0206b96cf1a57462fb0ff7d4dec5beb6.ppt
- Количество слайдов: 30
 Date: May 06, 2012
Date: May 06, 2012
 Introduction : §Industry that produces chlorine (Cl 2) and sodium hydroxide (Na. OH) or potassium hydroxide (KOH) –Chlor-alkali industry. § sodium hydroxide today are used to chemical industry, metallurgy, alumina/aluminium industry, pulp and paper industry, textile industry, soaps, surfactants, water treatment and consumer products. §Chlorine is largely used to produce PVC, , chloromethane, phosgene, trichloroethene, tetrachloroethene, oxygenated derivatives, sodium hypochlorite, hydrochloric acid, metal chlorides, bromine . Direct usage of chlorine is limited to pulp, paper industry and in waste water treatment.
Introduction : §Industry that produces chlorine (Cl 2) and sodium hydroxide (Na. OH) or potassium hydroxide (KOH) –Chlor-alkali industry. § sodium hydroxide today are used to chemical industry, metallurgy, alumina/aluminium industry, pulp and paper industry, textile industry, soaps, surfactants, water treatment and consumer products. §Chlorine is largely used to produce PVC, , chloromethane, phosgene, trichloroethene, tetrachloroethene, oxygenated derivatives, sodium hypochlorite, hydrochloric acid, metal chlorides, bromine . Direct usage of chlorine is limited to pulp, paper industry and in waste water treatment.
 Manufacture of Na. OH • Previously made by Causticization of soda ash with lime • Only 10% Na. OH solution obtained • Electrolysis of Brine – Most popular method adopted nowadays. Chlorine at Anode; Hydrogen along with alkali hydroxide at cathode. Three types of cell exist: • Mercury Cell • Diaphragm Cell • Membrane Cell Raw Materials 1. Brine (Na. Cl) 2. Electricity
Manufacture of Na. OH • Previously made by Causticization of soda ash with lime • Only 10% Na. OH solution obtained • Electrolysis of Brine – Most popular method adopted nowadays. Chlorine at Anode; Hydrogen along with alkali hydroxide at cathode. Three types of cell exist: • Mercury Cell • Diaphragm Cell • Membrane Cell Raw Materials 1. Brine (Na. Cl) 2. Electricity
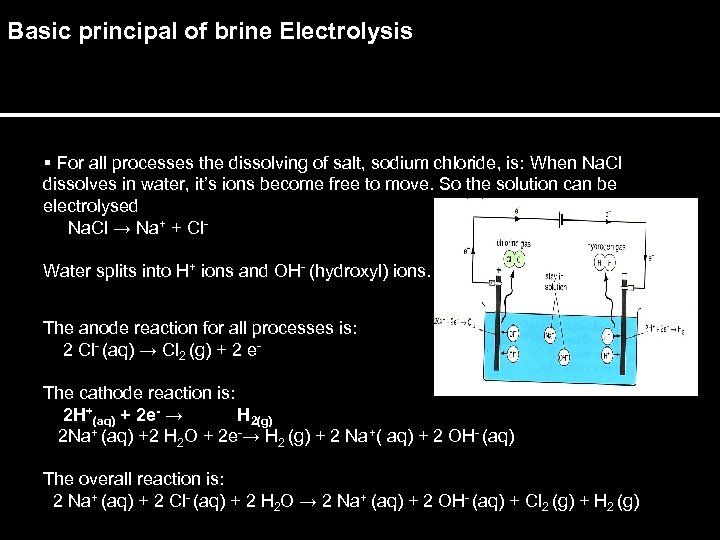 Basic principal of brine Electrolysis § For all processes the dissolving of salt, sodium chloride, is: When Na. Cl dissolves in water, it’s ions become free to move. So the solution can be electrolysed Na. Cl → Na+ + Cl. Water splits into H+ ions and OH- (hydroxyl) ions. The anode reaction for all processes is: 2 Cl- (aq) → Cl 2 (g) + 2 e. The cathode reaction is: 2 H+(aq) + 2 e- → H 2(g) 2 Na+ (aq) +2 H 2 O + 2 e-→ H 2 (g) + 2 Na+( aq) + 2 OH- (aq) The overall reaction is: 2 Na+ (aq) + 2 Cl- (aq) + 2 H 2 O → 2 Na+ (aq) + 2 OH- (aq) + Cl 2 (g) + H 2 (g)
