7fa66011747e8ad7d5f84759979a4ee4.ppt
- Количество слайдов: 34

Debate #2: For Now, We Still Need Randomized Controlled Clinical Trials: Pro Jeffrey J. Popma, MD Director, Innovations in Interventional Cardiology Senior Attending Physician Beth Israel Deaconess Medical Center Associate Professor of Medicine Harvard Medical School Boston, MA

DISCLOSURES Jeffrey J. Popma, MD Grants/Contracted Research – Abbott Vascular, Boston Scientific Corporation, Cordis, a Johnson & Johnson company, Medtronic Cardio. Vascular, Inc. Honoraria – Abbott Vascular, Boston Scientific Corporation, Cordis, a Johnson & Johnson company I intend to reference unlabeled/unapproved uses of drugs or devices in my presentation. I intend to reference transcatheter aortic valves.

Disclaimer: I am not a statistician. . Control is required for study validity, to avoid the fallacy of post hoc ergo propter hoc reasoning. All who drink of this treatment recover in a short time; Control is required for study validity, to avoid the fallacy of post hoc ergo propter hoc reasoning. Except those whom it does not help, who all die. It is obvious, therefore, that it fails only in incurable cases. ”
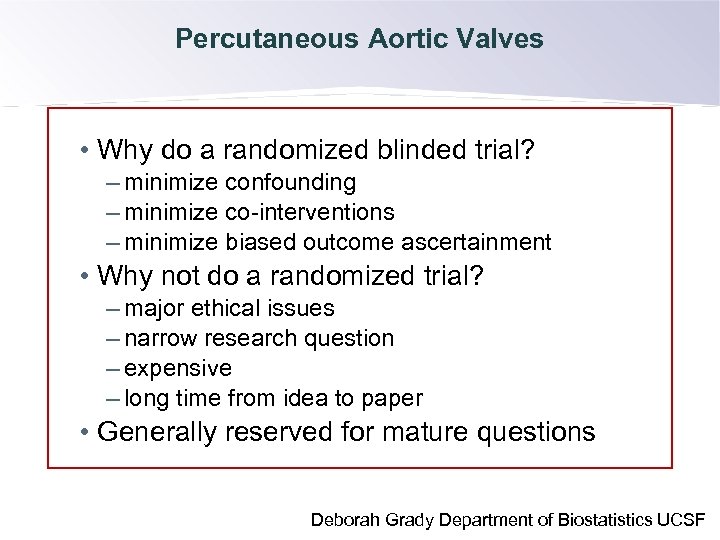
Percutaneous Aortic Valves • Why do a randomized blinded trial? – minimize confounding – minimize co-interventions – minimize biased outcome ascertainment Control is required for study validity, to avoid the fallacy • Why not do a randomized trial? of post hoc ergo propter hoc reasoning. – major ethical issues – narrow research question – expensive – long time from idea to paper • Generally reserved for mature questions Deborah Grady Department of Biostatistics UCSF

