6051c77c4de885aacce6b47c385e638a.ppt
- Количество слайдов: 171
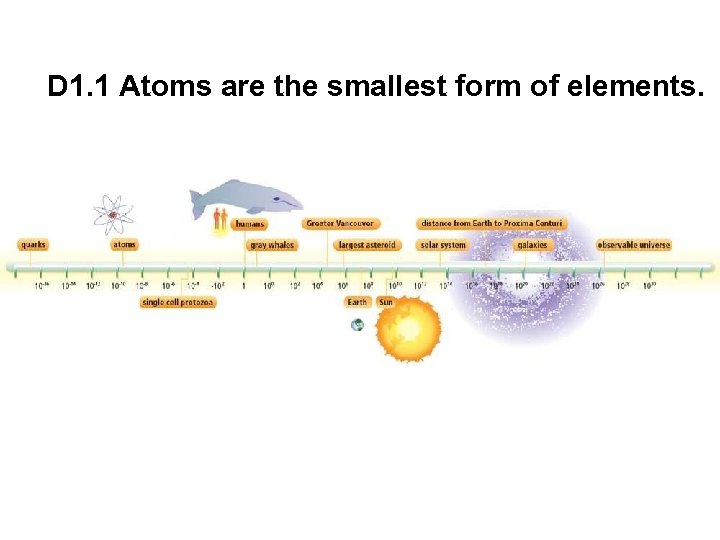 D 1. 1 Atoms are the smallest form of elements.
D 1. 1 Atoms are the smallest form of elements.
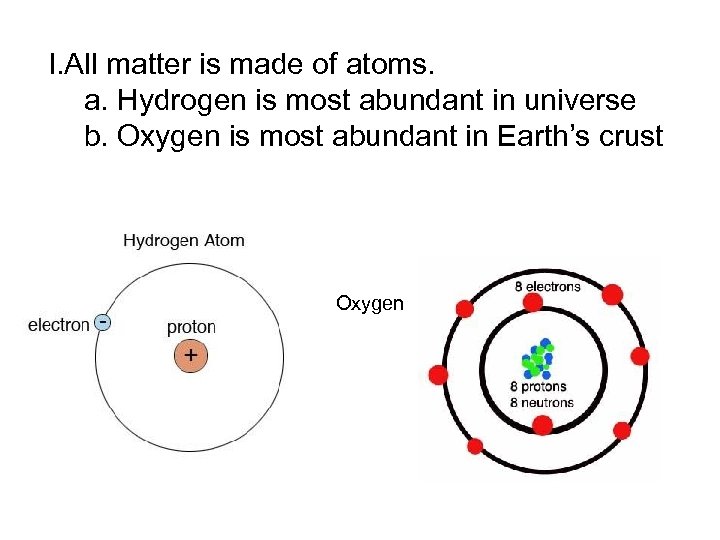 I. All matter is made of atoms. a. Hydrogen is most abundant in universe b. Oxygen is most abundant in Earth’s crust Oxygen
I. All matter is made of atoms. a. Hydrogen is most abundant in universe b. Oxygen is most abundant in Earth’s crust Oxygen
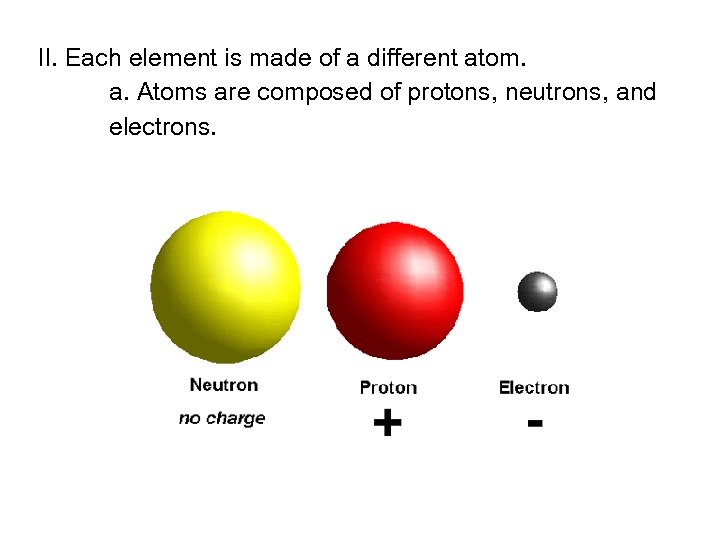 II. Each element is made of a different atom. a. Atoms are composed of protons, neutrons, and electrons.
II. Each element is made of a different atom. a. Atoms are composed of protons, neutrons, and electrons.
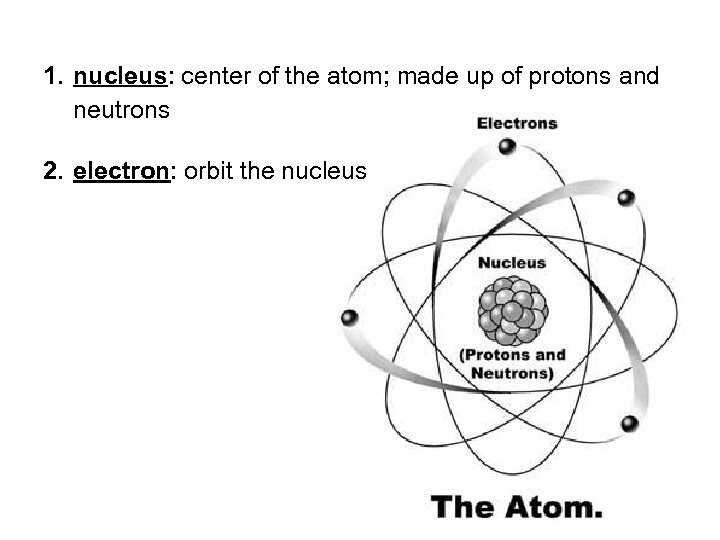 1. nucleus: center of the atom; made up of protons and neutrons 2. electron: orbit the nucleus
1. nucleus: center of the atom; made up of protons and neutrons 2. electron: orbit the nucleus
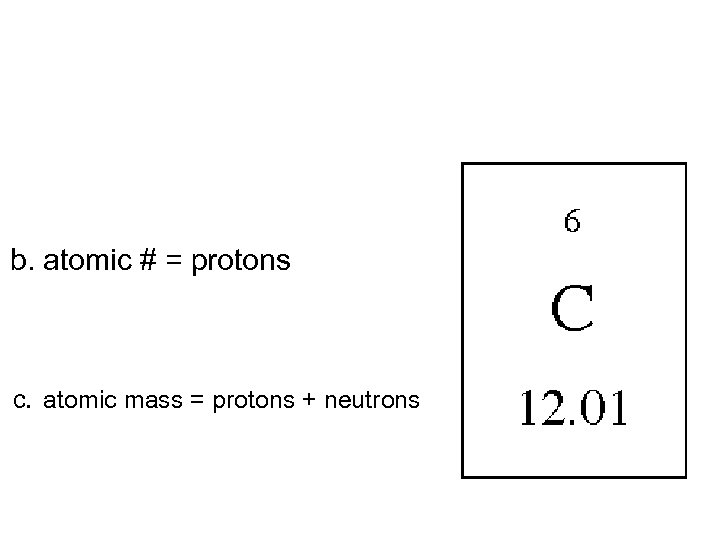 b. atomic # = protons c. atomic mass = protons + neutrons
b. atomic # = protons c. atomic mass = protons + neutrons
 Page?
Page?
 Pages R 58 -9
Pages R 58 -9

 Go S. T. E. M. !
Go S. T. E. M. !
 Formula: # of neutrons = atomic mass - atomic # *Always round atomic mass to the whole number
Formula: # of neutrons = atomic mass - atomic # *Always round atomic mass to the whole number
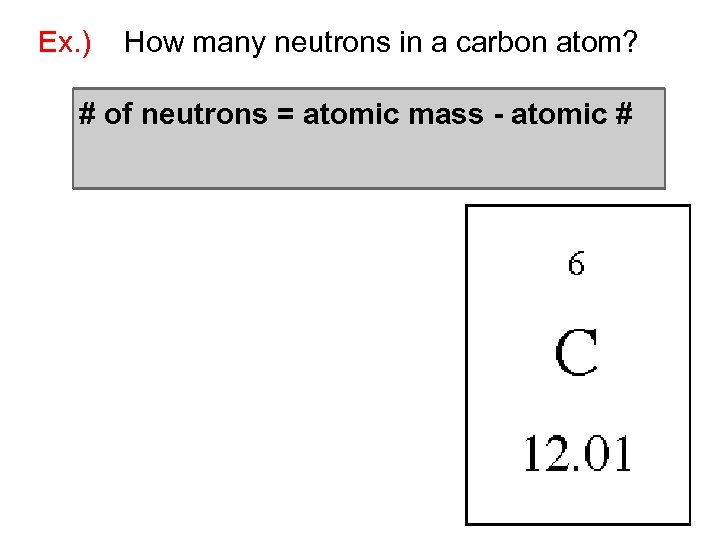 Ex. ) How many neutrons in a carbon atom? # of neutrons = atomic mass - atomic #
Ex. ) How many neutrons in a carbon atom? # of neutrons = atomic mass - atomic #
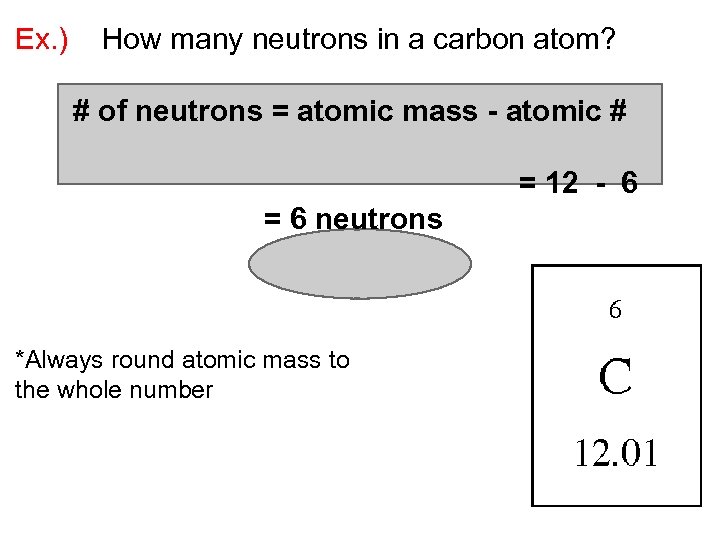 Ex. ) How many neutrons in a carbon atom? # of neutrons = atomic mass - atomic # = 12 - 6 = 6 neutrons *Always round atomic mass to the whole number
Ex. ) How many neutrons in a carbon atom? # of neutrons = atomic mass - atomic # = 12 - 6 = 6 neutrons *Always round atomic mass to the whole number
 How many neutrons in a nitrogen atom?
How many neutrons in a nitrogen atom?
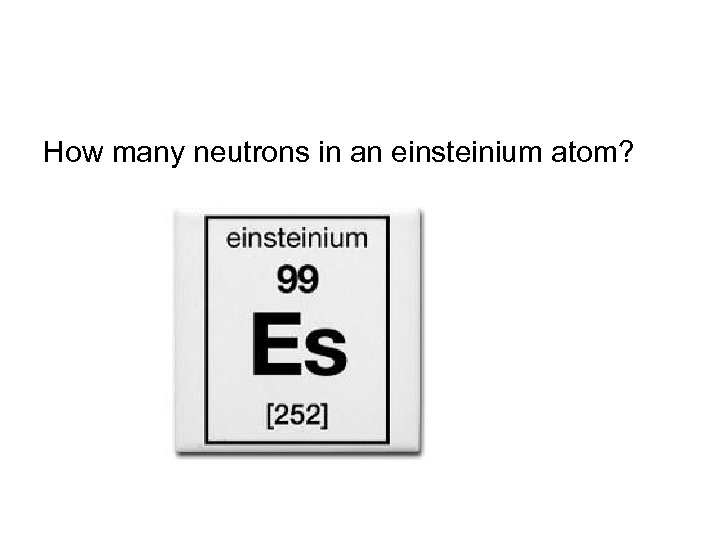 How many neutrons in an einsteinium atom?
How many neutrons in an einsteinium atom?

 Calculate the number of neutrons in an iron atom. Round atomic mass to the whole number.
Calculate the number of neutrons in an iron atom. Round atomic mass to the whole number.
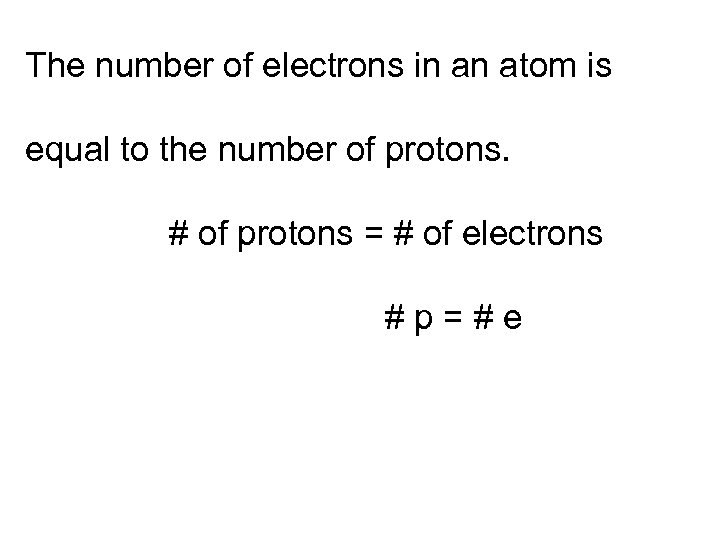 The number of electrons in an atom is equal to the number of protons. # of protons = # of electrons #p=#e
The number of electrons in an atom is equal to the number of protons. # of protons = # of electrons #p=#e
 Calculate the number of neutrons in an oxygen atom. Sketch in your notebook the structure of this atom.
Calculate the number of neutrons in an oxygen atom. Sketch in your notebook the structure of this atom.
 Calculate the number of neutrons in an oxygen atom. Sketch in your notebook the structure of this atom.
Calculate the number of neutrons in an oxygen atom. Sketch in your notebook the structure of this atom.
 Calculate the number of neutrons in an oxygen atom. Sketch in your notebook the structure of this atom.
Calculate the number of neutrons in an oxygen atom. Sketch in your notebook the structure of this atom.