Basic principal of brine Electrolysis § For all processes the dissolving of salt, sodium chloride, is: When Na. Cl dissolves in water, it’s ions become free to move. So the solution can be electrolysed Na. Cl → Na+ + Cl. Water splits into H+ ions and OH- (hydroxyl) ions. The anode reaction for all processes is: 2 Cl- (aq) → Cl 2 (g) + 2 e. The cathode reaction is: 2 H+(aq) + 2 e- → H 2(g) 2 Na+ (aq) +2 H 2 O + 2 e-→ H 2 (g) + 2 Na+( aq) + 2 OH- (aq) The overall reaction is: 2 Na+ (aq) + 2 Cl- (aq) + 2 H 2 O → 2 Na+ (aq) + 2 OH- (aq) + Cl 2 (g) + H 2 (g)
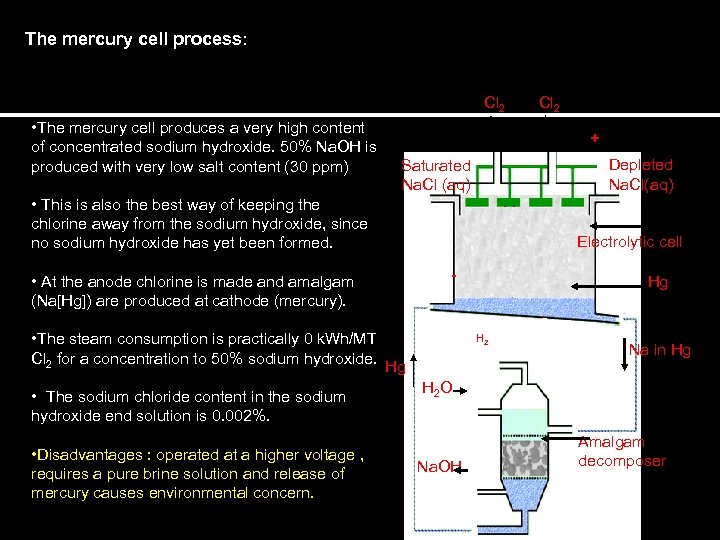 The mercury cell process: Cl 2 • The mercury cell produces a very high content of concentrated sodium hydroxide. 50% Na. OH is produced with very low salt content (30 ppm) + Depleted Na. Cl(aq) Saturated Na. Cl (aq) • This is also the best way of keeping the chlorine away from the sodium hydroxide, since no sodium hydroxide has yet been formed. Electrolytic cell - • At the anode chlorine is made and amalgam (Na[Hg]) are produced at cathode (mercury). • The steam consumption is practically 0 k. Wh/MT Cl 2 for a concentration to 50% sodium hydroxide. • The sodium chloride content in the sodium hydroxide end solution is 0. 002%. • Disadvantages : operated at a higher voltage , requires a pure brine solution and release of mercury causes environmental concern. Cl 2 Hg H 2 Hg Na in Hg H 2 O Na. OH Amalgam decomposer
The mercury cell process: Cl 2 • The mercury cell produces a very high content of concentrated sodium hydroxide. 50% Na. OH is produced with very low salt content (30 ppm) + Depleted Na. Cl(aq) Saturated Na. Cl (aq) • This is also the best way of keeping the chlorine away from the sodium hydroxide, since no sodium hydroxide has yet been formed. Electrolytic cell - • At the anode chlorine is made and amalgam (Na[Hg]) are produced at cathode (mercury). • The steam consumption is practically 0 k. Wh/MT Cl 2 for a concentration to 50% sodium hydroxide. • The sodium chloride content in the sodium hydroxide end solution is 0. 002%. • Disadvantages : operated at a higher voltage , requires a pure brine solution and release of mercury causes environmental concern. Cl 2 Hg H 2 Hg Na in Hg H 2 O Na. OH Amalgam decomposer
 The mercury cell process: ØAnode (positive electrode): 2 Cl-(aq) -----> Cl 2(g) + 2 e- ØAt the cathode the sodium ion (Na+) gains an electron to form a neutral atom Na. + Na (aq) + e -----> Na(s) ØAs the sodium atom is produced it dissolves in mercury to form an amalgam. 2 Na+ + 2 e- -----> 2 Na(Hg) Hg ØThe amalgam is sprayed into water where sodium reacts rapidly. 2 Na(Hg) + 2 H 2 O(l) -----> 2 Na. OH(aq) + H 2(g) + Hg ØOver-all reaction for the chlor-alkali process is as follows: 2 Na. Cl(aq) + 2 H 2 O(l) -----> 2 Na. OH(aq) + Cl 2(g) + H 2(g) operated at 70 -80 ºC