Problems with Uncontrolled Trials • When a new treatment comes to be, an adventurous and enthusiastic investigator may initially study this new treatment without any direct comparison to a similar group of patients on more standard therapy. Control is required for study validity, to avoid the fallacy of post hoc ergo propter hoc reasoning. • Uncontrolled trials have the potential to provide a very distorted view of therapy. • Uncontrolled studies are more likely to lead to enthusiastic recommendation of the treatment compared with properly controlled trials. Matthew S. Mayo, Ph. D. Director, Center for Biostatistics and Advanced Informatics Professor, Preventive Medicine and Public Health

Percutaneous Aortic Valves: Why Randomize? § Unique Disease Substrate for Randomization § Surgical Therapy is the “Gold Standard” § Heterogeneous Factors Determine Prognosis § Uncertain 30 Day and One Year Event Rates - Medical therapy, surgery, PAVI § Potential for Confounders § Novel But Disparate Complications § Ethical Dilemna for Non Operative Groups § New PAVI Designs
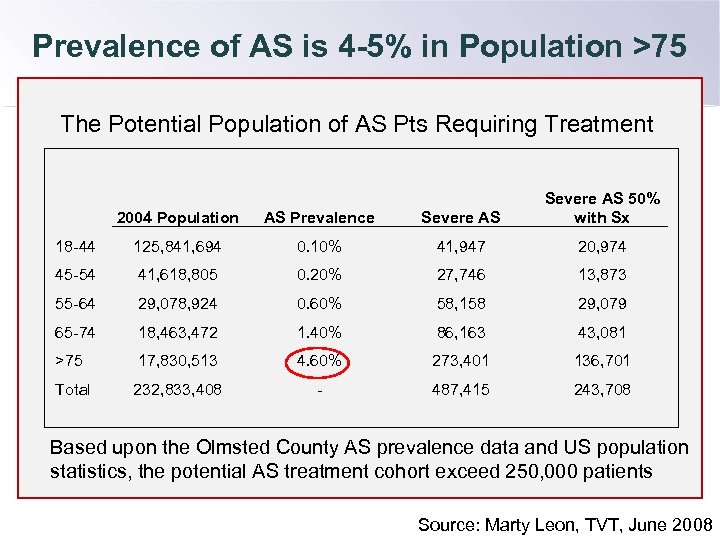
Prevalence of AS is 4 -5% in Population >75 The Potential Population of AS Pts Requiring Treatment 2004 Population AS Prevalence Severe AS 50% with Sx 18 -44 125, 841, 694 0. 10% 41, 947 20, 974 45 -54 41, 618, 805 0. 