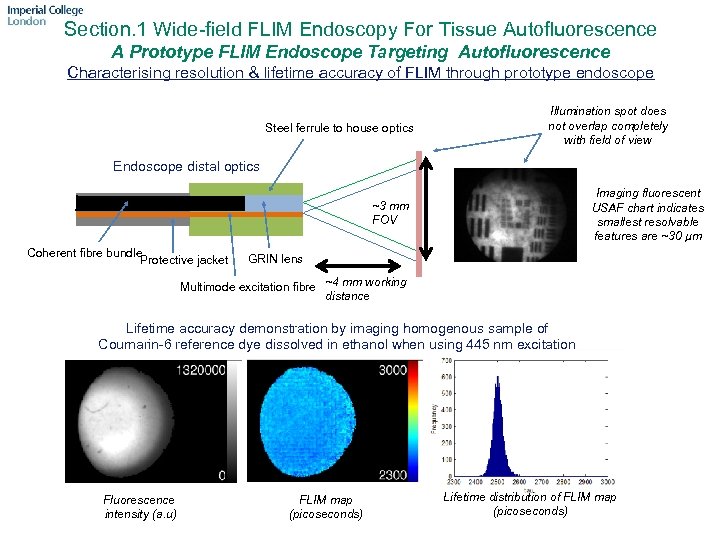
Section. 1 Wide-field FLIM Endoscopy For Tissue Autofluorescence A Prototype FLIM Endoscope Targeting Autofluorescence Characterising resolution & lifetime accuracy of FLIM through prototype endoscope Steel ferrule to house optics Illumination spot does not overlap completely with field of view Endoscope distal optics Imaging fluorescent USAF chart indicates smallest resolvable features are ~30 µm ~3 mm FOV Coherent fibre bundle Protective jacket GRIN lens Multimode excitation fibre ~4 mm working distance Lifetime accuracy demonstration by imaging homogenous sample of Coumarin-6 reference dye dissolved in ethanol when using 445 nm excitation Fluorescence intensity (a. u) FLIM map (picoseconds) Lifetime distribution of FLIM map (picoseconds)
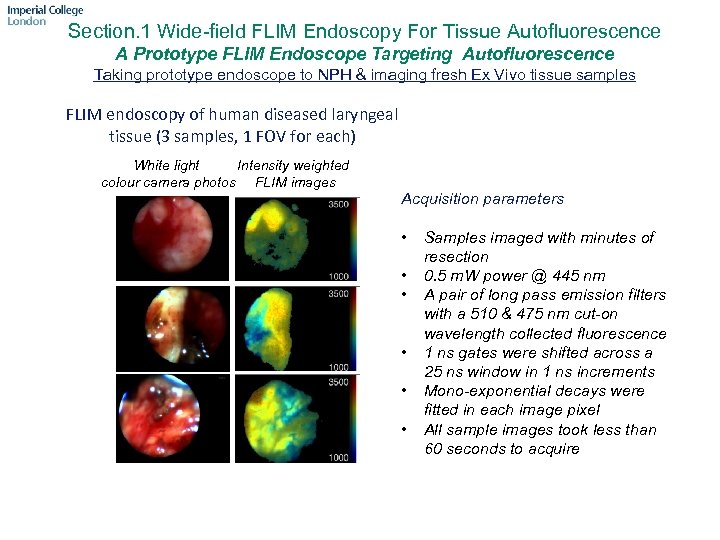
Section. 1 Wide-field FLIM Endoscopy For Tissue Autofluorescence A Prototype FLIM Endoscope Targeting Autofluorescence Taking prototype endoscope to NPH & imaging fresh Ex Vivo tissue samples FLIM endoscopy of human diseased laryngeal tissue (3 samples, 1 FOV for each) White light Intensity weighted colour camera photos FLIM images Acquisition parameters • • • Samples imaged with minutes of resection 0. 5 m. W power @ 445 nm A pair of long pass emission filters with a 510 & 475 nm cut-on wavelength collected fluorescence 1 ns gates were shifted across a 25 ns window in 1 ns increments Mono-exponential decays were fitted in each image pixel All sample images took less than 60 seconds to acquire

Section. 1 Wide-field FLIM Endoscopy For Tissue Autofluorescence Summary Demonstrated wide-field time-gated FLIM through a flexible endoscope that can fit down the working channel of commercial endoscopes Demonstrated FLIM of human diseased laryngeal tissue under blue light (445 nm) in less than 60 seconds using a compact laser diode Blue light is less phototoxic compared to ultra-violet light. Future outlook Investigate whether FLIM contrast correlates with tissue state by imaging animal models of cancer. Investigate optimum excitation light wavelength for clinical applications Investigate whether in vivo FLIM contrast correlates with tissue state in humans Reduce acquisition time by increasing laser powers and implementing rapid lifetime acquisition strategies