The mercury cell process: ØAnode (positive electrode): 2 Cl-(aq) -----> Cl 2(g) + 2 e- ØAt the cathode the sodium ion (Na+) gains an electron to form a neutral atom Na. + Na (aq) + e -----> Na(s) ØAs the sodium atom is produced it dissolves in mercury to form an amalgam. 2 Na+ + 2 e- -----> 2 Na(Hg) Hg ØThe amalgam is sprayed into water where sodium reacts rapidly. 2 Na(Hg) + 2 H 2 O(l) -----> 2 Na. OH(aq) + H 2(g) + Hg ØOver-all reaction for the chlor-alkali process is as follows: 2 Na. Cl(aq) + 2 H 2 O(l) -----> 2 Na. OH(aq) + Cl 2(g) + H 2(g) operated at 70 -80 ºC
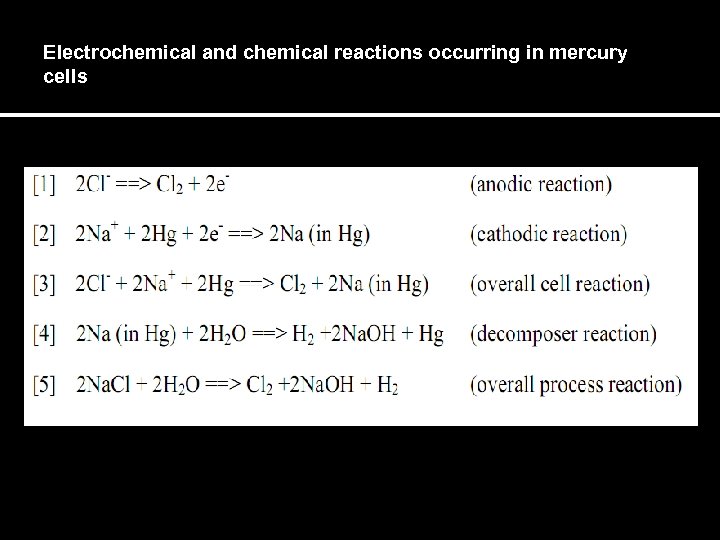 Electrochemical and chemical reactions occurring in mercury cells
Electrochemical and chemical reactions occurring in mercury cells
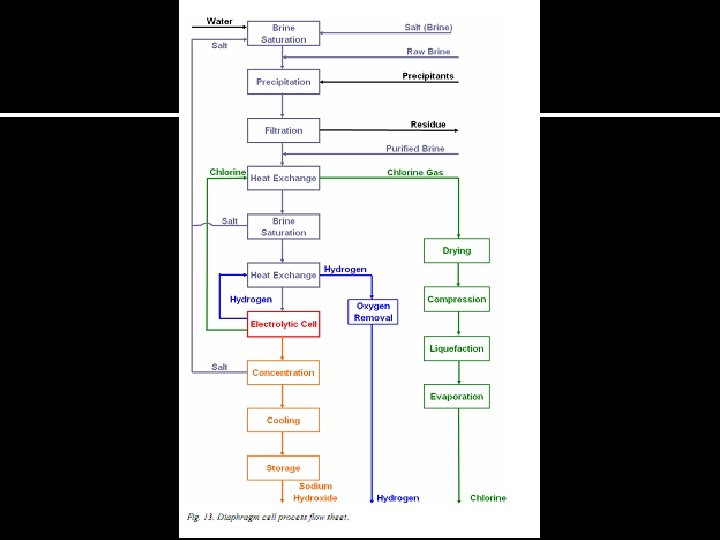
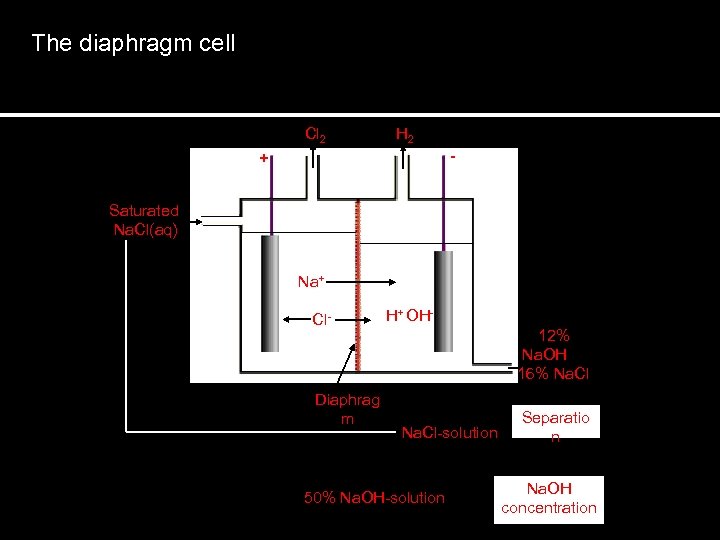 The diaphragm cell Cl 2 H 2 - + Saturated Na. Cl(aq) Na+ Cl- H+ OH 12% Na. OH 16% Na. Cl Diaphrag m Na. Cl-solution 50% Na. OH-solution Separatio n Na. OH concentration
The diaphragm cell Cl 2 H 2 - + Saturated Na. Cl(aq) Na+ Cl- H+ OH 12% Na. OH 16% Na. Cl Diaphrag m Na. Cl-solution 50% Na. OH-solution Separatio n Na. OH concentration
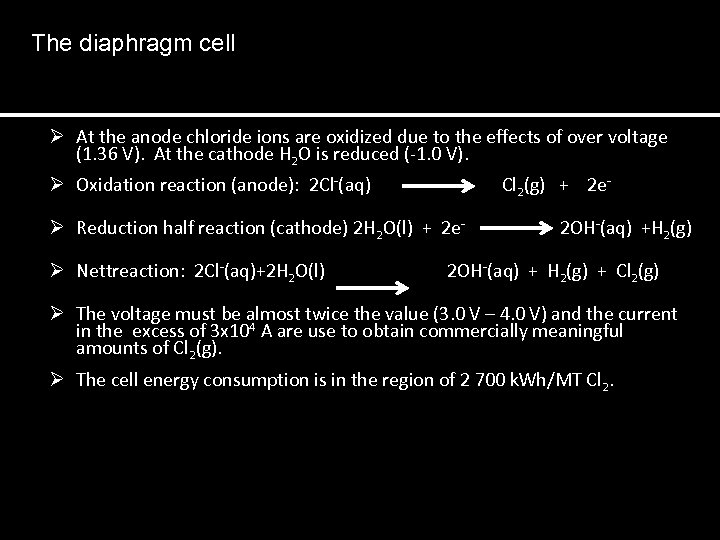 The diaphragm cell Ø At the anode chloride ions are oxidized due to the effects of over voltage (1. 36 V). At the cathode H 2 O is reduced (-1. 0 V). Ø Ø Oxidation reaction (anode): 2 Cl-(aq) Cl 2(g) + 2 e- Reduction half reaction (cathode) 2 H 2 O(l) + 2 e- 2 OH-(aq) +H 2(g) Nettreaction: 2 Cl-(aq)+2 H 2 O(l) 2 OH-(aq) + H 2(g) + Cl 2(g) The voltage must be almost twice the value (3. 0 V – 4. 0 V) and the current in the excess of 3 x 104 A are use to obtain commercially meaningful amounts of Cl 2(g). Ø The cell energy consumption is in the region of 2 700 k. Wh/MT Cl 2.
The diaphragm cell Ø At the anode chloride ions are oxidized due to the effects of over voltage (1. 36 V). At the cathode H 2 O is reduced (-1. 0 V). Ø Ø Oxidation reaction (anode): 2 Cl-(aq) Cl 2(g) + 2 e- Reduction half reaction (cathode) 2 H 2 O(l) + 2 e- 2 OH-(aq) +H 2(g) Nettreaction: 2 Cl-(aq)+2 H 2 O(l) 2 OH-(aq) + H 2(g) + Cl 2(g) The voltage must be almost twice the value (3. 0 V – 4. 0 V) and the current in the excess of 3 x 104 A are use to obtain commercially meaningful amounts of Cl 2(g). Ø The cell energy consumption is in the region of 2 700 k. Wh/MT Cl 2.
