204f132226422386c39efe77224accec.ppt
- Количество слайдов: 123
 Detection and Dosimetry of Ionising Radiation MSc-REP Lecture Notes Paddy Regan p. regan@surrey. ac. uk http: //www. ph. surrey. ac. uk/~phs 1 pr/lecture_notes MSc-REP Regan Dosimetry 1
Detection and Dosimetry of Ionising Radiation MSc-REP Lecture Notes Paddy Regan p. regan@surrey. ac. uk http: //www. ph. surrey. ac. uk/~phs 1 pr/lecture_notes MSc-REP Regan Dosimetry 1
 Course text book, Radiation Biophysics by E. L. Alpen, Academic Press 2 nd Edition, (1990) Important chapters for this course, Chapter 1: Quantities and Units Chapter 4: Radiation/Matter interactions. Chapter 5: Energy Transfer Processes Chapter 16: Dose, Dose Equivalent Also, refer to Radiation Detection and Measurement, G. F. Knoll, 2 nd Edition. Introduction to Health Physics, H. Cember and T. E. Johnson, 4 th Edition (Mc. Graw Hill) MSc-REP Regan Dosimetry 2
Course text book, Radiation Biophysics by E. L. Alpen, Academic Press 2 nd Edition, (1990) Important chapters for this course, Chapter 1: Quantities and Units Chapter 4: Radiation/Matter interactions. Chapter 5: Energy Transfer Processes Chapter 16: Dose, Dose Equivalent Also, refer to Radiation Detection and Measurement, G. F. Knoll, 2 nd Edition. Introduction to Health Physics, H. Cember and T. E. Johnson, 4 th Edition (Mc. Graw Hill) MSc-REP Regan Dosimetry 2
 Some Useful Web Pages Dosimetry definitions etc. • http: //www. physics. isu. edu/radinf/terms. htm • http: //www. hps. org/publicinformation/radfactsheets/ Also, good notes on basic dosimetry terms etc. can be found at • http: //www. physics. mtsu. edu/~phys 2020/index. html (chapter 11) • http: //www. physics. isu. edu/radinf/index. html International Commission in Radiation Protection (ICRP) web site • http: //www. icrp. org/ Stopping powers, attenuation coeffs of x-rays, e-s, ps & s from the USA National Institute for Standards and Technology • http: //physics. nist. gov/Phys. Ref. Data/contents-radi. html • http: //physics. nist. gov/Phys. Ref. Data/Xray. Mass. Coeff/ (see also Seltzer Radiation Research 136 (1993) p 147) • http: //www. nist. gov/physlab/data/xcom/index. cfm • http: //www. srim. org/ (for charged particles) MSc-REP Regan Dosimetry 3
Some Useful Web Pages Dosimetry definitions etc. • http: //www. physics. isu. edu/radinf/terms. htm • http: //www. hps. org/publicinformation/radfactsheets/ Also, good notes on basic dosimetry terms etc. can be found at • http: //www. physics. mtsu. edu/~phys 2020/index. html (chapter 11) • http: //www. physics. isu. edu/radinf/index. html International Commission in Radiation Protection (ICRP) web site • http: //www. icrp. org/ Stopping powers, attenuation coeffs of x-rays, e-s, ps & s from the USA National Institute for Standards and Technology • http: //physics. nist. gov/Phys. Ref. Data/contents-radi. html • http: //physics. nist. gov/Phys. Ref. Data/Xray. Mass. Coeff/ (see also Seltzer Radiation Research 136 (1993) p 147) • http: //www. nist. gov/physlab/data/xcom/index. cfm • http: //www. srim. org/ (for charged particles) MSc-REP Regan Dosimetry 3
 Relationship Between Detectors and Dosimetry – Physical and Chemical Effects of Ionising Radiation. – General Concepts and Units. – Radiation Quantities and Definitions – Absolute Methods of Dosimetry MSc-REP Regan Dosimetry 4
Relationship Between Detectors and Dosimetry – Physical and Chemical Effects of Ionising Radiation. – General Concepts and Units. – Radiation Quantities and Definitions – Absolute Methods of Dosimetry MSc-REP Regan Dosimetry 4
 Physical and Chemical Effects of Ionising Radiation Incident ionising radiation cause the following effects on matter (which can, therefore conversely be used to measure the amount of radiation imparted): • Ionisation (i. e. , electrons removed from atoms) • Excitation (atoms/molecules raised to excited states) • Chemical effects (changes in the structure of molecules which can lead to molecular disassociation resulting in biological changes). • Radiation damage to the crystalline structure in solids. • Thermal effects (radiation causes increase in temperature) • Nuclear excitations and/or transmutations. MSc-REP Regan Dosimetry 5
Physical and Chemical Effects of Ionising Radiation Incident ionising radiation cause the following effects on matter (which can, therefore conversely be used to measure the amount of radiation imparted): • Ionisation (i. e. , electrons removed from atoms) • Excitation (atoms/molecules raised to excited states) • Chemical effects (changes in the structure of molecules which can lead to molecular disassociation resulting in biological changes). • Radiation damage to the crystalline structure in solids. • Thermal effects (radiation causes increase in temperature) • Nuclear excitations and/or transmutations. MSc-REP Regan Dosimetry 5
 Radiation Damage in Biological Systems • In biological organisms, radiation damage occurs due to the ionisation of atoms and molecules in cells. • The production of ions can result in chemical reactions which break molecular bonds in proteins and other important biological molecules. • Typically 1 -> 40 e. V of energy is needed to ionize a molecule or atom, thus radiations such as , b and , which can have energies in the 100 ke. V to few Me. V range, can individually result in the ionisation of thousands of atoms or molecules. • Biological damage can subsequently result either by cells being killed or mutating (which can result in cancer). A large enough dose will destroy sufficient numbers of cells to kill the organism. MSc-REP Regan Dosimetry 6
Radiation Damage in Biological Systems • In biological organisms, radiation damage occurs due to the ionisation of atoms and molecules in cells. • The production of ions can result in chemical reactions which break molecular bonds in proteins and other important biological molecules. • Typically 1 -> 40 e. V of energy is needed to ionize a molecule or atom, thus radiations such as , b and , which can have energies in the 100 ke. V to few Me. V range, can individually result in the ionisation of thousands of atoms or molecules. • Biological damage can subsequently result either by cells being killed or mutating (which can result in cancer). A large enough dose will destroy sufficient numbers of cells to kill the organism. MSc-REP Regan Dosimetry 6
 There are 2 main types of radiation damage in biological systems: Somatic Damage (also known as ‘radiation sickness’): This refers to damage to cells which are not associated with reproduction. The degree of somatic damage depends on the organ exposed and the age of the individual (younger = more susceptible to somatic damage). Effects of somatic damage include: • reddening of the skin, • hair loss, • ulceration, • reduction of white blood cells, • cataracts in the eyes, • fibrosis of the lungs. Genetic Damage: This refers to damage to cells associated with reproduction which can lead to genetic mutations in the offspring. MSc-REP Regan Dosimetry 7
There are 2 main types of radiation damage in biological systems: Somatic Damage (also known as ‘radiation sickness’): This refers to damage to cells which are not associated with reproduction. The degree of somatic damage depends on the organ exposed and the age of the individual (younger = more susceptible to somatic damage). Effects of somatic damage include: • reddening of the skin, • hair loss, • ulceration, • reduction of white blood cells, • cataracts in the eyes, • fibrosis of the lungs. Genetic Damage: This refers to damage to cells associated with reproduction which can lead to genetic mutations in the offspring. MSc-REP Regan Dosimetry 7
 Some Terms Related to Dose • • Chronic Dose: dose received over an extended period of time. Acute Dose: dose received in a short period of time. Somatic Effects: effects seen in an individual exposed to the dose. Genetic Effects: effects in the offspring of the individual exposed to the radiation due to a pre-conception exposure of the offspring. • Teratogenic Effects: are effects in the offspring of the individual who experienced the dose during gestation. • Stochastic Effects: are effects which occur on a random basis. Such effects have no effective threshold, but the chances of such an effect are increased with dose. Cancer is a stochastic effect. • Non-Stochastic Effects: can be directly related to the size of the dose received. They often have a dose threshold below which the effect does not occur. Skin burning from radiation is a nonstochastic effect. MSc-REP Regan Dosimetry 8
Some Terms Related to Dose • • Chronic Dose: dose received over an extended period of time. Acute Dose: dose received in a short period of time. Somatic Effects: effects seen in an individual exposed to the dose. Genetic Effects: effects in the offspring of the individual exposed to the radiation due to a pre-conception exposure of the offspring. • Teratogenic Effects: are effects in the offspring of the individual who experienced the dose during gestation. • Stochastic Effects: are effects which occur on a random basis. Such effects have no effective threshold, but the chances of such an effect are increased with dose. Cancer is a stochastic effect. • Non-Stochastic Effects: can be directly related to the size of the dose received. They often have a dose threshold below which the effect does not occur. Skin burning from radiation is a nonstochastic effect. MSc-REP Regan Dosimetry 8
 Basis of Detector/Dosimetry Systems Dosimetry/radiation detection systems can then be designed and operated using these effects. Basic systems include, • Calorimetry, based of thermal effects and increases in temperature. This provides the most basic and accurate ‘primary standard’. • Chemical dosimeters, based on chemical effects and molecular changes, a good and accurate, ‘secondary standard’ • Ionisation chambers - electronic ionisation. • Proportional Counters/Geiger-Mueller detectors - electronic ionisation and atomic/molecular excitation in a gas medium. • Semiconductor detectors (silicon, germanium, Cd. Te) - ionisation MSc-REP Regan Dosimetry 9
Basis of Detector/Dosimetry Systems Dosimetry/radiation detection systems can then be designed and operated using these effects. Basic systems include, • Calorimetry, based of thermal effects and increases in temperature. This provides the most basic and accurate ‘primary standard’. • Chemical dosimeters, based on chemical effects and molecular changes, a good and accurate, ‘secondary standard’ • Ionisation chambers - electronic ionisation. • Proportional Counters/Geiger-Mueller detectors - electronic ionisation and atomic/molecular excitation in a gas medium. • Semiconductor detectors (silicon, germanium, Cd. Te) - ionisation MSc-REP Regan Dosimetry 9
 Basis of Detector/Dosimetry Systems (cont. ) • Scintillation counters (e. g. , Na. I(Tl), Ba. F 2) - scintillation light emitted following molecular excitations. (see Knoll p 221, p 231) • Solid state integrating dosimeters - radiation damage in solids • Photographic methods - radiation damage in solids • Solid state track detectors - radiation damage in solids • Activation detectors - nuclear transmutation (for neutrons usually via (n, ), (n, p) or (n, ) reactions). Slow (thermal) neutrons detection can use (see Knoll p 483 ff & 707 ff) 10 B(n, ) 7 Li (Q=2. 310, 94%, E(7 Li) = 0. 84 Me. V, E( ) = 1. 47 Me. V). 6 Li(n, )3 H (Q=4. 78 Me. V, E(3 H) = 2. 73 Me. V, E( ) = 2. 05 Me. V) 55 Mn(n, )56 Mn T =2. 6 h ; 59 Co(n, )60 Co T =10. 4 min & 5. 3 y ; 1/2 109 Ag(n, )110 Ag, T =24 secs; 164 Dy(n, )165 m. Dy, T =1. 3 mins. 1/2 Threshold activation detectors (for fast neutrons) include 59 Co(n, )56 Mn, T =2. 56 h ; 23 Na(n, )20 F (in a Na. I(Tl) detector) 1/2 MSc-REP Regan Dosimetry 10
Basis of Detector/Dosimetry Systems (cont. ) • Scintillation counters (e. g. , Na. I(Tl), Ba. F 2) - scintillation light emitted following molecular excitations. (see Knoll p 221, p 231) • Solid state integrating dosimeters - radiation damage in solids • Photographic methods - radiation damage in solids • Solid state track detectors - radiation damage in solids • Activation detectors - nuclear transmutation (for neutrons usually via (n, ), (n, p) or (n, ) reactions). Slow (thermal) neutrons detection can use (see Knoll p 483 ff & 707 ff) 10 B(n, ) 7 Li (Q=2. 310, 94%, E(7 Li) = 0. 84 Me. V, E( ) = 1. 47 Me. V). 6 Li(n, )3 H (Q=4. 78 Me. V, E(3 H) = 2. 73 Me. V, E( ) = 2. 05 Me. V) 55 Mn(n, )56 Mn T =2. 6 h ; 59 Co(n, )60 Co T =10. 4 min & 5. 3 y ; 1/2 109 Ag(n, )110 Ag, T =24 secs; 164 Dy(n, )165 m. Dy, T =1. 3 mins. 1/2 Threshold activation detectors (for fast neutrons) include 59 Co(n, )56 Mn, T =2. 56 h ; 23 Na(n, )20 F (in a Na. I(Tl) detector) 1/2 MSc-REP Regan Dosimetry 10
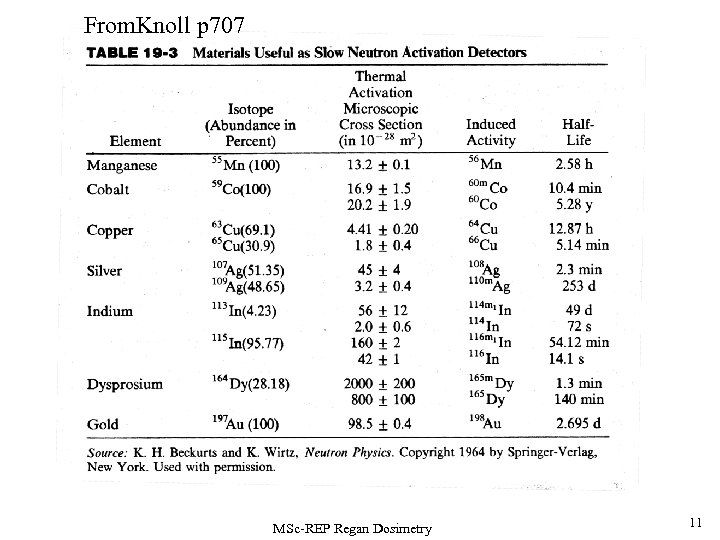 From. Knoll p 707 MSc-REP Regan Dosimetry 11
From. Knoll p 707 MSc-REP Regan Dosimetry 11
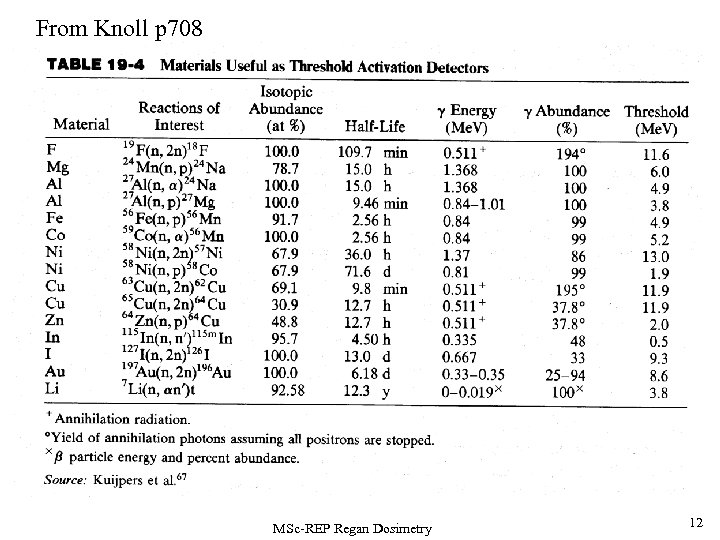 From Knoll p 708 MSc-REP Regan Dosimetry 12
From Knoll p 708 MSc-REP Regan Dosimetry 12
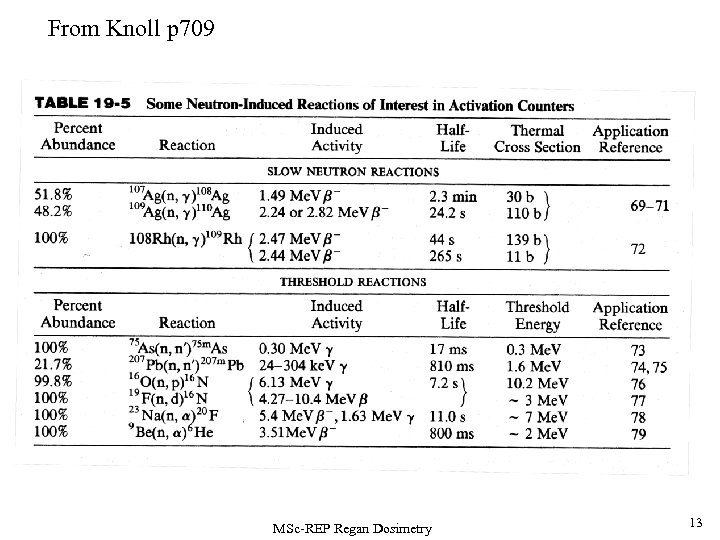 From Knoll p 709 MSc-REP Regan Dosimetry 13
From Knoll p 709 MSc-REP Regan Dosimetry 13
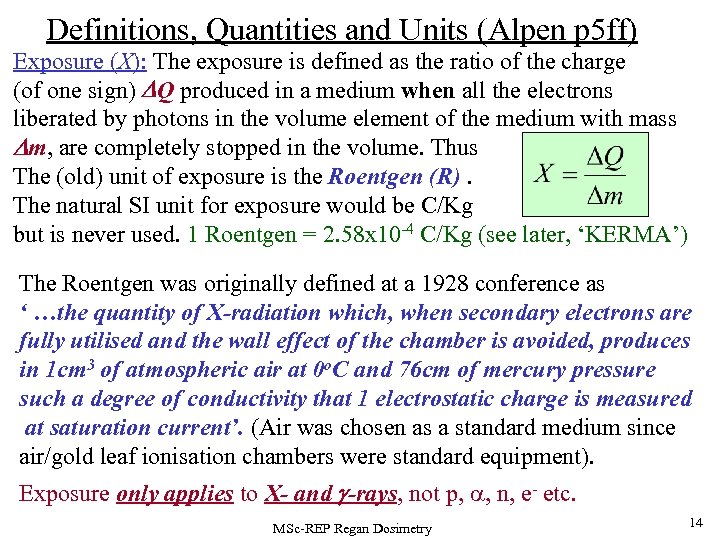 Definitions, Quantities and Units (Alpen p 5 ff) Exposure (X): The exposure is defined as the ratio of the charge (of one sign) DQ produced in a medium when all the electrons liberated by photons in the volume element of the medium with mass Dm, are completely stopped in the volume. Thus The (old) unit of exposure is the Roentgen (R). The natural SI unit for exposure would be C/Kg but is never used. 1 Roentgen = 2. 58 x 10 -4 C/Kg (see later, ‘KERMA’) The Roentgen was originally defined at a 1928 conference as ‘ …the quantity of X-radiation which, when secondary electrons are fully utilised and the wall effect of the chamber is avoided, produces in 1 cm 3 of atmospheric air at 0 o. C and 76 cm of mercury pressure such a degree of conductivity that 1 electrostatic charge is measured at saturation current’. (Air was chosen as a standard medium since air/gold leaf ionisation chambers were standard equipment). Exposure only applies to X- and g-rays, not p, , n, e- etc. MSc-REP Regan Dosimetry 14
Definitions, Quantities and Units (Alpen p 5 ff) Exposure (X): The exposure is defined as the ratio of the charge (of one sign) DQ produced in a medium when all the electrons liberated by photons in the volume element of the medium with mass Dm, are completely stopped in the volume. Thus The (old) unit of exposure is the Roentgen (R). The natural SI unit for exposure would be C/Kg but is never used. 1 Roentgen = 2. 58 x 10 -4 C/Kg (see later, ‘KERMA’) The Roentgen was originally defined at a 1928 conference as ‘ …the quantity of X-radiation which, when secondary electrons are fully utilised and the wall effect of the chamber is avoided, produces in 1 cm 3 of atmospheric air at 0 o. C and 76 cm of mercury pressure such a degree of conductivity that 1 electrostatic charge is measured at saturation current’. (Air was chosen as a standard medium since air/gold leaf ionisation chambers were standard equipment). Exposure only applies to X- and g-rays, not p, , n, e- etc. MSc-REP Regan Dosimetry 14
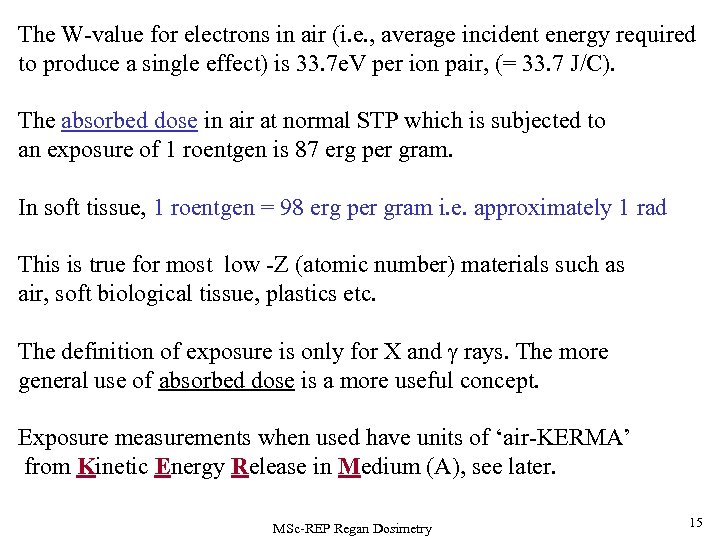 The W-value for electrons in air (i. e. , average incident energy required to produce a single effect) is 33. 7 e. V per ion pair, (= 33. 7 J/C). The absorbed dose in air at normal STP which is subjected to an exposure of 1 roentgen is 87 erg per gram. In soft tissue, 1 roentgen = 98 erg per gram i. e. approximately 1 rad This is true for most low -Z (atomic number) materials such as air, soft biological tissue, plastics etc. The definition of exposure is only for X and rays. The more general use of absorbed dose is a more useful concept. Exposure measurements when used have units of ‘air-KERMA’ from Kinetic Energy Release in Medium (A), see later. MSc-REP Regan Dosimetry 15