20% 27, 746 13, 873 55 -64 29, 078, 924 0. 60% 58, 158 29, 079 65 -74 18, 463, 472 1. 40% 86, 163 43, 081 >75 17, 830, 513 4. 60% 273, 401 136, 701 Total 232, 833, 408 - 487, 415 243, 708 Based upon the Olmsted County AS prevalence data and US population statistics, the potential AS treatment cohort exceed 250, 000 patients Source: Marty Leon, TVT, June 2008
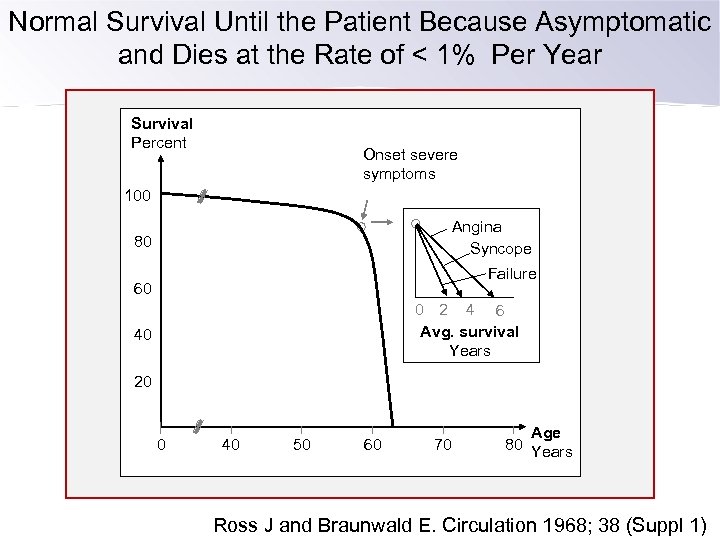
Normal Survival Until the Patient Because Asymptomatic and Dies at the Rate of < 1% Per Year Survival Percent Onset severe symptoms 100 Angina Syncope 80 Failure 60 0 2 4 6 Avg. survival Years 40 20 0 40 50 60 70 Age 80 Years Ross J and Braunwald E. Circulation 1968; 38 (Suppl 1)
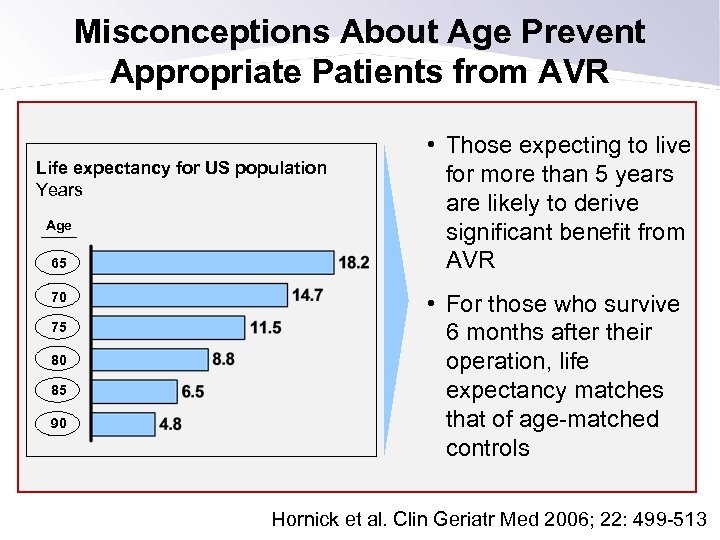
Misconceptions About Age Prevent Appropriate Patients from AVR Life expectancy for US population Years Age 65 70 75 80 85 90 • Those expecting to live for more than 5 years are likely to derive significant benefit from AVR • For those who survive 6 months after their operation, life expectancy matches that of age-matched controls Hornick et al. Clin Geriatr Med 2006; 22: 499 -513

Percutaneous Aortic Valves: Why Randomize? § Unique Disease Substrate for Randomization § Surgical Therapy is the “Gold Standard” § Heterogeneous Factors Determine Prognosis § Uncertain 30 Day and One Year Event Rates - Medical therapy, surgery, PAVI § Potential for Confounders § Novel But Disparate Complications § Ethical Dilemna for Non Operative Groups § New PAVI Designs
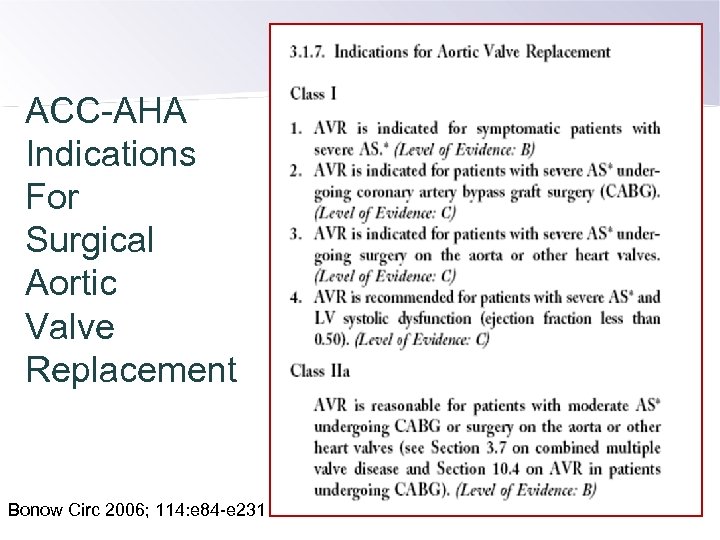