 The diaphragm cell ØContain a diaphragm made of asbestos fibers to separate anode from cathode. Due to good chemical stability and it is a relatively inexpensive and abundant material. Asbestos diaphragms began to be replaced by diaphragms containing 75% asbestos and 25% of fibrous fluorocarbon polymer of high chemical resistance. ØAllows ions to pass through by migration ØTItanium anode and cast iron cathode ØPurified brine enters the anode compartment and percolates through the diaphragm into the cathode chamber. Flow rate is maintained by keeping anode level higher. ØOperated at 80 -99 ºC. ØChlorine is made at the anode and OH- ions are produced at cathode with the liberation of H 2(g). Ø To stimulate the flow of OH- ions away from the anode, a small pressure difference is create by having a constant head of liquid in the anode department. Ø A continuous-flow process is operated by the cell and the product of sodium hydroxide is removed from the cathode region.
The diaphragm cell ØContain a diaphragm made of asbestos fibers to separate anode from cathode. Due to good chemical stability and it is a relatively inexpensive and abundant material. Asbestos diaphragms began to be replaced by diaphragms containing 75% asbestos and 25% of fibrous fluorocarbon polymer of high chemical resistance. ØAllows ions to pass through by migration ØTItanium anode and cast iron cathode ØPurified brine enters the anode compartment and percolates through the diaphragm into the cathode chamber. Flow rate is maintained by keeping anode level higher. ØOperated at 80 -99 ºC. ØChlorine is made at the anode and OH- ions are produced at cathode with the liberation of H 2(g). Ø To stimulate the flow of OH- ions away from the anode, a small pressure difference is create by having a constant head of liquid in the anode department. Ø A continuous-flow process is operated by the cell and the product of sodium hydroxide is removed from the cathode region.
 The diaphragm cell ØMajor Advantage – Can run on dilute (20%), fairly impure brine ØDilute brine, Na. Cl 15%, produces Na. OH 11% ØDisadvantage : ürelease of asbestos üConsumes lot of energy for evaporation üFor 1 ton of 50% caustic need 2600 kg of water to be evaporated. üSome amount of Chloride ion remains and is highly objectionable to some industries
The diaphragm cell ØMajor Advantage – Can run on dilute (20%), fairly impure brine ØDilute brine, Na. Cl 15%, produces Na. OH 11% ØDisadvantage : ürelease of asbestos üConsumes lot of energy for evaporation üFor 1 ton of 50% caustic need 2600 kg of water to be evaporated. üSome amount of Chloride ion remains and is highly objectionable to some industries
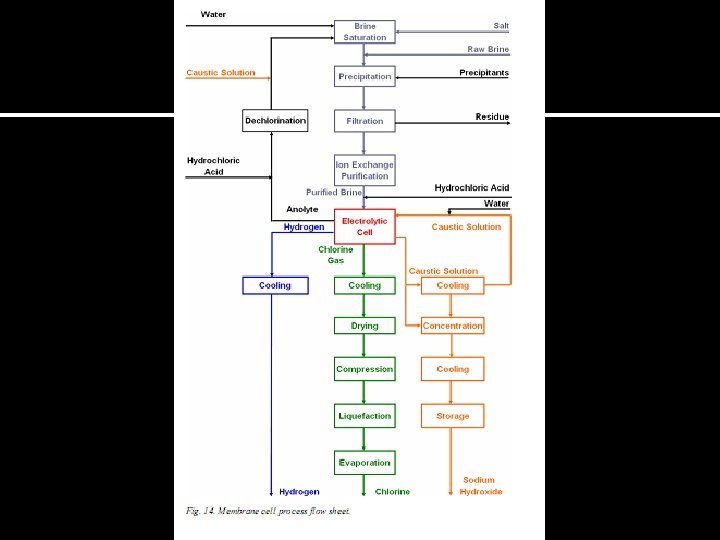
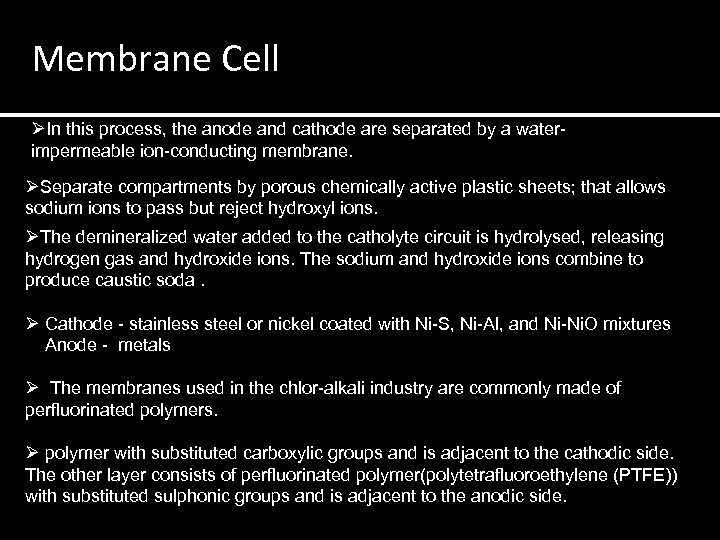 Membrane Cell ØIn this process, the anode and cathode are separated by a waterimpermeable ion-conducting membrane. ØSeparate compartments by porous chemically active plastic sheets; that allows sodium ions to pass but reject hydroxyl ions. ØThe demineralized water added to the catholyte circuit is hydrolysed, releasing hydrogen gas and hydroxide ions. The sodium and hydroxide ions combine to produce caustic soda. Ø Cathode - stainless steel or nickel coated with Ni-S, Ni-Al, and Ni-Ni. O mixtures Anode - metals Ø The membranes used in the chlor-alkali industry are commonly made of perfluorinated polymers. Ø polymer with substituted carboxylic groups and is adjacent to the cathodic side. The other layer consists of perfluorinated polymer(polytetrafluoroethylene (PTFE)) with substituted sulphonic groups and is adjacent to the anodic side.
