63e7bd5de37d3f2d261518129ad39e81.ppt
- Количество слайдов: 36

 Def. – A measure of average Kinetic Energy of the molecules in a substance The higher the temperature, the faster the particles are moving! Temp = KE
Def. – A measure of average Kinetic Energy of the molecules in a substance The higher the temperature, the faster the particles are moving! Temp = KE
 There are three temperature scales: • Celsius (°C) • Fahrenheit (°F) • Kelvin (K) –On the Kelvin scale, there is a theoretical point called Absolute Zero where all molecular motion stops
There are three temperature scales: • Celsius (°C) • Fahrenheit (°F) • Kelvin (K) –On the Kelvin scale, there is a theoretical point called Absolute Zero where all molecular motion stops
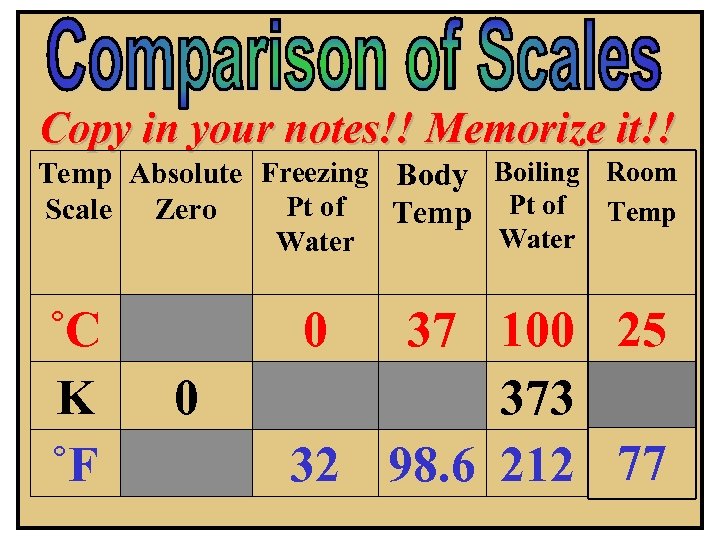 Copy in your notes!! Memorize it!! Temp Absolute Freezing Body Boiling Room Pt of Scale Zero Temp Pt of Temp Water ˚C K ˚F 37 100 25 373 32 98. 6 212 77 0 0
Copy in your notes!! Memorize it!! Temp Absolute Freezing Body Boiling Room Pt of Scale Zero Temp Pt of Temp Water ˚C K ˚F 37 100 25 373 32 98. 6 212 77 0 0
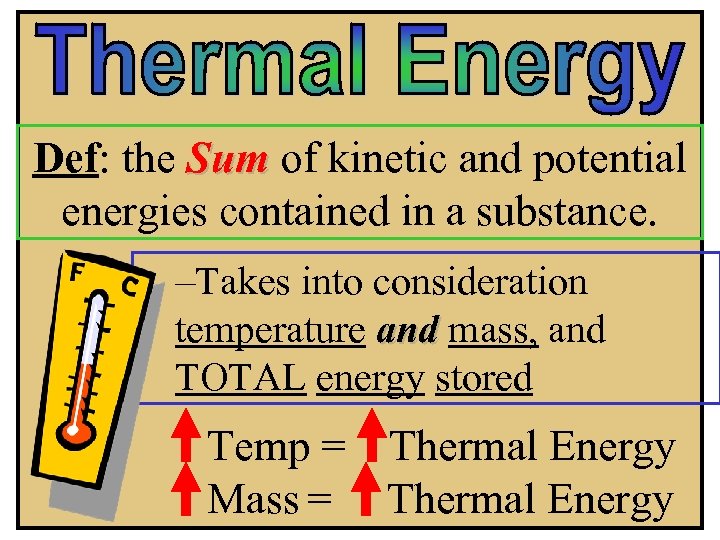 Def: the Sum of kinetic and potential energies contained in a substance. –Takes into consideration temperature and mass, and TOTAL energy stored Temp = Thermal Energy Mass = Thermal Energy
Def: the Sum of kinetic and potential energies contained in a substance. –Takes into consideration temperature and mass, and TOTAL energy stored Temp = Thermal Energy Mass = Thermal Energy
 Each particle has about 10 J of energy in both objects. Which has the higher temp? Same Which has more thermal energy? Box 2
Each particle has about 10 J of energy in both objects. Which has the higher temp? Same Which has more thermal energy? Box 2