ACC-AHA Indications For Surgical Aortic Valve Replacement Bonow Circ 2006; 114: e 84 -e 231
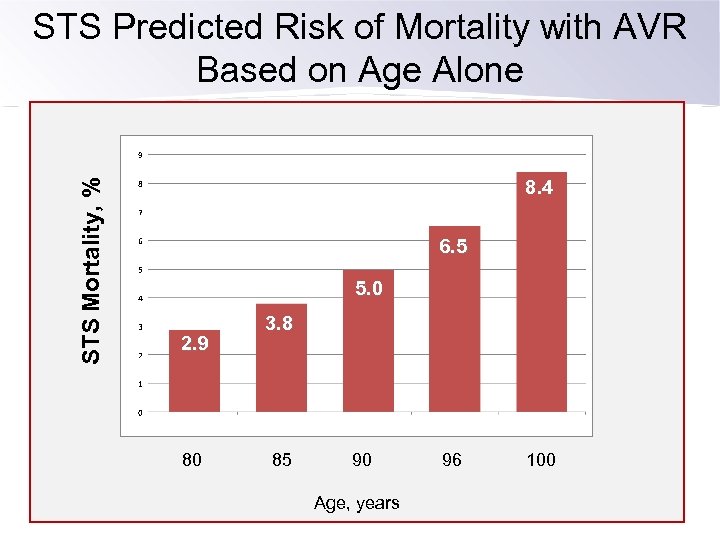
STS Mortality, % STS Predicted Risk of Mortality with AVR Based on Age Alone 8. 4 6. 5 5. 0 2. 9 80 3. 8 85 90 Age, years 96 100

Don’t Mess With the Guidelines – or the Surgeons

Percutaneous Aortic Valves: Why Randomize? § Unique Disease Substrate for Randomization § Surgical Therapy is the “Gold Standard” § Heterogeneous Factors Determine Prognosis § Uncertain 30 Day and One Year Event Rates - Medical therapy, surgery, PAVI § Potential for Confounders § Novel But Disparate Complications § Ethical Dilemna for Non Operative Groups § New PAVI Designs
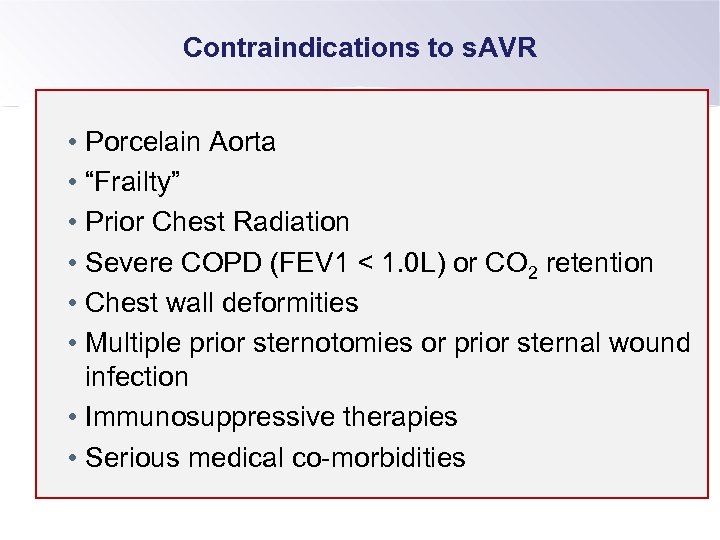
Contraindications to s. AVR • Porcelain Aorta • “Frailty” • Prior Chest Radiation • Severe COPD (FEV 1 < 1. 0 L) or CO 2 retention • Chest wall deformities • Multiple prior sternotomies or prior sternal wound infection • Immunosuppressive therapies • Serious medical co-morbidities
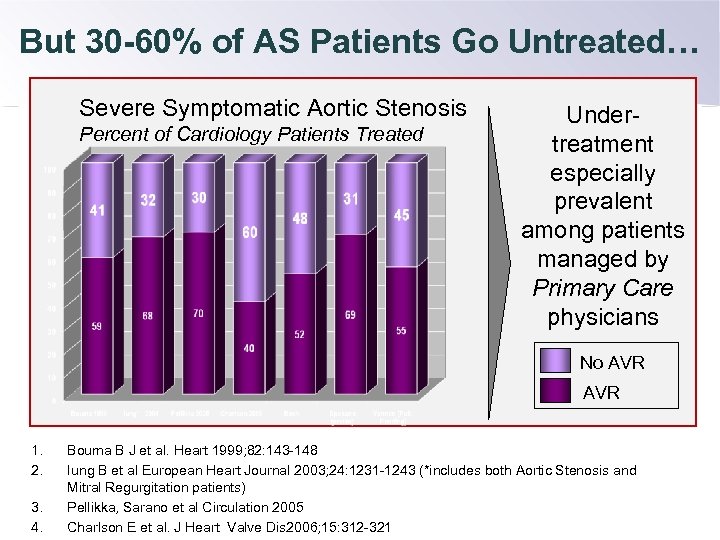
But 30 -60% of AS Patients Go Untreated… Severe Symptomatic Aortic Stenosis Percent of Cardiology Patients Treated Undertreatment especially prevalent among patients managed by Primary Care physicians No AVR 1. 2. 3. 4. Bouma B J et al. Heart 1999; 82: 143 -148 Iung B et al European Heart Journal 2003; 24: 1231 -1243 (*includes both Aortic Stenosis and Mitral Regurgitation patients) Pellikka, Sarano et al Circulation 2005 Charlson E et al. J Heart Valve Dis 2006; 15: 312 -321