The W-value for electrons in air (i. e. , average incident energy required to produce a single effect) is 33. 7 e. V per ion pair, (= 33. 7 J/C). The absorbed dose in air at normal STP which is subjected to an exposure of 1 roentgen is 87 erg per gram. In soft tissue, 1 roentgen = 98 erg per gram i. e. approximately 1 rad This is true for most low -Z (atomic number) materials such as air, soft biological tissue, plastics etc. The definition of exposure is only for X and rays. The more general use of absorbed dose is a more useful concept. Exposure measurements when used have units of ‘air-KERMA’ from Kinetic Energy Release in Medium (A), see later. MSc-REP Regan Dosimetry 15
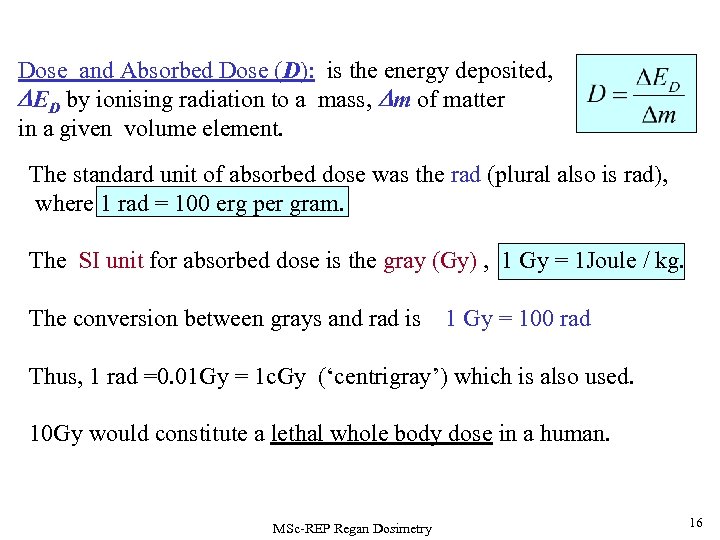 Dose and Absorbed Dose (D): is the energy deposited, DED by ionising radiation to a mass, Dm of matter in a given volume element. The standard unit of absorbed dose was the rad (plural also is rad), where 1 rad = 100 erg per gram. The SI unit for absorbed dose is the gray (Gy) , 1 Gy = 1 Joule / kg. The conversion between grays and rad is 1 Gy = 100 rad Thus, 1 rad =0. 01 Gy = 1 c. Gy (‘centrigray’) which is also used. 10 Gy would constitute a lethal whole body dose in a human. MSc-REP Regan Dosimetry 16
Dose and Absorbed Dose (D): is the energy deposited, DED by ionising radiation to a mass, Dm of matter in a given volume element. The standard unit of absorbed dose was the rad (plural also is rad), where 1 rad = 100 erg per gram. The SI unit for absorbed dose is the gray (Gy) , 1 Gy = 1 Joule / kg. The conversion between grays and rad is 1 Gy = 100 rad Thus, 1 rad =0. 01 Gy = 1 c. Gy (‘centrigray’) which is also used. 10 Gy would constitute a lethal whole body dose in a human. MSc-REP Regan Dosimetry 16
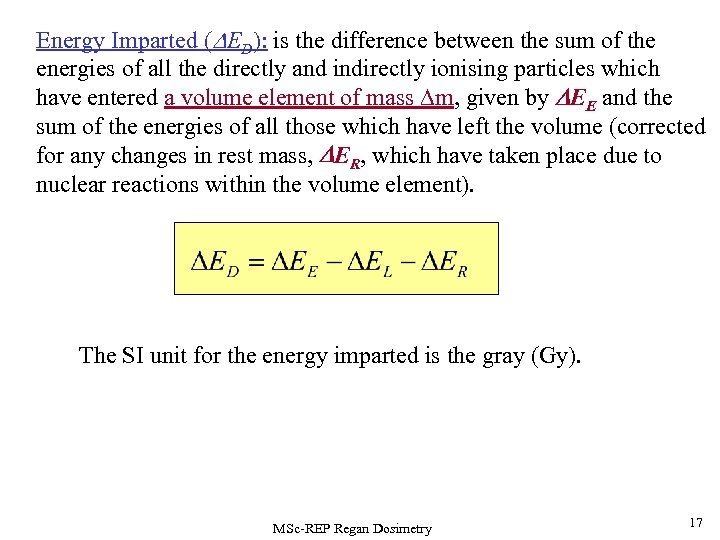 Energy Imparted (DED): is the difference between the sum of the energies of all the directly and indirectly ionising particles which have entered a volume element of mass m, given by DEE and the sum of the energies of all those which have left the volume (corrected for any changes in rest mass, DER, which have taken place due to nuclear reactions within the volume element). The SI unit for the energy imparted is the gray (Gy). MSc-REP Regan Dosimetry 17
Energy Imparted (DED): is the difference between the sum of the energies of all the directly and indirectly ionising particles which have entered a volume element of mass m, given by DEE and the sum of the energies of all those which have left the volume (corrected for any changes in rest mass, DER, which have taken place due to nuclear reactions within the volume element). The SI unit for the energy imparted is the gray (Gy). MSc-REP Regan Dosimetry 17
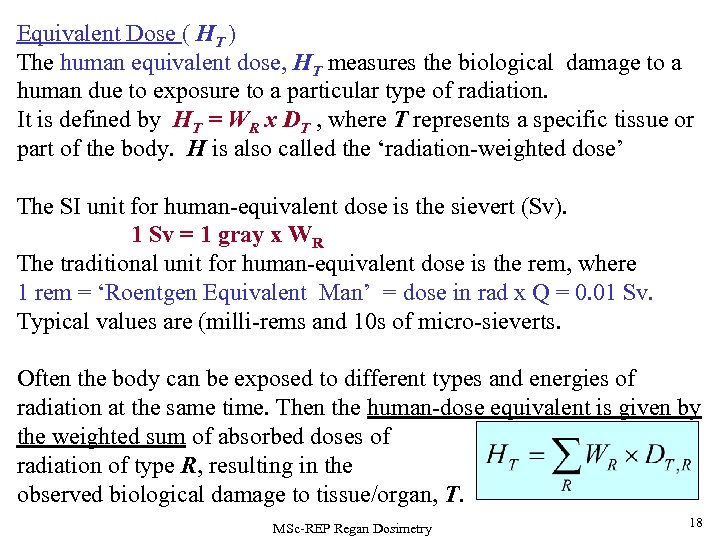 Equivalent Dose ( HT ) The human equivalent dose, HT measures the biological damage to a human due to exposure to a particular type of radiation. It is defined by HT = WR x DT , where T represents a specific tissue or part of the body. H is also called the ‘radiation-weighted dose’ The SI unit for human-equivalent dose is the sievert (Sv). 1 Sv = 1 gray x WR The traditional unit for human-equivalent dose is the rem, where 1 rem = ‘Roentgen Equivalent Man’ = dose in rad x Q = 0. 01 Sv. Typical values are (milli-rems and 10 s of micro-sieverts. Often the body can be exposed to different types and energies of radiation at the same time. Then the human-dose equivalent is given by the weighted sum of absorbed doses of radiation of type R, resulting in the observed biological damage to tissue/organ, T. MSc-REP Regan Dosimetry 18
Equivalent Dose ( HT ) The human equivalent dose, HT measures the biological damage to a human due to exposure to a particular type of radiation. It is defined by HT = WR x DT , where T represents a specific tissue or part of the body. H is also called the ‘radiation-weighted dose’ The SI unit for human-equivalent dose is the sievert (Sv). 1 Sv = 1 gray x WR The traditional unit for human-equivalent dose is the rem, where 1 rem = ‘Roentgen Equivalent Man’ = dose in rad x Q = 0. 01 Sv. Typical values are (milli-rems and 10 s of micro-sieverts. Often the body can be exposed to different types and energies of radiation at the same time. Then the human-dose equivalent is given by the weighted sum of absorbed doses of radiation of type R, resulting in the observed biological damage to tissue/organ, T. MSc-REP Regan Dosimetry 18
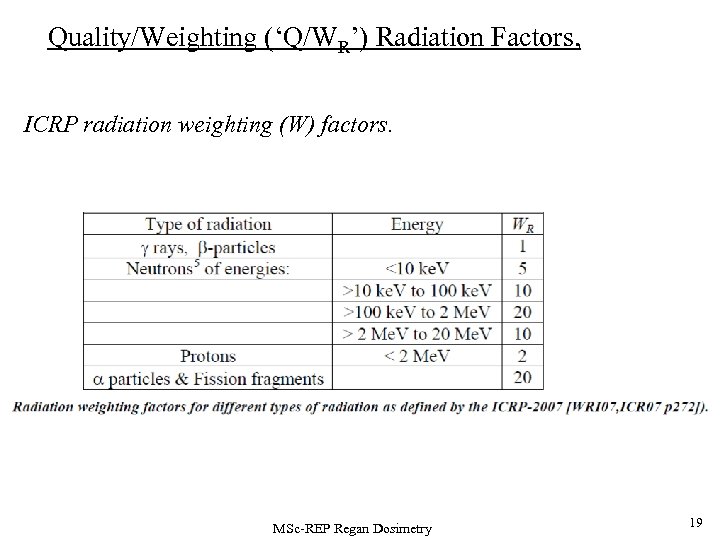 Quality/Weighting (‘Q/WR’) Radiation Factors, ICRP radiation weighting (W) factors. MSc-REP Regan Dosimetry 19
Quality/Weighting (‘Q/WR’) Radiation Factors, ICRP radiation weighting (W) factors. MSc-REP Regan Dosimetry 19
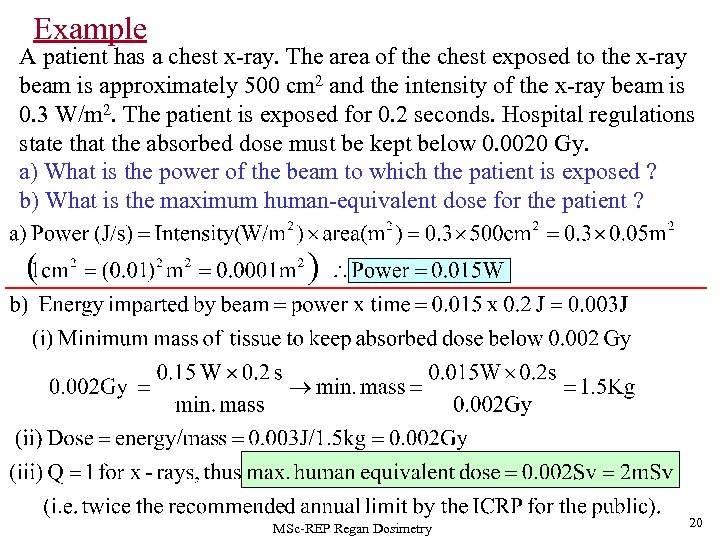 Example A patient has a chest x-ray. The area of the chest exposed to the x-ray beam is approximately 500 cm 2 and the intensity of the x-ray beam is 0. 3 W/m 2. The patient is exposed for 0. 2 seconds. Hospital regulations state that the absorbed dose must be kept below 0. 0020 Gy. a) What is the power of the beam to which the patient is exposed ? b) What is the maximum human-equivalent dose for the patient ? MSc-REP Regan Dosimetry 20
Example A patient has a chest x-ray. The area of the chest exposed to the x-ray beam is approximately 500 cm 2 and the intensity of the x-ray beam is 0. 3 W/m 2. The patient is exposed for 0. 2 seconds. Hospital regulations state that the absorbed dose must be kept below 0. 0020 Gy. a) What is the power of the beam to which the patient is exposed ? b) What is the maximum human-equivalent dose for the patient ? MSc-REP Regan Dosimetry 20
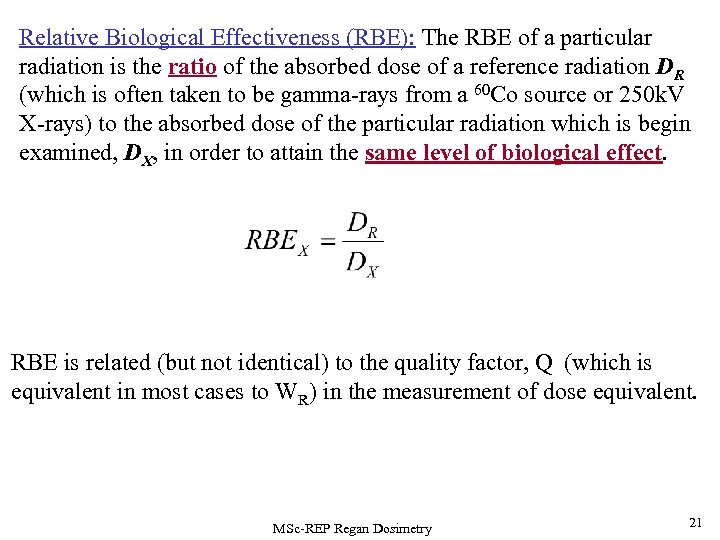 Relative Biological Effectiveness (RBE): The RBE of a particular radiation is the ratio of the absorbed dose of a reference radiation DR (which is often taken to be gamma-rays from a 60 Co source or 250 k. V X-rays) to the absorbed dose of the particular radiation which is begin examined, DX, in order to attain the same level of biological effect. RBE is related (but not identical) to the quality factor, Q (which is equivalent in most cases to WR) in the measurement of dose equivalent. MSc-REP Regan Dosimetry 21
Relative Biological Effectiveness (RBE): The RBE of a particular radiation is the ratio of the absorbed dose of a reference radiation DR (which is often taken to be gamma-rays from a 60 Co source or 250 k. V X-rays) to the absorbed dose of the particular radiation which is begin examined, DX, in order to attain the same level of biological effect. RBE is related (but not identical) to the quality factor, Q (which is equivalent in most cases to WR) in the measurement of dose equivalent. MSc-REP Regan Dosimetry 21
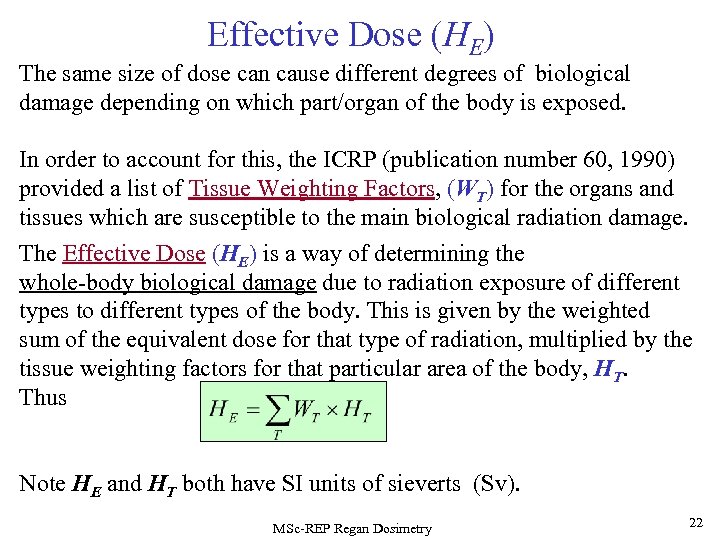 Effective Dose (HE) The same size of dose can cause different degrees of biological damage depending on which part/organ of the body is exposed. In order to account for this, the ICRP (publication number 60, 1990) provided a list of Tissue Weighting Factors, (WT) for the organs and tissues which are susceptible to the main biological radiation damage. The Effective Dose (HE) is a way of determining the whole-body biological damage due to radiation exposure of different types to different types of the body. This is given by the weighted sum of the equivalent dose for that type of radiation, multiplied by the tissue weighting factors for that particular area of the body, HT. Thus Note HE and HT both have SI units of sieverts (Sv). MSc-REP Regan Dosimetry 22
Effective Dose (HE) The same size of dose can cause different degrees of biological damage depending on which part/organ of the body is exposed. In order to account for this, the ICRP (publication number 60, 1990) provided a list of Tissue Weighting Factors, (WT) for the organs and tissues which are susceptible to the main biological radiation damage. The Effective Dose (HE) is a way of determining the whole-body biological damage due to radiation exposure of different types to different types of the body. This is given by the weighted sum of the equivalent dose for that type of radiation, multiplied by the tissue weighting factors for that particular area of the body, HT. Thus Note HE and HT both have SI units of sieverts (Sv). MSc-REP Regan Dosimetry 22
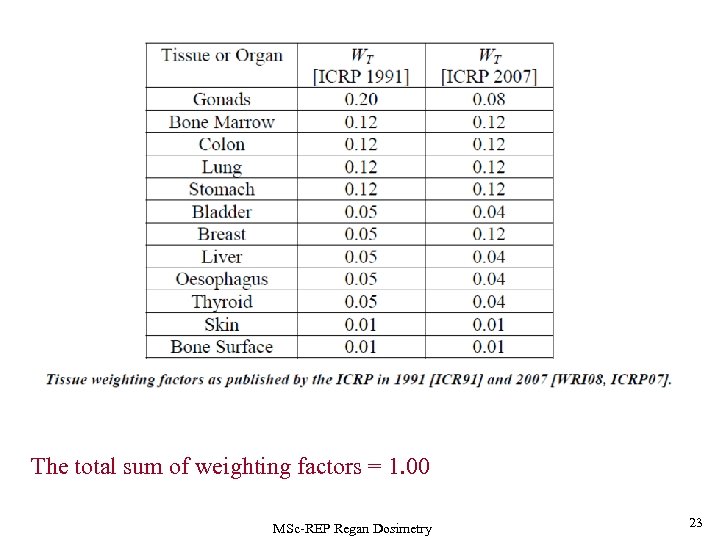 The total sum of weighting factors = 1. 00 MSc-REP Regan Dosimetry 23
The total sum of weighting factors = 1. 00 MSc-REP Regan Dosimetry 23
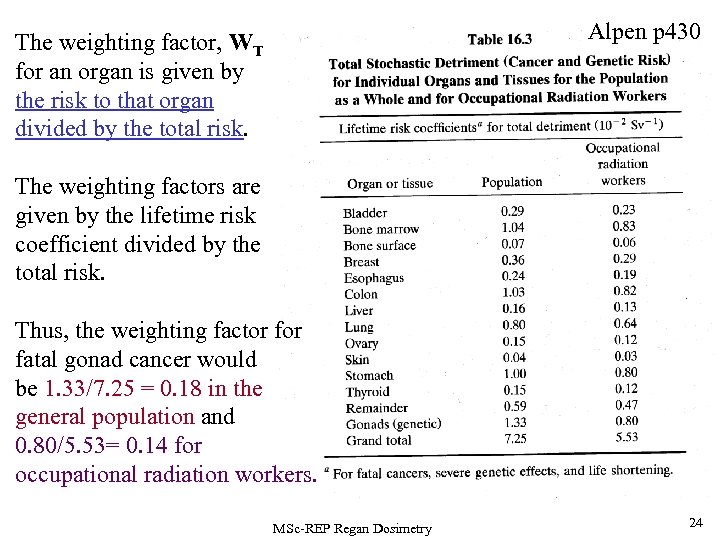 Alpen p 430 The weighting factor, WT for an organ is given by the risk to that organ divided by the total risk. The weighting factors are given by the lifetime risk coefficient divided by the total risk. Thus, the weighting factor fatal gonad cancer would be 1. 33/7. 25 = 0. 18 in the general population and 0. 80/5. 53= 0. 14 for occupational radiation workers. MSc-REP Regan Dosimetry 24
Alpen p 430 The weighting factor, WT for an organ is given by the risk to that organ divided by the total risk. The weighting factors are given by the lifetime risk coefficient divided by the total risk. Thus, the weighting factor fatal gonad cancer would be 1. 33/7. 25 = 0. 18 in the general population and 0. 80/5. 53= 0. 14 for occupational radiation workers. MSc-REP Regan Dosimetry 24
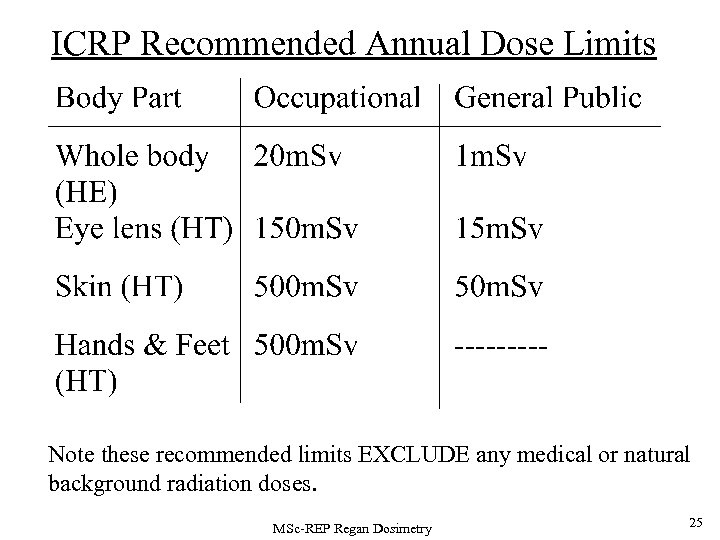 ICRP Recommended Annual Dose Limits Note these recommended limits EXCLUDE any medical or natural background radiation doses. MSc-REP Regan Dosimetry 25
ICRP Recommended Annual Dose Limits Note these recommended limits EXCLUDE any medical or natural background radiation doses. MSc-REP Regan Dosimetry 25
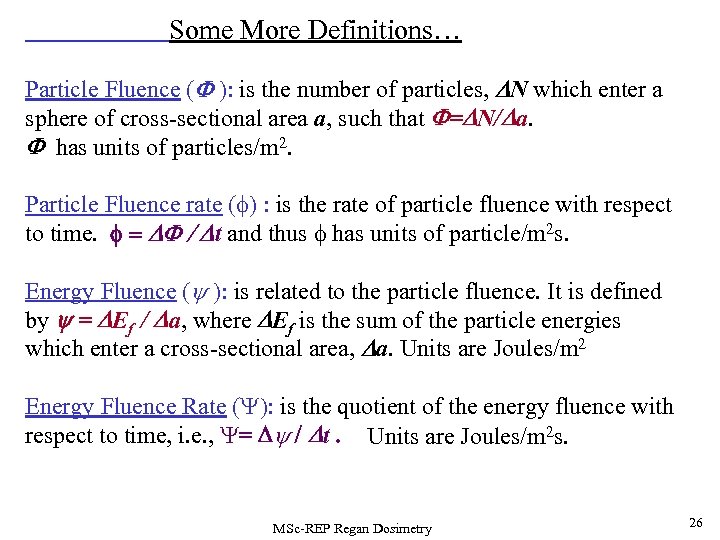 Some More Definitions… Particle Fluence (F ): is the number of particles, DN which enter a sphere of cross-sectional area a, such that F=DN/Da. F has units of particles/m 2. Particle Fluence rate (f) : is the rate of particle fluence with respect to time. f = DF / Dt and thus f has units of particle/m 2 s. Energy Fluence (y ): is related to the particle fluence. It is defined by y = DEf / Da, where DEf is the sum of the particle energies which enter a cross-sectional area, Da. Units are Joules/m 2 Energy Fluence Rate (Y): is the quotient of the energy fluence with respect to time, i. e. , Y= Dy / Dt. Units are Joules/m 2 s. MSc-REP Regan Dosimetry 26
Some More Definitions… Particle Fluence (F ): is the number of particles, DN which enter a sphere of cross-sectional area a, such that F=DN/Da. F has units of particles/m 2. Particle Fluence rate (f) : is the rate of particle fluence with respect to time. f = DF / Dt and thus f has units of particle/m 2 s. Energy Fluence (y ): is related to the particle fluence. It is defined by y = DEf / Da, where DEf is the sum of the particle energies which enter a cross-sectional area, Da. Units are Joules/m 2 Energy Fluence Rate (Y): is the quotient of the energy fluence with respect to time, i. e. , Y= Dy / Dt. Units are Joules/m 2 s. MSc-REP Regan Dosimetry 26
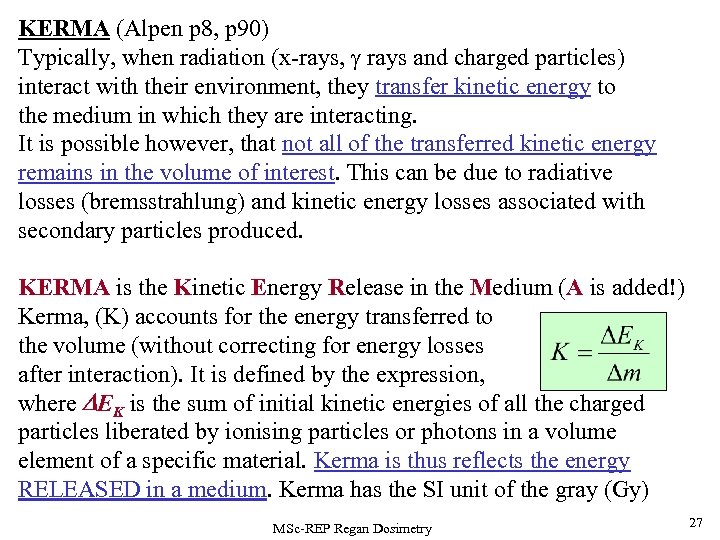 KERMA (Alpen p 8, p 90) Typically, when radiation (x-rays, rays and charged particles) interact with their environment, they transfer kinetic energy to the medium in which they are interacting. It is possible however, that not all of the transferred kinetic energy remains in the volume of interest. This can be due to radiative losses (bremsstrahlung) and kinetic energy losses associated with secondary particles produced. KERMA is the Kinetic Energy Release in the Medium (A is added!) Kerma, (K) accounts for the energy transferred to the volume (without correcting for energy losses after interaction). It is defined by the expression, where DEK is the sum of initial kinetic energies of all the charged particles liberated by ionising particles or photons in a volume element of a specific material. Kerma is thus reflects the energy RELEASED in a medium. Kerma has the SI unit of the gray (Gy) MSc-REP Regan Dosimetry 27
KERMA (Alpen p 8, p 90) Typically, when radiation (x-rays, rays and charged particles) interact with their environment, they transfer kinetic energy to the medium in which they are interacting. It is possible however, that not all of the transferred kinetic energy remains in the volume of interest. This can be due to radiative losses (bremsstrahlung) and kinetic energy losses associated with secondary particles produced. KERMA is the Kinetic Energy Release in the Medium (A is added!) Kerma, (K) accounts for the energy transferred to the volume (without correcting for energy losses after interaction). It is defined by the expression, where DEK is the sum of initial kinetic energies of all the charged particles liberated by ionising particles or photons in a volume element of a specific material. Kerma is thus reflects the energy RELEASED in a medium. Kerma has the SI unit of the gray (Gy) MSc-REP Regan Dosimetry 27
 Charged Particle Equilibrium (CPE) : Charged particle equilibrium is said to exist at a point p, centred in a volume V, if each charged particle carrying out a certain energy from this volume is replaced by another identical particle which carrying the same energy into the volume. If CPE exists at a point, the dose = kerma (D = K) at that point (provided that the secondary radiation losses by the charged particles such as bremsstrahlung are negligible). Dose is the energy absorbed in the unit volume, while kerma is the energy transferred from the original particle (or photon) in the same unit volume. MSc-REP Regan Dosimetry 28
Charged Particle Equilibrium (CPE) : Charged particle equilibrium is said to exist at a point p, centred in a volume V, if each charged particle carrying out a certain energy from this volume is replaced by another identical particle which carrying the same energy into the volume. If CPE exists at a point, the dose = kerma (D = K) at that point (provided that the secondary radiation losses by the charged particles such as bremsstrahlung are negligible). Dose is the energy absorbed in the unit volume, while kerma is the energy transferred from the original particle (or photon) in the same unit volume. MSc-REP Regan Dosimetry 28
 Absolute Methods of Dosimetry Absolute methods of dosimetry can provide measurements for the absorbed dose without the instrument (‘dosemeter’) being calibrated in a known radiation field. (Most instruments give measurements relative to calibrated primary or secondary standards). It is however possible to calibrate certain detector media which can then be placed inside the sensitive volume of specific dosemeters. These instruments then posses an effective ‘internal calibration’ and as such can be described as absolute dosemeters. For any radiation phenomenon (e. g. , gammas neutrons etc. ) to be used for radiation dosimetry, we need to know 1) the fraction (f) of the absorbed dose (D) which is channelled into a given effect, and 2) the average energy (H) needed to produce a given effect (e. g. , ionisation, chemical changes, nuclear reactions etc. ) MSc-REP Regan Dosimetry 29
Absolute Methods of Dosimetry Absolute methods of dosimetry can provide measurements for the absorbed dose without the instrument (‘dosemeter’) being calibrated in a known radiation field. (Most instruments give measurements relative to calibrated primary or secondary standards). It is however possible to calibrate certain detector media which can then be placed inside the sensitive volume of specific dosemeters. These instruments then posses an effective ‘internal calibration’ and as such can be described as absolute dosemeters. For any radiation phenomenon (e. g. , gammas neutrons etc. ) to be used for radiation dosimetry, we need to know 1) the fraction (f) of the absorbed dose (D) which is channelled into a given effect, and 2) the average energy (H) needed to produce a given effect (e. g. , ionisation, chemical changes, nuclear reactions etc. ) MSc-REP Regan Dosimetry 29