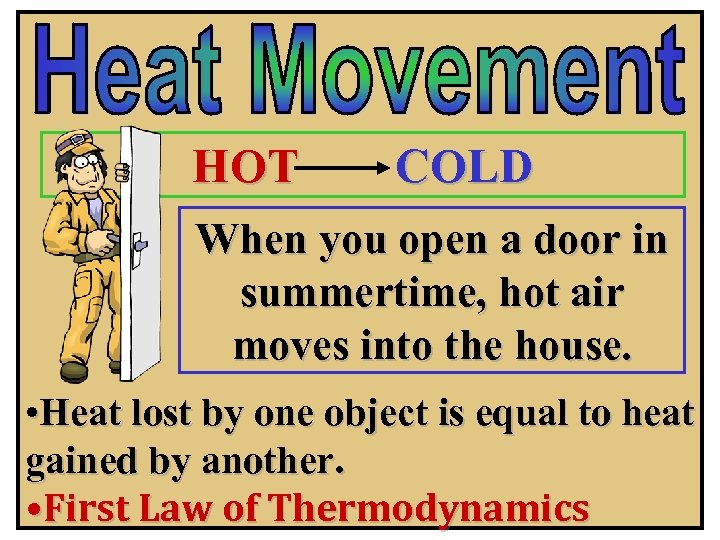 HOT COLD When you open a door in summertime, hot air moves into the house. • Heat lost by one object is equal to heat gained by another. • First Law of Thermodynamics
HOT COLD When you open a door in summertime, hot air moves into the house. • Heat lost by one object is equal to heat gained by another. • First Law of Thermodynamics
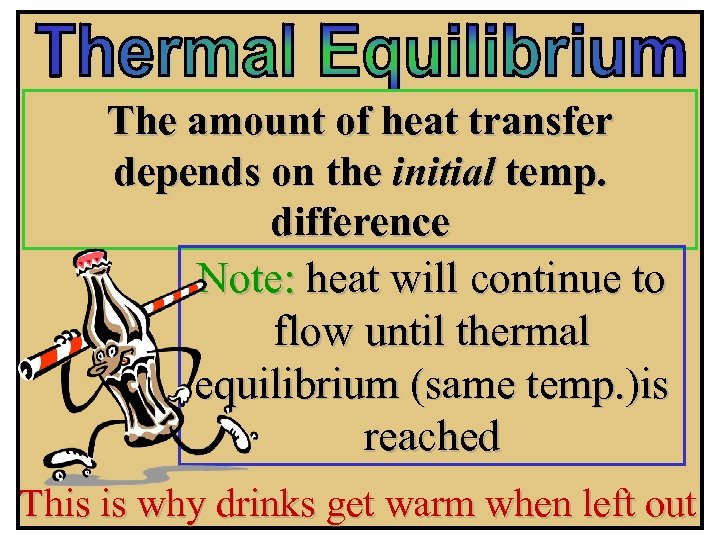 The amount of heat transfer depends on the initial temp. difference Note: heat will continue to flow until thermal equilibrium (same temp. )is reached This is why drinks get warm when left out
The amount of heat transfer depends on the initial temp. difference Note: heat will continue to flow until thermal equilibrium (same temp. )is reached This is why drinks get warm when left out
 Three Methods: 1. Conduction 2. Convection 3. Radiation
Three Methods: 1. Conduction 2. Convection 3. Radiation
 1. Conduction: transfer of heat by direct contact (TOUCH) Solids, liquids, & gases Ex. – Burning your hand on a hot stove
1. Conduction: transfer of heat by direct contact (TOUCH) Solids, liquids, & gases Ex. – Burning your hand on a hot stove

 Thermal Conductors: heat flows easily through these substances • Solids (i. e. – metals) are generally the best conductors Thermal Insulators: Materials that decrease the transfer of heat. • Styrofoam, wood, plastic are examples of insulators
Thermal Conductors: heat flows easily through these substances • Solids (i. e. – metals) are generally the best conductors Thermal Insulators: Materials that decrease the transfer of heat. • Styrofoam, wood, plastic are examples of insulators
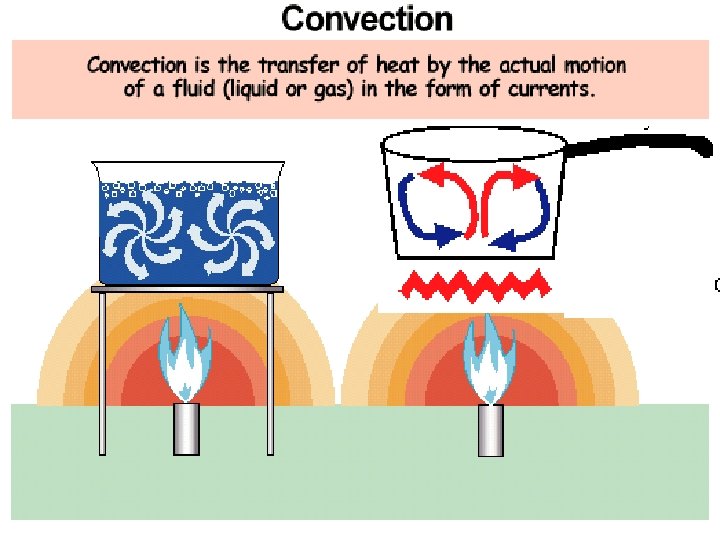