Membrane Cell ØIn this process, the anode and cathode are separated by a waterimpermeable ion-conducting membrane. ØSeparate compartments by porous chemically active plastic sheets; that allows sodium ions to pass but reject hydroxyl ions. ØThe demineralized water added to the catholyte circuit is hydrolysed, releasing hydrogen gas and hydroxide ions. The sodium and hydroxide ions combine to produce caustic soda. Ø Cathode - stainless steel or nickel coated with Ni-S, Ni-Al, and Ni-Ni. O mixtures Anode - metals Ø The membranes used in the chlor-alkali industry are commonly made of perfluorinated polymers. Ø polymer with substituted carboxylic groups and is adjacent to the cathodic side. The other layer consists of perfluorinated polymer(polytetrafluoroethylene (PTFE)) with substituted sulphonic groups and is adjacent to the anodic side.
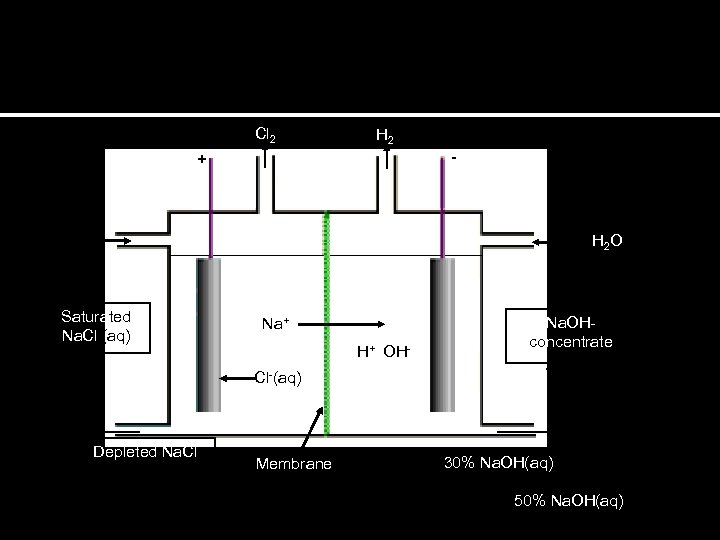 Cl 2 H 2 - + H 2 O Saturated Na. Cl (aq) Na+ H+ OH- Na. OHconcentrate Cl-(aq) Depleted Na. Cl Membrane 30% Na. OH(aq) 50% Na. OH(aq)
Cl 2 H 2 - + H 2 O Saturated Na. Cl (aq) Na+ H+ OH- Na. OHconcentrate Cl-(aq) Depleted Na. Cl Membrane 30% Na. OH(aq) 50% Na. OH(aq)
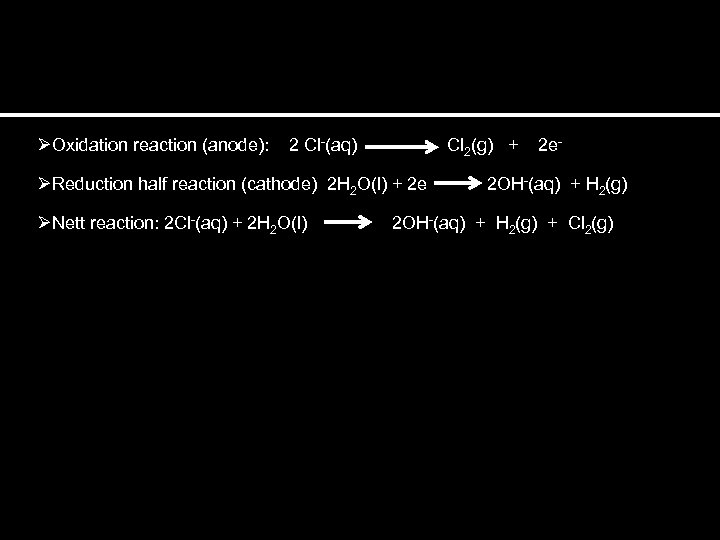 ØOxidation reaction (anode): 2 Cl-(aq) Cl 2(g) + 2 e- ØReduction half reaction (cathode) 2 H 2 O(l) + 2 e 2 OH-(aq) + H 2(g) ØNett reaction: 2 Cl-(aq) + 2 H 2 O(l) 2 OH-(aq) + H 2(g) + Cl 2(g)
