137f281442341eb7bd84856a9d111dc4.ppt
- Количество слайдов: 32
de Broglie & “matter waves” de Broglie argued…since light can display wave & particle properties, then matter can also be both a particle and a wave A normal wave is continuous But a photon or free moving electron, can be thought of as a wave packet, having both wave-like properties and also the single position & size associated with a particle Linked 1 Link 2
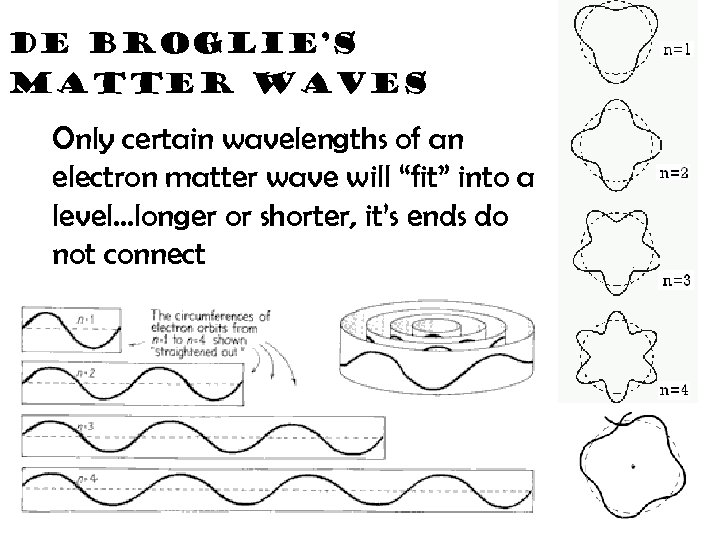
de Broglie’s matter waves Only certain wavelengths of an electron matter wave will “fit” into a level…longer or shorter, it’s ends do not connect
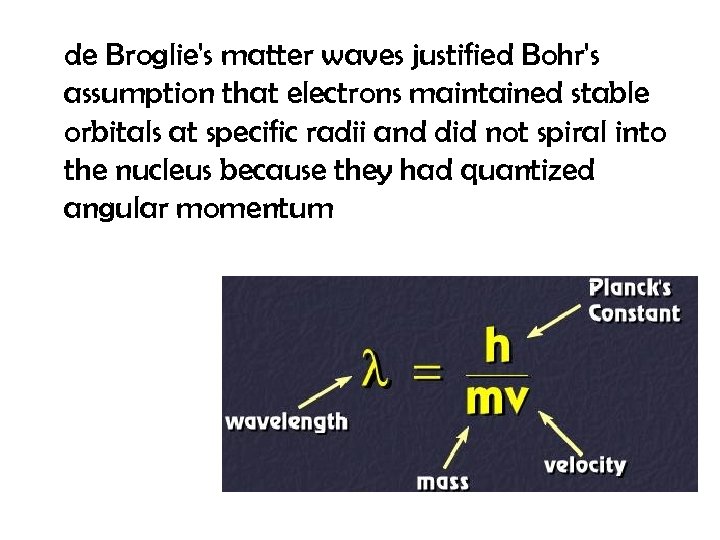
de Broglie's matter waves justified Bohr's assumption that electrons maintained stable orbitals at specific radii and did not spiral into the nucleus because they had quantized angular momentum