 2. Convection: transfer of heat by the motion of a fluid in the form of Liquids & gases ONLY currents Forced Convection: the process by which mechanical device moves a fluid or gas to transfer heat Ex - weather, ocean currents, heating homes
2. Convection: transfer of heat by the motion of a fluid in the form of Liquids & gases ONLY currents Forced Convection: the process by which mechanical device moves a fluid or gas to transfer heat Ex - weather, ocean currents, heating homes
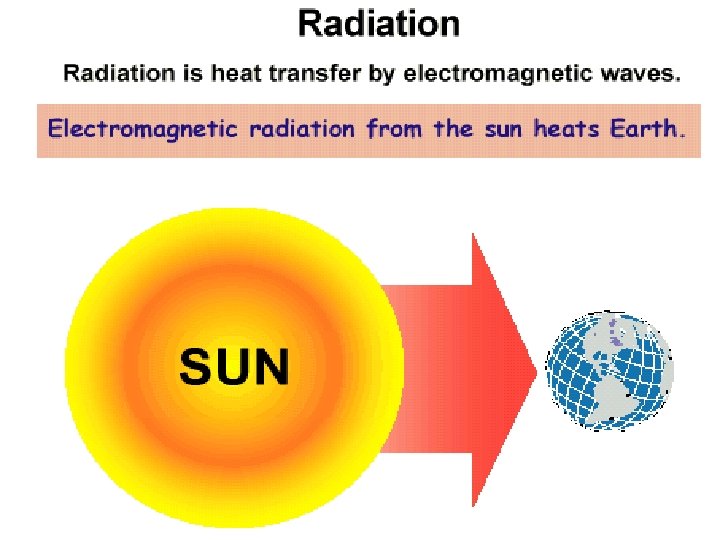
 3. Radiation: transfer of heat OUTWARD by electromagnetic waves Ex – heat radiating from a fireplace
3. Radiation: transfer of heat OUTWARD by electromagnetic waves Ex – heat radiating from a fireplace
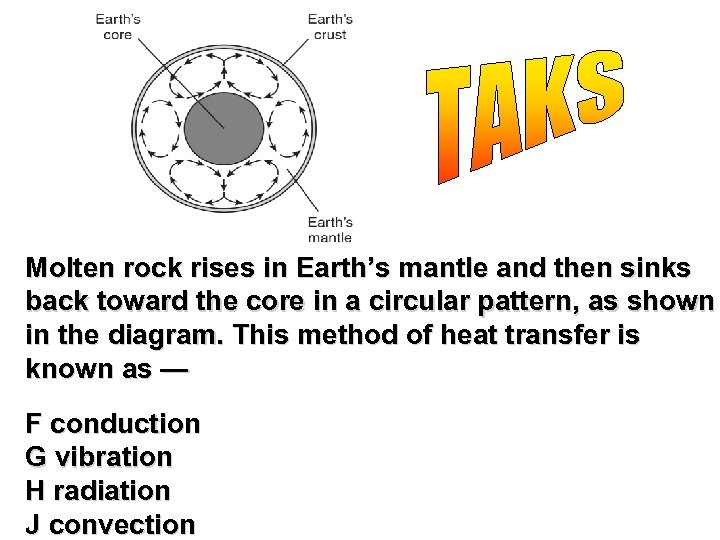 Molten rock rises in Earth’s mantle and then sinks back toward the core in a circular pattern, as shown in the diagram. This method of heat transfer is known as — F conduction G vibration H radiation J convection
Molten rock rises in Earth’s mantle and then sinks back toward the core in a circular pattern, as shown in the diagram. This method of heat transfer is known as — F conduction G vibration H radiation J convection
 Absorbers: Absorb radiation easily & emit heat quickly Reflectors: Reflect radiation & emit heat slowly
Absorbers: Absorb radiation easily & emit heat quickly Reflectors: Reflect radiation & emit heat slowly

 The following are all commonly used units of measurement for Heat: 1. Joules 2. Calories and calories 3. BTU’s
The following are all commonly used units of measurement for Heat: 1. Joules 2. Calories and calories 3. BTU’s
 Since heat is a type of Energy Transfer, the standard unit for heat is the JOULE.