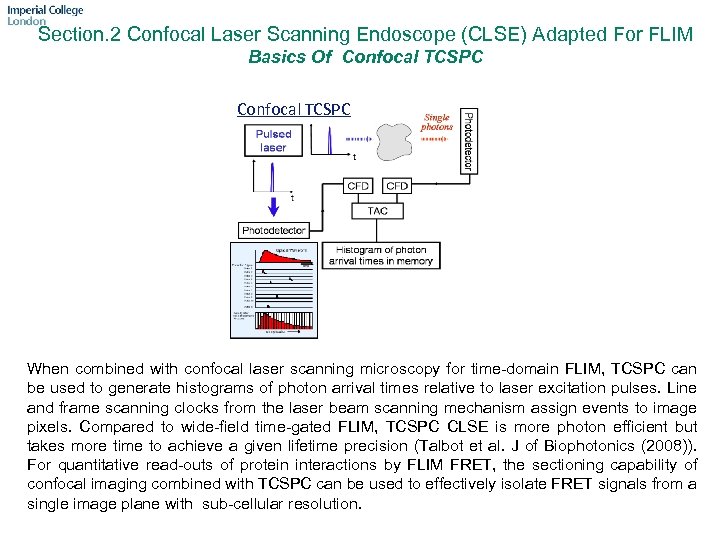
Section. 2 Confocal Laser Scanning Endoscope (CLSE) Adapted For FLIM Basics Of Confocal TCSPC When combined with confocal laser scanning microscopy for time-domain FLIM, TCSPC can be used to generate histograms of photon arrival times relative to laser excitation pulses. Line and frame scanning clocks from the laser beam scanning mechanism assign events to image pixels. Compared to wide-field time-gated FLIM, TCSPC CLSE is more photon efficient but takes more time to achieve a given lifetime precision (Talbot et al. J of Biophotonics (2008)). For quantitative read-outs of protein interactions by FLIM FRET, the sectioning capability of confocal imaging combined with TCSPC can be used to effectively isolate FRET signals from a single image plane with sub-cellular resolution.
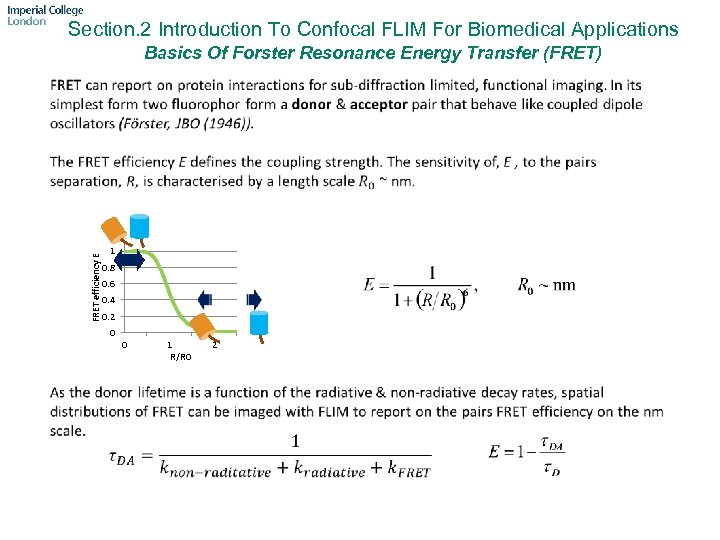
Section. 2 Introduction To Confocal FLIM For Biomedical Applications Basics Of Forster Resonance Energy Transfer (FRET) FRET efficiency E 1 0. 8 0. 6 0. 4 0. 2 0 0 1 R/R 0 2
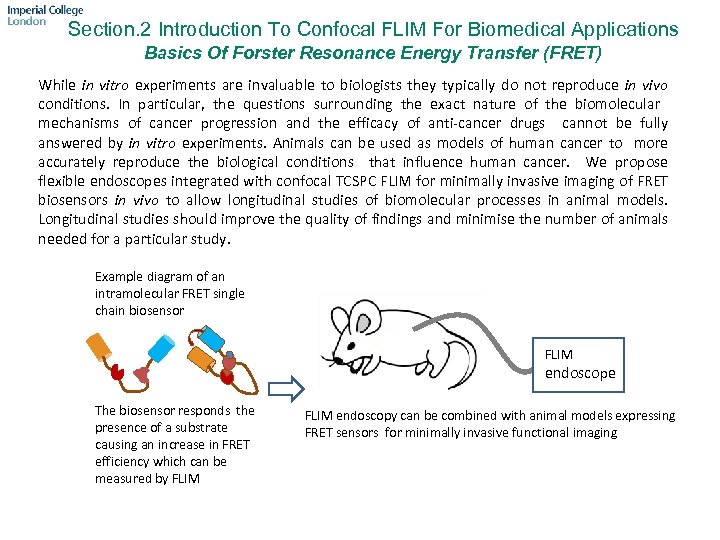
Section. 2 Introduction To Confocal FLIM For Biomedical Applications Basics Of Forster Resonance Energy Transfer (FRET) While in vitro experiments are invaluable to biologists they typically do not reproduce in vivo conditions. In particular, the questions surrounding the exact nature of the biomolecular mechanisms of cancer progression and the efficacy of anti-cancer drugs cannot be fully answered by in vitro experiments. Animals can be used as models of human cancer to more accurately reproduce the biological conditions that influence human cancer. We propose flexible endoscopes integrated with confocal TCSPC FLIM for minimally invasive imaging of FRET biosensors in vivo to allow longitudinal studies of biomolecular processes in animal models. Longitudinal studies should improve the quality of findings and minimise the number of animals needed for a particular study. Example diagram of an intramolecular FRET single chain biosensor FLIM endoscope The biosensor responds the presence of a substrate causing an increase in FRET efficiency which can be measured by FLIM endoscopy can be combined with animal models expressing FRET sensors for minimally invasive functional imaging