Practice • What is the wavelength of an electron moving with a speed of 5. 97 x 106 m/s? (mass of e- = 9. 11 x 10 -28 g. ) • Protons can be accelerated to speeds of near that of light in particle accelerators. Estimate the wavelength (in nm) of such a proton moving at 2. 90 x 108 m/s. (mass of proton = 1. 675 x 10 -24 g)
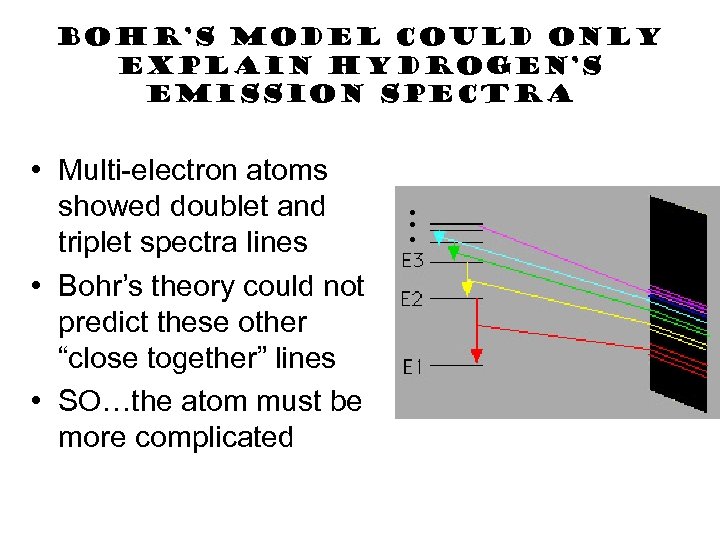
Bohr’s Model could only explain Hydrogen’s emission spectra • Multi-electron atoms showed doublet and triplet spectra lines • Bohr’s theory could not predict these other “close together” lines • SO…the atom must be more complicated
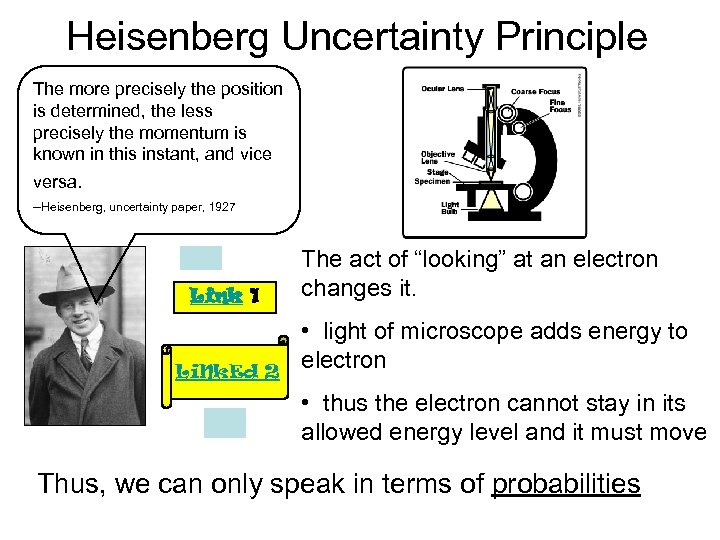
Heisenberg Uncertainty Principle The more precisely the position is determined, the less precisely the momentum is known in this instant, and vice versa. --Heisenberg, uncertainty paper, 1927 Link 1 Li. Nk. Ed 2 The act of “looking” at an electron changes it. • light of microscope adds energy to electron • thus the electron cannot stay in its allowed energy level and it must move Thus, we can only speak in terms of probabilities