Since heat is a type of Energy Transfer, the standard unit for heat is the JOULE.
 Typically used to measure the heat (energy) content in foods Calorie (kilocalorie): quantity of heat needed to raise temp. of 1 g of water 1ºC. 1 C = 1000 c P. 446
Typically used to measure the heat (energy) content in foods Calorie (kilocalorie): quantity of heat needed to raise temp. of 1 g of water 1ºC. 1 C = 1000 c P. 446
 British Thermal Unit Describes heat from heating systems Btu: quantity of heat needed to raise 1 lb. of water 1ºF.
British Thermal Unit Describes heat from heating systems Btu: quantity of heat needed to raise 1 lb. of water 1ºF.
 Def. - The amount of heat needed to raise the temperature of one gram of a substance one degree Celsius When thermal heat is transferred (flows) it raises the temp of some substances more than others!
Def. - The amount of heat needed to raise the temperature of one gram of a substance one degree Celsius When thermal heat is transferred (flows) it raises the temp of some substances more than others!
 Water is frequently used as a coolant because of its high specific heat. It takes: 1 calorie of Heat to raise the temp of 1 gram of H 2 O 1°C Equal to. . 4. 186 joules of Heat to raise the temp of 1 gram of H 2 O 1°C
Water is frequently used as a coolant because of its high specific heat. It takes: 1 calorie of Heat to raise the temp of 1 gram of H 2 O 1°C Equal to. . 4. 186 joules of Heat to raise the temp of 1 gram of H 2 O 1°C

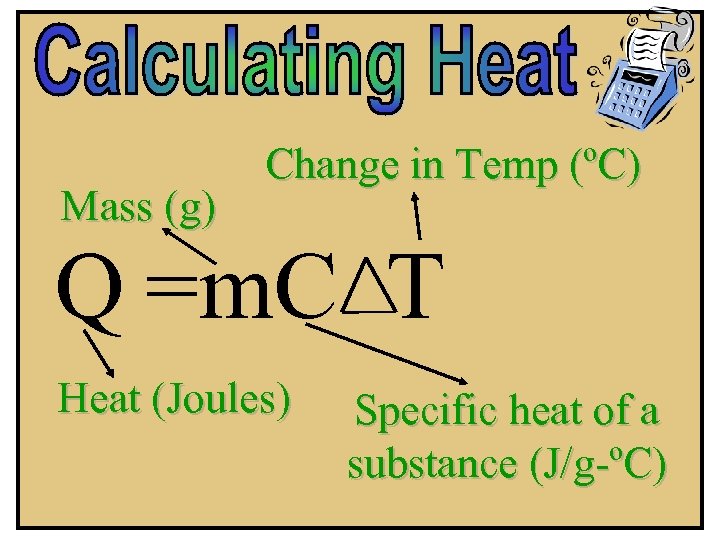 Mass (g) Change in Temp (ºC) Q =m. C T Heat (Joules) Specific heat of a substance (J/g-ºC)
Mass (g) Change in Temp (ºC) Q =m. C T Heat (Joules) Specific heat of a substance (J/g-ºC)