ØOxidation reaction (anode): 2 Cl-(aq) Cl 2(g) + 2 e- ØReduction half reaction (cathode) 2 H 2 O(l) + 2 e 2 OH-(aq) + H 2(g) ØNett reaction: 2 Cl-(aq) + 2 H 2 O(l) 2 OH-(aq) + H 2(g) + Cl 2(g)
 Advantages of Membrane Cell ØPurpose of membrane is to exclude OH- and Cl- ions from anode chamber üThus making the product far lower in salt than that from a diaphragm cell ØMembrane cells operate using more concentrated brine and produce purer, more concentrated product ü(30 -35% Na. OH containing 50 ppm of Na. Cl) ØRequires only 715 kg of water to be evaporated to produce 1 M ton of 50% Na. OH ØBecause of difficulty and expense of concentration and purification, only large diaphragm cells are feasible ØMembrane cells produce conc Na. OH ü considerable saving in energy (Evaporation) üoperate to the point of caustic use ØSmall, efficient units may cause a revolution in the distribution of the chlor-alkali industry, particularly if efficiencies remain high Disadvantages----the caustic soda produced may need to be evaporated to increase concentration, chlorine gas contains some oxygen and Membranes are more readily clogged than diaphragms, so some of savings are lost, bcos of necessity to pretreat the brine fed in order to remove Ca and Mg before electrolysis brine should be of high purity