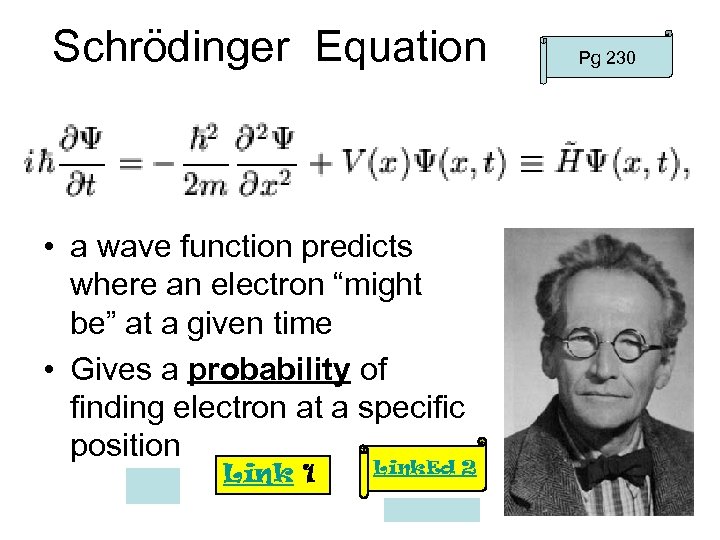
Schrödinger Equation • a wave function predicts where an electron “might be” at a given time • Gives a probability of finding electron at a specific position Link 1 Link. Ed 2 Pg 230
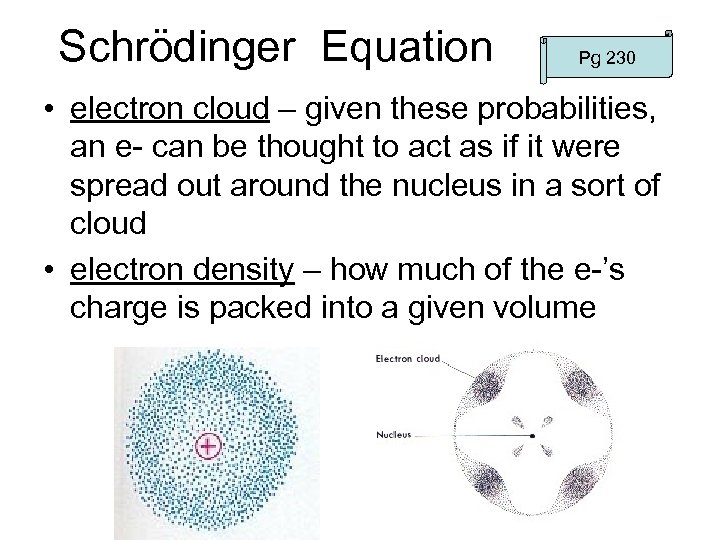
Schrödinger Equation Pg 230 • electron cloud – given these probabilities, an e- can be thought to act as if it were spread out around the nucleus in a sort of cloud • electron density – how much of the e-’s charge is packed into a given volume
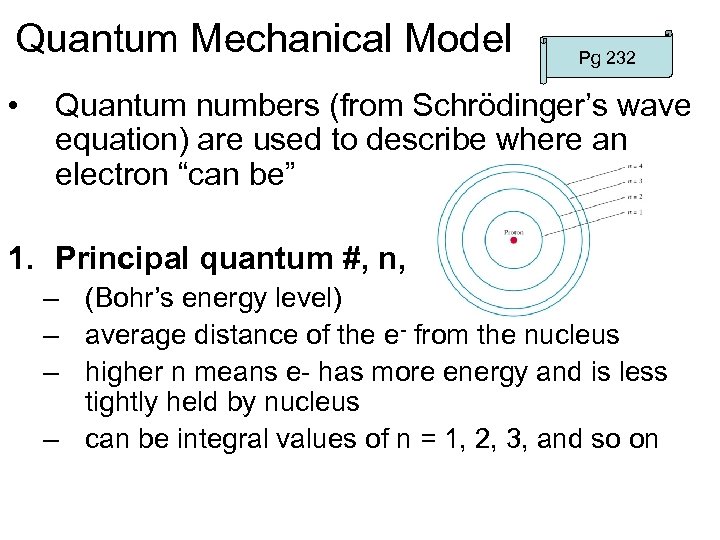
Quantum Mechanical Model • Pg 232 Quantum numbers (from Schrödinger’s wave equation) are used to describe where an electron “can be” 1. Principal quantum #, n, – (Bohr’s energy level) – average distance of the e- from the nucleus – higher n means e- has more energy and is less tightly held by nucleus – can be integral values of n = 1, 2, 3, and so on
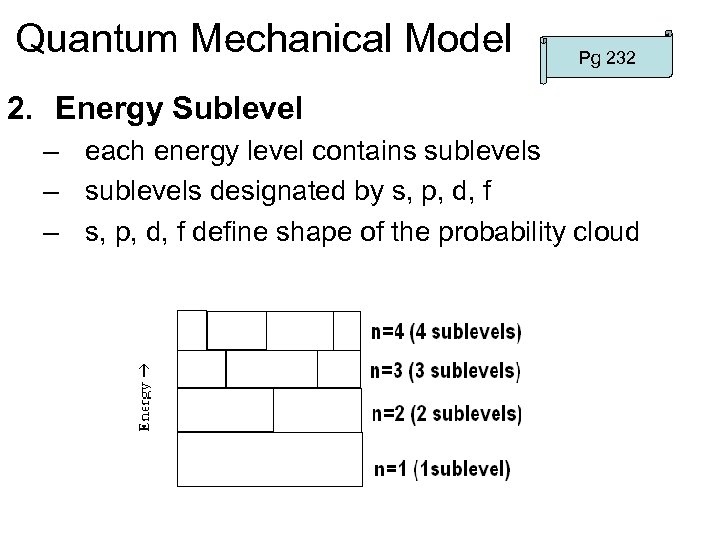