 Thus, the energy per unit mass going to any specific effect = f. D. If this causes Ne subsequent effects per unit mass, and the average energy required to produce the unit effect is H, then the total energy required is the product of Ne and H, ie. Ne. H = f. D Thus, since D = (H/f). Ne, , , if (H / f) is a constant and known, the number of effects induced by the radiation (Ne) is proportional to the absorbed dose. Thus the dose can be obtained by measuring Ne. Consider a charged particle of energy E, coming rest inside a medium. If a fraction fi of the particle’s kinetic energy produces ionisation in the medium, assuming that the energy required to cause ionisation by radiation induced collisions is Hi, we have fi. E=Ni Hi and the number of ions produced is Ni=(fi. E)/Hi . Now, (E / Ni)=(Hi / f. I) is the average energy required to produce one electron-ion pair, which is known as the W-value. (NOTE, This is NOT the same as WR mentioned earlier). MSc-REP Regan Dosimetry 30
Thus, the energy per unit mass going to any specific effect = f. D. If this causes Ne subsequent effects per unit mass, and the average energy required to produce the unit effect is H, then the total energy required is the product of Ne and H, ie. Ne. H = f. D Thus, since D = (H/f). Ne, , , if (H / f) is a constant and known, the number of effects induced by the radiation (Ne) is proportional to the absorbed dose. Thus the dose can be obtained by measuring Ne. Consider a charged particle of energy E, coming rest inside a medium. If a fraction fi of the particle’s kinetic energy produces ionisation in the medium, assuming that the energy required to cause ionisation by radiation induced collisions is Hi, we have fi. E=Ni Hi and the number of ions produced is Ni=(fi. E)/Hi . Now, (E / Ni)=(Hi / f. I) is the average energy required to produce one electron-ion pair, which is known as the W-value. (NOTE, This is NOT the same as WR mentioned earlier). MSc-REP Regan Dosimetry 30
 Therefore, Thus, if we know W, we have the calibration factor (Hi / fi) without having to know Hi or fi individually. In the case of dosimetry based on calorimetry, Ht is the energy required to raise the temperature of a unit mass of the radiation absorber by 1 K, which is also the definition of the specific heat, S of the material. Here the ‘measured effect’ is the temperature rise in degrees kelvin which is caused by the induced radiation. MSc-REP Regan Dosimetry 31
Therefore, Thus, if we know W, we have the calibration factor (Hi / fi) without having to know Hi or fi individually. In the case of dosimetry based on calorimetry, Ht is the energy required to raise the temperature of a unit mass of the radiation absorber by 1 K, which is also the definition of the specific heat, S of the material. Here the ‘measured effect’ is the temperature rise in degrees kelvin which is caused by the induced radiation. MSc-REP Regan Dosimetry 31
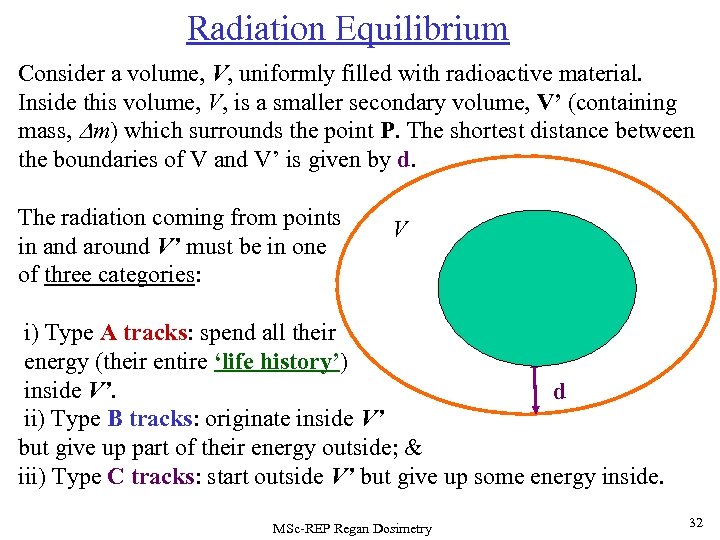 Radiation Equilibrium Consider a volume, V, uniformly filled with radioactive material. Inside this volume, V, is a smaller secondary volume, V’ (containing mass, Dm) which surrounds the point P. The shortest distance between the boundaries of V and V’ is given by d. The radiation coming from points in and around V’ must be in one of three categories: V C V’(Dm) P B C A i) Type A tracks: spend all their energy (their entire ‘life history’) inside V’. d ii) Type B tracks: originate inside V’ but give up part of their energy outside; & iii) Type C tracks: start outside V’ but give up some energy inside. MSc-REP Regan Dosimetry 32
Radiation Equilibrium Consider a volume, V, uniformly filled with radioactive material. Inside this volume, V, is a smaller secondary volume, V’ (containing mass, Dm) which surrounds the point P. The shortest distance between the boundaries of V and V’ is given by d. The radiation coming from points in and around V’ must be in one of three categories: V C V’(Dm) P B C A i) Type A tracks: spend all their energy (their entire ‘life history’) inside V’. d ii) Type B tracks: originate inside V’ but give up part of their energy outside; & iii) Type C tracks: start outside V’ but give up some energy inside. MSc-REP Regan Dosimetry 32
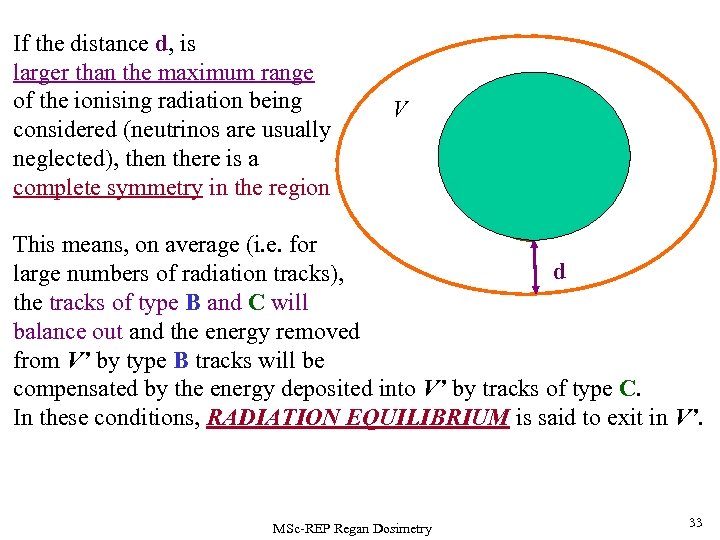 If the distance d, is larger than the maximum range of the ionising radiation being considered (neutrinos are usually neglected), then there is a complete symmetry in the region V’. V C V’(Dm) P B C A This means, on average (i. e. for d large numbers of radiation tracks), the tracks of type B and C will balance out and the energy removed from V’ by type B tracks will be compensated by the energy deposited into V’ by tracks of type C. In these conditions, RADIATION EQUILIBRIUM is said to exit in V’. MSc-REP Regan Dosimetry 33
If the distance d, is larger than the maximum range of the ionising radiation being considered (neutrinos are usually neglected), then there is a complete symmetry in the region V’. V C V’(Dm) P B C A This means, on average (i. e. for d large numbers of radiation tracks), the tracks of type B and C will balance out and the energy removed from V’ by type B tracks will be compensated by the energy deposited into V’ by tracks of type C. In these conditions, RADIATION EQUILIBRIUM is said to exit in V’. MSc-REP Regan Dosimetry 33
 A more complete definition of absorbed dose is given in Radiation Dosimetry volume 1 (1968) pp 32 -33, edited by Attix and Roesch. ‘ The energy imparted to matter by ionising radiation per unit mass is called the absorbed dose. By energy imparted to matter, we mean that which appears as ionisation or excitation, increase in chemical energy or crystal lattice energy etc. in the material. The energy that goes into changes in rest mass of the material or in the radiation itself (pair production) is excluded by definition; in some cases this energy can be comparable with absorbed dose, but does not produce important extra-nuclear effects. ’ Thus, the energy absorbed can be split into 2 components, causing: 1) changes in atomic/molecular/lattice energy states and 2) changes in the rest mass. MSc-REP Regan Dosimetry 34
A more complete definition of absorbed dose is given in Radiation Dosimetry volume 1 (1968) pp 32 -33, edited by Attix and Roesch. ‘ The energy imparted to matter by ionising radiation per unit mass is called the absorbed dose. By energy imparted to matter, we mean that which appears as ionisation or excitation, increase in chemical energy or crystal lattice energy etc. in the material. The energy that goes into changes in rest mass of the material or in the radiation itself (pair production) is excluded by definition; in some cases this energy can be comparable with absorbed dose, but does not produce important extra-nuclear effects. ’ Thus, the energy absorbed can be split into 2 components, causing: 1) changes in atomic/molecular/lattice energy states and 2) changes in the rest mass. MSc-REP Regan Dosimetry 34
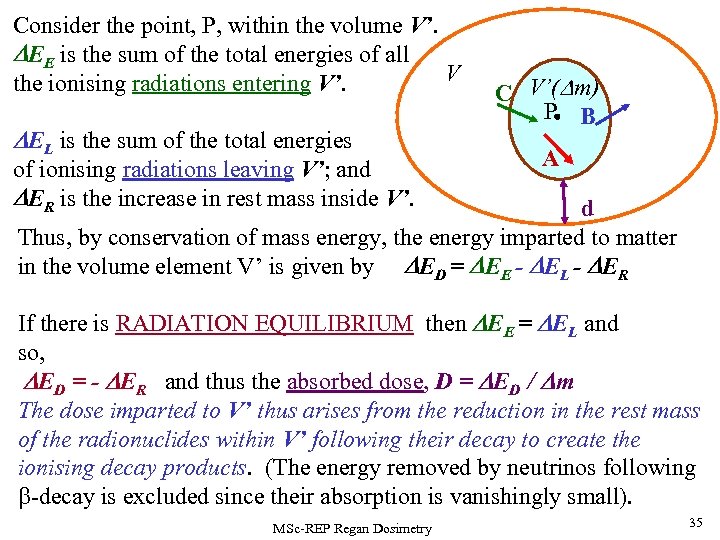 Consider the point, P, within the volume V’. DEE is the sum of the total energies of all V the ionising radiations entering V’. DEL is the sum of the total energies of ionising radiations leaving V’; and DER is the increase in rest mass inside V’. C V’(Dm) P B C A d Thus, by conservation of mass energy, the energy imparted to matter in the volume element V’ is given by DED = DEE - DEL - DER If there is RADIATION EQUILIBRIUM then DEE = DEL and so, DED = - DER and thus the absorbed dose, D = DED / Dm The dose imparted to V’ thus arises from the reduction in the rest mass of the radionuclides within V’ following their decay to create the ionising decay products. (The energy removed by neutrinos following b-decay is excluded since their absorption is vanishingly small). MSc-REP Regan Dosimetry 35
Consider the point, P, within the volume V’. DEE is the sum of the total energies of all V the ionising radiations entering V’. DEL is the sum of the total energies of ionising radiations leaving V’; and DER is the increase in rest mass inside V’. C V’(Dm) P B C A d Thus, by conservation of mass energy, the energy imparted to matter in the volume element V’ is given by DED = DEE - DEL - DER If there is RADIATION EQUILIBRIUM then DEE = DEL and so, DED = - DER and thus the absorbed dose, D = DED / Dm The dose imparted to V’ thus arises from the reduction in the rest mass of the radionuclides within V’ following their decay to create the ionising decay products. (The energy removed by neutrinos following b-decay is excluded since their absorption is vanishingly small). MSc-REP Regan Dosimetry 35
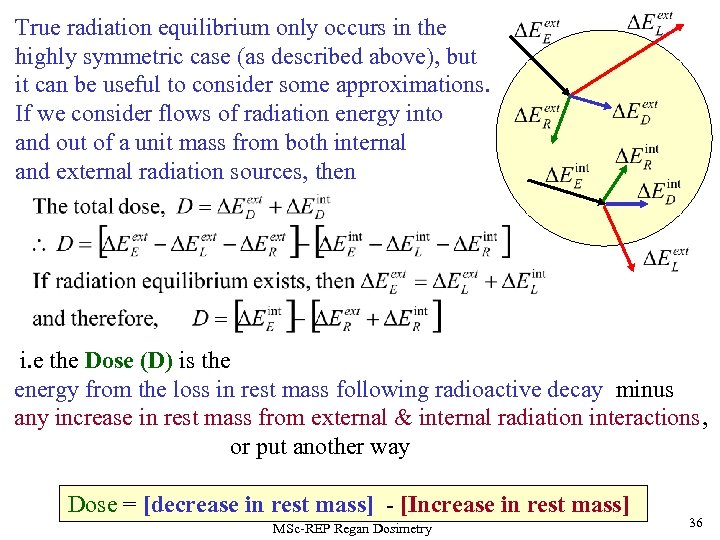 True radiation equilibrium only occurs in the highly symmetric case (as described above), but it can be useful to consider some approximations. If we consider flows of radiation energy into and out of a unit mass from both internal and external radiation sources, then i. e the Dose (D) is the energy from the loss in rest mass following radioactive decay minus any increase in rest mass from external & internal radiation interactions, or put another way Dose = [decrease in rest mass] - [Increase in rest mass] MSc-REP Regan Dosimetry 36
True radiation equilibrium only occurs in the highly symmetric case (as described above), but it can be useful to consider some approximations. If we consider flows of radiation energy into and out of a unit mass from both internal and external radiation sources, then i. e the Dose (D) is the energy from the loss in rest mass following radioactive decay minus any increase in rest mass from external & internal radiation interactions, or put another way Dose = [decrease in rest mass] - [Increase in rest mass] MSc-REP Regan Dosimetry 36
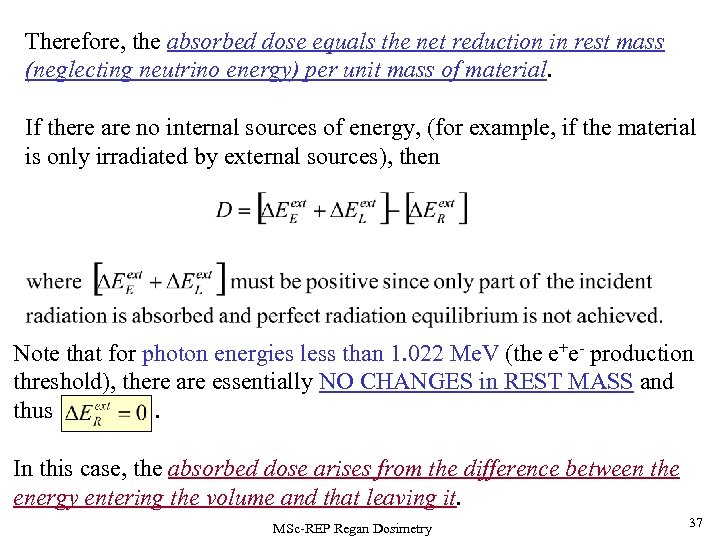 Therefore, the absorbed dose equals the net reduction in rest mass (neglecting neutrino energy) per unit mass of material. If there are no internal sources of energy, (for example, if the material is only irradiated by external sources), then Note that for photon energies less than 1. 022 Me. V (the e+e- production threshold), there are essentially NO CHANGES in REST MASS and thus . In this case, the absorbed dose arises from the difference between the energy entering the volume and that leaving it. MSc-REP Regan Dosimetry 37
Therefore, the absorbed dose equals the net reduction in rest mass (neglecting neutrino energy) per unit mass of material. If there are no internal sources of energy, (for example, if the material is only irradiated by external sources), then Note that for photon energies less than 1. 022 Me. V (the e+e- production threshold), there are essentially NO CHANGES in REST MASS and thus . In this case, the absorbed dose arises from the difference between the energy entering the volume and that leaving it. MSc-REP Regan Dosimetry 37
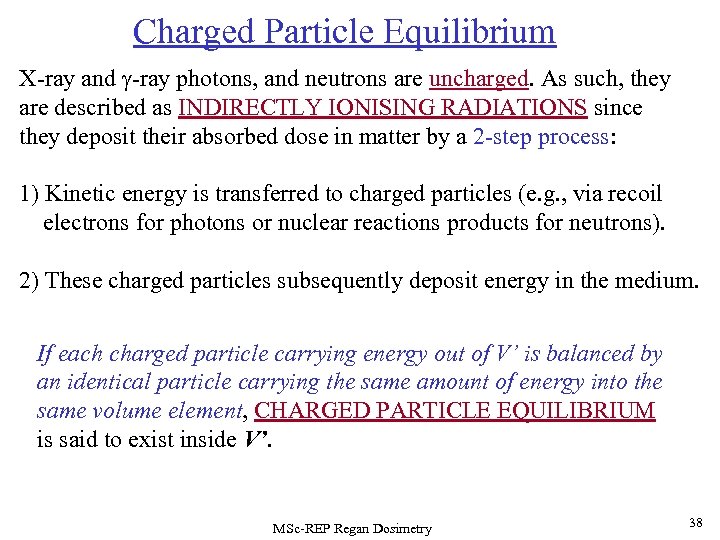 Charged Particle Equilibrium X-ray and -ray photons, and neutrons are uncharged. As such, they are described as INDIRECTLY IONISING RADIATIONS since they deposit their absorbed dose in matter by a 2 -step process: 1) Kinetic energy is transferred to charged particles (e. g. , via recoil electrons for photons or nuclear reactions products for neutrons). 2) These charged particles subsequently deposit energy in the medium. If each charged particle carrying energy out of V’ is balanced by an identical particle carrying the same amount of energy into the same volume element, CHARGED PARTICLE EQUILIBRIUM is said to exist inside V’. MSc-REP Regan Dosimetry 38
Charged Particle Equilibrium X-ray and -ray photons, and neutrons are uncharged. As such, they are described as INDIRECTLY IONISING RADIATIONS since they deposit their absorbed dose in matter by a 2 -step process: 1) Kinetic energy is transferred to charged particles (e. g. , via recoil electrons for photons or nuclear reactions products for neutrons). 2) These charged particles subsequently deposit energy in the medium. If each charged particle carrying energy out of V’ is balanced by an identical particle carrying the same amount of energy into the same volume element, CHARGED PARTICLE EQUILIBRIUM is said to exist inside V’. MSc-REP Regan Dosimetry 38
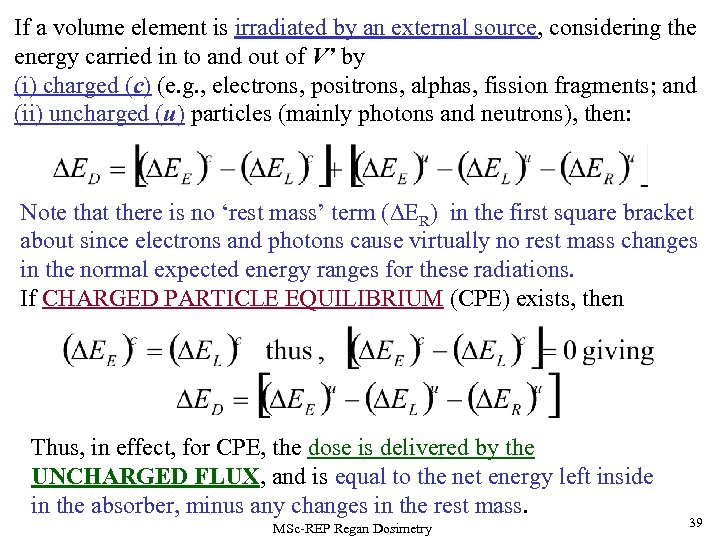 If a volume element is irradiated by an external source, considering the energy carried in to and out of V’ by (i) charged (c) (e. g. , electrons, positrons, alphas, fission fragments; and (ii) uncharged (u) particles (mainly photons and neutrons), then: Note that there is no ‘rest mass’ term ( ER) in the first square bracket about since electrons and photons cause virtually no rest mass changes in the normal expected energy ranges for these radiations. If CHARGED PARTICLE EQUILIBRIUM (CPE) exists, then Thus, in effect, for CPE, the dose is delivered by the UNCHARGED FLUX, and is equal to the net energy left inside in the absorber, minus any changes in the rest mass. MSc-REP Regan Dosimetry 39
If a volume element is irradiated by an external source, considering the energy carried in to and out of V’ by (i) charged (c) (e. g. , electrons, positrons, alphas, fission fragments; and (ii) uncharged (u) particles (mainly photons and neutrons), then: Note that there is no ‘rest mass’ term ( ER) in the first square bracket about since electrons and photons cause virtually no rest mass changes in the normal expected energy ranges for these radiations. If CHARGED PARTICLE EQUILIBRIUM (CPE) exists, then Thus, in effect, for CPE, the dose is delivered by the UNCHARGED FLUX, and is equal to the net energy left inside in the absorber, minus any changes in the rest mass. MSc-REP Regan Dosimetry 39
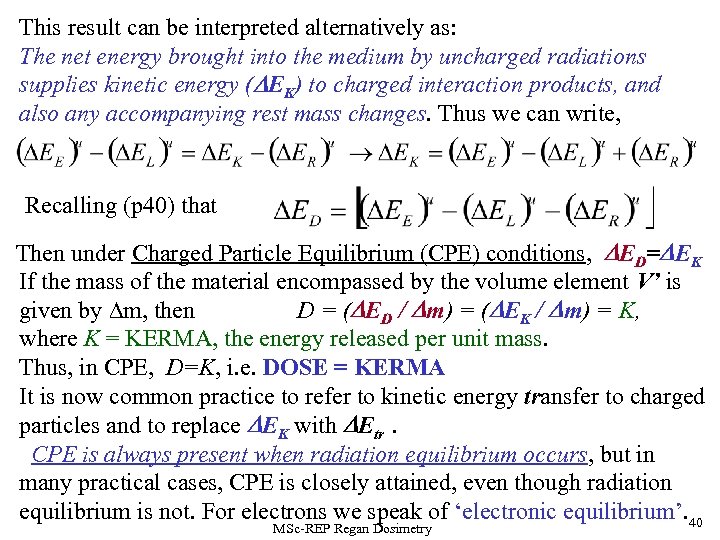 This result can be interpreted alternatively as: The net energy brought into the medium by uncharged radiations supplies kinetic energy (DEK) to charged interaction products, and also any accompanying rest mass changes. Thus we can write, Recalling (p 40) that Then under Charged Particle Equilibrium (CPE) conditions, DED=DEK If the mass of the material encompassed by the volume element V’ is given by m, then D = (DED / Dm) = (DEK / Dm) = K, where K = KERMA, the energy released per unit mass. Thus, in CPE, D=K, i. e. DOSE = KERMA It is now common practice to refer to kinetic energy transfer to charged particles and to replace DEK with DEtr. CPE is always present when radiation equilibrium occurs, but in many practical cases, CPE is closely attained, even though radiation equilibrium is not. For electrons we speak of ‘electronic equilibrium’. 40 MSc-REP Regan Dosimetry
This result can be interpreted alternatively as: The net energy brought into the medium by uncharged radiations supplies kinetic energy (DEK) to charged interaction products, and also any accompanying rest mass changes. Thus we can write, Recalling (p 40) that Then under Charged Particle Equilibrium (CPE) conditions, DED=DEK If the mass of the material encompassed by the volume element V’ is given by m, then D = (DED / Dm) = (DEK / Dm) = K, where K = KERMA, the energy released per unit mass. Thus, in CPE, D=K, i. e. DOSE = KERMA It is now common practice to refer to kinetic energy transfer to charged particles and to replace DEK with DEtr. CPE is always present when radiation equilibrium occurs, but in many practical cases, CPE is closely attained, even though radiation equilibrium is not. For electrons we speak of ‘electronic equilibrium’. 40 MSc-REP Regan Dosimetry
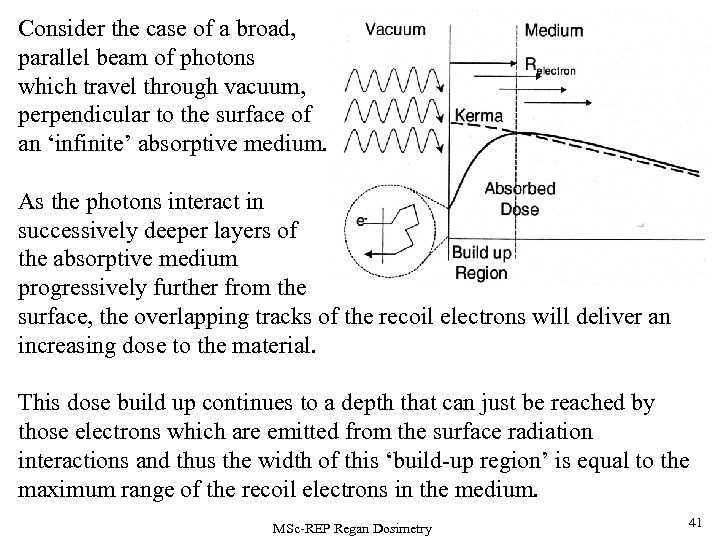 Consider the case of a broad, parallel beam of photons which travel through vacuum, perpendicular to the surface of an ‘infinite’ absorptive medium. As the photons interact in successively deeper layers of the absorptive medium progressively further from the surface, the overlapping tracks of the recoil electrons will deliver an increasing dose to the material. This dose build up continues to a depth that can just be reached by those electrons which are emitted from the surface radiation interactions and thus the width of this ‘build-up region’ is equal to the maximum range of the recoil electrons in the medium. MSc-REP Regan Dosimetry 41
Consider the case of a broad, parallel beam of photons which travel through vacuum, perpendicular to the surface of an ‘infinite’ absorptive medium. As the photons interact in successively deeper layers of the absorptive medium progressively further from the surface, the overlapping tracks of the recoil electrons will deliver an increasing dose to the material. This dose build up continues to a depth that can just be reached by those electrons which are emitted from the surface radiation interactions and thus the width of this ‘build-up region’ is equal to the maximum range of the recoil electrons in the medium. MSc-REP Regan Dosimetry 41
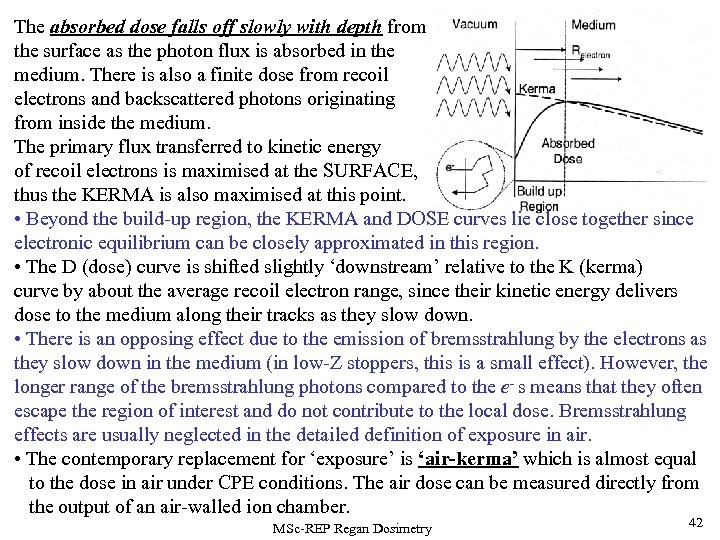 The absorbed dose falls off slowly with depth from the surface as the photon flux is absorbed in the medium. There is also a finite dose from recoil electrons and backscattered photons originating from inside the medium. The primary flux transferred to kinetic energy of recoil electrons is maximised at the SURFACE, thus the KERMA is also maximised at this point. • Beyond the build-up region, the KERMA and DOSE curves lie close together since electronic equilibrium can be closely approximated in this region. • The D (dose) curve is shifted slightly ‘downstream’ relative to the K (kerma) curve by about the average recoil electron range, since their kinetic energy delivers dose to the medium along their tracks as they slow down. • There is an opposing effect due to the emission of bremsstrahlung by the electrons as they slow down in the medium (in low-Z stoppers, this is a small effect). However, the longer range of the bremsstrahlung photons compared to the e- s means that they often escape the region of interest and do not contribute to the local dose. Bremsstrahlung effects are usually neglected in the detailed definition of exposure in air. • The contemporary replacement for ‘exposure’ is ‘air-kerma’ which is almost equal to the dose in air under CPE conditions. The air dose can be measured directly from the output of an air-walled ion chamber. MSc-REP Regan Dosimetry 42
The absorbed dose falls off slowly with depth from the surface as the photon flux is absorbed in the medium. There is also a finite dose from recoil electrons and backscattered photons originating from inside the medium. The primary flux transferred to kinetic energy of recoil electrons is maximised at the SURFACE, thus the KERMA is also maximised at this point. • Beyond the build-up region, the KERMA and DOSE curves lie close together since electronic equilibrium can be closely approximated in this region. • The D (dose) curve is shifted slightly ‘downstream’ relative to the K (kerma) curve by about the average recoil electron range, since their kinetic energy delivers dose to the medium along their tracks as they slow down. • There is an opposing effect due to the emission of bremsstrahlung by the electrons as they slow down in the medium (in low-Z stoppers, this is a small effect). However, the longer range of the bremsstrahlung photons compared to the e- s means that they often escape the region of interest and do not contribute to the local dose. Bremsstrahlung effects are usually neglected in the detailed definition of exposure in air. • The contemporary replacement for ‘exposure’ is ‘air-kerma’ which is almost equal to the dose in air under CPE conditions. The air dose can be measured directly from the output of an air-walled ion chamber. MSc-REP Regan Dosimetry 42
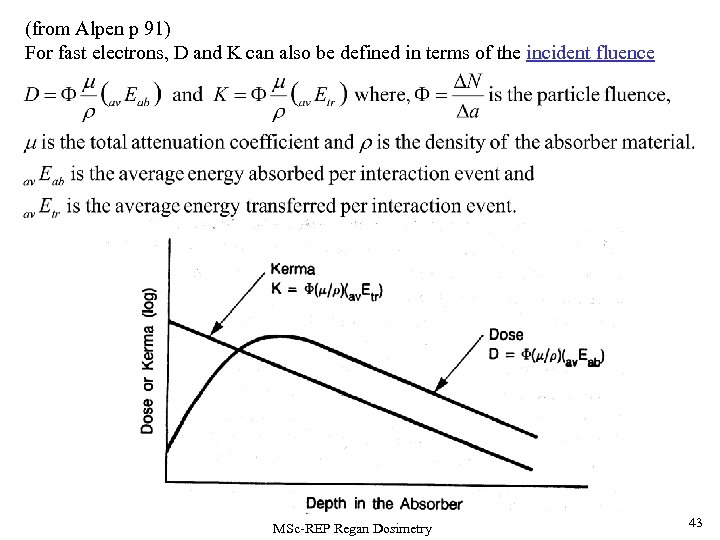 (from Alpen p 91) For fast electrons, D and K can also be defined in terms of the incident fluence MSc-REP Regan Dosimetry 43
(from Alpen p 91) For fast electrons, D and K can also be defined in terms of the incident fluence MSc-REP Regan Dosimetry 43
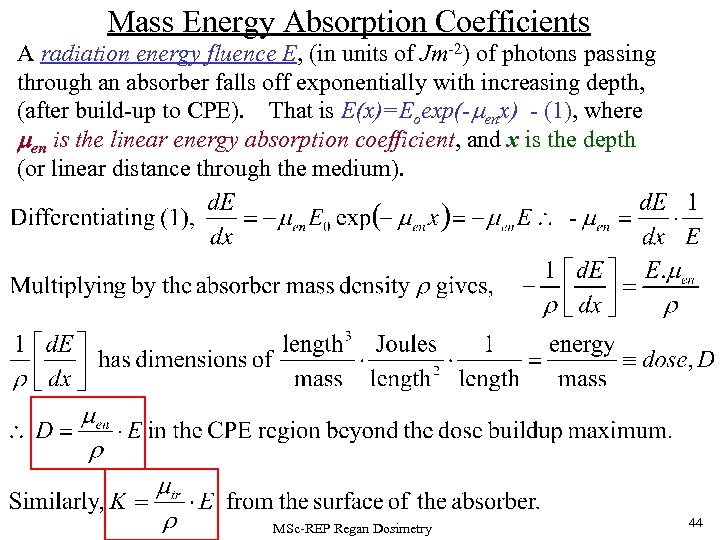 Mass Energy Absorption Coefficients A radiation energy fluence E, (in units of Jm-2) of photons passing through an absorber falls off exponentially with increasing depth, (after build-up to CPE). That is E(x)=Eoexp(- enx) - (1), where men is the linear energy absorption coefficient, and x is the depth (or linear distance through the medium). MSc-REP Regan Dosimetry 44
Mass Energy Absorption Coefficients A radiation energy fluence E, (in units of Jm-2) of photons passing through an absorber falls off exponentially with increasing depth, (after build-up to CPE). That is E(x)=Eoexp(- enx) - (1), where men is the linear energy absorption coefficient, and x is the depth (or linear distance through the medium). MSc-REP Regan Dosimetry 44
![Note men/r = mass energy absorption coefficient [units of cm 2. g-1] and mtr/r Note men/r = mass energy absorption coefficient [units of cm 2. g-1] and mtr/r](https://present5.com/presentation/204f132226422386c39efe77224accec/image-45.jpg) Note men/r = mass energy absorption coefficient [units of cm 2. g-1] and mtr/r = mass energy transfer coefficient [units of m 2. kg-1] The two coefficients can be obtained from the slopes of the dose-depth and kerma-depth curves respectively, and are both very closely related (but not identical!) to the underlying mass attenuation coefficient. For a photon fluence I, which enters a small absorber element of thickness dx in which the fluence is reduced by an amount d. I, the LINEAR ATTENUATION COEFFICIENT (m) is given by The mass attenuation coefficient is defined by (m /r ) , where r is the density of the absorber material MSc-REP Regan Dosimetry 45
Note men/r = mass energy absorption coefficient [units of cm 2. g-1] and mtr/r = mass energy transfer coefficient [units of m 2. kg-1] The two coefficients can be obtained from the slopes of the dose-depth and kerma-depth curves respectively, and are both very closely related (but not identical!) to the underlying mass attenuation coefficient. For a photon fluence I, which enters a small absorber element of thickness dx in which the fluence is reduced by an amount d. I, the LINEAR ATTENUATION COEFFICIENT (m) is given by The mass attenuation coefficient is defined by (m /r ) , where r is the density of the absorber material MSc-REP Regan Dosimetry 45
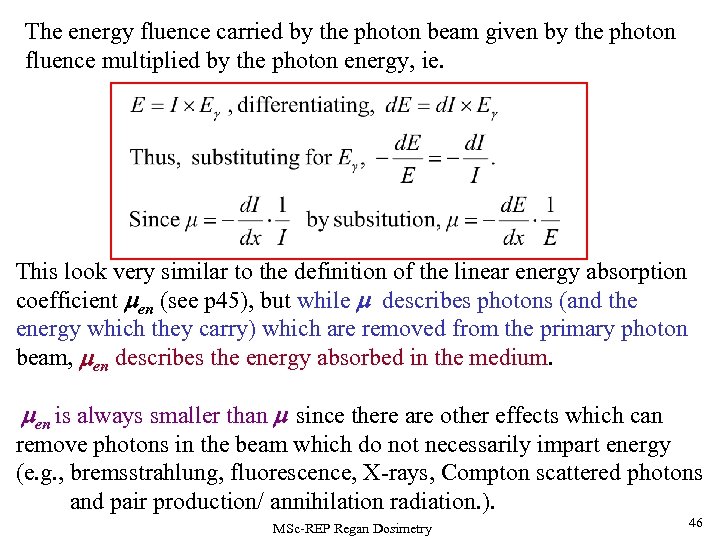 The energy fluence carried by the photon beam given by the photon fluence multiplied by the photon energy, ie. This look very similar to the definition of the linear energy absorption coefficient men (see p 45), but while m describes photons (and the energy which they carry) which are removed from the primary photon beam, men describes the energy absorbed in the medium. men is always smaller than m since there are other effects which can remove photons in the beam which do not necessarily impart energy (e. g. , bremsstrahlung, fluorescence, X-rays, Compton scattered photons and pair production/ annihilation radiation. ). MSc-REP Regan Dosimetry 46
The energy fluence carried by the photon beam given by the photon fluence multiplied by the photon energy, ie. This look very similar to the definition of the linear energy absorption coefficient men (see p 45), but while m describes photons (and the energy which they carry) which are removed from the primary photon beam, men describes the energy absorbed in the medium. men is always smaller than m since there are other effects which can remove photons in the beam which do not necessarily impart energy (e. g. , bremsstrahlung, fluorescence, X-rays, Compton scattered photons and pair production/ annihilation radiation. ). MSc-REP Regan Dosimetry 46
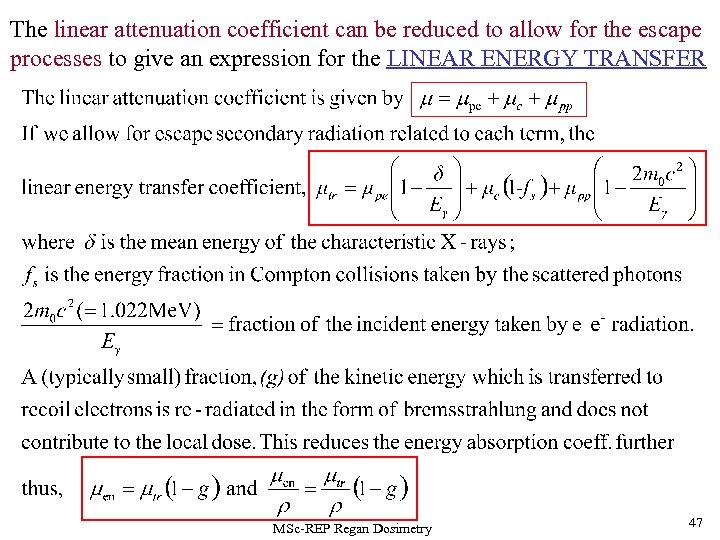 The linear attenuation coefficient can be reduced to allow for the escape processes to give an expression for the LINEAR ENERGY TRANSFER MSc-REP Regan Dosimetry 47
The linear attenuation coefficient can be reduced to allow for the escape processes to give an expression for the LINEAR ENERGY TRANSFER MSc-REP Regan Dosimetry 47
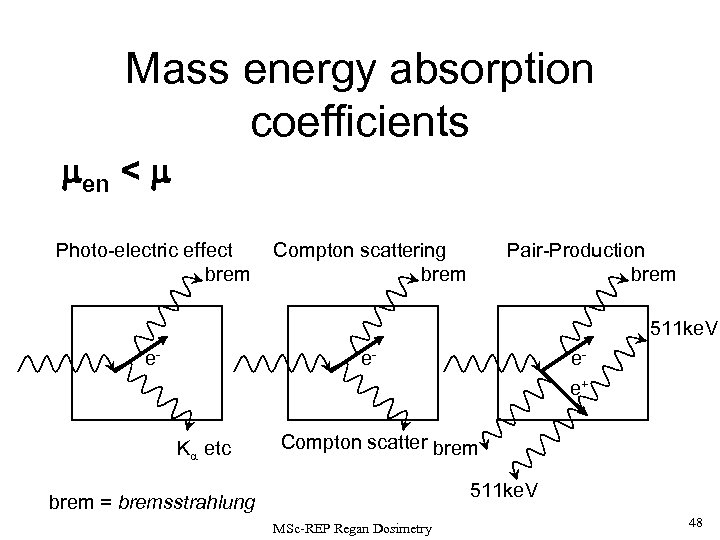 Mass energy absorption coefficients en < Photo-electric effect brem Pair-Production brem Compton scattering brem 511 ke. V e- e- K etc ee+ Compton scatter brem 511 ke. V brem = bremsstrahlung MSc-REP Regan Dosimetry 48
Mass energy absorption coefficients en < Photo-electric effect brem Pair-Production brem Compton scattering brem 511 ke. V e- e- K etc ee+ Compton scatter brem 511 ke. V brem = bremsstrahlung MSc-REP Regan Dosimetry 48
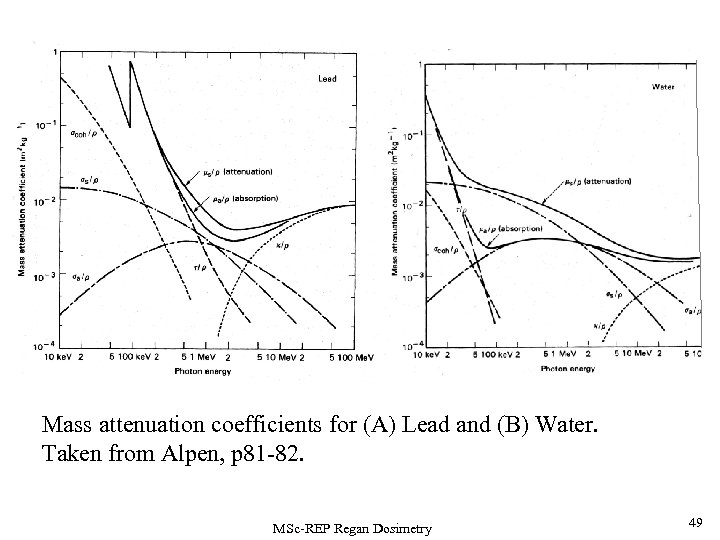 Mass attenuation coefficients for (A) Lead and (B) Water. Taken from Alpen, p 81 -82. MSc-REP Regan Dosimetry 49
Mass attenuation coefficients for (A) Lead and (B) Water. Taken from Alpen, p 81 -82. MSc-REP Regan Dosimetry 49
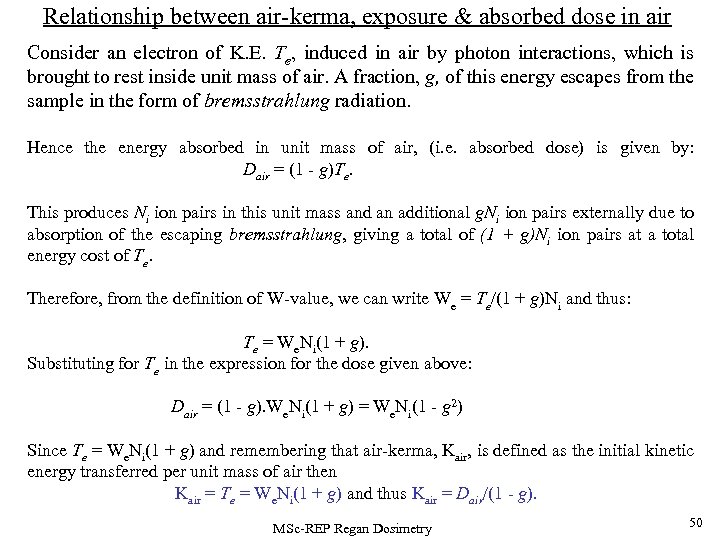 Relationship between air-kerma, exposure & absorbed dose in air Consider an electron of K. E. Te, induced in air by photon interactions, which is brought to rest inside unit mass of air. A fraction, g, of this energy escapes from the sample in the form of bremsstrahlung radiation. Hence the energy absorbed in unit mass of air, (i. e. absorbed dose) is given by: Dair = (1 - g)Te. This produces Ni ion pairs in this unit mass and an additional g. Ni ion pairs externally due to absorption of the escaping bremsstrahlung, giving a total of (1 + g)Ni ion pairs at a total energy cost of Te. Therefore, from the definition of W-value, we can write We = Te/(1 + g)Ni and thus: Te = We. Ni(1 + g). Substituting for Te in the expression for the dose given above: Dair = (1 - g). We. Ni(1 + g) = We. Ni(1 - g 2) Since Te = We. Ni(1 + g) and remembering that air-kerma, Kair, is defined as the initial kinetic energy transferred per unit mass of air then Kair = Te = We. Ni(1 + g) and thus Kair = Dair/(1 - g). MSc-REP Regan Dosimetry 50
Relationship between air-kerma, exposure & absorbed dose in air Consider an electron of K. E. Te, induced in air by photon interactions, which is brought to rest inside unit mass of air. A fraction, g, of this energy escapes from the sample in the form of bremsstrahlung radiation. Hence the energy absorbed in unit mass of air, (i. e. absorbed dose) is given by: Dair = (1 - g)Te. This produces Ni ion pairs in this unit mass and an additional g. Ni ion pairs externally due to absorption of the escaping bremsstrahlung, giving a total of (1 + g)Ni ion pairs at a total energy cost of Te. Therefore, from the definition of W-value, we can write We = Te/(1 + g)Ni and thus: Te = We. Ni(1 + g). Substituting for Te in the expression for the dose given above: Dair = (1 - g). We. Ni(1 + g) = We. Ni(1 - g 2) Since Te = We. Ni(1 + g) and remembering that air-kerma, Kair, is defined as the initial kinetic energy transferred per unit mass of air then Kair = Te = We. Ni(1 + g) and thus Kair = Dair/(1 - g). MSc-REP Regan Dosimetry 50
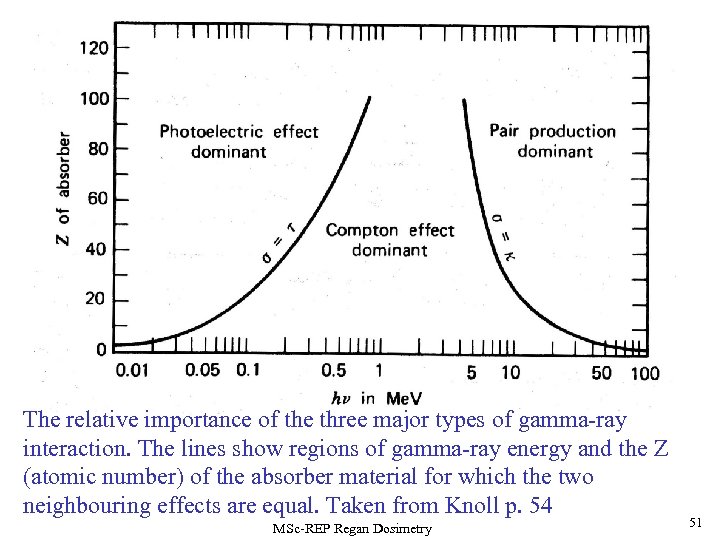 The relative importance of the three major types of gamma-ray interaction. The lines show regions of gamma-ray energy and the Z (atomic number) of the absorber material for which the two neighbouring effects are equal. Taken from Knoll p. 54 MSc-REP Regan Dosimetry 51
The relative importance of the three major types of gamma-ray interaction. The lines show regions of gamma-ray energy and the Z (atomic number) of the absorber material for which the two neighbouring effects are equal. Taken from Knoll p. 54 MSc-REP Regan Dosimetry 51
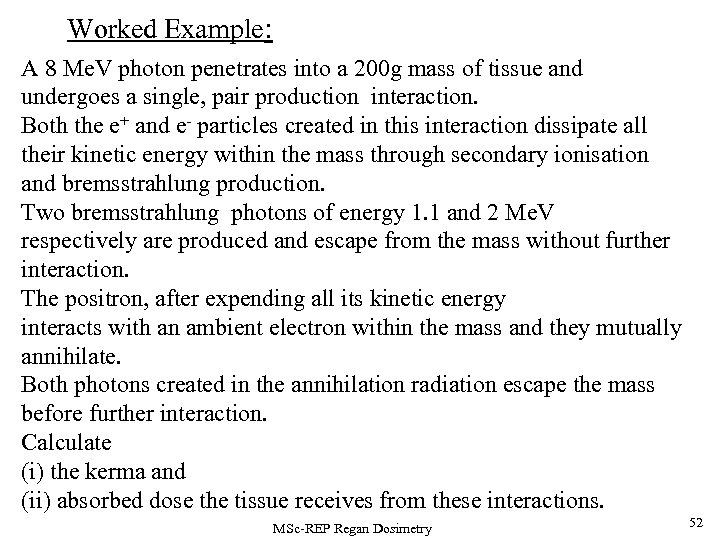 Worked Example: A 8 Me. V photon penetrates into a 200 g mass of tissue and undergoes a single, pair production interaction. Both the e+ and e- particles created in this interaction dissipate all their kinetic energy within the mass through secondary ionisation and bremsstrahlung production. Two bremsstrahlung photons of energy 1. 1 and 2 Me. V respectively are produced and escape from the mass without further interaction. The positron, after expending all its kinetic energy interacts with an ambient electron within the mass and they mutually annihilate. Both photons created in the annihilation radiation escape the mass before further interaction. Calculate (i) the kerma and (ii) absorbed dose the tissue receives from these interactions. MSc-REP Regan Dosimetry 52
Worked Example: A 8 Me. V photon penetrates into a 200 g mass of tissue and undergoes a single, pair production interaction. Both the e+ and e- particles created in this interaction dissipate all their kinetic energy within the mass through secondary ionisation and bremsstrahlung production. Two bremsstrahlung photons of energy 1. 1 and 2 Me. V respectively are produced and escape from the mass without further interaction. The positron, after expending all its kinetic energy interacts with an ambient electron within the mass and they mutually annihilate. Both photons created in the annihilation radiation escape the mass before further interaction. Calculate (i) the kerma and (ii) absorbed dose the tissue receives from these interactions. MSc-REP Regan Dosimetry 52
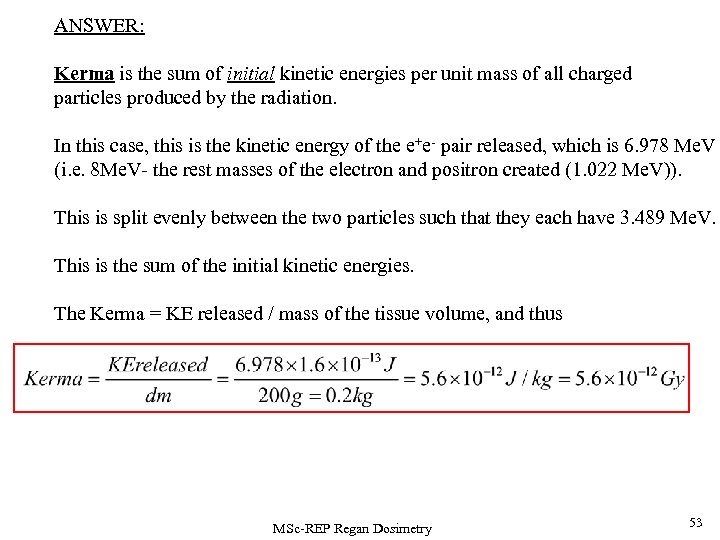 ANSWER: Kerma is the sum of initial kinetic energies per unit mass of all charged particles produced by the radiation. In this case, this is the kinetic energy of the e+e- pair released, which is 6. 978 Me. V (i. e. 8 Me. V- the rest masses of the electron and positron created (1. 022 Me. V)). This is split evenly between the two particles such that they each have 3. 489 Me. V. This is the sum of the initial kinetic energies. The Kerma = KE released / mass of the tissue volume, and thus MSc-REP Regan Dosimetry 53
ANSWER: Kerma is the sum of initial kinetic energies per unit mass of all charged particles produced by the radiation. In this case, this is the kinetic energy of the e+e- pair released, which is 6. 978 Me. V (i. e. 8 Me. V- the rest masses of the electron and positron created (1. 022 Me. V)). This is split evenly between the two particles such that they each have 3. 489 Me. V. This is the sum of the initial kinetic energies. The Kerma = KE released / mass of the tissue volume, and thus MSc-REP Regan Dosimetry 53
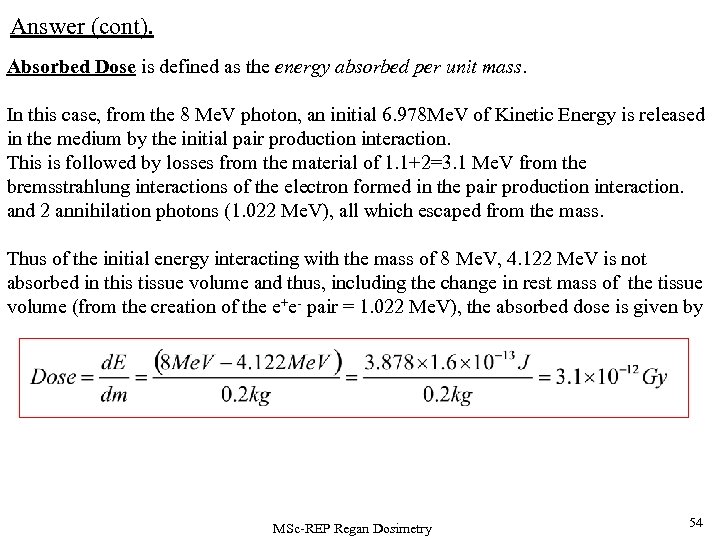 Answer (cont). Absorbed Dose is defined as the energy absorbed per unit mass. In this case, from the 8 Me. V photon, an initial 6. 978 Me. V of Kinetic Energy is released in the medium by the initial pair production interaction. This is followed by losses from the material of 1. 1+2=3. 1 Me. V from the bremsstrahlung interactions of the electron formed in the pair production interaction. and 2 annihilation photons (1. 022 Me. V), all which escaped from the mass. Thus of the initial energy interacting with the mass of 8 Me. V, 4. 122 Me. V is not absorbed in this tissue volume and thus, including the change in rest mass of the tissue volume (from the creation of the e+e- pair = 1. 022 Me. V), the absorbed dose is given by MSc-REP Regan Dosimetry 54
Answer (cont). Absorbed Dose is defined as the energy absorbed per unit mass. In this case, from the 8 Me. V photon, an initial 6. 978 Me. V of Kinetic Energy is released in the medium by the initial pair production interaction. This is followed by losses from the material of 1. 1+2=3. 1 Me. V from the bremsstrahlung interactions of the electron formed in the pair production interaction. and 2 annihilation photons (1. 022 Me. V), all which escaped from the mass. Thus of the initial energy interacting with the mass of 8 Me. V, 4. 122 Me. V is not absorbed in this tissue volume and thus, including the change in rest mass of the tissue volume (from the creation of the e+e- pair = 1. 022 Me. V), the absorbed dose is given by MSc-REP Regan Dosimetry 54
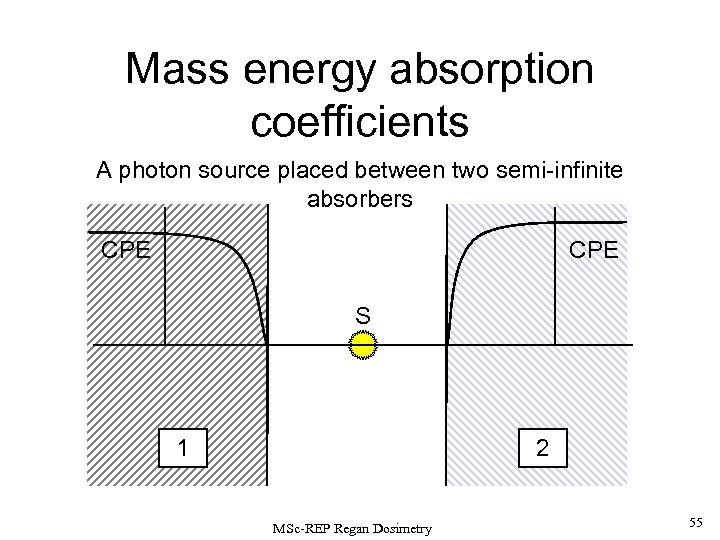 Mass energy absorption coefficients A photon source placed between two semi-infinite absorbers CPE S 1 2 MSc-REP Regan Dosimetry 55
Mass energy absorption coefficients A photon source placed between two semi-infinite absorbers CPE S 1 2 MSc-REP Regan Dosimetry 55
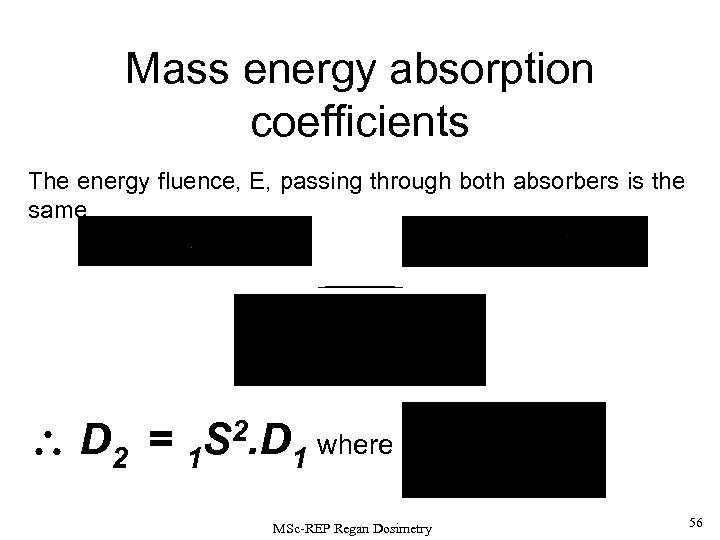 Mass energy absorption coefficients The energy fluence, E, passing through both absorbers is the same D 2 = 1 S 2. D 1 where MSc-REP Regan Dosimetry 56
Mass energy absorption coefficients The energy fluence, E, passing through both absorbers is the same D 2 = 1 S 2. D 1 where MSc-REP Regan Dosimetry 56
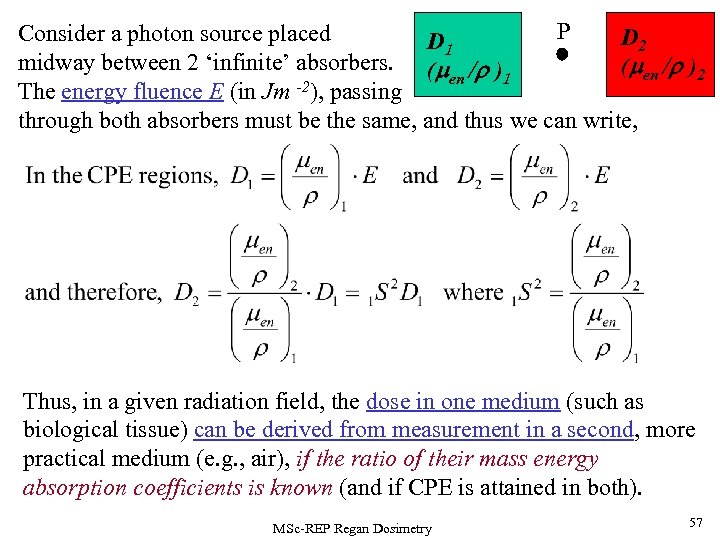 P Consider a photon source placed D 2 D 1 midway between 2 ‘infinite’ absorbers. (m /r ) (men /r )2 en 1 The energy fluence E (in Jm -2), passing through both absorbers must be the same, and thus we can write, Thus, in a given radiation field, the dose in one medium (such as biological tissue) can be derived from measurement in a second, more practical medium (e. g. , air), if the ratio of their mass energy absorption coefficients is known (and if CPE is attained in both). MSc-REP Regan Dosimetry 57
P Consider a photon source placed D 2 D 1 midway between 2 ‘infinite’ absorbers. (m /r ) (men /r )2 en 1 The energy fluence E (in Jm -2), passing through both absorbers must be the same, and thus we can write, Thus, in a given radiation field, the dose in one medium (such as biological tissue) can be derived from measurement in a second, more practical medium (e. g. , air), if the ratio of their mass energy absorption coefficients is known (and if CPE is attained in both). MSc-REP Regan Dosimetry 57
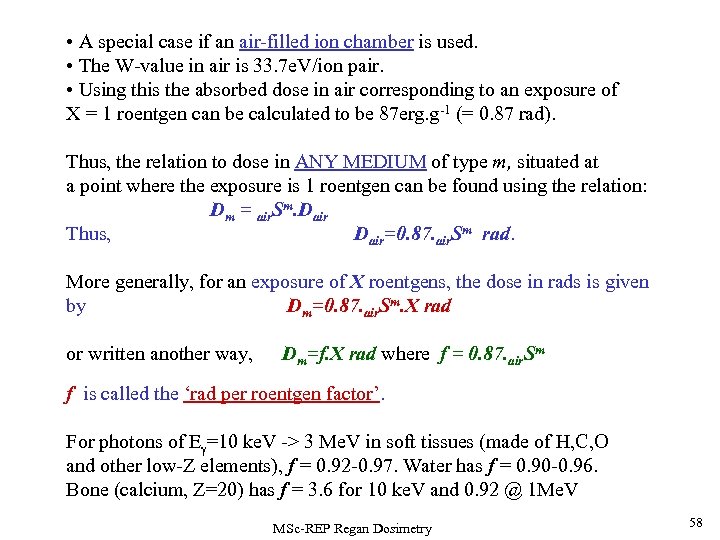 • A special case if an air-filled ion chamber is used. • The W-value in air is 33. 7 e. V/ion pair. • Using this the absorbed dose in air corresponding to an exposure of X = 1 roentgen can be calculated to be 87 erg. g-1 (= 0. 87 rad). Thus, the relation to dose in ANY MEDIUM of type m, situated at a point where the exposure is 1 roentgen can be found using the relation: Dm = air. Sm. Dair Thus, Dair=0. 87. air. Sm rad. More generally, for an exposure of X roentgens, the dose in rads is given by Dm=0. 87. air. Sm. X rad or written another way, Dm=f. X rad where f = 0. 87. air. Sm f is called the ‘rad per roentgen factor’. For photons of E =10 ke. V -> 3 Me. V in soft tissues (made of H, C, O and other low-Z elements), f = 0. 92 -0. 97. Water has f = 0. 90 -0. 96. Bone (calcium, Z=20) has f = 3. 6 for 10 ke. V and 0. 92 @ 1 Me. V MSc-REP Regan Dosimetry 58
• A special case if an air-filled ion chamber is used. • The W-value in air is 33. 7 e. V/ion pair. • Using this the absorbed dose in air corresponding to an exposure of X = 1 roentgen can be calculated to be 87 erg. g-1 (= 0. 87 rad). Thus, the relation to dose in ANY MEDIUM of type m, situated at a point where the exposure is 1 roentgen can be found using the relation: Dm = air. Sm. Dair Thus, Dair=0. 87. air. Sm rad. More generally, for an exposure of X roentgens, the dose in rads is given by Dm=0. 87. air. Sm. X rad or written another way, Dm=f. X rad where f = 0. 87. air. Sm f is called the ‘rad per roentgen factor’. For photons of E =10 ke. V -> 3 Me. V in soft tissues (made of H, C, O and other low-Z elements), f = 0. 92 -0. 97. Water has f = 0. 90 -0. 96. Bone (calcium, Z=20) has f = 3. 6 for 10 ke. V and 0. 92 @ 1 Me. V MSc-REP Regan Dosimetry 58
 Effective Atomic Numbers and’ Matching’ The dose in higher Z materials is much larger than in air (and other low-Z materials) for lower X-ray energies due to the strong Z-dependence of the photoelectric effect cross-section (spe~Zn/E 4 -5) The photon attenuation coefficients, and the related energy absorption coefficients are complex functions of E and atomic no. (Z), but over a RESTRICTED RANGE of these two parameters, it is possible to represent the dosimetric behaviour of a mixture of elements (such as in biological tissue) with the use of a single parameter, called the EFFECTIVE ATOMIC NUMBER, Zeff. For soft tissues over the range of diagnostic X-rays ( E=10 -250 ke. V, Zeff=7. 8). This concept can be useful for estimating the degree of equivalence (or ‘matching’) between real biological tissue and possible dosimetric media. Although PERFECT MATCHING (i. e. , 1 S 2=1. 0) is only true for identical atomic compositions, approximate matching is often good enough for radiation protection purposes. For example, tissue-equivalent gas mixtures can be used inside tissue-equivalent and used inside natural body apertures to accurate dose measurements in radiotherapy. MSc-REP Regan Dosimetry 59
Effective Atomic Numbers and’ Matching’ The dose in higher Z materials is much larger than in air (and other low-Z materials) for lower X-ray energies due to the strong Z-dependence of the photoelectric effect cross-section (spe~Zn/E 4 -5) The photon attenuation coefficients, and the related energy absorption coefficients are complex functions of E and atomic no. (Z), but over a RESTRICTED RANGE of these two parameters, it is possible to represent the dosimetric behaviour of a mixture of elements (such as in biological tissue) with the use of a single parameter, called the EFFECTIVE ATOMIC NUMBER, Zeff. For soft tissues over the range of diagnostic X-rays ( E=10 -250 ke. V, Zeff=7. 8). This concept can be useful for estimating the degree of equivalence (or ‘matching’) between real biological tissue and possible dosimetric media. Although PERFECT MATCHING (i. e. , 1 S 2=1. 0) is only true for identical atomic compositions, approximate matching is often good enough for radiation protection purposes. For example, tissue-equivalent gas mixtures can be used inside tissue-equivalent and used inside natural body apertures to accurate dose measurements in radiotherapy. MSc-REP Regan Dosimetry 59
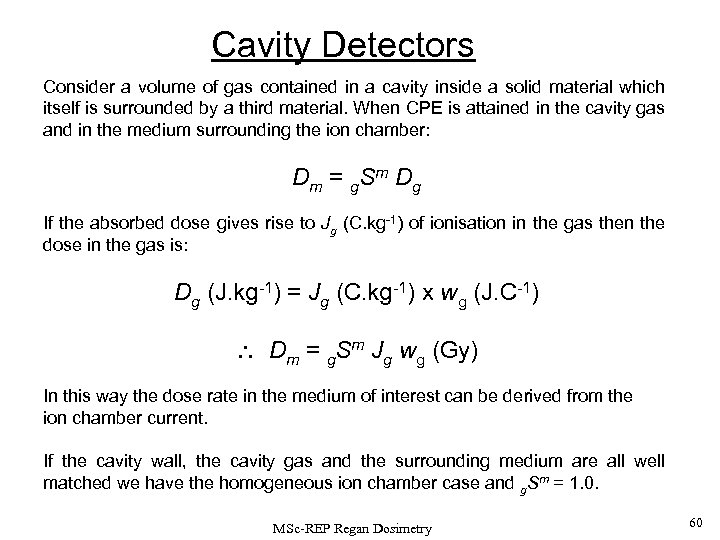 Cavity Detectors Consider a volume of gas contained in a cavity inside a solid material which itself is surrounded by a third material. When CPE is attained in the cavity gas and in the medium surrounding the ion chamber: Dm = g. Sm Dg If the absorbed dose gives rise to Jg (C. kg-1) of ionisation in the gas then the dose in the gas is: Dg (J. kg-1) = Jg (C. kg-1) x wg (J. C-1) Dm = g. Sm Jg wg (Gy) In this way the dose rate in the medium of interest can be derived from the ion chamber current. If the cavity wall, the cavity gas and the surrounding medium are all well matched we have the homogeneous ion chamber case and g. Sm = 1. 0. MSc-REP Regan Dosimetry 60
Cavity Detectors Consider a volume of gas contained in a cavity inside a solid material which itself is surrounded by a third material. When CPE is attained in the cavity gas and in the medium surrounding the ion chamber: Dm = g. Sm Dg If the absorbed dose gives rise to Jg (C. kg-1) of ionisation in the gas then the dose in the gas is: Dg (J. kg-1) = Jg (C. kg-1) x wg (J. C-1) Dm = g. Sm Jg wg (Gy) In this way the dose rate in the medium of interest can be derived from the ion chamber current. If the cavity wall, the cavity gas and the surrounding medium are all well matched we have the homogeneous ion chamber case and g. Sm = 1. 0. MSc-REP Regan Dosimetry 60
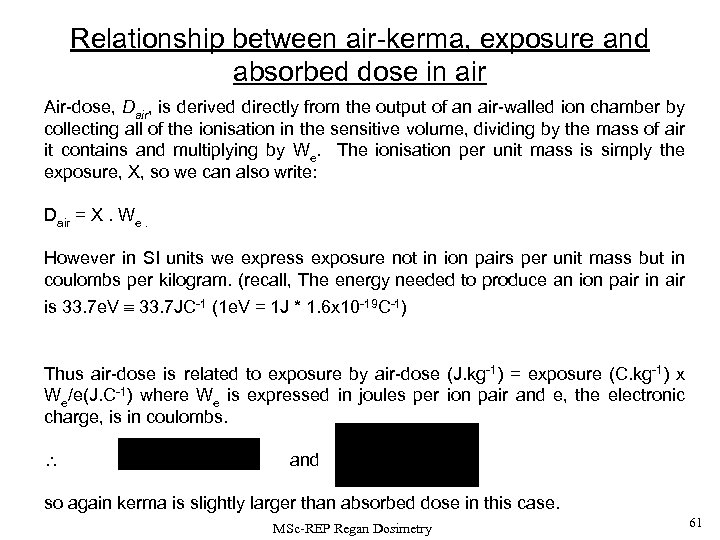 Relationship between air-kerma, exposure and absorbed dose in air Air-dose, Dair, is derived directly from the output of an air-walled ion chamber by collecting all of the ionisation in the sensitive volume, dividing by the mass of air it contains and multiplying by We. The ionisation per unit mass is simply the exposure, X, so we can also write: Dair = X. We. However in SI units we express exposure not in ion pairs per unit mass but in coulombs per kilogram. (recall, The energy needed to produce an ion pair in air is 33. 7 e. V 33. 7 JC-1 (1 e. V = 1 J * 1. 6 x 10 -19 C-1) Thus air-dose is related to exposure by air-dose (J. kg-1) = exposure (C. kg-1) x We/e(J. C-1) where We is expressed in joules per ion pair and e, the electronic charge, is in coulombs. and so again kerma is slightly larger than absorbed dose in this case. MSc-REP Regan Dosimetry 61
Relationship between air-kerma, exposure and absorbed dose in air Air-dose, Dair, is derived directly from the output of an air-walled ion chamber by collecting all of the ionisation in the sensitive volume, dividing by the mass of air it contains and multiplying by We. The ionisation per unit mass is simply the exposure, X, so we can also write: Dair = X. We. However in SI units we express exposure not in ion pairs per unit mass but in coulombs per kilogram. (recall, The energy needed to produce an ion pair in air is 33. 7 e. V 33. 7 JC-1 (1 e. V = 1 J * 1. 6 x 10 -19 C-1) Thus air-dose is related to exposure by air-dose (J. kg-1) = exposure (C. kg-1) x We/e(J. C-1) where We is expressed in joules per ion pair and e, the electronic charge, is in coulombs. and so again kerma is slightly larger than absorbed dose in this case. MSc-REP Regan Dosimetry 61
 The Bragg-Gray principle says that the amount of ionization produced in a small gas-filled volume cavity surrounded by a larger, solid absorbing medium is directly proportional to the radiation energy absorbed by the solid. The gas cavity must be small enough relative to the mass of the solid absorber to leave the angular and velocity distributions of the primary electrons unchanged. The cavity must be surrounded of a solid with sufficient thickness to achieve electronic equilibrium. MSc-REP Regan Dosimetry 62
The Bragg-Gray principle says that the amount of ionization produced in a small gas-filled volume cavity surrounded by a larger, solid absorbing medium is directly proportional to the radiation energy absorbed by the solid. The gas cavity must be small enough relative to the mass of the solid absorber to leave the angular and velocity distributions of the primary electrons unchanged. The cavity must be surrounded of a solid with sufficient thickness to achieve electronic equilibrium. MSc-REP Regan Dosimetry 62
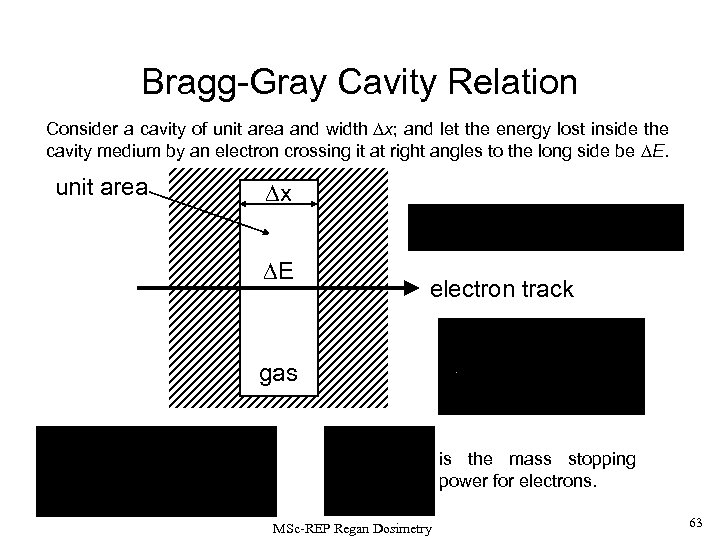 Bragg-Gray Cavity Relation Consider a cavity of unit area and width x; and let the energy lost inside the cavity medium by an electron crossing it at right angles to the long side be E. unit area x E electron track gas is the mass stopping power for electrons. MSc-REP Regan Dosimetry 63
Bragg-Gray Cavity Relation Consider a cavity of unit area and width x; and let the energy lost inside the cavity medium by an electron crossing it at right angles to the long side be E. unit area x E electron track gas is the mass stopping power for electrons. MSc-REP Regan Dosimetry 63
 Bragg-Gray Cavity Relation Dg mass stopping power, and for the same electron fluence in the surrounding medium we have. By taking the ratio of these two expressions we find that, where gsm is the ratio of mass stopping powers of the medium and the cavity gas respectively. By measuring the ionisation per unit mass in the cavity, Jg: MSc-REP Regan Dosimetry 64
Bragg-Gray Cavity Relation Dg mass stopping power, and for the same electron fluence in the surrounding medium we have. By taking the ratio of these two expressions we find that, where gsm is the ratio of mass stopping powers of the medium and the cavity gas respectively. By measuring the ionisation per unit mass in the cavity, Jg: MSc-REP Regan Dosimetry 64
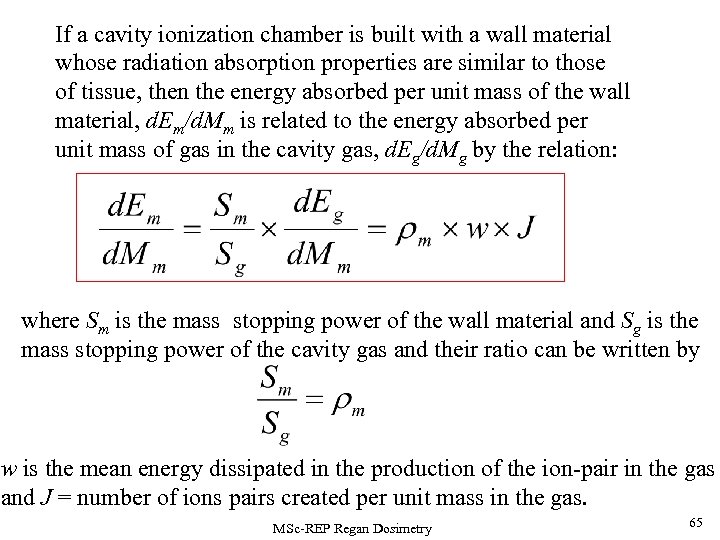 If a cavity ionization chamber is built with a wall material whose radiation absorption properties are similar to those of tissue, then the energy absorbed per unit mass of the wall material, d. Em/d. Mm is related to the energy absorbed per unit mass of gas in the cavity gas, d. Eg/d. Mg by the relation: where Sm is the mass stopping power of the wall material and Sg is the mass stopping power of the cavity gas and their ratio can be written by w is the mean energy dissipated in the production of the ion-pair in the gas and J = number of ions pairs created per unit mass in the gas. MSc-REP Regan Dosimetry 65
If a cavity ionization chamber is built with a wall material whose radiation absorption properties are similar to those of tissue, then the energy absorbed per unit mass of the wall material, d. Em/d. Mm is related to the energy absorbed per unit mass of gas in the cavity gas, d. Eg/d. Mg by the relation: where Sm is the mass stopping power of the wall material and Sg is the mass stopping power of the cavity gas and their ratio can be written by w is the mean energy dissipated in the production of the ion-pair in the gas and J = number of ions pairs created per unit mass in the gas. MSc-REP Regan Dosimetry 65
 Example Question: Calculate the absorbed dose measured in a tissue-equivalent chamber with a 1 cm 3 air filled cavity within the chamber which is exposed to a source of 60 Co gamma rays for 10 minutes. A total of 2. 25 x 109 electrons are collected from the chamber as a result of ionization events in this period and the average mass stopping power ratio of tissue to air is given by: for 60 Co gamma rays USEFUL INFORMATION: Average energy required to form an e--ion pair in air at STP = 34 e. V. 1 e. V = 1. 6 x 10 -19 J ; the density of air at STP, ρair = 1. 293 x 10 -6 kg/cm 3 SOLUTION: MSc-REP Regan Dosimetry 66
Example Question: Calculate the absorbed dose measured in a tissue-equivalent chamber with a 1 cm 3 air filled cavity within the chamber which is exposed to a source of 60 Co gamma rays for 10 minutes. A total of 2. 25 x 109 electrons are collected from the chamber as a result of ionization events in this period and the average mass stopping power ratio of tissue to air is given by: for 60 Co gamma rays USEFUL INFORMATION: Average energy required to form an e--ion pair in air at STP = 34 e. V. 1 e. V = 1. 6 x 10 -19 J ; the density of air at STP, ρair = 1. 293 x 10 -6 kg/cm 3 SOLUTION: MSc-REP Regan Dosimetry 66
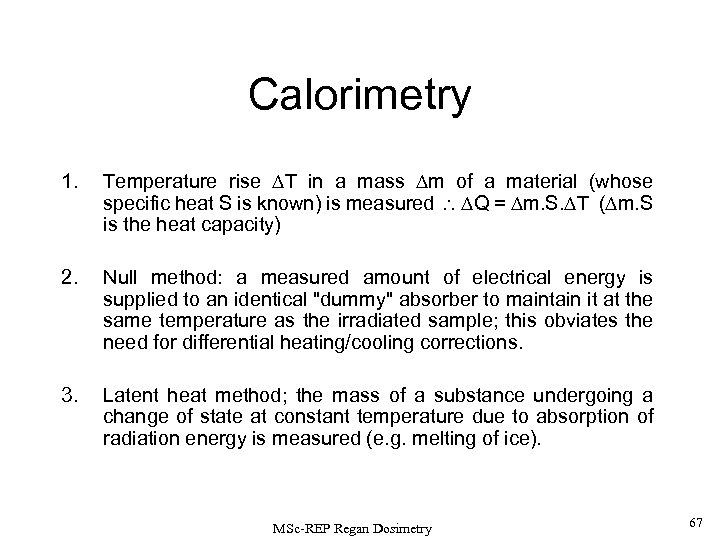 Calorimetry 1. Temperature rise T in a mass m of a material (whose specific heat S is known) is measured Q = m. S. T ( m. S is the heat capacity) 2. Null method: a measured amount of electrical energy is supplied to an identical "dummy" absorber to maintain it at the same temperature as the irradiated sample; this obviates the need for differential heating/cooling corrections. 3. Latent heat method; the mass of a substance undergoing a change of state at constant temperature due to absorption of radiation energy is measured (e. g. melting of ice). MSc-REP Regan Dosimetry 67
Calorimetry 1. Temperature rise T in a mass m of a material (whose specific heat S is known) is measured Q = m. S. T ( m. S is the heat capacity) 2. Null method: a measured amount of electrical energy is supplied to an identical "dummy" absorber to maintain it at the same temperature as the irradiated sample; this obviates the need for differential heating/cooling corrections. 3. Latent heat method; the mass of a substance undergoing a change of state at constant temperature due to absorption of radiation energy is measured (e. g. melting of ice). MSc-REP Regan Dosimetry 67
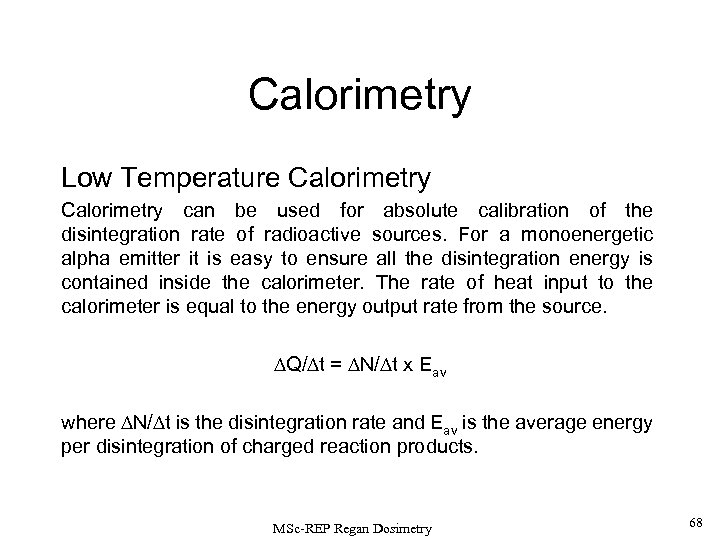 Calorimetry Low Temperature Calorimetry can be used for absolute calibration of the disintegration rate of radioactive sources. For a monoenergetic alpha emitter it is easy to ensure all the disintegration energy is contained inside the calorimeter. The rate of heat input to the calorimeter is equal to the energy output rate from the source. Q/ t = N/ t x Eav where N/ t is the disintegration rate and Eav is the average energy per disintegration of charged reaction products. MSc-REP Regan Dosimetry 68
Calorimetry Low Temperature Calorimetry can be used for absolute calibration of the disintegration rate of radioactive sources. For a monoenergetic alpha emitter it is easy to ensure all the disintegration energy is contained inside the calorimeter. The rate of heat input to the calorimeter is equal to the energy output rate from the source. Q/ t = N/ t x Eav where N/ t is the disintegration rate and Eav is the average energy per disintegration of charged reaction products. MSc-REP Regan Dosimetry 68
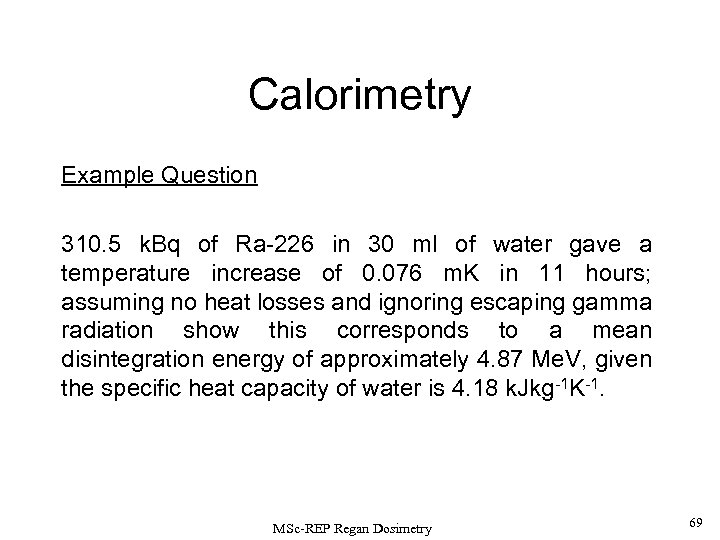 Calorimetry Example Question 310. 5 k. Bq of Ra-226 in 30 ml of water gave a temperature increase of 0. 076 m. K in 11 hours; assuming no heat losses and ignoring escaping gamma radiation show this corresponds to a mean disintegration energy of approximately 4. 87 Me. V, given the specific heat capacity of water is 4. 18 k. Jkg-1 K-1. MSc-REP Regan Dosimetry 69
Calorimetry Example Question 310. 5 k. Bq of Ra-226 in 30 ml of water gave a temperature increase of 0. 076 m. K in 11 hours; assuming no heat losses and ignoring escaping gamma radiation show this corresponds to a mean disintegration energy of approximately 4. 87 Me. V, given the specific heat capacity of water is 4. 18 k. Jkg-1 K-1. MSc-REP Regan Dosimetry 69
 Example Answer 310. 5 k. Bq of Ra-226 emits an alpha 100% of N times in 11 hours: N = 310. 5 x 103 x 11 x 60 = 1. 23 x 1010 The total energy released is: Q = m S T = 0. 03 kg x 4. 18 x 103 Jkg-1 K-1 x 7. 6 x 10 -5 K = 0. 00958 J The energy of each emission is therefore: E = Q / N = 0. 00958 / 1. 23 x 1010 = 7. 79 x 10 -13 J / 1. 6 x 10 -19 e. VJ-1 = 4. 87 Me. V MSc-REP Regan Dosimetry 70
Example Answer 310. 5 k. Bq of Ra-226 emits an alpha 100% of N times in 11 hours: N = 310. 5 x 103 x 11 x 60 = 1. 23 x 1010 The total energy released is: Q = m S T = 0. 03 kg x 4. 18 x 103 Jkg-1 K-1 x 7. 6 x 10 -5 K = 0. 00958 J The energy of each emission is therefore: E = Q / N = 0. 00958 / 1. 23 x 1010 = 7. 79 x 10 -13 J / 1. 6 x 10 -19 e. VJ-1 = 4. 87 Me. V MSc-REP Regan Dosimetry 70
 Calorimetry Thermometry Semiconductors (thermistors) have temperature coefficients of resistance of ~ -2 to -4 % per K. Platinum resistance thermometers are more stable and reproducible but are less sensitive (~ +0. 4 % per degree). Using thermistors, in order to measure T 10 -3 K to ~ 1 % we need to measure resistance changes of ~ 100 parts per billion using a sensitive Wheatstone bridge. Alternatively thermocouples such as copper/constantan with V = 50 V per K can be employed and the output voltage measured with a potentiometer; several thermocouples in series generate a larger potential difference. MSc-REP Regan Dosimetry 71
Calorimetry Thermometry Semiconductors (thermistors) have temperature coefficients of resistance of ~ -2 to -4 % per K. Platinum resistance thermometers are more stable and reproducible but are less sensitive (~ +0. 4 % per degree). Using thermistors, in order to measure T 10 -3 K to ~ 1 % we need to measure resistance changes of ~ 100 parts per billion using a sensitive Wheatstone bridge. Alternatively thermocouples such as copper/constantan with V = 50 V per K can be employed and the output voltage measured with a potentiometer; several thermocouples in series generate a larger potential difference. MSc-REP Regan Dosimetry 71
 Calorimetry Low Temperature Calorimetry (1) Heat transfer by radiation ( T 4) is reduced so that thermal isolation of the calorimeter from its surroundings is easier to attain. (2) Specific heat capacity decreases to give a much larger temperature change for a given radiation dose. (3) Thermistor sensitivity increases to ~ 10 % per K. In combination these improvements in sensitivity enable dose rates as low as ~30 m. Gy per minute to be measured to an accuracy of 25 % in a one hour run. MSc-REP Regan Dosimetry 72
Calorimetry Low Temperature Calorimetry (1) Heat transfer by radiation ( T 4) is reduced so that thermal isolation of the calorimeter from its surroundings is easier to attain. (2) Specific heat capacity decreases to give a much larger temperature change for a given radiation dose. (3) Thermistor sensitivity increases to ~ 10 % per K. In combination these improvements in sensitivity enable dose rates as low as ~30 m. Gy per minute to be measured to an accuracy of 25 % in a one hour run. MSc-REP Regan Dosimetry 72
 Solid State Integrating Detectors In alkali halides, such as Li. F, Na. I, etc (which form simple cubic lattices) the outer shell electron in the alkali is donated to the halide to produce an inert gas like in both atoms, and produces an insulator. In the lattice F-centres and H-centres are regions of positive and negative charge which can bind to wandering electrons and holes respectively. Impurity (activator) atoms can be added to the lattice in interstitial positions to create trapping centres. Ionising radiation can damage the lattice if they are very energetic (recoil energy of ~10 -20 e. V is needed) or may give rise to free charges which can become semi-permanently trapped. The number of trapped charges represents some measure of the absorbed dose, this phenomenon is used in (a) Radio. Photo. Luminescence and (b) Thermo. Luminescence. MSc-REP Regan Dosimetry 73
Solid State Integrating Detectors In alkali halides, such as Li. F, Na. I, etc (which form simple cubic lattices) the outer shell electron in the alkali is donated to the halide to produce an inert gas like in both atoms, and produces an insulator. In the lattice F-centres and H-centres are regions of positive and negative charge which can bind to wandering electrons and holes respectively. Impurity (activator) atoms can be added to the lattice in interstitial positions to create trapping centres. Ionising radiation can damage the lattice if they are very energetic (recoil energy of ~10 -20 e. V is needed) or may give rise to free charges which can become semi-permanently trapped. The number of trapped charges represents some measure of the absorbed dose, this phenomenon is used in (a) Radio. Photo. Luminescence and (b) Thermo. Luminescence. MSc-REP Regan Dosimetry 73
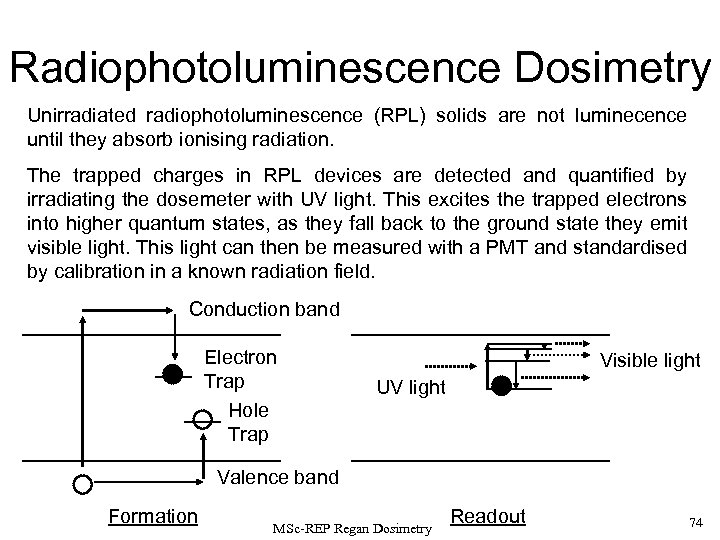 Radiophotoluminescence Dosimetry Unirradiated radiophotoluminescence (RPL) solids are not luminecence until they absorb ionising radiation. The trapped charges in RPL devices are detected and quantified by irradiating the dosemeter with UV light. This excites the trapped electrons into higher quantum states, as they fall back to the ground state they emit visible light. This light can then be measured with a PMT and standardised by calibration in a known radiation field. Conduction band Electron Trap Hole Trap Visible light UV light Valence band Formation MSc-REP Regan Dosimetry Readout 74
Radiophotoluminescence Dosimetry Unirradiated radiophotoluminescence (RPL) solids are not luminecence until they absorb ionising radiation. The trapped charges in RPL devices are detected and quantified by irradiating the dosemeter with UV light. This excites the trapped electrons into higher quantum states, as they fall back to the ground state they emit visible light. This light can then be measured with a PMT and standardised by calibration in a known radiation field. Conduction band Electron Trap Hole Trap Visible light UV light Valence band Formation MSc-REP Regan Dosimetry Readout 74
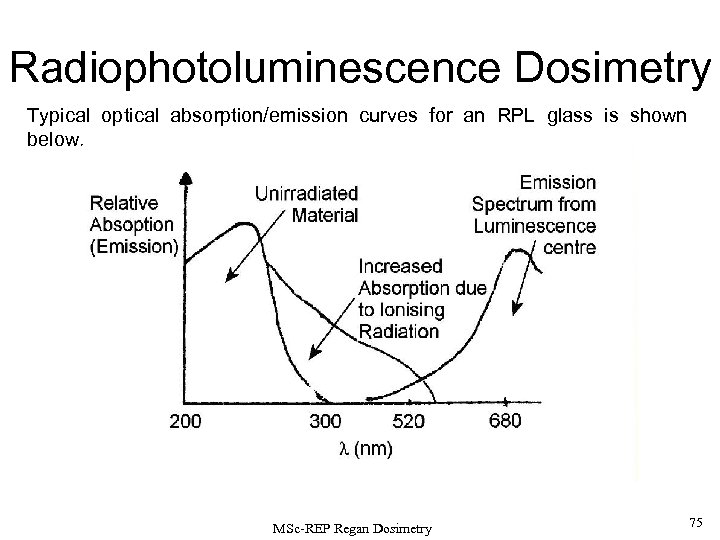 Radiophotoluminescence Dosimetry Typical optical absorption/emission curves for an RPL glass is shown below. MSc-REP Regan Dosimetry 75
Radiophotoluminescence Dosimetry Typical optical absorption/emission curves for an RPL glass is shown below. MSc-REP Regan Dosimetry 75
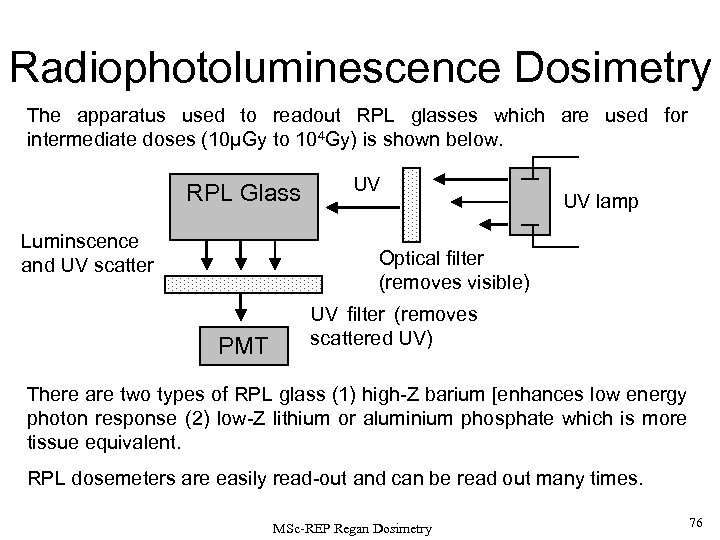 Radiophotoluminescence Dosimetry The apparatus used to readout RPL glasses which are used for intermediate doses (10μGy to 104 Gy) is shown below. RPL Glass Luminscence and UV scatter UV UV lamp Optical filter (removes visible) PMT UV filter (removes scattered UV) There are two types of RPL glass (1) high-Z barium [enhances low energy photon response (2) low-Z lithium or aluminium phosphate which is more tissue equivalent. RPL dosemeters are easily read-out and can be read out many times. MSc-REP Regan Dosimetry 76
Radiophotoluminescence Dosimetry The apparatus used to readout RPL glasses which are used for intermediate doses (10μGy to 104 Gy) is shown below. RPL Glass Luminscence and UV scatter UV UV lamp Optical filter (removes visible) PMT UV filter (removes scattered UV) There are two types of RPL glass (1) high-Z barium [enhances low energy photon response (2) low-Z lithium or aluminium phosphate which is more tissue equivalent. RPL dosemeters are easily read-out and can be read out many times. MSc-REP Regan Dosimetry 76
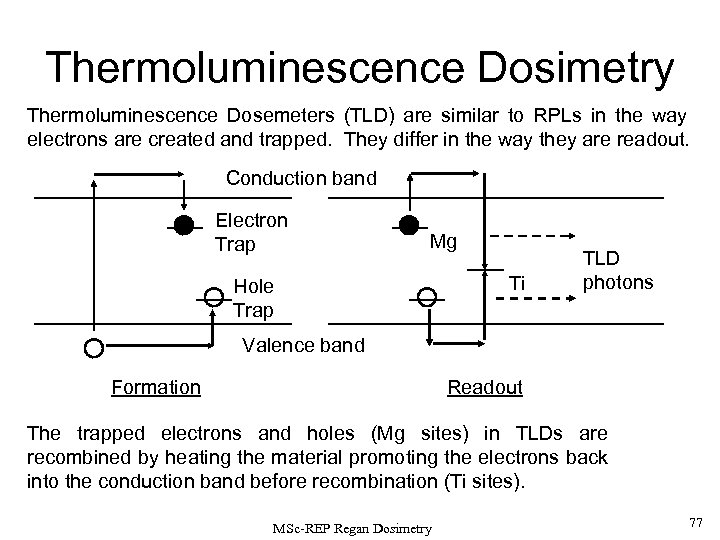 Thermoluminescence Dosimetry Thermoluminescence Dosemeters (TLD) are similar to RPLs in the way electrons are created and trapped. They differ in the way they are readout. Conduction band Electron Trap Mg Hole Trap Ti TLD photons Valence band Formation Readout The trapped electrons and holes (Mg sites) in TLDs are recombined by heating the material promoting the electrons back into the conduction band before recombination (Ti sites). MSc-REP Regan Dosimetry 77
Thermoluminescence Dosimetry Thermoluminescence Dosemeters (TLD) are similar to RPLs in the way electrons are created and trapped. They differ in the way they are readout. Conduction band Electron Trap Mg Hole Trap Ti TLD photons Valence band Formation Readout The trapped electrons and holes (Mg sites) in TLDs are recombined by heating the material promoting the electrons back into the conduction band before recombination (Ti sites). MSc-REP Regan Dosimetry 77
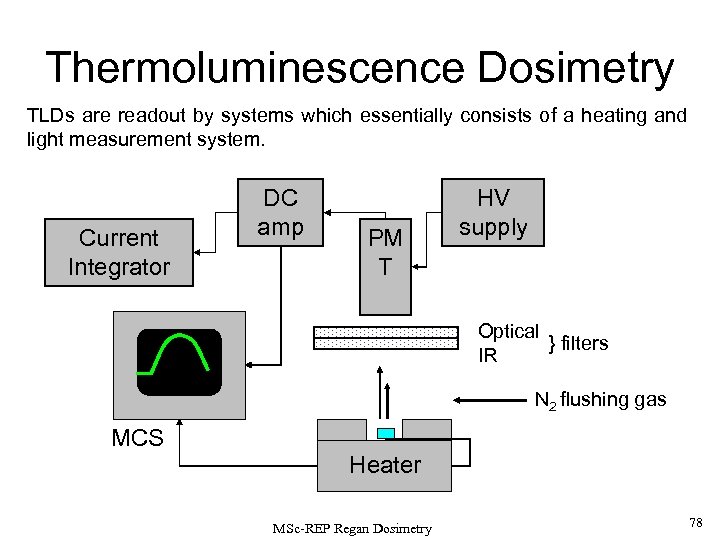 Thermoluminescence Dosimetry TLDs are readout by systems which essentially consists of a heating and light measurement system. Current Integrator DC amp PM T HV supply Optical } filters IR N 2 flushing gas MCS Heater MSc-REP Regan Dosimetry 78
Thermoluminescence Dosimetry TLDs are readout by systems which essentially consists of a heating and light measurement system. Current Integrator DC amp PM T HV supply Optical } filters IR N 2 flushing gas MCS Heater MSc-REP Regan Dosimetry 78
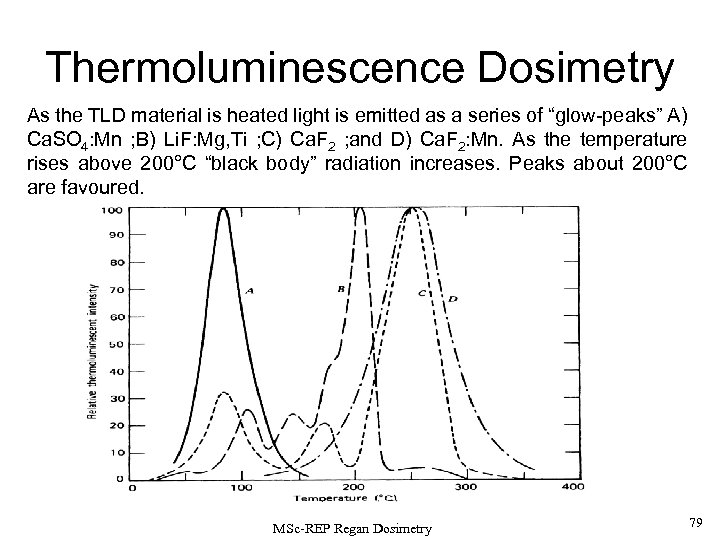 Thermoluminescence Dosimetry As the TLD material is heated light is emitted as a series of “glow-peaks” A) Ca. SO 4: Mn ; B) Li. F: Mg, Ti ; C) Ca. F 2 ; and D) Ca. F 2: Mn. As the temperature rises above 200°C “black body” radiation increases. Peaks about 200°C are favoured. MSc-REP Regan Dosimetry 79
Thermoluminescence Dosimetry As the TLD material is heated light is emitted as a series of “glow-peaks” A) Ca. SO 4: Mn ; B) Li. F: Mg, Ti ; C) Ca. F 2 ; and D) Ca. F 2: Mn. As the temperature rises above 200°C “black body” radiation increases. Peaks about 200°C are favoured. MSc-REP Regan Dosimetry 79
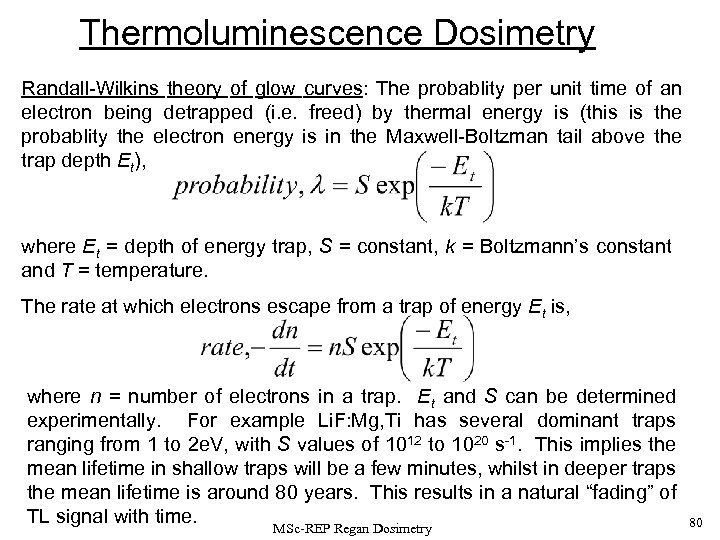 Thermoluminescence Dosimetry Randall-Wilkins theory of glow curves: The probablity per unit time of an electron being detrapped (i. e. freed) by thermal energy is (this is the probablity the electron energy is in the Maxwell-Boltzman tail above the trap depth Et), where Et = depth of energy trap, S = constant, k = Boltzmann’s constant and T = temperature. The rate at which electrons escape from a trap of energy Et is, where n = number of electrons in a trap. Et and S can be determined experimentally. For example Li. F: Mg, Ti has several dominant traps ranging from 1 to 2 e. V, with S values of 1012 to 1020 s-1. This implies the mean lifetime in shallow traps will be a few minutes, whilst in deeper traps the mean lifetime is around 80 years. This results in a natural “fading” of TL signal with time. MSc-REP Regan Dosimetry 80
Thermoluminescence Dosimetry Randall-Wilkins theory of glow curves: The probablity per unit time of an electron being detrapped (i. e. freed) by thermal energy is (this is the probablity the electron energy is in the Maxwell-Boltzman tail above the trap depth Et), where Et = depth of energy trap, S = constant, k = Boltzmann’s constant and T = temperature. The rate at which electrons escape from a trap of energy Et is, where n = number of electrons in a trap. Et and S can be determined experimentally. For example Li. F: Mg, Ti has several dominant traps ranging from 1 to 2 e. V, with S values of 1012 to 1020 s-1. This implies the mean lifetime in shallow traps will be a few minutes, whilst in deeper traps the mean lifetime is around 80 years. This results in a natural “fading” of TL signal with time. MSc-REP Regan Dosimetry 80
 Additional revision slides, examples and additional material MSc-REP Regan Dosimetry 81
Additional revision slides, examples and additional material MSc-REP Regan Dosimetry 81
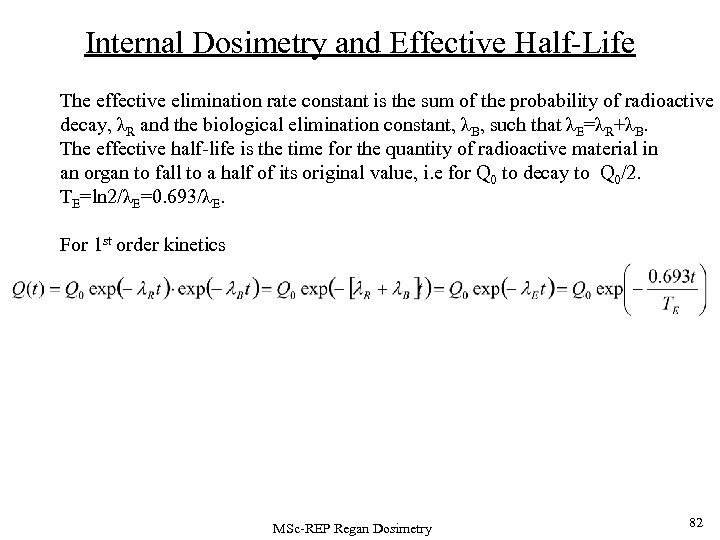 Internal Dosimetry and Effective Half-Life The effective elimination rate constant is the sum of the probability of radioactive decay, λR and the biological elimination constant, λB, such that λE=λR+λB. The effective half-life is the time for the quantity of radioactive material in an organ to fall to a half of its original value, i. e for Q 0 to decay to Q 0/2. TE=ln 2/λE=0. 693/λE. For 1 st order kinetics MSc-REP Regan Dosimetry 82
Internal Dosimetry and Effective Half-Life The effective elimination rate constant is the sum of the probability of radioactive decay, λR and the biological elimination constant, λB, such that λE=λR+λB. The effective half-life is the time for the quantity of radioactive material in an organ to fall to a half of its original value, i. e for Q 0 to decay to Q 0/2. TE=ln 2/λE=0. 693/λE. For 1 st order kinetics MSc-REP Regan Dosimetry 82
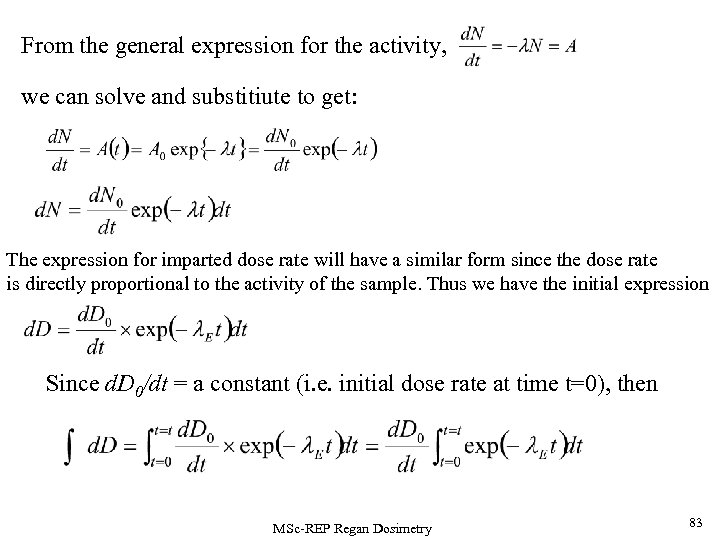 From the general expression for the activity, we can solve and substitiute to get: The expression for imparted dose rate will have a similar form since the dose rate is directly proportional to the activity of the sample. Thus we have the initial expression Since d. D 0/dt = a constant (i. e. initial dose rate at time t=0), then MSc-REP Regan Dosimetry 83
From the general expression for the activity, we can solve and substitiute to get: The expression for imparted dose rate will have a similar form since the dose rate is directly proportional to the activity of the sample. Thus we have the initial expression Since d. D 0/dt = a constant (i. e. initial dose rate at time t=0), then MSc-REP Regan Dosimetry 83
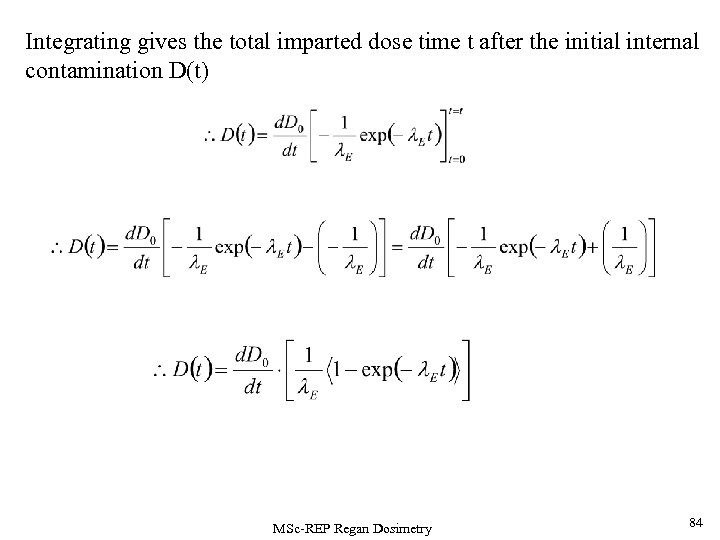 Integrating gives the total imparted dose time t after the initial internal contamination D(t) MSc-REP Regan Dosimetry 84
Integrating gives the total imparted dose time t after the initial internal contamination D(t) MSc-REP Regan Dosimetry 84
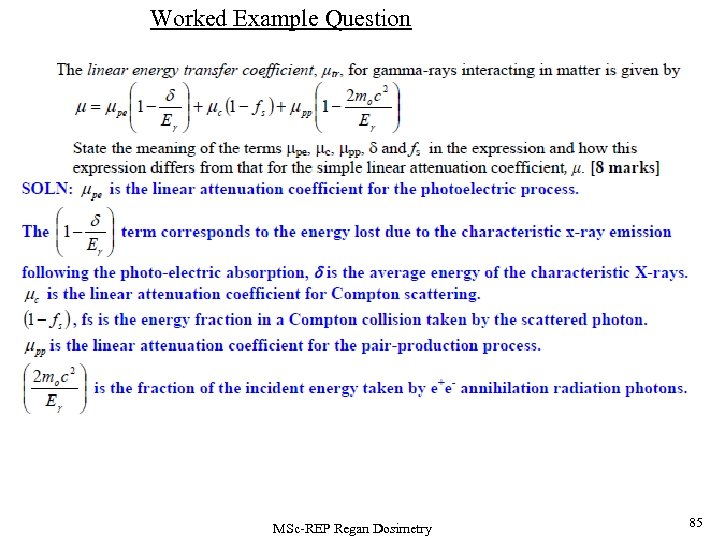 Worked Example Question MSc-REP Regan Dosimetry 85
Worked Example Question MSc-REP Regan Dosimetry 85
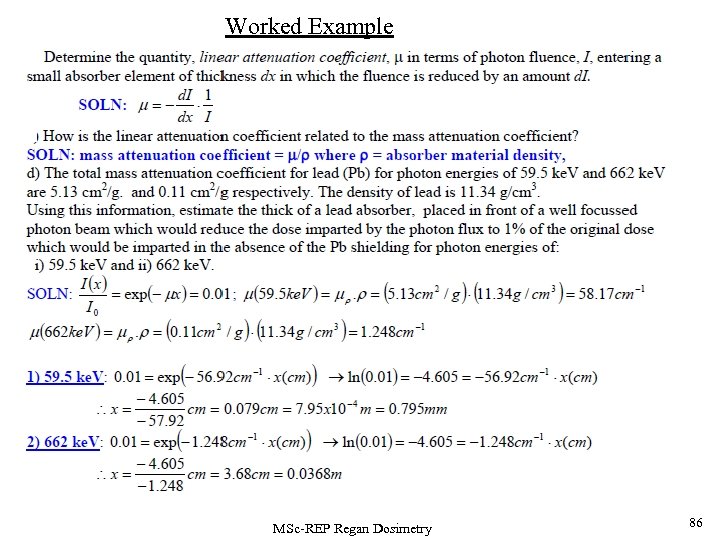 Worked Example MSc-REP Regan Dosimetry 86
Worked Example MSc-REP Regan Dosimetry 86
 Example examination question (2009): MSc-REP Regan Dosimetry 87
Example examination question (2009): MSc-REP Regan Dosimetry 87
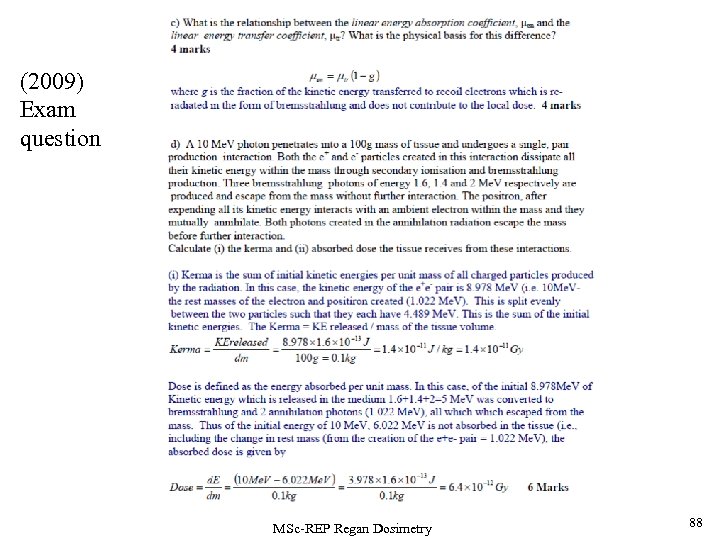 (2009) Exam question MSc-REP Regan Dosimetry 88
(2009) Exam question MSc-REP Regan Dosimetry 88
 Example exam question (2009) MSc-REP Regan Dosimetry 89
Example exam question (2009) MSc-REP Regan Dosimetry 89
 Example exam question (2009) MSc-REP Regan Dosimetry 90
Example exam question (2009) MSc-REP Regan Dosimetry 90
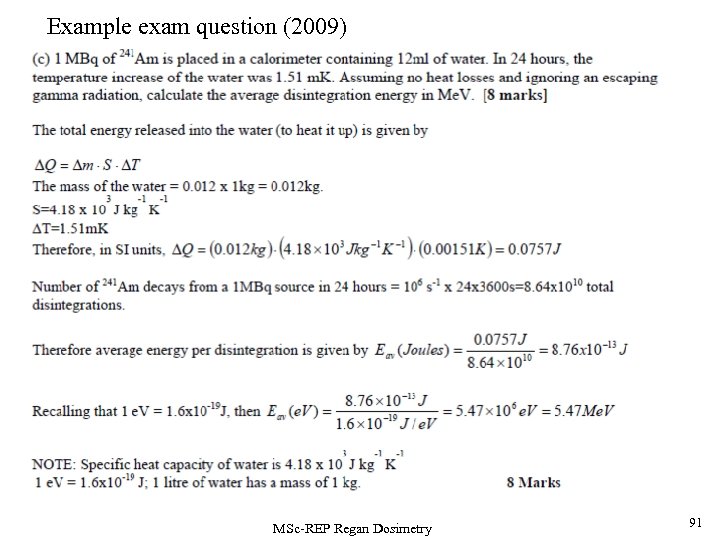 Example exam question (2009) MSc-REP Regan Dosimetry 91
Example exam question (2009) MSc-REP Regan Dosimetry 91
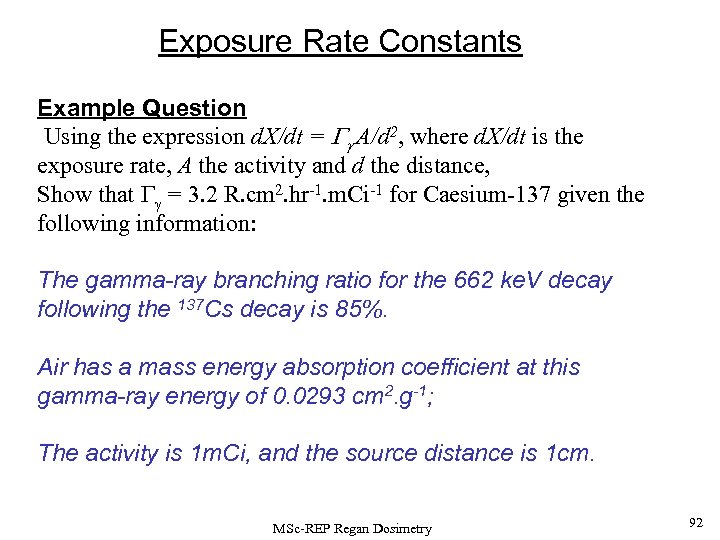 Exposure Rate Constants Example Question Using the expression d. X/dt = A/d 2, where d. X/dt is the exposure rate, A the activity and d the distance, Show that = 3. 2 R. cm 2. hr-1. m. Ci-1 for Caesium-137 given the following information: The gamma-ray branching ratio for the 662 ke. V decay following the 137 Cs decay is 85%. Air has a mass energy absorption coefficient at this gamma-ray energy of 0. 0293 cm 2. g-1; The activity is 1 m. Ci, and the source distance is 1 cm. MSc-REP Regan Dosimetry 92
Exposure Rate Constants Example Question Using the expression d. X/dt = A/d 2, where d. X/dt is the exposure rate, A the activity and d the distance, Show that = 3. 2 R. cm 2. hr-1. m. Ci-1 for Caesium-137 given the following information: The gamma-ray branching ratio for the 662 ke. V decay following the 137 Cs decay is 85%. Air has a mass energy absorption coefficient at this gamma-ray energy of 0. 0293 cm 2. g-1; The activity is 1 m. Ci, and the source distance is 1 cm. MSc-REP Regan Dosimetry 92
 Example Answer If activity A is 1 m. Ci and the distance is 1 cm. Therefore = d. X/dt. The Dose per second is given by energy photon fluence multiplied by the mass energy absorption coefficient. [remember 1 Ci = 3. 7 x 1010 Bq] Dose per second = 0. 85 x 3. 7 x 107 m. Ci– 1 x (0. 662)x 106 e. V x 1. 6 x 10 -19 J. e. V-1 x 0. 0293 cm 2. g-1 4 x 1 s = 7. 77 x 10 -9 J. s-1. g-1. cm 2. m. Ci-1 in 1 hour (1 hr = 3600 s) = 2. 80 x 10 -5 J. g-1. cm 2. hr-1. m. Ci-1 for 1 kg (1 kg = 1000 g) = 2. 80 x 10 -2 J. kg-1. cm 2. hr-1. m. Ci-1 (or in units Gy. cm 2. hr-1. m. Ci-1) in rads (1 Gy = 100 rad) = 2. 80 rad. cm 2. hr-1. m. Ci-1 in Roentgen (1 R = 0. 87 rad) = 3. 2 R. cm 2. hr-1. m. Ci-1 MSc-REP Regan Dosimetry 93
Example Answer If activity A is 1 m. Ci and the distance is 1 cm. Therefore = d. X/dt. The Dose per second is given by energy photon fluence multiplied by the mass energy absorption coefficient. [remember 1 Ci = 3. 7 x 1010 Bq] Dose per second = 0. 85 x 3. 7 x 107 m. Ci– 1 x (0. 662)x 106 e. V x 1. 6 x 10 -19 J. e. V-1 x 0. 0293 cm 2. g-1 4 x 1 s = 7. 77 x 10 -9 J. s-1. g-1. cm 2. m. Ci-1 in 1 hour (1 hr = 3600 s) = 2. 80 x 10 -5 J. g-1. cm 2. hr-1. m. Ci-1 for 1 kg (1 kg = 1000 g) = 2. 80 x 10 -2 J. kg-1. cm 2. hr-1. m. Ci-1 (or in units Gy. cm 2. hr-1. m. Ci-1) in rads (1 Gy = 100 rad) = 2. 80 rad. cm 2. hr-1. m. Ci-1 in Roentgen (1 R = 0. 87 rad) = 3. 2 R. cm 2. hr-1. m. Ci-1 MSc-REP Regan Dosimetry 93
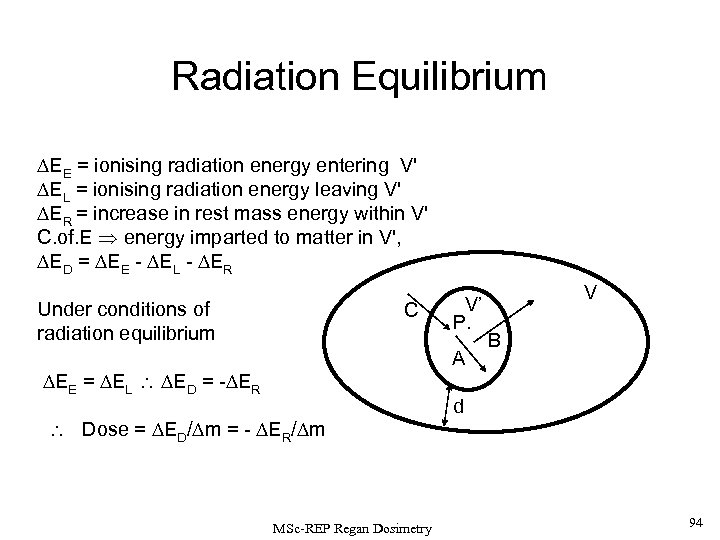 Radiation Equilibrium EE = ionising radiation energy entering V' EL = ionising radiation energy leaving V' ER = increase in rest mass energy within V' C. of. E energy imparted to matter in V', ED = EE - EL - ER V’ Under conditions of C P. radiation equilibrium B A EE = EL ED = - ER d Dose = ED/ m = - ER/ m MSc-REP Regan Dosimetry V 94
Radiation Equilibrium EE = ionising radiation energy entering V' EL = ionising radiation energy leaving V' ER = increase in rest mass energy within V' C. of. E energy imparted to matter in V', ED = EE - EL - ER V’ Under conditions of C P. radiation equilibrium B A EE = EL ED = - ER d Dose = ED/ m = - ER/ m MSc-REP Regan Dosimetry V 94
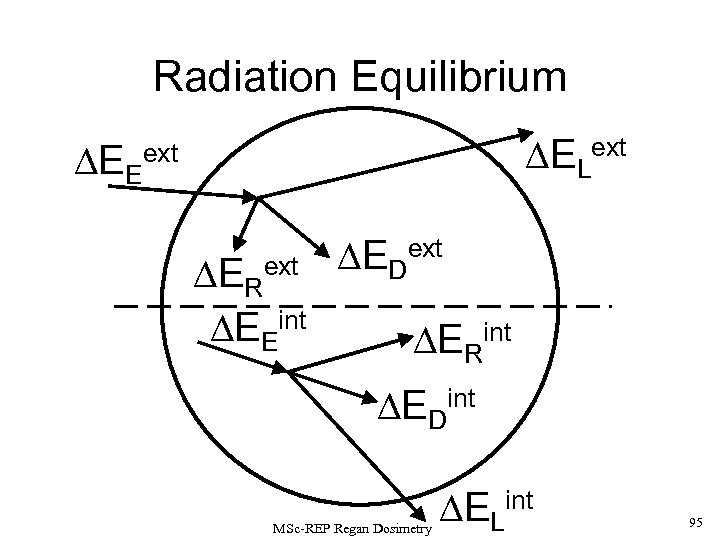 Radiation Equilibrium EE ELext ERext EEint EDext ERint EDint MSc-REP Regan Dosimetry ELint 95
Radiation Equilibrium EE ELext ERext EEint EDext ERint EDint MSc-REP Regan Dosimetry ELint 95
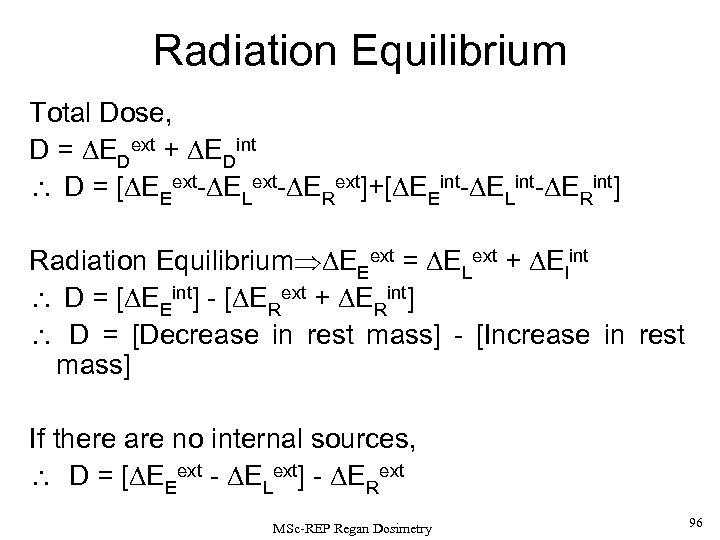 Radiation Equilibrium Total Dose, D = EDext + EDint D = [ EEext- ELext- ERext]+[ EEint- ELint- ERint] Radiation Equilibrium EEext = ELext + Elint D = [ EEint] - [ ERext + ERint] D = [Decrease in rest mass] - [Increase in rest mass] If there are no internal sources, D = [ EEext - ELext] - ERext MSc-REP Regan Dosimetry 96
Radiation Equilibrium Total Dose, D = EDext + EDint D = [ EEext- ELext- ERext]+[ EEint- ELint- ERint] Radiation Equilibrium EEext = ELext + Elint D = [ EEint] - [ ERext + ERint] D = [Decrease in rest mass] - [Increase in rest mass] If there are no internal sources, D = [ EEext - ELext] - ERext MSc-REP Regan Dosimetry 96
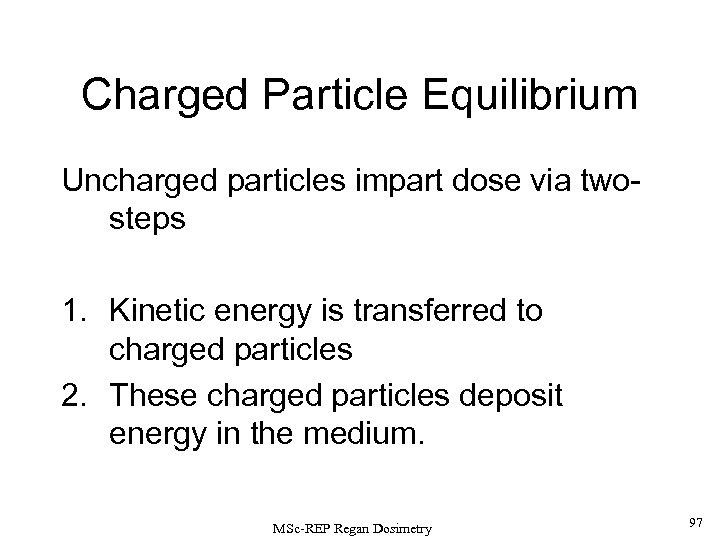 Charged Particle Equilibrium Uncharged particles impart dose via twosteps 1. Kinetic energy is transferred to charged particles 2. These charged particles deposit energy in the medium. MSc-REP Regan Dosimetry 97
Charged Particle Equilibrium Uncharged particles impart dose via twosteps 1. Kinetic energy is transferred to charged particles 2. These charged particles deposit energy in the medium. MSc-REP Regan Dosimetry 97
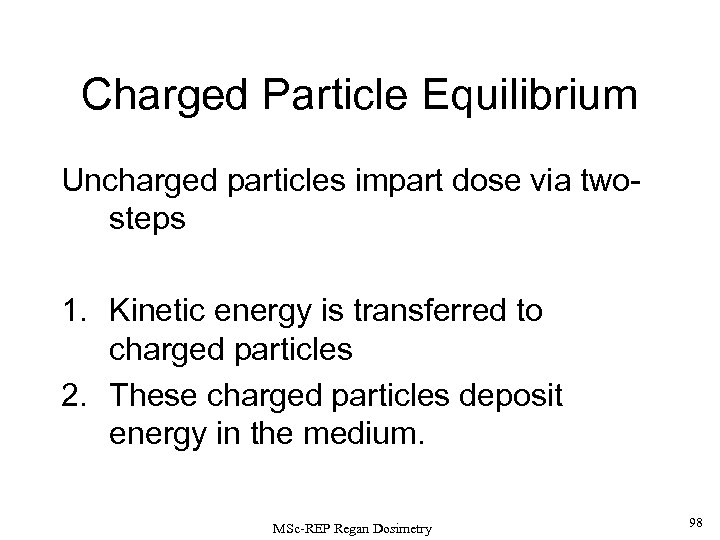 Charged Particle Equilibrium Uncharged particles impart dose via twosteps 1. Kinetic energy is transferred to charged particles 2. These charged particles deposit energy in the medium. MSc-REP Regan Dosimetry 98
Charged Particle Equilibrium Uncharged particles impart dose via twosteps 1. Kinetic energy is transferred to charged particles 2. These charged particles deposit energy in the medium. MSc-REP Regan Dosimetry 98
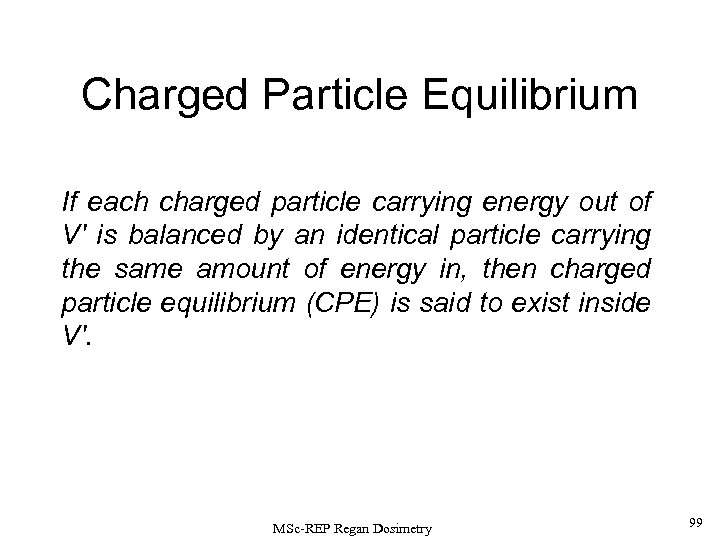 Charged Particle Equilibrium If each charged particle carrying energy out of V' is balanced by an identical particle carrying the same amount of energy in, then charged particle equilibrium (CPE) is said to exist inside V'. MSc-REP Regan Dosimetry 99
Charged Particle Equilibrium If each charged particle carrying energy out of V' is balanced by an identical particle carrying the same amount of energy in, then charged particle equilibrium (CPE) is said to exist inside V'. MSc-REP Regan Dosimetry 99
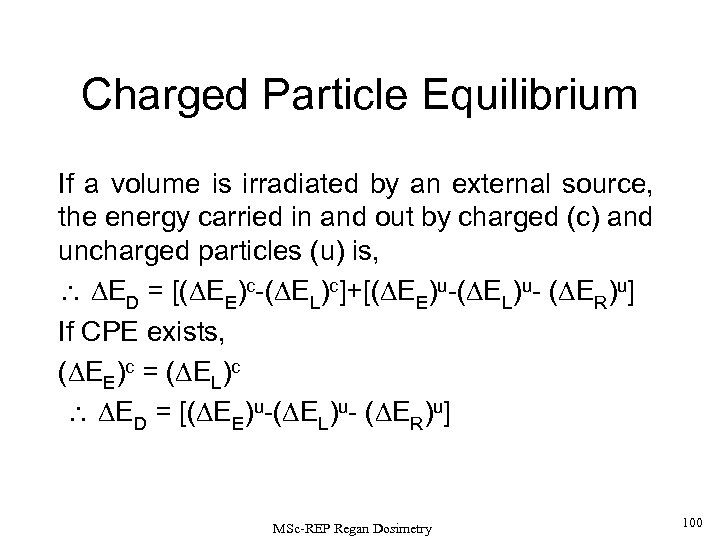 Charged Particle Equilibrium If a volume is irradiated by an external source, the energy carried in and out by charged (c) and uncharged particles (u) is, ED = [( EE)c-( EL)c]+[( EE)u-( EL)u- ( ER)u] If CPE exists, ( EE)c = ( EL)c ED = [( EE)u-( EL)u- ( ER)u] MSc-REP Regan Dosimetry 100
Charged Particle Equilibrium If a volume is irradiated by an external source, the energy carried in and out by charged (c) and uncharged particles (u) is, ED = [( EE)c-( EL)c]+[( EE)u-( EL)u- ( ER)u] If CPE exists, ( EE)c = ( EL)c ED = [( EE)u-( EL)u- ( ER)u] MSc-REP Regan Dosimetry 100
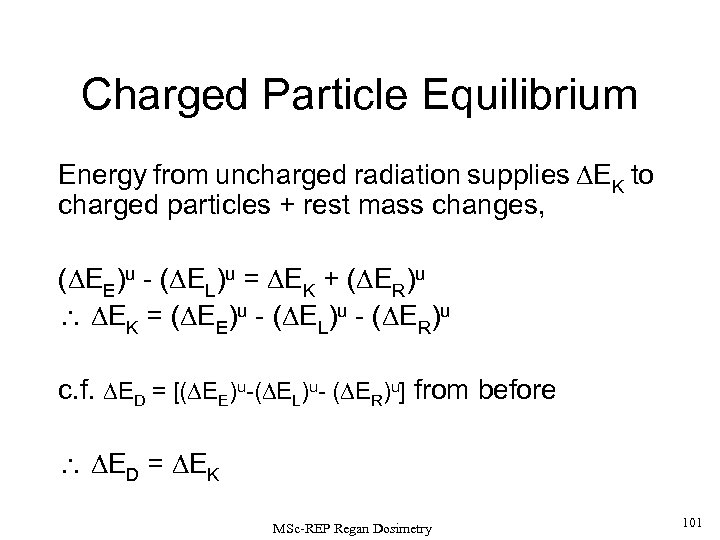 Charged Particle Equilibrium Energy from uncharged radiation supplies EK to charged particles + rest mass changes, ( EE)u - ( EL)u = EK + ( ER)u EK = ( EE)u - ( EL)u - ( ER)u c. f. ED = [( EE)u-( EL)u- ( ER)u] from before ED = EK MSc-REP Regan Dosimetry 101
Charged Particle Equilibrium Energy from uncharged radiation supplies EK to charged particles + rest mass changes, ( EE)u - ( EL)u = EK + ( ER)u EK = ( EE)u - ( EL)u - ( ER)u c. f. ED = [( EE)u-( EL)u- ( ER)u] from before ED = EK MSc-REP Regan Dosimetry 101
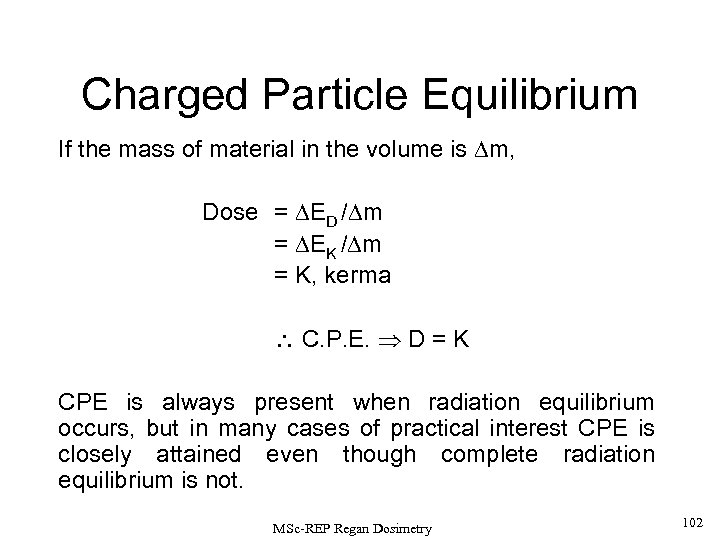 Charged Particle Equilibrium If the mass of material in the volume is m, Dose = ED / m = EK / m = K, kerma C. P. E. D = K CPE is always present when radiation equilibrium occurs, but in many cases of practical interest CPE is closely attained even though complete radiation equilibrium is not. MSc-REP Regan Dosimetry 102
Charged Particle Equilibrium If the mass of material in the volume is m, Dose = ED / m = EK / m = K, kerma C. P. E. D = K CPE is always present when radiation equilibrium occurs, but in many cases of practical interest CPE is closely attained even though complete radiation equilibrium is not. MSc-REP Regan Dosimetry 102
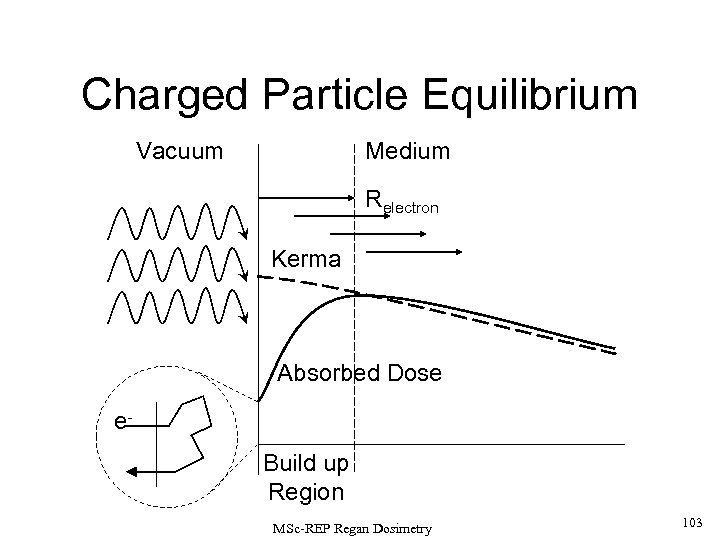 Charged Particle Equilibrium Vacuum Medium Relectron Kerma Absorbed Dose e. Build up Region MSc-REP Regan Dosimetry 103
Charged Particle Equilibrium Vacuum Medium Relectron Kerma Absorbed Dose e. Build up Region MSc-REP Regan Dosimetry 103
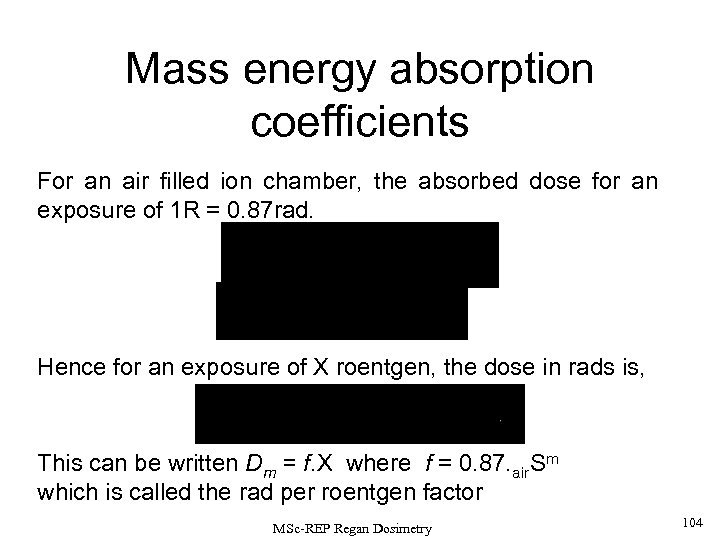 Mass energy absorption coefficients For an air filled ion chamber, the absorbed dose for an exposure of 1 R = 0. 87 rad. Hence for an exposure of X roentgen, the dose in rads is, This can be written Dm = f. X where f = 0. 87. air. Sm which is called the rad per roentgen factor MSc-REP Regan Dosimetry 104
Mass energy absorption coefficients For an air filled ion chamber, the absorbed dose for an exposure of 1 R = 0. 87 rad. Hence for an exposure of X roentgen, the dose in rads is, This can be written Dm = f. X where f = 0. 87. air. Sm which is called the rad per roentgen factor MSc-REP Regan Dosimetry 104
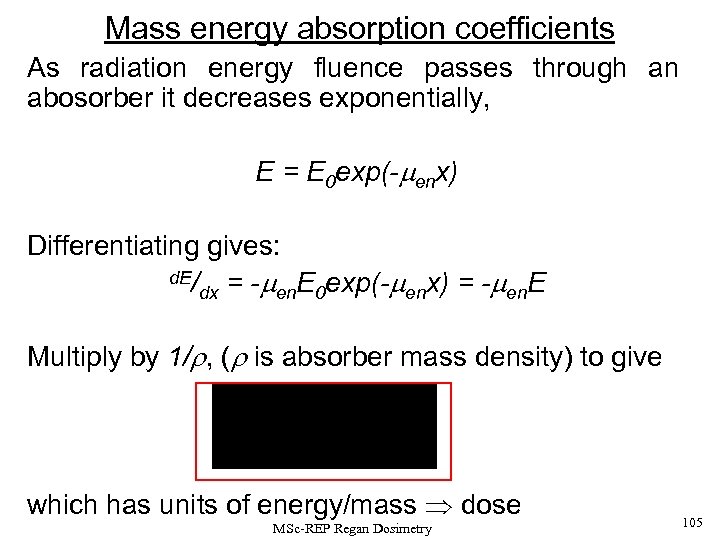 Mass energy absorption coefficients As radiation energy fluence passes through an abosorber it decreases exponentially, E = E 0 exp(- enx) Differentiating gives: d. E/ = - E exp(- x) = - E dx en 0 en en Multiply by 1/ , ( is absorber mass density) to give which has units of energy/mass dose MSc-REP Regan Dosimetry 105
Mass energy absorption coefficients As radiation energy fluence passes through an abosorber it decreases exponentially, E = E 0 exp(- enx) Differentiating gives: d. E/ = - E exp(- x) = - E dx en 0 en en Multiply by 1/ , ( is absorber mass density) to give which has units of energy/mass dose MSc-REP Regan Dosimetry 105
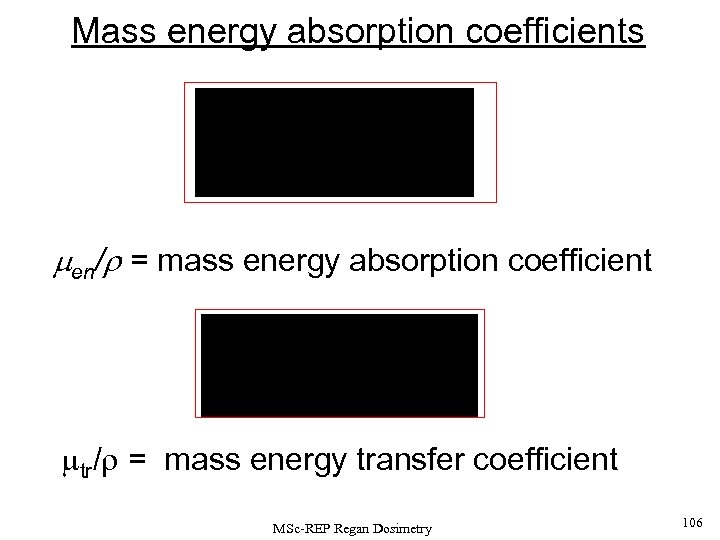 Mass energy absorption coefficients en/ = mass energy absorption coefficient tr/ = mass energy transfer coefficient MSc-REP Regan Dosimetry 106
Mass energy absorption coefficients en/ = mass energy absorption coefficient tr/ = mass energy transfer coefficient MSc-REP Regan Dosimetry 106
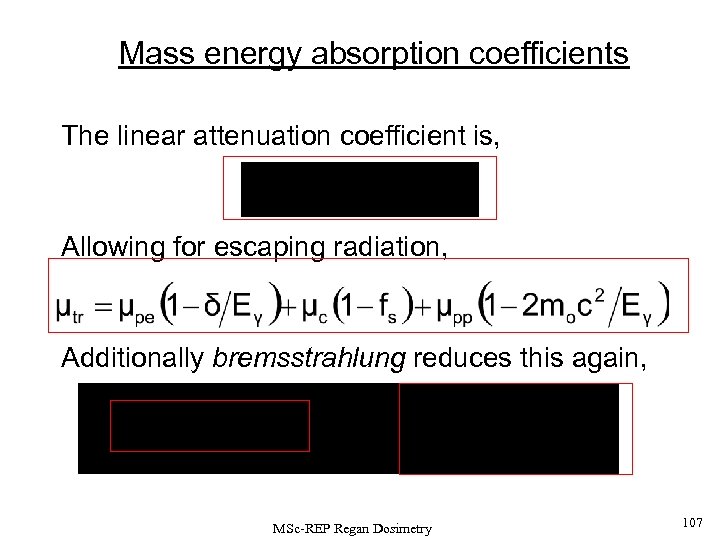 Mass energy absorption coefficients The linear attenuation coefficient is, Allowing for escaping radiation, Additionally bremsstrahlung reduces this again, MSc-REP Regan Dosimetry 107
Mass energy absorption coefficients The linear attenuation coefficient is, Allowing for escaping radiation, Additionally bremsstrahlung reduces this again, MSc-REP Regan Dosimetry 107
 Exposure Rate Constants Consider a point source of photons in air of activity A m. Ci situated at distance d from the point of interest where the exposure rate is d. X/dt R. h-1. The exposure rate depends only on the activity of the source and the inverse square of the distance from the source. Hence d. X/dt = . A/d 2 where is called the exposure rate constant. Using the above units has units R. cm 2. h-1. m. Ci-1. MSc-REP Regan Dosimetry 108
Exposure Rate Constants Consider a point source of photons in air of activity A m. Ci situated at distance d from the point of interest where the exposure rate is d. X/dt R. h-1. The exposure rate depends only on the activity of the source and the inverse square of the distance from the source. Hence d. X/dt = . A/d 2 where is called the exposure rate constant. Using the above units has units R. cm 2. h-1. m. Ci-1. MSc-REP Regan Dosimetry 108
 Additional Information on Personal Dosimetry / TLDs MSc-REP Regan Dosimetry 109
Additional Information on Personal Dosimetry / TLDs MSc-REP Regan Dosimetry 109
 Thermoluminescence Dosimetry Real TLD materials have a dose range of about 0. 1μGy to in excess of 10 Gy. As well as specifically made TLD materials, suitable environmental samples (such as the roof tiles in Hiroshima) can also be used. TLD measurements though are subject to two types of spurious luminescence (1) triboluminescence – arising from trapping of charges generated by friction between loose grains, reduced by encapsulating the TL crystals in a PFTE binder (2) chemiluminescence – arising from oxygen induced surface reactions, reduced by N 2 flushing. In addition UV light can cause the TLD to prematurely fade and can be reduced by keeping the TLD in light-tight packaging. There also two related techniques, Thermally Stimulated Electron Emission (TSEE) and Thermally Stimulated Conductivity (TSC) which are alternative readout methods. MSc-REP Regan Dosimetry 110
Thermoluminescence Dosimetry Real TLD materials have a dose range of about 0. 1μGy to in excess of 10 Gy. As well as specifically made TLD materials, suitable environmental samples (such as the roof tiles in Hiroshima) can also be used. TLD measurements though are subject to two types of spurious luminescence (1) triboluminescence – arising from trapping of charges generated by friction between loose grains, reduced by encapsulating the TL crystals in a PFTE binder (2) chemiluminescence – arising from oxygen induced surface reactions, reduced by N 2 flushing. In addition UV light can cause the TLD to prematurely fade and can be reduced by keeping the TLD in light-tight packaging. There also two related techniques, Thermally Stimulated Electron Emission (TSEE) and Thermally Stimulated Conductivity (TSC) which are alternative readout methods. MSc-REP Regan Dosimetry 110
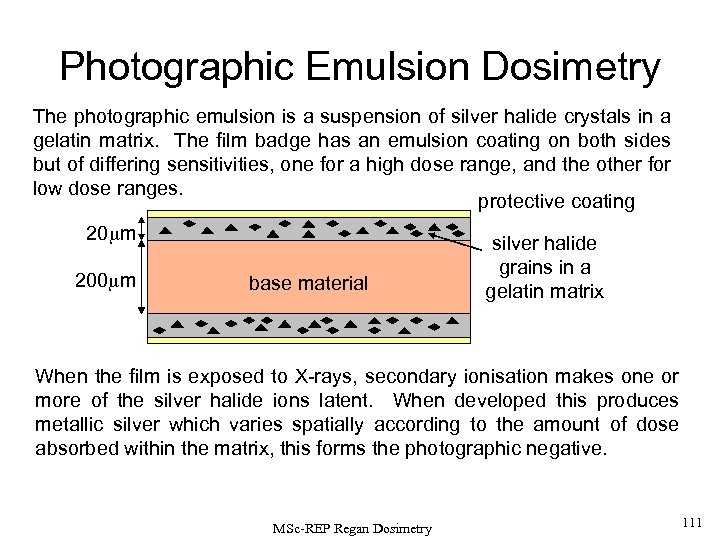 Photographic Emulsion Dosimetry The photographic emulsion is a suspension of silver halide crystals in a gelatin matrix. The film badge has an emulsion coating on both sides but of differing sensitivities, one for a high dose range, and the other for low dose ranges. protective coating 20 m 200 m base material silver halide grains in a gelatin matrix When the film is exposed to X-rays, secondary ionisation makes one or more of the silver halide ions latent. When developed this produces metallic silver which varies spatially according to the amount of dose absorbed within the matrix, this forms the photographic negative. MSc-REP Regan Dosimetry 111
Photographic Emulsion Dosimetry The photographic emulsion is a suspension of silver halide crystals in a gelatin matrix. The film badge has an emulsion coating on both sides but of differing sensitivities, one for a high dose range, and the other for low dose ranges. protective coating 20 m 200 m base material silver halide grains in a gelatin matrix When the film is exposed to X-rays, secondary ionisation makes one or more of the silver halide ions latent. When developed this produces metallic silver which varies spatially according to the amount of dose absorbed within the matrix, this forms the photographic negative. MSc-REP Regan Dosimetry 111
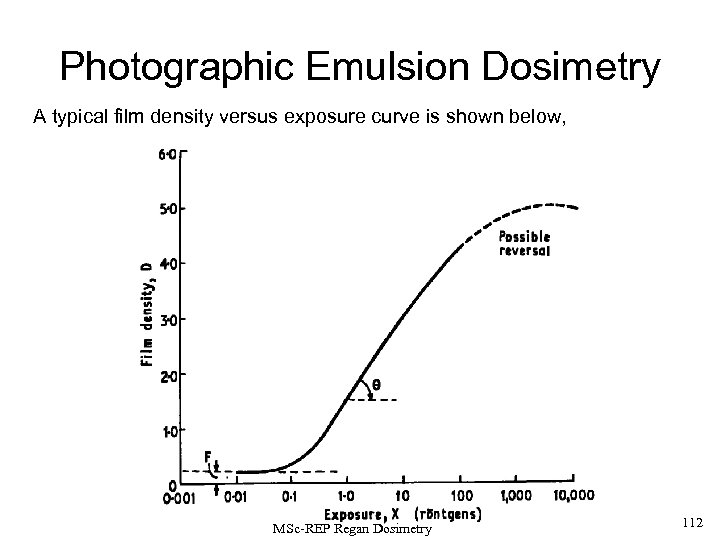 Photographic Emulsion Dosimetry A typical film density versus exposure curve is shown below, MSc-REP Regan Dosimetry 112
Photographic Emulsion Dosimetry A typical film density versus exposure curve is shown below, MSc-REP Regan Dosimetry 112
 Photographic Emulsion Dosimetry Despite some of the drawbacks described above photographic dosimetry still has some advantages over alternatives like TLD. (a) In fine grain emulsions individual particle tracks can be recognised under a microscope and the nature and energy of the incident radiation can be deduced. In radiation protection this has been used to record proton recoil tracks arising in the surrounding gelatin matrix as result of fast neutrons passing through the film badge. (b) The technique produces a permanent visible record so that exposures can be readily rechecked. For example radioactive contamination of a film badge can be distinguished from a "normal" uniform exposure to more distant radiation sources. The self-indicating feature is advantageous if required as legal evidence. (c) The technique is relatively "low-tech" compared with TLD for example and needs less costly equipment which may make it more practicable in some cases. Although it is not as readily automated as a TLD system this may not be important if the workforce being monitored is fairly small. MSc-REP Regan Dosimetry 113