Advantages of Membrane Cell ØPurpose of membrane is to exclude OH- and Cl- ions from anode chamber üThus making the product far lower in salt than that from a diaphragm cell ØMembrane cells operate using more concentrated brine and produce purer, more concentrated product ü(30 -35% Na. OH containing 50 ppm of Na. Cl) ØRequires only 715 kg of water to be evaporated to produce 1 M ton of 50% Na. OH ØBecause of difficulty and expense of concentration and purification, only large diaphragm cells are feasible ØMembrane cells produce conc Na. OH ü considerable saving in energy (Evaporation) üoperate to the point of caustic use ØSmall, efficient units may cause a revolution in the distribution of the chlor-alkali industry, particularly if efficiencies remain high Disadvantages----the caustic soda produced may need to be evaporated to increase concentration, chlorine gas contains some oxygen and Membranes are more readily clogged than diaphragms, so some of savings are lost, bcos of necessity to pretreat the brine fed in order to remove Ca and Mg before electrolysis brine should be of high purity
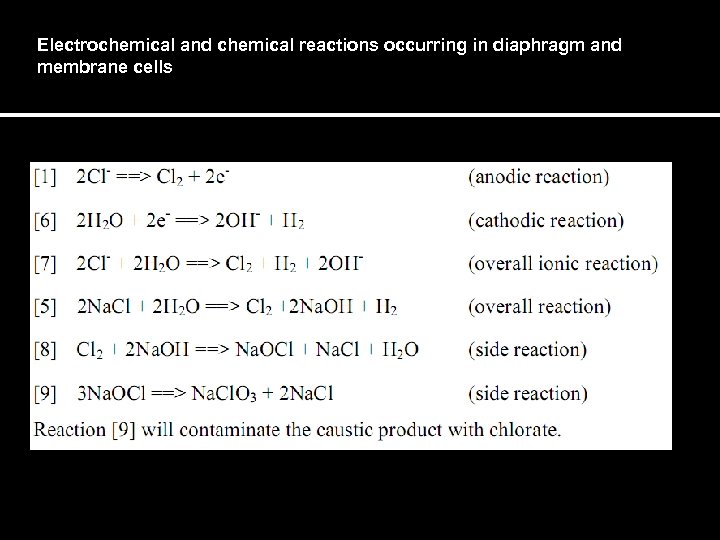 Electrochemical and chemical reactions occurring in diaphragm and membrane cells
Electrochemical and chemical reactions occurring in diaphragm and membrane cells