Quantum Mechanical Model Pg 232 2. Energy Sublevel – each energy level contains sublevels – sublevels designated by s, p, d, f – s, p, d, f define shape of the probability cloud
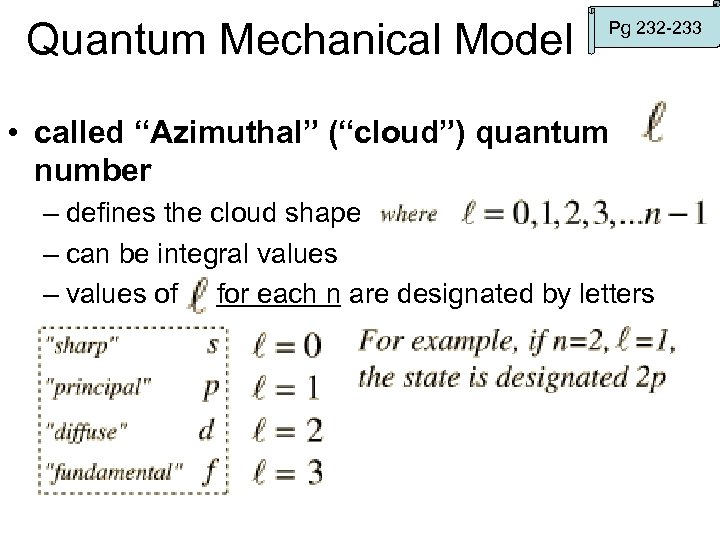
Quantum Mechanical Model Pg 232 -233 • called “Azimuthal” (“cloud”) quantum number – defines the cloud shape – can be integral values – values of for each n are designated by letters
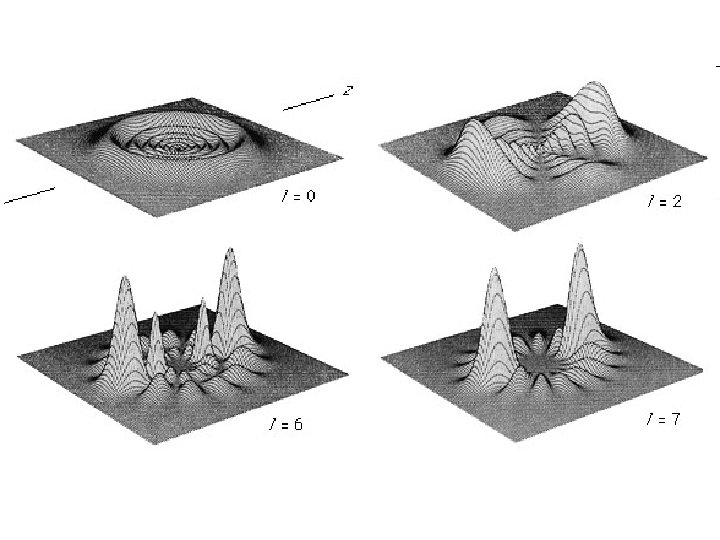
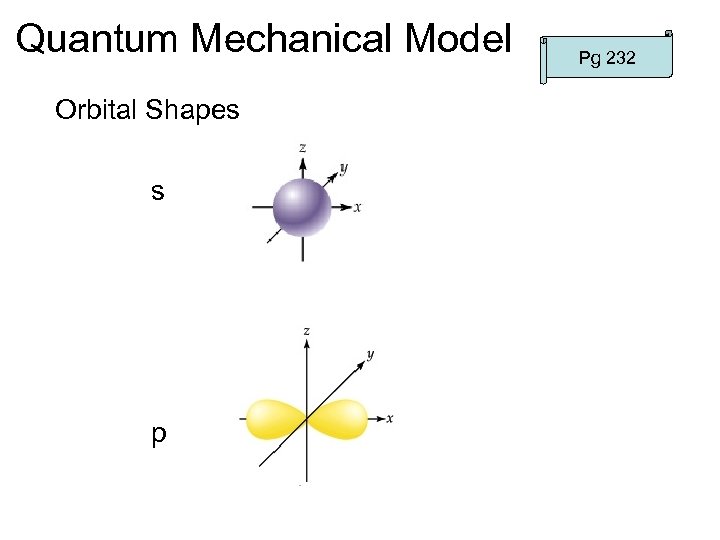
Quantum Mechanical Model Orbital Shapes s p Pg 232
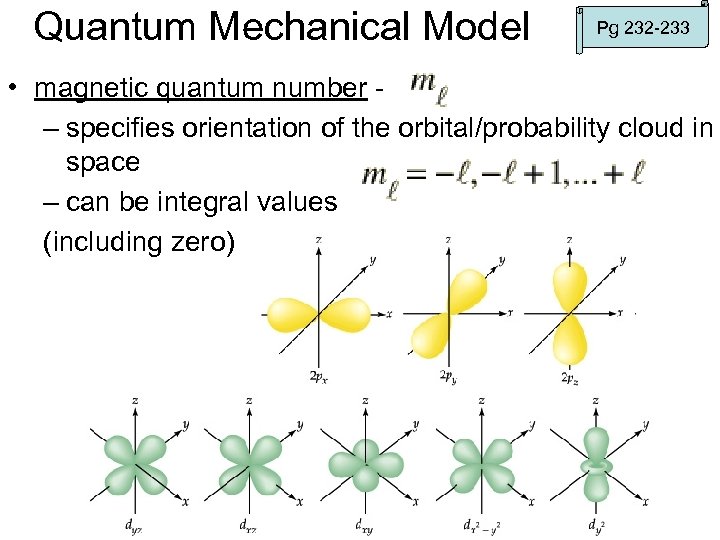
Quantum Mechanical Model Pg 232 -233 • magnetic quantum number – specifies orientation of the orbital/probability cloud in space – can be integral values (including zero)
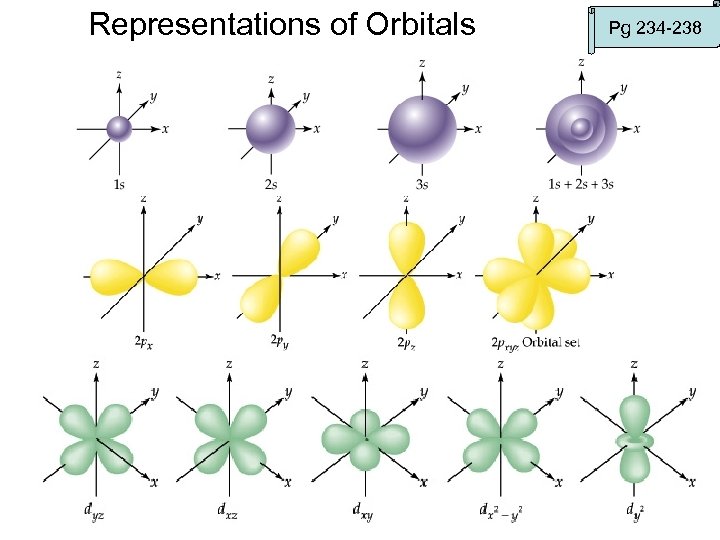
Representations of Orbitals Pg 234 -238
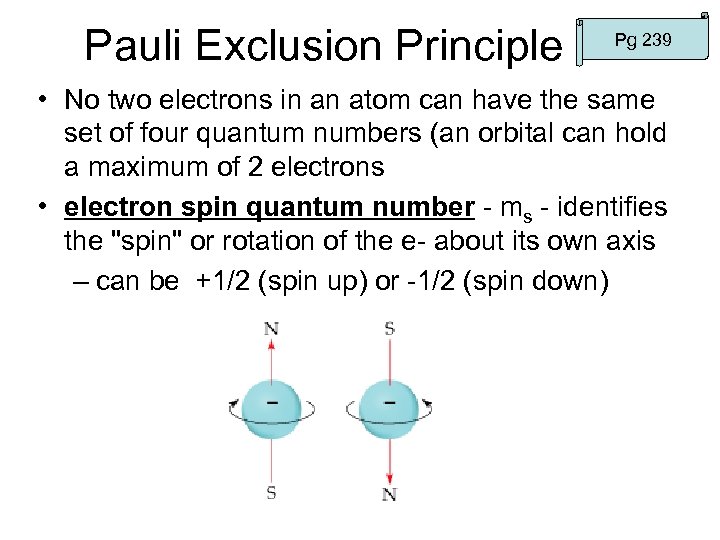