Photographic Emulsion Dosimetry Despite some of the drawbacks described above photographic dosimetry still has some advantages over alternatives like TLD. (a) In fine grain emulsions individual particle tracks can be recognised under a microscope and the nature and energy of the incident radiation can be deduced. In radiation protection this has been used to record proton recoil tracks arising in the surrounding gelatin matrix as result of fast neutrons passing through the film badge. (b) The technique produces a permanent visible record so that exposures can be readily rechecked. For example radioactive contamination of a film badge can be distinguished from a "normal" uniform exposure to more distant radiation sources. The self-indicating feature is advantageous if required as legal evidence. (c) The technique is relatively "low-tech" compared with TLD for example and needs less costly equipment which may make it more practicable in some cases. Although it is not as readily automated as a TLD system this may not be important if the workforce being monitored is fairly small. MSc-REP Regan Dosimetry 113
 Personal Dosemeters Film Badges and TLDs MSc-REP Regan Dosimetry 114
Personal Dosemeters Film Badges and TLDs MSc-REP Regan Dosimetry 114
 Personal Dosemeter Badges Photographic film dosemeter (a. k. a. “film badge”) Film is worn in a holder containing several different filters. When developed the film darkens in proportion to the amount of radiation energy received. Due to the differing amounts of filtration we can gain information on the energy of radiation causing the dose. Radioactive contamination of the film can be readily identified. Thermoluminescent dosemeter (a. k. a. “TLD”) This is a reusable dosemeter which uses lithium fluoride to measure radiation dose. It stores dose information until heated to over 250°C when it gives out light the amount of which is proportional to the dose received. It is environmentally robust and excellent for use in all working environments. Extremity dosemeter (a. k. a. “finger TLD”) This is a miniature TLD which can be supplied in different forms to suit your needs (stalls, straps or rings). The finger stall is most commonly used and is worn like the finger of a glove. MSc-REP Regan Dosimetry 115
Personal Dosemeter Badges Photographic film dosemeter (a. k. a. “film badge”) Film is worn in a holder containing several different filters. When developed the film darkens in proportion to the amount of radiation energy received. Due to the differing amounts of filtration we can gain information on the energy of radiation causing the dose. Radioactive contamination of the film can be readily identified. Thermoluminescent dosemeter (a. k. a. “TLD”) This is a reusable dosemeter which uses lithium fluoride to measure radiation dose. It stores dose information until heated to over 250°C when it gives out light the amount of which is proportional to the dose received. It is environmentally robust and excellent for use in all working environments. Extremity dosemeter (a. k. a. “finger TLD”) This is a miniature TLD which can be supplied in different forms to suit your needs (stalls, straps or rings). The finger stall is most commonly used and is worn like the finger of a glove. MSc-REP Regan Dosimetry 115
 Hp (0. 07) and Hp (10) A dosemeter often measures two quantities. The first is the personal dose equivalent Hp(10), which is often referred to as the “whole body” dose which results from penetrating radiation. The second quantity is the personal dose equivalent Hp(0. 07) which is an assessment of the dose equivalent to the skin from both weakly and strongly penetrating radiations. The “H” of Hp is used generally to signify the Equivalent Dose and is in units of Sieverts. The “p” is used to signify it is personal equivalent dose. The definition of Hp(10) is the dose equivalent at a depth of 10 mm into a human body. Similarly Hp(0. 07) is 0. 07 mm into the body. Other measurements of equivalent dose may not give accurate values of the Hp equivalent dose as it is the absorption and scattering effects of the human body throughout the different response to the energy of the radiation which give true Hp readings. MSc-REP Regan Dosimetry 116
Hp (0. 07) and Hp (10) A dosemeter often measures two quantities. The first is the personal dose equivalent Hp(10), which is often referred to as the “whole body” dose which results from penetrating radiation. The second quantity is the personal dose equivalent Hp(0. 07) which is an assessment of the dose equivalent to the skin from both weakly and strongly penetrating radiations. The “H” of Hp is used generally to signify the Equivalent Dose and is in units of Sieverts. The “p” is used to signify it is personal equivalent dose. The definition of Hp(10) is the dose equivalent at a depth of 10 mm into a human body. Similarly Hp(0. 07) is 0. 07 mm into the body. Other measurements of equivalent dose may not give accurate values of the Hp equivalent dose as it is the absorption and scattering effects of the human body throughout the different response to the energy of the radiation which give true Hp readings. MSc-REP Regan Dosimetry 116
 Film Badge Dosemeters For many decades the standard method personal dosimetry involved the use of photographic emulsions in the form of film badge dosemeters. Although they are increasingly being replaced by other methods, they are still in use as dosemeters for X-, beta and gamma radiation. To cover the required dose range the film incorporates two emulsions, of different sensitivities. Additionally the film badge holder has a number of different filters to sort out the various components of radiation exposure. Detection Gamma rays X-rays Dose range measured 0. 1 m. Sv to 10 Sv 0. 1 m. Sv to 400 m. Sv Energy range detected 10 ke. V to 7 Me. V for Hp (0. 07) 20 ke. V to 7 Me. V for Hp (10) Beta particles 0. 1 m. Sv to 10 Sv 10 ke. V to 7 Me. V 700 ke. V to 3. 5 for Hp (0. 07) Me. V (Emax) for 20 ke. V to 7 Me. V Hp (0. 07) for Hp (10) MSc-REP Regan Dosimetry 117
Film Badge Dosemeters For many decades the standard method personal dosimetry involved the use of photographic emulsions in the form of film badge dosemeters. Although they are increasingly being replaced by other methods, they are still in use as dosemeters for X-, beta and gamma radiation. To cover the required dose range the film incorporates two emulsions, of different sensitivities. Additionally the film badge holder has a number of different filters to sort out the various components of radiation exposure. Detection Gamma rays X-rays Dose range measured 0. 1 m. Sv to 10 Sv 0. 1 m. Sv to 400 m. Sv Energy range detected 10 ke. V to 7 Me. V for Hp (0. 07) 20 ke. V to 7 Me. V for Hp (10) Beta particles 0. 1 m. Sv to 10 Sv 10 ke. V to 7 Me. V 700 ke. V to 3. 5 for Hp (0. 07) Me. V (Emax) for 20 ke. V to 7 Me. V Hp (0. 07) for Hp (10) MSc-REP Regan Dosimetry 117
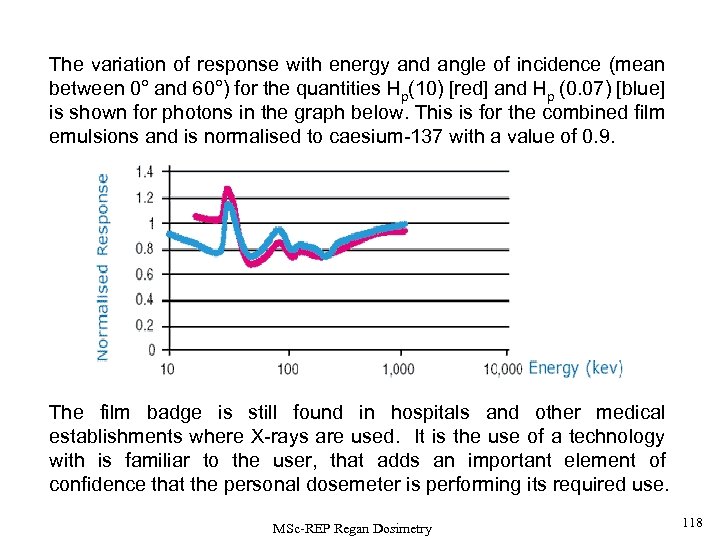 The variation of response with energy and angle of incidence (mean between 0° and 60°) for the quantities Hp(10) [red] and Hp (0. 07) [blue] is shown for photons in the graph below. This is for the combined film emulsions and is normalised to caesium-137 with a value of 0. 9. The film badge is still found in hospitals and other medical establishments where X-rays are used. It is the use of a technology with is familiar to the user, that adds an important element of confidence that the personal dosemeter is performing its required use. MSc-REP Regan Dosimetry 118
The variation of response with energy and angle of incidence (mean between 0° and 60°) for the quantities Hp(10) [red] and Hp (0. 07) [blue] is shown for photons in the graph below. This is for the combined film emulsions and is normalised to caesium-137 with a value of 0. 9. The film badge is still found in hospitals and other medical establishments where X-rays are used. It is the use of a technology with is familiar to the user, that adds an important element of confidence that the personal dosemeter is performing its required use. MSc-REP Regan Dosimetry 118
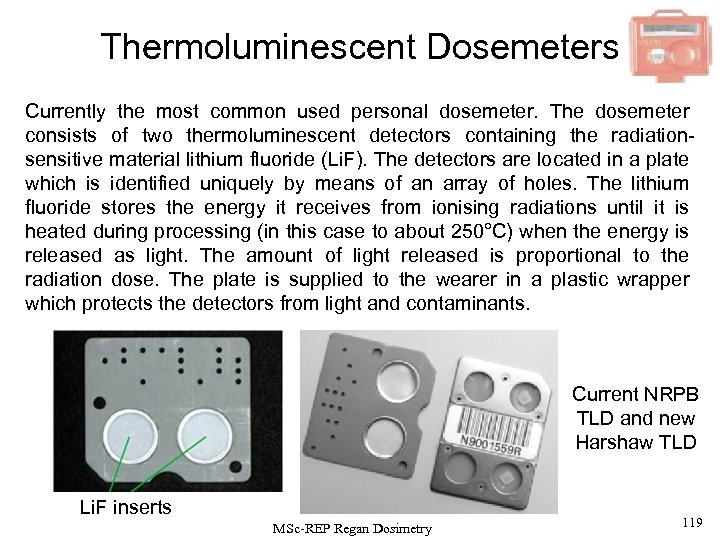 Thermoluminescent Dosemeters Currently the most common used personal dosemeter. The dosemeter consists of two thermoluminescent detectors containing the radiationsensitive material lithium fluoride (Li. F). The detectors are located in a plate which is identified uniquely by means of an array of holes. The lithium fluoride stores the energy it receives from ionising radiations until it is heated during processing (in this case to about 250°C) when the energy is released as light. The amount of light released is proportional to the radiation dose. The plate is supplied to the wearer in a plastic wrapper which protects the detectors from light and contaminants. Current NRPB TLD and new Harshaw TLD Li. F inserts MSc-REP Regan Dosimetry 119
Thermoluminescent Dosemeters Currently the most common used personal dosemeter. The dosemeter consists of two thermoluminescent detectors containing the radiationsensitive material lithium fluoride (Li. F). The detectors are located in a plate which is identified uniquely by means of an array of holes. The lithium fluoride stores the energy it receives from ionising radiations until it is heated during processing (in this case to about 250°C) when the energy is released as light. The amount of light released is proportional to the radiation dose. The plate is supplied to the wearer in a plastic wrapper which protects the detectors from light and contaminants. Current NRPB TLD and new Harshaw TLD Li. F inserts MSc-REP Regan Dosimetry 119
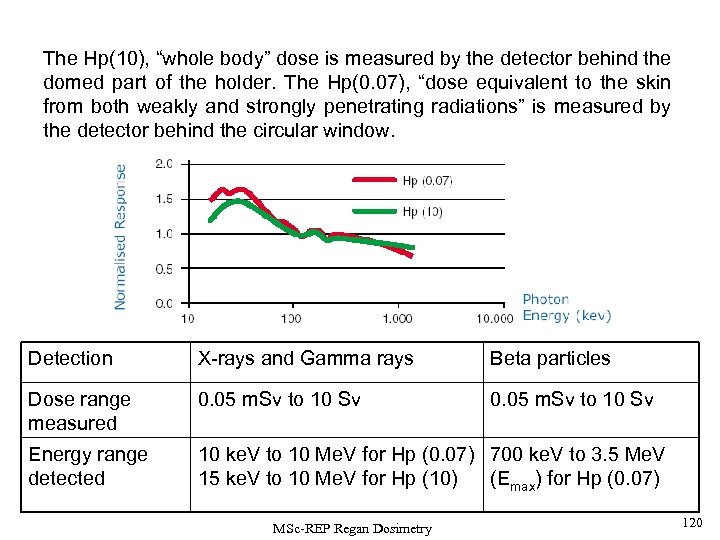 The Hp(10), “whole body” dose is measured by the detector behind the domed part of the holder. The Hp(0. 07), “dose equivalent to the skin from both weakly and strongly penetrating radiations” is measured by the detector behind the circular window. Detection X-rays and Gamma rays Beta particles Dose range measured 0. 05 m. Sv to 10 Sv Energy range detected 10 ke. V to 10 Me. V for Hp (0. 07) 700 ke. V to 3. 5 Me. V 15 ke. V to 10 Me. V for Hp (10) (Emax) for Hp (0. 07) MSc-REP Regan Dosimetry 120
The Hp(10), “whole body” dose is measured by the detector behind the domed part of the holder. The Hp(0. 07), “dose equivalent to the skin from both weakly and strongly penetrating radiations” is measured by the detector behind the circular window. Detection X-rays and Gamma rays Beta particles Dose range measured 0. 05 m. Sv to 10 Sv Energy range detected 10 ke. V to 10 Me. V for Hp (0. 07) 700 ke. V to 3. 5 Me. V 15 ke. V to 10 Me. V for Hp (10) (Emax) for Hp (0. 07) MSc-REP Regan Dosimetry 120
 Optically Stimulated Luminesence Dosemeters (OSLDs) measures radiation through a thin layer of aluminum oxide. During analysis, the aluminum oxide is stimulated with selected frequencies of laser light causing it to become luminescent in proportion to the amount of radiation exposure. OSLDs gives accurate readings down to 1 Sv. This degree of sensitivity is ideal for employees working in low-radiation environments and for pregnant employees. The Al 203 (aluminum oxide) detector can be restimulated numerous times to confirm the accuracy of a radiation dose measurement. OSLDs be used for up to one year. They are unaffected by heat, moisture, and pressure when the clear blister packaging is uncompromised. OSLD readers do not require heaters or gas flow. MSc-REP Regan Dosimetry 121
Optically Stimulated Luminesence Dosemeters (OSLDs) measures radiation through a thin layer of aluminum oxide. During analysis, the aluminum oxide is stimulated with selected frequencies of laser light causing it to become luminescent in proportion to the amount of radiation exposure. OSLDs gives accurate readings down to 1 Sv. This degree of sensitivity is ideal for employees working in low-radiation environments and for pregnant employees. The Al 203 (aluminum oxide) detector can be restimulated numerous times to confirm the accuracy of a radiation dose measurement. OSLDs be used for up to one year. They are unaffected by heat, moisture, and pressure when the clear blister packaging is uncompromised. OSLD readers do not require heaters or gas flow. MSc-REP Regan Dosimetry 121
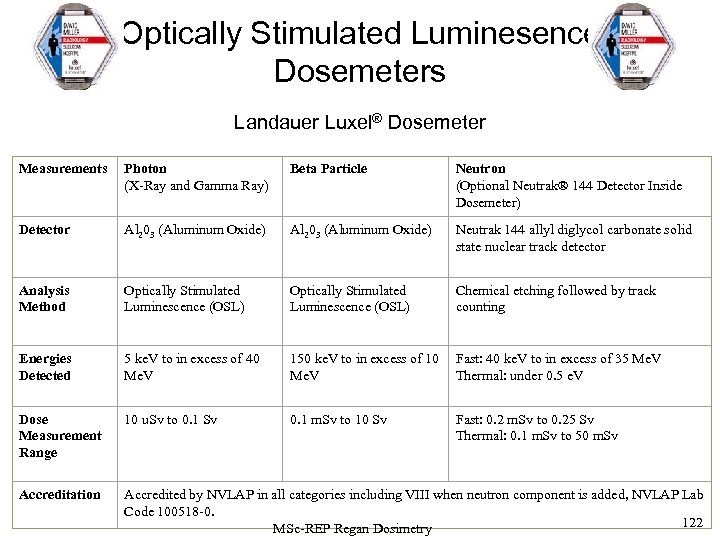 Optically Stimulated Luminesence Dosemeters Landauer Luxel® Dosemeter Measurements Photon (X-Ray and Gamma Ray) Beta Particle Neutron (Optional Neutrak® 144 Detector Inside Dosemeter) Detector Al 203 (Aluminum Oxide) Neutrak 144 allyl diglycol carbonate solid state nuclear track detector Analysis Method Optically Stimulated Luminescence (OSL) Chemical etching followed by track counting Energies Detected 5 ke. V to in excess of 40 Me. V 150 ke. V to in excess of 10 Fast: 40 ke. V to in excess of 35 Me. V Thermal: under 0. 5 e. V Dose Measurement Range 10 u. Sv to 0. 1 Sv 0. 1 m. Sv to 10 Sv Accreditation Accredited by NVLAP in all categories including VIII when neutron component is added, NVLAP Lab Code 100518 -0. 122 MSc-REP Regan Dosimetry Fast: 0. 2 m. Sv to 0. 25 Sv Thermal: 0. 1 m. Sv to 50 m. Sv
Optically Stimulated Luminesence Dosemeters Landauer Luxel® Dosemeter Measurements Photon (X-Ray and Gamma Ray) Beta Particle Neutron (Optional Neutrak® 144 Detector Inside Dosemeter) Detector Al 203 (Aluminum Oxide) Neutrak 144 allyl diglycol carbonate solid state nuclear track detector Analysis Method Optically Stimulated Luminescence (OSL) Chemical etching followed by track counting Energies Detected 5 ke. V to in excess of 40 Me. V 150 ke. V to in excess of 10 Fast: 40 ke. V to in excess of 35 Me. V Thermal: under 0. 5 e. V Dose Measurement Range 10 u. Sv to 0. 1 Sv 0. 1 m. Sv to 10 Sv Accreditation Accredited by NVLAP in all categories including VIII when neutron component is added, NVLAP Lab Code 100518 -0. 122 MSc-REP Regan Dosimetry Fast: 0. 2 m. Sv to 0. 25 Sv Thermal: 0. 1 m. Sv to 50 m. Sv
 Neutron Dosemeters The poly-allyl diglycol carbonate (PADC) neutron dosemeter is designed to measure doses from neutron radiation in terms of the radiation quantities specified by the Health and Safety Executive (HSE). The PADC neutron service is approved by the HSE under Regulation 35 of the Ionising Radiations Regulations 1999. The dosemeter is a passive device for the detection of thermal, epithermal and fast neutrons. It is insensitive to other radiations (gamma, X- and beta), is relatively unaffected by environmental factors such as heat and humidity and has a very low radon sensitivity. Detection Neutron radiation Dose range measured 0. 2 m. Sv to 250 m. Sv Energy range detected Thermal, epithermal and fast (144 ke. V to 15 Me. V) neutrons for Hp (10) MSc-REP Regan Dosimetry 123
Neutron Dosemeters The poly-allyl diglycol carbonate (PADC) neutron dosemeter is designed to measure doses from neutron radiation in terms of the radiation quantities specified by the Health and Safety Executive (HSE). The PADC neutron service is approved by the HSE under Regulation 35 of the Ionising Radiations Regulations 1999. The dosemeter is a passive device for the detection of thermal, epithermal and fast neutrons. It is insensitive to other radiations (gamma, X- and beta), is relatively unaffected by environmental factors such as heat and humidity and has a very low radon sensitivity. Detection Neutron radiation Dose range measured 0. 2 m. Sv to 250 m. Sv Energy range detected Thermal, epithermal and fast (144 ke. V to 15 Me. V) neutrons for Hp (10) MSc-REP Regan Dosimetry 123