Section. 2 Developing CLSE FLIM endoscopy for FLIM FRET A CLSE FLIM Endoscope Optically-sectioned subcellular resolution FLIM endoscopy system Ø Commercial laser scanning confocal endomicroscope (CLSE), (Mauna Kea technologies, Cellvizio®) adapted for TCSPC. Ø Frequency-doubled tunable (355 - 495 nm) Tai-Sapphire laser (Spectra-Physics, BB Mai Tai). coupled a Cellvizio® scanning unit via a single-mode optical fibre (acting as a pinhole). Ø Dichroic beam splitter transmits fluorescence to the photomultiplier for TCSPC (Becker & Hickl, SPC-830). Ø TCSPC assigns photons to arrival times relative to laser pulses times at the sample plane Ø The endoscopic probe comprises a coherent fibre optic imaging bundle with a miniature objective at the distal end that provides a 60 µm working distance & 250 µm field of view. Ø Line & frame clocks from the scanning unit register photons to pixels. Ø FLIM data is acquired in FIFO mode to generate “preview” FLIM images in real time based on mean arrival time. Post acquisition processing permits more detailed non-linear fitting analysis.
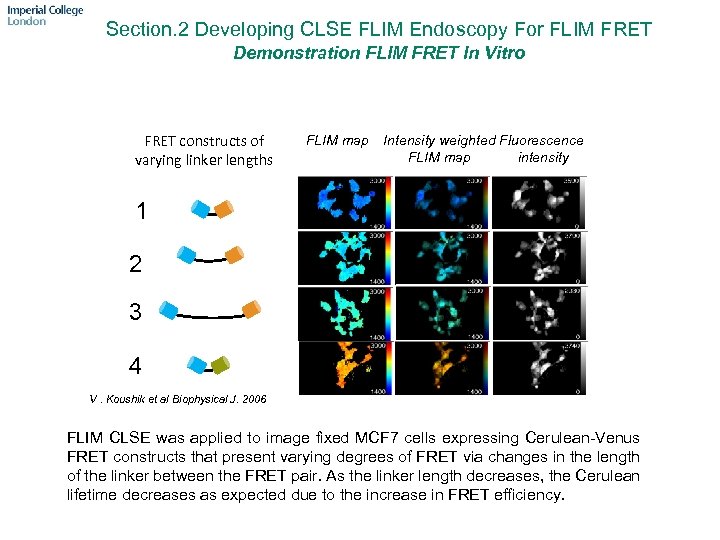
Section. 2 Developing CLSE FLIM Endoscopy For FLIM FRET Demonstration FLIM FRET In Vitro FRET constructs of varying linker lengths FLIM map Intensity weighted Fluorescence FLIM map intensity 1 2 3 4 V. Koushik et al Biophysical J. 2006 FLIM CLSE was applied to image fixed MCF 7 cells expressing Cerulean-Venus FRET constructs that present varying degrees of FRET via changes in the length of the linker between the FRET pair. As the linker length decreases, the Cerulean lifetime decreases as expected due to the increase in FRET efficiency.

Section. 2 Developing CLSE FLIM endoscopy for FLIM FRET Summary • • CLSE adapted for optically sectioned FLIM FRET with subcellular resolution Demonstration of potential to read out protein interactions by FRET using FRET standards Future outlook • Apply instrument to in vivo imaging of FRET sensors to investigate value of method to biomedical research

“Thank you” Kentech Instruments
c36e86e6a06690ae45e1f22fc6181089.ppt