Pauli Exclusion Principle Pg 239 • No two electrons in an atom can have the same set of four quantum numbers (an orbital can hold a maximum of 2 electrons • electron spin quantum number - ms - identifies the "spin" or rotation of the e- about its own axis – can be +1/2 (spin up) or -1/2 (spin down)
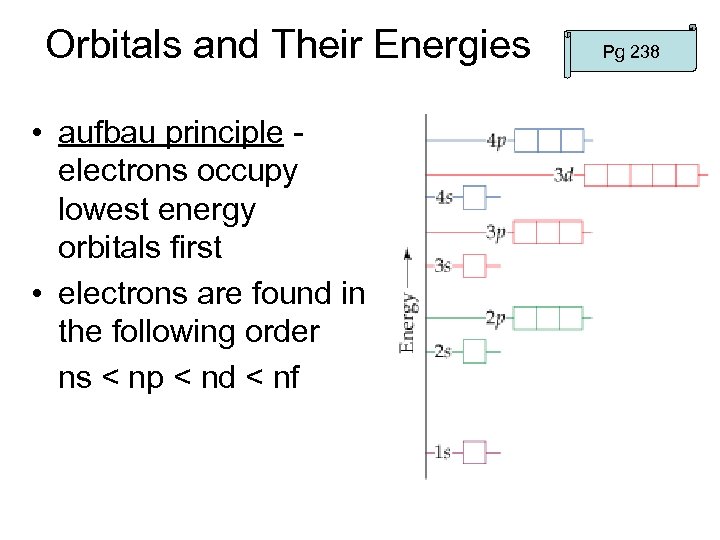
Orbitals and Their Energies • aufbau principle electrons occupy lowest energy orbitals first • electrons are found in the following order ns < np < nd < nf Pg 238
Practice • What is the distinction between “orbit” and “orbital”? • Which orbital in each of the following has the higher energy? a) 2 s or 3 s b) 2 px or 3 px c) 2 px or 2 py • State the maximum number of electrons that can occupy the following: a) 1 s b) 2 p c) 3 d d) 4 f
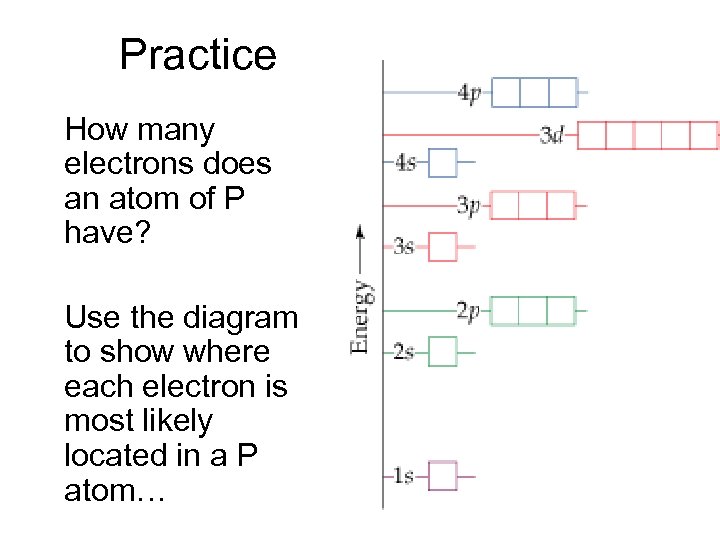
Practice How many electrons does an atom of P have? Use the diagram to show where each electron is most likely located in a P atom…
If it were a house model…where will the electrons live?