Percutaneous Aortic Valves: Why Randomize? § Unique Disease Substrate for Randomization § Surgical Therapy is the “Gold Standard” § Heterogeneous Factors Determine Prognosis § Uncertain 30 Day and One Year Event Rates - Medical therapy, surgery, PAVI § Potential for Confounders § Novel But Disparate Complications § Ethical Dilemna for Non Operative Groups § New PAVI Designs
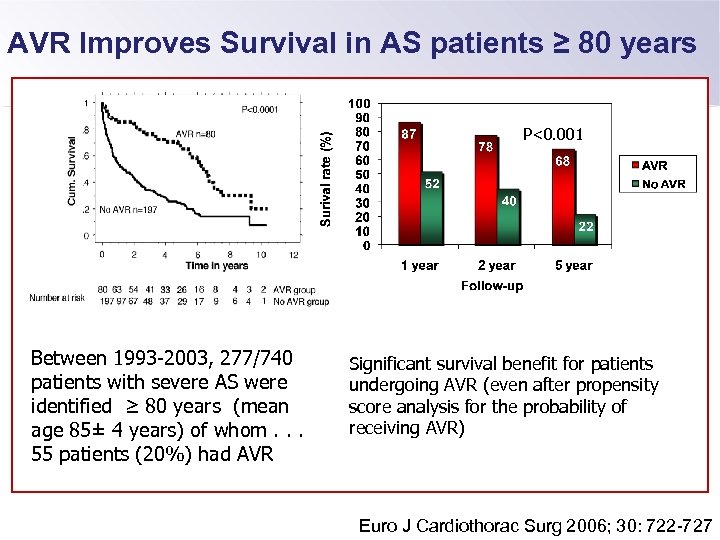
AVR Improves Survival in AS patients ≥ 80 years P<0. 001 Between 1993 -2003, 277/740 patients with severe AS were identified ≥ 80 years (mean age 85± 4 years) of whom. . . 55 patients (20%) had AVR Significant survival benefit for patients undergoing AVR (even after propensity score analysis for the probability of receiving AVR) Euro J Cardiothorac Surg 2006; 30: 722 -727
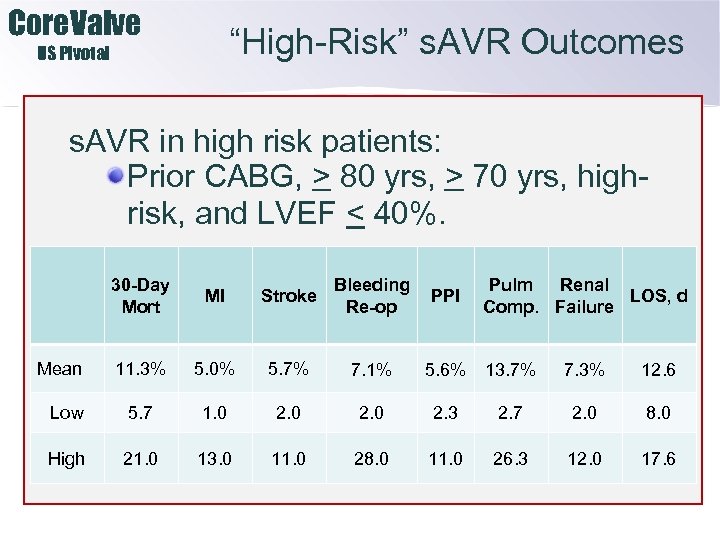
Core. Valve “High-Risk” s. AVR Outcomes US Pivotal s. AVR in high risk patients: Prior CABG, > 80 yrs, > 70 yrs, highrisk, and LVEF < 40%. 30 -Day Mort MI Stroke Bleeding Re-op 11. 3% 5. 0% 5. 7% 7. 1% Low 5. 7 1. 0 2. 3 High 21. 0 13. 0 11. 0 28. 0 11. 0 Mean PPI Pulm Renal LOS, d Comp. Failure 5. 6% 13. 7% 7. 3% 12. 6 2. 7 2. 0 8. 0 26. 3 12. 0 17. 6
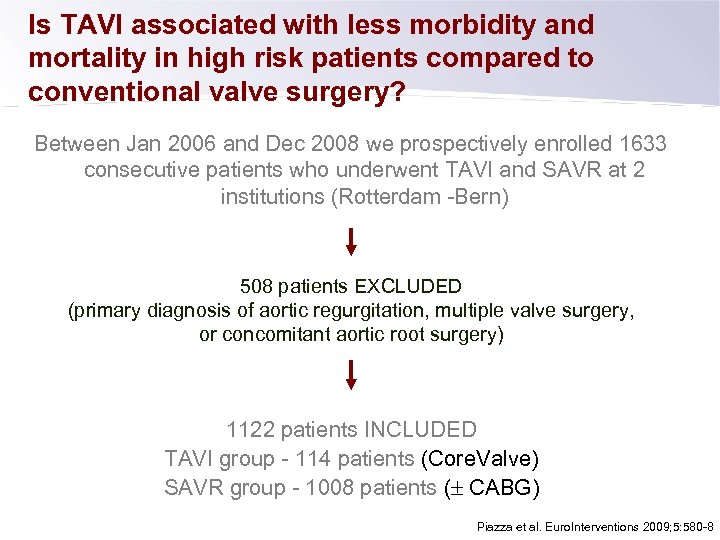
Is TAVI associated with less morbidity and mortality in high risk patients compared to conventional valve surgery? Between Jan 2006 and Dec 2008 we prospectively enrolled 1633 consecutive patients who underwent TAVI and SAVR at 2 institutions (Rotterdam -Bern) 508 patients EXCLUDED (primary diagnosis of aortic regurgitation, multiple valve surgery, or concomitant aortic root surgery) 1122 patients INCLUDED TAVI group - 114 patients (Core. Valve) SAVR group - 1008 patients ( CABG) Piazza et al. Euro. Interventions 2009; 5: 580 -8
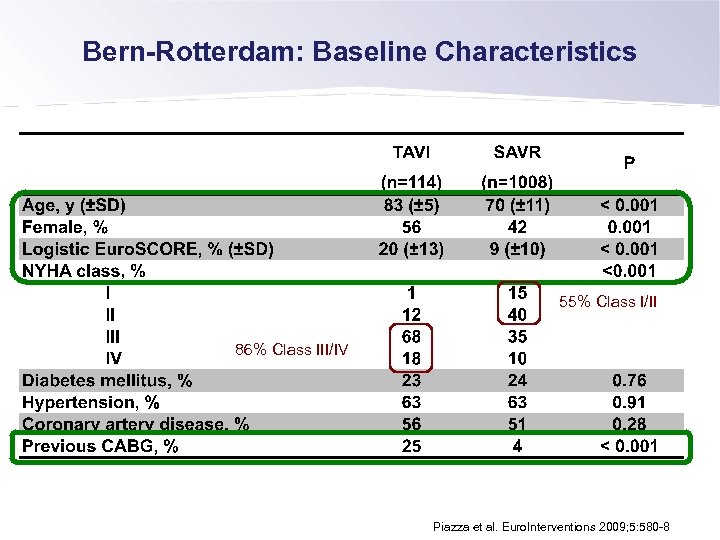
Bern-Rotterdam: Baseline Characteristics 55% Class I/II 86% Class III/IV Piazza et al. Euro. Interventions 2009; 5: 580 -8
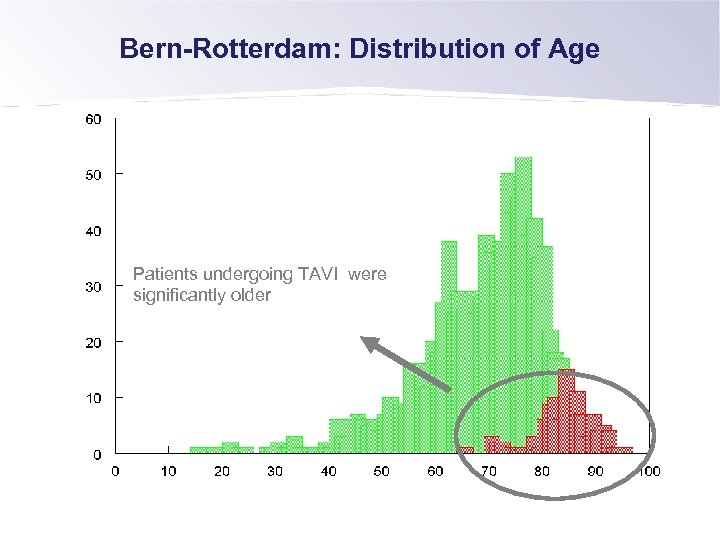
Bern-Rotterdam: Distribution of Age Patients undergoing TAVI were significantly older
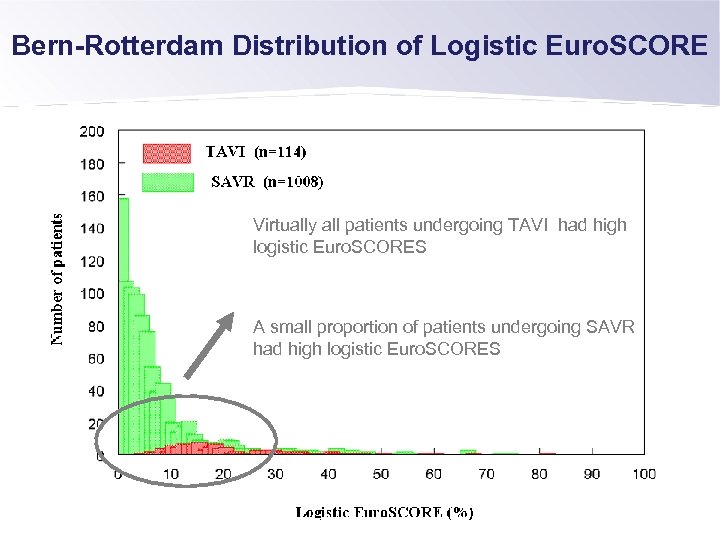
Bern-Rotterdam Distribution of Logistic Euro. SCORE Virtually all patients undergoing TAVI had high logistic Euro. SCORES A small proportion of patients undergoing SAVR had high logistic Euro. SCORES
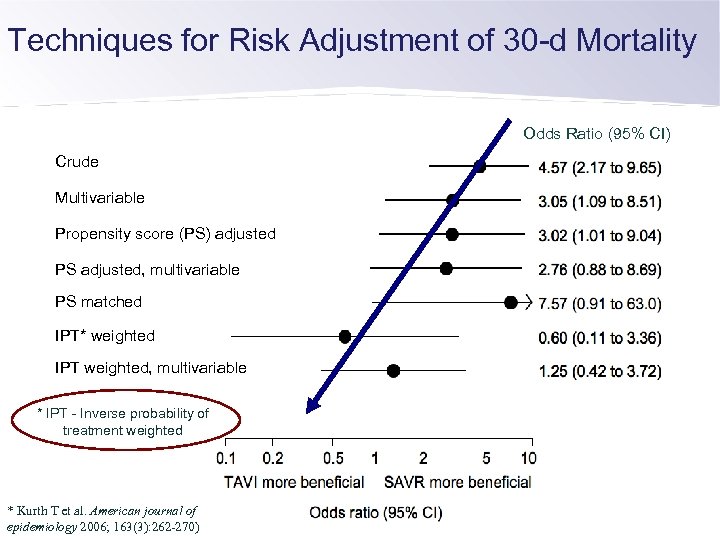
Techniques for Risk Adjustment of 30 -d Mortality Odds Ratio (95% CI) Crude Multivariable Propensity score (PS) adjusted PS adjusted, multivariable PS matched IPT* weighted IPT weighted, multivariable * IPT - Inverse probability of treatment weighted * Kurth T et al. American journal of epidemiology 2006; 163(3): 262 -270)

Bern-Rotterdam: Conclusion Based on these results and the currently available data, there is a need for a randomized controlled trial Furthermore, if the practice does not become evidenced-based and taking into account health economic factors, TAVI will not be considered legitimate or justified in the eyes of the health authorities and therefore not reimbursed.