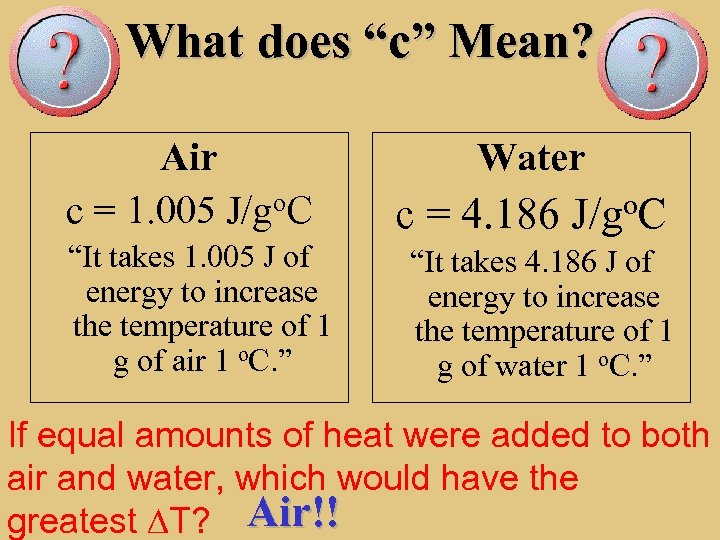 What does “c” Mean? Air c = 1. 005 J/go. C “It takes 1. 005 J of energy to increase the temperature of 1 g of air 1 o. C. ” Water c = 4. 186 J/go. C “It takes 4. 186 J of energy to increase the temperature of 1 g of water 1 o. C. ” If equal amounts of heat were added to both air and water, which would have the greatest DT? Air!!
What does “c” Mean? Air c = 1. 005 J/go. C “It takes 1. 005 J of energy to increase the temperature of 1 g of air 1 o. C. ” Water c = 4. 186 J/go. C “It takes 4. 186 J of energy to increase the temperature of 1 g of water 1 o. C. ” If equal amounts of heat were added to both air and water, which would have the greatest DT? Air!!
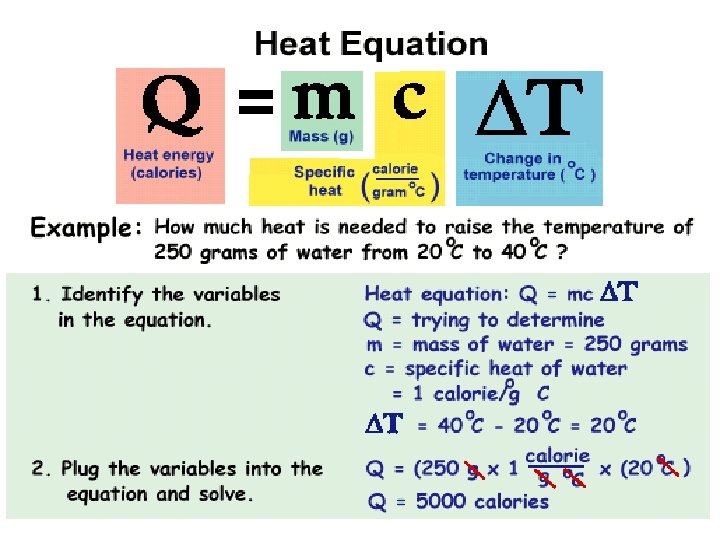
 Calculating Heat 1. An IPC student wants to raise the temperature of a 13 g sample of copper from -30°C to 10°C. If the specific heat of copper is 0. 385 J/g-°C, how much heat needs to be added?
Calculating Heat 1. An IPC student wants to raise the temperature of a 13 g sample of copper from -30°C to 10°C. If the specific heat of copper is 0. 385 J/g-°C, how much heat needs to be added?
 Calculating Heat 2. A 50 g sample of a material required 60 calories of heat to have its temperature raised from 20°C to 80°C. What is the specific heat of the material?
Calculating Heat 2. A 50 g sample of a material required 60 calories of heat to have its temperature raised from 20°C to 80°C. What is the specific heat of the material?
 Calculating Heat 3. A 200 g sample of ethanol is provided with 1500 J of heat energy. What will be the change in temperature of this sample if the specific heat is 2. 44 J/g-°C?
Calculating Heat 3. A 200 g sample of ethanol is provided with 1500 J of heat energy. What will be the change in temperature of this sample if the specific heat is 2. 44 J/g-°C?