Hund’s Rule Hund’s rule – for orbitals of equal energy, the lowest energy is attained when the number of electrons with the same spin is maximized – “Put one electron in each orbital before you start doubling up…” Pg 242 -243
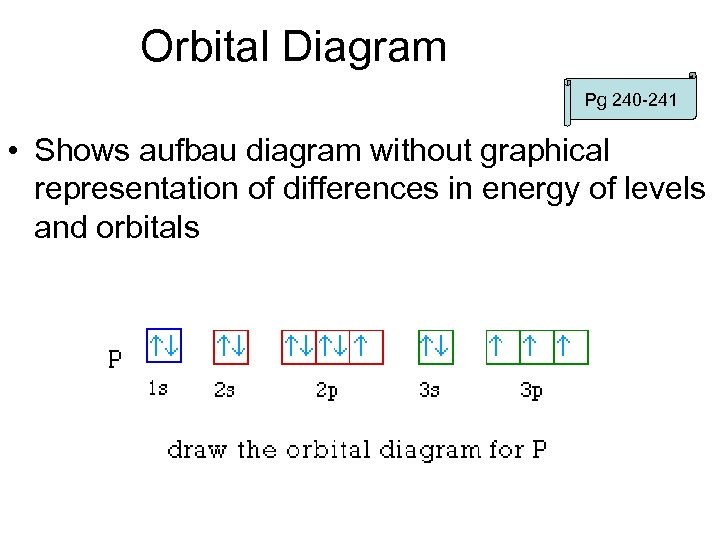
Orbital Diagram Pg 240 -241 • Shows aufbau diagram without graphical representation of differences in energy of levels and orbitals
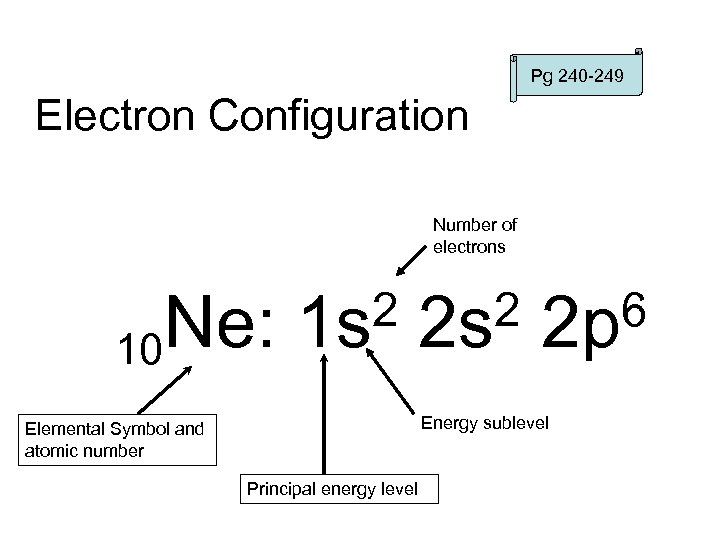
Pg 240 -249 Electron Configuration Number of electrons Ne: 10 2 1 s 2 2 s 6 2 p Energy sublevel Elemental Symbol and atomic number Principal energy level
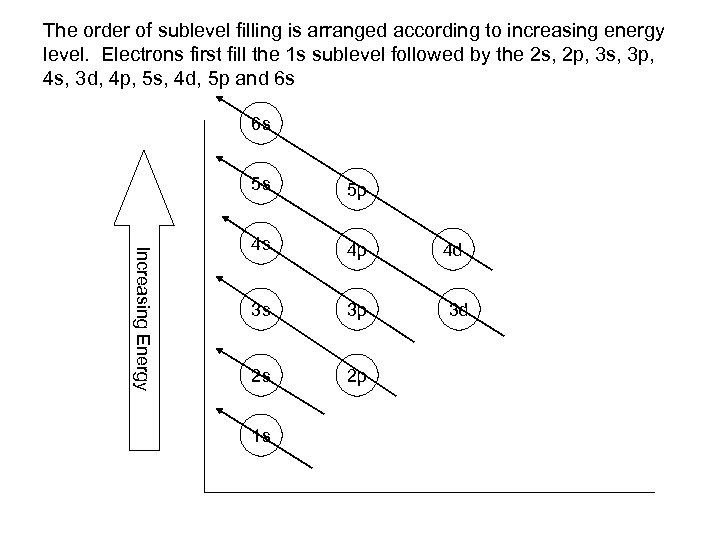
The order of sublevel filling is arranged according to increasing energy level. Electrons first fill the 1 s sublevel followed by the 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, 5 p and 6 s 6 s Increasing Energy 5 s 5 p 4 s 4 p 3 s 3 p 2 s 2 p 1 s 4 d 3 d