Percutaneous Aortic Valves: Why Randomize? § Unique Disease Substrate for Randomization § Surgical Therapy is the “Gold Standard” § Heterogeneous Factors Determine Prognosis § Uncertain 30 Day and One Year Event Rates - Medical therapy, surgery, PAVI § Potential for Confounders § Novel But Disparate Complications § Ethical Dilemna for Non Operative Groups § New PAVI Designs
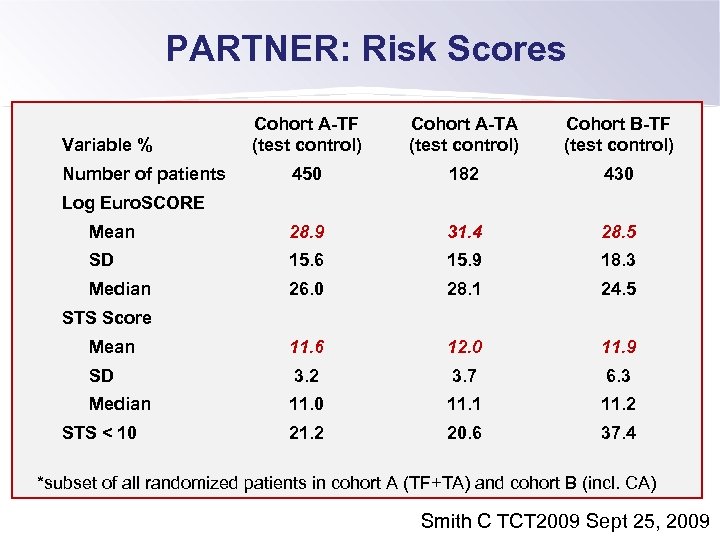
PARTNER: Risk Scores Cohort A-TF (test control) Cohort A-TA (test control) Cohort B-TF (test control) 450 182 430 Mean 28. 9 31. 4 28. 5 SD 15. 6 15. 9 18. 3 Median 26. 0 28. 1 24. 5 Mean 11. 6 12. 0 11. 9 SD 3. 2 3. 7 6. 3 Median 11. 0 11. 1 11. 2 20. 6 37. 4 Variable % Number of patients Log Euro. SCORE STS Score STS < 10 *subset of all randomized patients in cohort A (TF+TA) and cohort B (incl. CA) Smith C TCT 2009 Sept 25, 2009

Percutaneous Aortic Valves: Why Randomize? § Unique Disease Substrate for Randomization § Surgical Therapy is the “Gold Standard” § Heterogeneous Factors Determine Prognosis § Uncertain 30 Day and One Year Event Rates - Medical therapy, surgery, PAVI § Potential for Confounders § Novel But Disparate Complications § Ethical Dilemna for Non Operative Groups § New PAVI Designs
Core. Valve US Pivotal Study Endpoint: All Cause Mortality Study population has severe co-morbidities that predispose to non-cardiac mortality at one year – so there are challenges in identifying the specific effect of the valve treatment vs. the “noise” of the co-morbidities in these high risk populations • Time: 24 hours, 30 days, 90 days, 12+ months • Place: OR, in Hospital, Post-discharge • Type: Cardiovascular, and non cardiovascular, valve related 29 | MDT Confidential 29 Title | Description
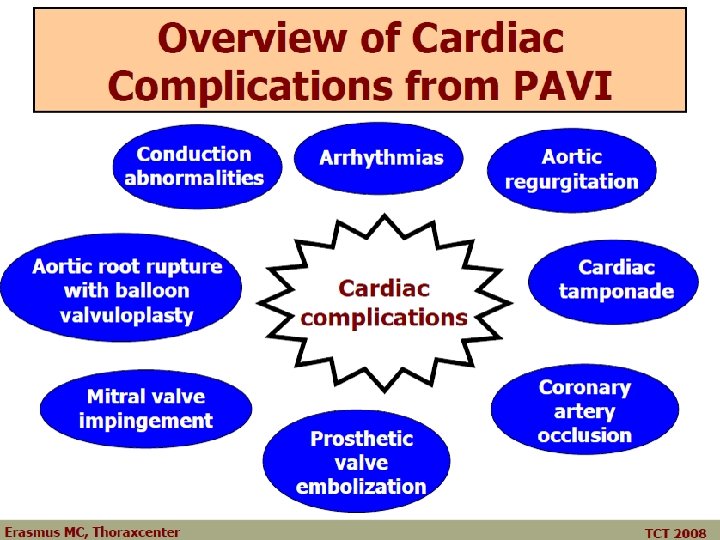

