d4abe7c08dfde0dd0a5dae2fae05a029.ppt
- Количество слайдов: 38
 Detection of Organic Peroxide Explosives Through The Fenton Reaction I. Francis Cheng, Derek F. Laine, Christopher Roske University of Idaho Moscow, ID 83844 -2343 Email: ifcheng@uidaho. edu Tel. : 208 -885 -6387 Fax: 208 -885 -6173 Homepage: http: //www. chem. uidaho. edu/faculty/ifcheng/ Acknowledgement: NSF-SGER 1 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Detection of Organic Peroxide Explosives Through The Fenton Reaction I. Francis Cheng, Derek F. Laine, Christopher Roske University of Idaho Moscow, ID 83844 -2343 Email: ifcheng@uidaho. edu Tel. : 208 -885 -6387 Fax: 208 -885 -6173 Homepage: http: //www. chem. uidaho. edu/faculty/ifcheng/ Acknowledgement: NSF-SGER 1 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
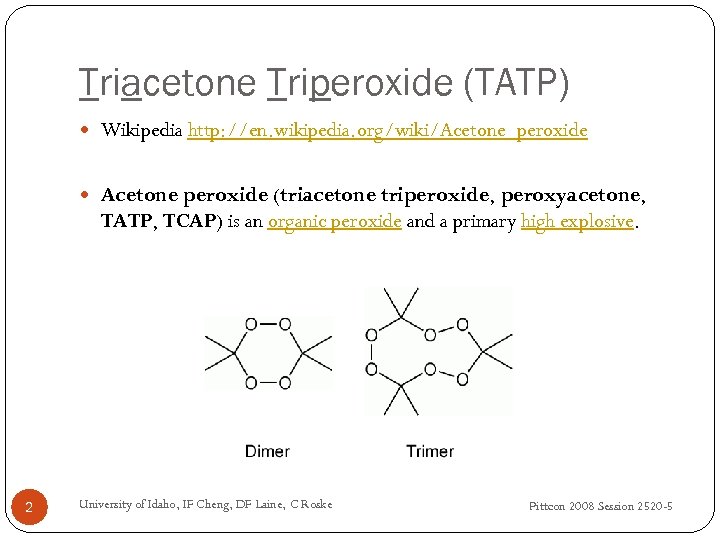 Triacetone Triperoxide (TATP) Wikipedia http: //en. wikipedia. org/wiki/Acetone_peroxide Acetone peroxide (triacetone triperoxide, peroxyacetone, TATP, TCAP) is an organic peroxide and a primary high explosive. 2 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Triacetone Triperoxide (TATP) Wikipedia http: //en. wikipedia. org/wiki/Acetone_peroxide Acetone peroxide (triacetone triperoxide, peroxyacetone, TATP, TCAP) is an organic peroxide and a primary high explosive. 2 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
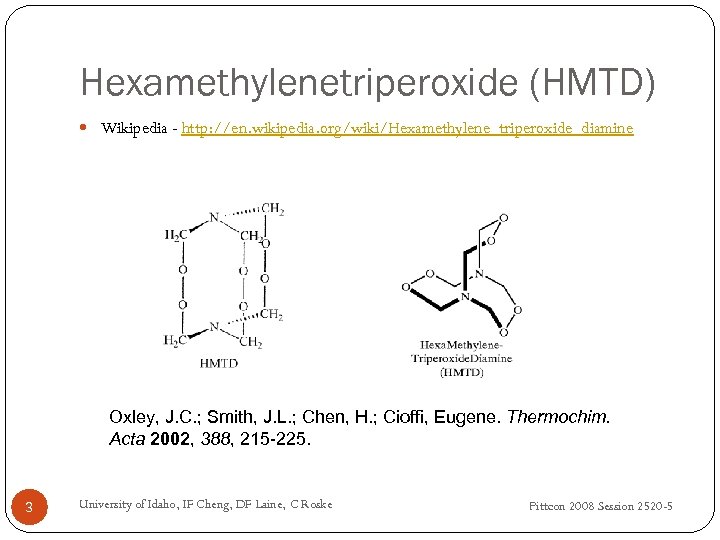 Hexamethylenetriperoxide (HMTD) Wikipedia - http: //en. wikipedia. org/wiki/Hexamethylene_triperoxide_diamine Oxley, J. C. ; Smith, J. L. ; Chen, H. ; Cioffi, Eugene. Thermochim. Acta 2002, 388, 215 -225. 3 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Hexamethylenetriperoxide (HMTD) Wikipedia - http: //en. wikipedia. org/wiki/Hexamethylene_triperoxide_diamine Oxley, J. C. ; Smith, J. L. ; Chen, H. ; Cioffi, Eugene. Thermochim. Acta 2002, 388, 215 -225. 3 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 Outline Background Dangers Recent News Need for Detection Systems Fast Field Portable (handheld) Selective and LOD Electrochemical Detection Via Fenton Reaction 4 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Outline Background Dangers Recent News Need for Detection Systems Fast Field Portable (handheld) Selective and LOD Electrochemical Detection Via Fenton Reaction 4 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 TATP & HMTD – the threat • Due to the cost and ease with which the precursors can be obtained, acetone peroxide is commonly manufactured by those without the resources needed to manufacture or buy more sophisticated explosives. When the reaction is carried out without proper equipment the risk of an accident is significant. • 5 http: //en. wikipedia. org/wiki/Acetone_peroxide University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP & HMTD – the threat • Due to the cost and ease with which the precursors can be obtained, acetone peroxide is commonly manufactured by those without the resources needed to manufacture or buy more sophisticated explosives. When the reaction is carried out without proper equipment the risk of an accident is significant. • 5 http: //en. wikipedia. org/wiki/Acetone_peroxide University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
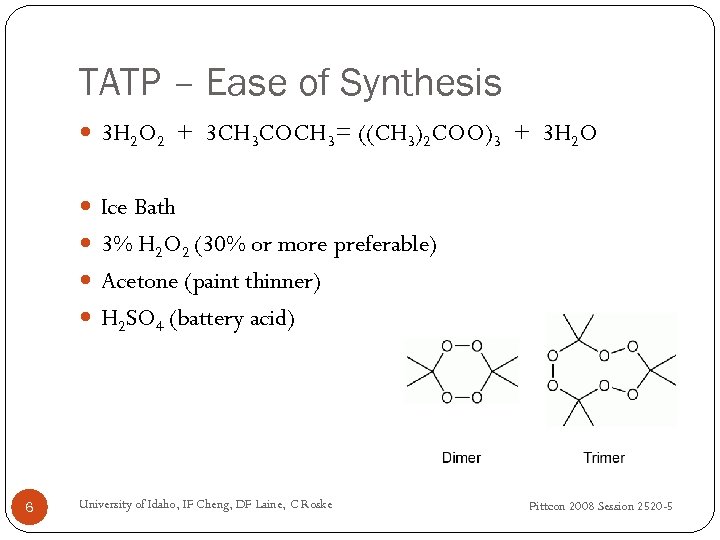 TATP – Ease of Synthesis 3 H 2 O 2 + 3 CH 3 COCH 3= ((CH 3)2 COO)3 + 3 H 2 O Ice Bath 3% H 2 O 2 (30% or more preferable) Acetone (paint thinner) H 2 SO 4 (battery acid) 6 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP – Ease of Synthesis 3 H 2 O 2 + 3 CH 3 COCH 3= ((CH 3)2 COO)3 + 3 H 2 O Ice Bath 3% H 2 O 2 (30% or more preferable) Acetone (paint thinner) H 2 SO 4 (battery acid) 6 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 Ease of HMTD Synthesis http: //business. fortunecity. com/executive/674/hmtd. html Hexamethylenetetramine + Citric Acid + H 2 O 2 HMTD 7 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Ease of HMTD Synthesis http: //business. fortunecity. com/executive/674/hmtd. html Hexamethylenetetramine + Citric Acid + H 2 O 2 HMTD 7 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
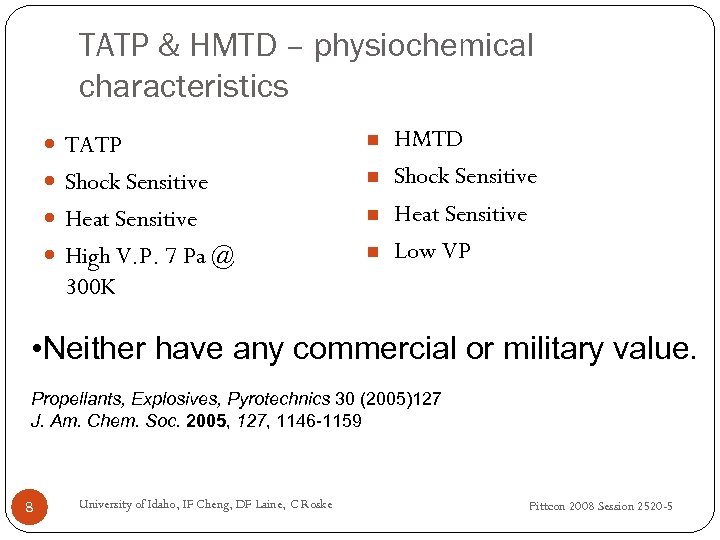 TATP & HMTD – physiochemical characteristics TATP n Shock Sensitive n Heat Sensitive n High V. P. 7 Pa @ n HMTD Shock Sensitive Heat Sensitive Low VP 300 K • Neither have any commercial or military value. Propellants, Explosives, Pyrotechnics 30 (2005)127 J. Am. Chem. Soc. 2005, 127, 1146 -1159 8 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP & HMTD – physiochemical characteristics TATP n Shock Sensitive n Heat Sensitive n High V. P. 7 Pa @ n HMTD Shock Sensitive Heat Sensitive Low VP 300 K • Neither have any commercial or military value. Propellants, Explosives, Pyrotechnics 30 (2005)127 J. Am. Chem. Soc. 2005, 127, 1146 -1159 8 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 TATP – Most Recent News NY Times Sept. 5, 2007 FRANKFURT, Sept. 5 — The German police have arrested three Islamic militants suspected of planning large-scale terrorist attacks against several sites frequented by Americans, including discos, bars, airports, and military installations. She said the suspects had amassed large amounts of hydrogen peroxide, the main chemical used to manufacture the explosives used in the suicide bombings in London in July 2005. 9 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP – Most Recent News NY Times Sept. 5, 2007 FRANKFURT, Sept. 5 — The German police have arrested three Islamic militants suspected of planning large-scale terrorist attacks against several sites frequented by Americans, including discos, bars, airports, and military installations. She said the suspects had amassed large amounts of hydrogen peroxide, the main chemical used to manufacture the explosives used in the suicide bombings in London in July 2005. 9 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 TATP & HMTD – London Subway Bombings July 7, 2005 http: //news. bbc. co. uk/nol/shared/spl/hi/pop_ups/05/uk_enl_1121567244/img/1. jpg 10 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP & HMTD – London Subway Bombings July 7, 2005 http: //news. bbc. co. uk/nol/shared/spl/hi/pop_ups/05/uk_enl_1121567244/img/1. jpg 10 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 TATP & HMTD Incidents 2006 – London airline bombing plot – HMTD 2005 - Joel Henry Hinrichs III – University of Oklahoma. – TATP. 2001 - Richard Reid, Shoe Bomber – TATP 1999 - Millennium bomber Ahmed Ressam. 124 pounds of HMTD 1994/95 – Bojinka Plot – TATP? HMTD? 1994 – Philippines Airlines - TATP 1980’s – present - West Bank Israel – TATP “Mother of Satan” 11 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP & HMTD Incidents 2006 – London airline bombing plot – HMTD 2005 - Joel Henry Hinrichs III – University of Oklahoma. – TATP. 2001 - Richard Reid, Shoe Bomber – TATP 1999 - Millennium bomber Ahmed Ressam. 124 pounds of HMTD 1994/95 – Bojinka Plot – TATP? HMTD? 1994 – Philippines Airlines - TATP 1980’s – present - West Bank Israel – TATP “Mother of Satan” 11 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 TATP – TSA Fluid Ban Effective November 10, 2006, the TSA has advised that travelers may now carry through security checkpoints travel-size toiletries (3. 4 ounces/100 ml or less) that fit comfortably in ONE, QUARTSIZE, clear plastic re-sealable bag. The 3 -1 -1 Kit contains six 2 -1/2 oz and four 1 -1/2 oz flexible squeeze tubes, plus one 13/4 oz Envirosprayer. Kit is also compliant with the new International Security Measures Accord. http: //www. easytravelerinc. com/ 12 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP – TSA Fluid Ban Effective November 10, 2006, the TSA has advised that travelers may now carry through security checkpoints travel-size toiletries (3. 4 ounces/100 ml or less) that fit comfortably in ONE, QUARTSIZE, clear plastic re-sealable bag. The 3 -1 -1 Kit contains six 2 -1/2 oz and four 1 -1/2 oz flexible squeeze tubes, plus one 13/4 oz Envirosprayer. Kit is also compliant with the new International Security Measures Accord. http: //www. easytravelerinc. com/ 12 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 TATP & HMTD Detection - The Challenge The Need for a Fast Portable Detector Innocuous Appearing White Powder Dogs are only moderately successful at detection of TATP & HMTD - Expensive Lacks Chromophoric Groups (not detectable by UV-vis absorbance) 13 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP & HMTD Detection - The Challenge The Need for a Fast Portable Detector Innocuous Appearing White Powder Dogs are only moderately successful at detection of TATP & HMTD - Expensive Lacks Chromophoric Groups (not detectable by UV-vis absorbance) 13 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 TATP & HMTD – Detector Requirements Unknown Materials – Public Safety, e. g. Airports High Selectivity – Low Limits of Detection not Required Air Samples, e. g. Airports Moderate Selectivity– Low Limits of Detection Required Debris at Post-Explosion Sites High Selectivity– Low Detection Limits Field Portability Schulte-Ladbeck, R. ; Vogel, M. ; Karst, U Recent methods for the determination of peroxide-based explosives Anal. Bioanal. Chem. 386 559 -565 (2006) 14 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP & HMTD – Detector Requirements Unknown Materials – Public Safety, e. g. Airports High Selectivity – Low Limits of Detection not Required Air Samples, e. g. Airports Moderate Selectivity– Low Limits of Detection Required Debris at Post-Explosion Sites High Selectivity– Low Detection Limits Field Portability Schulte-Ladbeck, R. ; Vogel, M. ; Karst, U Recent methods for the determination of peroxide-based explosives Anal. Bioanal. Chem. 386 559 -565 (2006) 14 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 TATP & HMTD - Detectors IR-Raman High Selectivity – Relatively High LOD Fluorescence/UV-vis Absorbance Low LOD requires tagging Ion Mobility Good Selectivity, moderate LOD HPLC or GC Excellent Selectivity and LOD 15 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP & HMTD - Detectors IR-Raman High Selectivity – Relatively High LOD Fluorescence/UV-vis Absorbance Low LOD requires tagging Ion Mobility Good Selectivity, moderate LOD HPLC or GC Excellent Selectivity and LOD 15 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 TATP & HMTD – State of Detectors Costs Lack of Field Portability Ideal – Handheld Sensor May Require Knowledgeable User e. g. Commercial Glucose Sensors, electrochemical devices 16 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP & HMTD – State of Detectors Costs Lack of Field Portability Ideal – Handheld Sensor May Require Knowledgeable User e. g. Commercial Glucose Sensors, electrochemical devices 16 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
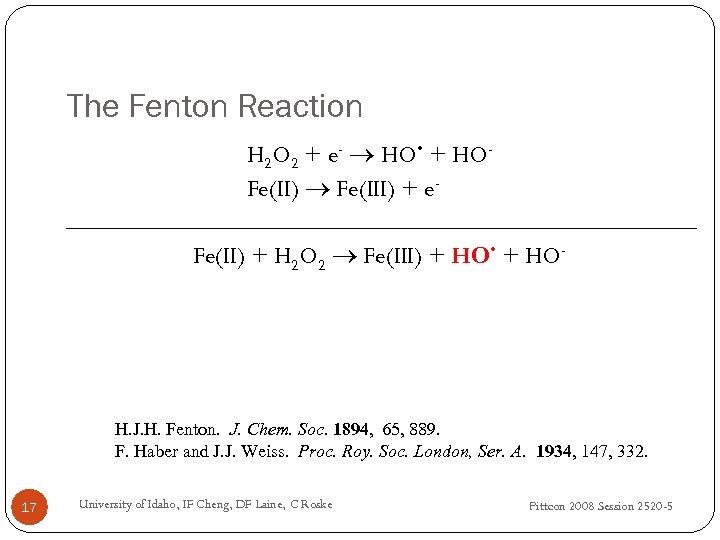 The Fenton Reaction H 2 O 2 + e- HO • + HOFe(II) Fe(III) + e. Fe(II) + H 2 O 2 Fe(III) + HO • + HO- H. J. H. Fenton. J. Chem. Soc. 1894, 65, 889. F. Haber and J. J. Weiss. Proc. Roy. Soc. London, Ser. A. 1934, 147, 332. 17 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
The Fenton Reaction H 2 O 2 + e- HO • + HOFe(II) Fe(III) + e. Fe(II) + H 2 O 2 Fe(III) + HO • + HO- H. J. H. Fenton. J. Chem. Soc. 1894, 65, 889. F. Haber and J. J. Weiss. Proc. Roy. Soc. London, Ser. A. 1934, 147, 332. 17 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
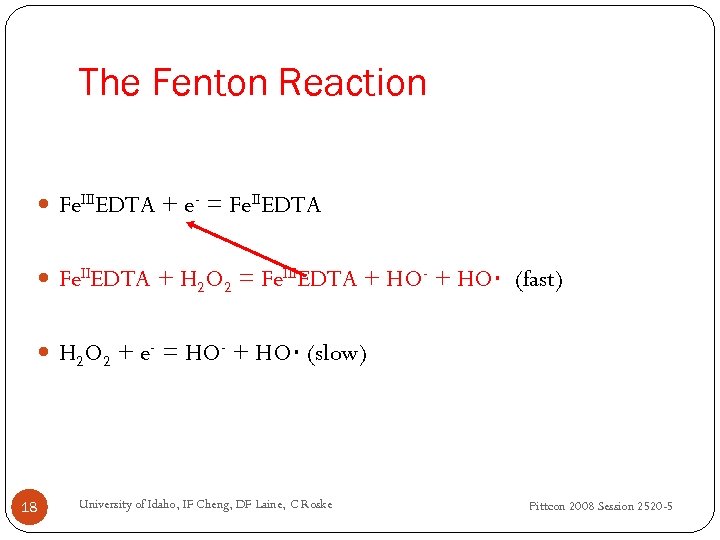 The Fenton Reaction Fe. IIIEDTA + e- = Fe. IIEDTA + H 2 O 2 = Fe. IIIEDTA + HO- + HO∙ (fast) H 2 O 2 + e- = HO- + HO∙ (slow) 18 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
The Fenton Reaction Fe. IIIEDTA + e- = Fe. IIEDTA + H 2 O 2 = Fe. IIIEDTA + HO- + HO∙ (fast) H 2 O 2 + e- = HO- + HO∙ (slow) 18 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
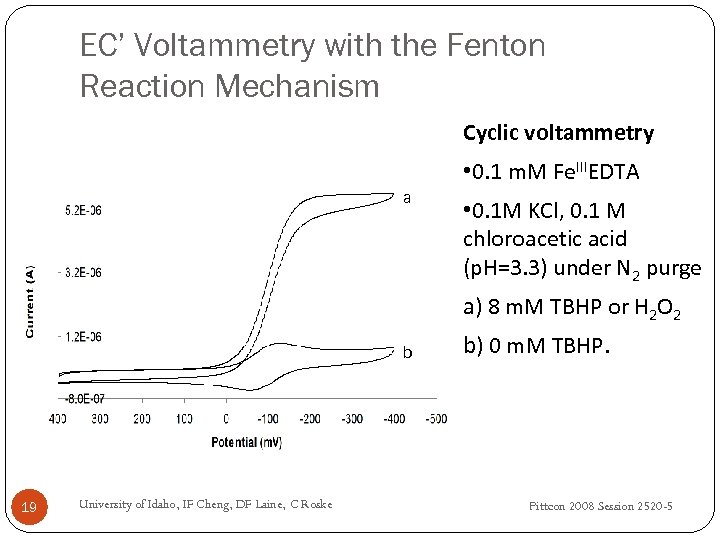 EC’ Voltammetry with the Fenton Reaction Mechanism Cyclic voltammetry • 0. 1 m. M Fe. IIIEDTA a • 0. 1 M KCl, 0. 1 M chloroacetic acid (p. H=3. 3) under N 2 purge a) 8 m. M TBHP or H 2 O 2 b 19 University of Idaho, IF Cheng, DF Laine, C Roske b) 0 m. M TBHP. Pittcon 2008 Session 2520 -5
EC’ Voltammetry with the Fenton Reaction Mechanism Cyclic voltammetry • 0. 1 m. M Fe. IIIEDTA a • 0. 1 M KCl, 0. 1 M chloroacetic acid (p. H=3. 3) under N 2 purge a) 8 m. M TBHP or H 2 O 2 b 19 University of Idaho, IF Cheng, DF Laine, C Roske b) 0 m. M TBHP. Pittcon 2008 Session 2520 -5
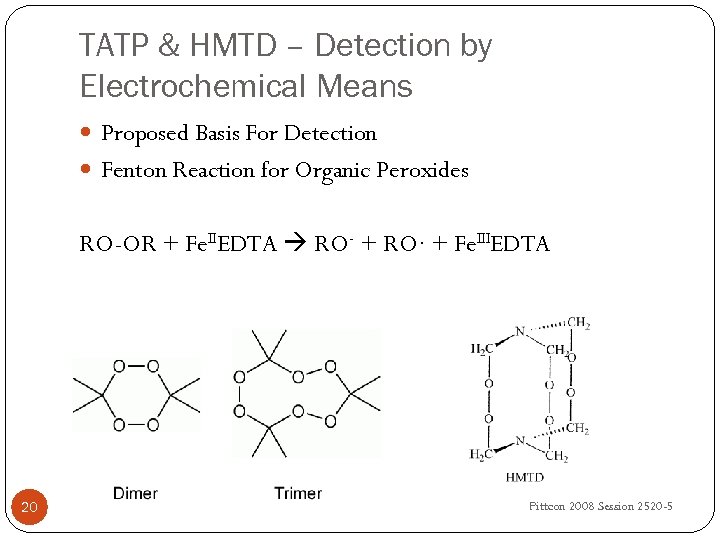 TATP & HMTD – Detection by Electrochemical Means Proposed Basis For Detection Fenton Reaction for Organic Peroxides RO-OR + Fe. IIEDTA RO- + RO∙ + Fe. IIIEDTA 20 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP & HMTD – Detection by Electrochemical Means Proposed Basis For Detection Fenton Reaction for Organic Peroxides RO-OR + Fe. IIEDTA RO- + RO∙ + Fe. IIIEDTA 20 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
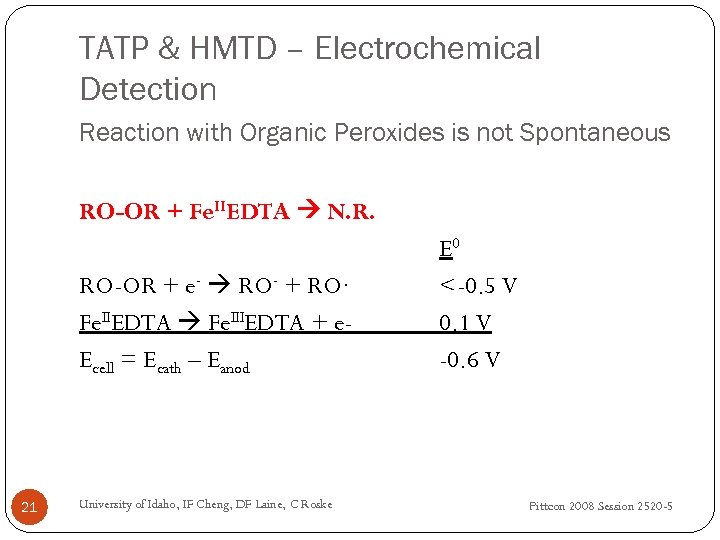 TATP & HMTD – Electrochemical Detection Reaction with Organic Peroxides is not Spontaneous RO-OR + Fe. IIEDTA N. R. RO-OR + e- RO- + RO∙ Fe. IIEDTA Fe. IIIEDTA + e. Ecell = Ecath – Eanod 21 University of Idaho, IF Cheng, DF Laine, C Roske E 0 <-0. 5 V 0. 1 V -0. 6 V Pittcon 2008 Session 2520 -5
TATP & HMTD – Electrochemical Detection Reaction with Organic Peroxides is not Spontaneous RO-OR + Fe. IIEDTA N. R. RO-OR + e- RO- + RO∙ Fe. IIEDTA Fe. IIIEDTA + e. Ecell = Ecath – Eanod 21 University of Idaho, IF Cheng, DF Laine, C Roske E 0 <-0. 5 V 0. 1 V -0. 6 V Pittcon 2008 Session 2520 -5
 TATP & HMTD – Electrochemical Detection Reaction with Peroxides and Hydroperoxides is Spontaneous E 0 RO-OH + e- RO- + HO∙ ≈0. 4 V HO-OH + e- HO- + HO∙ 0. 8 V Fe. IIEDTA + RO-OH/HO-OH Fe. IIIEDTA + RO∙/H+ Requires that TATP & HMTD be degraded 22 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP & HMTD – Electrochemical Detection Reaction with Peroxides and Hydroperoxides is Spontaneous E 0 RO-OH + e- RO- + HO∙ ≈0. 4 V HO-OH + e- HO- + HO∙ 0. 8 V Fe. IIEDTA + RO-OH/HO-OH Fe. IIIEDTA + RO∙/H+ Requires that TATP & HMTD be degraded 22 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 TATP – Degradation to HOOH/ROOH Acid degradation TATP + H+ H 2 O 2 + Products Concentrated HCl 1 -10 minutes 23 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP – Degradation to HOOH/ROOH Acid degradation TATP + H+ H 2 O 2 + Products Concentrated HCl 1 -10 minutes 23 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 HMTD Degradation HMTD products + H 2 O 2 Rapid (almost immediate) & Spontaneous With addition of Fe. IIIEDTA p. H effect – 2. 1 24 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
HMTD Degradation HMTD products + H 2 O 2 Rapid (almost immediate) & Spontaneous With addition of Fe. IIIEDTA p. H effect – 2. 1 24 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
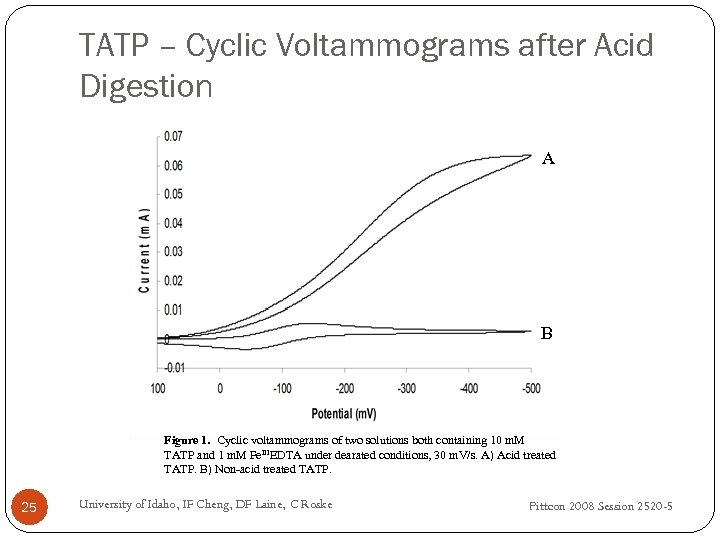 TATP – Cyclic Voltammograms after Acid Digestion A B Figure 1. Cyclic voltammograms of two solutions both containing 10 m. M TATP and 1 m. M Fe. IIIEDTA under dearated conditions, 30 m. V/s. A) Acid treated TATP. B) Non-acid treated TATP. 25 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP – Cyclic Voltammograms after Acid Digestion A B Figure 1. Cyclic voltammograms of two solutions both containing 10 m. M TATP and 1 m. M Fe. IIIEDTA under dearated conditions, 30 m. V/s. A) Acid treated TATP. B) Non-acid treated TATP. 25 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
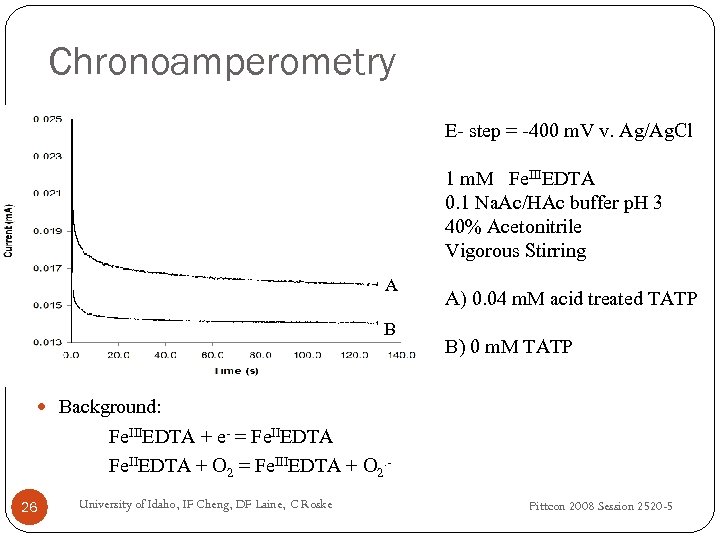 Chronoamperometry E- step = -400 m. V v. Ag/Ag. Cl 1 m. M Fe. IIIEDTA 0. 1 Na. Ac/HAc buffer p. H 3 40% Acetonitrile Vigorous Stirring A B A) 0. 04 m. M acid treated TATP B) 0 m. M TATP Background: Fe. IIIEDTA + e- = Fe. IIEDTA + O 2 = Fe. IIIEDTA + O 2. 26 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Chronoamperometry E- step = -400 m. V v. Ag/Ag. Cl 1 m. M Fe. IIIEDTA 0. 1 Na. Ac/HAc buffer p. H 3 40% Acetonitrile Vigorous Stirring A B A) 0. 04 m. M acid treated TATP B) 0 m. M TATP Background: Fe. IIIEDTA + e- = Fe. IIEDTA + O 2 = Fe. IIIEDTA + O 2. 26 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
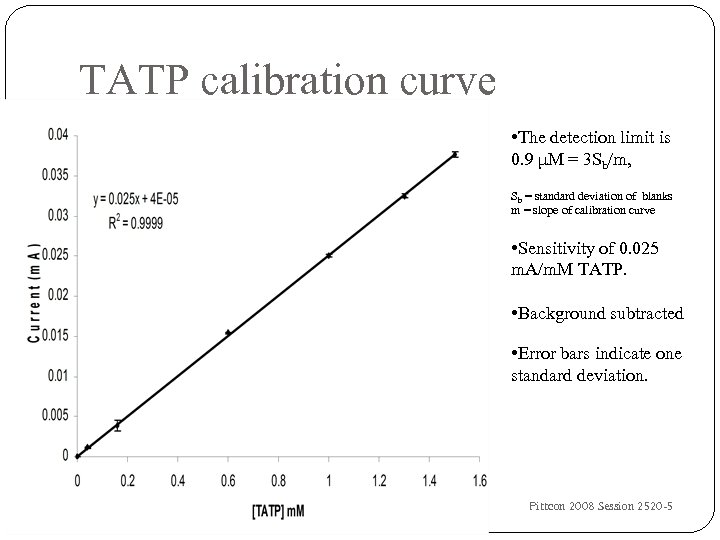 TATP calibration curve • The detection limit is 0. 9 μM = 3 Sb/m, Sb = standard deviation of blanks m = slope of calibration curve • Sensitivity of 0. 025 m. A/m. M TATP. • Background subtracted • Error bars indicate one standard deviation. 27 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
TATP calibration curve • The detection limit is 0. 9 μM = 3 Sb/m, Sb = standard deviation of blanks m = slope of calibration curve • Sensitivity of 0. 025 m. A/m. M TATP. • Background subtracted • Error bars indicate one standard deviation. 27 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
![HMTD analysis Increasing [HMTD] E- step = -400 m. V v. Ag/Ag. Cl 1 HMTD analysis Increasing [HMTD] E- step = -400 m. V v. Ag/Ag. Cl 1](https://present5.com/presentation/d4abe7c08dfde0dd0a5dae2fae05a029/image-28.jpg) HMTD analysis Increasing [HMTD] E- step = -400 m. V v. Ag/Ag. Cl 1 m. M Fe. IIIEDTA Vigorous Stirring • Detection limit 30 μM = 3 Sb/m, Sb = standard deviation of blanks m = slope of calibration curve • Error bars indicate one standard deviation. 28 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
HMTD analysis Increasing [HMTD] E- step = -400 m. V v. Ag/Ag. Cl 1 m. M Fe. IIIEDTA Vigorous Stirring • Detection limit 30 μM = 3 Sb/m, Sb = standard deviation of blanks m = slope of calibration curve • Error bars indicate one standard deviation. 28 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 Detection of TATP in technical mixtures Significant concentrations of HOOH and ROOH. Provides Target for the Detection of Technical Mixtures TATP purification requires Me. OH Recrystallization – More Stable than Technical Mixtures 29 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Detection of TATP in technical mixtures Significant concentrations of HOOH and ROOH. Provides Target for the Detection of Technical Mixtures TATP purification requires Me. OH Recrystallization – More Stable than Technical Mixtures 29 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
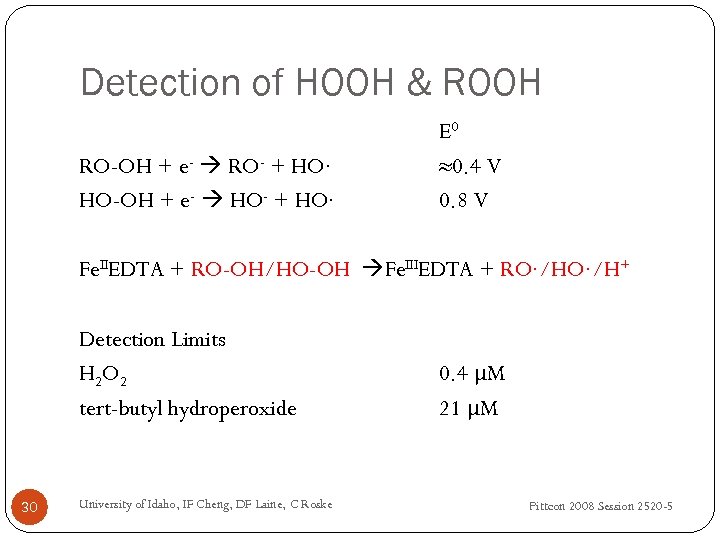 Detection of HOOH & ROOH RO-OH + e- RO- + HO∙ HO-OH + e- HO- + HO∙ E 0 ≈0. 4 V 0. 8 V Fe. IIEDTA + RO-OH/HO-OH Fe. IIIEDTA + RO∙/H+ Detection Limits H 2 O 2 tert-butyl hydroperoxide 30 University of Idaho, IF Cheng, DF Laine, C Roske 0. 4 μM 21 μM Pittcon 2008 Session 2520 -5
Detection of HOOH & ROOH RO-OH + e- RO- + HO∙ HO-OH + e- HO- + HO∙ E 0 ≈0. 4 V 0. 8 V Fe. IIEDTA + RO-OH/HO-OH Fe. IIIEDTA + RO∙/H+ Detection Limits H 2 O 2 tert-butyl hydroperoxide 30 University of Idaho, IF Cheng, DF Laine, C Roske 0. 4 μM 21 μM Pittcon 2008 Session 2520 -5
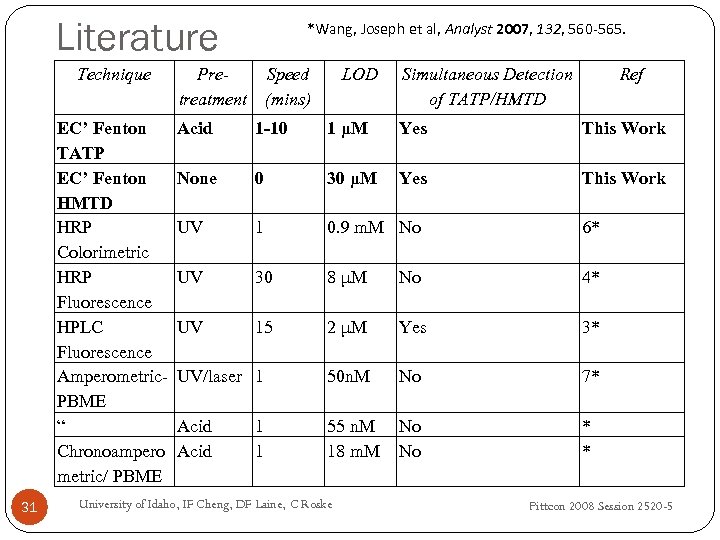 Literature Technique EC’ Fenton TATP EC’ Fenton HMTD HRP Colorimetric HRP Fluorescence HPLC Fluorescence Amperometric. PBME “ Chronoampero metric/ PBME 31 *Wang, Joseph et al, Analyst 2007, 132, 560 -565. Pre. Speed treatment (mins) LOD Simultaneous Detection of TATP/HMTD Ref Acid 1 -10 1 μM Yes This Work None 0 30 μM Yes This Work UV 1 0. 9 m. M No 6* UV 30 8 μM No 4* UV 15 2 μM Yes 3* UV/laser 1 50 n. M No 7* Acid 55 n. M 18 m. M No No * * 1 1 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Literature Technique EC’ Fenton TATP EC’ Fenton HMTD HRP Colorimetric HRP Fluorescence HPLC Fluorescence Amperometric. PBME “ Chronoampero metric/ PBME 31 *Wang, Joseph et al, Analyst 2007, 132, 560 -565. Pre. Speed treatment (mins) LOD Simultaneous Detection of TATP/HMTD Ref Acid 1 -10 1 μM Yes This Work None 0 30 μM Yes This Work UV 1 0. 9 m. M No 6* UV 30 8 μM No 4* UV 15 2 μM Yes 3* UV/laser 1 50 n. M No 7* Acid 55 n. M 18 m. M No No * * 1 1 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 Other Needs for H 2 O 2 Detection Glucose Detector Glucose + H 2 O + O 2 Gluconic Acid + H 2 O 2 H 2 O 2 2 H+ + O 2 + 2 e(slow) Immobilized HRP Limited Linear Range to 3 m. M 32 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Other Needs for H 2 O 2 Detection Glucose Detector Glucose + H 2 O + O 2 Gluconic Acid + H 2 O 2 H 2 O 2 2 H+ + O 2 + 2 e(slow) Immobilized HRP Limited Linear Range to 3 m. M 32 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 Advantages of H 2 O 2 via Fenton Reaction This Work: Fe. IIIEDTA + e- = Fe. IIEDTA (fast) Fe. IIEDTA + H 2 O 2 = Fe. IIIEDTA + HO- + HO∙ (fast) Does not require immobilization of enzymes Less Expensive Linear Range LOD – 100 m. M 33 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Advantages of H 2 O 2 via Fenton Reaction This Work: Fe. IIIEDTA + e- = Fe. IIEDTA (fast) Fe. IIEDTA + H 2 O 2 = Fe. IIIEDTA + HO- + HO∙ (fast) Does not require immobilization of enzymes Less Expensive Linear Range LOD – 100 m. M 33 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
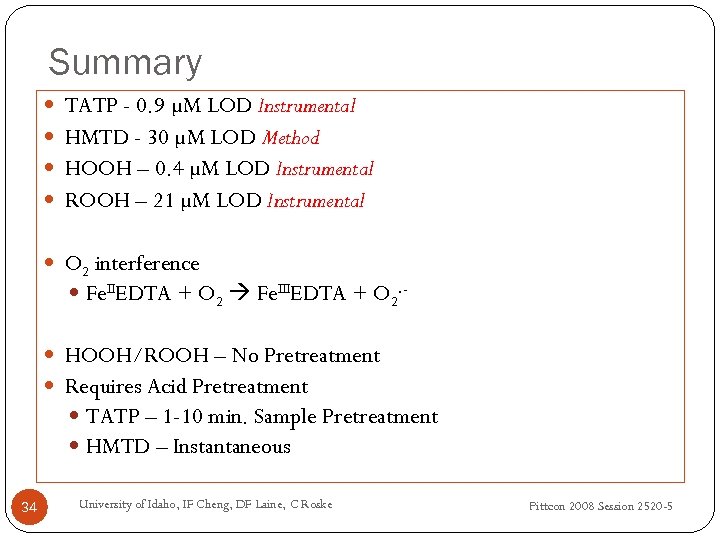 Summary TATP - 0. 9 µM LOD Instrumental HMTD - 30 µM LOD Method HOOH – 0. 4 µM LOD Instrumental ROOH – 21 µM LOD Instrumental O 2 interference Fe. IIEDTA + O 2 Fe. IIIEDTA + O 2. HOOH/ROOH – No Pretreatment Requires Acid Pretreatment TATP – 1 -10 min. Sample Pretreatment HMTD – Instantaneous 34 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Summary TATP - 0. 9 µM LOD Instrumental HMTD - 30 µM LOD Method HOOH – 0. 4 µM LOD Instrumental ROOH – 21 µM LOD Instrumental O 2 interference Fe. IIEDTA + O 2 Fe. IIIEDTA + O 2. HOOH/ROOH – No Pretreatment Requires Acid Pretreatment TATP – 1 -10 min. Sample Pretreatment HMTD – Instantaneous 34 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 Summary Proof of concept No modified electrodes or enzymes required. Reagents can stand up to long term storage. Allows for development of simple, handheld & inexpensive devices, e. g. glucose sensors Not a stand-off detection device High TATP VP may allow for gas phase sensor 35 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Summary Proof of concept No modified electrodes or enzymes required. Reagents can stand up to long term storage. Allows for development of simple, handheld & inexpensive devices, e. g. glucose sensors Not a stand-off detection device High TATP VP may allow for gas phase sensor 35 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 Future Work Elimination of O 2 interference Metal Complex Reduction Potential Kinetics of H 2 O 2 vs. O 2 reduction Optimal Hydrolysis Design of probes Air Samples Liquid Sample 36 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Future Work Elimination of O 2 interference Metal Complex Reduction Potential Kinetics of H 2 O 2 vs. O 2 reduction Optimal Hydrolysis Design of probes Air Samples Liquid Sample 36 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 Acknowledgements National Science Foundation 37 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Acknowledgements National Science Foundation 37 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
 Abstract - Detection of Organic Peroxide Explosives through the Fenton Reaction There is an urgent need for methods and techniques that are able to detect quantitatively and qualitatively peroxide based explosives, especially triacetone triperoxide or TATP. The basic chemistries for such endeavor have not been fully described. This investigation will examine the electrochemical mediation of the Fenton Reaction as a basis for detection of this class of explosives. The mediation takes place as a result of the homogeneous Fenton Reaction and the electro-reduction of an Fe. III complex to Fe. II followed by oxidation by either a hydroperoxide or hydrogen peroxide: Fe. IIcomplex + RO-OH Fe. IIIcomplex + RO- + HO∙ Fe. IIcomplex + HO-OH Fe. IIIcomplex + HO- + HO∙ The current due to the electro-reduction of the Fe. III complex is proportional to the square root of the peroxide concentration. The process is expected to be rapid, robust, and inexpensive. We will report on the detection limits, kinetics, optimal conditions for the degradation of TATP to hydroperoxides and H 2 O , and the role of the 2 chelate of that iron complex. The latter is based on considerations of the structure-activity relationships developed by cyclic voltammetric studies. 38 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5
Abstract - Detection of Organic Peroxide Explosives through the Fenton Reaction There is an urgent need for methods and techniques that are able to detect quantitatively and qualitatively peroxide based explosives, especially triacetone triperoxide or TATP. The basic chemistries for such endeavor have not been fully described. This investigation will examine the electrochemical mediation of the Fenton Reaction as a basis for detection of this class of explosives. The mediation takes place as a result of the homogeneous Fenton Reaction and the electro-reduction of an Fe. III complex to Fe. II followed by oxidation by either a hydroperoxide or hydrogen peroxide: Fe. IIcomplex + RO-OH Fe. IIIcomplex + RO- + HO∙ Fe. IIcomplex + HO-OH Fe. IIIcomplex + HO- + HO∙ The current due to the electro-reduction of the Fe. III complex is proportional to the square root of the peroxide concentration. The process is expected to be rapid, robust, and inexpensive. We will report on the detection limits, kinetics, optimal conditions for the degradation of TATP to hydroperoxides and H 2 O , and the role of the 2 chelate of that iron complex. The latter is based on considerations of the structure-activity relationships developed by cyclic voltammetric studies. 38 University of Idaho, IF Cheng, DF Laine, C Roske Pittcon 2008 Session 2520 -5