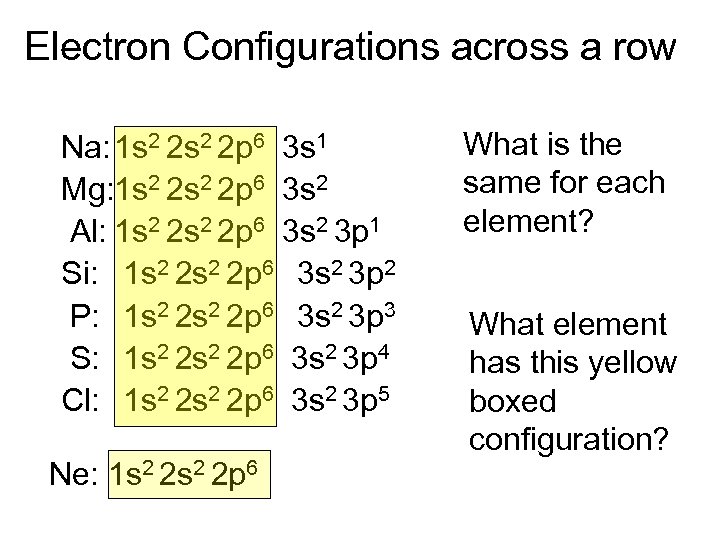
Electron Configurations across a row Na: 1 s 2 2 p 6 3 s 1 Mg: 1 s 2 2 p 6 3 s 2 Al: 1 s 2 2 p 6 3 s 2 3 p 1 Si: 1 s 2 2 p 6 3 s 2 3 p 2 P: 1 s 2 2 p 6 3 s 2 3 p 3 S: 1 s 2 2 p 6 3 s 2 3 p 4 Cl: 1 s 2 2 p 6 3 s 2 3 p 5 Ne: 1 s 2 2 p 6 What is the same for each element? What element has this yellow boxed configuration?
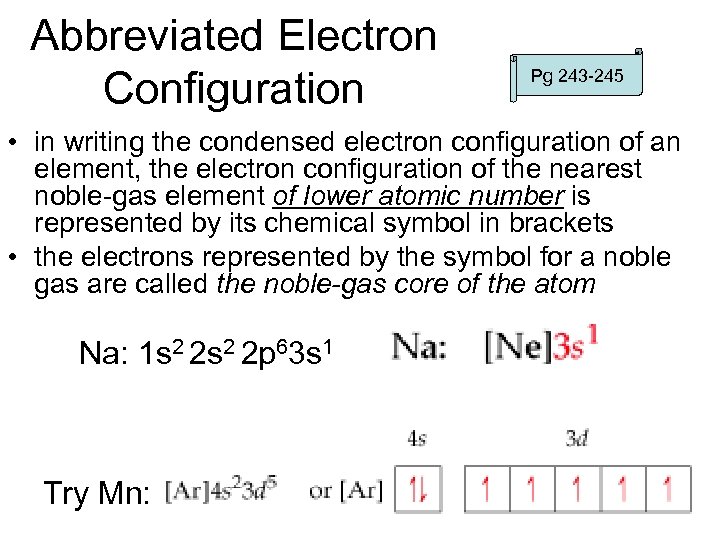
Abbreviated Electron Configuration Pg 243 -245 • in writing the condensed electron configuration of an element, the electron configuration of the nearest noble-gas element of lower atomic number is represented by its chemical symbol in brackets • the electrons represented by the symbol for a noble gas are called the noble-gas core of the atom Na: 1 s 2 2 p 63 s 1 Try Mn:
What part of a car is damaged in a fender bender?