 In your notebook, calculate the number neutrons, for the following elements. 1. 2. 3. 4. 5. Copper (Cu) Arsenic (As) Selenium (Se) Mercury (Hg) Plutonium (Pu)
In your notebook, calculate the number neutrons, for the following elements. 1. 2. 3. 4. 5. Copper (Cu) Arsenic (As) Selenium (Se) Mercury (Hg) Plutonium (Pu)
 In your notebook, calculate the number of protons, neutrons, and elements, for the following elements. 1. Copper (Cu) 2. Arsenic (As) 3. Selenium (Se) 4. Mercury (Hg) 5. Plutonium (Pu) 6. Barium (Ba) 7. Xenon (Xe) 8. Krypton (Kr) 9. Potassium (K) 10. Americium (Am)
In your notebook, calculate the number of protons, neutrons, and elements, for the following elements. 1. Copper (Cu) 2. Arsenic (As) 3. Selenium (Se) 4. Mercury (Hg) 5. Plutonium (Pu) 6. Barium (Ba) 7. Xenon (Xe) 8. Krypton (Kr) 9. Potassium (K) 10. Americium (Am)
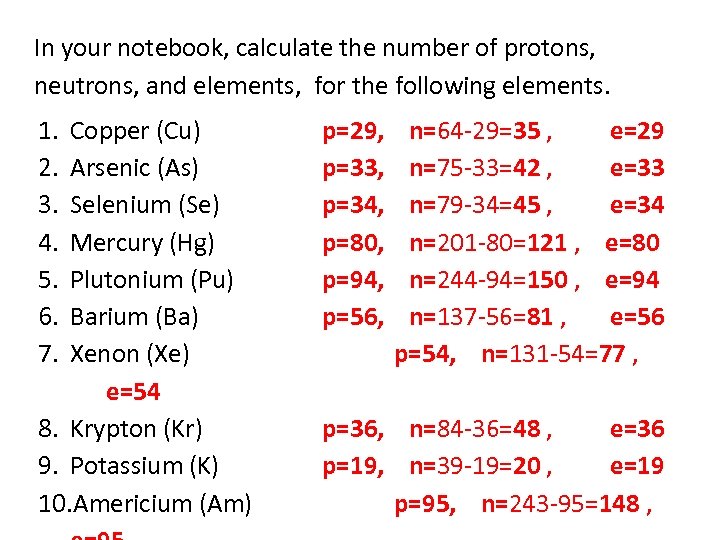 In your notebook, calculate the number of protons, neutrons, and elements, for the following elements. 1. 2. 3. 4. 5. 6. 7. Copper (Cu) Arsenic (As) Selenium (Se) Mercury (Hg) Plutonium (Pu) Barium (Ba) Xenon (Xe) e=54 8. Krypton (Kr) 9. Potassium (K) 10. Americium (Am) p=29, p=33, p=34, p=80, p=94, p=56, n=64 -29=35 , e=29 n=75 -33=42 , e=33 n=79 -34=45 , e=34 n=201 -80=121 , e=80 n=244 -94=150 , e=94 n=137 -56=81 , e=56 p=54, n=131 -54=77 , p=36, n=84 -36=48 , e=36 p=19, n=39 -19=20 , e=19 p=95, n=243 -95=148 ,
In your notebook, calculate the number of protons, neutrons, and elements, for the following elements. 1. 2. 3. 4. 5. 6. 7. Copper (Cu) Arsenic (As) Selenium (Se) Mercury (Hg) Plutonium (Pu) Barium (Ba) Xenon (Xe) e=54 8. Krypton (Kr) 9. Potassium (K) 10. Americium (Am) p=29, p=33, p=34, p=80, p=94, p=56, n=64 -29=35 , e=29 n=75 -33=42 , e=33 n=79 -34=45 , e=34 n=201 -80=121 , e=80 n=244 -94=150 , e=94 n=137 -56=81 , e=56 p=54, n=131 -54=77 , p=36, n=84 -36=48 , e=36 p=19, n=39 -19=20 , e=19 p=95, n=243 -95=148 ,
 DO NOW: In your notebook, 1. Calculate the number of neutrons in the element Tellurium (Te). Put a box around the formula. 2. Calculate the number of particles in each element. Draw the atom. -Magnesium (Mg) -Aluminum (Al)
DO NOW: In your notebook, 1. Calculate the number of neutrons in the element Tellurium (Te). Put a box around the formula. 2. Calculate the number of particles in each element. Draw the atom. -Magnesium (Mg) -Aluminum (Al)
 QUIZ: On a piece of paper: 1. Calculate the number of neutrons in the element Silver (Ag). Put a box around the formula. 2. Calculate the number of particles in each element. Draw the atom. -Helium (He) -Beryllium (Be) -Fluorine (F) -Sodium (Na)
QUIZ: On a piece of paper: 1. Calculate the number of neutrons in the element Silver (Ag). Put a box around the formula. 2. Calculate the number of particles in each element. Draw the atom. -Helium (He) -Beryllium (Be) -Fluorine (F) -Sodium (Na)
 QUIZ: On a piece of paper: 1. Calculate the number of particles in each element. Draw the atom. -Fluorine (F) -Sodium (Na)
QUIZ: On a piece of paper: 1. Calculate the number of particles in each element. Draw the atom. -Fluorine (F) -Sodium (Na)
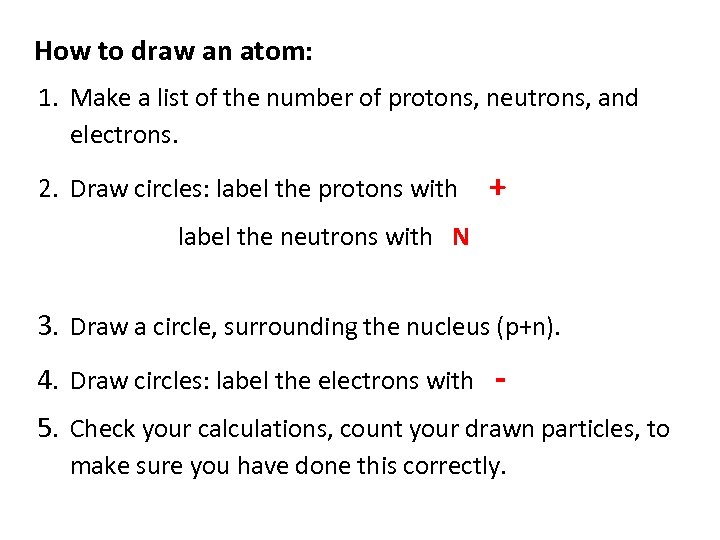 How to draw an atom: 1. Make a list of the number of protons, neutrons, and electrons. 2. Draw circles: label the protons with + label the neutrons with N 3. Draw a circle, surrounding the nucleus (p+n). 4. Draw circles: label the electrons with 5. Check your calculations, count your drawn particles, to make sure you have done this correctly.
How to draw an atom: 1. Make a list of the number of protons, neutrons, and electrons. 2. Draw circles: label the protons with + label the neutrons with N 3. Draw a circle, surrounding the nucleus (p+n). 4. Draw circles: label the electrons with 5. Check your calculations, count your drawn particles, to make sure you have done this correctly.
 Practice Drawing Atoms: Draw these atoms, in your notebook. 1. Lithium 2. Boron 3. Carbon 4. Nitrogen 5. Oxygen 6. Hydrogen
Practice Drawing Atoms: Draw these atoms, in your notebook. 1. Lithium 2. Boron 3. Carbon 4. Nitrogen 5. Oxygen 6. Hydrogen
 Drawing Atoms parts 214: https: //www. youtube. com/watch? v=j. ZO 6 W-DEVLk Molecule basics: https: //www. youtube. com/watch? v=vl. SOESXQI 7 o quarks: https: //www. youtube. com/watch? v=SMgi 2 j 9 Ks 9 k Electron on photon: https: //www. youtube. com/watch? v=ofp. OHIq 6 Wo&feature=endscreen&NR=1
Drawing Atoms parts 214: https: //www. youtube. com/watch? v=j. ZO 6 W-DEVLk Molecule basics: https: //www. youtube. com/watch? v=vl. SOESXQI 7 o quarks: https: //www. youtube. com/watch? v=SMgi 2 j 9 Ks 9 k Electron on photon: https: //www. youtube. com/watch? v=ofp. OHIq 6 Wo&feature=endscreen&NR=1
 DO NOW: In your notebook, Calculate the number of particles in each element. Draw the atom. Carbon (C) Helium (He)
DO NOW: In your notebook, Calculate the number of particles in each element. Draw the atom. Carbon (C) Helium (He)
 Why study atoms? Bonds examples, reactions. https: //m. youtube. com/watch? v=yjge 1 Wd. CFPs Intro bonds ted vid https: //m. youtube. com/watch? v=Ng. D 9 y. HSJ 29 I Chem reactions : https: //m. youtube. com/watch? v=fr 6 QGmef. VBI Song Types bonds https: //m. youtube. com/watch? v=QIf. TT-_-x. Lo
Why study atoms? Bonds examples, reactions. https: //m. youtube. com/watch? v=yjge 1 Wd. CFPs Intro bonds ted vid https: //m. youtube. com/watch? v=Ng. D 9 y. HSJ 29 I Chem reactions : https: //m. youtube. com/watch? v=fr 6 QGmef. VBI Song Types bonds https: //m. youtube. com/watch? v=QIf. TT-_-x. Lo
 Why study atoms? Bonds examples, reactions. https: //m. youtube. com/watch? v=yjge 1 Wd. CFPs Intro bonds ted vid https: //m. youtube. com/watch? v=Ng. D 9 y. HSJ 29 I Chem reactions : https: //m. youtube. com/watch? v=fr 6 QGmef. VBI Song Types bonds https: //m. youtube. com/watch? v=QIf. TT-_-x. Lo
Why study atoms? Bonds examples, reactions. https: //m. youtube. com/watch? v=yjge 1 Wd. CFPs Intro bonds ted vid https: //m. youtube. com/watch? v=Ng. D 9 y. HSJ 29 I Chem reactions : https: //m. youtube. com/watch? v=fr 6 QGmef. VBI Song Types bonds https: //m. youtube. com/watch? v=QIf. TT-_-x. Lo
 “Happy” atoms rule: An atom is least reactive when the outer orbital shell is full.
“Happy” atoms rule: An atom is least reactive when the outer orbital shell is full.
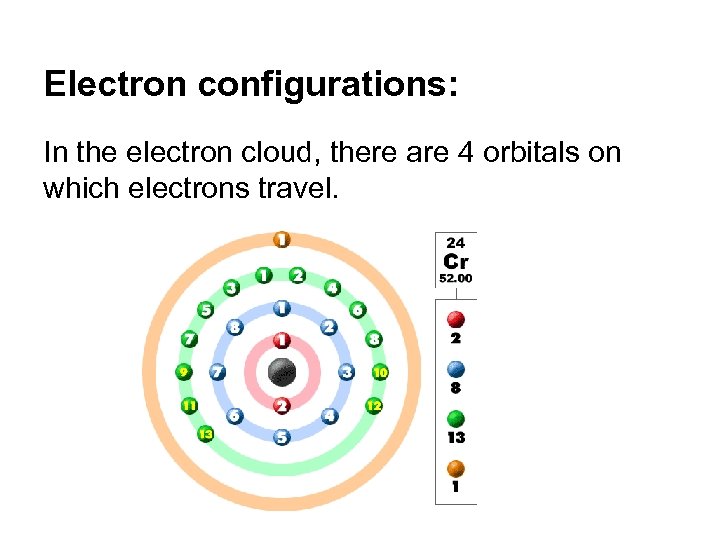 Electron configurations: In the electron cloud, there are 4 orbitals on which electrons travel.
Electron configurations: In the electron cloud, there are 4 orbitals on which electrons travel.
 Practice: a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 1. Boron (B)
Practice: a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 1. Boron (B)
 Practice: a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 2. Fluorine (F)
Practice: a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 2. Fluorine (F)
 Practice: a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 3. Chlorine (Cl)
Practice: a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 3. Chlorine (Cl)
 Practice: a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 4. Calcium (Ca) 5. Germanium (Ge)
Practice: a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 4. Calcium (Ca) 5. Germanium (Ge)
 Practice: a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 1. Titanium (Ti)
Practice: a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 1. Titanium (Ti)
 Date: Pop Quiz a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 1. Lithium (Li) 2. Neon (Ne) 3. Potassium (K)
Date: Pop Quiz a. Draw the Periodic Table Box. b. Calculate the number of particles in the atom. c. Draw the atom. Electrons should be in the correct energy level. Subtract electrons as you sit them. d. Is this atom happy? 1. Lithium (Li) 2. Neon (Ne) 3. Potassium (K)
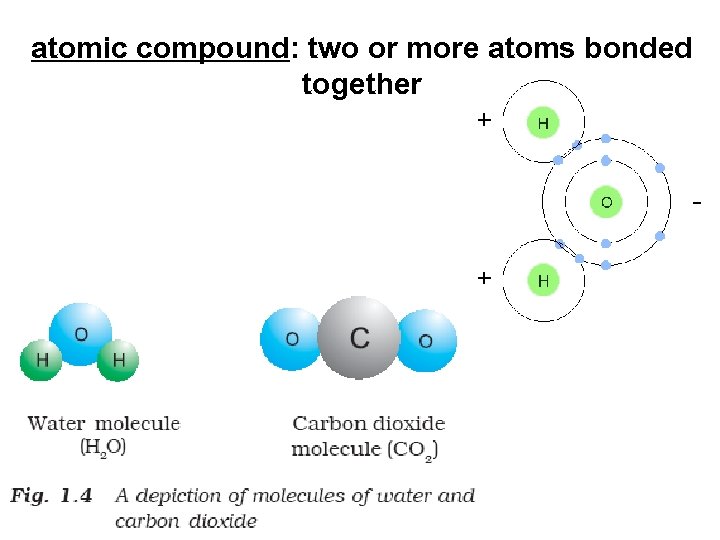
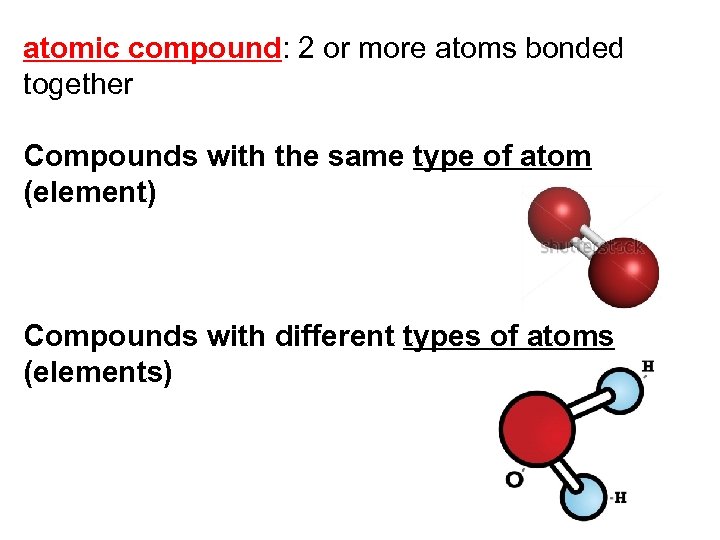 atomic compound: 2 or more atoms bonded together Compounds with the same type of atom (element) Compounds with different types of atoms (elements)
atomic compound: 2 or more atoms bonded together Compounds with the same type of atom (element) Compounds with different types of atoms (elements)
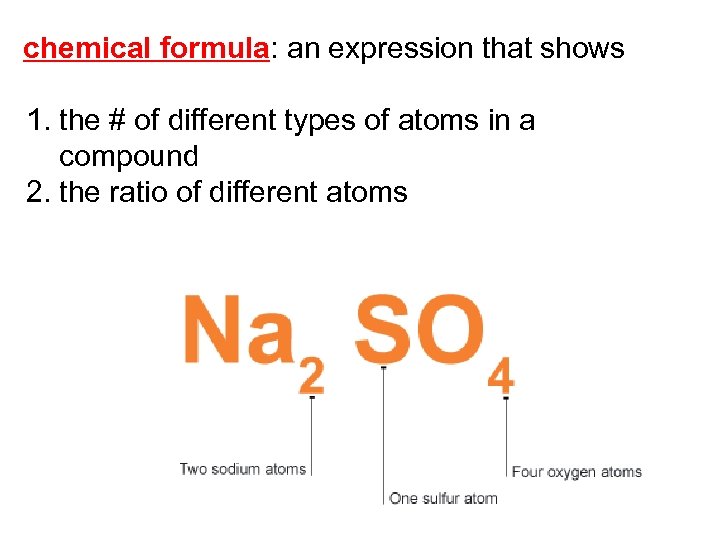 chemical formula: an expression that shows 1. the # of different types of atoms in a compound 2. the ratio of different atoms
chemical formula: an expression that shows 1. the # of different types of atoms in a compound 2. the ratio of different atoms
 chemical formula: Videos: 1. 25: https: //www. youtube. com/watch? v=SRR-4 BLz. Nus Ted atom size vid: https: //www. youtube. com/watch? v=y. QP 4 UJh. Nn 0 I
chemical formula: Videos: 1. 25: https: //www. youtube. com/watch? v=SRR-4 BLz. Nus Ted atom size vid: https: //www. youtube. com/watch? v=y. QP 4 UJh. Nn 0 I
 ratio: the number of one object in relation to the number of another object Examples: 10 apples per 1 bag 1 calculator per 3 students 2 hydrogen per 1 oxygen
ratio: the number of one object in relation to the number of another object Examples: 10 apples per 1 bag 1 calculator per 3 students 2 hydrogen per 1 oxygen
 ratio: the number of one object in relation to the number of another object Examples: 2 hydrogen per 1 oxygen
ratio: the number of one object in relation to the number of another object Examples: 2 hydrogen per 1 oxygen
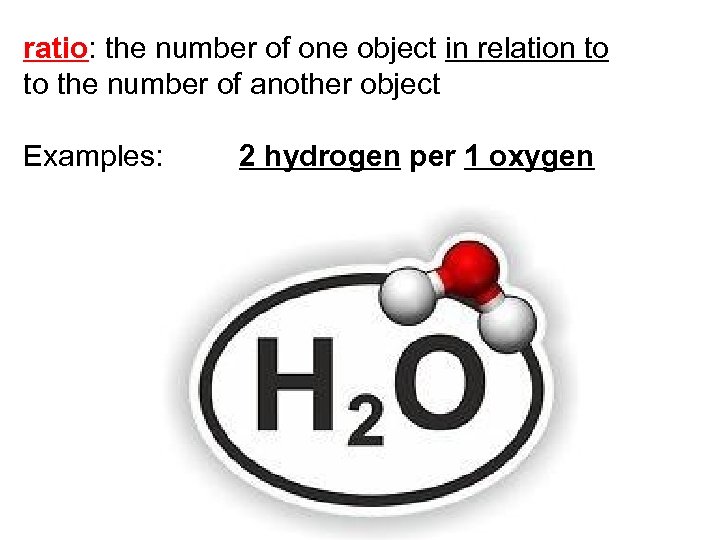 ratio: the number of one object in relation to to the number of another object Examples: 2 hydrogen per 1 oxygen
ratio: the number of one object in relation to to the number of another object Examples: 2 hydrogen per 1 oxygen
 Modeling Atomic Compounds Procedure: 1. Gather materials. 2. Read the board to find out which compound you are to build. 2. Read the board to find out which color = which element. 2. Build a model of the compound, using toothpicks to connect the atoms.
Modeling Atomic Compounds Procedure: 1. Gather materials. 2. Read the board to find out which compound you are to build. 2. Read the board to find out which color = which element. 2. Build a model of the compound, using toothpicks to connect the atoms.
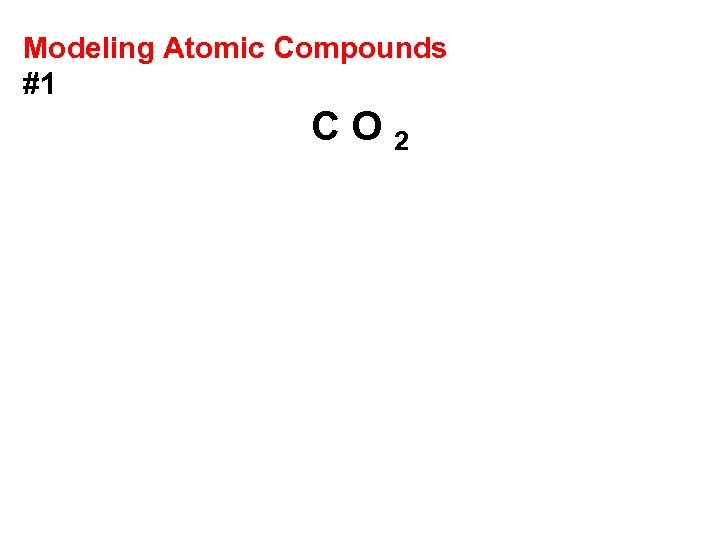 Modeling Atomic Compounds #1 CO 2
Modeling Atomic Compounds #1 CO 2
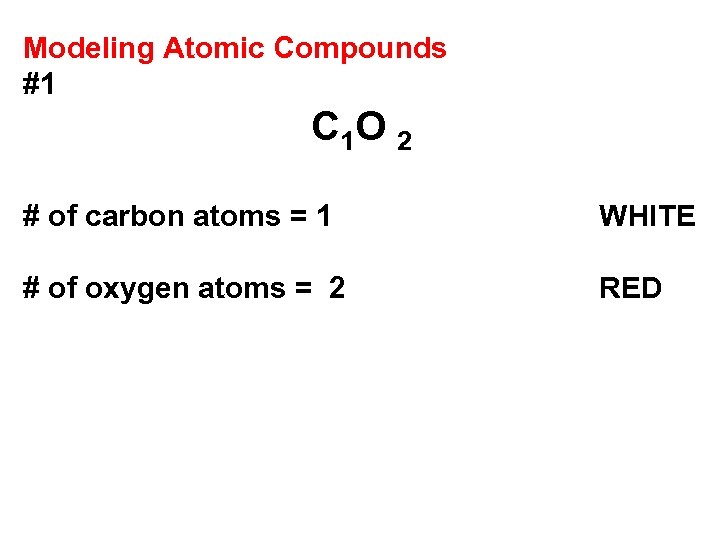 Modeling Atomic Compounds #1 C 1 O 2 # of carbon atoms = 1 WHITE # of oxygen atoms = 2 RED
Modeling Atomic Compounds #1 C 1 O 2 # of carbon atoms = 1 WHITE # of oxygen atoms = 2 RED
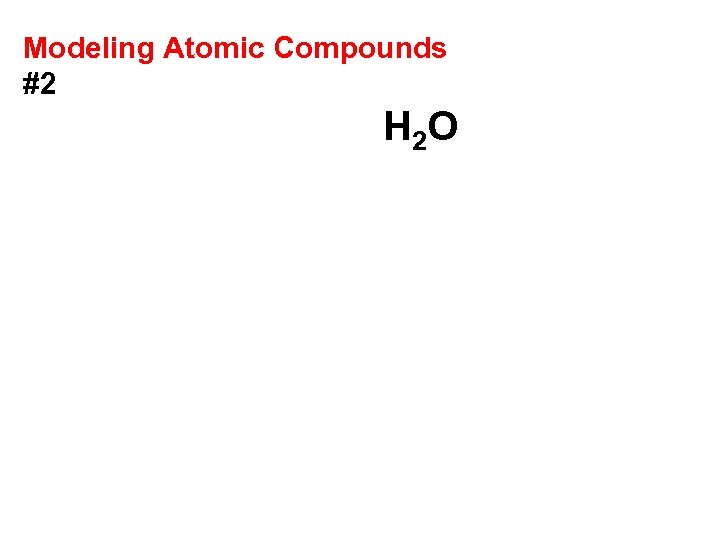 Modeling Atomic Compounds #2 H 2 O
Modeling Atomic Compounds #2 H 2 O
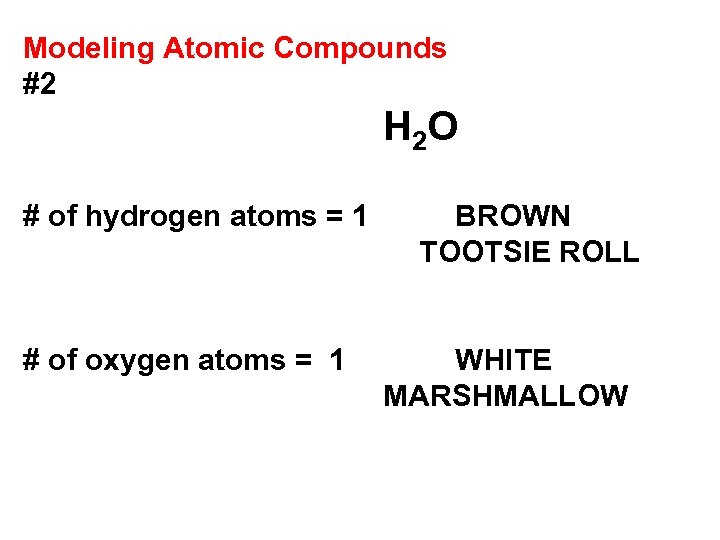 Modeling Atomic Compounds #2 H 2 O # of hydrogen atoms = 1 # of oxygen atoms = 1 BROWN TOOTSIE ROLL WHITE MARSHMALLOW
Modeling Atomic Compounds #2 H 2 O # of hydrogen atoms = 1 # of oxygen atoms = 1 BROWN TOOTSIE ROLL WHITE MARSHMALLOW
 Modeling Atomic Compounds #3 Na Cl
Modeling Atomic Compounds #3 Na Cl
 Modeling Atomic Compounds #3 Na Cl # of sodium atoms = 1 CANDY CORN TRIANGLE # of chlorine atoms = 1 BROWN TOOTSIE ROLL
Modeling Atomic Compounds #3 Na Cl # of sodium atoms = 1 CANDY CORN TRIANGLE # of chlorine atoms = 1 BROWN TOOTSIE ROLL
 Modeling Atomic Compounds #4 H 2 SO 4
Modeling Atomic Compounds #4 H 2 SO 4
 Modeling Atomic Compounds #4 H 2 SO 4 # of hydrogen atoms = 2 CANDY CORN # of sulfur atoms = 1 MARSHMALLOW # of oxygen atoms = 4 WHITE RED TWIZZLER
Modeling Atomic Compounds #4 H 2 SO 4 # of hydrogen atoms = 2 CANDY CORN # of sulfur atoms = 1 MARSHMALLOW # of oxygen atoms = 4 WHITE RED TWIZZLER
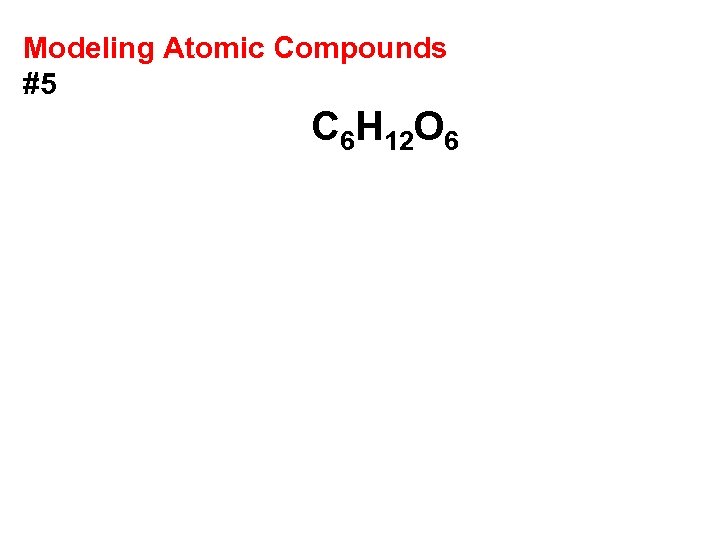 Modeling Atomic Compounds #5 C 6 H 12 O 6
Modeling Atomic Compounds #5 C 6 H 12 O 6
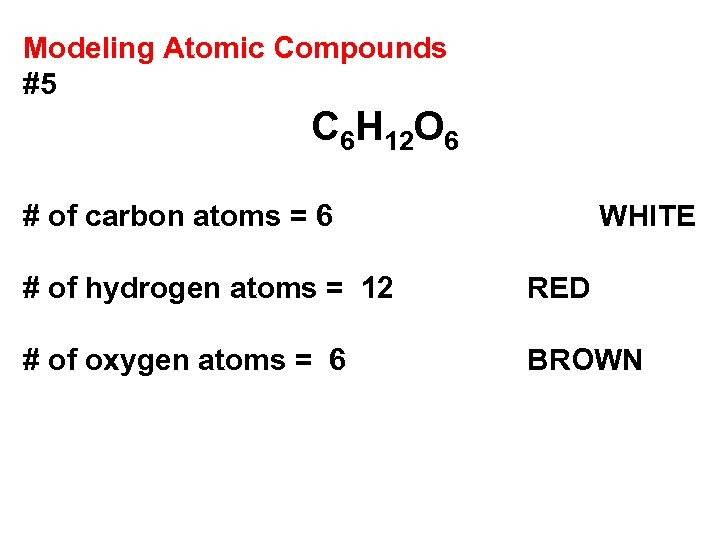 Modeling Atomic Compounds #5 C 6 H 12 O 6 # of carbon atoms = 6 WHITE # of hydrogen atoms = 12 RED # of oxygen atoms = 6 BROWN
Modeling Atomic Compounds #5 C 6 H 12 O 6 # of carbon atoms = 6 WHITE # of hydrogen atoms = 12 RED # of oxygen atoms = 6 BROWN
 Modeling Atomic Compounds #6 O 2 Use 2 gummy bears of different colors. This compound is made up of 2 oxygen isotopes.
Modeling Atomic Compounds #6 O 2 Use 2 gummy bears of different colors. This compound is made up of 2 oxygen isotopes.
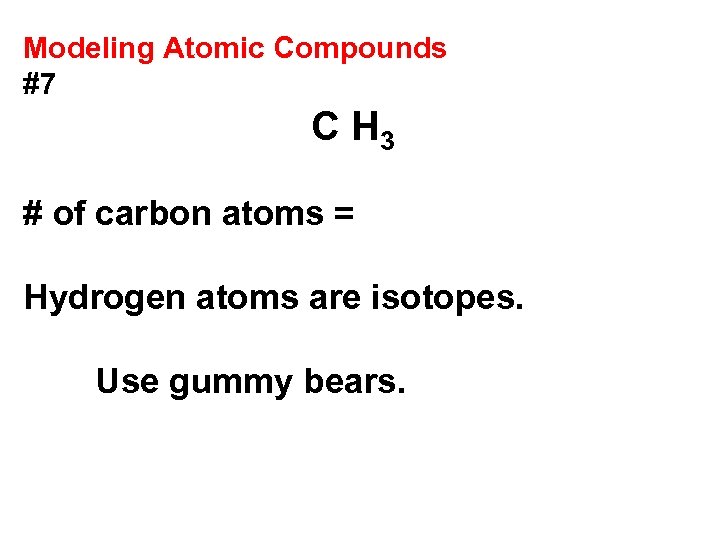 Modeling Atomic Compounds #7 C H 3 # of carbon atoms = Hydrogen atoms are isotopes. Use gummy bears.
Modeling Atomic Compounds #7 C H 3 # of carbon atoms = Hydrogen atoms are isotopes. Use gummy bears.
 Modeling Atomic Compounds #7 C H 3 # of carbon atoms = 1 Hydrogen atoms are isotopes. Use gummy bears.
Modeling Atomic Compounds #7 C H 3 # of carbon atoms = 1 Hydrogen atoms are isotopes. Use gummy bears.
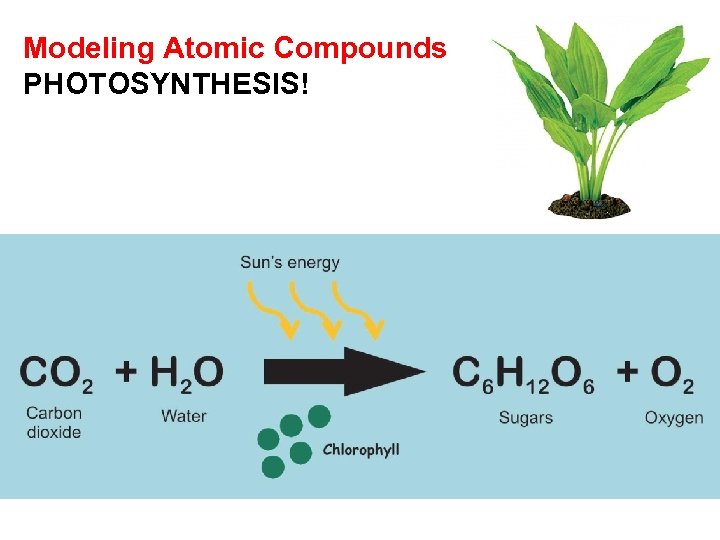 Modeling Atomic Compounds PHOTOSYNTHESIS!
Modeling Atomic Compounds PHOTOSYNTHESIS!
 Modeling Atomic Compounds #7 C H 3 # of carbon atoms = 1 Hydrogen atoms are isotopes. Use gummy bears.
Modeling Atomic Compounds #7 C H 3 # of carbon atoms = 1 Hydrogen atoms are isotopes. Use gummy bears.
 Modeling Atomic Compounds #8 6 H 20 # of hydrogen atoms = # of oxygen atoms =
Modeling Atomic Compounds #8 6 H 20 # of hydrogen atoms = # of oxygen atoms =
 Modeling Atomic Compounds #8 6 H 20 # of hydrogen atoms =12 # of oxygen atoms = 6 BROWN TOOTSIE ROLLS No isotopes. RED
Modeling Atomic Compounds #8 6 H 20 # of hydrogen atoms =12 # of oxygen atoms = 6 BROWN TOOTSIE ROLLS No isotopes. RED
 D 1. 1 (1 -5), Restate the Qs 1. The two atoms most common in Earth’s crust are oxygen and silicon. The two atoms most common in the human body are oxygen and carbon.
D 1. 1 (1 -5), Restate the Qs 1. The two atoms most common in Earth’s crust are oxygen and silicon. The two atoms most common in the human body are oxygen and carbon.
 D 1. 1 (1 -5), Restate the Qs 2. The particles that make up an atom are protons, neutrons, and electrons.
D 1. 1 (1 -5), Restate the Qs 2. The particles that make up an atom are protons, neutrons, and electrons.
 D 1. 1 (1 -5), Restate the Qs 3. When an atom forms an ion, it gains or loses an electron.
D 1. 1 (1 -5), Restate the Qs 3. When an atom forms an ion, it gains or loses an electron.
 D 1. 1 (1 -5), Restate the Qs 4. The size of a magnesium ion with a 2+ charge can be compared with that of a sodium ion with a single + charge. A 2+ charge means the atom has lost 2 electrons. A single + charge means the atom has lost 1 electron. A 2+ atom (ion) is smaller than a (1)+ atom (ion).
D 1. 1 (1 -5), Restate the Qs 4. The size of a magnesium ion with a 2+ charge can be compared with that of a sodium ion with a single + charge. A 2+ charge means the atom has lost 2 electrons. A single + charge means the atom has lost 1 electron. A 2+ atom (ion) is smaller than a (1)+ atom (ion).
 D 1. 1 (1 -5), Restate the Qs 5. Potassium-39 differs from potassium-41 because they have a different number of neutrons. Potassium-41 has two more neutrons than Potassium-39.
D 1. 1 (1 -5), Restate the Qs 5. Potassium-39 differs from potassium-41 because they have a different number of neutrons. Potassium-41 has two more neutrons than Potassium-39.
 How big is an atom? Tell us, Bill Nye! https: //www. youtube. com/watch? v=MZ 6 -dpx. Lq. Uk On a separate sheet of paper, write 10 facts and 1 question.
How big is an atom? Tell us, Bill Nye! https: //www. youtube. com/watch? v=MZ 6 -dpx. Lq. Uk On a separate sheet of paper, write 10 facts and 1 question.
 Other videos Sci show: https: //www. youtube. com/watch? v=thn. Dx. Fdkz. Zs Aurora sci show particle accelerator: https: //www. youtube. com/watch? v=YFNhu. QPVw. Xk bill nye: https: //www. youtube. com/watch? v=cn. XV 7 Ph 3 WPk&list=PL 2 m. VBA_uqi. Ucn 0 P 5 U_NQHWOu. Y-JANQM 5 Q chanel: https: //www. youtube. com/watch? v=bw 5 TE 5 o 7 Jt. E&list=PLPUFU 9 ubs 44 g 79 q. Q 6 bj. TPbic. CDTfv 6 IEI
Other videos Sci show: https: //www. youtube. com/watch? v=thn. Dx. Fdkz. Zs Aurora sci show particle accelerator: https: //www. youtube. com/watch? v=YFNhu. QPVw. Xk bill nye: https: //www. youtube. com/watch? v=cn. XV 7 Ph 3 WPk&list=PL 2 m. VBA_uqi. Ucn 0 P 5 U_NQHWOu. Y-JANQM 5 Q chanel: https: //www. youtube. com/watch? v=bw 5 TE 5 o 7 Jt. E&list=PLPUFU 9 ubs 44 g 79 q. Q 6 bj. TPbic. CDTfv 6 IEI
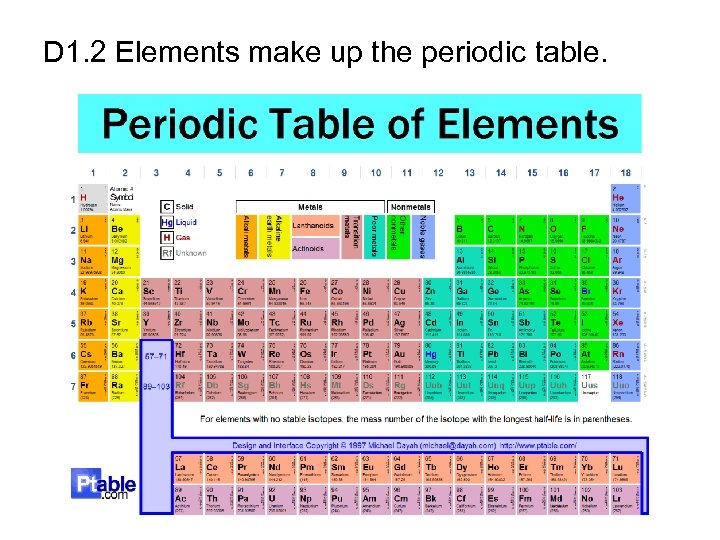 D 1. 2 Elements make up the periodic table.
D 1. 2 Elements make up the periodic table.
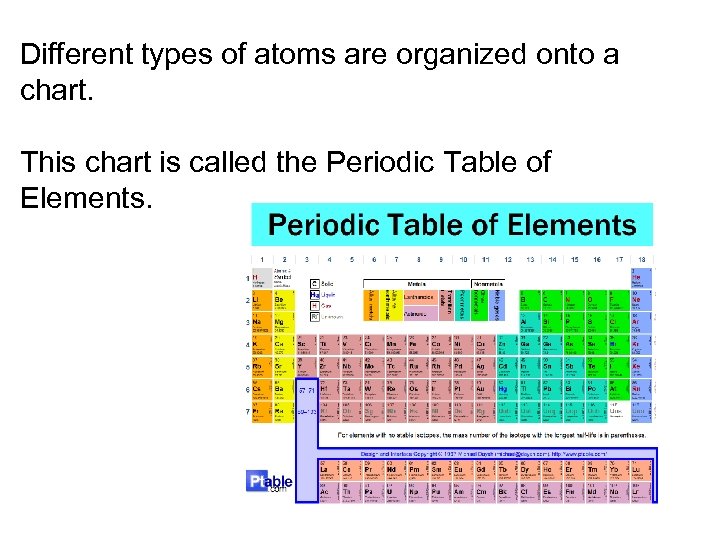 Different types of atoms are organized onto a chart. This chart is called the Periodic Table of Elements.
Different types of atoms are organized onto a chart. This chart is called the Periodic Table of Elements.
 Science + Music = Awesome Asap 4. 2 https: //www. youtube. com/watch? v=-I 7 l 8 Tgtu. LQ Faster 3: https: //www. youtube. com/watch? v=z. UDDi. Wt. Ft. EM&list=RDz. UDDi. Wt. Ft EM
Science + Music = Awesome Asap 4. 2 https: //www. youtube. com/watch? v=-I 7 l 8 Tgtu. LQ Faster 3: https: //www. youtube. com/watch? v=z. UDDi. Wt. Ft. EM&list=RDz. UDDi. Wt. Ft EM
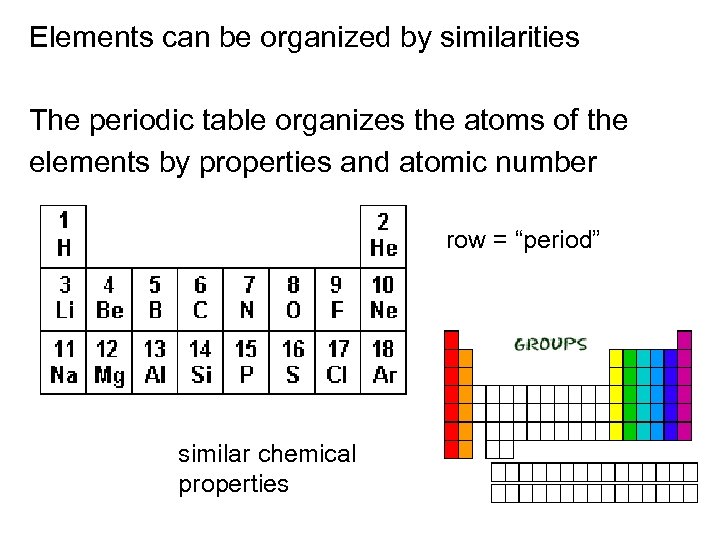 Elements can be organized by similarities The periodic table organizes the atoms of the elements by properties and atomic number row = “period” similar chemical properties
Elements can be organized by similarities The periodic table organizes the atoms of the elements by properties and atomic number row = “period” similar chemical properties
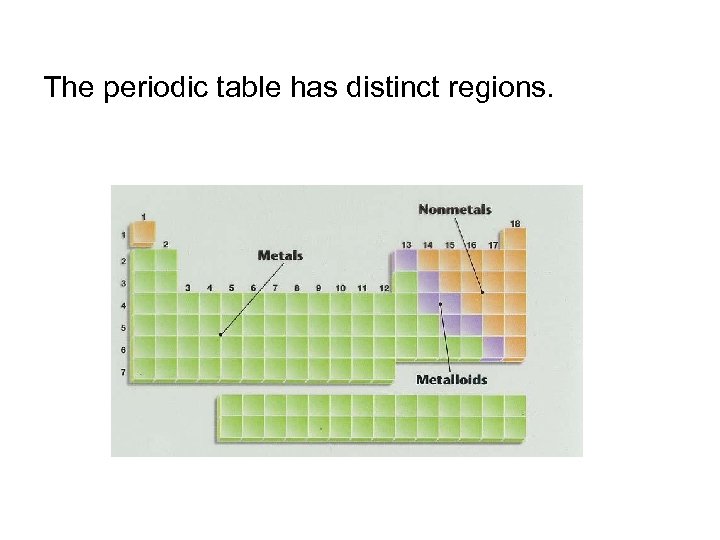 The periodic table has distinct regions.
The periodic table has distinct regions.
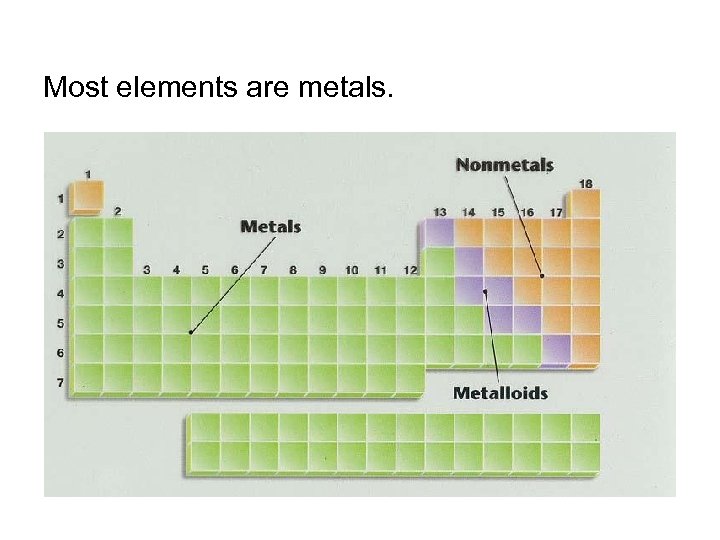 Most elements are metals.
Most elements are metals.
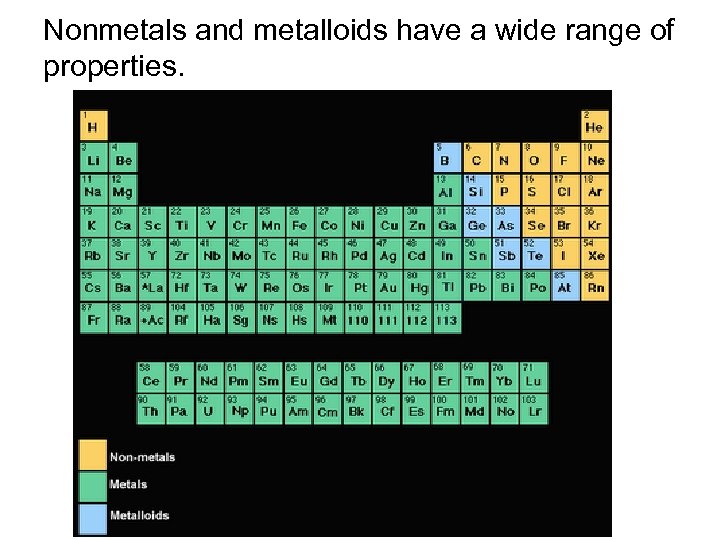 Nonmetals and metalloids have a wide range of properties.
Nonmetals and metalloids have a wide range of properties.
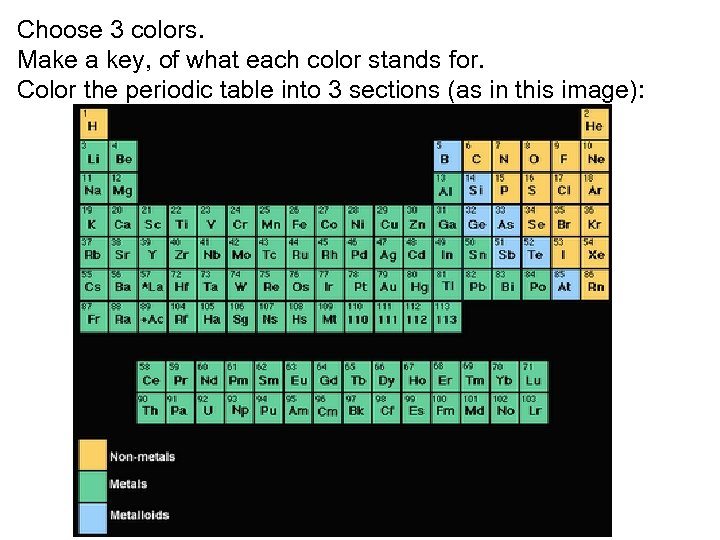 Choose 3 colors. Make a key, of what each color stands for. Color the periodic table into 3 sections (as in this image):
Choose 3 colors. Make a key, of what each color stands for. Color the periodic table into 3 sections (as in this image):
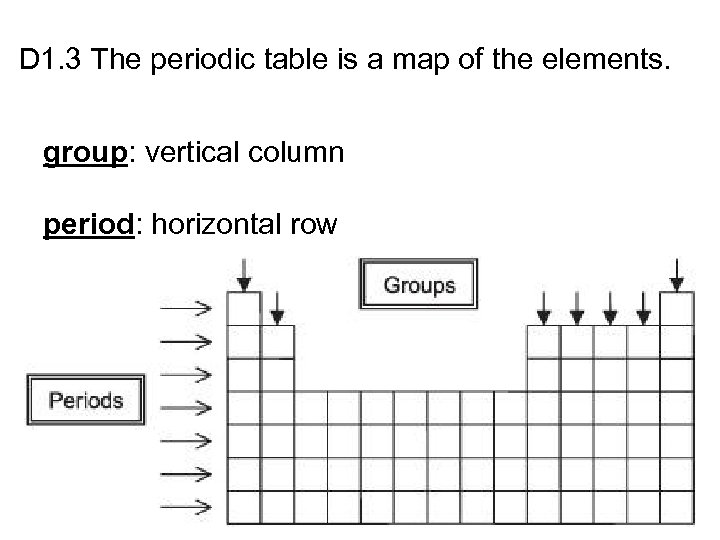 D 1. 3 The periodic table is a map of the elements. group: vertical column period: horizontal row
D 1. 3 The periodic table is a map of the elements. group: vertical column period: horizontal row
 Videos Sci Show: creation 11 http: //www. youtube. com/watch? v=0 RRVV 4 Diomg Periodic table parts: http: //www. youtube. com/watch? v=5 MMWpe. J 5 dn 4
Videos Sci Show: creation 11 http: //www. youtube. com/watch? v=0 RRVV 4 Diomg Periodic table parts: http: //www. youtube. com/watch? v=5 MMWpe. J 5 dn 4
 Video 4. 5 min reactions Chemical http: //www. youtube. com/watch? v=l. Qap 3 c. Rn. P 28 +reverse: http: //www. youtube. com/watch? v=C 5 t. OEBm. BAHg another: http: //www. youtube. com/watch? v=p-g_0 wyh. V 9 E reactions: http: //www. youtube. com/watch? v=Uk. Bh. W 8 Kj 3 r 8 w/ heat: http: //www. youtube. com/watch? v=ritaljhhk 7 s Nye: https: //www. youtube. com/watch? v=smk. Vzf. Zm. DIU 23: http: //www. youtube. com/watch? v=Plwuxp. Mh 8 nk
Video 4. 5 min reactions Chemical http: //www. youtube. com/watch? v=l. Qap 3 c. Rn. P 28 +reverse: http: //www. youtube. com/watch? v=C 5 t. OEBm. BAHg another: http: //www. youtube. com/watch? v=p-g_0 wyh. V 9 E reactions: http: //www. youtube. com/watch? v=Uk. Bh. W 8 Kj 3 r 8 w/ heat: http: //www. youtube. com/watch? v=ritaljhhk 7 s Nye: https: //www. youtube. com/watch? v=smk. Vzf. Zm. DIU 23: http: //www. youtube. com/watch? v=Plwuxp. Mh 8 nk
 Alka Seltzer Chemical reaction Why fizz? https: //www. youtube. com/watch? v=TKp. MTz 06 qr. U
Alka Seltzer Chemical reaction Why fizz? https: //www. youtube. com/watch? v=TKp. MTz 06 qr. U
 atomic compound: two or more atoms bonded together
atomic compound: two or more atoms bonded together
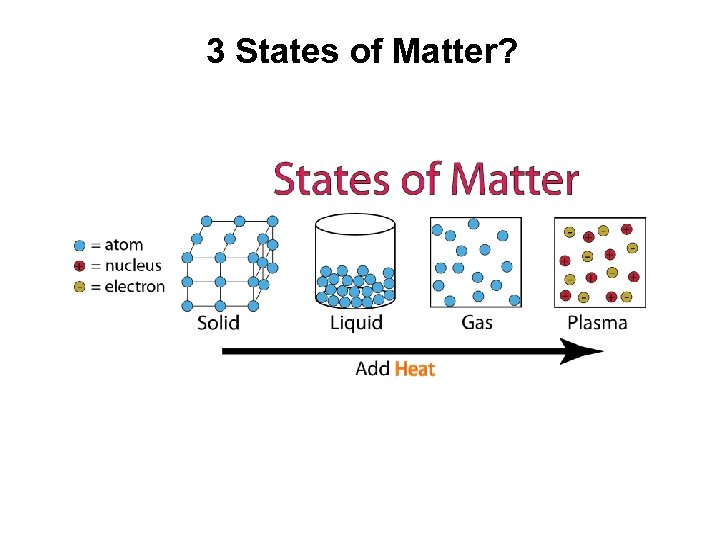 3 States of Matter?
3 States of Matter?
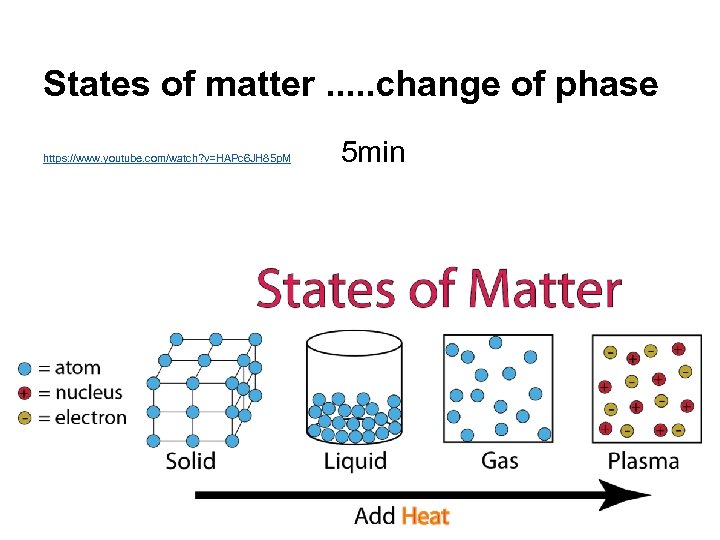 States of matter. . . change of phase https: //www. youtube. com/watch? v=HAPc 6 JH 85 p. M 5 min
States of matter. . . change of phase https: //www. youtube. com/watch? v=HAPc 6 JH 85 p. M 5 min
 non-Newtonian fluid: a state of matter; substance acts like a liquid when at rest, like a solid when pressure is applied pool: http: //www. youtube. com/watch? v=f 2 XQ 97 XHj. Vw over speaker: http: //www. youtube. com/watch? v=3 zo. TKXXNQIU Slow Mo: http: //www. youtube. com/watch? v=G 1 Op_1 y. G 6 l. Q science party: http: //www. youtube. com/watch? v=D-wxn. ID 2 q 4 A
non-Newtonian fluid: a state of matter; substance acts like a liquid when at rest, like a solid when pressure is applied pool: http: //www. youtube. com/watch? v=f 2 XQ 97 XHj. Vw over speaker: http: //www. youtube. com/watch? v=3 zo. TKXXNQIU Slow Mo: http: //www. youtube. com/watch? v=G 1 Op_1 y. G 6 l. Q science party: http: //www. youtube. com/watch? v=D-wxn. ID 2 q 4 A
 More non-newtonian vids: Mythbusters: http: //www. youtube. com/watch? v=D 2 a. B 3 n. Cm. III Oobleck Dubb step: http: //www. youtube. com/watch? v=yo. B 0 p. M 2 VASg
More non-newtonian vids: Mythbusters: http: //www. youtube. com/watch? v=D 2 a. B 3 n. Cm. III Oobleck Dubb step: http: //www. youtube. com/watch? v=yo. B 0 p. M 2 VASg
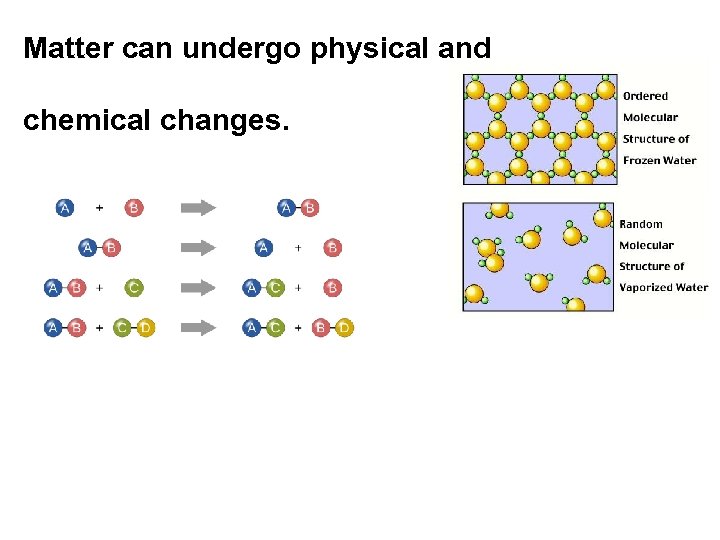 Matter can undergo physical and chemical changes.
Matter can undergo physical and chemical changes.
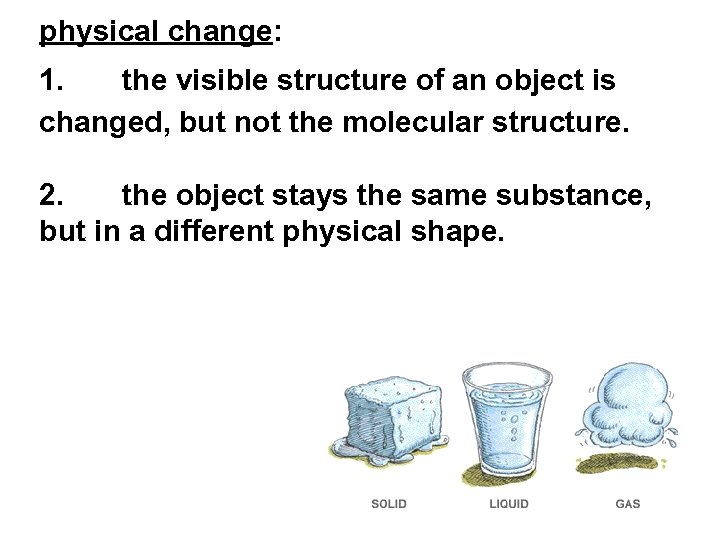 physical change: 1. the visible structure of an object is changed, but not the molecular structure. 2. the object stays the same substance, but in a different physical shape.
physical change: 1. the visible structure of an object is changed, but not the molecular structure. 2. the object stays the same substance, but in a different physical shape.
 chemical change: 1. the molecular structure of an object is changed into a new substance. 2. all or some signs are present: -production of an odor -change in temperature -change in color -formation of bubbles -formation of a solid VID: https: //www. youtube. com/watch? v=qqqm. FFCwd 7 k
chemical change: 1. the molecular structure of an object is changed into a new substance. 2. all or some signs are present: -production of an odor -change in temperature -change in color -formation of bubbles -formation of a solid VID: https: //www. youtube. com/watch? v=qqqm. FFCwd 7 k
 Videos: https: //www. youtube. com/watch? v=hcun. Qqb. NEMQ how plastic made: http: //www. youtube. com/watch? v=6 e. Ct 0 VDg-Kc how recyled bottles made: http: //www. youtube. com/watch? v=8 Qkxp. QT 967 w chem reactions: https: //www. youtube. com/watch? v=Uk. Bh. W 8 Kj 3 r 8 https: //www. youtube. com/watch? v=Bjy. XIZtl. HFo bill Nye: https: //www. youtube. com/watch? v=Plwuxp. Mh 8 nk changes: https: //www. youtube. com/watch? v=hcun. Qqb. NEMQ Bozeman: https: //www. youtube. com/watch? v=X 328 AWa. JXv. I chem reactions: Chem. Reactions 4 min Beautiful reactions: https: //www. youtube. com/watch? v=T 14 D 61 Pd Yko
Videos: https: //www. youtube. com/watch? v=hcun. Qqb. NEMQ how plastic made: http: //www. youtube. com/watch? v=6 e. Ct 0 VDg-Kc how recyled bottles made: http: //www. youtube. com/watch? v=8 Qkxp. QT 967 w chem reactions: https: //www. youtube. com/watch? v=Uk. Bh. W 8 Kj 3 r 8 https: //www. youtube. com/watch? v=Bjy. XIZtl. HFo bill Nye: https: //www. youtube. com/watch? v=Plwuxp. Mh 8 nk changes: https: //www. youtube. com/watch? v=hcun. Qqb. NEMQ Bozeman: https: //www. youtube. com/watch? v=X 328 AWa. JXv. I chem reactions: Chem. Reactions 4 min Beautiful reactions: https: //www. youtube. com/watch? v=T 14 D 61 Pd Yko
 energy: the ability to cause a change
energy: the ability to cause a change
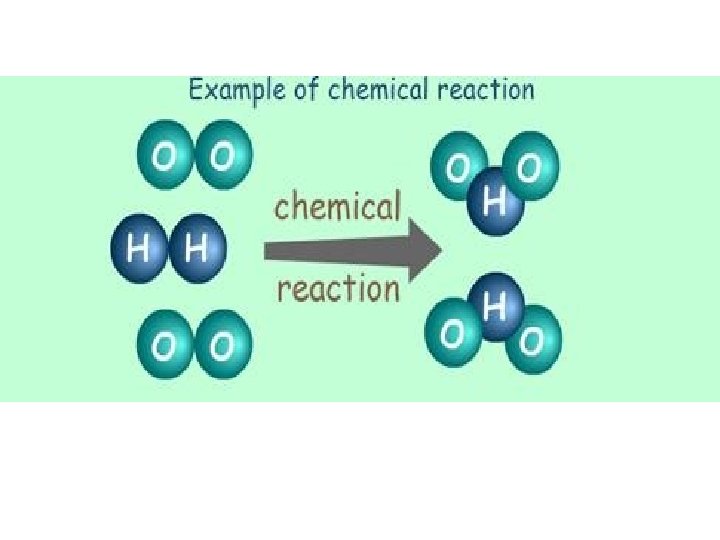
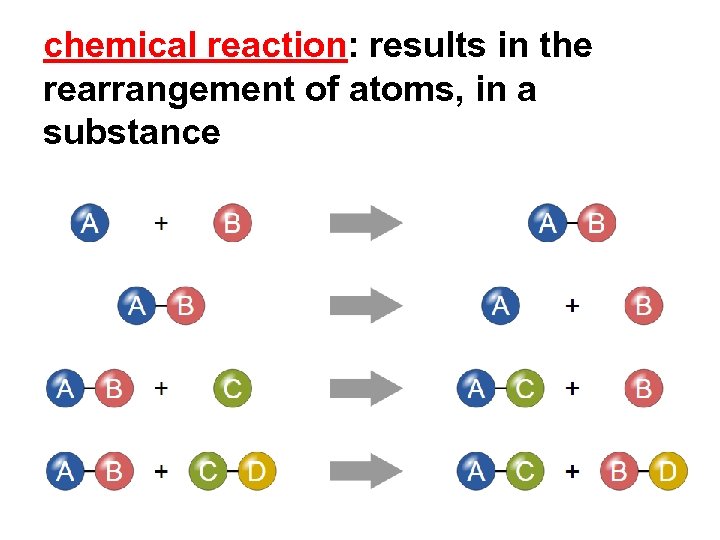 chemical reaction: results in the rearrangement of atoms, in a substance
chemical reaction: results in the rearrangement of atoms, in a substance
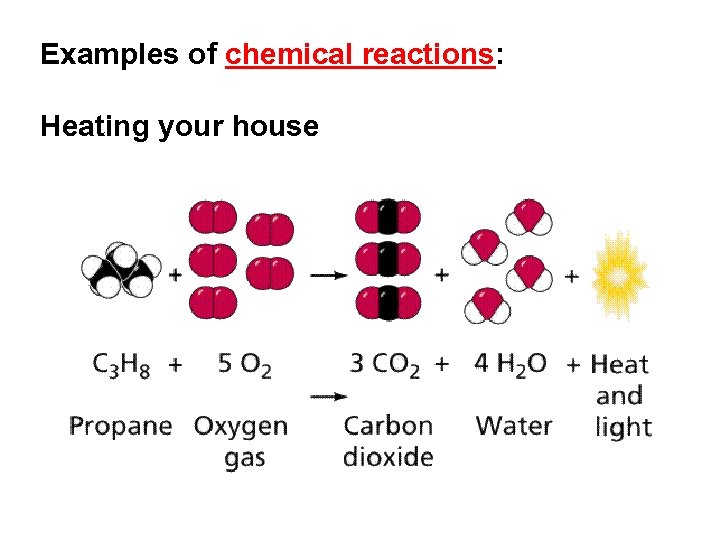 Examples of chemical reactions: Heating your house
Examples of chemical reactions: Heating your house
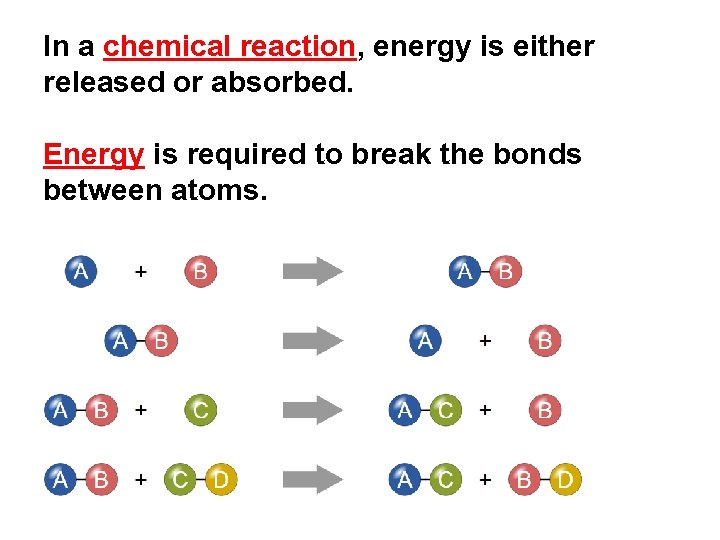 In a chemical reaction, energy is either released or absorbed. Energy is required to break the bonds between atoms.
In a chemical reaction, energy is either released or absorbed. Energy is required to break the bonds between atoms.
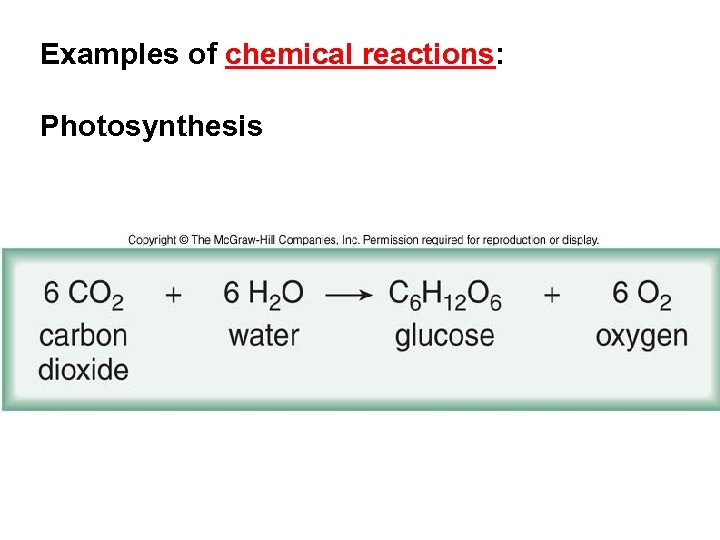 Examples of chemical reactions: Photosynthesis
Examples of chemical reactions: Photosynthesis
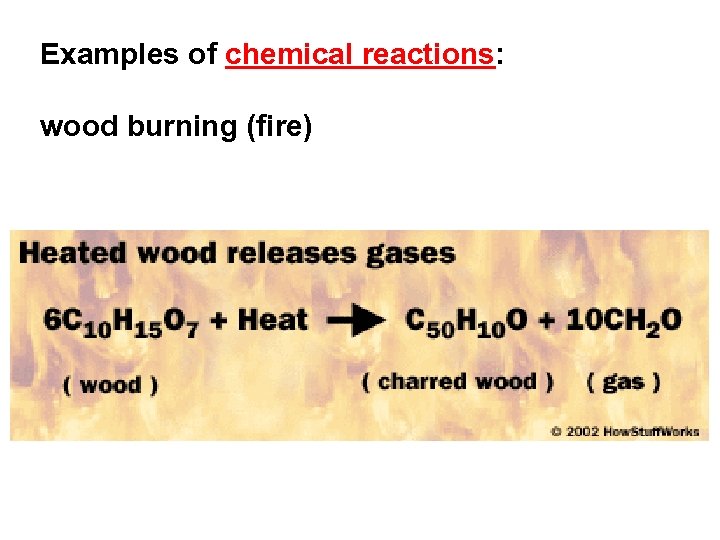 Examples of chemical reactions: wood burning (fire)
Examples of chemical reactions: wood burning (fire)
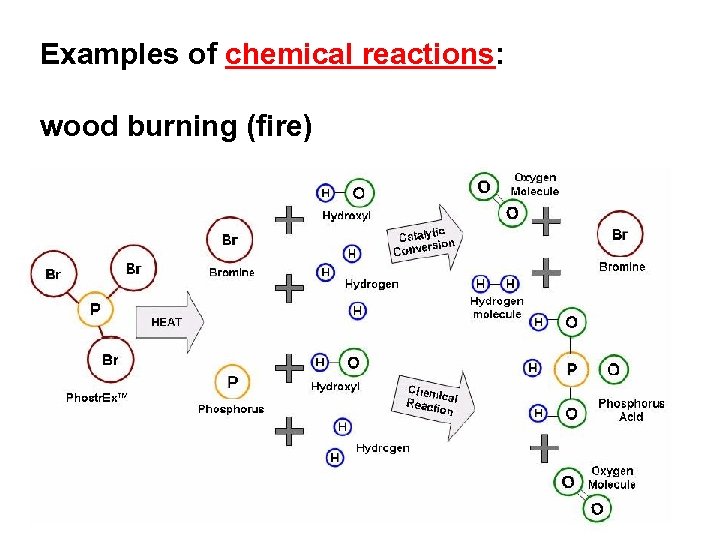 Examples of chemical reactions: wood burning (fire)
Examples of chemical reactions: wood burning (fire)
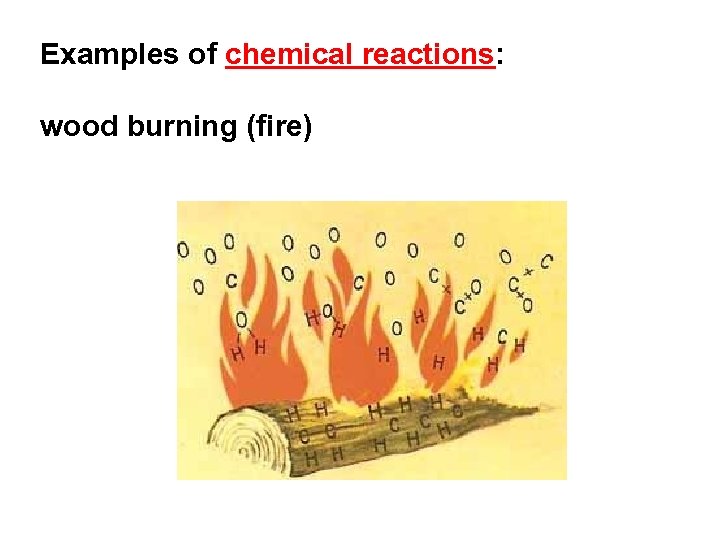 Examples of chemical reactions: wood burning (fire)
Examples of chemical reactions: wood burning (fire)
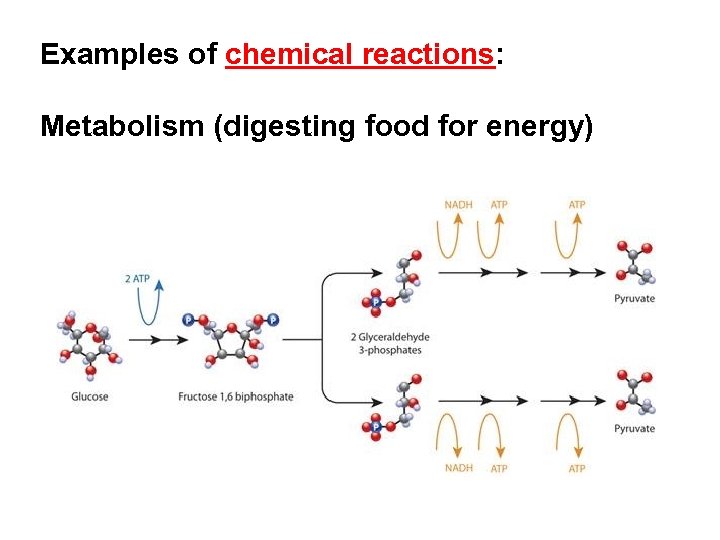 Examples of chemical reactions: Metabolism (digesting food for energy)
Examples of chemical reactions: Metabolism (digesting food for energy)
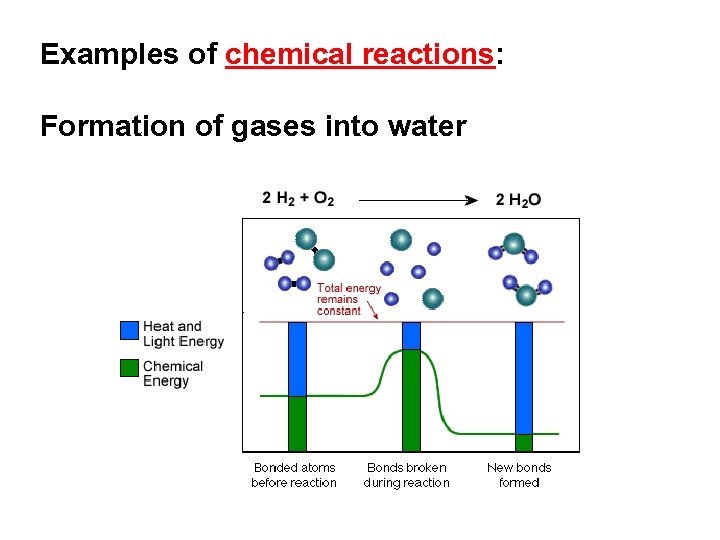 Examples of chemical reactions: Formation of gases into water
Examples of chemical reactions: Formation of gases into water
 Write 5 sentences: Describe a chemical reaction that you have observed in everyday life. Underline: 3 pieces of quantitative info Underline: 5 pieces of qualitative info How did the chemical reaction start? What did it look like? What was the end result? How did you know it was a chemical reaction?
Write 5 sentences: Describe a chemical reaction that you have observed in everyday life. Underline: 3 pieces of quantitative info Underline: 5 pieces of qualitative info How did the chemical reaction start? What did it look like? What was the end result? How did you know it was a chemical reaction?
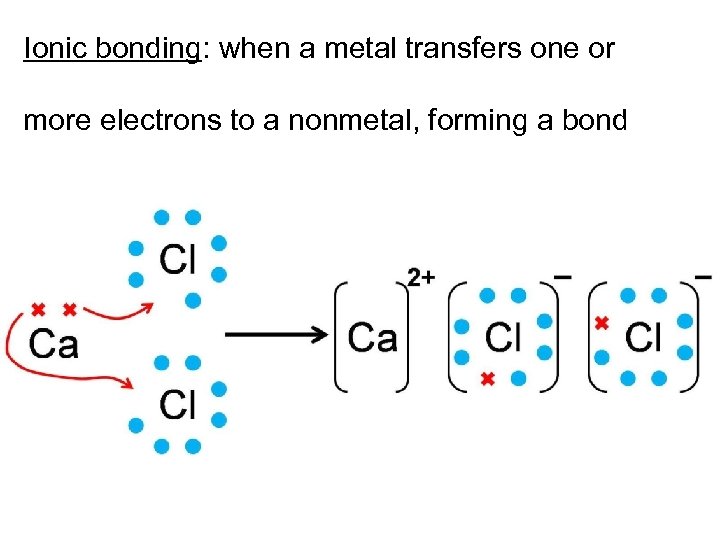 Ionic bonding: when a metal transfers one or more electrons to a nonmetal, forming a bond video: http: //www. youtube. com/watch? v=Qf 07 -8 Jhhpc
Ionic bonding: when a metal transfers one or more electrons to a nonmetal, forming a bond video: http: //www. youtube. com/watch? v=Qf 07 -8 Jhhpc
 Study by watching videos http: //www. youtube. com/watch? v=Qf 07 -8 Jhhpc Balancing equations: http: //www. youtube. com/watch? v=UGf 60 kq_ZDI Sodium Chloride reaction atoms: https: //www. youtube. com/watch? v=2 Ljxw. UNSd FA
Study by watching videos http: //www. youtube. com/watch? v=Qf 07 -8 Jhhpc Balancing equations: http: //www. youtube. com/watch? v=UGf 60 kq_ZDI Sodium Chloride reaction atoms: https: //www. youtube. com/watch? v=2 Ljxw. UNSd FA
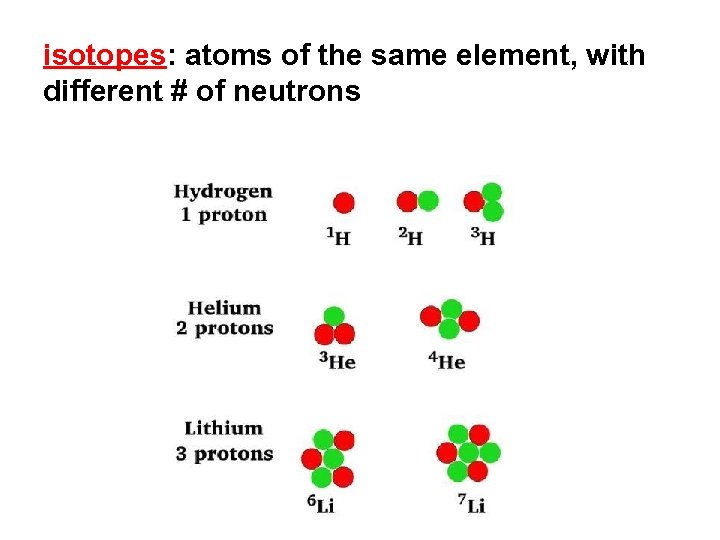 isotopes: atoms of the same element, with different # of neutrons
isotopes: atoms of the same element, with different # of neutrons
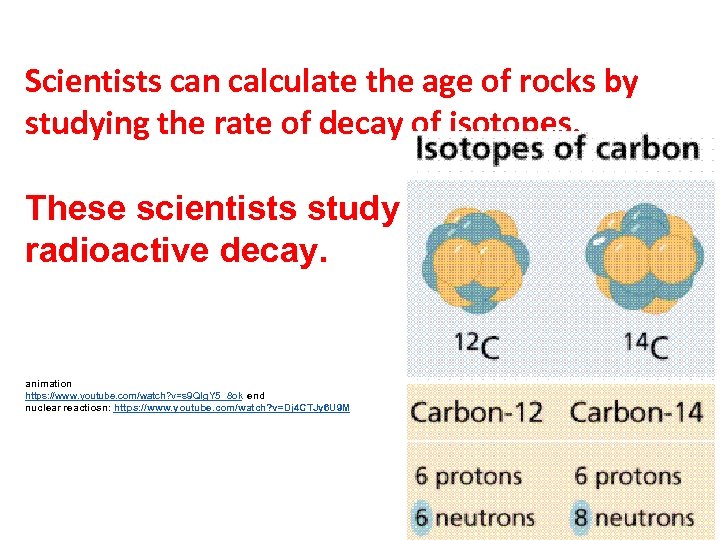 Scientists can calculate the age of rocks by studying the rate of decay of isotopes. These scientists study radioactive decay. animation https: //www. youtube. com/watch? v=s 9 QIg. Y 5_8 ok end nuclear reactiosn: https: //www. youtube. com/watch? v=Dj 4 CTJy 6 U 9 M
Scientists can calculate the age of rocks by studying the rate of decay of isotopes. These scientists study radioactive decay. animation https: //www. youtube. com/watch? v=s 9 QIg. Y 5_8 ok end nuclear reactiosn: https: //www. youtube. com/watch? v=Dj 4 CTJy 6 U 9 M
 4. Some atoms can change their identity
4. Some atoms can change their identity
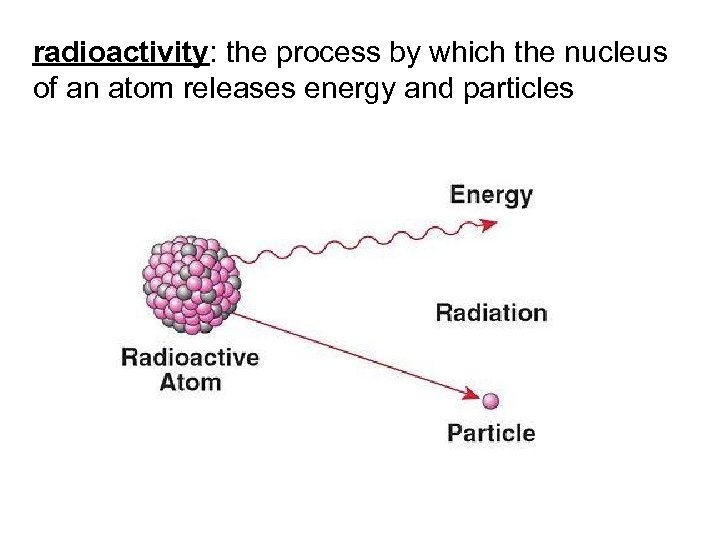 radioactivity: the process by which the nucleus of an atom releases energy and particles
radioactivity: the process by which the nucleus of an atom releases energy and particles
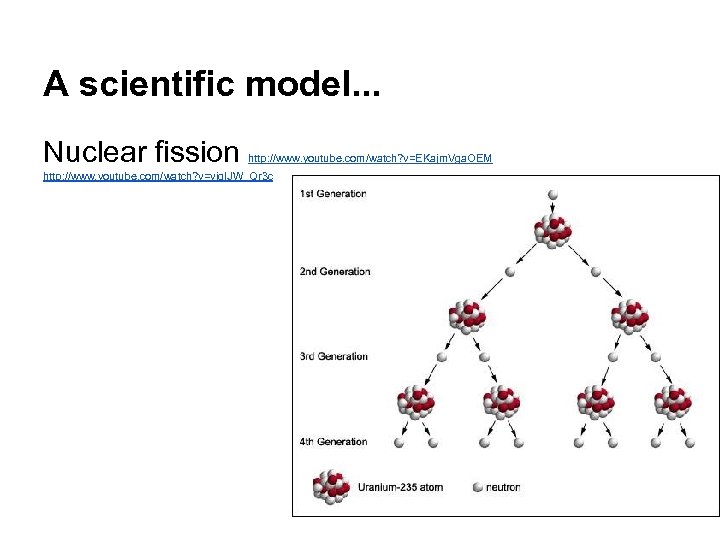 A scientific model. . . Nuclear fission http: //www. youtube. com/watch? v=EKajm. Vga. OEM http: //www. youtube. com/watch? v=vjq. IJW_Qr 3 c
A scientific model. . . Nuclear fission http: //www. youtube. com/watch? v=EKajm. Vga. OEM http: //www. youtube. com/watch? v=vjq. IJW_Qr 3 c
 Radioactive decay 1. the process by which the neutrons in an atom change over time; 2. a method used to find out the age of fossils How vid: http: //science. howstuffworks. com/29296 -100 -greatest-discoveries-radioactivity-video. htm
Radioactive decay 1. the process by which the neutrons in an atom change over time; 2. a method used to find out the age of fossils How vid: http: //science. howstuffworks. com/29296 -100 -greatest-discoveries-radioactivity-video. htm
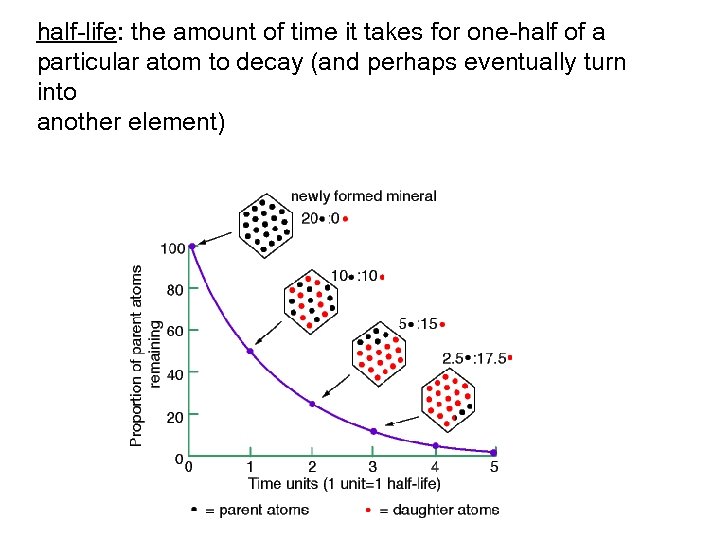 half-life: the amount of time it takes for one-half of a particular atom to decay (and perhaps eventually turn into another element)
half-life: the amount of time it takes for one-half of a particular atom to decay (and perhaps eventually turn into another element)
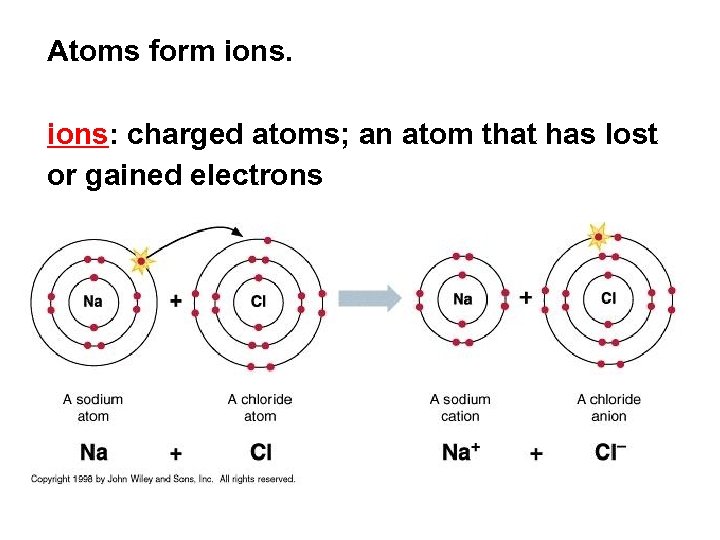 Atoms form ions: charged atoms; an atom that has lost or gained electrons
Atoms form ions: charged atoms; an atom that has lost or gained electrons
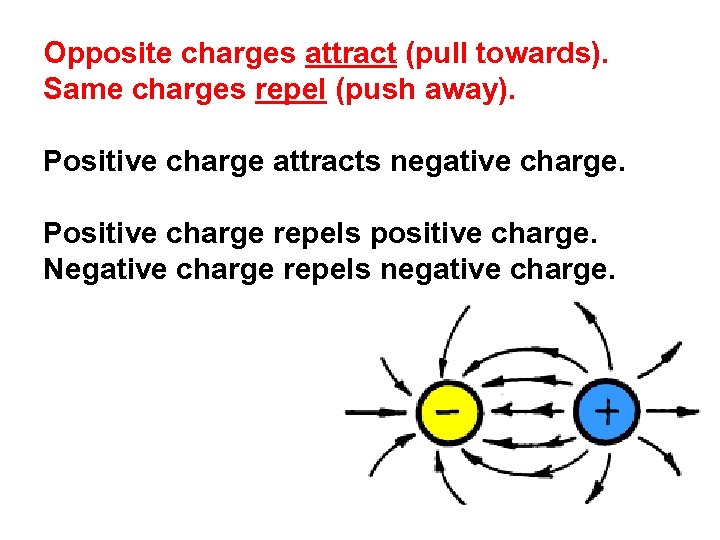 Opposite charges attract (pull towards). Same charges repel (push away). Positive charge attracts negative charge. Positive charge repels positive charge. Negative charge repels negative charge.
Opposite charges attract (pull towards). Same charges repel (push away). Positive charge attracts negative charge. Positive charge repels positive charge. Negative charge repels negative charge.
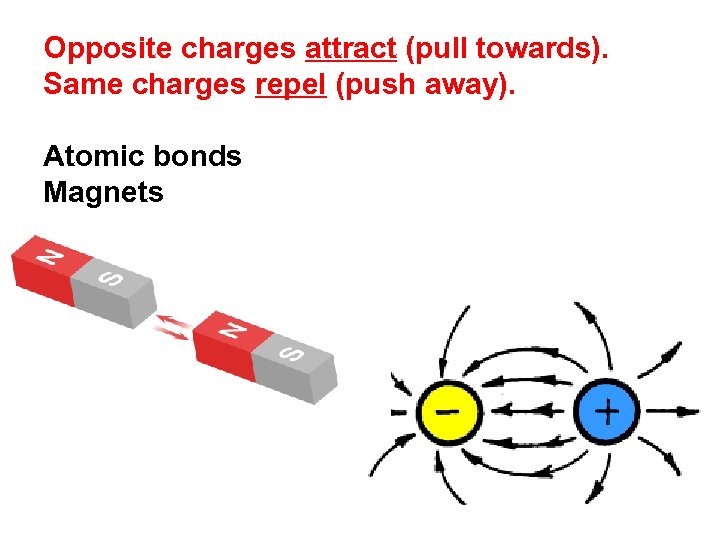 Opposite charges attract (pull towards). Same charges repel (push away). Atomic bonds Magnets
Opposite charges attract (pull towards). Same charges repel (push away). Atomic bonds Magnets
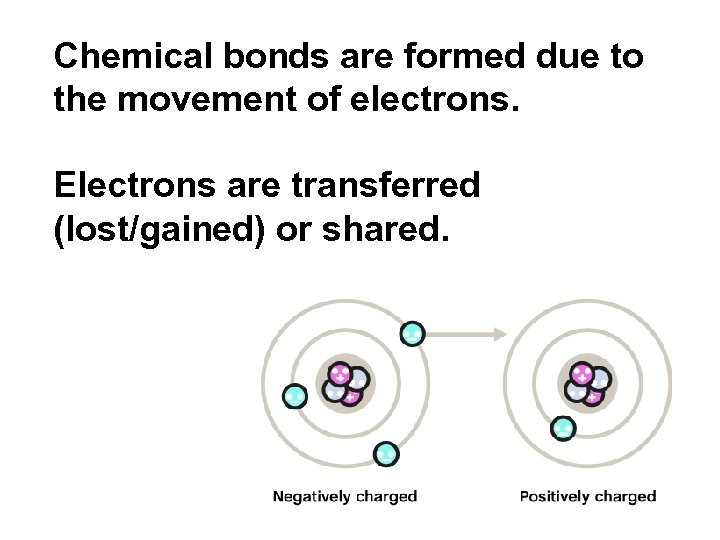 Chemical bonds are formed due to the movement of electrons. Electrons are transferred (lost/gained) or shared.
Chemical bonds are formed due to the movement of electrons. Electrons are transferred (lost/gained) or shared.
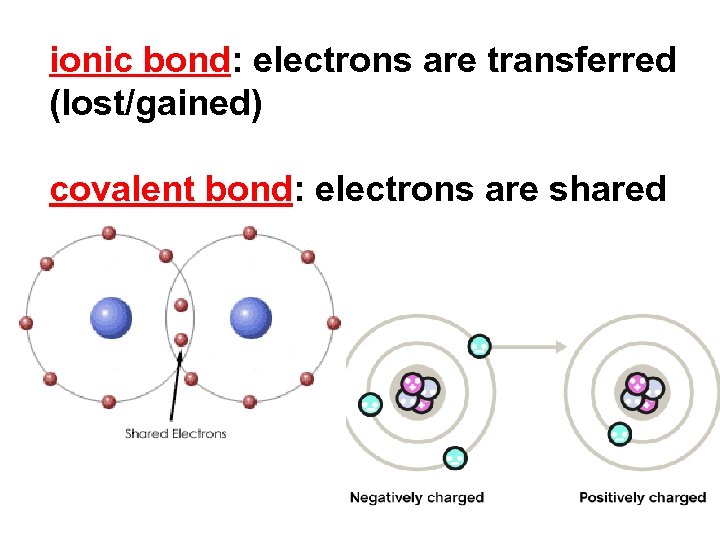 ionic bond: electrons are transferred (lost/gained) covalent bond: electrons are shared
ionic bond: electrons are transferred (lost/gained) covalent bond: electrons are shared
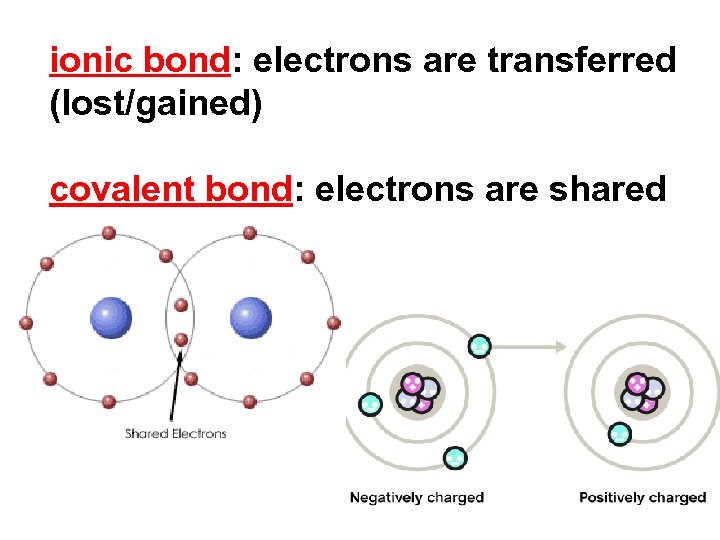 ionic bond: electrons are transferred (lost/gained) covalent bond: electrons are shared
ionic bond: electrons are transferred (lost/gained) covalent bond: electrons are shared
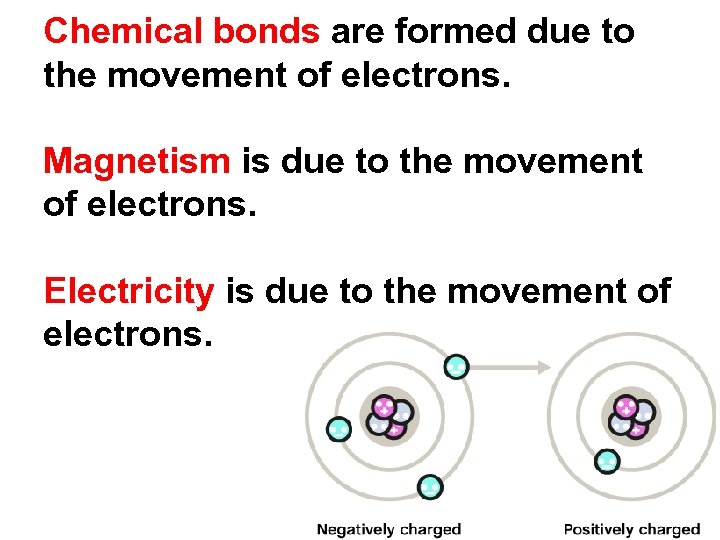 Chemical bonds are formed due to the movement of electrons. Magnetism is due to the movement of electrons. Electricity is due to the movement of electrons.
Chemical bonds are formed due to the movement of electrons. Magnetism is due to the movement of electrons. Electricity is due to the movement of electrons.
 Radioactive dating: the method of studying radioactive decay rates in order to measure the age of rocks info: https: //www. youtube. com/watch? v=v. Yv. USro 7 YUM
Radioactive dating: the method of studying radioactive decay rates in order to measure the age of rocks info: https: //www. youtube. com/watch? v=v. Yv. USro 7 YUM
 How to express ratios verbally. 3: 1 three to one. . . book example: hydrogen to nitrogen H 3 N This is NOT the same as 1: 3
How to express ratios verbally. 3: 1 three to one. . . book example: hydrogen to nitrogen H 3 N This is NOT the same as 1: 3
 1: 2 CO 2 in words? ratio of elements?
1: 2 CO 2 in words? ratio of elements?
 1: 2 CO 2 one to two ratio of elements?
1: 2 CO 2 one to two ratio of elements?
 1: 2 CO 2 one to two carbon to oxygen
1: 2 CO 2 one to two carbon to oxygen
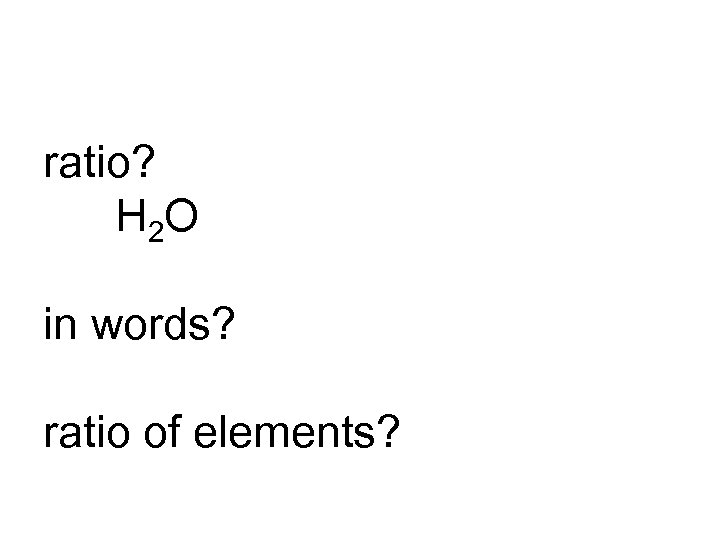 ratio? H 2 O in words? ratio of elements?
ratio? H 2 O in words? ratio of elements?
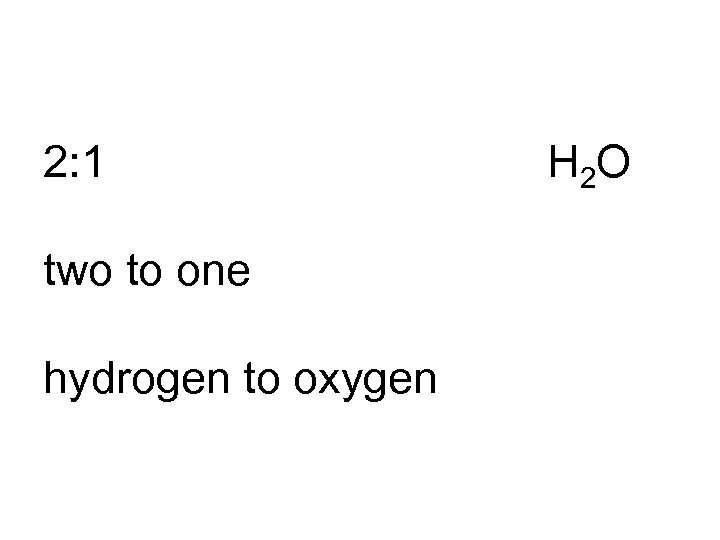 2: 1 two to one hydrogen to oxygen H 2 O
2: 1 two to one hydrogen to oxygen H 2 O
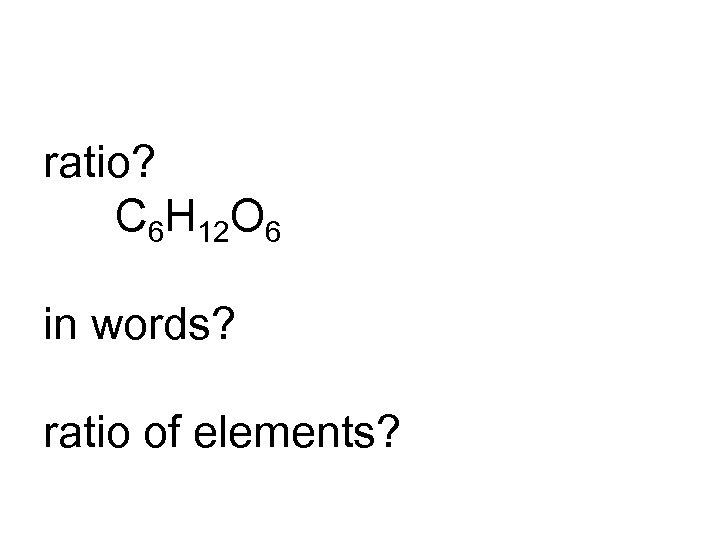 ratio? C 6 H 12 O 6 in words? ratio of elements?
ratio? C 6 H 12 O 6 in words? ratio of elements?
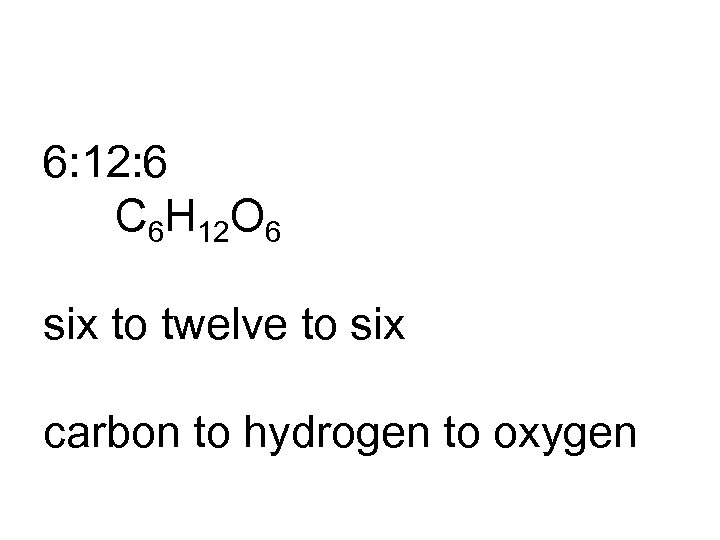 6: 12: 6 C 6 H 12 O 6 six to twelve to six carbon to hydrogen to oxygen
6: 12: 6 C 6 H 12 O 6 six to twelve to six carbon to hydrogen to oxygen
 Unit D Section 2. 1 I. Elements combine to form compounds
Unit D Section 2. 1 I. Elements combine to form compounds
 Unit D Section 2. 1 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them.
Unit D Section 2. 1 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them.
 Unit D Section 2. 1 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. i. compound: a substance made of atoms of 2 or more different elements
Unit D Section 2. 1 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. i. compound: a substance made of atoms of 2 or more different elements
 Unit D Section 2. 1 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. i. compound: a substance made of atoms of 2 or more different elements ii. chemical bonds: hold atoms together in large networks or small groups; determine the properties of a compound.
Unit D Section 2. 1 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. i. compound: a substance made of atoms of 2 or more different elements ii. chemical bonds: hold atoms together in large networks or small groups; determine the properties of a compound.
 Unit D Section 2. 1 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. i. compound: a substance made of atoms of 2 or more different elements ii. chemical bonds: hold atoms together in large networks or small groups; determine the properties of a compound. iii. compound properties: depend upon type of atoms (element) and shape (arrangement of atoms)
Unit D Section 2. 1 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. i. compound: a substance made of atoms of 2 or more different elements ii. chemical bonds: hold atoms together in large networks or small groups; determine the properties of a compound. iii. compound properties: depend upon type of atoms (element) and shape (arrangement of atoms)
 Unit D Section 2. 1 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. i. compound: a substance made of atoms of 2 or more different elements ii. chemical bonds: hold atoms together in large networks or small groups; determine the properties of a compound. iii. compound properties: depend upon type of atoms (element) and shape (arrangement of atoms) iv. properties of compound may be different than the substances they make-up
Unit D Section 2. 1 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. i. compound: a substance made of atoms of 2 or more different elements ii. chemical bonds: hold atoms together in large networks or small groups; determine the properties of a compound. iii. compound properties: depend upon type of atoms (element) and shape (arrangement of atoms) iv. properties of compound may be different than the substances they make-up
 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. b. Atoms combine in predictable numbers.
I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. b. Atoms combine in predictable numbers.
 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. b. Atoms combine in predictable numbers. i. particular compounds contain a particular ratio of atoms; this ratio determines the substance
I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. b. Atoms combine in predictable numbers. i. particular compounds contain a particular ratio of atoms; this ratio determines the substance
 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. b. Atoms combine in predictable numbers. i. particular compounds contain a particular ratio of atoms; this ratio determines the substance c. Chemical formulas i. chemical formula: represents the ratio of atoms in a chemical compound using element symbols
I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. b. Atoms combine in predictable numbers. i. particular compounds contain a particular ratio of atoms; this ratio determines the substance c. Chemical formulas i. chemical formula: represents the ratio of atoms in a chemical compound using element symbols
 I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. b. Atoms combine in predictable numbers. i. particular compounds contain a particular ratio of atoms; this ratio determines the substance c. Chemical formulas i. chemical formula: represents the ratio of atoms in a chemical compound using element symbols ii. steps to writing a chemical formula
I. Elements combine to form compounds a. Compounds have different properties form the elements that make them. b. Atoms combine in predictable numbers. i. particular compounds contain a particular ratio of atoms; this ratio determines the substance c. Chemical formulas i. chemical formula: represents the ratio of atoms in a chemical compound using element symbols ii. steps to writing a chemical formula
 1. find the symbols for the element types 2. include a subscript to note how many atoms of each type of element; (the subscript 1 is never used)
1. find the symbols for the element types 2. include a subscript to note how many atoms of each type of element; (the subscript 1 is never used)
 d. Same elements, different compounds i. different compounds can be formed of atoms of the same elements
d. Same elements, different compounds i. different compounds can be formed of atoms of the same elements
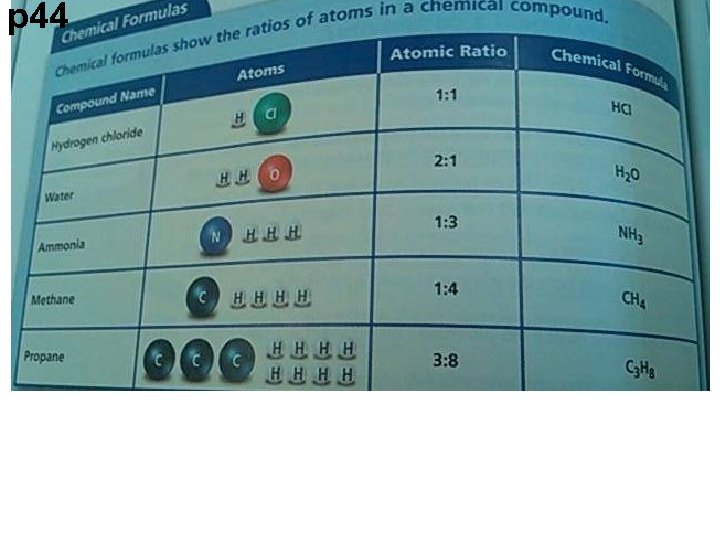 p 44
p 44
 2. 1 Review Questions 1. In many cases, they are different.
2. 1 Review Questions 1. In many cases, they are different.
 2. 1 Review Questions 1. In many cases, they are different. 2. 12 carbon atoms + 22 oxygen atoms + 11 oxygen atoms = 45 total atoms
2. 1 Review Questions 1. In many cases, they are different. 2. 12 carbon atoms + 22 oxygen atoms + 11 oxygen atoms = 45 total atoms
 2. 1 Review Questions 1. In many cases, they are different. 2. 12 carbon atoms + 22 oxygen atoms + 11 oxygen atoms = 45 total atoms 3. Atoms can combine in many different ratios.
2. 1 Review Questions 1. In many cases, they are different. 2. 12 carbon atoms + 22 oxygen atoms + 11 oxygen atoms = 45 total atoms 3. Atoms can combine in many different ratios.
 2. 1 Review Questions 4. All atoms are in a 1: 1 ratio. 5. Compare how they react with another substance 6. The carbon and oxygen atoms are in different ratios in the compounds.
2. 1 Review Questions 4. All atoms are in a 1: 1 ratio. 5. Compare how they react with another substance 6. The carbon and oxygen atoms are in different ratios in the compounds.
 Videos Molecule basics: https: //www. youtube. com/watch? v=vl. SOESXQI 7 o 2. 5 What the Bleep: Water mediation 6 min Water Strider types of bonds (cov+ionic) another Hydrogen Bonding (Polar) FUnction of neurotransmitters solute into solvent Acid Rain Bonds happy#: https: //www. youtube. com/watch? v=Qqjc. Cvz. Wwww dogs: https: //www. youtube. com/watch? v=_M 9 khs 87 x. Q 8
Videos Molecule basics: https: //www. youtube. com/watch? v=vl. SOESXQI 7 o 2. 5 What the Bleep: Water mediation 6 min Water Strider types of bonds (cov+ionic) another Hydrogen Bonding (Polar) FUnction of neurotransmitters solute into solvent Acid Rain Bonds happy#: https: //www. youtube. com/watch? v=Qqjc. Cvz. Wwww dogs: https: //www. youtube. com/watch? v=_M 9 khs 87 x. Q 8
 quantitative: (adj) expressed as a quantity; information as expressed through numbers
quantitative: (adj) expressed as a quantity; information as expressed through numbers

 qualitative: (adj) expressed in a description; information as expressed through words (especially adjectives and adverbs)
qualitative: (adj) expressed in a description; information as expressed through words (especially adjectives and adverbs)

 What is the charge of an electron? What is an ion?
What is the charge of an electron? What is an ion?
 What is the charge of an electron? negative What is an ion? an atom with a (positive or negative) charge
What is the charge of an electron? negative What is an ion? an atom with a (positive or negative) charge
 What determines an ion’s charge? If an atom loses an electron, then it becomes less negative and has a positive charge. If an atom gains an electron, then it becomes more negative and has a negative charge.
What determines an ion’s charge? If an atom loses an electron, then it becomes less negative and has a positive charge. If an atom gains an electron, then it becomes more negative and has a negative charge.
 Visual reminder: http: //www. youtube. com/watch? v=zpa. HPXVR 8 WU
Visual reminder: http: //www. youtube. com/watch? v=zpa. HPXVR 8 WU
 MAGNETS! How’s it made, magnets http: //www. youtube. com/watch? v=no. GGcy. PHtd. I Vinegar battery: http: //www. youtube. com/watch? v=V_P 27 iln 1 Qk Solar cell: http: //www. youtube. com/watch? v=Caf 1 JIz 4 X 2 I Earth’s magnetic field: https: //www. youtube. com/watch? v=izlqo. HYZWqg Ferrofluid: http: //www. youtube. com/watch? v=1 Euy. Z 5 Lml 4 k
MAGNETS! How’s it made, magnets http: //www. youtube. com/watch? v=no. GGcy. PHtd. I Vinegar battery: http: //www. youtube. com/watch? v=V_P 27 iln 1 Qk Solar cell: http: //www. youtube. com/watch? v=Caf 1 JIz 4 X 2 I Earth’s magnetic field: https: //www. youtube. com/watch? v=izlqo. HYZWqg Ferrofluid: http: //www. youtube. com/watch? v=1 Euy. Z 5 Lml 4 k
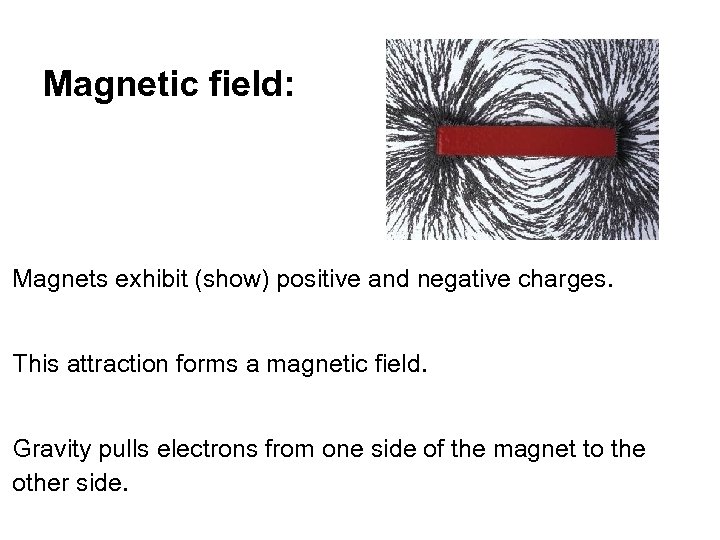 Magnetic field: Magnets exhibit (show) positive and negative charges. This attraction forms a magnetic field. Gravity pulls electrons from one side of the magnet to the other side.
Magnetic field: Magnets exhibit (show) positive and negative charges. This attraction forms a magnetic field. Gravity pulls electrons from one side of the magnet to the other side.
 Vocabulary: magnetic field: invisible force that travels from positive to negative north pole: end of magnet that is positively charged south pole: end of magnet that is negatively charged
Vocabulary: magnetic field: invisible force that travels from positive to negative north pole: end of magnet that is positively charged south pole: end of magnet that is negatively charged
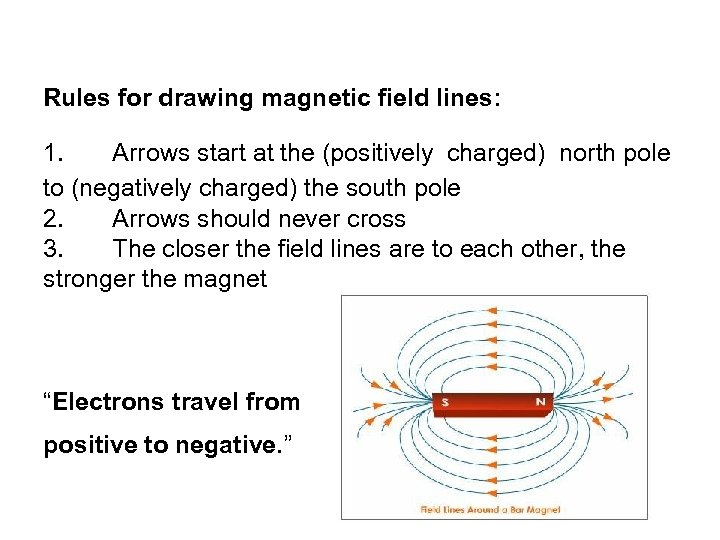 Rules for drawing magnetic field lines: 1. Arrows start at the (positively charged) north pole to (negatively charged) the south pole 2. Arrows should never cross 3. The closer the field lines are to each other, the stronger the magnet “Electrons travel from positive to negative. ”
Rules for drawing magnetic field lines: 1. Arrows start at the (positively charged) north pole to (negatively charged) the south pole 2. Arrows should never cross 3. The closer the field lines are to each other, the stronger the magnet “Electrons travel from positive to negative. ”
 Vocabulary electricity: presence and motion of charged particles electric current: flow of electrons around a closed path (electric circuit) circuit: closed path on which electrons travel
Vocabulary electricity: presence and motion of charged particles electric current: flow of electrons around a closed path (electric circuit) circuit: closed path on which electrons travel
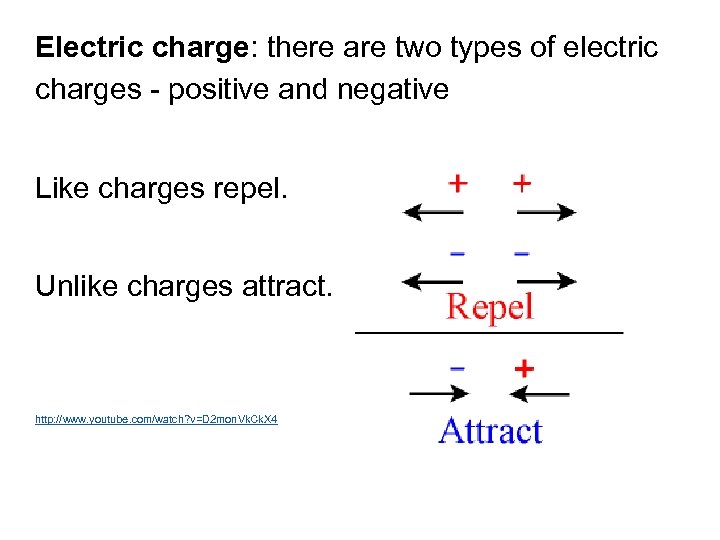 Electric charge: there are two types of electric charges - positive and negative Like charges repel. Unlike charges attract. http: //www. youtube. com/watch? v=D 2 mon. Vk. Ck. X 4
Electric charge: there are two types of electric charges - positive and negative Like charges repel. Unlike charges attract. http: //www. youtube. com/watch? v=D 2 mon. Vk. Ck. X 4
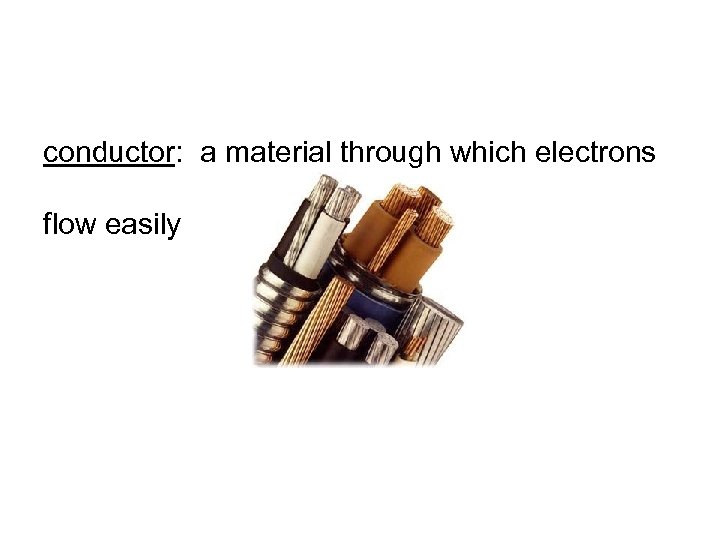 conductor: a material through which electrons flow easily
conductor: a material through which electrons flow easily
 insulator: a material that resists the flow of electrons
insulator: a material that resists the flow of electrons
 Let’s practice making more circuits!
Let’s practice making more circuits!
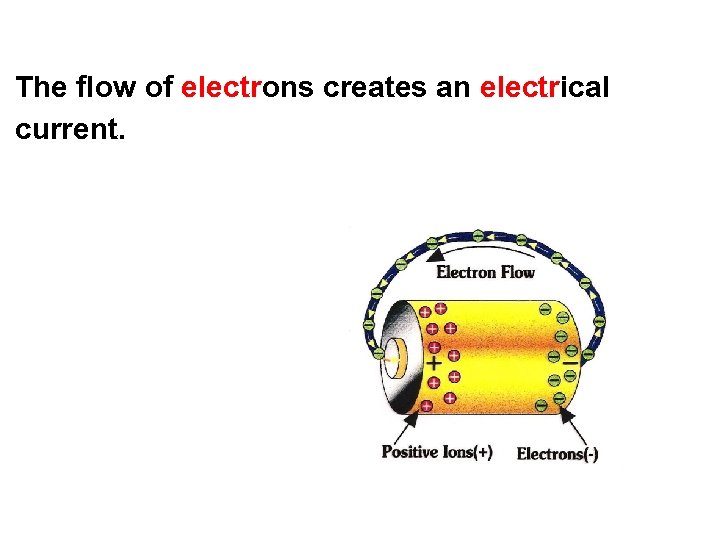 The flow of electrons creates an electrical current.
The flow of electrons creates an electrical current.
 How many circuit combinations can you make?
How many circuit combinations can you make?
 Electricity: Intro w/ vocb: http: //www. youtube. com/watch? v=EJe. Au. Q 7 pkpc
Electricity: Intro w/ vocb: http: //www. youtube. com/watch? v=EJe. Au. Q 7 pkpc
 What are the four qualities of a scientist? -creative -curious -skeptical -observant
What are the four qualities of a scientist? -creative -curious -skeptical -observant
 What are the four qualities of a scientist? -creative -curious -skeptical -observant These qualities require self-direction, a personal responsibility to learn.
What are the four qualities of a scientist? -creative -curious -skeptical -observant These qualities require self-direction, a personal responsibility to learn.
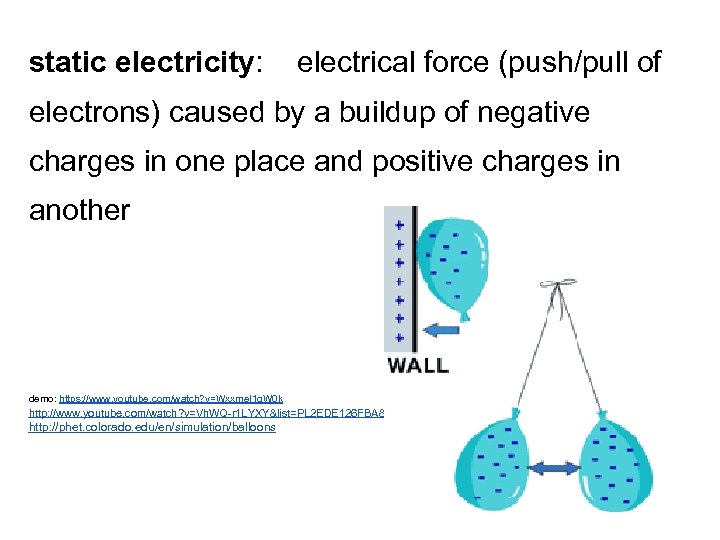 static electricity: electrical force (push/pull of electrons) caused by a buildup of negative charges in one place and positive charges in another demo: https: //www. youtube. com/watch? v=Wxxme. I 1 g. W 0 k http: //www. youtube. com/watch? v=Vh. WQ-r 1 LYXY&list=PL 2 EDE 126 FBA 8519 EF http: //phet. colorado. edu/en/simulation/balloons
static electricity: electrical force (push/pull of electrons) caused by a buildup of negative charges in one place and positive charges in another demo: https: //www. youtube. com/watch? v=Wxxme. I 1 g. W 0 k http: //www. youtube. com/watch? v=Vh. WQ-r 1 LYXY&list=PL 2 EDE 126 FBA 8519 EF http: //phet. colorado. edu/en/simulation/balloons
 Static Electricity Lab - Observations 1. Interaction between balloon and wall Provide space between numbers for qualitative and quantitative data. -For each: 3 pieces of qualitative data, 3 pieces of quantitative data 2. Interaction between balloon and hair 3. Interaction between 2 pieces of tape, not touching 4. Interaction between 2 pieces of tape, overlapping 5. Interaction between pen and paper scraps
Static Electricity Lab - Observations 1. Interaction between balloon and wall Provide space between numbers for qualitative and quantitative data. -For each: 3 pieces of qualitative data, 3 pieces of quantitative data 2. Interaction between balloon and hair 3. Interaction between 2 pieces of tape, not touching 4. Interaction between 2 pieces of tape, overlapping 5. Interaction between pen and paper scraps