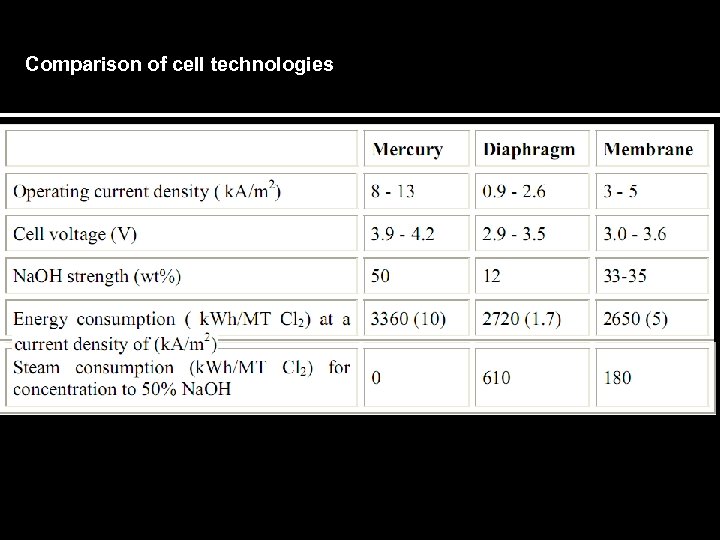 Comparison of cell technologies
Comparison of cell technologies
 Unit Operations and Chemical Conversions ØBrine Purification Section ØBrine Electrolysis ØPurification and processing of Caustic, Chlorine and Hydrogen
Unit Operations and Chemical Conversions ØBrine Purification Section ØBrine Electrolysis ØPurification and processing of Caustic, Chlorine and Hydrogen
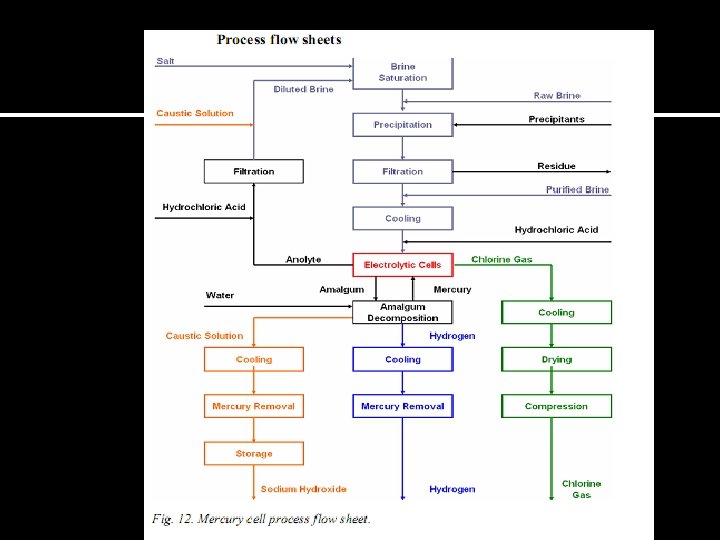
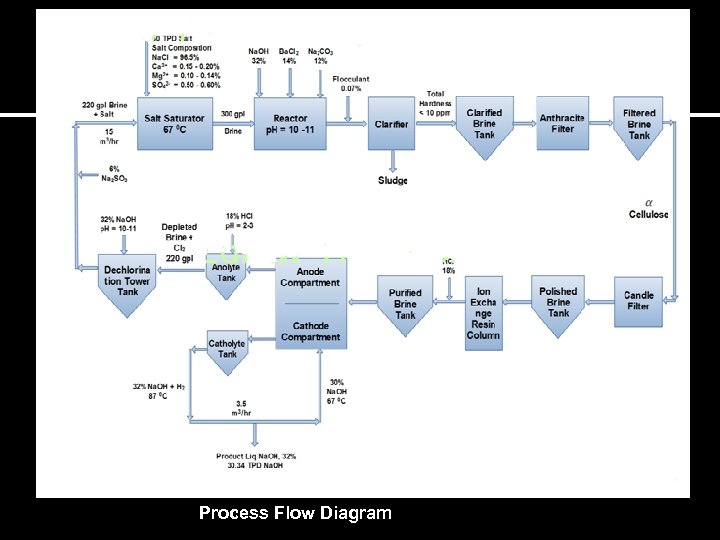 Process Flow Diagram
Process Flow Diagram
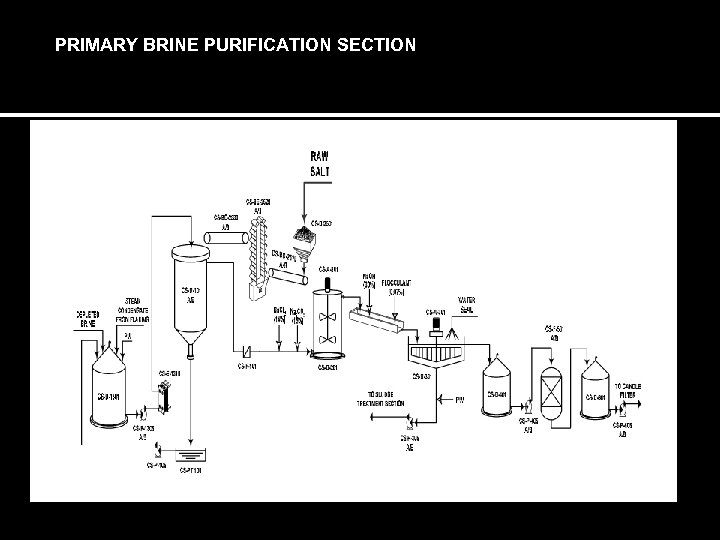 PRIMARY BRINE PURIFICATION SECTION
PRIMARY BRINE PURIFICATION SECTION
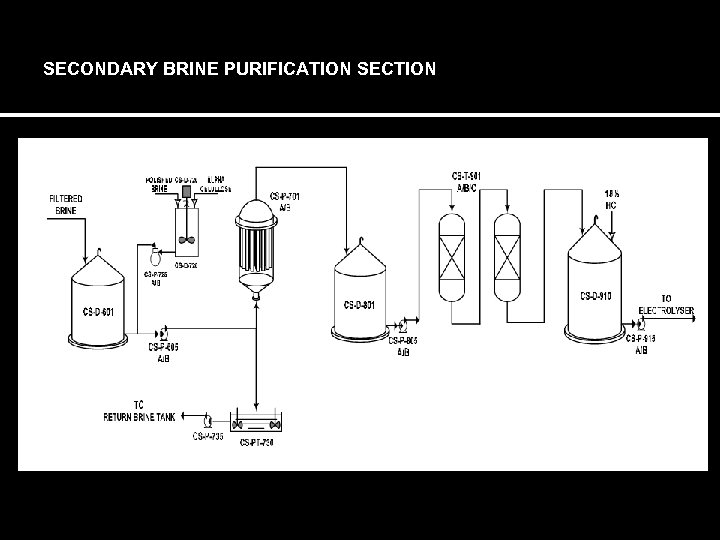 SECONDARY BRINE PURIFICATION SECTION
SECONDARY BRINE PURIFICATION SECTION
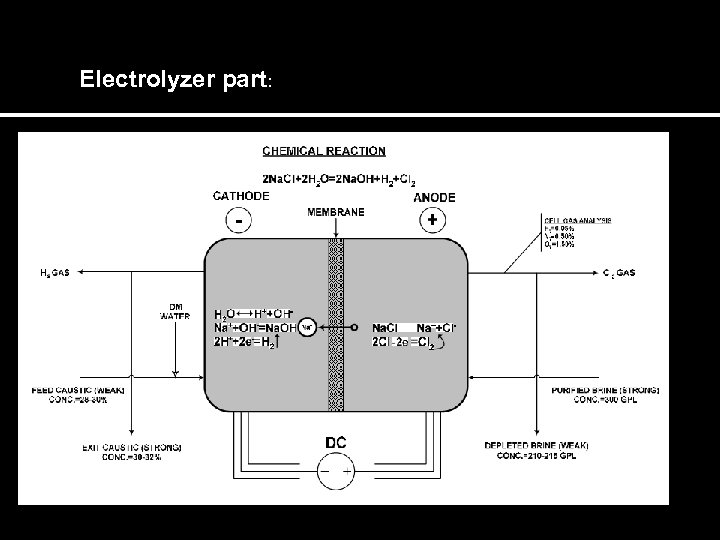 Electrolyzer part:
Electrolyzer part:
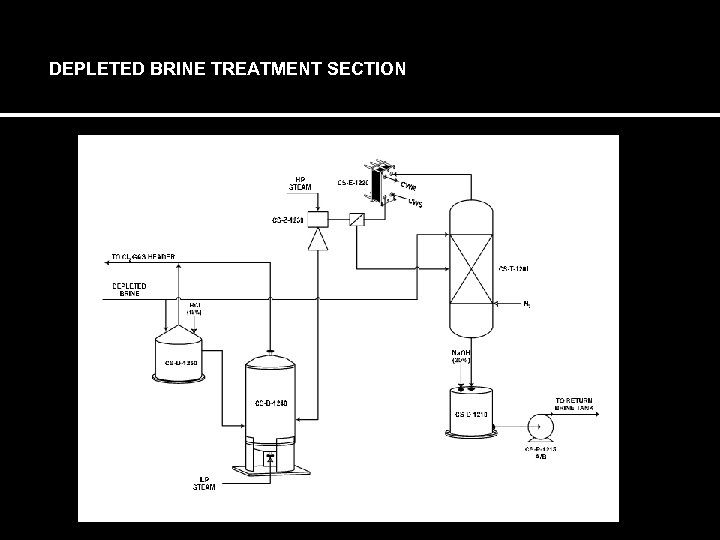 DEPLETED BRINE TREATMENT SECTION
DEPLETED BRINE TREATMENT SECTION
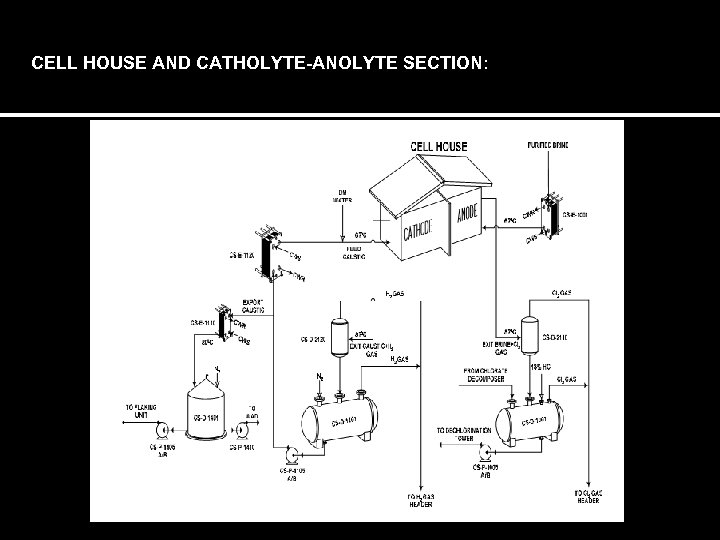 CELL HOUSE AND CATHOLYTE-ANOLYTE SECTION:
CELL HOUSE AND CATHOLYTE-ANOLYTE SECTION:
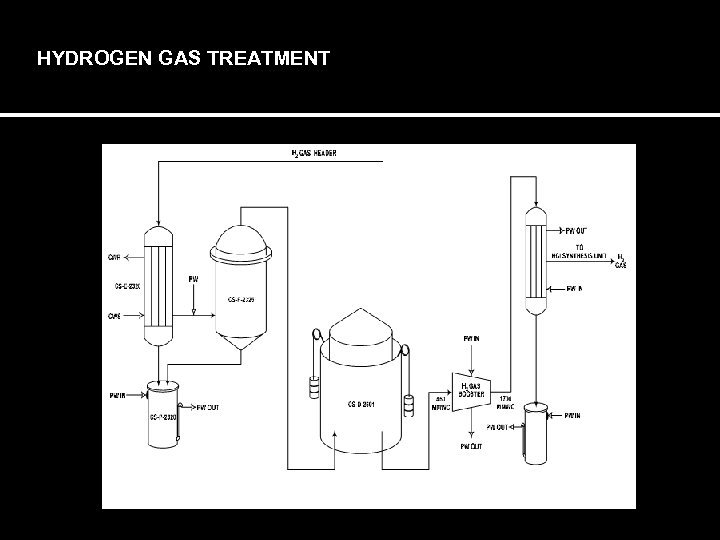 HYDROGEN GAS TREATMENT
HYDROGEN GAS TREATMENT
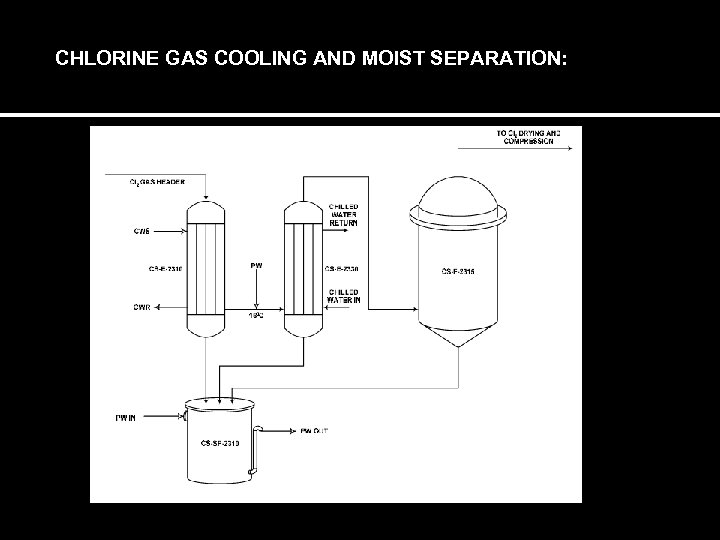 CHLORINE GAS COOLING AND MOIST SEPARATION:
CHLORINE GAS COOLING AND MOIST SEPARATION:



