a20cd628dbfd296cc5346da0b6650d2c.ppt
- Количество слайдов: 13
 Detection methods of Ig. E : a critical view Allergy School 21 st-24 th September 2007 Laurence Guilloux 1
Detection methods of Ig. E : a critical view Allergy School 21 st-24 th September 2007 Laurence Guilloux 1
 Detection method of Ig. E § The diagnostic algorithm for human allergic disorders begins with • A thorough clinical history • And a physical examination § Temporal associations between allergic symptoms and allergen exposures lead to a high degree of suspicion that the patient has an allergic disorder. § Confirmatory testing for Ig. E antibody (AB) is often performed to strengthen the probability that the working diagnosis is correct. • This involve in vivo methods (skin, provocation tests) • Laboratory-based in vitro analysis Ø Cell-based technology Ø Serum-based technology 2
Detection method of Ig. E § The diagnostic algorithm for human allergic disorders begins with • A thorough clinical history • And a physical examination § Temporal associations between allergic symptoms and allergen exposures lead to a high degree of suspicion that the patient has an allergic disorder. § Confirmatory testing for Ig. E antibody (AB) is often performed to strengthen the probability that the working diagnosis is correct. • This involve in vivo methods (skin, provocation tests) • Laboratory-based in vitro analysis Ø Cell-based technology Ø Serum-based technology 2
 Serum-based technology History § 1960, identification of human « reagin » as Ig. E § Preparation of Ig. E Fc fragment and production of polyclonal AB specific for the e heavy chain § 1967, first total Ig. E and allergen-specific Ig. E RAST were developed (Wide, Lancet) § 1974, first generation semi-quantitative Ig. E AB assay (birch specific Ig. E calibration curve and results expressed in classes) was first made commercially available by Pharmacia Laboratories. § Over the past decade there have been improvements in all aspects of technology with • Second-generation quantitative Ig. E assays • Third-generation automated Ig. E assays with a detection limit of 0. 1 k. U/L (Li CCA 2005) • Biochip technology, microarrays (Wiltshire, Clin Chem 2000; Kim, Exp Mol Med 2002) 3
Serum-based technology History § 1960, identification of human « reagin » as Ig. E § Preparation of Ig. E Fc fragment and production of polyclonal AB specific for the e heavy chain § 1967, first total Ig. E and allergen-specific Ig. E RAST were developed (Wide, Lancet) § 1974, first generation semi-quantitative Ig. E AB assay (birch specific Ig. E calibration curve and results expressed in classes) was first made commercially available by Pharmacia Laboratories. § Over the past decade there have been improvements in all aspects of technology with • Second-generation quantitative Ig. E assays • Third-generation automated Ig. E assays with a detection limit of 0. 1 k. U/L (Li CCA 2005) • Biochip technology, microarrays (Wiltshire, Clin Chem 2000; Kim, Exp Mol Med 2002) 3
 The assay of specific Ig. E AB is considerably more complicated than most other immunoassays § Firstly, the concentration of the Ig. E in the blood is extremely low, (0. 004 mg/100 ml; Ig. G: 1200 mg/100 ml) § Secondly, each main allergen, contains a large number of different allergenic components, § The assay must be sensitive enough to capture Ig. E AB to all relevant components but still with a very low level of non-specific binding. Today The two most representative classes of immunoassays for the measurement of specific Ig. E are based on the use of § Solid phase allergens § Liquid phase allergens (the fluid phase allergen is bound to the solid phase only after the allergen has complexed with fluid phase Ig. E AB). 4
The assay of specific Ig. E AB is considerably more complicated than most other immunoassays § Firstly, the concentration of the Ig. E in the blood is extremely low, (0. 004 mg/100 ml; Ig. G: 1200 mg/100 ml) § Secondly, each main allergen, contains a large number of different allergenic components, § The assay must be sensitive enough to capture Ig. E AB to all relevant components but still with a very low level of non-specific binding. Today The two most representative classes of immunoassays for the measurement of specific Ig. E are based on the use of § Solid phase allergens § Liquid phase allergens (the fluid phase allergen is bound to the solid phase only after the allergen has complexed with fluid phase Ig. E AB). 4
 « Architecture » of specific Ig. E immunoassays The analytical performances of immunoassays depend on the whole « architecture » of the method and particularly on some fundamental variables. 1. 2. 3. 4. Allergen-containing reagent and binding chemistry Anti Ig. E Calibration system And also the tracer and the signal detection system influence the sensitivity and quality of final results 5
« Architecture » of specific Ig. E immunoassays The analytical performances of immunoassays depend on the whole « architecture » of the method and particularly on some fundamental variables. 1. 2. 3. 4. Allergen-containing reagent and binding chemistry Anti Ig. E Calibration system And also the tracer and the signal detection system influence the sensitivity and quality of final results 5
 1. Allergen-containing reagent and binding chemistry § The solid-phase (allergosorbent) or liquid-phase labelled allergen reagent is the principal component of the assay that confers specificity on the Ig. E AB assay. § The most complex and highly variable component partly due to: 1 -1) the heterogeneity of most allergen extracts 1 -2) the different supports 1 -3) and the different chemical methods used to insolubilise or label the allergenic material 6
1. Allergen-containing reagent and binding chemistry § The solid-phase (allergosorbent) or liquid-phase labelled allergen reagent is the principal component of the assay that confers specificity on the Ig. E AB assay. § The most complex and highly variable component partly due to: 1 -1) the heterogeneity of most allergen extracts 1 -2) the different supports 1 -3) and the different chemical methods used to insolubilise or label the allergenic material 6
 1 -1) Heterogeneity of « allergens » n Whole extract Heterogeneity has many possible causes, even when the same manufacturer uses; • • n the same extract source (variable stability, inherent biological variation of the raw material, contamination…) the same chemical and manufacturing procedures (extraction process, variable binding of allergen, variable stability…. ) Purified native and recombinant allergenic molecules The advent of these allergenic molecules offers new perspectives: • r. Allergens produced in almost unlimited quantities will avoid the variability • • of natural allergenic extracts and allow standardisation (but different of the native form). Allergenic molecules are essential for the development of innovative techniques using microchip technologies and to improve our understanding of problems related to cross-reactivity of Ig. E AB to allergens. Spiking of allergen extract with purified native or recombinant allergenic molecules (known to be clinically relevant), can improve the sensitivity of the 7 diagnostic tests.
1 -1) Heterogeneity of « allergens » n Whole extract Heterogeneity has many possible causes, even when the same manufacturer uses; • • n the same extract source (variable stability, inherent biological variation of the raw material, contamination…) the same chemical and manufacturing procedures (extraction process, variable binding of allergen, variable stability…. ) Purified native and recombinant allergenic molecules The advent of these allergenic molecules offers new perspectives: • r. Allergens produced in almost unlimited quantities will avoid the variability • • of natural allergenic extracts and allow standardisation (but different of the native form). Allergenic molecules are essential for the development of innovative techniques using microchip technologies and to improve our understanding of problems related to cross-reactivity of Ig. E AB to allergens. Spiking of allergen extract with purified native or recombinant allergenic molecules (known to be clinically relevant), can improve the sensitivity of the 7 diagnostic tests.
 1 -2) Different supports Solid phase allergens (allergosorbent) § Insoluble dextran § Cellulose • filter paper disc (RAST) • Minicolumns (CAP) • Thread (CLA) § § Mycrocrystalline particles Nitocellulose (dot blot) Polystyrene tube (FAST) Microtiter well activated silica chip (ISAC) Liquid phase allergens § Biotin-labelled allergens (Advia Centaur) § The ligand (biotin) is covalently attached to a backbone (soluble polymer and amino acid copolymer) to wich the allergen molecules are also attached (Immulite 2000) 8
1 -2) Different supports Solid phase allergens (allergosorbent) § Insoluble dextran § Cellulose • filter paper disc (RAST) • Minicolumns (CAP) • Thread (CLA) § § Mycrocrystalline particles Nitocellulose (dot blot) Polystyrene tube (FAST) Microtiter well activated silica chip (ISAC) Liquid phase allergens § Biotin-labelled allergens (Advia Centaur) § The ligand (biotin) is covalently attached to a backbone (soluble polymer and amino acid copolymer) to wich the allergen molecules are also attached (Immulite 2000) 8
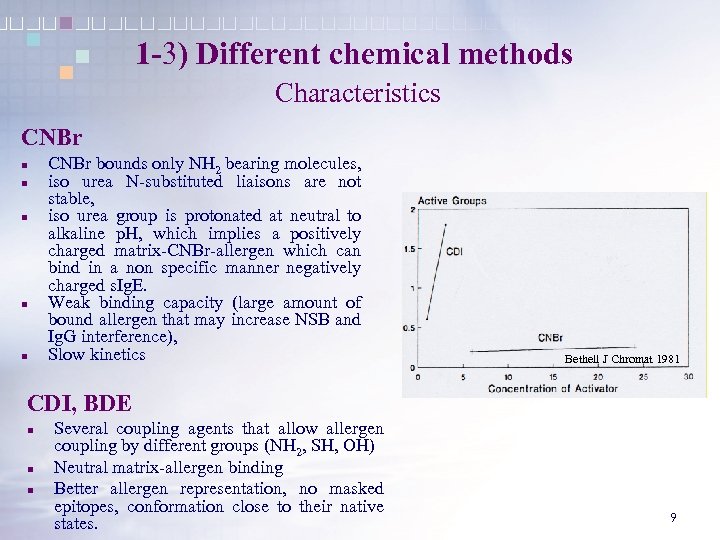 1 -3) Different chemical methods Characteristics CNBr n n n CNBr bounds only NH 2 bearing molecules, iso urea N-substituted liaisons are not stable, iso urea group is protonated at neutral to alkaline p. H, which implies a positively charged matrix-CNBr-allergen which can bind in a non specific manner negatively charged s. Ig. E. Weak binding capacity (large amount of bound allergen that may increase NSB and Ig. G interference), Slow kinetics Bethell J Chromat 1981 CDI, BDE n n n Several coupling agents that allow allergen coupling by different groups (NH 2, SH, OH) Neutral matrix-allergen binding Better allergen representation, no masked epitopes, conformation close to their native states. 9
1 -3) Different chemical methods Characteristics CNBr n n n CNBr bounds only NH 2 bearing molecules, iso urea N-substituted liaisons are not stable, iso urea group is protonated at neutral to alkaline p. H, which implies a positively charged matrix-CNBr-allergen which can bind in a non specific manner negatively charged s. Ig. E. Weak binding capacity (large amount of bound allergen that may increase NSB and Ig. G interference), Slow kinetics Bethell J Chromat 1981 CDI, BDE n n n Several coupling agents that allow allergen coupling by different groups (NH 2, SH, OH) Neutral matrix-allergen binding Better allergen representation, no masked epitopes, conformation close to their native states. 9
 2. Anti - Ig. E Highly specific for unique determinants on e heavy chains Used as both capture and detection reagents § Subjected to chemical modification (radio-labelled, enzyme-labelled, chemical and physical immobilisation on solid-phase matrices § Initially polyclonal reagents § Monoclonal reagents are specific and lack batch-to-batch variations, however as they have low affinity and avidity, mixtures of mono and polyclonal antisera are often used § 3. Calibration system (NCCLS 1997) n Homologous interpolation methods This is a term that defines a calibration scheme in which the standard or reference (calibration) dose-response curve is constructed using reagents that have the same specificity from those being used to measure the analyte of interest. n Heterologous interpolation methods This is a term that defines a calibration scheme in which the standard or reference (calibration) dose-response curve is constructed using reagents that have a different specificity from those being used to measure the analyte of interest. In the contexte of specific Ig. E assays, this mean that a total serum Ig. E calibration curve in IU/ml of Ig. E, which is calibrated back to an Ig. E primary standard, is used to 10 interpolate unknown allergen-specific Ig. E response data.
2. Anti - Ig. E Highly specific for unique determinants on e heavy chains Used as both capture and detection reagents § Subjected to chemical modification (radio-labelled, enzyme-labelled, chemical and physical immobilisation on solid-phase matrices § Initially polyclonal reagents § Monoclonal reagents are specific and lack batch-to-batch variations, however as they have low affinity and avidity, mixtures of mono and polyclonal antisera are often used § 3. Calibration system (NCCLS 1997) n Homologous interpolation methods This is a term that defines a calibration scheme in which the standard or reference (calibration) dose-response curve is constructed using reagents that have the same specificity from those being used to measure the analyte of interest. n Heterologous interpolation methods This is a term that defines a calibration scheme in which the standard or reference (calibration) dose-response curve is constructed using reagents that have a different specificity from those being used to measure the analyte of interest. In the contexte of specific Ig. E assays, this mean that a total serum Ig. E calibration curve in IU/ml of Ig. E, which is calibrated back to an Ig. E primary standard, is used to 10 interpolate unknown allergen-specific Ig. E response data.
 Classification of specific Ig. E antibody immunoassays Is dependent on the degree with which the assay result accurately reflects the quantity of s. Ig. E AB in the test specimen, and the assay’s precision requirements (according to NCCLS approved guidelines) n Qualitative assays (no calibration curve) Positive/negative based on pre-assigned positive threshold n Semi-quantitative assays The most diffuse and typical illustration is the multiallergen screening test in which different allergen specificities are present in a single reagent The variations in the positive signal detected are commonly presented • • n in terms of a series of increasing grades (e. g. , I to IV; low to high) or classes in arbitrary units (U/ml) determined relative to a supplier-specific, heterologous dose-response curve The results produced or an end-point dilution to which the signal becomes negative (e. g. , titer), are not traceable to any comon reference or in comparison to a qualitative grading scheme (e. g. , colour chart) Quantitative assay (multipoint calibration curve) material. A quantitative assay provides an accurate and reproducible estimate of the concentration of Ig. E and fulfills the analytical criteria for quantification including: • Parallelism, recovery, precision and • Linearity across the whole assay’s working range 11
Classification of specific Ig. E antibody immunoassays Is dependent on the degree with which the assay result accurately reflects the quantity of s. Ig. E AB in the test specimen, and the assay’s precision requirements (according to NCCLS approved guidelines) n Qualitative assays (no calibration curve) Positive/negative based on pre-assigned positive threshold n Semi-quantitative assays The most diffuse and typical illustration is the multiallergen screening test in which different allergen specificities are present in a single reagent The variations in the positive signal detected are commonly presented • • n in terms of a series of increasing grades (e. g. , I to IV; low to high) or classes in arbitrary units (U/ml) determined relative to a supplier-specific, heterologous dose-response curve The results produced or an end-point dilution to which the signal becomes negative (e. g. , titer), are not traceable to any comon reference or in comparison to a qualitative grading scheme (e. g. , colour chart) Quantitative assay (multipoint calibration curve) material. A quantitative assay provides an accurate and reproducible estimate of the concentration of Ig. E and fulfills the analytical criteria for quantification including: • Parallelism, recovery, precision and • Linearity across the whole assay’s working range 11
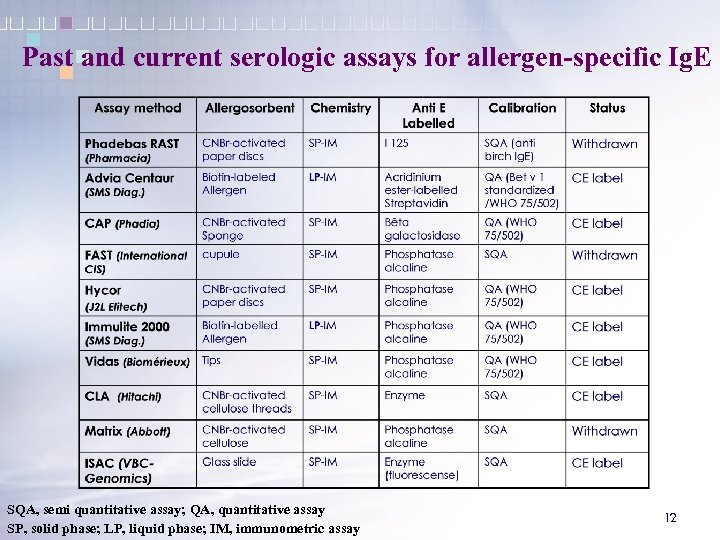 Past and current serologic assays for allergen-specific Ig. E SQA, semi quantitative assay; QA, quantitative assay SP, solid phase; LP, liquid phase; IM, immunometric assay 12
Past and current serologic assays for allergen-specific Ig. E SQA, semi quantitative assay; QA, quantitative assay SP, solid phase; LP, liquid phase; IM, immunometric assay 12
 Conclusion n Excellent analytical performances are generally achieved by third generation laboratory systems for s. Ig. E as CAP System, IMMULITE 2000 3 g and ADVIA Centaur in term of quantitative results. • n Third generation laboratory systems for s. Ig. E demonstrate acceptably high concordance with objective clinical tests (skin, provocation tests). • n On account of differences in term of k. U/L results obtained with the different systems and without the avaibality of the « true » value of s. Ig. E we should keep in mind that the different technics are not interchangeable (thresholds using ROC curves, probability curves are not identical). It’s a general rule for immunoassays (ex. tumor markers). Discrepant results have to be carefully analysed in term of sensitivity and specificity. Immunoassays for the s. Ig. E AB have undergone significant improvements with, the use of more purified extacts, the capacity to quantitate Ig. E AB in mass units, and total automation of assay system. However, laboratory information should be totally integrated in rational clinical pathways. 13
Conclusion n Excellent analytical performances are generally achieved by third generation laboratory systems for s. Ig. E as CAP System, IMMULITE 2000 3 g and ADVIA Centaur in term of quantitative results. • n Third generation laboratory systems for s. Ig. E demonstrate acceptably high concordance with objective clinical tests (skin, provocation tests). • n On account of differences in term of k. U/L results obtained with the different systems and without the avaibality of the « true » value of s. Ig. E we should keep in mind that the different technics are not interchangeable (thresholds using ROC curves, probability curves are not identical). It’s a general rule for immunoassays (ex. tumor markers). Discrepant results have to be carefully analysed in term of sensitivity and specificity. Immunoassays for the s. Ig. E AB have undergone significant improvements with, the use of more purified extacts, the capacity to quantitate Ig. E AB in mass units, and total automation of assay system. However, laboratory information should be totally integrated in rational clinical pathways. 13