Percutaneous Aortic Valves: Why Randomize? § Unique Disease Substrate for Randomization § Surgical Therapy is the “Gold Standard” § Heterogeneous Factors Determine Prognosis § Uncertain 30 Day and One Year Event Rates - Medical therapy, surgery, PAVI § Potential for Confounders § Novel But Disparate Complications § Ethical Dilemna for Non Operative Groups § New PAVI Designs
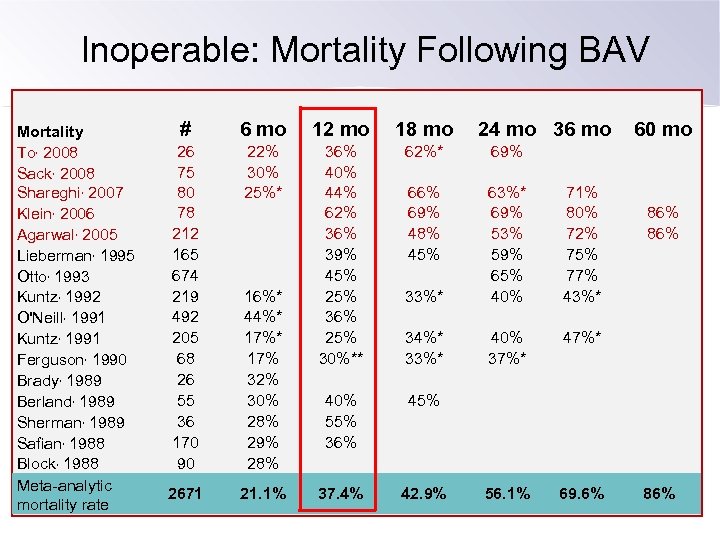
Inoperable: Mortality Following BAV Mortality To, 2008 Sack, 2008 Shareghi, 2007 Klein, 2006 Agarwal, 2005 Lieberman, 1995 Otto, 1993 Kuntz, 1992 O'Neill, 1991 Kuntz, 1991 Ferguson, 1990 Brady, 1989 Berland, 1989 Sherman, 1989 Safian, 1988 Block, 1988 Meta-analytic mortality rate # 6 mo 12 mo 18 mo 26 75 80 78 212 165 674 219 492 205 68 26 55 36 170 90 22% 30% 25%* 36% 40% 44% 62% 36% 39% 45% 25% 36% 25% 30%** 62%* 69% 66% 69% 48% 45% 33%* 69% 53% 59% 65% 40% 71% 80% 72% 75% 77% 43%* 34%* 33%* 40% 37%* 40% 55% 36% 45% 2671 21. 1% 37. 4% 42. 9% 56. 1% 69. 6% 16%* 44%* 17% 32% 30% 28% 29% 28% 24 mo 36 mo 60 mo 86% 86%

Percutaneous Aortic Valves: Why Randomize? § Unique Disease Substrate for Randomization § Surgical Therapy is the “Gold Standard” § Heterogeneous Factors Determine Prognosis § Uncertain 30 Day and One Year Event Rates - Medical therapy, surgery, PAVI § Potential for Confounders § Novel But Disparate Complications § Ethical Dilemna for Non Operative Groups § Clinical Equipoise for New PAVI Designs
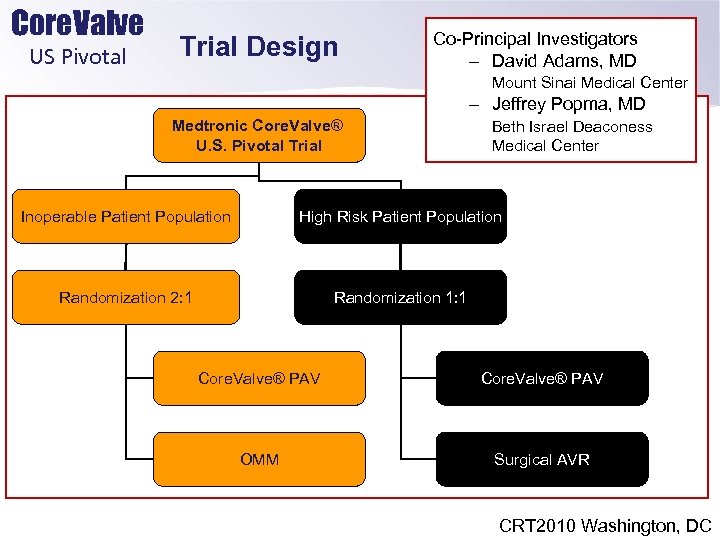
Core. Valve US Pivotal Trial Design Co-Principal Investigators – David Adams, MD Mount Sinai Medical Center – Jeffrey Popma, MD Medtronic Core. Valve® U. S. Pivotal Trial Beth Israel Deaconess Medical Center Inoperable Patient Population High Risk Patient Population Randomization 2: 1 Randomization 1: 1 Core. Valve® PAV OMM Surgical AVR CRT 2010 Washington, DC
7fa66011747e8ad7d5f84759979a4ee4.ppt