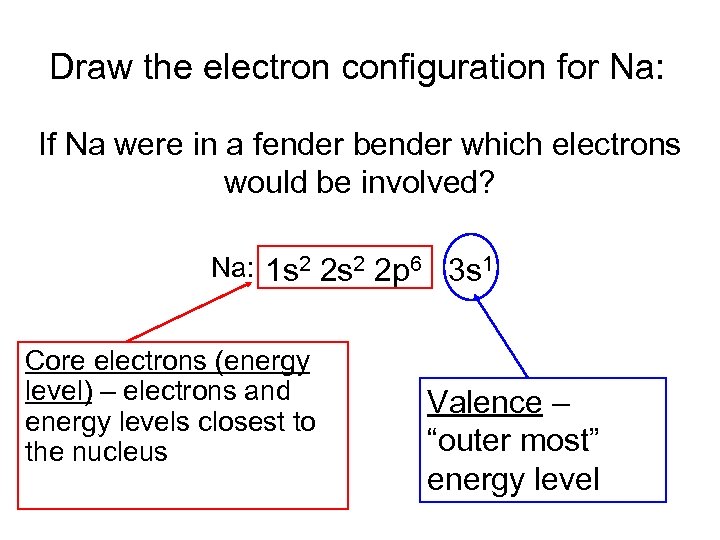
Draw the electron configuration for Na: If Na were in a fender bender which electrons would be involved? Na: 1 s 2 2 p 6 3 s 1 Core electrons (energy level) – electrons and energy levels closest to the nucleus Valence – “outer most” energy level
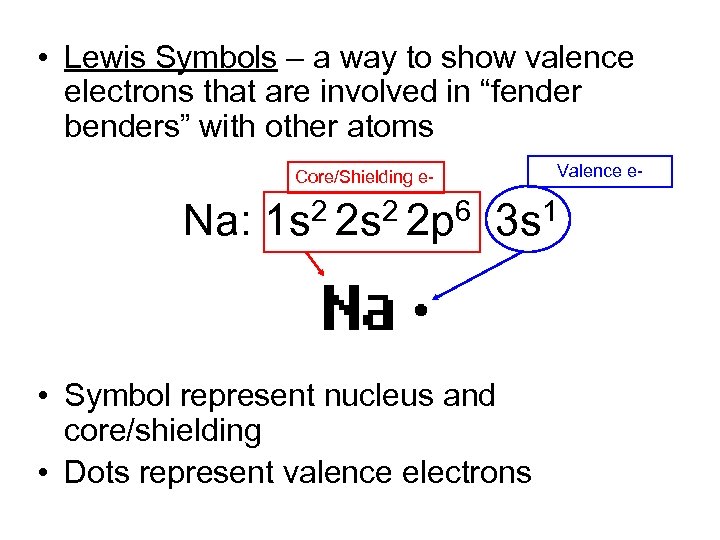
• Lewis Symbols – a way to show valence electrons that are involved in “fender benders” with other atoms Core/Shielding e- Valence e- Na: 1 s 2 2 p 6 3 s 1 • Symbol represent nucleus and core/shielding • Dots represent valence electrons
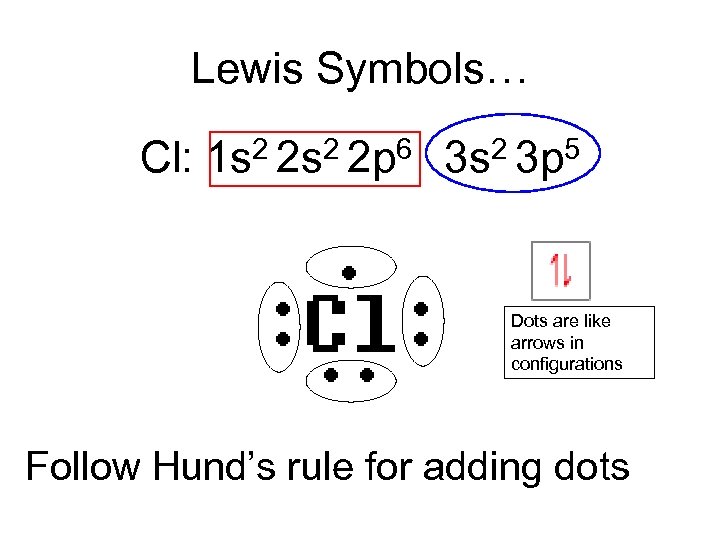
Lewis Symbols… Cl: 2 2 s 2 2 p 6 1 s 2 3 p 5 3 s Dots are like arrows in configurations Follow Hund’s rule for adding dots
Practice – use electron configuration to write Lewis symbols for the following Be Rb Si Te P As
137f281442341eb7bd84856a9d111dc4.ppt